Oxidative Stress and Oocyte Cryopreservation: Recent Advances in Mitigation Strategies Involving Antioxidants
Abstract
1. Introduction
2. ROS and Oxidative Stress
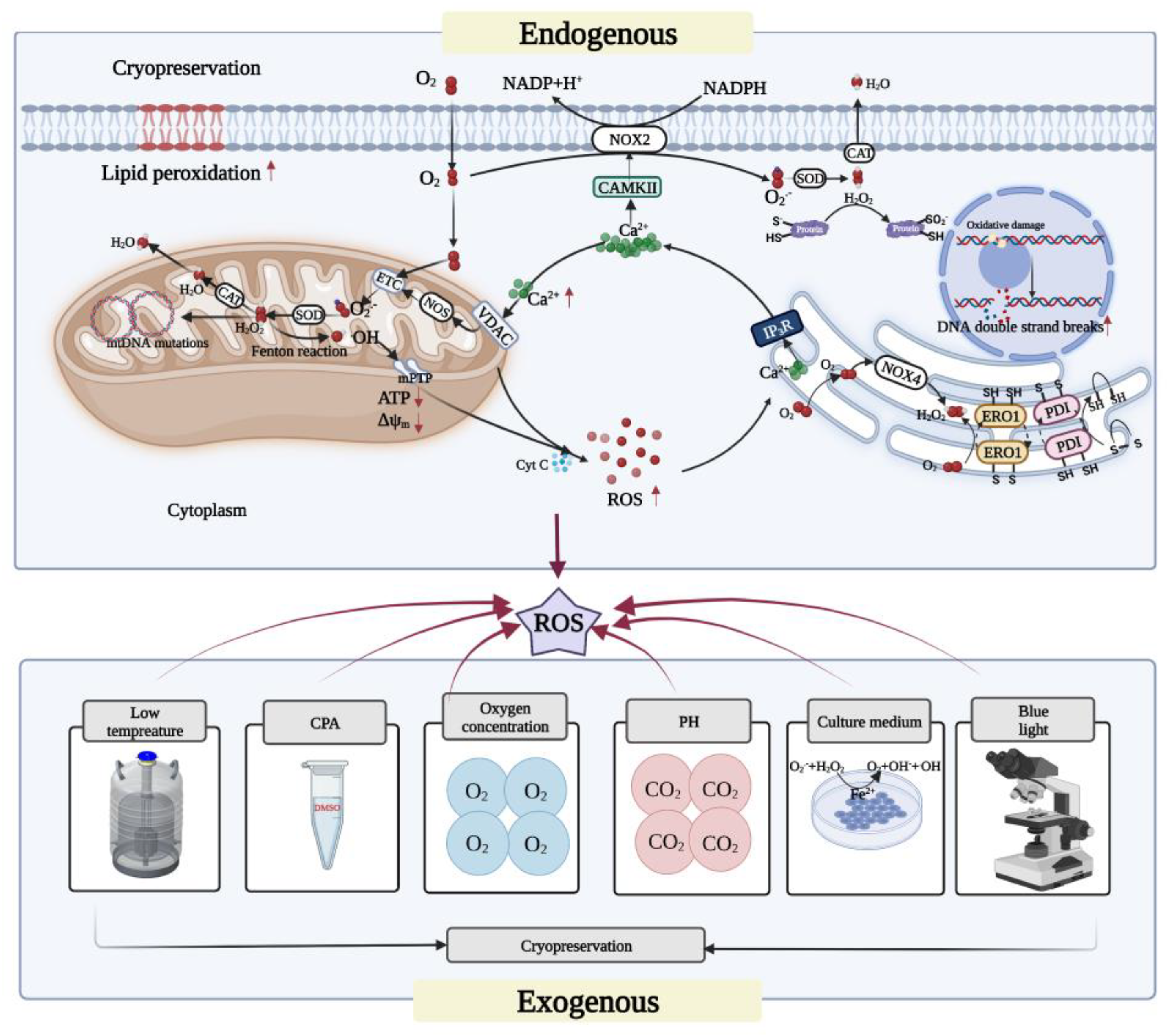
3. Sources of ROS in Oocyte Cryopreservation
4. Cryopreservation-Induced Oxidative Stress in Oocytes
4.1. Mitochondrial Oxidative Stress
4.2. Endoplasmic-Reticulum-Related Oxidative Stress
5. Antioxidant Mechanisms in Oocytes
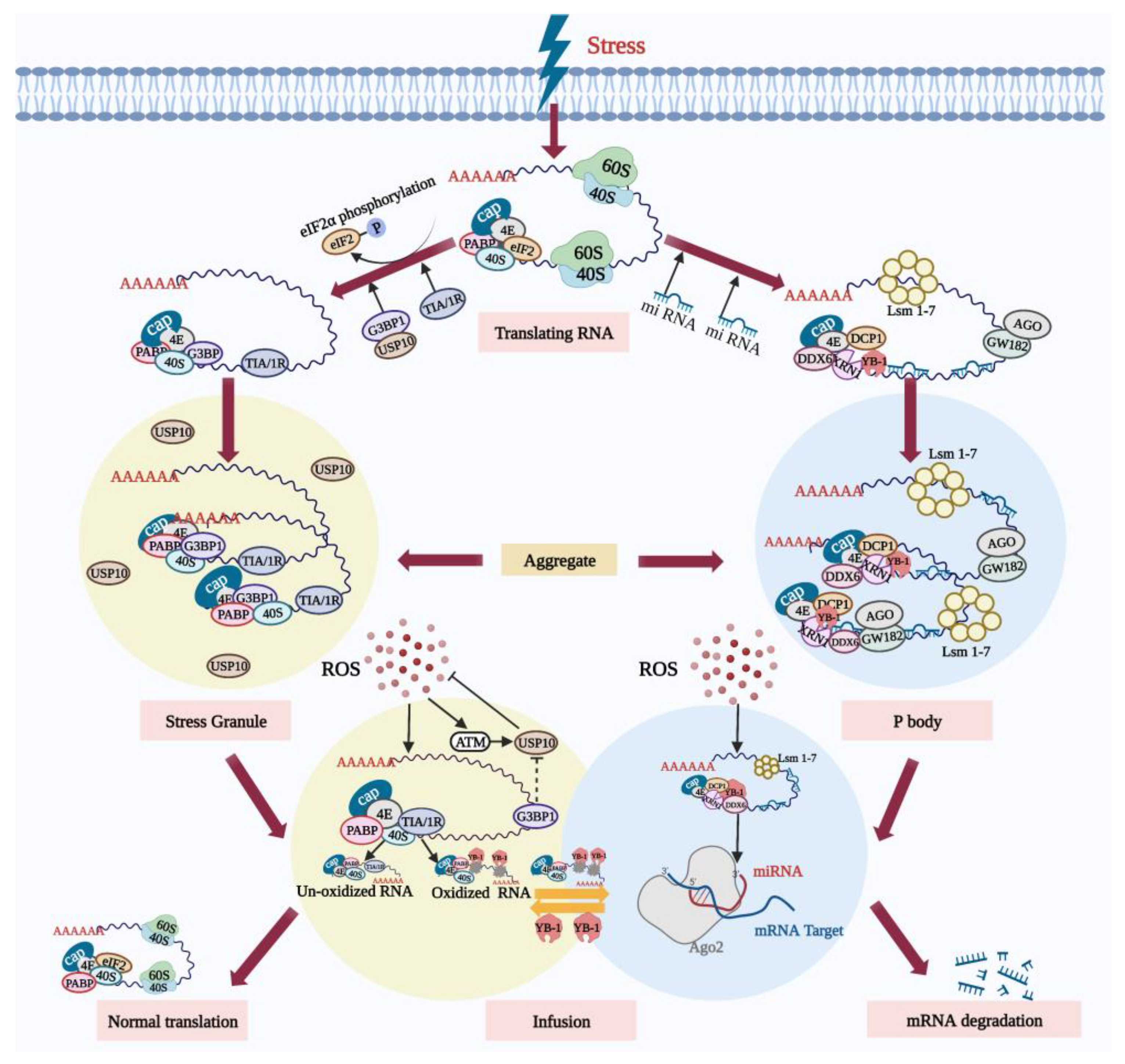
5.1. Ribonucleoprotein Particles Can Reduce Oxidative Damage and Protect Cells
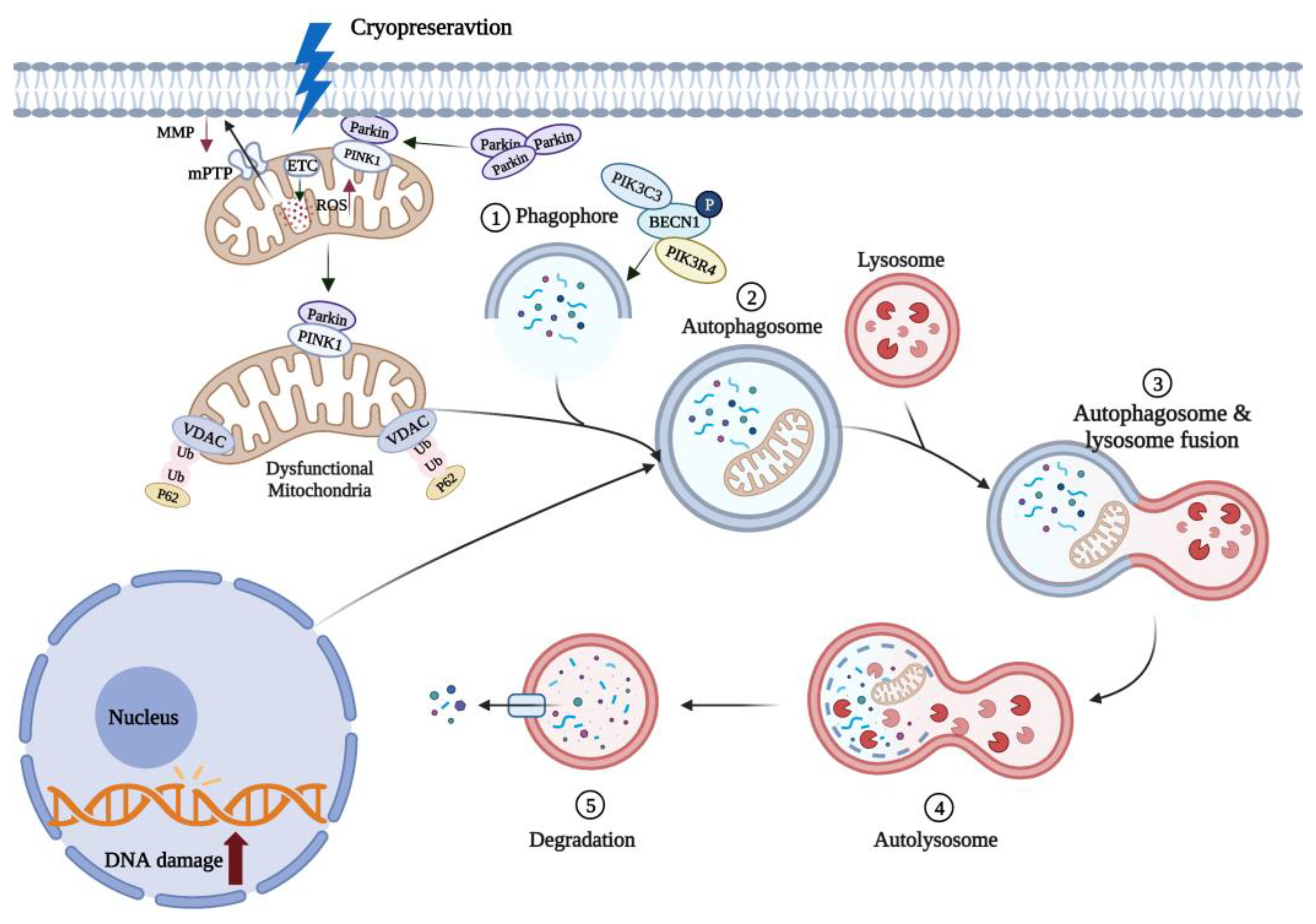
5.2. Mitophagy Can Reduce Oocyte Apoptosis Induced by Oxidative Stress
6. Oocyte Cryopreservation and Antioxidants
6.1. Endogenous Antioxidants Resist Oxidative Damage Induced by Oocyte Cryopreservation
6.2. Exogenous Non-Enzymatic Antioxidants Protect Oocytes from Oxidative Damage Induced by Cryopreservation
7. Mechanism of Exogenous Non-Enzymatic Antioxidants and Their Implication in Reducing Oxidative Damage Following Oocyte Cryopreservation
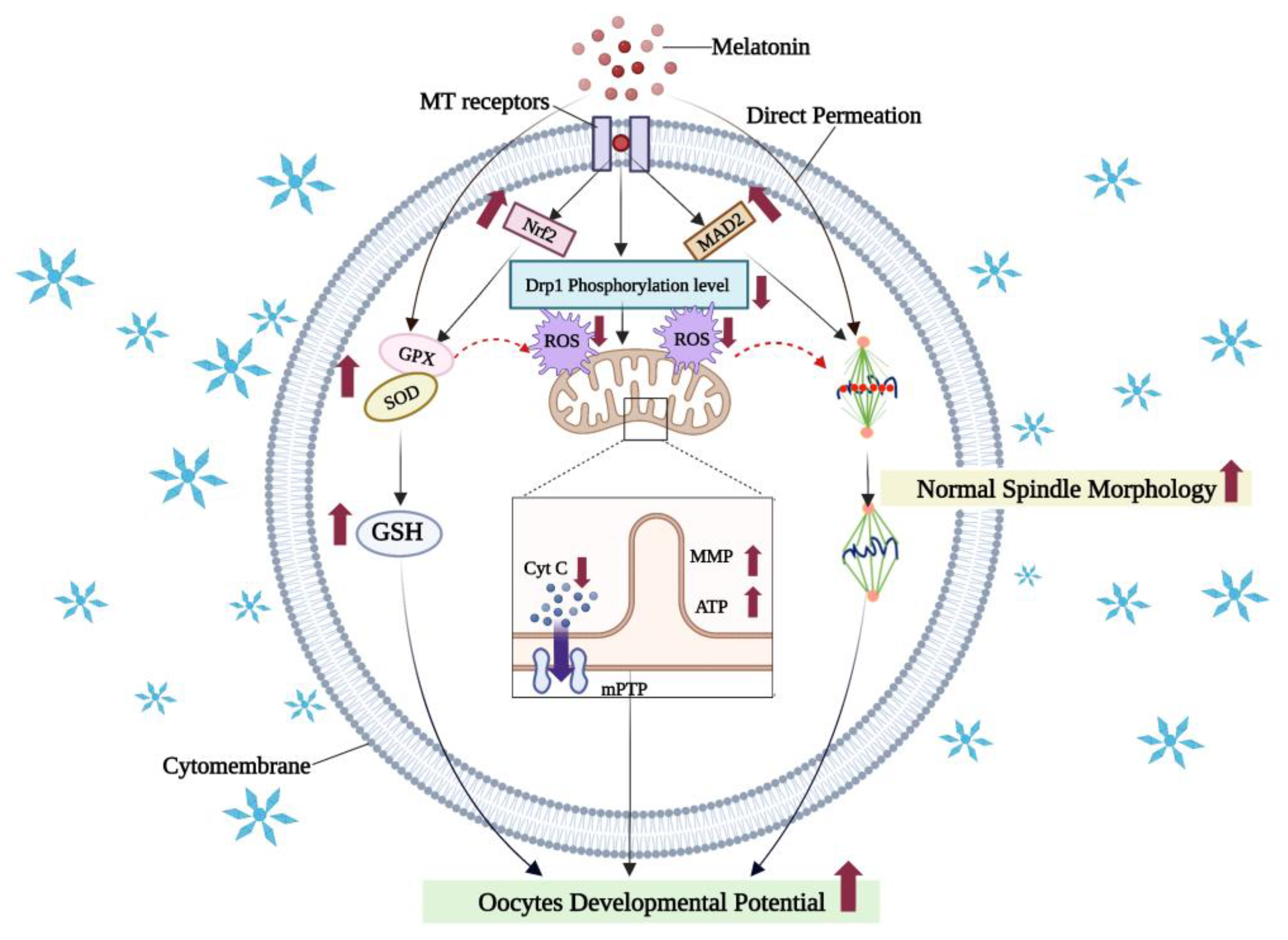
7.1. Melatonin Reduces Oxidative Damage in Vitrified-Warmed Oocytes by Activating Antioxidant Signaling Pathways and Maintaining Organelle Morphology
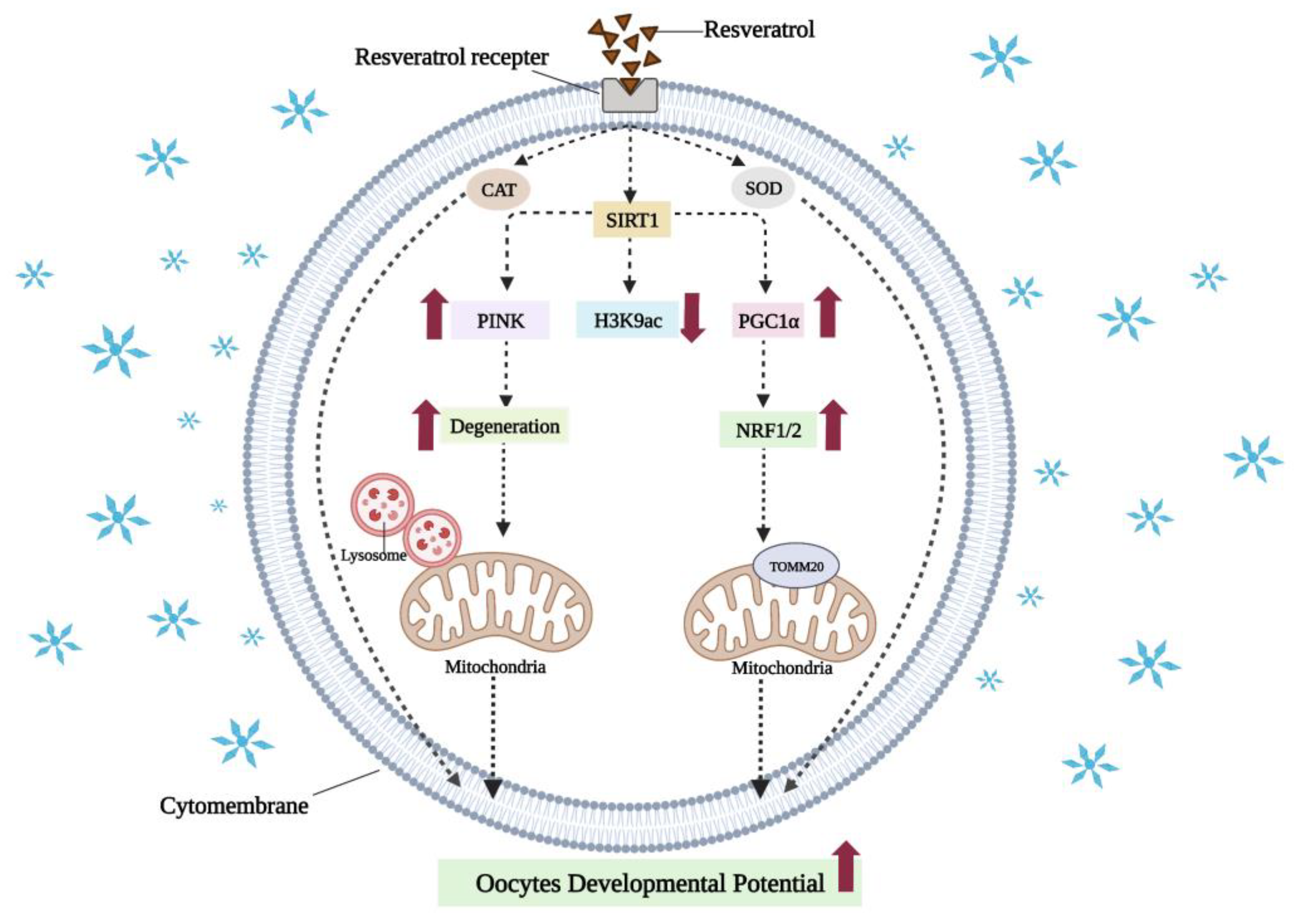
7.2. Resveratrol Can Reduce Oxidative Damage in Vitrified-Warmed Oocytes by Activating SIRT1 to Mediate Mitochondrial Function
7.3. Other Antioxidants Can Also Eliminate Reactive Oxygen Species to Reduce Oxidative Damage Directly or Iindirectly in Vitrified-Warmed Oocytes
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polge, C.; Smith, A.U.; Parkes, A.S. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 1949, 164, 666. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, D.G.; Leibo, S.P.; Mazur, P. Survival of mouse embryos frozen to −196° and −269 °C. Science 1972, 178, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, D.G. Fertilization in vitro and development to term of unfertilized mouse oocytes previously stored at—96 °C. J. Reprod. Fertil. 1977, 49, 89–94. [Google Scholar] [CrossRef]
- Rall, W.F.; Fahy, G.M. Ice-free cryopreservation of mouse embryos at −196 °C by vitrification. Nature 1985, 313, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Kasai, M.; Iritani, A.; Chang, M.C. Fertilization in vitro of rat ovarian oocytes after freezing and thawing. Biol. Reprod. 1979, 21, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Siebzehnruebl, E.R.; Todorow, S.; van Uem, J.; Koch, R.; Wildt, L.; Lang, N. Cryopreservation of human and rabbit oocytes and one-cell embryos: A comparison of DMSO and propanediol. Hum. Reprod. 1989, 4, 312–317. [Google Scholar] [CrossRef]
- DeMayo, F.J.; Rawlins, R.G.; Dukelow, W.R. Xenogenous and in vitro fertilization of frozen/thawed primate oocytes and blastomere separation of embryos. Fertil. Steril. 1985, 43, 295–300. [Google Scholar] [CrossRef]
- Chen, C. Pregnancy after human oocyte cryopreservation. Lancet 1986, 1, 884–886. [Google Scholar] [CrossRef]
- Argyle, C.E.; Harper, J.C.; Davies, M.C. Oocyte cryopreservation: Where are we now? Hum. Reprod. Update 2016, 22, 440–449. [Google Scholar] [CrossRef]
- Abedpour, N.; Rajaei, F. Vitrification by Cryotop and the Maturation, Fertilization, and Developmental Rates of Mouse Oocytes. Iran. Red Crescent Med. J. 2015, 17, e18172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hou, Y.P.; Dai, Y.P.; Zhu, S.E.; Zhu, H.B.; Wu, T.Y.; Gong, G.C.; Wang, H.P.; Wang, L.L.; Liu, Y.; Li, R.; et al. Bovine oocytes vitrified by the open pulled straw method and used for somatic cell cloning supported development to term. Theriogenology 2005, 64, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Critser, J.K. Mechanisms of cryoinjury in living cells. ILAR J. 2000, 41, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, C.; Zhang, J.; Sui, X.; Zhu, Y.; Wen, C.; Zhang, L. Exploring the Potential of Biocompatible Osmoprotectants as Highly Efficient Cryoprotectants. ACS Appl. Mater. Interfaces 2017, 9, 42516–42524. [Google Scholar] [CrossRef]
- Bernard, A.; Fuller, B.J. Cryopreservation of human oocytes: A review of current problems and perspectives. Hum. Reprod. Update 1996, 2, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Len, J.S.; Koh, W.S.D.; Tan, S.X. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 2019, 39, BSR20191601. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Petry, A.; Görlach, A. Regulation of NADPH Oxidases by G Protein-Coupled Receptors. Antioxid. Redox Signal. 2019, 30, 74–94. [Google Scholar] [CrossRef]
