Abstract
Antimicrobial-resistant (AMR) pathogens are a significant threat to public health worldwide. However, the primary carrier of AMR genes, particularly against last-resort antibiotics, is still only partially studied in Chinese hospitals. In a sentinel hospital in China, we collected 157 E. coli strains from patients between January and July 2021. One blaNDM-1-, nine blaNDM-5-, and one mcr-1-positive E. coli recovered from inpatients were identified as resistant to meropenem and colistin. There are 37 virulence genes discovered in the 11 strains, including astA in strain EC21Z-147 (O128: H4), which belongs to the enteroaggregative E. coli (EAEC). The blaNDM gene is distributed into distinct ST types, including ST48, ST616, ST410, ST711, and ST2003, while the mcr-1 gene was identified in ST117. The conjugative plasmids IncX3, IncI1-I, and IncI2 mediated the blaNDM-5 and mcr-1 genes detected among inpatients. Notably, the youngest age at which mcr-1-positive E. coli has been reported was at one day old, in a child in which the strain is closely related to strains with animal origins. Hospitals are major environments for the spread and dissemination of critical virulence and AMR genes, which requires active monitoring systems at the genome level to surveil the spread of virulence and AMR.
1. Introduction
Antimicrobial resistance (AMR) has become a global public health concern with the spread of multidrug resistance (MDR) bacteria worldwide. Carbapenems are the most critical antimicrobials for treating clinical Gram-negative bacterial infections. The carbapenem-resistant Enterobacteriaceae (CRE), defined as a superbug that carried the blaNDM-1 gene, was identified in 2009 [1]. blaNDM is a metallo-β-lactamase (MBL) type gene that could hydrolyze most β-lactam antimicrobials [2]. Hence, they are considered last-resort antimicrobials against serious infections caused by CRE [3]. Moreover, colistin is considered among the last resort for treating MDR Enterobacteriaceae; however, its extensive use in veterinary practice has led to the development of resistance in a wide range of pathogens recovered from animals, food, the environment, and human samples [4,5,6,7]. On the other hand, tigecycline is a last-resort antibiotic used to treat severe infections caused by extensively drug-resistant pathogenic bacteria [8]. Hence, resistance to these last-resort antimicrobial drugs threatens the healthcare systems.
Resistance to these antimicrobials is encoded by the antimicrobial resistance gene (ARG) carried on transferable plasmids, further complicating the situation and accelerating their dissemination worldwide. So far, thirty-one variants of blaNDM, ten variants of mcr, and dozens of tet(X) variants encoding resistance to carbapenem, colistin, and tigecycline have been identified in various bacterial species globally [9,10,11,12,13]. These ARGs have been reported in several incompatibility groups of plasmids, including IncF, IncFII, IncN, IncHI, IncX, and others [14,15].
Hospital-associated and community-acquired infections are important routes of transmission of ARGs [16]. Escherichia coli is considered one of the most common bacterial species responsible for the horizontal transfer of ARGs [17]. Hence, to estimate the trend and transfer mechanisms of carbapenems, polymyxins, and tigecycline resistance genes in patients, here, we analyzed the prevalence of meropenem-, colistin- and tigecycline-resistant E. coli strains for inpatients and compared them with outpatients in the same period in a sentinel hospital in Zhejiang province, China. The molecular characteristics and the capability of horizontally transferring the blaNDM and mcr-1 genes were also assessed.
2. Materials and Methods
2.1. Bacteria Isolation and Identification
A total of 157 E. coli strains from 139 patients were retrospectively collected from outpatients (n = 69) and inpatients (n = 88) between January and July 2021 at a sentinel hospital in Zhejiang province, China. The isolated strains were confirmed with MALDI-TOF Mass Spectrometry. These strains were recovered from blood (n = 65), urine (n = 48), stool (n = 14), bile (n = 23), and lung (n = 7) samples. The patients’ ages were between 1 day and 100 years old.
2.2. Antimicrobial Susceptibility Testing
The E. coli isolates were first tested against meropenem (2 mg/L), colistin (2 mg/L), and tigecycline (2 mg/L) to select the resistant strains by using the diffusion method on Mueller–Hinton agar (MHA). The genes encoding resistance to carbapenem (blaNDM) and colistin (mcr-1) were then amplified using the primers previously described [18,19]. Thereafter, the minimum inhibitory concentrations (MICs) were determined for 11 meropenem and/or colistin-resistant strains from 14 antimicrobial agents, including ampicillin (AMP), amoxicillin/clavulanate (A/C), gentamicin (GEN), spectinomycin (SPT), florfenicol (FFC), sulfisoxazole (SF), trimethoprim/sulfamethoxazole (SXT), ceftiofur (CEF), ceftazidime (CAZ), enrofloxacin (ENR), ofloxacin (OFL), tetracycline (TET), colistin (COL), and meropenem (MEM) using the broth microdilution method according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [20].
2.3. Whole-Genome Sequencing and Annotation
The genomic DNA of the 11 colistin- or meropenem-resistant E. coli strains was extracted using a DNA Extraction Kit (Genray, Shanghai, China). Whole-genome sequencing was performed by the Oxford Nanopore GridION platform for long sequencing reads and Illumina HiSeq-PE150 for High-Throughput Sequencing reads. The genome sequences were hybrid assembled using Unicycler v0.4.4. The sequences were automatically annotated by the RAST [21]. The ARGs were identified using the ResFinder 4.1 (>90% identity and >80% coverage) (https://cge.food.dtu.dk/services/ResFinder/) (accessed on 15 June 2022). The virulence genes were identified using the VirulenceFinder 2.0 (>90% identity and >80% coverage) (https://cge.food.dtu.dk/services/VirulenceFinder/) (accessed on 15 June 2022), and the incompatibility (Inc) group of plasmids were identified using the PlasmidFinder 2.1 (>95% identity and >80% coverage) (https://cge.food.dtu.dk/services/PlasmidFinder/) (accessed on 15 June 2022), as reported in previous studies [22,23,24,25,26]. Insertion sequences were identified by ISFinder [27].
2.4. S1-Pulsed-Field Gel Electrophoresis (PFGE) and Southern Blot
As described in a previous study, the 11 E. coli strains were subjected to S1-PFGE to identify the number and size of plasmids [28,29]. The overnight cultures were washed with PBS buffer and then embedded in agarose plugs. The plugs were digested with proteinase K at 37 °C, shaking at 120 rpm for 2 h, and were restricted with S1 nuclease. Salmonella H9812 was restricted with XbaI, which was used as the size marker. A Southern blot was used to confirm the positioning of the mcr-1 or blaNDM genes on the plasmids. The DNA fragments were transferred to a positively charged nylon membrane (Millipore, USA) by wet transfer and then hybridized according to the protocol as the DIG-High Prime DNA Labeling and Detection Starter Kit I (Roche, Germany).
2.5. Conjugation Test
Colistin- or meropenem-resistant E. coli containing the mcr-1, blaNDM-1, or blaNDM-5 genes was used as the donor strain, and E. coli J53 (sodium azide-resistant) was used as the recipient. Fresh cultures of the donor and recipient strains were adjusted in concentration and co-incubated on the LB agar plate at 37 °C overnight. The mixed cultures were collected and diluted with PBS. Then, the mixture was inoculated onto MHA plates containing 2 µg/mL of colistin with NaN3 (100 µg/mL) or 2 µg/mL meropenem with NaN3 (100 µg/mL) at 37 °C overnight. The number of transconjugants and recipients was recorded, which was used to calculate the transfer frequency as described previously [18].
