Human Vδ2 T Cells and Their Versatility for Immunotherapeutic Approaches
Abstract
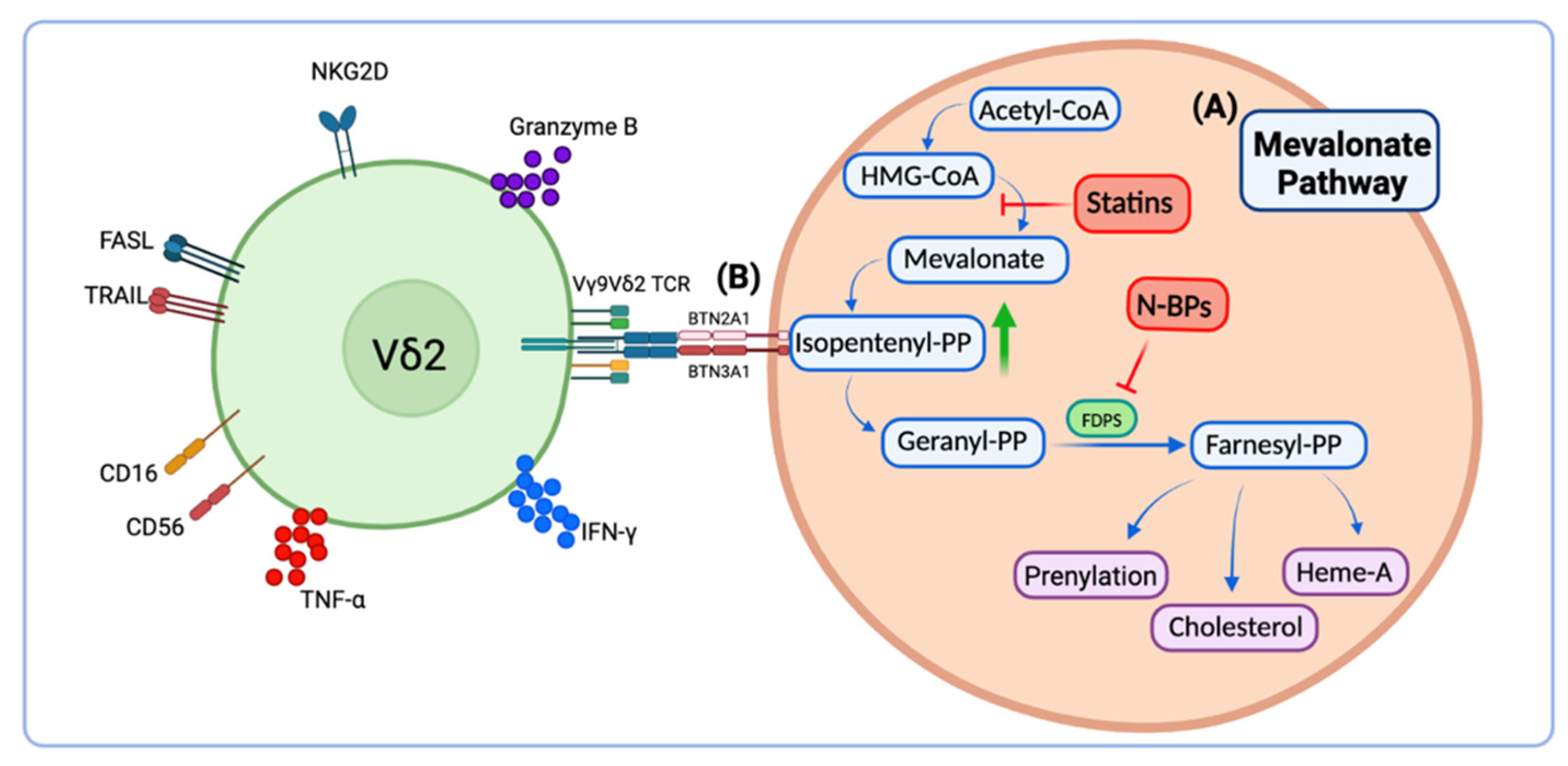
1. Background
2. Phosphoantigens for Ex Vivo Vδ2 T Cell Activation
3. Clinical Applications of Vδ2 T Cell Immunotherapy
3.1. Immunotherapy for Infectious Diseases
3.2. Immunotherapy for Cancer
3.3. Lessons Learned from the Clinic
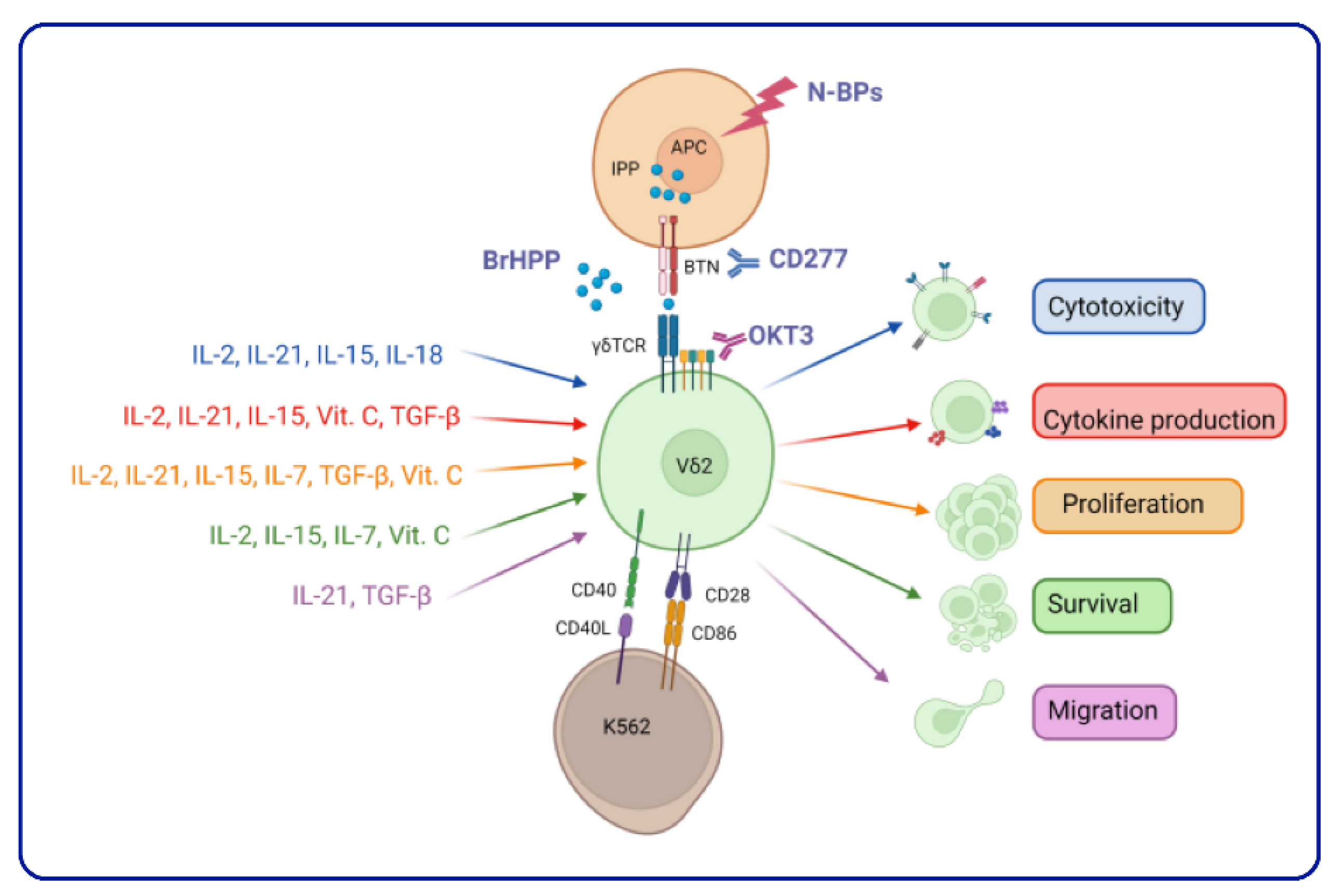
3.4. Enhancing Vδ2 T Cell Cytotoxic Effector Functions
3.5. Vδ2 T Cell Expansion: Combining the Cytokine Cocktail
4. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vantourout, P.; Hayday, A. Six-of-the-best: Unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 2013, 13, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santos, B.; Serre, K.; Norell, H. γδ T cells in cancer. Nat. Rev. Immunol. 2015, 15, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, P.; Qiu, F.; Wei, Q.; Huang, J. Human γδT-cell subsets and their involvement in tumor immunity. Cell Mol. Immunol. 2017, 14, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Takihara, Y.; Reimann, J.; Michalopoulos, E.; Ciccone, E.; Moretta, L.; Mak, T.W. Diversity and structure of human T cell receptor delta chain genes in peripheral blood gamma/delta-bearing T lymphocytes. J. Exp. Med. 1989, 169, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Ding, Y.P.; Tanaka, Y.; Shen, L.W.; Wei, C.H.; Minato, N.; Zhang, W. γδ T cells and their potential for immunotherapy. Int. J. Biol. Sci. 2014, 10, 119–135. [Google Scholar] [CrossRef]
- Ryan, P.L.; Sumaria, N.; Holland, C.J.; Bradford, C.M.; Izotova, N.; Grandjean, C.L.; Jawad, A.S.; Bergmeier, L.A.; Pennington, D.J. Heterogeneous yet stable Vδ2(+) T-cell profiles define distinct cytotoxic effector potentials in healthy human individuals. Proc. Natl. Acad. Sci. USA 2016, 113, 14378–14383. [Google Scholar] [CrossRef]
- Peters, C.; Oberg, H.H.; Kabelitz, D.; Wesch, D. Phenotype and regulation of immunosuppressive Vδ2-expressing γδ T cells. Cell Mol. Life Sci. 2014, 71, 1943–1960. [Google Scholar] [CrossRef]
- Kabelitz, D.; Serrano, R.; Kouakanou, L.; Peters, C.; Kalyan, S. Cancer immunotherapy with γδ T cells: Many paths ahead of us. Cell. Mol. Immunol. 2020, 17, 925–939. [Google Scholar] [CrossRef]
- Wu, J.; Groh, V.; Spies, T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J. Immunol. 2002, 169, 1236–1240. [Google Scholar] [CrossRef]
- Rincon-Orozco, B.; Kunzmann, V.; Wrobel, P.; Kabelitz, D.; Steinle, A.; Herrmann, T. Activation of V gamma 9V delta 2 T cells by NKG2D. J. Immunol. 2005, 175, 2144–2151. [Google Scholar] [CrossRef]
- Dalton, J.E.; Howell, G.; Pearson, J.; Scott, P.; Carding, S.R. Fas-Fas ligand interactions are essential for the binding to and killing of activated macrophages by gamma delta T cells. J. Immunol. 2004, 173, 3660–3667. [Google Scholar] [CrossRef]
- Ponomarev, E.D.; Dittel, B.N. Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J. Immunol. 2005, 174, 4678–4687. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, W.; Wong, W.M.; Ward, E.; Thrasher, A.J.; Goldblatt, D.; Osman, M.; Digard, P.; Canaday, D.H.; Gustafsson, K. Human gamma delta T cells: A lymphoid lineage cell capable of professional phagocytosis. J. Immunol. 2009, 183, 5622–5629. [Google Scholar] [CrossRef]
- Chen, Z.; Freedman, M.S. CD16+ gammadelta T cells mediate antibody dependent cellular cytotoxicity: Potential mechanism in the pathogenesis of multiple sclerosis. Clin. Immunol. 2008, 128, 219–227. [Google Scholar] [CrossRef]
- Di Lorenzo, B.; Simões, A.E.; Caiado, F.; Tieppo, P.; Correia, D.V.; Carvalho, T.; da Silva, M.G.; Déchanet-Merville, J.; Schumacher, T.N.; Prinz, I.; et al. Broad Cytotoxic Targeting of Acute Myeloid Leukemia by Polyclonal Delta One T Cells. Cancer Immunol. Res. 2019, 7, 552–558. [Google Scholar] [CrossRef]
- Almeida, A.R.; Correia, D.V.; Fernandes-Platzgummer, A.; da Silva, C.L.; da Silva, M.G.; Anjos, D.R.; Silva-Santos, B. Delta One T Cells for Immunotherapy of Chronic Lymphocytic Leukemia: Clinical-Grade Expansion/Differentiation and Preclinical Proof of Concept. Clin. Cancer Res. 2016, 22, 5795–5804. [Google Scholar] [CrossRef]
- Kouakanou, L.; Xu, Y.; Peters, C.; He, J.; Wu, Y.; Yin, Z.; Kabelitz, D. Vitamin C promotes the proliferation and effector functions of human γδ T cells. Cell. Mol. Immunol. 2020, 17, 462–473. [Google Scholar] [CrossRef]
- Peters, C.; Meyer, A.; Kouakanou, L.; Feder, J.; Schricker, T.; Lettau, M.; Janssen, O.; Wesch, D.; Kabelitz, D. TGF-β enhances the cytotoxic activity of Vδ2 T cells. Oncoimmunology 2019, 8, e1522471. [Google Scholar] [CrossRef]
- Cazzetta, V.; Bruni, E.; Terzoli, S.; Carenza, C.; Franzese, S.; Piazza, R.; Marzano, P.; Donadon, M.; Torzilli, G.; Cimino, M.; et al. NKG2A expression identifies a subset of human Vδ2 T cells exerting the highest antitumor effector functions. Cell Rep. 2021, 37, 109871. [Google Scholar] [CrossRef]
- Constant, P.; Davodeau, F.; Peyrat, M.A.; Poquet, Y.; Puzo, G.; Bonneville, M.; Fournié, J.J. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science 1994, 264, 267–270. [Google Scholar] [CrossRef]
- Tanaka, Y.; Morita, C.T.; Tanaka, Y.; Nieves, E.; Brenner, M.B.; Bloom, B.R. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 1995, 375, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Hintz, M.; Reichenberg, A.; Altincicek, B.; Bahr, U.; Gschwind, R.M.; Kollas, A.K.; Beck, E.; Wiesner, J.; Eberl, M.; Jomaa, H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett. 2001, 509, 317–322. [Google Scholar] [CrossRef]
- Jomaa, H.; Feurle, J.; Luhs, K.; Kunzmann, V.; Tony, H.P.; Herderich, M.; Wilhelm, M. Vgamma9/Vdelta2 T cell activation induced by bacterial low molecular mass compounds depends on the 1-deoxy-D-xylulose 5-phosphate pathway of isoprenoid biosynthesis. FEMS Immunol. Med. Microbiol. 1999, 25, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Amslinger, S.; Kis, K.; Hecht, S.; Adam, P.; Rohdich, F.; Arigoni, D.; Bacher, A.; Eisenreich, W. Biosynthesis of terpenes. Preparation of (E)-1-hydroxy-2-methyl-but-2-enyl 4-diphosphate, an intermediate of the deoxyxylulose phosphate pathway. J. Org. Chem. 2002, 67, 4590–4594. [Google Scholar] [CrossRef] [PubMed]
- Reichenberg, A.; Hintz, M.; Kletschek, Y.; Kuhl, T.; Haug, C.; Engel, R.; Moll, J.; Ostrovsky, D.N.; Jomaa, H.; Eberl, M. Replacing the pyrophosphate group of HMB-PP by a diphosphonate function abrogates Its potential to activate human gammadelta T cells but does not lead to competitive antagonism. Bioorg. Med. Chem. Lett. 2003, 13, 1257–1260. [Google Scholar] [CrossRef]
- Fox, D.T.; Poulter, C.D. Synthesis of (E)-4-hydroxydimethylallyl diphosphate. An intermediate in the methyl erythritol phosphate branch of the isoprenoid pathway. J. Org. Chem. 2002, 67, 5009–5010. [Google Scholar] [CrossRef]
- Eberl, M.; Engel, R.; Beck, E.; Jomaa, H. Differentiation of human gamma-delta T cells towards distinct memory phenotypes. Cell. Immunol. 2002, 218, 1–6. [Google Scholar] [CrossRef]