- Sawyer, D.T.; Sobkowiak, A.; Matsushita, T. Metal [MLx; M = Fe, Cu, Co, Mn]/Hydroperoxide-Induced Activation of Dioxygen for the Oxygenation of Hydrocarbons: Oxygenated Fenton Chemistry. Acc. Chem. Res. 1996, 29, 409–416. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Chang, H.; Chen, H.; Zhang, L.; Wang, Y.; Xie, X.; Zhang, Y.; Quan, F. Effect of oocyte vitrification on DNA damage in metaphase II oocytes and the resulting preimplantation embryos. Mol. Reprod. Dev. 2019, 86, 1603–1614. [Google Scholar] [CrossRef]
- Zribi, N.; Feki Chakroun, N.; El Euch, H.; Gargouri, J.; Bahloul, A.; Ammar Keskes, L. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil. Steril. 2010, 93, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cowley, S.; Flaim, C.J.; James, W.; Seymour, L.; Cui, Z. The roles of apoptotic pathways in the low recovery rate after cryopreservation of dissociated human embryonic stem cells. Biotechnol. Prog. 2010, 26, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Hockberger, P.E.; Skimina, T.A.; Centonze, V.E.; Lavin, C.; Chu, S.; Dadras, S.; Reddy, J.K.; White, J.G. Activation of flavin-containing oxidases underlies light-induced production of H2O2 in mammalian cells. Proc. Natl. Acad. Sci. USA 1999, 96, 6255–6260. [Google Scholar] [CrossRef] [PubMed]
- Squirrell, J.M.; Wokosin, D.L.; White, J.G.; Bavister, B.D. Long-term two-photon fluorescence imaging of mammalian embryos without compromising viability. Nat. Biotechnol. 1999, 17, 763–767. [Google Scholar] [CrossRef]
- Ottosen, L.D.M.; Hindkjær, J.; Ingerslev, J. Light exposure of the ovum and preimplantation embryo during ART procedures. J. Assist. Reprod. Genet. 2007, 24, 99–103. [Google Scholar] [CrossRef]
- Beehler, B.C.; Przybyszewski, J.; Box, H.B.; Kulesz-Martin, M.F. Formation of 8-hydroxydeoxyguanosine within DNA of mouse keratinocytes exposed in culture to UVB and H2O2. Carcinogenesis 1992, 13, 2003–2007. [Google Scholar] [CrossRef]
- Goto, Y.; Noda, Y.; Mori, T.; Nakano, M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free. Radic. Biol. Med. 1993, 15, 69–75. [Google Scholar] [CrossRef]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Zhou, T.; Prather, E.R.; Garrison, D.E.; Zuo, L. Interplay between ROS and Antioxidants during Ischemia-Reperfusion Injuries in Cardiac and Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 417. [Google Scholar] [CrossRef]
- Martín-Maestro, A.; Sánchez-Ajofrín, I.; Maside, C.; Peris-Frau, P.; Medina-Chávez, D.A.; Cardoso, B.; Navarro, J.C.; Fernández-Santos, M.R.; Garde, J.J.; Soler, A.J. Cellular and Molecular Events that Occur in the Oocyte during Prolonged Ovarian Storage in Sheep. Animals 2020, 10, 2414. [Google Scholar] [CrossRef] [PubMed]
- de Barros, F.R.O.; Paula-Lopes, F.F. Cellular and epigenetic changes induced by heat stress in bovine preimplantation embryos. Mol. Reprod. Dev. 2018, 85, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Teng, S.; Saunders, P.T. A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol. Reprod. 2009, 80, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Widjiati, W.; Faizah, Z.; Darsini, N.; Hendrawan, V.F.; Karima, H.N.; Chotimah, C.; Sumitro, S.B.; Yustinasari, L.R.; Kasman, A.; Ntoruru, J.M.; et al. Calcium (Ca2+) expression and intensity in cumulus-oocyte complex (COCs)in Kacang goat after vitrification. Pol. J. Vet. Sci. 2022, 25, 19–26. [Google Scholar] [CrossRef]
- Zorov, D.B.; Filburn, C.R.; Klotz, L.O.; Zweier, J.L.; Sollott, S.J. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000, 192, 1001–1014. [Google Scholar] [CrossRef]
- Ischiropoulos, H.; Beckman, J.S. Oxidative stress and nutrition in neurodegeneration: Cause, effect, or association? J. Clin. Investig. 2003, 111, 163. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free. Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Thomas, C.; Mackey, M.M.; Diaz, A.A.; Cox, D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. 2009, 14, 102–108. [Google Scholar] [CrossRef]