3. Results
3.1. Bacterial Strains
A total of 157 E. coli strains were collected from outpatients and inpatients between January and July 2021 in Zhejiang province, China. Ten (6.37%, 10/157) meropenem-resistant strains were isolated from the stool and lungs of inpatients (Table 1). Among them, four strains (EC21Z-078, EC21Z-083, EC21Z-097, and EC21Z-101) were recovered from the same patient (No. 01262726) with diffuse large B-cell lymphoma at different times, and two strains (EC21Z-144 and EC21Z-151) were isolated from another patient (No. 01273986) with non-Hodgkin’s lymphoma. A total of nine (5.73%) blaNDM-5- and one (0.64%) blaNDM-1-harboring strains were detected, while only one (0.64%) colistin-resistant strain carrying the mcr-1 gene was isolated from the lung of a one-day-old inpatient child (Table 1). Importantly, screening of the studied isolates (n = 157) toward tigecycline showed that all the tested strains were susceptible. It is important to note that all 11 resistant strains were isolated from inpatients (12.5%, 11/88) instead of outpatients (0%, 0/69).

Table 1.
Source information of 11 E. coli isolates with meropenem- or colistin-resistant phenotype.
3.2. Antimicrobial Resistance Profile
The susceptibility testing results showed that all 11 strains (100%) were resistant to ampicillin, sulfisoxazole, and ceftiofur, and most of them exhibited resistance against amoxicillin/clavulanate (90.9%), ceftazidime (90.9%), spectinomycin (81.8%), florfenicol (81.8%), trimethoprim/sulfamethoxazole (81.8%), tetracycline (72.7%), enrofloxacin (72.7%), and ofloxacin (72.7%). However, only three (27.3%) strains were resistant to gentamicin (Table 2). Importantly, all 11 examined strains showed an MDR. It is noted that despite the strains EC21Z-078, EC21Z-083, EC21Z-097, and EC21Z-101 being isolated from one patient at a different time, they did not present identical antimicrobial resistance profiles.

Table 2.
Antimicrobial susceptibility test of 11 E. coli isolates against 14 antimicrobials.
3.3. Characterization of the blaNDM-1, blaNDM-5, and mcr-1 Genes
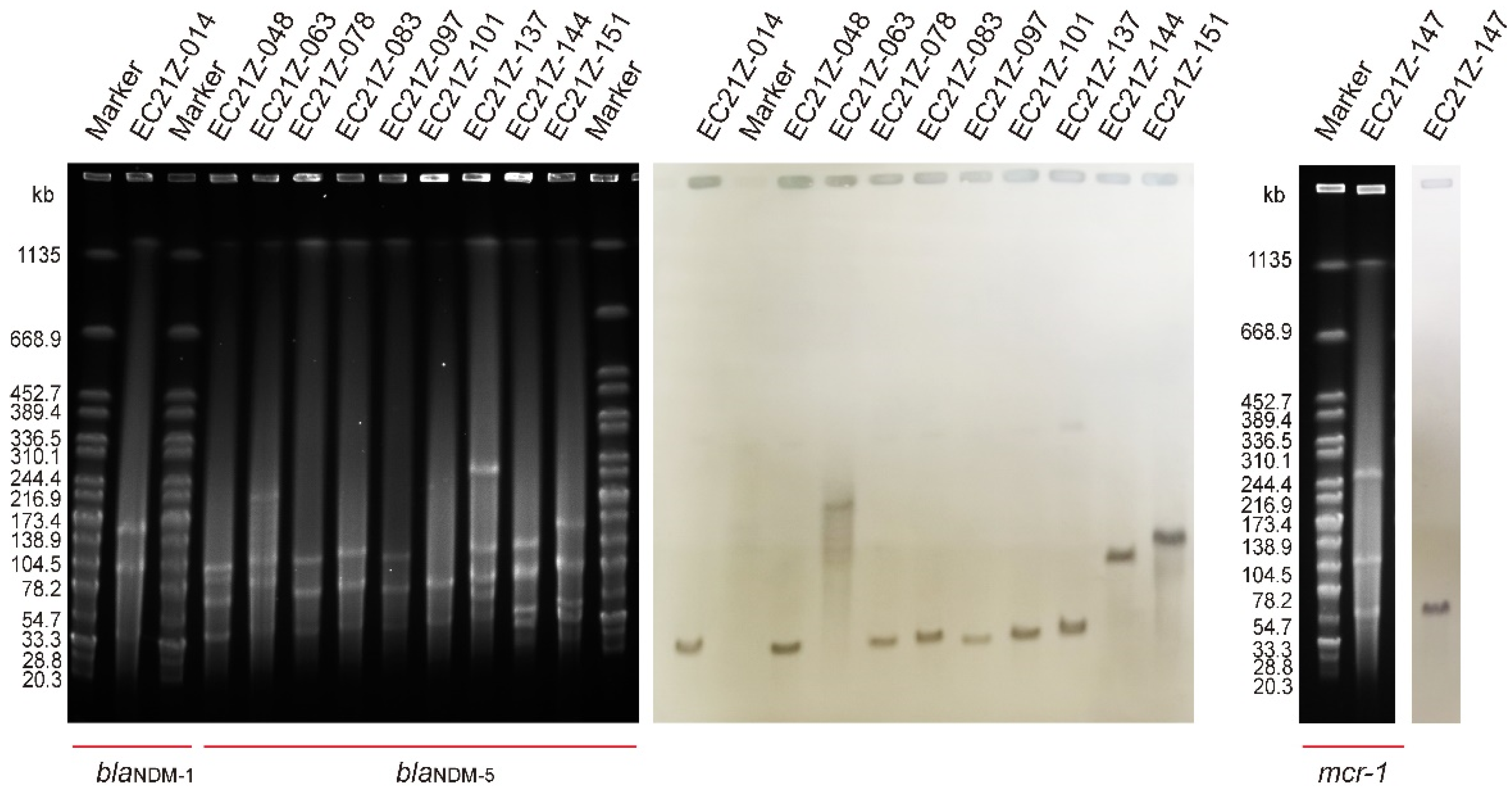
The results of S1-PFGE showed that all 11 strains contain more than two plasmids. The sizes of the plasmids were concentrated between 20.5 kb and 244.4 kb. One plasmid (~90 kb) was missed in the strain EC21Z-101 compared to the first three isolates from the same patient. The Southern blot analysis demonstrated that all blaNDM-1, blaNDM-5 and mcr-1 genes were located on the plasmids (Figure 1). Among them, the blaNDM-1, mcr-1, and most blaNDM-5 genes were found on similar size (~50 kb) plasmids that may have similar Inc types. Moreover, the strain EC21Z-063 contained a large plasmid (~200 kb) carrying blaNDM-5. The strain EC21Z-151 had a slightly larger plasmid than strain EC21Z-144, both isolated from a patient.
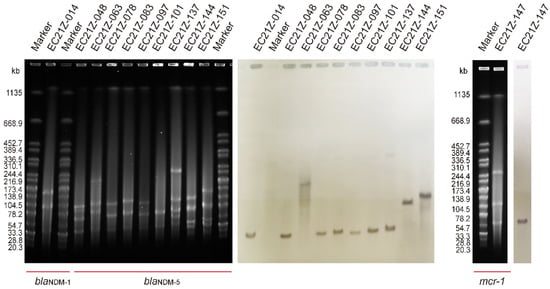
Figure 1.
S1-PFGE and Southern blot of blaNDM-1-, blaNDM-5-, and mcr-1-bearing E. coli strains (n = 11).
