Targeting PIM Kinases to Improve the Efficacy of Immunotherapy
Abstract
1. Introduction
2. Anti-Tumor Immune Responses
3. PIM in Immune Cells
3.1. B Cells
3.2. T Cells
3.3. Hematopoesis
4. PIM and Inflammation
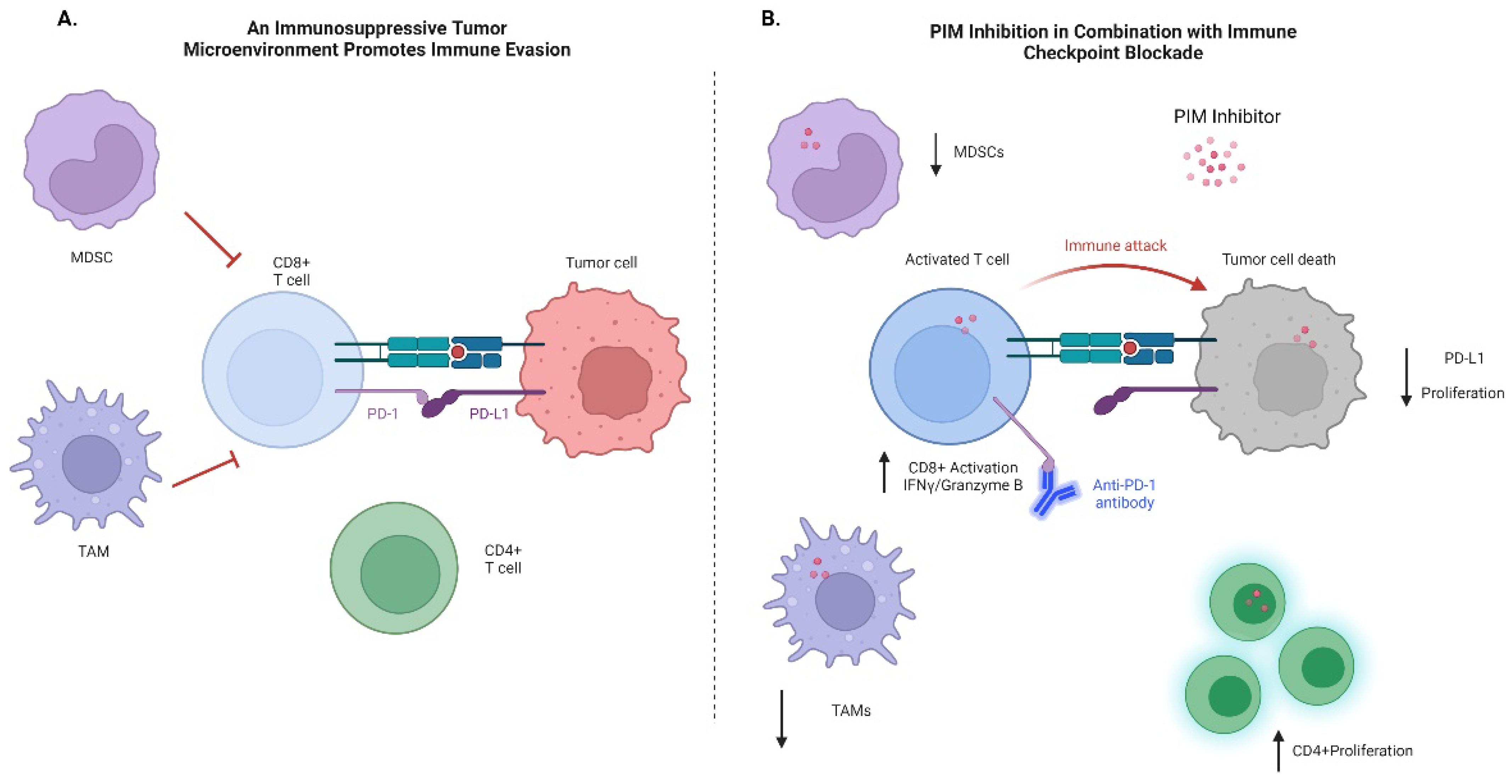
5. PIM and Immune Evasion
6. Immune Checkpoint Therapy
7. PIM Inhibitors in Combination with Immunotherapy
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santio, N.M.; Koskinen, P.J. PIM kinases: From survival factors to regulators of cell motility. Int. J. Biochem. Cell Biol. 2017, 93, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, A.; Yuan, L.; Bréant, C.; Alitalo, K.; Koskinen, P.J. Developmental expression of Pim kinases suggests functions also outside of the hematopoietic system. Oncogene 2000, 19, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Mikkers, H.; Nawijn, M.; Allen, J.; Brouwers, C.; Verhoeven, E.; Jonkers, J.; Berns, A. Mice Deficient for All PIM Kinases Display Reduced Body Size and Impaired Responses to Hematopoietic Growth Factors. Mol. Cell. Biol. 2004, 24, 6104–6115. [Google Scholar] [CrossRef]
- Qian, K.C.; Studts, J.; Wang, L.; Barringer, K.; Kronkaitis, A.; Peng, C.; Baptiste, A.; Lafrance, R.; Mische, S.; Farmer, B. Expression, purification, crystallization and preliminary crystallographic analysis of human Pim-1 kinase. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2004, 61, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Wernig, G.; Gonneville, J.R.; Crowley, B.J.; Rodrigues, M.S.; Reddy, M.M.; Hudon, H.E.; Walz, C.; Reiter, A.; Podar, K.; Royer, Y.; et al. The Jak2V617F oncogene associated with myeloproliferative diseases requires a functional FERM domain for transformation and for expression of the Myc and Pim proto-oncogenes. Blood 2008, 111, 3751–3759. [Google Scholar] [CrossRef]
- Shirogane, T.; Fukada, T.; Muller, J.M.; Shima, D.T.; Hibi, M.; Hirano, T. Synergistic Roles for Pim-1 and c-Myc in STAT3-Mediated Cell Cycle Progression and Antiapoptosis. Immunity 1999, 11, 709–719. [Google Scholar] [CrossRef]
- Chen, X.P.; Losman, J.A.; Cowan, S.; Donahue, E.; Fay, S.; Vuong, B.Q.; Nawijn, M.C.; Capece, D.; Cohan, V.L.; Rothman, P. Pim serine/threonine kinases regulate the stability of Socs-1 protein. Proc. Natl. Acad. Sci. USA 2002, 99, 2175–2180. [Google Scholar] [CrossRef]
- Jackson, L.J.; Pheneger, J.A.; Pheneger, T.J.; Davis, G.; Wright, A.D.; Robinson, J.E.; Allen, S.; Munson, M.C.; Carter, L.L. The role of PIM kinases in human and mouse CD4+ T cell activation and inflammatory bowel disease. Cell. Immunol. 2012, 272, 200–213. [Google Scholar] [CrossRef]
- Zhu, N.; Ramirez, L.M.; Lee, R.L.; Magnuson, N.S.; Bishop, G.A.; Gold, M.R. CD40 Signaling in B Cells Regulates the Expression of the Pim-1 Kinase Via the NF-κappa B Pathway. J. Immunol. 2002, 168, 744–754. [Google Scholar] [CrossRef]