- Iverson, S.L.; Orrenius, S. The cardiolipin-cytochrome c interaction and the mitochondrial regulation of apoptosis. Arch. Biochem. Biophys. 2004, 423, 37–46. [Google Scholar] [CrossRef]
- Fariss, M.W.; Chan, C.B.; Patel, M.; Van Houten, B.; Orrenius, S. Role of mitochondria in toxic oxidative stress. Mol. Interv. 2005, 5, 94–111. [Google Scholar] [CrossRef]
- Lenaz, G. Role of mitochondria in oxidative stress and ageing. Biochim. Biophys. Acta 1998, 1366, 53–67. [Google Scholar] [CrossRef]
- Wright, A.F.; Murphy, M.P.; Turnbull, D.M. Do organellar genomes function as long-term redox damage sensors? Trends Genet 2009, 25, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Katagiri, S.; Takahashi, Y. ATP content and maturational/developmental ability of bovine oocytes with various cytoplasmic morphologies. Zygote 2006, 14, 299–304. [Google Scholar] [CrossRef][Green Version]
- Duran, H.E.; Simsek-Duran, F.; Oehninger, S.C.; Jones, H.W., Jr.; Castora, F.J. The association of reproductive senescence with mitochondrial quantity, function, and DNA integrity in human oocytes at different stages of maturation. Fertil. Steril. 2011, 96, 384–388. [Google Scholar] [CrossRef]
- Dai, J.; Wu, C.; Muneri, C.W.; Niu, Y.; Zhang, S.; Rui, R.; Zhang, D. Changes in mitochondrial function in porcine vitrified MII-stage oocytes and their impacts on apoptosis and developmental ability. Cryobiology 2015, 71, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mu, Y.; Ding, D.; Zou, W.; Li, X.; Chen, B.; Leung, P.C.; Chang, H.M.; Zhu, Q.; Wang, K.; et al. Melatonin improves the effect of cryopreservation on human oocytes by suppressing oxidative stress and maintaining the permeability of the oolemma. J. Pineal Res. 2021, 70, e12707. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Van Blerkom, J.; Davis, P.; Toledo, A.A. Cryopreservation of metaphase II human oocytes effects mitochondrial membrane potential: Implications for developmental competence. Hum. Reprod. 2004, 19, 1861–1866. [Google Scholar] [CrossRef]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial Dysfunction and Oxidative Stress Caused by Cryopreservation in Reproductive Cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef]
- Zeeshan, H.M.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Bedard, K.; Lardy, B.; Krause, K.H. NOX family NADPH oxidases: Not just in mammals. Biochimie 2007, 89, 1107–1112. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, X.J.; Li, J.T.; Zhang, L.; Fu, Y.; Zhang, Y.J.; Chen, R.X.; Wei, X.Q.; Wang, R.; Wang, Y.; et al. Endoplasmic reticulum stress inhibition is a valid therapeutic strategy in vitrifying oocytes. Cryobiology 2015, 70, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Zhao, X.M.; Hao, H.S.; Du, W.H.; Zhao, S.J.; Wang, H.Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016, 60, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Mittag, T.; Parker, R. Multiple Modes of Protein-Protein Interactions Promote RNP Granule Assembly. J. Mol. Biol. 2018, 430, 4636–4649. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Kedersha, N. RNA granules. J. Cell Biol. 2006, 172, 803–808. [Google Scholar] [CrossRef]
- Cadena Sandoval, M.; Heberle, A.M.; Rehbein, U.; Barile, C.; Ramos Pittol, J.M.; Thedieck, K. mTORC1 Crosstalk with Stress Granules in Aging and Age-Related Diseases. Front. Aging 2021, 2, 761333. [Google Scholar] [CrossRef]
- Kedersha, N.; Anderson, P. Stress granules: Sites of mRNA triage that regulate mRNA stability and translatability. Biochem. Soc. Trans. 2002, 30, 963–969. [Google Scholar] [CrossRef]
- Parker, R.; Sheth, U. P bodies and the control of mRNA translation and degradation. Mol. Cell 2007, 25, 635–646. [Google Scholar] [CrossRef]
- Standart, N.; Minshall, N. Translational control in early development: CPEB, P-bodies and germinal granules. Biochem. Soc. Trans. 2008, 36, 671–676. [Google Scholar] [CrossRef]
- Riggs, C.L.; Kedersha, N.; Ivanov, P.; Anderson, P. Mammalian stress granules and P bodies at a glance. J. Cell Sci. 2020, 133, jcs242487. [Google Scholar] [CrossRef]