- Vermijlen, D.; Ellis, P.; Langford, C.; Klein, A.; Engel, R.; Willimann, K.; Jomaa, H.; Hayday, A.C.; Eberl, M. Distinct cytokine-driven responses of activated blood gammadelta T cells: Insights into unconventional T cell pleiotropy. J. Immunol. 2007, 178, 4304–4314. [Google Scholar] [CrossRef]
- Thedrez, A.; Harly, C.; Morice, A.; Salot, S.; Bonneville, M.; Scotet, E. IL-21-mediated potentiation of antitumor cytolytic and proinflammatory responses of human V gamma 9V delta 2 T cells for adoptive immunotherapy. J. Immunol. 2009, 182, 3423–3431. [Google Scholar] [CrossRef]
- Green, A.E.; Lissina, A.; Hutchinson, S.L.; Hewitt, R.E.; Temple, B.; James, D.; Boulter, J.M.; Price, D.A.; Sewell, A.K. Recognition of nonpeptide antigens by human V gamma 9V delta 2 T cells requires contact with cells of human origin. Clin. Exp. Immunol. 2004, 136, 472–482. [Google Scholar] [CrossRef]
- Gober, H.J.; Kistowska, M.; Angman, L.; Jenö, P.; Mori, L.; De Libero, G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef]
- Gossman, W.; Oldfield, E. Quantitative structure--activity relations for gammadelta T cell activation by phosphoantigens. J. Med. Chem. 2002, 45, 4868–4874. [Google Scholar] [CrossRef]
- Buhaescu, I.; Izzedine, H. Mevalonate pathway: A review of clinical and therapeutical implications. Clin. Biochem 2007, 40, 575–584. [Google Scholar] [CrossRef]
- Greenwood, J.; Steinman, L.; Zamvil, S.S. Statin therapy and autoimmune disease: From protein prenylation to immunomodulation. Nat. Rev. Immunol. 2006, 6, 358–370. [Google Scholar] [CrossRef]
- Guerra, B.; Recio, C.; Aranda-Tavío, H.; Guerra-Rodríguez, M.; García-Castellano, J.M.; Fernández-Pérez, L. The Mevalonate Pathway, a Metabolic Target in Cancer Therapy. Front. Oncol. 2021, 11, 626971. [Google Scholar] [CrossRef]
- Kunzmann, V.; Bauer, E.; Wilhelm, M. Gamma/delta T-cell stimulation by pamidronate. N. Engl. J. Med. 1999, 340, 737–738. [Google Scholar] [CrossRef]
- Tricarico, P.M.; Girardelli, M.; Kleiner, G.; Knowles, A.; Valencic, E.; Crovella, S.; Marcuzzi, A. Alendronate, a double-edged sword acting in the mevalonate pathway. Mol. Med. Rep. 2015, 12, 4238–4242. [Google Scholar] [CrossRef]
- Ebetino, F.H.; Sun, S.; Cherian, P.; Roshandel, S.; Neighbors, J.D.; Hu, E.; Dunford, J.E.; Sedghizadeh, P.P.; McKenna, C.E.; Srinivasan, V.; et al. Bisphosphonates: The role of chemistry in understanding their biological actions and structure-activity relationships, and new directions for their therapeutic use. Bone 2022, 156, 116289. [Google Scholar] [CrossRef]
- Thompson, K.; Rogers, M.J. Statins prevent bisphosphonate-induced γ,δ-T-cell proliferation and activation in vitro. J. Bone Miner. Res. 2004, 19, 278–288. [Google Scholar] [CrossRef]
- Moghadasian, M.H. Clinical pharmacology of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Life Sci. 1999, 65, 1329–1337. [Google Scholar] [CrossRef]
- van Beek, E.; Pieterman, E.; Cohen, L.; Löwik, C.; Papapoulos, S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem. Biophys. Res. Commun. 1999, 264, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Benzaïd, I.; Mönkkönen, H.; Stresing, V.; Bonnelye, E.; Green, J.; Mönkkönen, J.; Touraine, J.L.; Clézardin, P. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Res. 2011, 71, 4562–4572. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.K.; Fliesler, S.J. Mechanism of aminobisphosphonate action: Characterization of alendronate inhibition of the isoprenoid pathway. Biochem. Biophys. Res. Commun. 1999, 266, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Thiele, S.; Browne, A.J.; Rauner, M.; Zinna, V.M.; Hofbauer, L.C.; Rachner, T.D. Combined inhibition of the mevalonate pathway with statins and zoledronic acid potentiates their anti-tumor effects in human breast cancer cells. Cancer Lett. 2016, 375, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Nakamura, M.; Matsuda, R.; Nishimura, F.; Park, Y.S.; Motoyama, Y.; Hironaka, Y.; Nakagawa, I.; Yokota, H.; Yamada, S.; et al. Antitumor effects of minodronate, a third-generation nitrogen-containing bisphosphonate, in synergy with γδT cells in human glioblastoma in vitro and in vivo. J. Neurooncol. 2016, 129, 231–241. [Google Scholar] [CrossRef]
- Göbel, A.; Zinna, V.M.; Dell’Endice, S.; Jaschke, N.; Kuhlmann, J.D.; Wimberger, P.; Rachner, T.D. Anti-tumor effects of mevalonate pathway inhibition in ovarian cancer. BMC Cancer 2020, 20, 703. [Google Scholar] [CrossRef]
- Harly, C.; Guillaume, Y.; Nedellec, S.; Peigne, C.M.; Monkkonen, H.; Monkkonen, J.; Li, J.; Kuball, J.; Adams, E.J.; Netzer, S.; et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood 2012, 120, 2269–2279. [Google Scholar] [CrossRef]
- Rigau, M.; Ostrouska, S.; Fulford, T.S.; Johnson, D.N.; Woods, K.; Ruan, Z.; McWilliam, H.E.G.; Hudson, C.; Tutuka, C.; Wheatley, A.K.; et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 2020, 367, eaay5516. [Google Scholar] [CrossRef]
- Sandstrom, A.; Peigné, C.M.; Léger, A.; Crooks, J.E.; Konczak, F.; Gesnel, M.C.; Breathnach, R.; Bonneville, M.; Scotet, E.; Adams, E.J. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity 2014, 40, 490–500. [Google Scholar] [CrossRef]
- Vavassori, S.; Kumar, A.; Wan, G.S.; Ramanjaneyulu, G.S.; Cavallari, M.; El Daker, S.; Beddoe, T.; Theodossis, A.; Williams, N.K.; Gostick, E.; et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human gammadelta T cells. Nat. Immunol. 2013, 14, 908–916. [Google Scholar] [CrossRef]
- Gu, S.; Borowska, M.T.; Boughter, C.T.; Adams, E.J. Butyrophilin3A proteins and Vγ9Vδ2 T cell activation. Semin. Cell. Dev. Biol. 2018, 84, 65–74. [Google Scholar] [CrossRef]
- Arnett, H.A.; Viney, J.L. Immune modulation by butyrophilins. Nat. Rev. Immunol. 2014, 14, 559–569. [Google Scholar] [CrossRef]
- Jeong, J.; Rao, A.U.; Xu, J.; Ogg, S.L.; Hathout, Y.; Fenselau, C.; Mather, I.H. The PRY/SPRY/B30.2 domain of butyrophilin 1A1 (BTN1A1) binds to xanthine oxidoreductase: Implications for the function of BTN1A1 in the mammary gland and other tissues. J. Biol. Chem. 2009, 284, 22444–22456. [Google Scholar] [CrossRef]
- Gu, S.; Sachleben, J.R.; Boughter, C.T.; Nawrocka, W.I.; Borowska, M.T.; Tarrasch, J.T.; Skiniotis, G.; Roux, B.; Adams, E.J. Phosphoantigen-induced conformational change of butyrophilin 3A1 (BTN3A1) and its implication on Vγ9Vδ2 T cell activation. Proc. Natl. Acad. Sci. USA 2017, 114, E7311–E7320. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Yuan, L.; Zhou, X.; Duan, J.; Xiao, H.; Cai, N.; Han, S.; Ma, X.; Liu, W.; et al. A Structural Change in Butyrophilin upon Phosphoantigen Binding Underlies Phosphoantigen-Mediated Vγ9Vδ2 T Cell Activation. Immunity 2019, 50, 1043–1053.e1045. [Google Scholar] [CrossRef]
- Palakodeti, A.; Sandstrom, A.; Sundaresan, L.; Harly, C.; Nedellec, S.; Olive, D.; Scotet, E.; Bonneville, M.; Adams, E.J. The molecular basis for modulation of human Vγ9Vδ2 T cell responses by CD277/butyrophilin-3 (BTN3A)-specific antibodies. J. Biol. Chem. 2012, 287, 32780–32790. [Google Scholar] [CrossRef]