- Toth, R.K.; Solomon, R.; Warfel, N.A. Stabilization of PIM Kinases in Hypoxia Is Mediated by the Deubiquitinase USP. Cells 2022, 11, 1006. [Google Scholar] [CrossRef]
- Warfel, N.A.; Sainz, A.G.; Song, J.H.; Kraft, A.S. PIM Kinase Inhibitors Kill Hypoxic Tumor Cells by Reducing Nrf2 Signaling and Increasing Reactive Oxygen Species. Mol. Cancer Ther. 2016, 15, 1637–1647. [Google Scholar] [CrossRef]
- Cohen, A.M.; Grinblat, B.; Bessler, H.; Kristt, D.A.; Kremer, A.; Shalom, S.; Schwartz, A.; Halperin, M.; Merkel, D.; Don, J. Increased Expression of the hPim-2 Gene In Human Chronic lymphocytic Leukemia and Non-Hodgkin Lymphoma. Leuk. Lymphoma 2004, 45, 951–955. [Google Scholar] [CrossRef]
- Hüttmann, A.; Klein-Hitpass, L.; Thomale, J.; Deenen, R.; Carpinteiro, A.; Nückel, H.; Ebeling, P.; Führer, A.; Edelmann, J.; Sellmann, L.; et al. Gene expression signatures separate B-cell chronic lymphocytic leukaemia prognostic subgroups defined by ZAP-70 and CD38 expression status. Leukemia 2006, 20, 1774–1782. [Google Scholar] [CrossRef]
- Weirauch, U.; Beckmann, N.; Thomas, M.; Grünweller, A.; Huber, K.; Bracher, F.; Hartmann, R.K.; Aigner, A. Functional Role and Therapeutic Potential of the Pim-1 Kinase in Colon Carcinoma. Neoplasia 2013, 15, 783–794. [Google Scholar] [CrossRef]
- Cibull, T.L.; Jones, T.D.; Li, L.; Eble, J.N.; Baldridge, L.A.; Malott, S.R.; Luo, Y.; Cheng, L. Overexpression of Pim-1 during progression of prostatic adenocarcinoma. J. Clin. Pathol. 2006, 59, 285–288. [Google Scholar] [CrossRef]
- Brasó-Maristany, F.; Filosto, S.; Catchpole, S.; Marlow, R.; Quist, J.; Francesch-Domenech, E.; Plumb, D.A.; Zakka, L.; Gazinska, P.; Liccardi, G.; et al. PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Nat. Med. 2016, 22, 1303–1313. [Google Scholar] [CrossRef]
- Brunen, D.; de Vries, R.C.; Lieftink, C.; Beijersbergen, R.L.; Bernards, R. PIM Kinases Are a Potential Prognostic Biomarker and Therapeutic Target in Neuroblastoma. Mol. Cancer Ther. 2018, 17, 849–857. [Google Scholar] [CrossRef]
- Xu, J.; Xiong, G.; Cao, Z.; Huang, H.; Wang, T.; You, L.; Zhou, L.; Zheng, L.; Hu, Y.; Zhang, T.; et al. PIM-1 contributes to the malignancy of pancreatic cancer and displays diagnostic and prognostic value. J. Exp. Clin. Cancer Res. 2016, 35, 133. [Google Scholar] [CrossRef]
- Mumenthaler, S.M.; Ng, P.Y.; Hodge, A.; Bearss, D.; Berk, G.; Kanekal, S.; Redkar, S.; Taverna, P.; Agus, D.B.; Jain, A. Pharmacologic inhibition of Pim kinases alters prostate cancer cell growth and resensitizes chemoresistant cells to taxanes. Mol. Cancer Ther. 2009, 8, 2882–2893. [Google Scholar] [CrossRef]
- Kim, W.; Youn, H.; Seong, K.M.; Yang, H.J.; Yun, Y.J.; Kwon, T.; Kim, Y.H.; Lee, J.Y.; Jin, Y.-W.; Youn, B. PIM1-Activated PRAS40 Regulates Radioresistance in Non-small Cell Lung Cancer Cells through Interplay with FOXO3a, 14-3-3 and Protein Phosphatases. Radiat. Res. 2011, 176, 539–552. [Google Scholar] [CrossRef]
