Circular RNA hsa_circ_0051040 Promotes Hepatocellular Carcinoma Progression by Sponging miR-569 and Regulating ITGAV Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Tissue and Plasma Specimens
2.2. Cell Culture and Transfection
2.3. CircRNA Microarray Analysis
2.4. RNA Fluorescence In Situ Hybridization (FISH) and Nuclear and Cytoplasmic Extraction
2.5. 5-Ethynyl-20-Deoxyuridine (EdU) Incorporation Assay
2.6. RNA Preparation and qRT-PCR
2.7. Western Blotting
2.8. Wound-Healing Assay
2.9. Colony Formation Assay
2.10. Dual Luciferase Reporter Assay
2.11. Construction of Plasmids, SiRNAs, Lentiviral Vectors, and miRNA Mimics and Inhibitors
2.12. Biotin-Coupled Probe Pulldown Assay
2.13. Cell Migration and Invasion Assays
2.14. H&E and Immunohistochemical (IHC) Staining
2.15. Animal Experiments
2.16. Statistical Analysis
3. Results
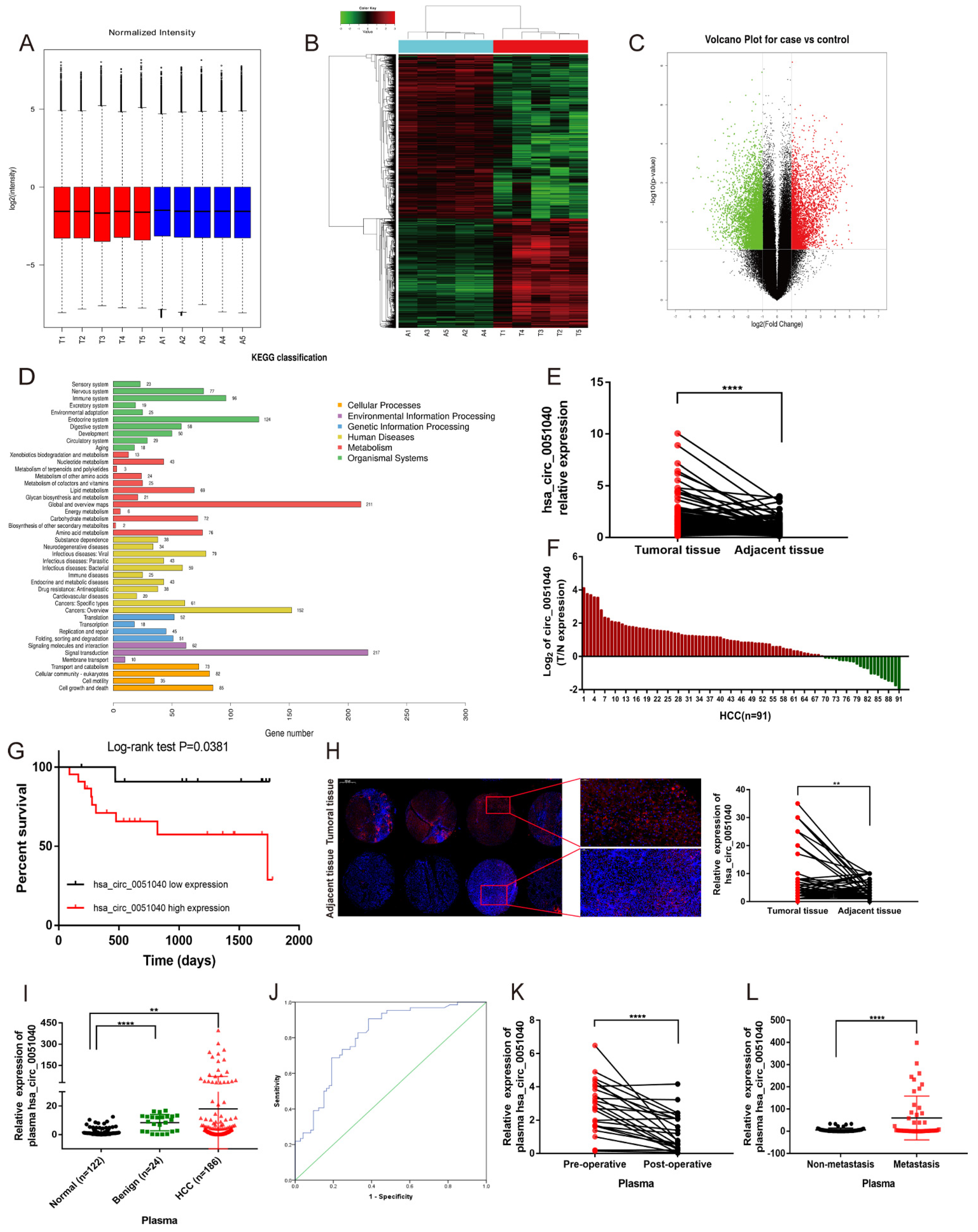
3.1. hsa_circ_0051040 Is Overexpressed in HCC Tissues and Correlates with Poor Prognosis
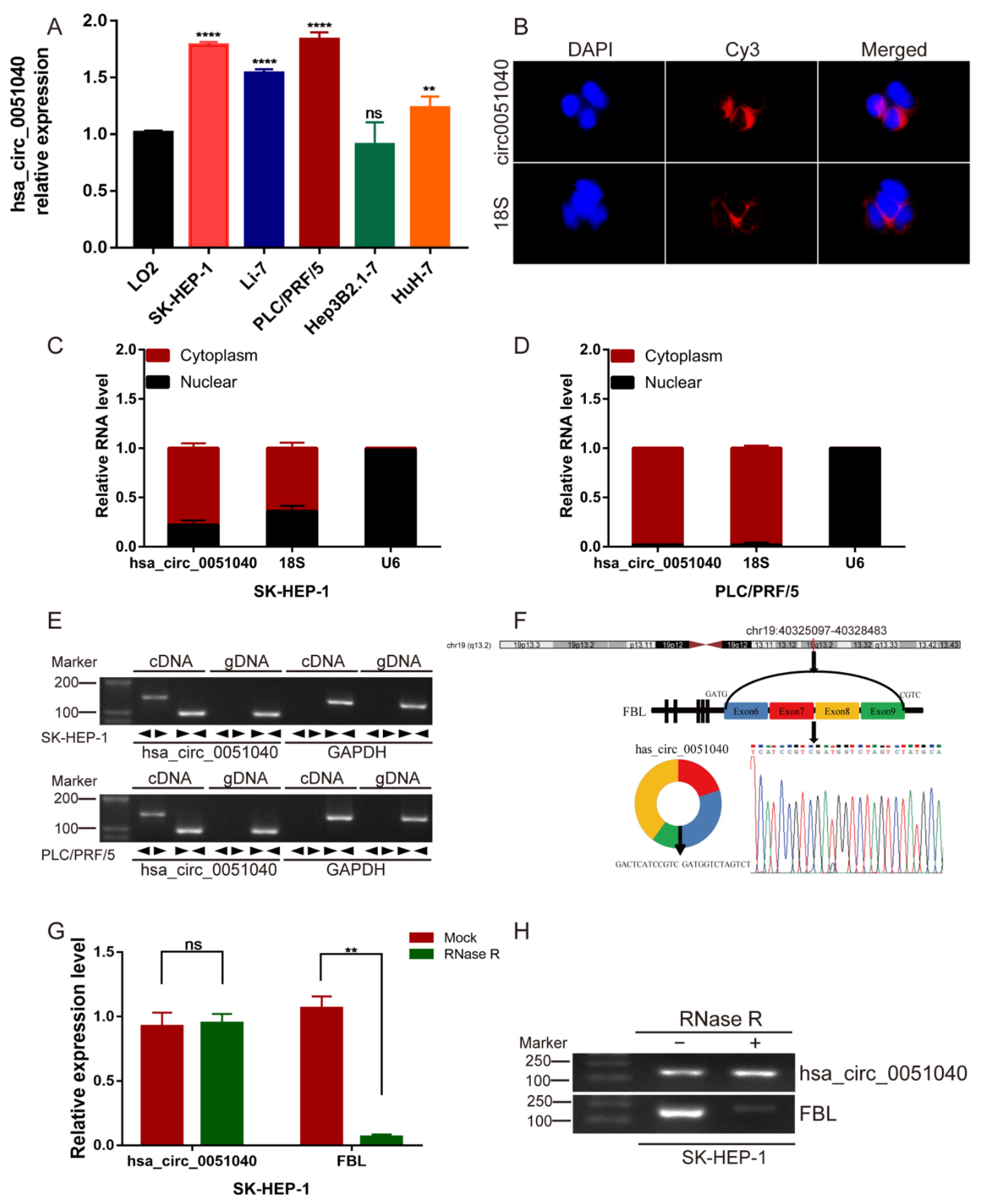
3.2. Identification and Characteristics of hsa_circ_0051040 in HCC Cells
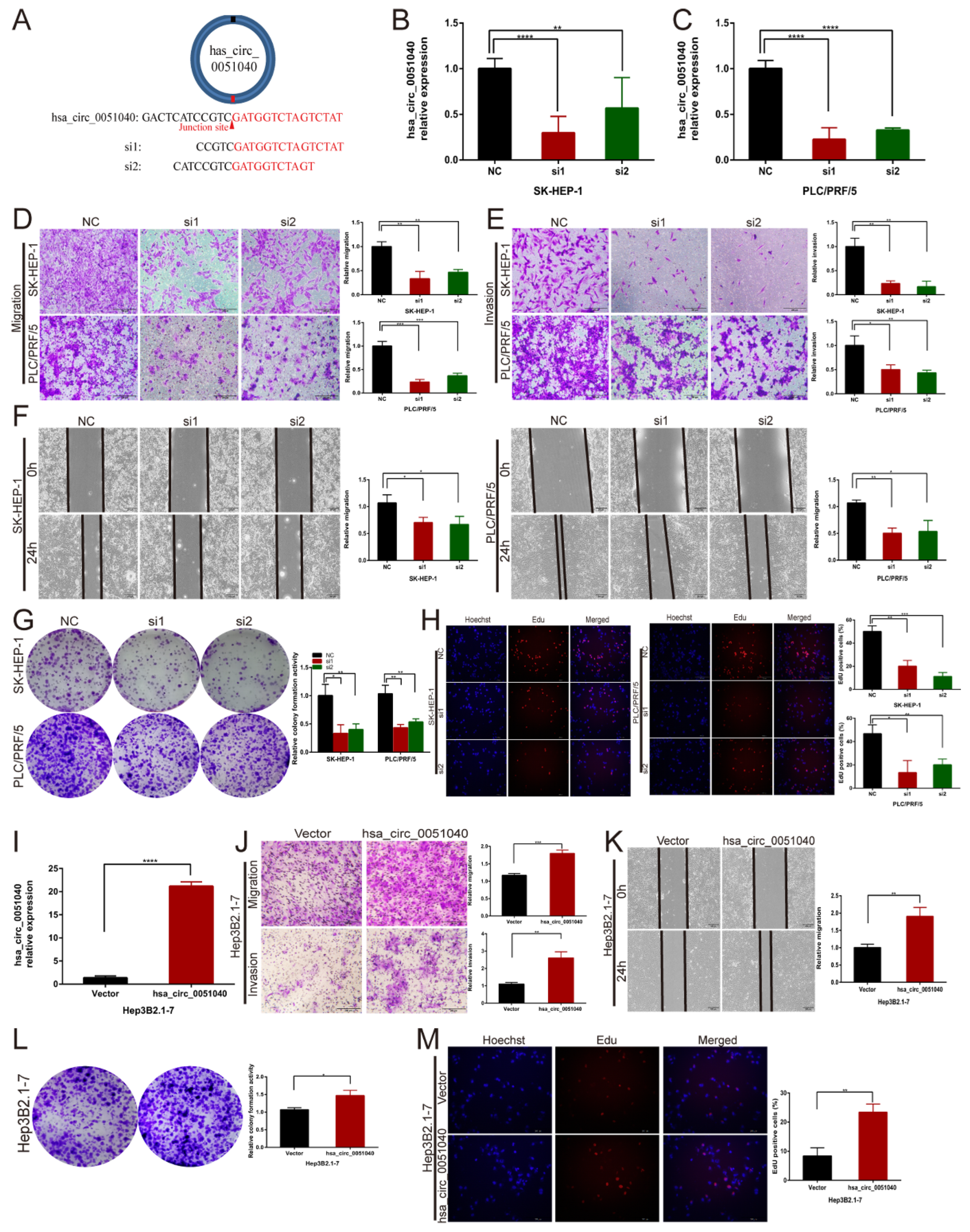
3.3. hsa_circ_0051040 Promotes the Migration, Invasion and Proliferation of HCC Cells In Vitro
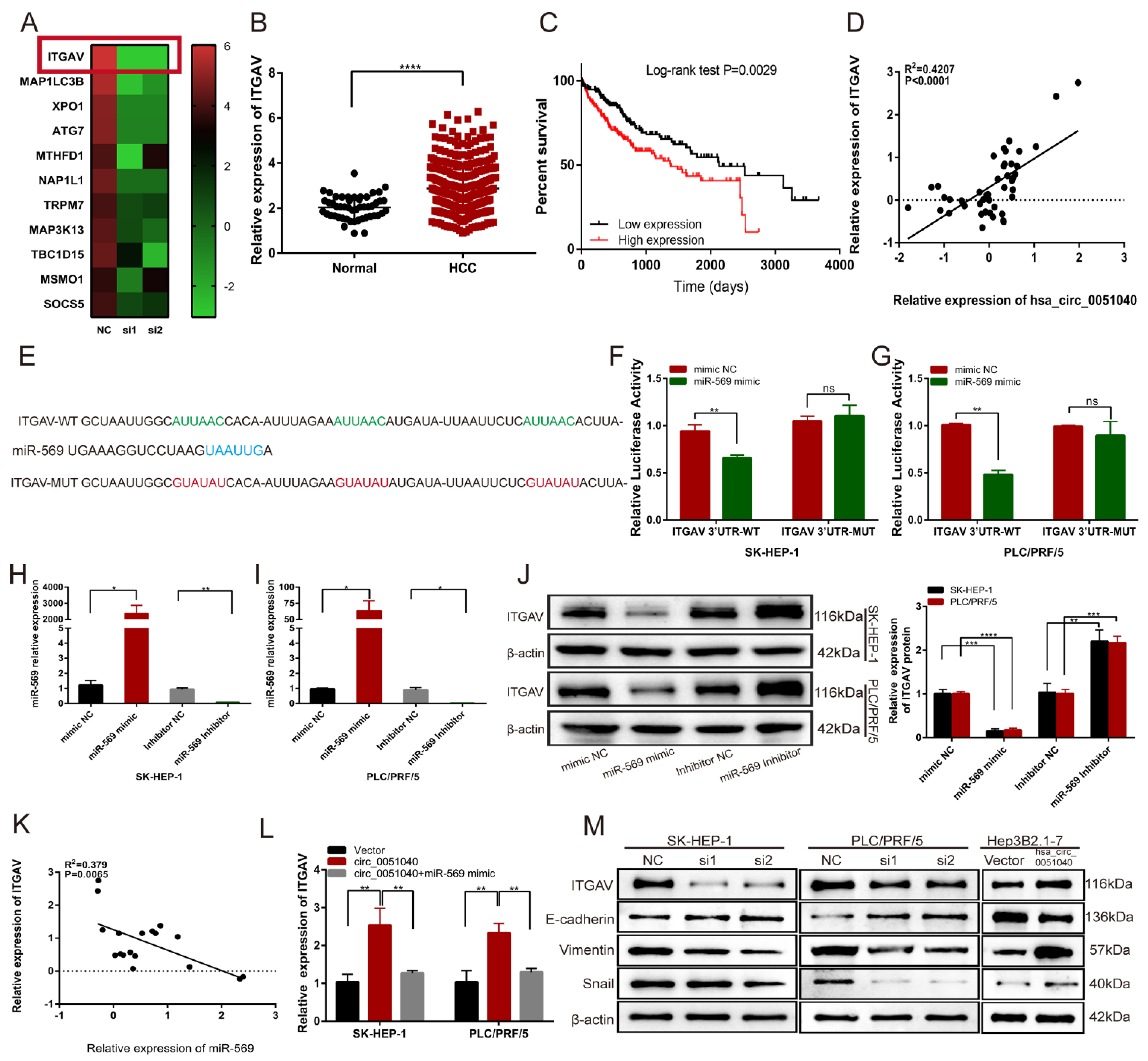