- Wang, H.; Henry, O.; Distefano, M.D.; Wang, Y.C.; Räikkönen, J.; Mönkkönen, J.; Tanaka, Y.; Morita, C.T. Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T cells. J. Immunol. 2013, 191, 1029–1042. [Google Scholar] [CrossRef]
- De Gassart, A.; Le, K.S.; Brune, P.; Agaugué, S.; Sims, J.; Goubard, A.; Castellano, R.; Joalland, N.; Scotet, E.; Collette, Y.; et al. Development of ICT01, a first-in-class, anti-BTN3A antibody for activating Vγ9Vδ2 T cell-mediated antitumor immune response. Sci. Transl. Med. 2021, 13, eabj0835. [Google Scholar] [CrossRef]
- Soriano-Sarabia, N.; Sandvold, H.; Jomaa, H.; Kubin, T.; Bein, G.; Hackstein, H. Primary MHC-class II+ cells are necessary to promote resting Vδ2 cell expansion in response to (E)-4-hydroxy-3-methyl-but-2-enyl-pyrophosphate and isopentenyl pyrophosphate. J. Immunol. 2012, 189, 5212–5222. [Google Scholar] [CrossRef]
- Landin, A.M.; Cox, C.; Yu, B.; Bejanyan, N.; Davila, M.; Kelley, L. Expansion and Enrichment of Gamma-Delta (γδ) T Cells from Apheresed Human Product. J. Vis. Exp. 2021, e62622. [Google Scholar] [CrossRef]
- Caccamo, N.; Dieli, F.; Wesch, D.; Jomaa, H.; Eberl, M. Sex-specific phenotypical and functional differences in peripheral human Vgamma9/Vdelta2 T cells. J. Leukoc. Biol. 2006, 79, 663–666. [Google Scholar] [CrossRef]
- Cairo, C.; Armstrong, C.L.; Cummings, J.S.; Deetz, C.O.; Tan, M.; Lu, C.; Davis, C.E.; Pauza, C.D. Impact of age, gender, and race on circulating γδ T cells. Hum. Immunol. 2010, 71, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Baker, F.L.; Bigley, A.B.; Agha, N.H.; Pedlar, C.R.; O’Connor, D.P.; Bond, R.A.; Bollard, C.M.; Katsanis, E.; Simpson, R.J. Systemic β-Adrenergic Receptor Activation Augments the ex vivo Expansion and Anti-Tumor Activity of Vγ9Vδ2 T-Cells. Front. Immunol. 2019, 10, 3082. [Google Scholar] [CrossRef] [PubMed]
- Clohosey, M.L.; Mann, B.T.; Ryan, P.L.; Apanasovich, T.V.; Maggirwar, S.B.; Pennington, D.J.; Soriano-Sarabia, N. Comparable Vδ2 Cell Functional Characteristics in Virally Suppressed People Living with HIV and Uninfected Individuals. Cells 2020, 9, 2568. [Google Scholar] [CrossRef] [PubMed]
- Burnham, R.E.; Zoine, J.T.; Story, J.Y.; Garimalla, S.N.; Gibson, G.; Rae, A.; Williams, E.; Bixby, L.; Archer, D.; Doering, C.B.; et al. Characterization of Donor Variability for γδ T Cell ex vivo Expansion and Development of an Allogeneic γδ T Cell Immunotherapy. Front. Med. 2020, 7, 588453. [Google Scholar] [CrossRef] [PubMed]
- Poccia, F.; Gioia, C.; Martini, F.; Sacchi, A.; Piacentini, P.; Tempestilli, M.; Agrati, C.; Amendola, A.; Abdeddaim, A.; Vlassi, C.; et al. Zoledronic acid and interleukin-2 treatment improves immunocompetence in HIV-infected persons by activating Vgamma9Vdelta2 T cells. Aids 2009, 23, 555–565. [Google Scholar] [CrossRef]
- Poonia, B. Adoptive transfer of aminobisphonate-expanded Vgamma9Vdelta2+ T cells does not control HIV replication in a humanized mouse model. Immunotherapy 2016, 8, 521–526. [Google Scholar] [CrossRef]
- Poonia, B.; Pauza, C.D. Gamma delta T cells from HIV+ donors can be expanded in vitro by zoledronate/interleukin-2 to become cytotoxic effectors for antibody-dependent cellular cytotoxicity. Cytotherapy 2012, 14, 173–181. [Google Scholar] [CrossRef]
- Wallace, M.; Bartz, S.R.; Chang, W.L.; Mackenzie, D.A.; Pauza, C.D.; Malkovsky, M. Gamma delta T lymphocyte responses to HIV. Clin. Exp. Immunol. 1996, 103, 177–184. [Google Scholar] [CrossRef]
- Ali, Z.; Yan, L.; Plagman, N.; Reichenberg, A.; Hintz, M.; Jomaa, H.; Villinger, F.; Chen, Z.W. Gammadelta T cell immune manipulation during chronic phase of simian-human immunodeficiency virus infection [corrected] confers immunological benefits. J. Immunol. 2009, 183, 5407–5417. [Google Scholar] [CrossRef]
- Garrido, C.; Clohosey, M.L.; Whitworth, C.P.; Hudgens, M.; Margolis, D.M.; Soriano-Sarabia, N. γδ T cells: An immunotherapeutic approach for HIV cure strategies. JCI Insight 2018, 3, e120121. [Google Scholar] [CrossRef]
- James, K.S.; Trumble, I.; Clohosey, M.L.; Moeser, M.; Roan, N.R.; Adimora, A.A.; Joseph, S.B.; Archin, N.M.; Hudgens, M.; Soriano-Sarabia, N. Measuring the contribution of gammadelta T cells to the persistent HIV reservoir. AIDS 2020, 34, 363–371. [Google Scholar] [CrossRef]
- Archin, N.M.; Sung, J.M.; Garrido, C.; Soriano-Sarabia, N.; Margolis, D.M. Eradicating HIV-1 infection: Seeking to clear a persistent pathogen. Nat. Rev. Microbiol. 2014, 12, 750–764. [Google Scholar] [CrossRef]
- Board, N.L.; Moskovljevic, M.; Wu, F.; Siliciano, R.F.; Siliciano, J.D. Engaging innate immunity in HIV-1 cure strategies. Nat. Rev. Immunol. 2021, 22, 499–512. [Google Scholar] [CrossRef]
- Fletcher, C.V.; Dyavar, S.R.; Acharya, A.; Byrareddy, S.N. The Contributions of Clinical Pharmacology to HIV Cure Research. Clin. Pharmacol. Ther. 2021, 110, 334–345. [Google Scholar] [CrossRef]
- Mann, B.T.; Sambrano, E., 3rd; Maggirwar, S.B.; Soriano-Sarabia, N. Boosting the Immune System for HIV Cure: A γδ T Cell Perspective. Front. Cell. Infect. Microbiol. 2020, 10, 221. [Google Scholar] [CrossRef]
- Bricker, K.M.; Chahroudi, A.; Mavigner, M. New Latency Reversing Agents for HIV-1 Cure: Insights from Nonhuman Primate Models. Viruses 2021, 13, 1560. [Google Scholar] [CrossRef]
- Chen, M.; Li, M.; Budai, M.M.; Rice, A.P.; Kimata, J.T.; Mohan, M.; Wang, J. Clearance of HIV-1 or SIV reservoirs by promotion of apoptosis and inhibition of autophagy: Targeting intracellular molecules in cure-directed strategies. J. Leukoc. Biol. 2022, 112, 1245–1259. [Google Scholar] [CrossRef]
- Caron, J.; Ridgley, L.A.; Bodman-Smith, M. How to Train Your Dragon: Harnessing Gamma Delta T Cells Antiviral Functions and Trained Immunity in a Pandemic Era. Front. Immunol. 2021, 12, 666983. [Google Scholar] [CrossRef]
- Wang, T.; Gao, Y.; Scully, E.; Davis, C.T.; Anderson, J.F.; Welte, T.; Ledizet, M.; Koski, R.; Madri, J.A.; Barrett, A.; et al. Gamma delta T cells facilitate adaptive immunity against West Nile virus infection in mice. J. Immunol. 2006, 177, 1825–1832. [Google Scholar] [CrossRef]
- Agrati, C.; Castilletti, C.; Cimini, E.; Romanelli, A.; Lapa, D.; Quartu, S.; Martini, F.; Capobianchi, M.R. Antiviral activity of human Vδ2 T-cells against WNV includes both cytolytic and non-cytolytic mechanisms. New Microbiol. 2016, 39, 139–142. [Google Scholar] [PubMed]
- Agrati, C.; Alonzi, T.; De Santis, R.; Castilletti, C.; Abbate, I.; Capobianchi, M.R.; D’Offizi, G.; Siepi, F.; Fimia, G.M.; Tripodi, M.; et al. Activation of Vgamma9Vdelta2 T cells by non-peptidic antigens induces the inhibition of subgenomic HCV replication. Int. Immunol. 2006, 18, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Cimini, E.; Bonnafous, C.; Bordoni, V.; Lalle, E.; Sicard, H.; Sacchi, A.; Berno, G.; Gioia, C.; D’Offizi, G.; Visco Comandini, U.; et al. Interferon-α improves phosphoantigen-induced Vγ9Vδ2 T-cells interferon-γ production during chronic HCV infection. PLoS ONE 2012, 7, e37014. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Yao, S.; Huang, D.; Wei, H.; Sicard, H.; Zeng, G.; Jomaa, H.; Larsen, M.H.; Jacobs, W.R., Jr.; Wang, R.; et al. Phosphoantigen/IL2 expansion and differentiation of Vγ2Vδ2 T cells increase resistance to tuberculosis in nonhuman primates. PLoS Pathog. 2013, 9, e1003501. [Google Scholar] [CrossRef] [PubMed]