- Casillas, A.L.; Toth, R.K.; Sainz, A.G.; Singh, N.; Desai, A.A.; Kraft, A.S.; Warfel, N.A. Hypoxia-Inducible PIM Kinase Expression Promotes Resistance to Antiangiogenic Agents. Clin. Cancer Res. 2018, 24, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Toth, R.K.; Warfel, N.A. Targeting PIM Kinases to Overcome Therapeutic Resistance in Cancer. Mol. Cancer Ther. 2021, 20, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. PIM kinase inhibitors: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2019, 172, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Alnabulsi, S.; Al-Hurani, E.A. Pim kinase inhibitors in cancer: Medicinal chemistry insights into their activity and selectivity. Drug Discov. Today 2020, 25, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Yuen, G.J.; Demissie, E.; Pillai, S. B Lymphocytes and Cancer: A Love–Hate Relationship. Trends Cancer 2016, 2, 747–757. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Gubin, M.M.; Vesely, M.D. Cancer Immunoediting in the Era of Immuno-oncology. Clin. Cancer Res. 2022, 28, 3917–3928. [Google Scholar] [CrossRef]
- An, N.; Kraft, A.S.; Kang, Y. Abnormal hematopoietic phenotypes in Pim kinase triple knockout mice. J. Hematol. Oncol. 2013, 6, 12. [Google Scholar] [CrossRef]
- Van Lohuizen, M.; Verbeek, S.; Krimpenfort, P.; Domen, J.; Saris, C.; Radaszkiewicz, T.; Berns, A. Predisposition to lymphomagenesis in pim-1 transgenic mice: Cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell 1989, 56, 673–682. [Google Scholar] [CrossRef]
- Verbeek, S.; van Lohuizen, M.; van der Valk, M.; Domen, J.; Kraal, G.; Berns, A. Mice bearing the E mu-myc and E mu-pim-1 transgenes develop pre-B-cell leukemia prenatally. Mol. Cell. Biol. 1991, 11, 1176–1179. [Google Scholar] [CrossRef]
- Bouquet, C.; Melchers, F. Pim1 and Myc reversibly transform murine precursor B lymphocytes but not mature B lymphocytes. Eur. J. Immunol. 2012, 42, 522–532. [Google Scholar] [CrossRef]
- Brault, L.; Menter, T.; Obermann, E.C.; Knapp, S.; Thommen, S.; Schwaller, J.; Tzankov, A. PIM kinases are progression markers and emerging therapeutic targets in diffuse large B-cell lymphoma. Br. J. Cancer 2012, 107, 491–500. [Google Scholar] [CrossRef]
- Mondello, P.; Tarantelli, C.; Cascione, L.; Arribas, A.; Rinaldi, A.; Younes, A.; Bertoni, F. Inhibition of PIM Kinases Targets Synthetic Vulnerabilities and Enhances Antigen Presentation in B-Cell Lymphoma. Blood 2019, 134, 2858. [Google Scholar] [CrossRef]
- Szydłowski, M.; Garbicz, F.; Jabłońska, E.; Górniak, P.; Komar, D.; Pyrzyńska, B.; Bojarczuk, K.; Prochorec-Sobieszek, M.; Szumera-Ciećkiewicz, A.; Rymkiewicz, G.; et al. Inhibition of PIM Kinases in DLBCL Targets MYC Transcriptional Program and Augments the Efficacy of Anti-CD20 Antibodies. Cancer Res. 2021, 81, 6029–6043. [Google Scholar] [CrossRef]