3.4. hsa_circ_0051040 Acts as a Sponge of MiR-569 in HCC Cells
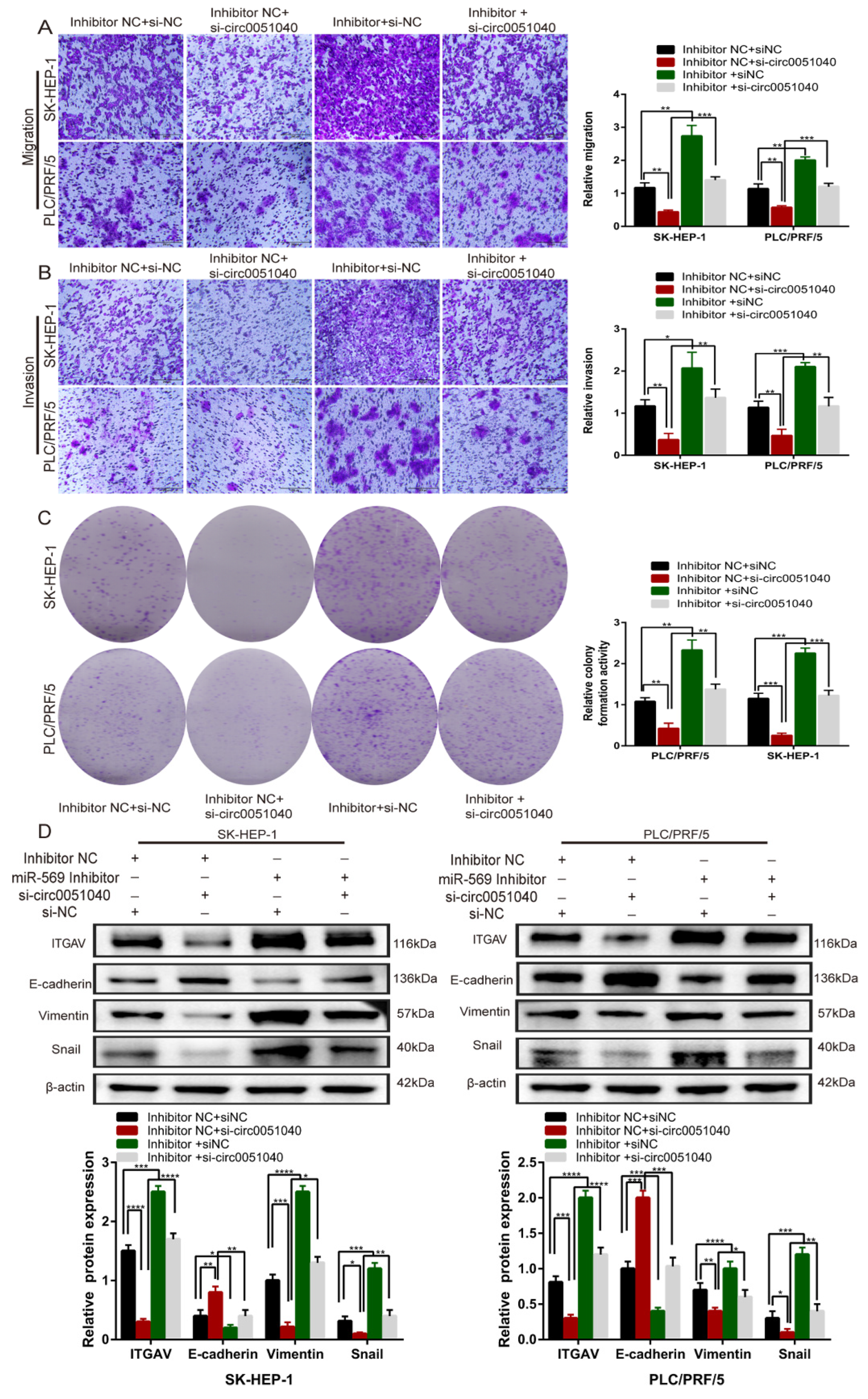
3.5. hsa_circ_0051040 Promotes ITGAV Expression by Interacting with MiR-569 in HCC Cells
3.6. hsa_circ_0051040 Knockdown Inhibits the Growth and Metastasis of HCC Cells In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.; Kelley, R.; Villanueva, A.; Singal, A.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Zhang, J.; Tao, K.; Hambly, B.D.; Bao, S. Inverse correlation between Interleukin-34 and gastric cancer, a potential biomarker for prognosis. Cell Biosci. 2020, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.; Gregersen, L.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Guo, Z.; Li, M.; Li, M.; Liu, S.; Liu, H.; Li, W.; Yin, X.; Tao, J.; et al. Emerging function and potential diagnostic value of circular RNAs in cancer. Mol. Cancer 2018, 17, 123. [Google Scholar] [CrossRef]
- Wang, S.; Tang, D.; Wang, W.; Yang, Y.; Wu, X.; Wang, L.; Wang, D. circLMTK2 acts as a sponge of miR-150-5p and promotes proliferation and metastasis in gastric cancer. Mol. Cancer 2019, 18, 162. [Google Scholar] [CrossRef]
- Su, M.; Xiao, Y.; Ma, J.; Tang, Y.; Tian, B.; Zhang, Y.; Li, X.; Wu, Z.; Yang, D.; Zhou, Y.; et al. Circular RNAs in Cancer: Emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Mol. Cancer 2019, 18, 90. [Google Scholar] [CrossRef]