- Qaqish, A.; Huang, D.; Chen, C.Y.; Zhang, Z.; Wang, R.; Li, S.; Yang, E.; Lu, Y.; Larsen, M.H.; Jacobs, W.R., Jr.; et al. Adoptive Transfer of Phosphoantigen-Specific γδ T Cell Subset Attenuates Mycobacterium tuberculosis Infection in Nonhuman Primates. J. Immunol. 2017, 198, 4753–4763. [Google Scholar] [CrossRef]
- Mazzola, T.N.; Da Silva, M.T.; Moreno, Y.M.; Lima, S.C.; Carniel, E.F.; Morcillo, A.M.; Antonio, M.A.; Zanolli, M.L.; Netto, A.A.; Blotta, M.H.; et al. Robust gammadelta+ T cell expansion in infants immunized at birth with BCG vaccine. Vaccine 2007, 25, 6313–6320. [Google Scholar] [CrossRef]
- Zufferey, C.; Germano, S.; Dutta, B.; Ritz, N.; Curtis, N. The contribution of non-conventional T cells and NK cells in the mycobacterial-specific IFNγ response in Bacille Calmette-Guérin (BCG)-immunized infants. PLoS ONE 2013, 8, e77334. [Google Scholar] [CrossRef]
- Shen, L.; Frencher, J.; Huang, D.; Wang, W.; Yang, E.; Chen, C.Y.; Zhang, Z.; Wang, R.; Qaqish, A.; Larsen, M.H.; et al. Immunization of Vγ2Vδ2 T cells programs sustained effector memory responses that control tuberculosis in nonhuman primates. Proc. Natl. Acad. Sci. USA 2019, 116, 6371–6378. [Google Scholar] [CrossRef]
- Hoft, D.F.; Brown, R.M.; Roodman, S.T. Bacille Calmette-Guérin vaccination enhances human gamma delta T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J. Immunol. 1998, 161, 1045–1054. [Google Scholar]
- Girardi, M.; Oppenheim, D.E.; Steele, C.R.; Lewis, J.M.; Glusac, E.; Filler, R.; Hobby, P.; Sutton, B.; Tigelaar, R.E.; Hayday, A.C. Regulation of cutaneous malignancy by gammadelta T cells. Science 2001, 294, 605–609. [Google Scholar] [CrossRef]
- Tosolini, M.; Pont, F.; Poupot, M.; Vergez, F.; Nicolau-Travers, M.L.; Vermijlen, D.; Sarry, J.E.; Dieli, F.; Fournie, J.J. Assessment of tumor-infiltrating TCRVgamma9Vdelta2 gammadelta lymphocyte abundance by deconvolution of human cancers microarrays. Oncoimmunology 2017, 6, e1284723. [Google Scholar] [CrossRef]
- Poccia, F.; Agrati, C.; Martini, F.; Capobianchi, M.R.; Wallace, M.; Malkovsky, M. Antiviral reactivities of gammadelta T cells. Microbes Infect. 2005, 7, 518–528. [Google Scholar] [CrossRef]
- Lafont, V.; Sanchez, F.; Laprevotte, E.; Michaud, H.A.; Gros, L.; Eliaou, J.F.; Bonnefoy, N. Plasticity of gammadelta T Cells: Impact on the Anti-Tumor Response. Front. Immunol. 2014, 5, 622. [Google Scholar] [CrossRef]
- Zou, C.; Zhao, P.; Xiao, Z.; Han, X.; Fu, F.; Fu, L. gammadelta T cells in cancer immunotherapy. Oncotarget 2017, 8, 8900–8909. [Google Scholar] [CrossRef]
- Zhao, Y.; Niu, C.; Cui, J. Gamma-delta (γδ) T cells: Friend or foe in cancer development? J. Transl. Med. 2018, 16, 3. [Google Scholar] [CrossRef]
- Silva-Santos, B.; Mensurado, S.; Coffelt, S.B. γδ T cells: Pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer 2019, 19, 392–404. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.K. Function of γδ T cells in tumor immunology and their application to cancer therapy. Exp. Mol. Med. 2021, 53, 318–327. [Google Scholar] [CrossRef]
- Shafi, S.; Vantourout, P.; Wallace, G.; Antoun, A.; Vaughan, R.; Stanford, M.; Hayday, A. An NKG2D-mediated human lymphoid stress surveillance response with high interindividual variation. Sci. Transl. Med. 2011, 3, 113ra124. [Google Scholar] [CrossRef]
- Correia, D.V.; Lopes, A.; Silva-Santos, B. Tumor cell recognition by γδ T lymphocytes: T-cell receptor vs. NK-cell receptors. Oncoimmunology 2013, 2, e22892. [Google Scholar] [CrossRef]
- Ochoa, M.C.; Minute, L.; Rodriguez, I.; Garasa, S.; Perez-Ruiz, E.; Inogés, S.; Melero, I.; Berraondo, P. Antibody-dependent cell cytotoxicity: Immunotherapy strategies enhancing effector NK cells. Immunol. Cell. Biol. 2017, 95, 347–355. [Google Scholar] [CrossRef]
- Tokuyama, H.; Hagi, T.; Mattarollo, S.R.; Morley, J.; Wang, Q.; So, H.F.; Moriyasu, F.; Nieda, M.; Nicol, A.J. V gamma 9 V delta 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs--rituximab and trastuzumab. Int. J. Cancer 2008, 122, 2526–2534. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, H.; Han, M.; Hwang, H.J.; Kim, H.; Im, H.J.; Kim, N.; Koh, K.N. Characteristics of Human Peripheral Blood γδ T Cells Expanded With Zoledronate. Anticancer Res. 2021, 41, 6031–6038. [Google Scholar] [CrossRef] [PubMed]
- Maniar, A.; Zhang, X.; Lin, W.; Gastman, B.R.; Pauza, C.D.; Strome, S.E.; Chapoval, A.I. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood 2010, 116, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Brandes, M.; Willimann, K.; Bioley, G.; Lévy, N.; Eberl, M.; Luo, M.; Tampé, R.; Lévy, F.; Romero, P.; Moser, B. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc. Natl. Acad. Sci. USA 2009, 106, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, V.; Bauer, E.; Feurle, J.; Weissinger, F.; Tony, H.P.; Wilhelm, M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000, 96, 384–392. [Google Scholar] [CrossRef]
- Wilhelm, M.; Kunzmann, V.; Eckstein, S.; Reimer, P.; Weissinger, F.; Ruediger, T.; Tony, H.P. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 2003, 102, 200–206. [Google Scholar] [CrossRef]
- Dieli, F.; Vermijlen, D.; Fulfaro, F.; Caccamo, N.; Meraviglia, S.; Cicero, G.; Roberts, A.; Buccheri, S.; D’Asaro, M.; Gebbia, N.; et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007, 67, 7450–7457. [Google Scholar] [CrossRef]
- Meraviglia, S.; Eberl, M.; Vermijlen, D.; Todaro, M.; Buccheri, S.; Cicero, G.; La Mendola, C.; Guggino, G.; D’Asaro, M.; Orlando, V.; et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin. Exp. Immunol. 2010, 161, 290–297. [Google Scholar] [CrossRef]
- Lang, J.M.; Kaikobad, M.R.; Wallace, M.; Staab, M.J.; Horvath, D.L.; Wilding, G.; Liu, G.; Eickhoff, J.C.; McNeel, D.G.; Malkovsky, M. Pilot trial of interleukin-2 and zoledronic acid to augment gammadelta T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol. Immunother. 2011, 60, 1447–1460. [Google Scholar] [CrossRef]
- Kunzmann, V.; Smetak, M.; Kimmel, B.; Weigang-Koehler, K.; Goebeler, M.; Birkmann, J.; Becker, J.; Schmidt-Wolf, I.G.; Einsele, H.; Wilhelm, M. Tumor-promoting versus tumor-antagonizing roles of gammadelta T cells in cancer immunotherapy: Results from a prospective phase I/II trial. J. Immunother. 2012, 35, 205–213. [Google Scholar] [CrossRef]