- Kedersha, N.; Anderson, P. Mammalian stress granules and processing bodies. Methods Enzymol. 2007, 431, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.; Stoecklin, G.; Ayodele, M.; Yacono, P.; Lykke-Andersen, J.; Fritzler, M.J.; Scheuner, D.; Kaufman, R.J.; Golan, D.E.; Anderson, P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 2005, 169, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Bloch, D.B. Probing the mRNA processing body using protein macroarrays and “autoantigenomics”. RNA 2007, 13, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Higuchi, M.; Matsuki, H.; Yoshita, M.; Ohsawa, T.; Oie, M.; Fujii, M. Stress granules inhibit apoptosis by reducing reactive oxygen species production. Mol. Cell. Biol. 2013, 33, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Emara, M.M.; Fujimura, K.; Sciaranghella, D.; Ivanova, V.; Ivanov, P.; Anderson, P. Hydrogen peroxide induces stress granule formation independent of eIF2α phosphorylation. Biochem. Biophys. Res. Commun. 2012, 423, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Rodriguez-Enriquez, S.; Lemasters, J.J. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 2007, 462, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, G. Autophagy in Mitochondrial Quality Control. In Autophagy: Biology and Diseases: Basic Science; Qin, Z.-H., Ed.; Springer: Singapore, 2019; pp. 421–434. [Google Scholar]
- Bang, S.; Shin, H.; Song, H.; Suh, C.S.; Lim, H.J. Autophagic activation in vitrified-warmed mouse oocytes. Reproduction 2014, 148, 11–19. [Google Scholar] [CrossRef]
- Ito, J.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Resveratrol treatment increases mitochondrial biogenesis and improves viability of porcine germinal-vesicle stage vitrified-warmed oocytes. Cryobiology 2020, 93, 37–43. [Google Scholar] [CrossRef]
- Xu, J.; Sun, L.; Wu, C.; Zhang, S.; Ju, S.; Rui, R.; Zhang, D.; Dai, J. Involvement of PINK1/Parkin-mediated mitophagy in mitochondrial functional disruption under oxidative stress in vitrified porcine oocytes. Theriogenology 2021, 174, 160–168. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, D.; Ju, S.; Sun, L.; Zhang, S.; Wu, C.; Rui, R.; Dai, J. Mitophagy is involved in the mitochondrial dysfunction of vitrified porcine oocytes. Mol. Reprod. Dev. 2021, 88, 427–436. [Google Scholar] [CrossRef]
- Kurihara, Y.; Kanki, T.; Aoki, Y.; Hirota, Y.; Saigusa, T.; Uchiumi, T.; Kang, D. Mitophagy plays an essential role in reducing mitochondrial production of reactive oxygen species and mutation of mitochondrial DNA by maintaining mitochondrial quantity and quality in yeast. J. Biol. Chem. 2012, 287, 3265–3272. [Google Scholar] [CrossRef] [PubMed]
- Yorimitsu, T.; Klionsky, D.J. Autophagy: Molecular machinery for self-eating. Cell Death Differ. 2005, 12 (Suppl. S2), 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.K.; Shin, H.; Lim, H.J. Rapamycin Influences the Efficiency of in vitro Fertilization and Development in the Mouse: A Role for Autophagic Activation. Asian-Australas. J. Anim. Sci. 2016, 29, 1102–1110. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef] [PubMed]
- Habibi, A.; Farrokhi, N.; Moreira da Silva, F.; Bettencourt, B.F.; Bruges-Armas, J.; Amidi, F.; Hosseini, A. The effects of vitrification on gene expression in mature mouse oocytes by nested quantitative PCR. J. Assist. Reprod Genet. 2010, 27, 599–604. [Google Scholar] [CrossRef][Green Version]
- Niu, Y.; Dai, J.; Wu, C.; Chen, Y.; Zhang, S.; Zhang, D. The application of apoptotic inhibitor in apoptotic pathways of MII stage porcine oocytes after vitrification. Reprod. Domest. Anim. = Zuchthyg. 2016, 51, 953–959. [Google Scholar] [CrossRef]
- Somfai, T.; Ozawa, M.; Noguchi, J.; Kaneko, H.; Kuriani Karja, N.W.; Farhudin, M.; Dinnyés, A.; Nagai, T.; Kikuchi, K. Developmental competence of in vitro-fertilized porcine oocytes after in vitro maturation and solid surface vitrification: Effect of cryopreservation on oocyte antioxidative system and cell cycle stage. Cryobiology 2007, 55, 115–126. [Google Scholar] [CrossRef]
- Dinara, S.; Sengoku, K.; Tamate, K.; Horikawa, M.; Ishikawa, M. Effects of supplementation with free radical scavengers on the survival and fertilization rates of mouse cryopreserved oocytes. Hum. Reprod. 2001, 16, 1976–1981. [Google Scholar] [CrossRef]
- Succu, S.; Gadau, S.D.; Serra, E.; Zinellu, A.; Carru, C.; Porcu, C.; Naitana, S.; Berlinguer, F.; Leoni, G.G. A recovery time after warming restores mitochondrial function and improves developmental competence of vitrified ovine oocytes. Theriogenology 2018, 110, 18–26. [Google Scholar] [CrossRef]