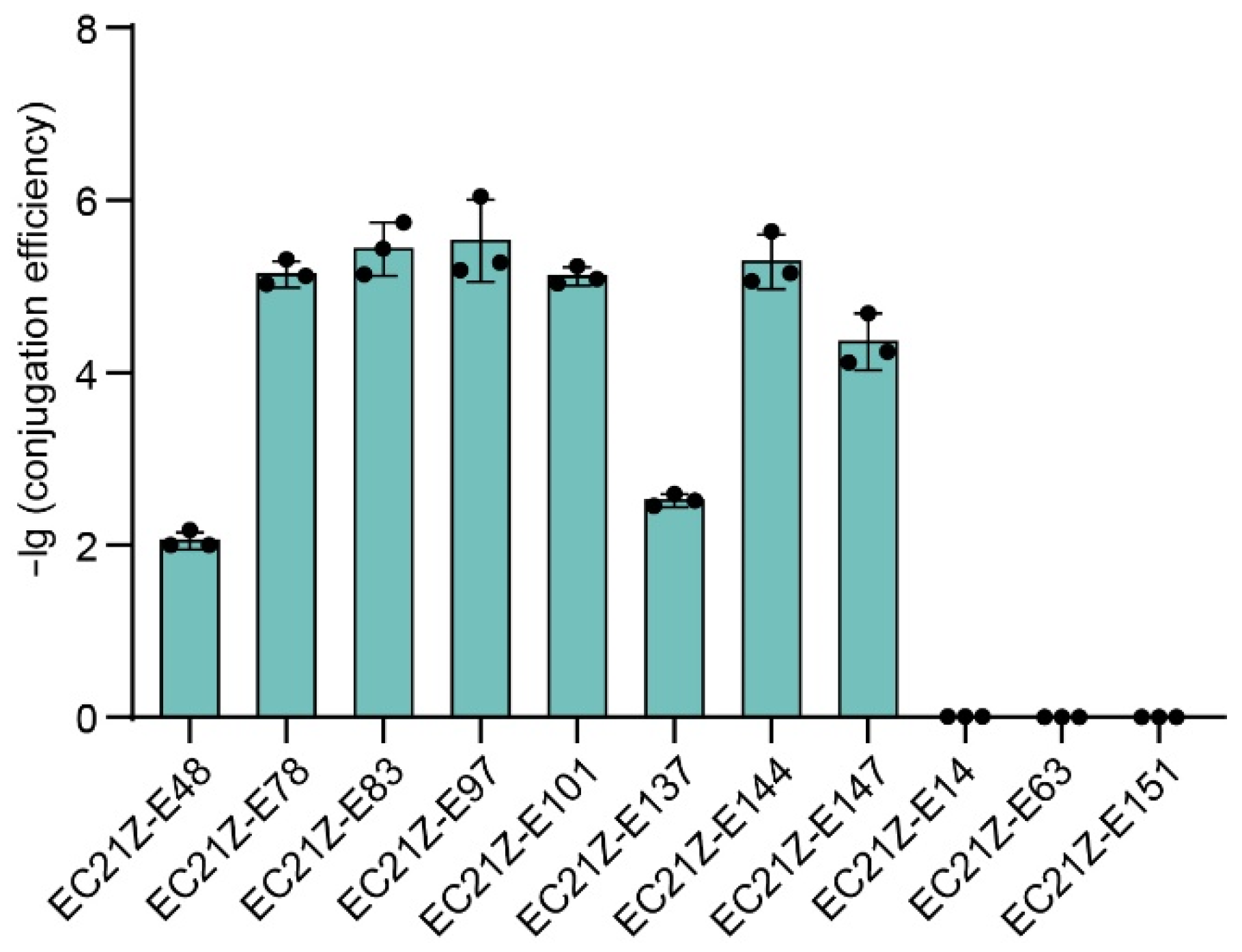
Conjugation experiments showed that the blaNDM-1 gene carried on the plasmid in strain EC21Z-014 could not be transferred to the recipient E. coli J53. Seven of nine plasmids carrying blaNDM-5 (strains EC21Z-048, EC21Z-078, EC21Z-083, EC21Z-097, EC21Z-101, EC21Z-137, and EC21Z-144) were successfully transferred to recipient E. coli J53 with a conjugation frequency in the range from 1 × 10−2 to 5 × 10−6 per recipient strain. Interestingly, the conjugation ability was different for different strains, EC21Z-144 and EC21Z-151, isolated from one patient. The plasmid carrying the mcr-1 gene from strain EC21Z-147 was transferred to the recipient at 4.79 × 10−5 conjugation frequency (Figure 2).
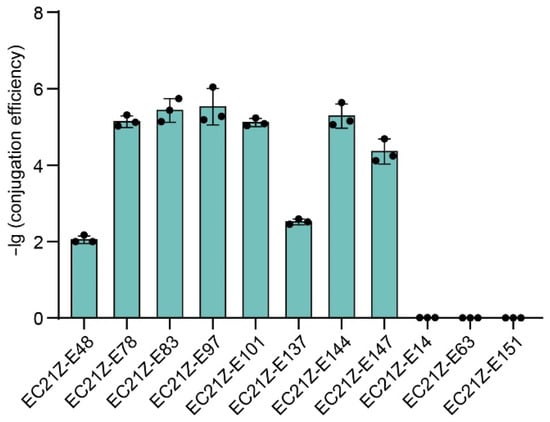
Figure 2.
The conjugation transfer frequency of 11 blaNDM- or mcr-positive E. coli strains.
3.4. Genomic Characteristics Analysis
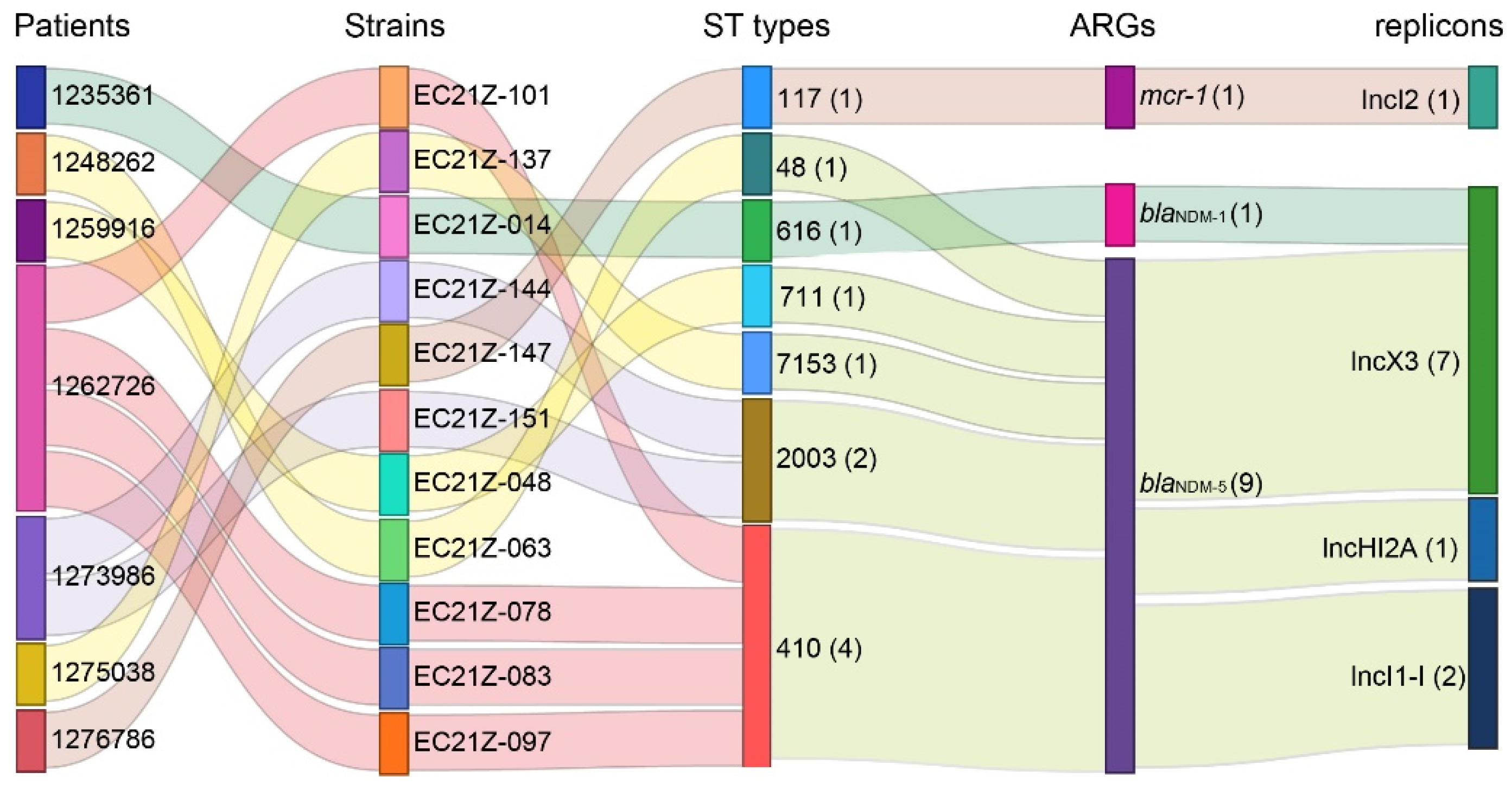
The genomes of all blaNDM-1-, blaNDM-5-, and mcr-1-positive E. coli strains were sequenced. The E. coli from different patients was assigned to ST48, ST117, ST616, ST410, ST711, and ST2003 according to the MLST scheme. The strains EC21Z-078, EC21Z-083, EC21Z-097, and EC21Z-101 isolated from the same patient belonged to ST410, and the strains EC21Z-144 and EC21Z-151 isolated from another patient belonged to ST2003. It should be noted that strains isolated from different patients presented different STs (Figure 3).