- Rong, D.; Lu, C.; Zhang, B.; Fu, K.; Zhao, S.; Tang, W.; Cao, H. CircPSMC3 suppresses the proliferation and metastasis of gastric cancer by acting as a competitive endogenous RNA through sponging miR-296-5p. Mol. Cancer 2019, 18, 25. [Google Scholar] [CrossRef]
- Tan, S.; Sun, D.; Pu, W.; Gou, Q.; Guo, C.; Gong, Y.; Li, J.; Wei, Y.; Liu, L.; Zhao, Y.; et al. Circular RNA F-circEA-2a derived from EML4-ALK fusion gene promotes cell migration and invasion in non-small cell lung cancer. Mol. Cancer 2018, 17, 138. [Google Scholar] [CrossRef]
- Zhong, Y.; Du, Y.; Yang, X.; Mo, Y.; Fan, C.; Xiong, F.; Ren, D.; Ye, X.; Li, C.; Wang, Y.; et al. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol. Cancer 2018, 17, 79. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, M.; Xing, L.; Yang, R.; Wang, X.; Jiang, R.; Zhang, L.; Chen, J. The circRNA circSEPT9 mediated by E2F1 and EIF4A3 facilitates the carcinogenesis and development of triple-negative breast cancer. Mol. Cancer 2020, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Nan, A.; Zhang, N.; Jia, Y.; Li, X.; Ling, Y.; Dai, J.; Zhang, S.; Yang, Q.; Yi, Y.; et al. Circular RNA 100146 functions as an oncogene through direct binding to miR-361-3p and miR-615-5p in non-small cell lung cancer. Mol. Cancer 2019, 18, 13. [Google Scholar] [CrossRef]