- Pressey, J.G.; Adams, J.; Harkins, L.; Kelly, D.; You, Z.; Lamb, L.S., Jr. In vivo expansion and activation of gammadelta T cells as immunotherapy for refractory neuroblastoma: A phase 1 study. Medicine 2016, 95, e4909. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tanaka, Y.; Yagi, J.; Osaka, Y.; Nakazawa, H.; Uchiyama, T.; Minato, N.; Toma, H. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: A pilot study. Cancer Immunol. Immunother. 2007, 56, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Bennouna, J.; Levy, V.; Sicard, H.; Senellart, H.; Audrain, M.; Hiret, S.; Rolland, F.; Bruzzoni-Giovanelli, H.; Rimbert, M.; Galea, C.; et al. Phase I study of bromohydrin pyrophosphate (BrHPP, IPH 1101), a Vgamma9Vdelta2 T lymphocyte agonist in patients with solid tumors. Cancer Immunol. Immunother. 2010, 59, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Bennouna, J.; Bompas, E.; Neidhardt, E.M.; Rolland, F.; Philip, I.; Galéa, C.; Salot, S.; Saiagh, S.; Audrain, M.; Rimbert, M.; et al. Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol. Immunother. 2008, 57, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Benzaïd, I.; Mönkkönen, H.; Bonnelye, E.; Mönkkönen, J.; Clézardin, P. In vivo phosphoantigen levels in bisphosphonate-treated human breast tumors trigger Vγ9Vδ2 T-cell antitumor cytotoxicity through ICAM-1 engagement. Clin. Cancer Res. 2012, 18, 6249–6259. [Google Scholar] [CrossRef]
- Abe, Y.; Muto, M.; Nieda, M.; Nakagawa, Y.; Nicol, A.; Kaneko, T.; Goto, S.; Yokokawa, K.; Suzuki, K. Clinical and immunological evaluation of zoledronate-activated Vgamma9gammadelta T-cell-based immunotherapy for patients with multiple myeloma. Exp. Hematol. 2009, 37, 956–968. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tanaka, Y.; Shimmura, H.; Minato, N.; Tanabe, K. Complete remission of lung metastasis following adoptive immunotherapy using activated autologous gammadelta T-cells in a patient with renal cell carcinoma. Anticancer Res. 2010, 30, 575–579. [Google Scholar]
- Nakajima, J.; Murakawa, T.; Fukami, T.; Goto, S.; Kaneko, T.; Yoshida, Y.; Takamoto, S.; Kakimi, K. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. Eur. J. Cardiothorac. Surg. 2010, 37, 1191–1197. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tanaka, Y.; Nakazawa, H.; Yagi, J.; Minato, N.; Tanabe, K. A new indicator of favorable prognosis in locally advanced renal cell carcinomas: Gamma delta T-cells in peripheral blood. Anticancer Res. 2011, 31, 1027–1031. [Google Scholar]
- Sakamoto, M.; Nakajima, J.; Murakawa, T.; Fukami, T.; Yoshida, Y.; Murayama, T.; Takamoto, S.; Matsushita, H.; Kakimi, K. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded γδTcells: A phase I clinical study. J. Immunother. 2011, 34, 202–211. [Google Scholar] [CrossRef]
- Nicol, A.J.; Tokuyama, H.; Mattarollo, S.R.; Hagi, T.; Suzuki, K.; Yokokawa, K.; Nieda, M. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br. J. Cancer 2011, 105, 778–786. [Google Scholar] [CrossRef]
- Fujimi, A.; Kamihara, Y.; Kanisawa, Y.; Hashimoto, A.; Nakajima, C.; Hayasaka, N.; Uemura, N.; Okuda, T.; Minami, S.; Iyama, S.; et al. Anti-erythropoietin receptor antibody-associated pure red cell aplasia accompanied by Coombs-negative autoimmune hemolytic anemia in a patient with T cell/histiocyte-rich large B cell lymphoma. Int. J. Hematol. 2014, 100, 490–493. [Google Scholar] [CrossRef]
- Aoki, T.; Matsushita, H.; Hoshikawa, M.; Hasegawa, K.; Kokudo, N.; Kakimi, K. Adjuvant combination therapy with gemcitabine and autologous γδ T-cell transfer in patients with curatively resected pancreatic cancer. Cytotherapy 2017, 19, 473–485. [Google Scholar] [CrossRef]
- Kakimi, K.; Matsushita, H.; Masuzawa, K.; Karasaki, T.; Kobayashi, Y.; Nagaoka, K.; Hosoi, A.; Ikemura, S.; Kitano, K.; Kawada, I.; et al. Adoptive transfer of zoledronate-expanded autologous Vγ9Vδ2 T-cells in patients with treatment-refractory non-small-cell lung cancer: A multicenter, open-label, single-arm, phase 2 study. J. Immunother. Cancer 2020, 8, e001185. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, X.; Liang, S.; Luo, H.; Alnaggar, M.; Liu, A.; Yin, Z.; Chen, J.; Niu, L.; Jiang, Y. Irreversible electroporation plus allogenic Vγ9Vδ2 T cells enhances antitumor effect for locally advanced pancreatic cancer patients. Signal Transduct. Target. Ther. 2020, 5, 215. [Google Scholar] [CrossRef]
- Wilhelm, M.; Smetak, M.; Schaefer-Eckart, K.; Kimmel, B.; Birkmann, J.; Einsele, H.; Kunzmann, V. Successful adoptive transfer and in vivo expansion of haploidentical γδ T cells. J. Transl. Med. 2014, 12, 45. [Google Scholar] [CrossRef]
- Alnaggar, M.; Xu, Y.; Li, J.; He, J.; Chen, J.; Li, M.; Wu, Q.; Lin, L.; Liang, Y.; Wang, X.; et al. Allogenic Vγ9Vδ2 T cell as new potential immunotherapy drug for solid tumor: A case study for cholangiocarcinoma. J. Immunother. Cancer 2019, 7, 36. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, J.; Niu, L.; Liu, Y.; Ye, G.; Jiang, M.; Qi, Z. Clinical Safety and Efficacy of Locoregional Therapy Combined with Adoptive Transfer of Allogeneic γδ T Cells for Advanced Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Vasc. Interv. Radiol. 2022, 33, 19–27.e13. [Google Scholar] [CrossRef]
- Xu, Y.; Xiang, Z.; Alnaggar, M.; Kouakanou, L.; Li, J.; He, J.; Yang, J.; Hu, Y.; Chen, Y.; Lin, L.; et al. Allogeneic Vgamma9Vdelta2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell. Mol. Immunol. 2021, 18, 427–439. [Google Scholar] [CrossRef]
- Jhita, N.; Raikar, S.S. Allogeneic gamma delta T cells as adoptive cellular therapy for hematologic malignancies. Explor. Immunol. 2022, 2, 334–350. [Google Scholar] [CrossRef]
- Kang, N.; Zhou, J.; Zhang, T.; Wang, L.; Lu, F.; Cui, Y.; Cui, L.; He, W. Adoptive immunotherapy of lung cancer with immobilized anti-TCRgammadelta antibody-expanded human gammadelta T-cells in peripheral blood. Cancer Biol. Ther. 2009, 8, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Meidenbauer, N.; Marienhagen, J.; Laumer, M.; Vogl, S.; Heymann, J.; Andreesen, R.; Mackensen, A. Survival and tumor localization of adoptively transferred Melan-A-specific T cells in melanoma patients. J. Immunol. 2003, 170, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Lamb, L.S., Jr.; Henslee-Downey, P.J.; Parrish, R.S.; Godder, K.; Thompson, J.; Lee, C.; Gee, A.P. Increased frequency of TCR gamma delta + T cells in disease-free survivors following T cell-depleted, partially mismatched, related donor bone marrow transplantation for leukemia. J. Hematother. 1996, 5, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Godder, K.T.; Henslee-Downey, P.J.; Mehta, J.; Park, B.S.; Chiang, K.Y.; Abhyankar, S.; Lamb, L.S. Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 2007, 39, 751–757. [Google Scholar] [CrossRef]
- Chauvin, C.; Joalland, N.; Perroteau, J.; Jarry, U.; Lafrance, L.; Willem, C.; Retière, C.; Oliver, L.; Gratas, C.; Gautreau-Rolland, L.; et al. NKG2D Controls Natural Reactivity of Vγ9Vδ2 T Lymphocytes against Mesenchymal Glioblastoma Cells. Clin. Cancer Res. 2019, 25, 7218–7228. [Google Scholar] [CrossRef]
- Ravens, S.; Fichtner, A.S.; Willers, M.; Torkornoo, D.; Pirr, S.; Schoning, J.; Deseke, M.; Sandrock, I.; Bubke, A.; Wilharm, A.; et al. Microbial exposure drives polyclonal expansion of innate gammadelta T cells immediately after birth. Proc. Natl. Acad. Sci. USA 2020, 117, 18649–18660. [Google Scholar] [CrossRef]