- Carvalho, A.A.; Faustino, L.R.; Silva, C.M.; Castro, S.V.; Lobo, C.H.; Santos, F.W.; Santos, R.R.; Campello, C.C.; Bordignon, V.; Figueiredo, J.R.; et al. Catalase addition to vitrification solutions maintains goat ovarian preantral follicles stability. Res. Vet. Sci. 2014, 97, 140–147. [Google Scholar] [CrossRef]
- Hammond, C.L.; Lee, T.K.; Ballatori, N. Novel roles for glutathione in gene expression, cell death, and membrane transport of organic solutes. J. Hepatol. 2001, 34, 946–954. [Google Scholar] [CrossRef]
- García-Martínez, T.; Vendrell-Flotats, M.; Martínez-Rodero, I.; Ordóñez-León, E.A.; Álvarez-Rodríguez, M.; López-Béjar, M.; Yeste, M.; Mogas, T. Glutathione Ethyl Ester Protects In Vitro-Maturing Bovine Oocytes against Oxidative Stress Induced by Subsequent Vitrification/Warming. Int. J. Mol. Sci. 2020, 21, 7547. [Google Scholar] [CrossRef] [PubMed]
- de Matos, D.G.; Furnus, C.C. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development: Effect of β-mercaptoethanol, cysteine and cystine. Theriogenology 2000, 53, 761–771. [Google Scholar] [CrossRef]
- de Matos, D.G.; Furnus, C.C.; Moses, D.F.; Baldassarre, H. Effect of cysteamine on glutathione level and developmental capacity of bovine oocyte matured in vitro. Mol. Reprod. Dev. 1995, 42, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Newton, G.L.; Gonick, G.; Fahey, R.C.; Ward, J.F. Radioprotection of DNA by thiols: Relationship between the net charge on a thiol and its ability to protect DNA. Radiat. Res. 1988, 114, 11–27. [Google Scholar] [CrossRef]
- Hara, H.; Yamane, I.; Noto, I.; Kagawa, N.; Kuwayama, M.; Hirabayashi, M.; Hochi, S. Microtubule assembly and in vitro development of bovine oocytes with increased intracellular glutathione level prior to vitrification and in vitro fertilization. Zygote 2014, 22, 476–482. [Google Scholar] [CrossRef]
- Yang, J.; Guo, S.; Pan, B.; Qazi, I.H.; Qin, J.; Zang, S.; Han, H.; Meng, Q.; Zhou, G. Melatonin promotes in vitro maturation of vitrified-warmed mouse GV oocytes potentially by modulating MAD2 protein expression of SAC component through MTRs. Cryobiology 2021, 102, 82–91. [Google Scholar] [CrossRef]
- Guo, S.; Yang, J.; Qin, J.; Qazi, I.H.; Pan, B.; Zang, S.; Lv, T.; Deng, S.; Fang, Y.; Zhou, G. Melatonin Promotes In Vitro Maturation of Vitrified-Warmed Mouse Germinal Vesicle Oocytes, Potentially by Reducing Oxidative Stress through the Nrf2 Pathway. Animals 2021, 11, 2324. [Google Scholar] [CrossRef]
- Giaretta, E.; Spinaci, M.; Bucci, D.; Tamanini, C.; Galeati, G. Effects of resveratrol on vitrified porcine oocytes. Oxid. Med. Cell. Longev. 2013, 2013, 920257. [Google Scholar] [CrossRef]
- Piras, A.R.; Ariu, F.; Falchi, L.; Zedda, M.T.; Pau, S.; Schianchi, E.; Paramio, M.; Bogliolo, L. Resveratrol treatment during maturation enhances developmental competence of oocytes after prolonged ovary storage at 4 °C in the domestic cat model. Theriogenology 2020, 144, 152–157. [Google Scholar] [CrossRef]
- Comizzoli, P.; Wildt, D.E.; Pukazhenthi, B.S. In Vitro compaction of germinal vesicle chromatin is beneficial to survival of vitrified cat oocytes. Reprod. Domest. Anim. = Zuchthyg. 2009, 44 (Suppl. S2), 269–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jia, C.; Cheng, W.; Zhang, T.; Tao, R.; Ma, Y.; Si, L.; Xu, Y.; Li, J. The Effect of L-Carnitine Additive During In Vitro Maturation on the Vitrification of Pig Oocytes. Cell. Reprogr. 2020, 22, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Zare, Z.; Rezaei, N.; Mohammadi, M. Treatment of mouse cumulus-oocyte complexes with L-carnitine during vitrification and in vitro maturation affects maturation and embryonic developmental rate after parthenogenetic activation. Anat. Histol. Embryol. 2022, 51, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Davoodian, N.; Kadivar, A.; Ahmadi, E.; Nazari, H.; Mehrban, H. Quercetin effect on the efficiency of ovine oocyte vitrification at GV stage. Theriogenology 2021, 174, 53–59. [Google Scholar] [CrossRef]