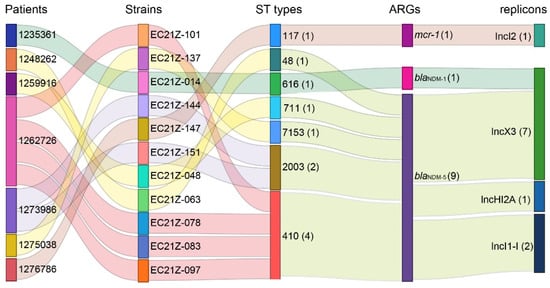
Figure 3.
The relationship of the resistant strains from the patient with the ST types, blaNDM-1, blaNDM-5, and mcr-1 genes and their bearing plasmids. The strain numbers are marked behind the information with parentheses.
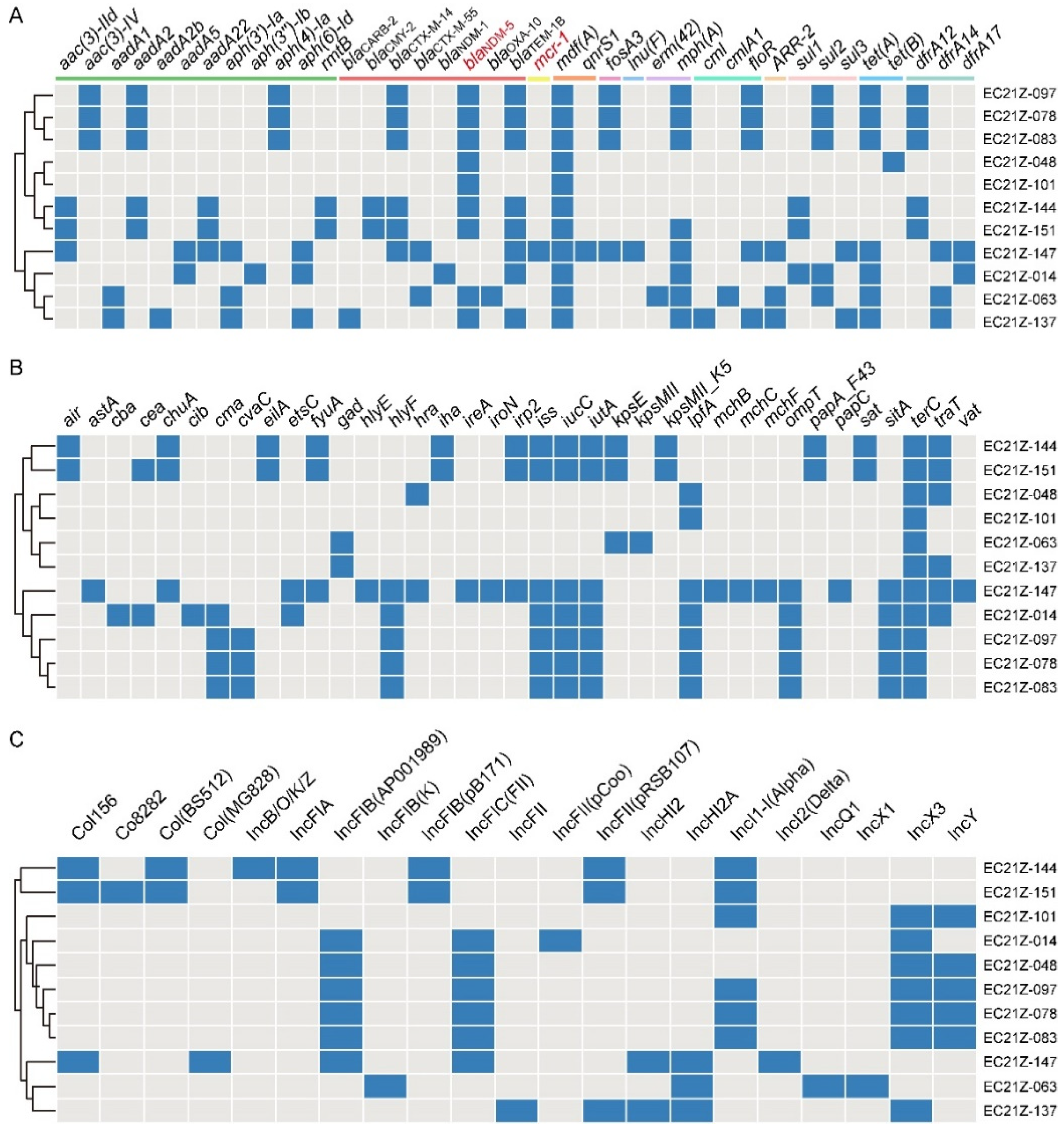
These strains carried various ARGs encoding resistance to aminoglycoside, β-lactam, colistin, phenicol, tetracycline, sulphonamides, trimethoprim, quinolones, and others (Figure 4A). The common ARGs were mdf(A) (100%), blaTEM-1B(81.82%), mph(A) (72.72%), tet(A) (63.64%), and blaCTX-M-14 (54.55%), besides blaNDM-5.
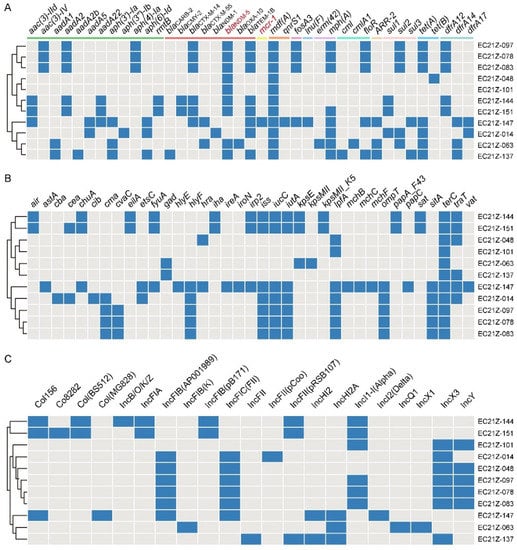
Figure 4.
The diagram of ARGs, virulence genes, and replicon types of the 11 strains. (A) The blue boxes indicate the positive ARGs. (B) The virulence genes in E. coli strains. (C) The various plasmid replicons in E. coli strains.
There are 37 virulence genes identified from the 11 strains, with the terC gene detected in all strains. The virulence genes in strains from different patients are less similar (Figure 4B). The astA gene encodes the enteroaggregative E. coli (EAEC) heat-stable enterotoxin 1 (EAST1) that causes diarrheal disease [30,31]. One EAEC EC21Z-147 carrying mcr-1 was identified from the one-day-old child. The serotype analysis showed the strain EC21Z-147 belongs to O128: H4.
Consistent with the result of S1-PFGE, several replicon types were identified in each E. coli strain, including B/O/K/Z, FIA, FIB, FIC, FII, HI2, HI2A, I1-I, I2, Q1, X1, X3, Y, Co8282, Col156, Col(BS512), and Col(MG828). These strains contained at least three replicons (EC21Z-101) and at most seven replicons (EC21Z-144, EC21Z-147, and EC21Z-151) (Figure 4C). The blaNDM-1 and mcr-1 genes were located in the IncX3 (45.7 kb) and IncI2 (62.3 kb) plasmids, respectively. The plasmid of IncX3 (~46 kb) was the primary vector for the blaNDM-5 gene. The IncHI2A (109.4 kb) and IncI1-1 (~120 kb) plasmids carrying the blaNDM-5 genes were also discovered (Figure 3).