- Dimova, T.; Brouwer, M.; Gosselin, F.; Tassignon, J.; Leo, O.; Donner, C.; Marchant, A.; Vermijlen, D. Effector Vγ9Vδ2 T cells dominate the human fetal γδ T-cell repertoire. Proc. Natl. Acad. Sci. USA 2015, 112, E556–E565. [Google Scholar] [CrossRef]
- Davey, M.S.; Willcox, C.R.; Hunter, S.; Kasatskaya, S.A.; Remmerswaal, E.B.M.; Salim, M.; Mohammed, F.; Bemelman, F.J.; Chudakov, D.M.; Oo, Y.H.; et al. The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9− subsets. Nat. Commun. 2018, 9, 1760. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Anguille, S.; Willemen, Y.; Van den Bergh, J.M.; Berneman, Z.N.; Lion, E.; Smits, E.L.; Van Tendeloo, V.F. Interleukin-15 enhances the proliferation, stimulatory phenotype, and antitumor effector functions of human gamma delta T cells. J. Hematol. Oncol. 2016, 9, 101. [Google Scholar] [CrossRef]
- Capsomidis, A.; Benthall, G.; Van Acker, H.H.; Fisher, J.; Kramer, A.M.; Abeln, Z.; Majani, Y.; Gileadi, T.; Wallace, R.; Gustafsson, K.; et al. Chimeric Antigen Receptor-Engineered Human Gamma Delta T Cells: Enhanced Cytotoxicity with Retention of Cross Presentation. Mol. Ther. 2018, 26, 354–365. [Google Scholar] [CrossRef]
- Mariani, S.; Muraro, M.; Pantaleoni, F.; Fiore, F.; Nuschak, B.; Peola, S.; Foglietta, M.; Palumbo, A.; Coscia, M.; Castella, B.; et al. Effector gammadelta T cells and tumor cells as immune targets of zoledronic acid in multiple myeloma. Leukemia 2005, 19, 664–670. [Google Scholar] [CrossRef]
- Fisher, J.P.; Yan, M.; Heuijerjans, J.; Carter, L.; Abolhassani, A.; Frosch, J.; Wallace, R.; Flutter, B.; Capsomidis, A.; Hubank, M.; et al. Neuroblastoma killing properties of Vδ2 and Vδ2-negative γδT cells following expansion by artificial antigen-presenting cells. Clin. Cancer Res. 2014, 20, 5720–5732. [Google Scholar] [CrossRef]
- Kondo, M.; Izumi, T.; Fujieda, N.; Kondo, A.; Morishita, T.; Matsushita, H.; Kakimi, K. Expansion of human peripheral blood γδ T cells using zoledronate. J. Vis. Exp. 2011, e3182. [Google Scholar] [CrossRef]
- Khan, M.W.A.; Otaibi, A.A.; Sherwani, S.; Alshammari, E.M.; Al-Zahrani, S.A.; Khan, W.A.; Alsukaibi, A.K.D.; Alouffi, S.; Khan, S.N. Optimization of methods for peripheral blood mononuclear cells isolation and expansion of human gamma delta T cells. Bioinformation 2021, 17, 460–469. [Google Scholar] [CrossRef]
- Schilbach, K.; Krickeberg, N.; Kaißer, C.; Mingram, S.; Kind, J.; Siegers, G.M.; Hashimoto, H. Suppressive activity of Vδ2+ γδ T cells on αβ T cells is licensed by TCR signaling and correlates with signal strength. Cancer Immunol. Immunother. 2020, 69, 593–610. [Google Scholar] [CrossRef]
- Duault, C.; Franchini, D.M.; Familliades, J.; Cayrol, C.; Roga, S.; Girard, J.P.; Fournié, J.J.; Poupot, M. TCRVγ9 γδ T Cell Response to IL-33: A CD4 T Cell-Dependent Mechanism. J. Immunol. 2016, 196, 493–502. [Google Scholar] [CrossRef]
- Jonus, H.C.; Burnham, R.E.; Ho, A.; Pilgrim, A.A.; Shim, J.; Doering, C.B.; Spencer, H.T.; Goldsmith, K.C. Dissecting the cellular components of ex vivo γδ T cell expansions to optimize selection of potent cell therapy donors for neuroblastoma immunotherapy trials. Oncoimmunology 2022, 11, 2057012. [Google Scholar] [CrossRef]
- Kouakanou, L.; Peters, C.; Sun, Q.; Floess, S.; Bhat, J.; Huehn, J.; Kabelitz, D. Vitamin C supports conversion of human gammadelta T cells into FOXP3-expressing regulatory cells by epigenetic regulation. Sci. Rep. 2020, 10, 6550. [Google Scholar] [CrossRef]
- Schilbach, K.; Welker, C.; Krickeberg, N.; Kaisser, C.; Schleicher, S.; Hashimoto, H. In the Absence of a TCR Signal IL-2/IL-12/18-Stimulated gammadelta T Cells Demonstrate Potent Anti-Tumoral Function Through Direct Killing and Senescence Induction in Cancer Cells. Cancers 2020, 12, 130. [Google Scholar] [CrossRef]
- Domae, E.; Hirai, Y.; Ikeo, T.; Goda, S.; Shimizu, Y. Cytokine-mediated activation of human ex vivo-expanded Vgamma9Vdelta2 T cells. Oncotarget 2017, 8, 45928–45942. [Google Scholar] [CrossRef] [PubMed]
- García, V.E.; Jullien, D.; Song, M.; Uyemura, K.; Shuai, K.; Morita, C.T.; Modlin, R.L. IL-15 enhances the response of human gamma delta T cells to nonpeptide [correction of nonpetide] microbial antigens. J. Immunol. 1998, 160, 4322–4329. [Google Scholar] [PubMed]
- Li, W.; Kubo, S.; Okuda, A.; Yamamoto, H.; Ueda, H.; Tanaka, T.; Nakamura, H.; Yamanishi, H.; Terada, N.; Okamura, H. Effect of IL-18 on expansion of gammadelta T cells stimulated by zoledronate and IL-2. J. Immunother. 2010, 33, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, O.; Gruenbacher, G.; Gander, H.; Komuczki, J.; Rahm, A.; Thurnher, M. Essential requirements of zoledronate-induced cytokine and γδ T cell proliferative responses. J. Immunol. 2013, 191, 1346–1355. [Google Scholar] [CrossRef]
- Hudspeth, K.; Fogli, M.; Correia, D.V.; Mikulak, J.; Roberto, A.; Della Bella, S.; Silva-Santos, B.; Mavilio, D. Engagement of NKp30 on Vδ1 T cells induces the production of CCL3, CCL4, and CCL5 and suppresses HIV-1 replication. Blood 2012, 119, 4013–4016. [Google Scholar] [CrossRef]
- Correia, D.V.; Fogli, M.; Hudspeth, K.; da Silva, M.G.; Mavilio, D.; Silva-Santos, B. Differentiation of human peripheral blood Vδ1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood 2011, 118, 992–1001. [Google Scholar] [CrossRef]
- Mikulak, J.; Oriolo, F.; Bruni, E.; Roberto, A.; Colombo, F.S.; Villa, A.; Bosticardo, M.; Bortolomai, I.; Lo Presti, E.; Meraviglia, S.; et al. NKp46-expressing human gut-resident intraepithelial Vδ1 T cell subpopulation exhibits high antitumor activity against colorectal cancer. JCI Insight 2019, 4, e125884. [Google Scholar] [CrossRef]
- von Lilienfeld-Toal, M.; Nattermann, J.; Feldmann, G.; Sievers, E.; Frank, S.; Strehl, J.; Schmidt-Wolf, I.G. Activated gammadelta T cells express the natural cytotoxicity receptor natural killer p 44 and show cytotoxic activity against myeloma cells. Clin. Exp. Immunol. 2006, 144, 528–533. [Google Scholar] [CrossRef]
- Groh, V.; Steinle, A.; Bauer, S.; Spies, T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 1998, 279, 1737–1740. [Google Scholar] [CrossRef]
- Chalupny, N.J.; Sutherland, C.L.; Lawrence, W.A.; Rein-Weston, A.; Cosman, D. ULBP4 is a novel ligand for human NKG2D. Biochem. Biophys. Res. Commun. 2003, 305, 129–135. [Google Scholar] [CrossRef]
- Bauer, S.; Groh, V.; Wu, J.; Steinle, A.; Phillips, J.H.; Lanier, L.L.; Spies, T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999, 285, 727–729. [Google Scholar] [CrossRef]
- Lamb, L.S., Jr.; Bowersock, J.; Dasgupta, A.; Gillespie, G.Y.; Su, Y.; Johnson, A.; Spencer, H.T. Engineered drug resistant γδ T cells kill glioblastoma cell lines during a chemotherapy challenge: A strategy for combining chemo- and immunotherapy. PLoS ONE 2013, 8, e51805. [Google Scholar] [CrossRef]