- Yashiro, I.; Tagiri, M.; Ogawa, H.; Tashima, K.; Takashima, S.; Hara, H.; Hirabayashi, M.; Hochi, S. High revivability of vitrified–warmed bovine mature oocytes after recovery culture with α-tocopherol. Reproduction 2015, 149, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Farzollahi, M.; Tayefi-Nasrabadi, H.; Mohammadnejad, D.; Abedelahi, A. Supplementation of culture media with vitamin E improves mouse antral follicle maturation and embryo development from vitrified ovarian tissue. J. Obstet. Gynaecol. Res. 2016, 42, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.C.; Jia, B.Y.; Fu, X.W.; Guo, J.X.; Hong, Q.H.; Quan, G.B.; Wu, G.Q. Role of astaxanthin as an efficient antioxidant on the in vitro maturation and vitrification of porcine oocytes. Theriogenology 2021, 167, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, X.; Yan, J.; Yan, L.Y.; Jin, X.H.; Zhu, X.H.; He, Z.Z.; Liu, J.; Li, R.; Qiao, J. L-proline: A highly effective cryoprotectant for mouse oocyte vitrification. Sci. Rep. 2016, 6, 26326. [Google Scholar] [CrossRef]
- Ruiz-Conca, M.; Vendrell, M.; Sabés-Alsina, M.; Mogas, T.; Lopez-Bejar, M. Coenzyme Q(10) supplementation during in vitro maturation of bovine oocytes (Bos taurus) helps to preserve oocyte integrity after vitrification. Reprod. Domest. Anim. = Zuchthyg. 2017, 52 (Suppl. S4), 52–54. [Google Scholar] [CrossRef]
- Shirzeyli, M.H.; Eini, F.; Shirzeyli, F.H.; Majd, S.A.; Ghahremani, M.; Joupari, M.D.; Novin, M.G. Assessment of Mitochondrial Function and Developmental Potential of Mouse Oocytes after Mitoquinone Supplementation during Vitrification. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2021, 60, 388–395. [Google Scholar] [CrossRef]
- Fang, L.; Bai, C.; Chen, Y.; Dai, J.; Xiang, Y.; Ji, X.; Huang, C.; Dong, Q. Inhibition of ROS production through mitochondria-targeted antioxidant and mitochondrial uncoupling increases post-thaw sperm viability in yellow catfish. Cryobiology 2014, 69, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Zorov, D.B. Pros and Cons of Use of Mitochondria-Targeted Antioxidants. Antioxidants 2019, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Verrugio, C.; Ruiz-Ortega, M.; Mosqueira, M.; Simon, F. Oxidative Stress in Disease and Aging: Mechanisms and Therapies. Oxidative Med. Cell. Longev. 2016, 2016, 8786564. [Google Scholar] [CrossRef] [PubMed]
- Meja, K.K.; Rajendrasozhan, S.; Adenuga, D.; Biswas, S.K.; Sundar, I.K.; Spooner, G.; Marwick, J.A.; Chakravarty, P.; Fletcher, D.; Whittaker, P.; et al. Curcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2. Am. J. Respir. Cell Mol. Biol. 2008, 39, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free. Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Chaudhuri, R.K.; Meyer, T.; Premi, S.; Brash, D. Acetyl zingerone: An efficacious multifunctional ingredient for continued protection against ongoing DNA damage in melanocytes after sun exposure ends. Int. J. Cosmet. Sci. 2020, 42, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; Lan, M.; Zang, X.-W.; Li, Y.-L.; Cui, X.-S.; Kim, N.-H.; Sun, S.-C. Melatonin protects oocytes from MEHP exposure-induced meiosis defects in porcine†. Biol. Reprod. 2018, 98, 286–298. [Google Scholar] [CrossRef]
- Li, W.D.; Yu, S.; Luo, S.M.; Shen, W.; Yin, S.; Sun, Q.Y. Melatonin defends mouse oocyte quality from benzo[ghi]perylene-induced deterioration. J. Cell. Physiol. 2019, 234, 6220–6229. [Google Scholar] [CrossRef]
- An, Q.; Peng, W.; Cheng, Y.; Lu, Z.; Zhou, C.; Zhang, Y.; Su, J. Melatonin supplementation during in vitro maturation of oocyte enhances subsequent development of bovine cloned embryos. J. Cell. Physiol. 2019, 234, 17370–17381. [Google Scholar] [CrossRef]
- Khor, S.P.; Yeow, L.C.; Poobathy, R.; Zakaria, R.; Chew, B.L.; Subramaniam, S. Droplet-vitrification of Aranda Broga Blue orchid: Role of ascorbic acid on the antioxidant system and genetic fidelity assessments via RAPD and SCoT markers. Biotechnol. Rep. 2020, 26, e00448. [Google Scholar] [CrossRef]
- Bisht, S.; Dada, R. Oxidative stress: Major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front. Biosci. Sch. Ed. 2017, 9, 420–447. [Google Scholar] [CrossRef]
- Aghaz, F.; Vaisi-Raygani, A.; Khazaei, M.; Arkan, E. Enhanced Cryoprotective Effect of Melatonin and Resveratrol by Coencapsulation: Improved In Vitro Development of Vitrified-Warmed Mouse Germinal Vesicle Oocytes. Biopreserv. Biobank. 2021, 19, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Limaye, L.S.; Kale, V.P. Cryopreservation of human hematopoietic cells with membrane stabilizers and bioantioxidants as additives in the conventional freezing medium. J. Hematother. Stem Cell Res. 2001, 10, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Fuentes-Broto, L. Melatonin: A multitasking molecule. Prog. Brain Res. 2010, 181, 127–151. [Google Scholar] [CrossRef]