- Fox, C.J.; Hammerman, P.S.; Thompson, C.B. The Pim kinases control rapamycin-resistant T cell survival and activation. J. Exp. Med. 2005, 201, 259–266. [Google Scholar] [CrossRef]
- Peperzak, V.; Veraar, E.A.M.; Keller, A.M.; Xiao, Y.; Borst, J. The Pim Kinase Pathway Contributes to Survival Signaling in Primed CD8+ T Cells upon CD27 Costimulation. J. Immunol. 2010, 185, 6670–6678. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Beharry, Z.; Hill, E.G.; Song, J.; Wang, W.; Xia, Z.; Zhang, Z.; Aplan, P.D.; Aster, J.C.; Smith, C.D.; et al. A small molecule inhibitor of Pim protein kinases blocks the growth of precursor T-cell lymphoblastic leukemia/lymphoma. Blood 2010, 115, 824–833. [Google Scholar] [CrossRef]
- Padi, S.K.; Luevano, L.A.; An, N.; Pandey, R.; Singh, N.; Song, J.H.; Aster, J.C.; Yu, X.-Z.; Mehrotra, S.; Kraft, A.S. Targeting the PIM protein kinases for the treatment of a T-cell acute lymphoblastic leukemia subset. Oncotarget 2017, 8, 30199–30216. [Google Scholar] [CrossRef]
- La Starza, R.; Messina, M.; Gianfelici, V.; Pierini, V.; Matteucci, C.; Pierini, T.; Limongi, M.Z.; Vitale, A.; Roti, G.; Chiaretti, S.; et al. High PIM1 expression is a biomarker of T-cell acute lymphoblastic leukemia with JAK/STAT activation or t(6;7)(p21;q34)/TRB@-PIM1 rearrangement. Leukemia 2018, 32, 1807–1810. [Google Scholar] [CrossRef]
- Lim, J.T.; Singh, N.; Leuvano, L.A.; Calvert, V.S.; Petricoin, E.F.; Teachey, D.T.; Lock, R.B.; Padi, M.; Kraft, A.S.; Padi, S.K.R. PIM Kinase Inhibitors Block the Growth of Primary T-cell Acute Lymphoblastic Leukemia: Resistance Pathways Identified by Network Modeling Analysis. Mol. Cancer Ther. 2020, 19, 1809–1821. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, R.; Morscio, J.; Reunes, L.; Roels, J.; Bardelli, V.; Lintermans, B.; van Loocke, W.; Almeida, A.; Cheung, L.C.; Kotecha, R.S.; et al. Targeting cytokine- and therapy-induced PIM1 activation in preclinical models of T-cell acute lymphoblastic leukemia and lymphoma. Blood 2020, 135, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy—New insights into old paradigms. Cancer Gene Ther. 2021, 28, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Daenthanasanmak, A.; Wu, Y.; Iamsawat, S.; Nguyen, H.D.; Bastian, D.; Zhang, M.; Sofi, M.H.; Chatterjee, S.; Hill, E.G.; Mehrotra, S.; et al. PIM-2 protein kinase negatively regulates T cell responses in transplantation and tumor immunity. J. Clin. Investig. 2018, 128, 2787–2801. [Google Scholar] [CrossRef] [PubMed]
- Aho, T.L.T.; Lund, R.J.; Ylikoski, E.K.; Matikainen, S.; Lahesmaa, R.; Koskinen, P. Expression of human pim family genes is selectively up-regulated by cytokines promoting T helper type 1, but not T helper type 2, cell differentiation. Immunology 2005, 116, 82–88. [Google Scholar] [CrossRef]