- Shimizu, T.; Tomogane, M.; Miyashita, M.; Ukimura, O.; Ashihara, E. Low dose gemcitabine increases the cytotoxicity of human Vγ9Vδ2 T cells in bladder cancer cells in vitro and in an orthotopic xenograft model. Oncoimmunology 2018, 7, e1424671. [Google Scholar] [CrossRef]
- Bhat, J.; Dubin, S.; Dananberg, A.; Quabius, E.S.; Fritsch, J.; Dowds, C.M.; Saxena, A.; Chitadze, G.; Lettau, M.; Kabelitz, D. Histone Deacetylase Inhibitor Modulates NKG2D Receptor Expression and Memory Phenotype of Human Gamma/Delta T Cells Upon Interaction With Tumor Cells. Front. Immunol. 2019, 10, 569. [Google Scholar] [CrossRef]
- Joncker, N.T.; Fernandez, N.C.; Treiner, E.; Vivier, E.; Raulet, D.H. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: The rheostat model. J. Immunol. 2009, 182, 4572–4580. [Google Scholar] [CrossRef]
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef]
- Abd Hamid, M.; Wang, R.Z.; Yao, X.; Fan, P.; Li, X.; Chang, X.M.; Feng, Y.; Jones, S.; Maldonado-Perez, D.; Waugh, C.; et al. Enriched HLA-E and CD94/NKG2A Interaction Limits Antitumor CD8+ Tumor-Infiltrating T Lymphocyte Responses. Cancer Immunol. Res. 2019, 7, 1293–1306. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Gooden, M.; Lampen, M.; Jordanova, E.S.; Leffers, N.; Trimbos, J.B.; van der Burg, S.H.; Nijman, H.; van Hall, T. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8⁺ T lymphocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 10656–10661. [Google Scholar] [CrossRef]
- Bossard, C.; Bézieau, S.; Matysiak-Budnik, T.; Volteau, C.; Laboisse, C.L.; Jotereau, F.; Mosnier, J.F. HLA-E/β2 microglobulin overexpression in colorectal cancer is associated with recruitment of inhibitory immune cells and tumor progression. Int. J. Cancer 2012, 131, 855–863. [Google Scholar] [CrossRef]
- Iwasaki, M.; Tanaka, Y.; Kobayashi, H.; Murata-Hirai, K.; Miyabe, H.; Sugie, T.; Toi, M.; Minato, N. Expression and function of PD-1 in human γδ T cells that recognize phosphoantigens. Eur. J. Immunol. 2011, 41, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, D.; Biswas, D.; Borkakoty, B.; Mahanta, J. Exposure to Plasmodium vivax is associated with the increased expression of exhaustion markers on γδ T lymphocytes. Parasite Immunol. 2018, 40, e12594. [Google Scholar] [CrossRef] [PubMed]
- Girard, P.; Charles, J.; Cluzel, C.; Degeorges, E.; Manches, O.; Plumas, J.; De Fraipont, F.; Leccia, M.T.; Mouret, S.; Chaperot, L.; et al. The features of circulating and tumor-infiltrating γδ T cells in melanoma patients display critical perturbations with prognostic impact on clinical outcome. Oncoimmunology 2019, 8, 1601483. [Google Scholar] [CrossRef] [PubMed]
- Cimini, E.; Grassi, G.; Beccacece, A.; Casetti, R.; Castilletti, C.; Capobianchi, M.R.; Nicastri, E.; Agrati, C. In Acute Dengue Infection, High TIM-3 Expression May Contribute to the Impairment of IFNγ Production by Circulating Vδ2 T Cells. Viruses 2022, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Hoeres, T.; Holzmann, E.; Smetak, M.; Birkmann, J.; Wilhelm, M. PD-1 signaling modulates interferon-γ production by Gamma Delta (γδ) T-Cells in response to leukemia. Oncoimmunology 2019, 8, 1550618. [Google Scholar] [CrossRef]
- Nada, M.H.; Wang, H.; Hussein, A.J.; Tanaka, Y.; Morita, C.T. PD-1 checkpoint blockade enhances adoptive immunotherapy by human Vγ2Vδ2 T cells against human prostate cancer. Oncoimmunology 2021, 10, 1989789. [Google Scholar] [CrossRef]
- Han, M.G.; Jang, B.S.; Kang, M.H.; Na, D.; Kim, I.A. PI3Kγδ inhibitor plus radiation enhances the antitumour immune effect of PD-1 blockade in syngenic murine breast cancer and humanised patient-derived xenograft model. Eur. J. Cancer 2021, 157, 450–463. [Google Scholar] [CrossRef]
- Li, X.; Lu, H.; Gu, Y.; Zhang, X.; Zhang, G.; Shi, T.; Chen, W. Tim-3 suppresses the killing effect of Vγ9Vδ2 T cells on colon cancer cells by reducing perforin and granzyme B expression. Exp. Cell. Res. 2020, 386, 111719. [Google Scholar] [CrossRef]
- Wistuba-Hamprecht, K.; Martens, A.; Haehnel, K.; Geukes Foppen, M.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; et al. Proportions of blood-borne Vδ1+ and Vδ2+ T-cells are associated with overall survival of melanoma patients treated with ipilimumab. Eur. J. Cancer 2016, 64, 116–126. [Google Scholar] [CrossRef]
- Zumwalde, N.A.; Sharma, A.; Xu, X.; Ma, S.; Schneider, C.L.; Romero-Masters, J.C.; Hudson, A.W.; Gendron-Fitzpatrick, A.; Kenney, S.C.; Gumperz, J.E. Adoptively transferred Vγ9Vδ2 T cells show potent antitumor effects in a preclinical B cell lymphomagenesis model. JCI Insight 2017, 2, e93179. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, P.; Zhang, Z.; Zhang, J.; Zhang, Z.; Hua, Y.; Han, B.; Li, N.; Zhao, X.; Hou, L. TIM-3 blockade combined with bispecific antibody MT110 enhances the anti-tumor effect of γδ T cells. Cancer Immunol. Immunother. 2020, 69, 2571–2587. [Google Scholar] [CrossRef]
- Akdis, M.; Burgler, S.; Crameri, R.; Eiwegger, T.; Fujita, H.; Gomez, E.; Klunker, S.; Meyer, N.; O’Mahony, L.; Palomares, O.; et al. Interleukins, from 1 to 37, and interferon-γ: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2011, 127, 701–721.e70. [Google Scholar] [CrossRef]
- Taniguchi, T.; Matsui, H.; Fujita, T.; Takaoka, C.; Kashima, N.; Yoshimoto, R.; Hamuro, J. Structure and expression of a cloned cDNA for human interleukin-2. Nature 1983, 302, 305–310. [Google Scholar] [CrossRef]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Pilipow, K.; Roberto, A.; Roederer, M.; Waldmann, T.A.; Mavilio, D.; Lugli, E. IL15 and T-cell Stemness in T-cell-Based Cancer Immunotherapy. Cancer Res. 2015, 75, 5187–5193. [Google Scholar] [CrossRef]
- Steel, J.C.; Waldmann, T.A.; Morris, J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012, 33, 35–41. [Google Scholar] [CrossRef]
- Viey, E.; Lucas, C.; Romagne, F.; Escudier, B.; Chouaib, S.; Caignard, A. Chemokine receptors expression and migration potential of tumor-infiltrating and peripheral-expanded Vgamma9Vdelta2 T cells from renal cell carcinoma patients. J. Immunother. 2008, 31, 313–323. [Google Scholar] [CrossRef]
- Wu, K.; Feng, J.; Xiu, Y.; Li, Z.; Lin, Z.; Zhao, H.; Zeng, H.; Xia, W.; Yu, L.; Xu, B. Vδ2 T cell subsets, defined by PD-1 and TIM-3 expression, present varied cytokine responses in acute myeloid leukemia patients. Int. Immunopharmacol. 2020, 80, 106122. [Google Scholar] [CrossRef]
- Wu, K.; Zhao, H.; Xiu, Y.; Li, Z.; Zhao, J.; Xie, S.; Zeng, H.; Zhang, H.; Yu, L.; Xu, B. IL-21-mediated expansion of Vγ9Vδ2 T cells is limited by the Tim-3 pathway. Int. Immunopharmacol. 2019, 69, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Tsutsi, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K.; et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995, 378, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin. Nephrol. 2007, 27, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Yoshimoto, T.; Tsutsui, H.; Okamura, H. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 2001, 19, 423–474. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Coker, R. Transforming growth factor-beta (TGF-beta). Int. J. Biochem. Cell. Biol. 1998, 30, 293–298. [Google Scholar] [CrossRef]