- Yang, X.; Ye, T.; Liu, H.; Lv, P.; Duan, C.; Wu, X.; Jiang, K.; Lu, H.; Xia, D.; Peng, E.; et al. Expression profiles, biological functions and clinical significance of circRNAs in bladder cancer. Mol. Cancer 2021, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Lin, Z.; Li, L.; Ma, M.; Lu, Z.; Jing, L.; Li, X.; Lin, C. Comprehensive landscape and future perspectives of circular RNAs in colorectal cancer. Mol. Cancer 2021, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, B.; Shu, C.; Ma, Q.; Wang, J. Functions and clinical significance of circular RNAs in glioma. Mol. Cancer 2020, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Yang, Z.; Jia, R.; Ge, S. The novel roles of circRNAs in human cancer. Mol. Cancer 2019, 18, 6. [Google Scholar] [CrossRef]
- Lei, M.; Zheng, G.; Ning, Q.; Zheng, J.; Dong, D. Translation and functional roles of circular RNAs in human cancer. Mol. Cancer 2020, 19, 30. [Google Scholar] [CrossRef]
- Ma, Z.; Shuai, Y.; Gao, X.; Wen, X.; Ji, J. Circular RNAs in the tumour microenvironment. Mol. Cancer 2020, 19, 8. [Google Scholar] [CrossRef]
- Fontemaggi, G.; Turco, C.; Esposito, G.; Di Agostino, S. New Molecular Mechanisms and Clinical Impact of circRNAs in Human Cancer. Cancers 2021, 13, 3154. [Google Scholar] [CrossRef]