- Tahvanainen, J.; Kyläniemi, M.; Kanduri, K.; Gupta, B.; Lähteenmäki, H.; Kallonen, T.; Rajavuori, A.; Rasool, O.; Koskinen, P.; Rao, K.V.S.; et al. Proviral Integration Site for Moloney Murine Leukemia Virus (PIM) Kinases Promote Human T Helper 1 Cell Differentiation. J. Biol. Chem. 2013, 288, 3048–3058. [Google Scholar] [CrossRef]
- An, N.; Lin, Y.-W.; Mahajan, S.; Kellner, J.N.; Wang, Y.; Li, Z.; Kraft, A.S.; Kang, Y. Pim1 Serine/Threonine Kinase Regulates the Number and Functions of Murine Hematopoietic Stem Cells. Stem Cells 2013, 31, 1202–1212. [Google Scholar] [CrossRef]
- Shen, Y.-M.; Zhao, Y.; Zeng, Y.; Yan, L.; Chen, B.-L.; Leng, A.-M.; Mu, Y.-B.; Zhang, G.-Y. Inhibition of Pim-1 Kinase Ameliorates Dextran Sodium Sulfate-Induced Colitis in Mice. Am. J. Dig. Dis. 2012, 57, 1822–1831. [Google Scholar] [CrossRef]
- Li, Z.; Lin, F.; Zhuo, C.; Deng, G.; Chen, Z.; Yin, S.; Gao, Z.; Piccioni, M.; Tsun, A.; Cai, S.; et al. PIM1 Kinase Phosphorylates the Human Transcription Factor FOXP3 at Serine 422 to Negatively Regulate Its Activity under Inflammation. J. Biol. Chem. 2014, 289, 26872–26881. [Google Scholar] [CrossRef]
- Deng, G.; Nagai, Y.; Xiao, Y.; Li, Z.; Dai, S.; Ohtani, T.; Banham, A.; Li, B.; Wu, S.-L.; Hancock, W.; et al. Pim-2 Kinase Influences Regulatory T Cell Function and Stability by Mediating Foxp3 Protein N-terminal Phosphorylation. J. Biol. Chem. 2015, 290, 20211–20220. [Google Scholar] [CrossRef]
- Nihira, K.; Ando, Y.; Yamaguchi, T.; Kagami, Y.; Miki, Y.; Yoshida, K. Pim-1 controls NF-κappa B signalling by stabilizing RelA/p65. Cell Death Differ. 2010, 17, 689–698. [Google Scholar] [CrossRef]
- Jiménez-García, M.-P.; Lucena-Cacace, A.; Frías, M.-J.R.; Ferrer, I.; Narlik-Grassow, M.; Blanco-Aparicio, C.; Carnero, A. Inflammation and stem markers association to PIM1/PIM2 kinase-induced tumors in breast and uterus. Oncotarget 2017, 8, 58872–58886. [Google Scholar] [CrossRef]
- Jiménez-García, M.P.; Lucena-Cacace, A.; Robles-Frías, M.J.; Narlik-Grassow, M.; Blanco-Aparicio, C.; Carnero, A. The role of PIM1/PIM2 kinases in tumors of the male reproductive system. Sci. Rep. 2016, 6, 38079. [Google Scholar] [CrossRef]
- Szydłowski, M.; Prochorec-Sobieszek, M.; Szumera-Ciećkiewicz, A.; Derezińska, E.; Hoser, G.; Wasilewska, D.; Szymańska-Giemza, O.; Jabłońska, E.; Białopiotrowicz, E.; Sewastianik, T.; et al. Expression of PIM kinases in Reed-Sternberg cells fosters immune privilege and tumor cell survival in Hodgkin lymphoma. Blood 2017, 130, 1418–1429. [Google Scholar] [CrossRef]
- Szydłowski, M.; Dębek, S.; Prochorec-Sobieszek, M.; Szołkowska, M.; Tomirotti, A.M.; Juszczyński, P.; Szumera-Ciećkiewicz, A. PIM Kinases Promote Survival and Immune Escape in Primary Mediastinal Large B-Cell Lymphoma through Modulation of JAK-STAT and NF-κB Activity. Am. J. Pathol. 2021, 191, 567–574. [Google Scholar] [CrossRef]