3.5. Plasmid Sequence and Comparative Analysis
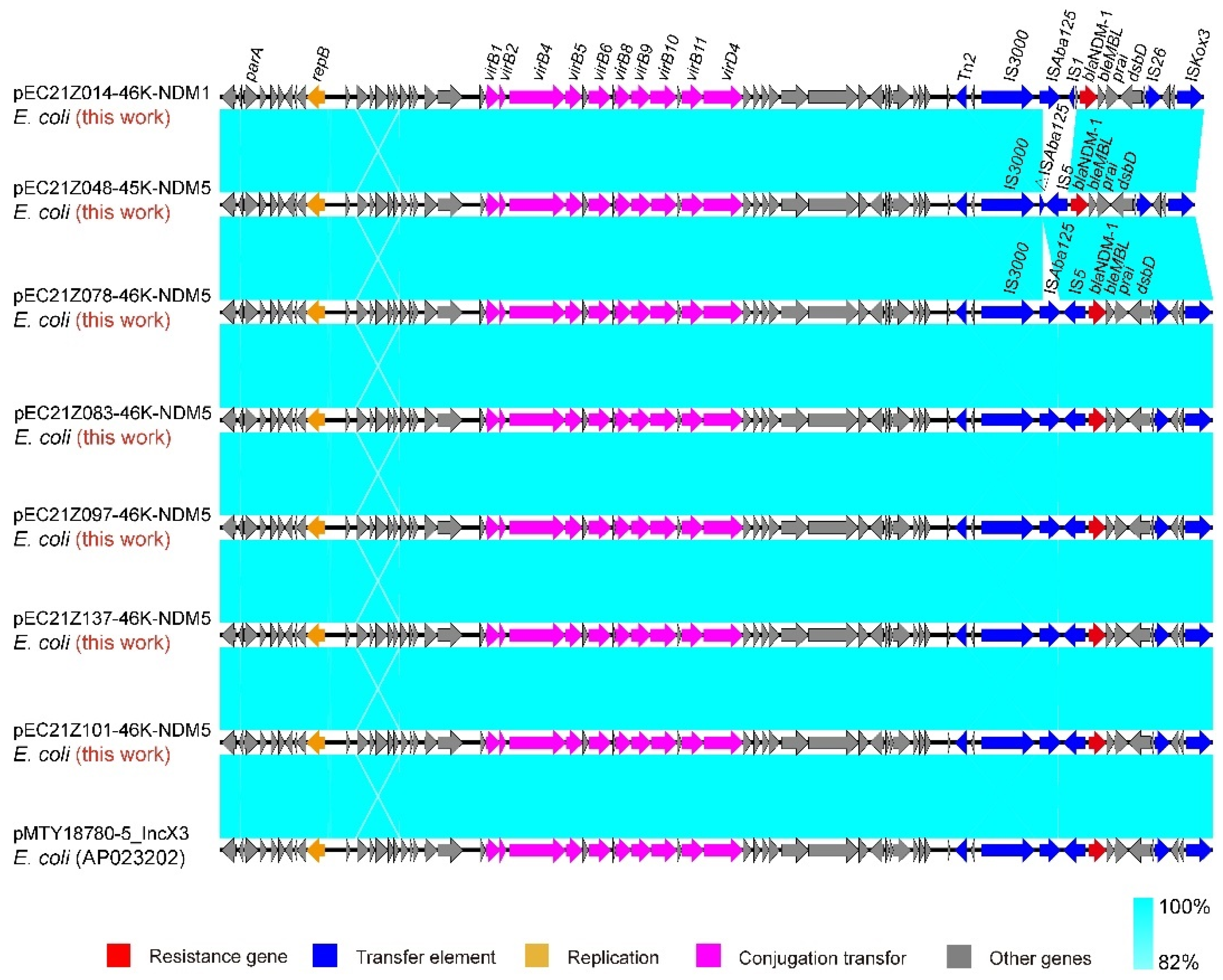
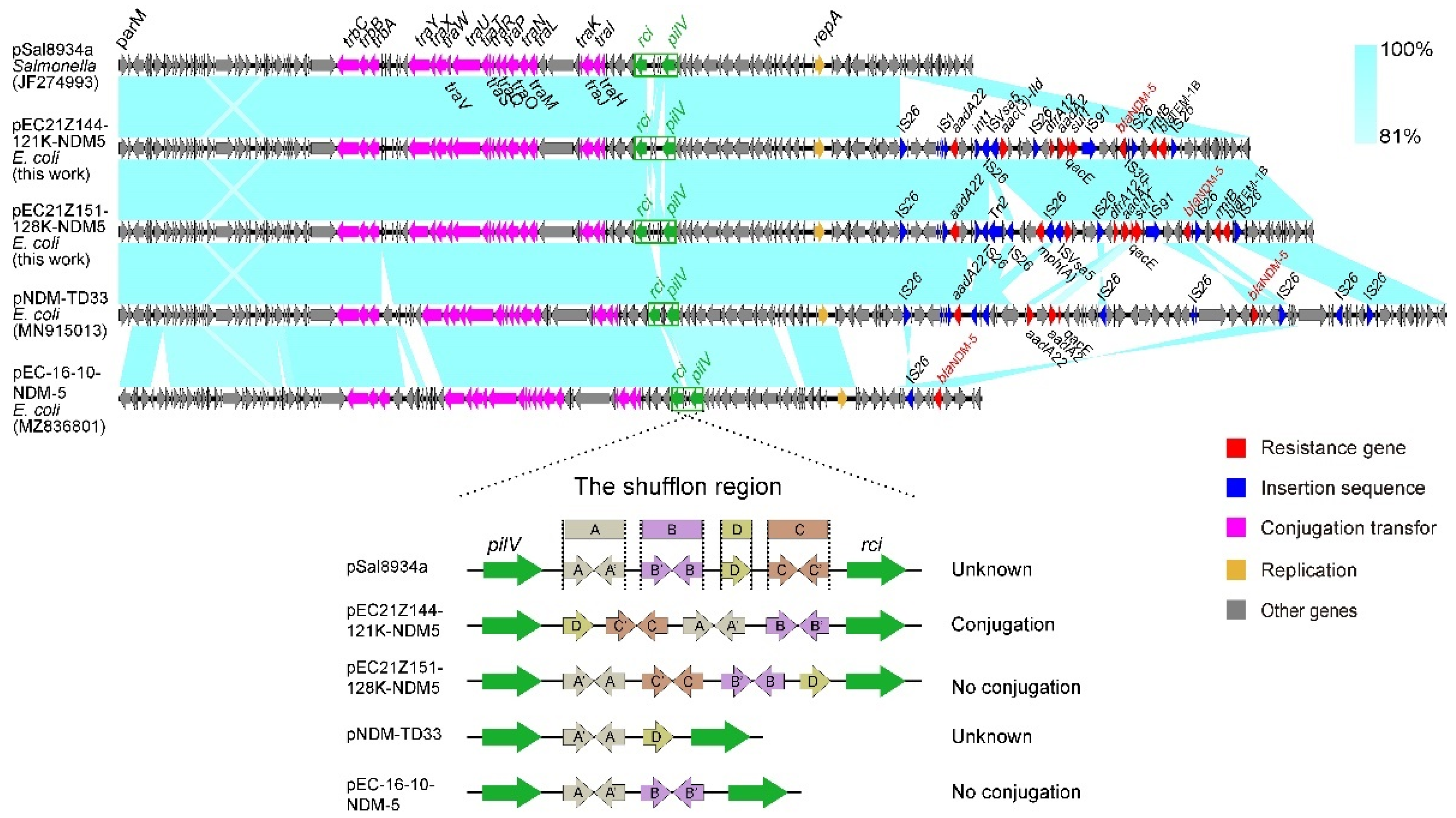
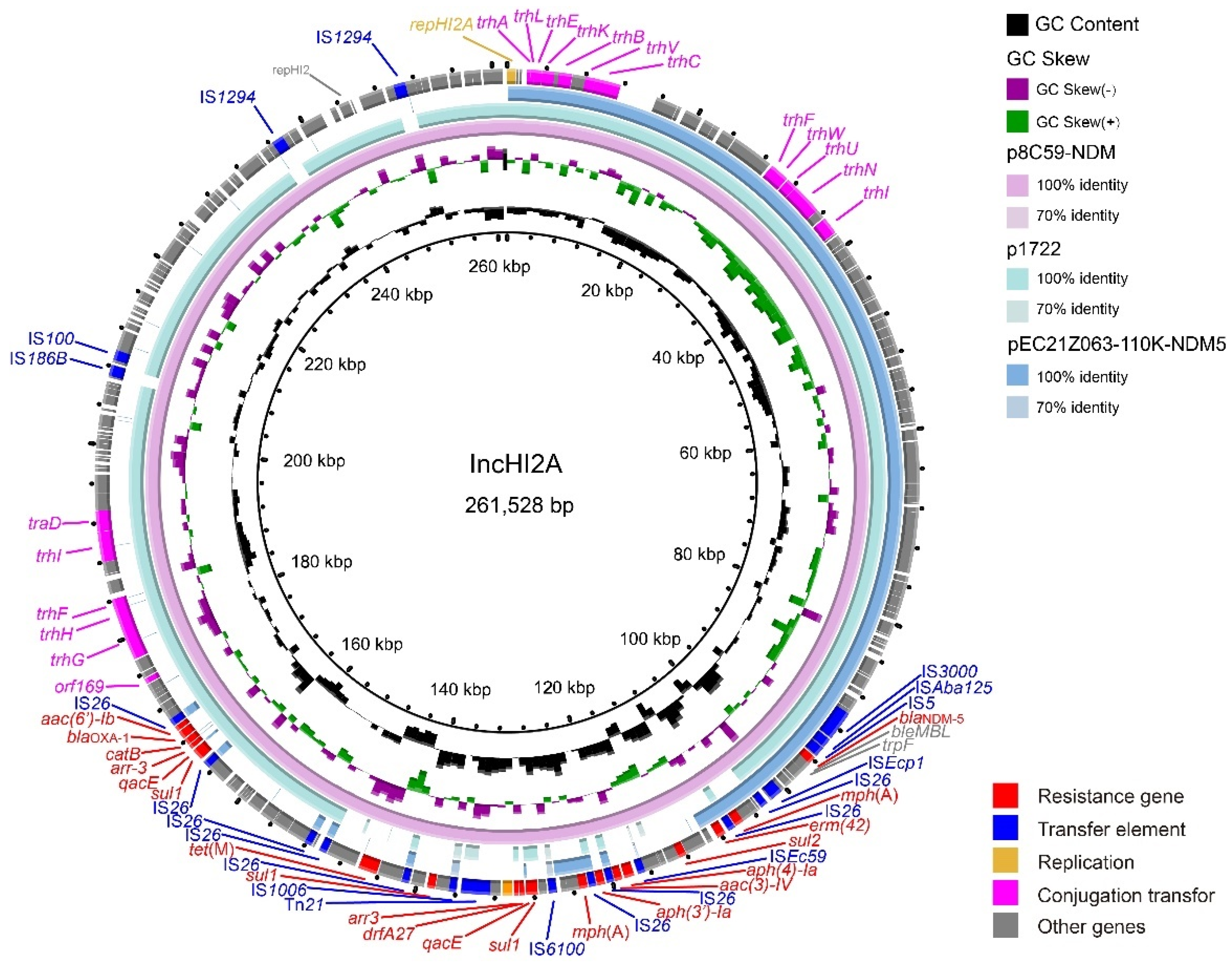
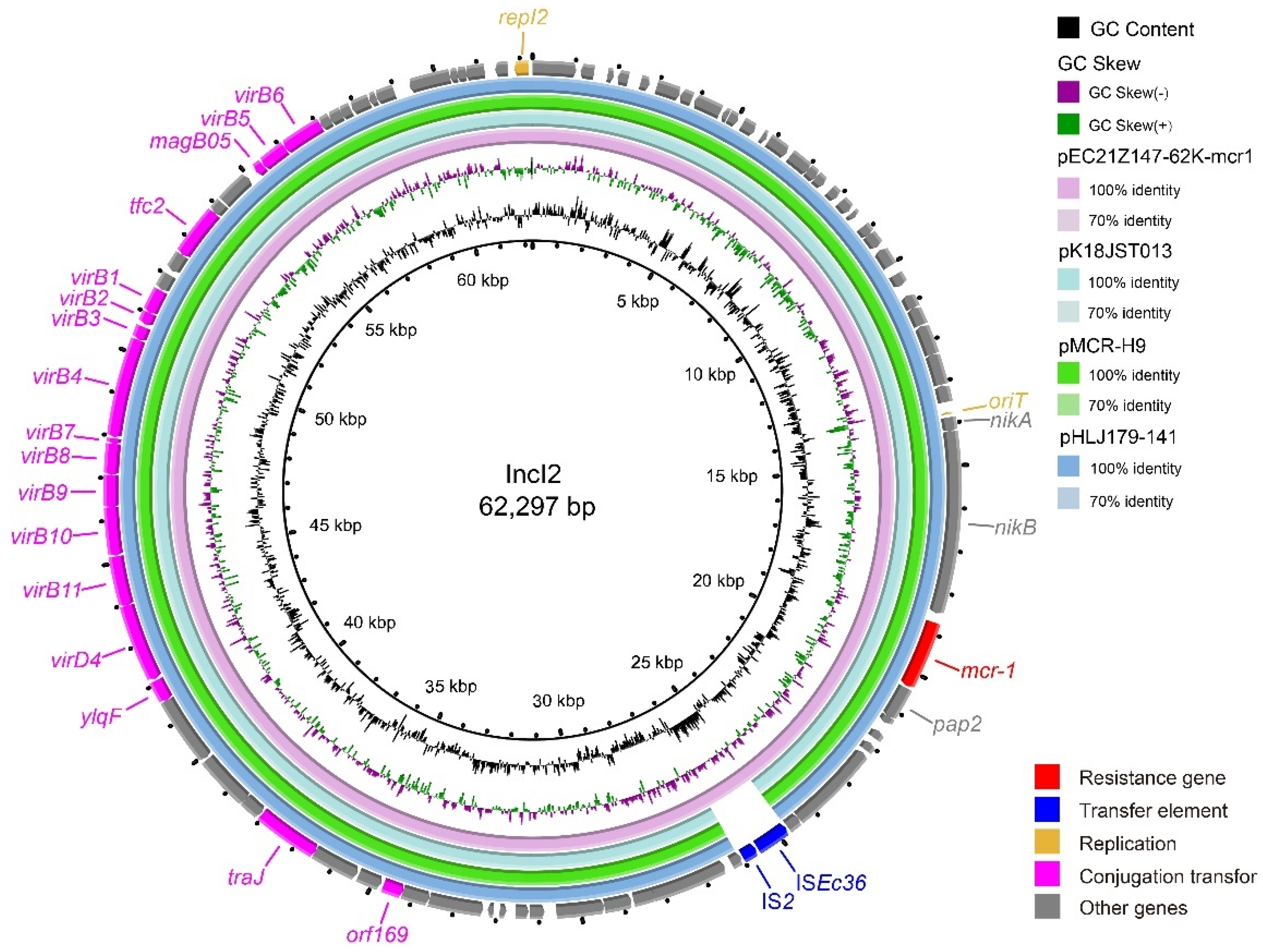
The IncX3 plasmids (pEC21Z014-46K-NDM1, pEC21Z048-45K-NDM5, pEC21Z078-46K-NDM5, pEC21Z083-46K-NDM5, pEC21Z097-46K-NDM5, pEC21Z101-46K-NDM5, and pEC21Z137-46K-NDM5) carrying the blaNDM-1 or blaNDM-5 genes showed a similar sequence. All of them contained the essential elements for the conjugational system, such as the origin of transfer, relaxases, type IV coupling proteins (T4CPs), and type IV secretion system (T4SS). They were similar to the blaNDM-5-positive IncX3 plasmid (pMTY18780-5_IncX3, AP023202) in the previous study (Figure 5) [32]. The IncI1-I plasmids pEC21Z144-121K-NDM5 (120.9 kb) and pEC21Z151-128K-NDM5 (127.8 kb) carrying blaNDM-5 were isolated from one patient. The pEC21Z151-128K-NDM5 obtained an insert fragment compared with pEC21Z144-121K-NDM5 that contained macrolide resistance genes (mph(A) and mrx(A)) and the transcriptional regulator (tetR/acrR). There are two IS26 flanked on both sides of the insert fragment that indicated the IS26 played an important role in transmission. Furthermore, the shufflon regions of the IncI1-1 plasmid exhibited differences between pEC21Z144-121K-NDM5 and pEC21Z151-128K-NDM5 that may have affected the ability of conjugation (Figure 6). An IncI1-1 plasmid (pSal8934, JF274993) from Salmonella enterica was found in GenBank and has a similar genetic backbone but without the ARG (Figure 6). The pEC21Z063-110K-NDM5 carrying the blaNDM-5 gene belonged to IncHI2A, which lacked the type IV coupling protein. The similar genetic backbone plasmid p8C59-NDM (MT407547) contained a complete conjugation element (Figure 7). The pEC21Z147-62K-mcr1 carrying mcr-1 gene belonged to IncI2, which has a typical genetic backbone and contains the essential elements for the conjugational system (Figure 8). Similar plasmids were identified in Salmonella (CP065423) and E. coli (MN232210, CP029184) from animals and humans.