- Tan, D.; Manchester, L.C.; Reiter, R.J.; Qi, W.; Hanes, M.A.; Farley, N.J. High physiological levels of melatonin in the bile of mammals. Life Sci. 1999, 65, 2523–2529. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamura, H.; Takayama, H.; Kato, H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil. Steril. 2003, 80, 1012–1016. [Google Scholar] [CrossRef]
- Mihajlović, A.I.; FitzHarris, G. Segregating Chromosomes in the Mammalian Oocyte. Curr. Biol. 2018, 28, R895–R907. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J.J.M.R. Mitochondria: The birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019, 2, 44–66. [Google Scholar] [CrossRef]
- Acua-Castroviejo, D.; Noguiera-Navarro, M.T.; Reiter, R.J.; Escames, G.J.M.R. Melatonin actions in the heart; more than a hormone. Melatonin Res. 2018, 1, 21–26. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, W.; Ma, Y.; Wang, D.; Zhao, X.; Zeng, C.; Zhang, M.; Zeng, X.; Meng, Q.; Zhou, G. Improved development by melatonin treatment after vitrification of mouse metaphase II oocytes. Cryobiology 2016, 73, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cheng, K.; Zhang, Y.; Meng, Q.; Zhu, S.; Zhou, G. No effect of exogenous melatonin on development of cryopreserved metaphase II oocytes in mouse. J. Anim. Sci. Biotechnol. 2015, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Le Du, A.; Kadoch, I.J.; Bourcigaux, N.; Doumerc, S.; Bourrier, M.C.; Chevalier, N.; Fanchin, R.; Chian, R.C.; Tachdjian, G.; Frydman, R.; et al. In Vitro oocyte maturation for the treatment of infertility associated with polycystic ovarian syndrome: The French experience. Hum. Reprod. 2005, 20, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Qazi, I.H.; Guo, S.; Yang, J.; Qin, J.; Lv, T.; Zang, S.; Zhang, Y.; Zeng, C.; Meng, Q.; et al. Melatonin improves the first cleavage of parthenogenetic embryos from vitrified-warmed mouse oocytes potentially by promoting cell cycle progression. J. Anim. Sci. Biotechnol. 2021, 12, 84. [Google Scholar] [CrossRef]
- Sahin, K.; Onderci, M.; Gursu, M.F.; Kucuk, O.; Sahin, N.J. Effect of Melatonin Supplementation on Biomarkers of Oxidative Stress and Serum Vitamin and Mineral Concentrations in Heat-Stressed Japanese Quail. J. Appl. Poult. Res. 2004, 13, 342–348. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, B.; Qazi, I.H.; Yang, H.; Guo, S.; Yang, J.; Zhang, Y.; Zeng, C.; Zhang, M.; Han, H.; et al. Melatonin Improves In Vitro Development of Vitrified-Warmed Mouse Germinal Vesicle Oocytes Potentially via Modulation of Spindle Assembly Checkpoint-Related Genes. Cells 2019, 8, 1009. [Google Scholar] [CrossRef]
- Gruhn, J.R.; Zielinska, A.P.; Shukla, V.; Blanshard, R.; Capalbo, A.; Cimadomo, D.; Nikiforov, D.; Chan, A.C.; Newnham, L.J.; Vogel, I.; et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science 2019, 365, 1466–1469. [Google Scholar] [CrossRef]
- Qin, J.; Guo, S.; Yang, J.; Qazi, I.H.; Pan, B.; Lv, T.; Zang, S.; Fang, Y.; Zhou, G. Melatonin Promotes in vitro Development of Vitrified-Warmed Mouse GV Oocytes, Potentially by Modulating Phosphorylation of Drp1. Front. Vet. Sci. 2021, 8, 752001. [Google Scholar] [CrossRef]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The Role of Resveratrol in Mammalian Reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef]
- Martinez, J.; Moreno, J.J. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem. Pharmacol. 2000, 59, 865–870. [Google Scholar] [CrossRef]
- Kwak, S.S.; Cheong, S.A.; Jeon, Y.; Lee, E.; Choi, K.C.; Jeung, E.B.; Hyun, S.H. The effects of resveratrol on porcine oocyte in vitro maturation and subsequent embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology 2012, 78, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites-Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, L.; Wang, Z.; Chang, H.; Xie, X.; Fu, L.; Zhang, Y.; Quan, F. Resveratrol improved the developmental potential of oocytes after vitrification by modifying the epigenetics. Mol. Reprod. Dev. 2019, 86, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Barrozo, L.G.; Paulino, L.; Silva, B.R.; Barbalho, E.C.; Nascimento, D.R.; Neto, M.F.L.; Silva, J.R.V. N-acetyl-cysteine and the control of oxidative stress during in vitro ovarian follicle growth, oocyte maturation, embryo development and cryopreservation. Anim. Reprod. Sci. 2021, 231, 106801. [Google Scholar] [CrossRef]