- Yang, T.; Ren, C.; Lu, C.; Qiao, P.; Han, X.; Wang, L.; Wang, D.; Lv, S.; Sun, Y.; Yu, Z. Phosphorylation of HSF1 by PIM2 Induces PD-L1 Expression and Promotes Tumor Growth in Breast Cancer. Cancer Res. 2019, 79, 5233–5244. [Google Scholar] [CrossRef]
- Guo, Q.; Lan, P.; Yu, X.; Han, Q.; Zhang, J.; Tian, Z.; Zhang, C. Immunotherapy for Hepatoma Using a Dual-Function Vector with Both Immunostimulatory and Pim-3–Silencing Effects. Mol. Cancer Ther. 2014, 13, 1503–1513. [Google Scholar] [CrossRef]
- Walunas, T.L.; Lenschow, D.J.; Bakker, C.Y.; Linsley, P.S.; Freeman, G.J.; Green, J.M.; Thompson, C.B.; Bluestone, J.A. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994, 1, 405–413. [Google Scholar] [CrossRef]
- Agata, Y.; Kawasaki, A.; Nishimura, H.; Ishida, Y.; Tsubat, T.; Yagita, H.; Honjo, T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996, 8, 765–772. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Sinha, P.; Clements, V.K.; Rodriguez, P.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells Inhibit T-Cell Activation by Depleting Cystine and Cysteine. Cancer Res 2010, 70, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Law, A.M.K.; Valdes-Mora, F.; Gallego-Ortega, D. Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer. Cells 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 Control over Foxp3+ Regulatory T Cell Function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies with Curative Potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. New Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Koblish, H.; Li, Y.-L.; Shin, N.; Hall, L.; Wang, Q.; Wang, K.; Covington, M.; Marando, C.; Bowman, K.; Boer, J.; et al. Preclinical characterization of INCB053914, a novel pan-PIM kinase inhibitor, alone and in combination with anticancer agents, in models of hematologic malignancies. PLoS ONE 2018, 13, e0199108. [Google Scholar] [CrossRef]
- Cortes, J.; Tamura, K.; DeAngelo, D.; de Bono, J.; Lorente, D.; Minden, M.; Uy, G.L.; Kantarjian, H.; Chen, L.S.; Gandhi, V.; et al. Phase I studies of AZD1208, a proviral integration Moloney virus kinase inhibitor in solid and haematological cancers. Br. J. Cancer 2018, 118, 1425–1433. [Google Scholar] [CrossRef]
- Raab, M.S.; Thomas, S.K.; Ocio, E.M.; Guenther, A.; Goh, Y.-T.; Talpaz, M.; Hohmann, N.; Zhao, S.; Xiang, F.; Simon, C.; et al. The first-in-human study of the pan-PIM kinase inhibitor PIM447 in patients with relapsed and/or refractory multiple myeloma. Leukemia 2019, 33, 2924–2933. [Google Scholar] [CrossRef]