- Xue, B.; Chuang, C.; Prosser, H.; Fuziwara, C.; Chan, C.; Sahasrabudhe, N.; Kühn, M.; Wu, Y.; Chen, J.; Biton, A.; et al. miR-200 deficiency promotes lung cancer metastasis by activating Notch signaling in cancer-associated fibroblasts. Genes Dev. 2021. [Google Scholar] [CrossRef]
- Muluhngwi, P.; Klinge, C. Identification and Roles of miR-29b-1-3p and miR29a-3p-Regulated and Non-Regulated lncRNAs in Endocrine-Sensitive and Resistant Breast Cancer Cells. Cancers 2021, 13, 3530. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Y.; Mackowiak, B.; Gao, B. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut 2021, 70, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.; Carvalho, R.; Justulin, L.; Cury, S.; Seiva, F.; Jardim-Perassi, B.; Zuccari, D.; Reiter, R. A meta-analysis of microRNA networks regulated by melatonin in cancer: Portrait of potential candidates for breast cancer treatment. J. Pineal Res. 2020, 69, e12693. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, L.; Gao, J.; Wang, Y. Tumor suppressive role of miR-569 in lung cancer. Oncol. Lett. 2018, 15, 4087–4092. [Google Scholar] [CrossRef]
- Li, L.; Guo, L.; Yin, G.; Yu, G.; Zhao, Y.; Pan, Y. Upregulation of circular RNA circ_0001721 predicts unfavorable prognosis in osteosarcoma and facilitates cell progression via sponging miR-569 and miR-599. Biomed. Pharm. 2019, 109, 226–232. [Google Scholar] [CrossRef]
- Kang, C.; Qi, B.; Cai, Q.; Fu, L.; Yang, Y.; Tang, C.; Zhu, P.; Chen, Q.; Pan, J.; Chen, M.; et al. ITGAVLncRNA AY promotes hepatocellular carcinoma metastasis by stimulating transcription. Theranostics 2019, 9, 4421–4436. [Google Scholar] [CrossRef]
- Cai, Q.; Liu, Y.; Zhu, P.; Kang, C.; Xu, H.; Qi, B.; Wang, R.; Dong, Y.; Wu, X. SIN3B promotes integrin αV subunit gene transcription and cell migration of hepatocellular carcinoma. J. Mol. Cell Biol. 2019, 11, 421–432. [Google Scholar] [CrossRef]


| Characteristics | No.(%) | has_circ_0051040 | p Value | |
|---|---|---|---|---|
| Low (%) | High (%) | |||
| Gender | 0.304 | |||
| Male | 66 | 29 | 37 | |
| Female | 25 | 14 | 11 | |
| Age | 0.875 | |||
| <60 | 41 | 19 | 22 | |
| ≥60 | 50 | 24 | 26 | |
| Tumor size (cm) | 0.024 | |||
| <5 | 59 | 33 | 26 | |
| ≥5 | 32 | 10 | 22 | |
| TNM stage | 0.178 | |||
| T1-T2 | 16 | 10 | 6 | |
| T3-T4 | 75 | 33 | 42 | |
| Lymph node metastasis | 0.002 | |||
| Positive | 20 | 3 | 17 | |
| Negative | 71 | 40 | 31 | |
| Serum AFP (ng/mL) | 0.156 | |||
| <400 | 61 | 32 | 29 | |