- Matilla, E.; Martín-Cano, F.E.; González-Fernández, L.; Sánchez-Margallo, F.M.; Álvarez, I.S.; Macías-García, B. N-acetylcysteine addition after vitrification improves oocyte mitochondrial polarization status and the quality of embryos derived from vitrified murine oocytes. BMC Vet. Res. 2019, 15, 31. [Google Scholar] [CrossRef]
- Yue, S.L.; Zhang, Y.T.; Wang, S.W.; Sun, M.; Xing, Y.C.; Wen, J.; Zhou, J.B. Effect of nac on mouse gv oocyte survival and subsequent embryonic development following vitrfication. Cryo Lett. 2016, 37, 295–302. [Google Scholar]
- Alrawaiq, N.S.; Abdullah, A.J. A Review of Flavonoid Quercetin: Metabolism, Bioactivity and Antioxidant Properties. Int. J. PharmTech Res. 2014, 6, 933–941. [Google Scholar]
- Jia, Y.; Lin, J.; Mi, Y.; Zhang, C. Quercetin attenuates cadmium-induced oxidative damage and apoptosis in granulosa cells from chicken ovarian follicles. Reprod. Toxicol. 2011, 31, 477–485. [Google Scholar] [CrossRef]
- Baghel, S.S.; Shrivastava, N.; Baghel, P.R.; Rajput, S. A review of quercetin: Antioxidant and anticancer properties. World J. Pharm. Pharmaceutical. Sci. 2012, 1, 146–160. [Google Scholar]
- Wang, W.; Wang, C.; Ding, X.Q.; Pan, Y.; Gu, T.T.; Wang, M.X.; Liu, Y.L.; Wang, F.M.; Wang, S.J.; Kong, L.D. Quercetin and allopurinol reduce liver thioredoxin-interacting protein to alleviate inflammation and lipid accumulation in diabetic rats. Br. J. Pharmacol. 2013, 169, 1352–1371. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef] [PubMed]
| Components | Concentration | Oocyte Types | Effects | References |
|---|---|---|---|---|
| Melatonin | 10−7 mol/L | Mouse GV-stage oocytes | (↓) ROS levels, spindle damage | [89] |
| 10−9 mol/L | Human MII-stage oocytes | (↑) Blastocyst formation rate, Oocyte maturation rate, MMP, ATP | [46] | |
| Resveratrol | 1.5 × 10−3 mol/L | Cat GV-stage oocytes | (↑) Oocyte maturation rate, cleavage rate, embryo developmental ratio | [92] |
| 5 × 10−6 mol/L | Cat COC complexes | (↓) ROS levels (↑) GSH levels | [91] | |
| 2 × 10−6 mol/L | Porcine MII-stage oocytes | (↓) Cell apoptosis | [90] | |
| Quercetin | 5 × 10−6 mol/L | Mouse GV-stage oocytes | (↑) Oocyte maturation rate, embryo developmental ratio | [95] |
| Vitamin E | 3 × 10−4 mol/L | Bovine MII-stage oocytes | (↑) Blastocyst formation rate | [96] |
| 10−4 mol/L | Mouse MII-stage oocytes | (↑) Oocyte morphology and ultrastructure | [97] | |
| Astaxanthin | 2.5 × 10−6 mol/L | Porcine GV-stage oocytes | (↑) GSH levels, lysosomal activity (↓) ROS levels, cathepsin B activity | [98] |
| Proline | 2 mol/L | Mouse MII-stage oocytes | (↑) Spindle and mitochondrial function | [99] |
| Coenzyme Q10 | 5 × 10−5 mol/L | Bovine COC complexes | (↑) Cell survival after vitrification (↑) Migration of cortical granule | [100] |
| L-carnitine | 0.8 × 10−3 mol/L | Porcine COC complexes | (↑) SOD1 gene expression | [93] |
| 0.6 g/mL | Mouse COC complexes | (↑) GSH levels | [94] | |
| MitoQ | 2 × 10−8 mol/L | Mouse MII-stage oocytes | (↑) MMP, cell survival | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, B.; Qin, J.; Pan, B.; Qazi, I.H.; Ye, J.; Fang, Y.; Zhou, G. Oxidative Stress and Oocyte Cryopreservation: Recent Advances in Mitigation Strategies Involving Antioxidants. Cells 2022, 11, 3573. https://doi.org/10.3390/cells11223573
Cao B, Qin J, Pan B, Qazi IH, Ye J, Fang Y, Zhou G. Oxidative Stress and Oocyte Cryopreservation: Recent Advances in Mitigation Strategies Involving Antioxidants. Cells. 2022; 11(22):3573. https://doi.org/10.3390/cells11223573
Chicago/Turabian StyleCao, Beijia, Jianpeng Qin, Bo Pan, Izhar Hyder Qazi, Jiangfeng Ye, Yi Fang, and Guangbin Zhou. 2022. "Oxidative Stress and Oocyte Cryopreservation: Recent Advances in Mitigation Strategies Involving Antioxidants" Cells 11, no. 22: 3573. https://doi.org/10.3390/cells11223573
APA StyleCao, B., Qin, J., Pan, B., Qazi, I. H., Ye, J., Fang, Y., & Zhou, G. (2022). Oxidative Stress and Oocyte Cryopreservation: Recent Advances in Mitigation Strategies Involving Antioxidants. Cells, 11(22), 3573. https://doi.org/10.3390/cells11223573