- Malone, T.; Schäfer, L.; Simon, N.; Heavey, S.; Cuffe, S.; Finn, S.; Moore, G.; Gately, K. Current perspectives on targeting PIM kinases to overcome mechanisms of drug resistance and immune evasion in cancer. Pharmacol. Ther. 2020, 207, 107454. [Google Scholar] [CrossRef]
- Luszczak, S.; Kumar, C.; Sathyadevan, V.K.; Simpson, B.S.; Gately, K.; Whitaker, H.C.; Heavey, S. PIM kinase inhibition: Co-targeted therapeutic approaches in prostate cancer. Signal Transduct. Target. Ther. 2020, 5, 7–10. [Google Scholar] [CrossRef]
- Sugiura, A.; Rathmell, J.C. Metabolic Barriers to T Cell Function in Tumors. J. Immunol. 2018, 200, 400–407. [Google Scholar] [CrossRef]
- Sukumar, M.; Liu, J.; Ji, Y.; Subramanian, M.; Crompton, J.G.; Yu, Z.; Roychoudhuri, R.; Palmer, D.C.; Muranski, P.; Karoly, E.D.; et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Investig. 2013, 123, 4479–4488. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chakraborty, P.; Daenthanasanmak, A.; Iamsawat, S.; Andrejeva, G.; Luevano, L.A.; Wolf, M.M.; Baliga, U.K.; Krieg, C.; Beeson, C.C.; et al. Targeting PIM Kinase with PD1 Inhibition Improves Immunotherapeutic Antitumor T-cell Response. Clin. Cancer Res. 2019, 25, 1036–1049. [Google Scholar] [CrossRef]
- Xin, G.; Chen, Y.; Topchyan, P.; Kasmani, M.Y.; Burns, R.; Volberding, P.J.; Wu, X.; Cohn, A.; Lin, C.W.; Ho, P.C.; et al. Targeting PIM1-Mediated Metabolism in Myeloid Suppressor Cells to Treat Cancer. Cancer Immunol. Res. 2021, 9, 454–469. [Google Scholar] [CrossRef]
- Wang, J.-C.; Chen, D.-P.; Lu, S.-X.; Chen, J.-B.; Wei, Y.; Liu, X.-C.; Tang, Y.-H.; Zhang, R.; Chen, J.-C.; Kan, A.; et al. PIM2 expression induced by proinflammatory macrophages suppresses immunotherapy efficacy in hepatocellular carcinoma. Cancer Res. 2022, 82, 3307–3320. [Google Scholar] [CrossRef]
| Isoform | Tissue Distribution | Isoform Specific Inhibitor | Pan-PIM Inhibitor | |
|---|---|---|---|---|
| PIM Kinases | PIM1 | Hematopoietic cells, gastric, head and neck, and prostate tumors | SGI-1776 TP-3654 SMI-4a | AZD1208 PIM447 (LGH447) INCB053914 SEL24/MEN1703 * CXR1002 CX-6258 DHPCC-9 GDC-0339 LGB321 AUM302 (IBL-302) * |
| PIM2 | Lymphoid Brain | SGI-1776 | ||
| PIM3 | Breast Kidney Brain | M-110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clements, A.N.; Warfel, N.A. Targeting PIM Kinases to Improve the Efficacy of Immunotherapy. Cells 2022, 11, 3700. https://doi.org/10.3390/cells11223700
Clements AN, Warfel NA. Targeting PIM Kinases to Improve the Efficacy of Immunotherapy. Cells. 2022; 11(22):3700. https://doi.org/10.3390/cells11223700
Chicago/Turabian StyleClements, Amber N., and Noel A. Warfel. 2022. "Targeting PIM Kinases to Improve the Efficacy of Immunotherapy" Cells 11, no. 22: 3700. https://doi.org/10.3390/cells11223700
APA StyleClements, A. N., & Warfel, N. A. (2022). Targeting PIM Kinases to Improve the Efficacy of Immunotherapy. Cells, 11(22), 3700. https://doi.org/10.3390/cells11223700