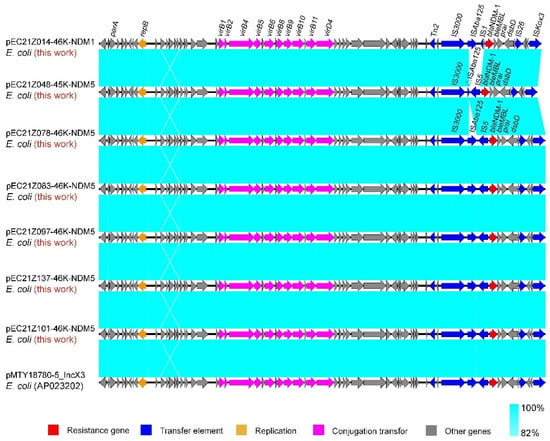
Figure 5.
Sequence comparison of the IncX3 plasmids carrying blaNDM-1 and blaNDM-5 genes with the common plasmid backbone (pMTY18780-5_IncX3) in GenBank. Sequence similarity is shown in light blue. Arrows and triangles represent genes of different functional categories (red: resistance genes; dark blue: transfer elements; orange: replication; pink: conjugation transfer, grey: other genes).
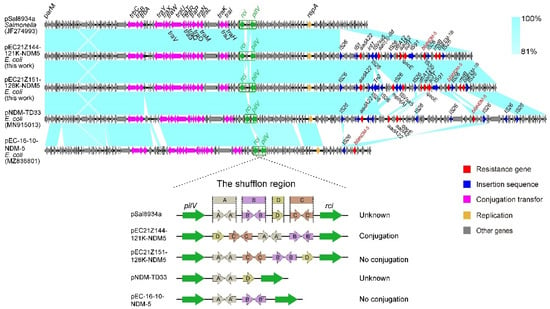
Figure 6.
Sequence comparison of the IncHI2A plasmids carrying blaNDM-5 genes from the strains EC21Z-144 and EC21Z-151. The original plasmid (pSal8934) is compared with the pEC21Z144-121K-NDM5 and pEC21Z151-128K-NDM5. The shufflon regions are marked by a green box, and the box marking A, B, C and D referred to the four segments of shufflon. The arrows and triangles represent genes from different functional categories (red: ARGs, dark blue: insertion sequence, orange: replication, pink: conjugation transfer, green: shufflon region, grey: other genes).
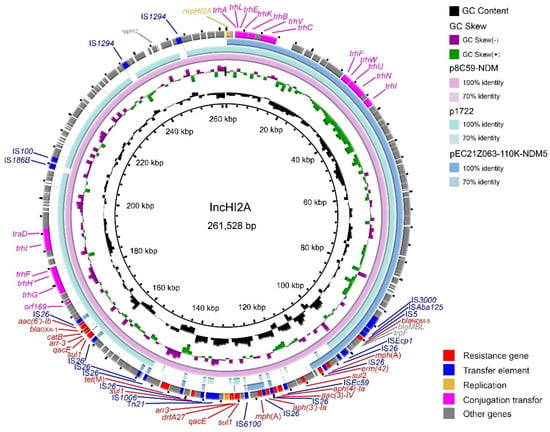
Figure 7.
Genetic characteristics of the blaNDM-5-positive plasmid pEC21Z063-110K-NDM5 identified in this study compared with similar plasmids from pathogenic bacteria in humans or animals. The arrows and triangles represent genes from different functional categories (red: ARGs, dark blue: insertion sequence, orange: replication, pink: conjugation transfer, grey: other genes).
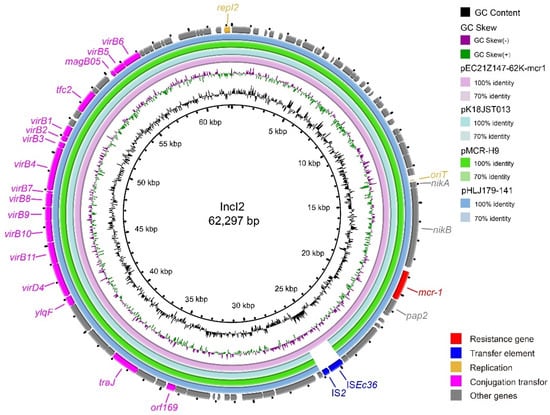
Figure 8.
Genetic characteristics of the mcr-1-positive plasmid (pEC21Z147-62K-mcr1) identified in this study compared with similar plasmids from pathogenic bacteria in humans or animals. The arrows and triangles represent genes from different functional categories (red: ARGs, dark blue: insertion sequence, orange: replication, pink: conjugation transfer, grey: other genes).
The blaNDM-5 gene on the IncX3 and IncHI2A plasmids has a conservative genome context IS3000-ISAba125-IS5-blaNDM-5-ble-prai-dsbD. The blaNDM-1 gene on the IncX3 plasmid has a similar context to IS3000-ISAba125-IS1-blaNDM-1-ble-prai-dsbD. However, the blaNDM-5 gene was located on the IncI1-1 plasmid as IS30-blaNDM-5-ble-prai-dsbD-ISCR1. The mcr-1 was located on IncI2 as a common genome context (nikA-nikB-mcr-1-pap2).
4. Discussion
Hospital and community settings are important reservoirs for the spread of pathogens and ARGs, such as ESBL, blaNDM, blaKPC, mcr, etc. Here, we investigated the prevalence of last-resort antimicrobial-resistant E. coli strains from inpatients in a hospital. Ten (6.37%) carbapenem-resistant-, one (0.64%) colistin-resistant-, and zero (0%) tigecycline resistant-strains were identified. All of them were found in inpatients instead of outpatients, indicating that the ARGs were spread in the hospital environment. Additionally, we observed that inpatients carry significantly more E. coli against last-resort antibiotics than outpatients.