- Peters, C.; Häsler, R.; Wesch, D.; Kabelitz, D. Human Vδ2 T cells are a major source of interleukin-9. Proc. Natl. Acad. Sci. USA 2016, 113, 12520–12525. [Google Scholar] [CrossRef]
- Siegers, G.M. Integral Roles for Integrins in γδ T Cell Function. Front. Immunol. 2018, 9, 521. [Google Scholar] [CrossRef]
- Beatson, R.E.; Parente-Pereira, A.C.; Halim, L.; Cozzetto, D.; Hull, C.; Whilding, L.M.; Martinez, O.; Taylor, C.A.; Obajdin, J.; Luu Hoang, K.N.; et al. TGF-beta1 potentiates Vgamma9Vdelta2 T cell adoptive immunotherapy of cancer. Cell Rep. Med. 2021, 2, 100473. [Google Scholar] [CrossRef]
- D’Aniello, C.; Cermola, F.; Patriarca, E.J.; Minchiotti, G. Vitamin C in Stem Cell Biology: Impact on Extracellular Matrix Homeostasis and Epigenetics. Stem Cells Int. 2017, 2017, 8936156. [Google Scholar] [CrossRef]
- Mastrangelo, D.; Pelosi, E.; Castelli, G.; Lo-Coco, F.; Testa, U. Mechanisms of anti-cancer effects of ascorbate: Cytotoxic activity and epigenetic modulation. Blood Cells Mol. Dis. 2018, 69, 57–64. [Google Scholar] [CrossRef]
- Tu, W.; Zheng, J.; Liu, Y.; Sia, S.F.; Liu, M.; Qin, G.; Ng, I.H.; Xiang, Z.; Lam, K.T.; Peiris, J.S.; et al. The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a gammadelta T cell population in humanized mice. J. Exp. Med. 2011, 208, 1511–1522. [Google Scholar] [CrossRef]
| Cancer Type | Approach | N | Expansion Method | Ref. | |
|---|---|---|---|---|---|
| Autologous | Advanced RCC | Adoptive therapy | 7 | 100 mM 2M3B1-PP + 100 U/mL IL-2, 14 days | [112] |
| Advanced RCC | Adoptive therapy + low dose IL-2 | 10 | 3 mM BrHPP + 20–60 ng/mL IL-2, 14 days | [114] | |
| MM | Adoptive therapy | 6 | 5 mM Zol + 1000 U/mL IL-2, 14 days | [116] | |
| RCC and multiple lung metastasis | Adoptive therapy + Zol + low dose IL-2 | 1 | 4 mg Zol + 1.4 million unit of IL-2 | [117] | |
| NSCLC* | Autologous gd infusion | 10 | 5 mM Zol + 1000 U/mL IL-2, 14 days | [118] | |
| Advanced RCC and lung metastasis | Adoptive therapy + Zol + low dose IL-2 | 41 | 100 mM 2M3B1-PP + 100 U/mL IL-2, 11 days | [119] | |
| NSCLC | Adoptive therapy | 15 | 5 mM Zol + 1000 U/mL IL-2, 14 days | [120] | |
| Advanced solid tumors | Adoptive therapy + Zol | 18 | 1 mM Zol + 700 U/mL IL-2 | [121] | |
| Gastric | Adoptive therapy + Zol | 7 | 5 mM Zol + 1000 U/mL IL-2, 14 days | [122] | |
| Pancreatic | Adoptive therapy + Gemcitabine | 56 | 5 mM Zol + 1000 U/mL IL-2, 14 days | [123] | |
| NSCLC | Adoptive therapy | 25 | 5 mM Zol + 1000 U/mL IL-2, 14 days | [124] | |
| Advanced pancreatic | Electroporation + adoptive therapy | 62 | 50 mM Zol + 10 ng/mL IL-2 | [125] | |
| Allogeneic | Cancer type | Approach | N | Expansion method | Ref. |
| Hematological malignancies | Haploidentical adoptive therapy | 4 | CD4 and CD8 depletion | [126] | |
| Cholangiocarcinoma, liver transplanted | Adoptive therapy | 1 | patent pending: not shown in paper | [127] | |
| Advanced HCC (N = 30)/ICC (N = 29) | Locoregional + adoptive therapy | 59 | 50 mM Zol + 100 U/mL IL-2 + 100 U/mL IL-15 + 70 mM Vit C, 12–14 days | [128] | |
| Late-stage lung or liver | Adoptive therapy | 18 | 50 mM Zol + 100 U/mL IL-2 + 100 U/mL IL-15 + 70 mM Vit C, 12–14 days | [129] |
| CK | Concentration | pAg or BP | Additional Stimuli | Condition | Effect in γδ T cells | Target | Ref. |
|---|---|---|---|---|---|---|---|
| IL-2 | 50 U/mL | Zol (2.5 µM) | TGF-ß | PBMCs | IFN, GrzA, GrzB, Perforin, TNF, adhesion molecules | Pancreas and colon tumor cells | [18] |
| 100 U/mL | Synthetic HMBPP | - | PBMCs | IFN, TNF, IL5, IL-13 | n/a | [28] | |
| 20 ng/mL | HDMAPP | - | PBMCs | Proliferation, CD56, NKG2D/A, GrzA/B, Perforin | RCC and Burkitt’s lymphoma | [29] | |
| 100 U/mL | IPP or ZOL | - | Isolated γδ | HLA-DR, CD69, CD56, CD16, IFN | Burkitt’s lymphoma and MM | [140] | |
| 100 U/mL | ZOL (40µg/mL) | - | PBMCs | CD25, CD69, CD94, CD152, ICAM NKG2D, IFN, TNF | Human Neuroblastoma LAN 1 | [141] | |
| 10 U/mL | ZOL (1µM) | - | PBMCs | IFN, TNF | MM | [142] | |
| 100 U/mL | IPP | aAPC, Anti-γδ TCR mAb | PBMCs/Isolated γδ | NKG2D, cytotoxicity | Neuroblastoma | [143] | |
| 1000 U/mL | Zol (5 µM) | - | PBMCs | NKG2D, CD69, IFN | n/a | [144] | |
| 100 U/mL | Zol (1µM) | IL-15 | PBMCs | CD69, HLA-DR, CD80, CD86 | n/a | [145] | |
| 6.5 U/ML | IPP or TGF-B | Anti-γδ TCR mAb | Isolated γδ | GrzB, perforin, CD107a | Glioblastoma and melanoma | [146] | |
| 400 U/mL | BrHPP (100 nM) | IL-33 | PBMCs | CD16, CD28, NKG2A, NKG2D, Perforin, IFN, TNF, GrzB | Burkitt´s lymphoma | [147] | |
| CXCR3, CD28, CCR5, Trail | |||||||
| 15.2 ng/mL | Zol (5 µM) | IL-15 | Isolated γδ | NKG2D, NKG2A | Neuroblastoma cell lines | [148] | |
| 50 U/mL | BrHPP (300 nM) | Vitamin C, TGF-ß | Isolated γδ | Proliferation | n/a | [149] | |
| IL-12 | 10 ng/mL | IMMU510 | IL2,IL-15,IL-18 | Isolated γδ | IFN, TNF, GrzB, Perforin | sarcoma, rhabdomyosarcoma, neuroblastoma | [150] |
| 10 ng/mL | IL-18 | PBMCs | IFN | n/a | [151] | ||
| IL-15 | 10 ng/mL | Zol (2.5 µM) | TGF-ß | PBMCs | IFN, GrzA, GrzB, Perforin, TNF, adhesion molecules | Pancreas and colon tumor cells | [18] |
| 10 ng/mL | HMBPP | - | PBMCs | Proliferation, TNF, IFN, CD16, CD94 | n/a | [27] | |
| 12.5 ng/mL | IPP or ZOL | - | Isolated γδ | Cytotoxicity, HLA-DR, CD69, CD56, CD16, IFN | Burkitt´s lymphoma and MM | [140] | |
| 50 ng/mL | IPP or TGF-B | Anti-γδ TCR mAb | Isolated γδ | GrzB, perforin, CD107a | Glioblastoma and melanoma | [146] | |
| 10 ng/mL | IPP (30µM) | IL-12 | PBMCs | Proliferation, IFN, | n/a | [152] | |
| IL-18 | 50 ng/mL | ZOL (1µM) | IL-2 | PBMCs | IFN, TNF, cytotoxicity | Mesothelioma | [153] |
| 200 ng/mL | Zol (10 or 30 µM) | IL-1β, IL-2 | PBMCs/Isolated γδ | Cytokine response | n/a | [154] | |
| IL-21 | 10 ng/mL | HMBPP | - | PBMCs | Proliferation, CD16, CD94 | n/a | [27] |
| 10 ng/mL | Synthetic HMBPP | - | PBMCs | CD25, CD27, CD69, NKG2D | n/a | [28] | |
| 10 ng/mL | HDMAPP | - | PBMCs | Proliferation, CD56, NKG2D, GrzA, GrzB, Perforin | RCC and Burkitt´s lymphoma | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz, M.; Mann, B.T.; Chitrakar, A.; Soriano-Sarabia, N. Human Vδ2 T Cells and Their Versatility for Immunotherapeutic Approaches. Cells 2022, 11, 3572. https://doi.org/10.3390/cells11223572
Sanz M, Mann BT, Chitrakar A, Soriano-Sarabia N. Human Vδ2 T Cells and Their Versatility for Immunotherapeutic Approaches. Cells. 2022; 11(22):3572. https://doi.org/10.3390/cells11223572
Chicago/Turabian StyleSanz, Marta, Brendan T. Mann, Alisha Chitrakar, and Natalia Soriano-Sarabia. 2022. "Human Vδ2 T Cells and Their Versatility for Immunotherapeutic Approaches" Cells 11, no. 22: 3572. https://doi.org/10.3390/cells11223572
APA StyleSanz, M., Mann, B. T., Chitrakar, A., & Soriano-Sarabia, N. (2022). Human Vδ2 T Cells and Their Versatility for Immunotherapeutic Approaches. Cells, 11(22), 3572. https://doi.org/10.3390/cells11223572