| ≥400 | 30 | 11 | 19 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, L.; Yao, M.; Lu, R.; Cao, Y.; Wang, H.; Yuan, L.; Xiao, F.; Shao, J.; Cai, W.; Chen, L.; et al. Circular RNA hsa_circ_0051040 Promotes Hepatocellular Carcinoma Progression by Sponging miR-569 and Regulating ITGAV Expression. Cells 2022, 11, 3571. https://doi.org/10.3390/cells11223571
Ju L, Yao M, Lu R, Cao Y, Wang H, Yuan L, Xiao F, Shao J, Cai W, Chen L, et al. Circular RNA hsa_circ_0051040 Promotes Hepatocellular Carcinoma Progression by Sponging miR-569 and Regulating ITGAV Expression. Cells. 2022; 11(22):3571. https://doi.org/10.3390/cells11223571
Chicago/Turabian StyleJu, Linling, Min Yao, Rujian Lu, Yali Cao, Huixuan Wang, Liuxia Yuan, Feng Xiao, Jianguo Shao, Weihua Cai, Lin Chen, and et al. 2022. "Circular RNA hsa_circ_0051040 Promotes Hepatocellular Carcinoma Progression by Sponging miR-569 and Regulating ITGAV Expression" Cells 11, no. 22: 3571. https://doi.org/10.3390/cells11223571
APA StyleJu, L., Yao, M., Lu, R., Cao, Y., Wang, H., Yuan, L., Xiao, F., Shao, J., Cai, W., Chen, L., & Bian, Z. (2022). Circular RNA hsa_circ_0051040 Promotes Hepatocellular Carcinoma Progression by Sponging miR-569 and Regulating ITGAV Expression. Cells, 11(22), 3571. https://doi.org/10.3390/cells11223571