The prevalence of blaNDM (6.37%, 10/157) was higher than the global prevalence in 55 countries (0.28%, 290/103,960) [15]. It is also higher than the prevalence of CREC (2.38%, 92/3895) in healthy people in healthcare centers located in 19 provinces across China [16]. The blaNDM gene was also detected from the specimens of various environments, vegetables (0.28%), companion animals (2.3%), and livestock and poultry (0.88%), the amounts of which were significantly lower than that in patients [33,34,35]. However, it has a similar prevalence in India (6.22%) [15]. Unlike blaNDM, the prevalence of the mcr and tet(X) genes in patients was significantly lower than that in animals, especially for the tet(X) gene. As expected, except the tet(X) gene identified in this study, these genes were more widespread in freshwater fishes (24.7%), chickens (23.6%), cattle (19.3%), and pigs (8.95%) than in patients (0.3%) [36,37]. Only one mcr-1-positive E. coli strain (0.64%) was identified in patients, similar to the positive rates in patients (0.62%, 36/5828) from several provinces of China [38]. This rate is significantly lower than those in livestock and poultry (14.81%, 270/1823), and environmental strains (5.43%, 5/92) [35,38]. It is important to note that this mcr-1-positive E. coli was isolated from the lungs in a one-day-old child. This child is the youngest reported case of mcr-1-positive E. coli infection.
The virulence gene co-located with ARGs in Enterobacteriaceae has received extensive attention in recent years. The virulence determinants were identified in various bacteria harboring mcr, blaNDM, or other genes from foods, animals, humans, and environments and caused a serious threat to public security [39,40,41]. The EAEC harboring heat-stable enterotoxin gene astA has been discovered in E. coli serotype O7: H4, O untypeable: H10, O4: H34, etc. The novel serotype O128: H4 E. coli harboring astA was identified in the mcr-1-positive E. coli EC21Z-14 belonging to ST117. The E.coli ST117 was an emerging foodborne zoonotic pathogen that has been isolated from the poultry, meat, human, and sea ecosystem [42,43,44,45]. A recent study showed that the ST117 strains isolated from different hosts had higher genetic similarity, suggesting that ST117 could transfer among different hosts [46]. In this study, the EC21Z-14 isolated from the one-day-old child was associated with the pathogenic E. coli from avian animals, reminding us that MDR pathogens from animals could spread in human and cause disease.
The blaNDM-1 and blaNDM-5 genes were first identified in Klebsiella pneumoniae in India in 2009 and in E. coli in the United Kingdom in 2011, respectively [1,47]. A higher prevalence of the blaNDM-1 gene (69%) than the blaNDM-5 gene (19%) was primarily reported in clinical settings [16]. However, the opposite was observed for the blaNDM-5 gene, which was dominant in CREC in China after 2016 [16]. It was observed in this study that 90% of CRECs bore the blaNDM-5 gene instead of the blaNDM-1 gene. The different hydrolysis activities may cause this to carbapenems [48]. The blaNDM-5 gene gradually replaced blaNDM-1 in China, and this phenomenon may have spread to other countries.
blaNDM-positive E. coli strains belonging to various STs have been reported. The most common CRECs worldwide were ST167, ST410, and ST617, and ST131 was the major CREC in China [15,48,49]. No obvious epidemic clones of NDM-positive E. coli were found, and only three ST410 strains were identified from one patient in this study. The strains isolated from different patients belonged to different STs. Furthermore, the mobile plasmid IncX3 is the primary replicon type for carrying various blaNDM (NDM-1, NDM-4, NDM-5, NDM-6, NDM-7, etc.) and was detected in several strains in this study [15]. IncX3 is dominant in blaNDM-positive plasmids and promote horizontal blaNDM gene transfer. It is pointed out that the IncX3 carrying blaNDM-1 unsuccessfully transferred to the recipient by conjugation, and no obvious mechanic was found for this abnormality. This may be a reason for the lower prevalence of the blaNDM-1 gene. IncHI2A and IncI1-1 were discovered as relatively uncommon replicon types for carrying blaNDM-5 [50]. Overall, the blaNDM-5 gene is the primarily genetic determinant for CREC, mainly spread by the IncX3 plasmids.
Multiple samples collected from two lymphoma patients at several points in time were further analyzed. The EC21Z-101 strains lost a plasmid (IncFIB-FIC) carrying several ARGs and virulence genes compared with the EC21Z-078, EC21Z-083, and EC21Z-097 strains from one patient that became sensitive to the antimicrobials. The mechanism behind the missing highly stable multidrug-resistant IncFIB plasmid is worth further studying [51]. On the other hand, the conjugation ability showed significant differences between two IncI1-1 plasmids (pEC21Z144-121K-NDM5 and pEC21Z151-128K-NDM5) carrying blaNDM-5 from one patient. Different shufflon regions were discovered between shufflon-specific DNA recombinase and PilV. It is widely known that shufflon is a multiple-inversion system that is closely related to the efficiency of the conjugation [52]. Shufflon comprises four segments (A, B, C, and D) which may be rearranged and inverted to influence the conjugation during liquid mating [53]. The rearrangement of shufflon usually reduces the transfer frequency instead of the loss [54]. The novel rearrangement in pEC21Z151-128K-NDM5 may provide a strategy to decrease plasmid dissemination, which is worth further investigating.
5. Conclusions
In summary, our data support that the hospital setting is a vital vehicle for spreading and disseminating last-resort AMR pathogens. Moreover, the acquisition of blaNDM and mcr-1 genes encoding resistance to carbapenem and colistin was mediated by horizontal transfer via plasmids. IncX3 carrying blaNDM-5 is the leading carrier of resistance in CREC. The ST117 E. coli is an emerging zoonotic pathogen carrying the mcr-1 gene transferred between humans and animals. Therefore, the horizontal transfer of ARGs among inpatients indicated the importance of preventing plasmids in clinical pathogens. The continuous surveillance of carbapenem and colistin resistance at the genome level in sentinel hospitals is highly prioritized to limit the transmission of last-resort pathogens.
Author Contributions
Conception and design of study: B.T., H.Y. and M.Y. Acquisition of data: M.Y. and A.E.-D. Data analysis and/or interpretation: B.T., J.M., J.W., A.E.-D. and Y.D. Drafting of manuscript and/or critical revision: B.T., J.M., J.L. and H.L. Approval of final version of manuscript: M.Y. and H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Research and Development Program of Hangzhou (202203A08), the Key Research and Development Program of Zhejiang Province (2022C02024, 2021C02008, 2020C02031), the State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products (2010DS700124-ZZ2102), China Postdoctoral Science Foundation (2021M702904), the Collaborative Extension Plan of Major Agricultural Technologies in Zhejiang Province (2021XTTGXM03), and State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products (No. 2021DG700024-KF202213).
Institutional Review Board Statement
The ethical approval was made by Zhejiang Provincial Center for Disease Control and Prevention (20190429).
Informed Consent Statement
Not applicable.
Data Availability Statement
The nucleotide sequences presented in this study were deposited at Project accession number PRJNA856840.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S.; et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef]
- Linciano, P.; Cendron, L.; Gianquinto, E.; Spyrakis, F.; Tondi, D. Ten years with New Delhi Metallo-β-lactamase-1 (NDM-1): From structural insights to inhibitor design. ACS Infect. Dis. 2019, 5, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Yin, Y.; Chen, H.; Jin, L.; Gu, B.; Xie, L.; Yang, C.; Ma, X.; Li, H.; et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: Report from the China CRE Network. Antimicrob. Agents Chemother. 2018, 62, e01882-17. [Google Scholar] [CrossRef] [PubMed]
- Elbediwi, M.; Pan, H.; Zhou, X.; Rankin, S.C.; Schifferli, D.M.; Yue, M. Detection of mcr-9-harbouring ESBL-producing Salmonella Newport isolated from an outbreak in a large-animal teaching hospital in the USA. J. Antimicrob. Chemother. 2021, 76, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Li, Y.; Elbediwi, M.; Yue, M. Emergence and dissemination of mcr-carrying clinically relevant Salmonella Typhimurium monophasic clone ST34. Microorganisms 2019, 7, 298. [Google Scholar] [CrossRef]
- Elbediwi, M.; Li, Y.; Paudyal, N.; Pan, H.; Li, X.; Xie, S.; Rajkovic, A.; Feng, Y.; Fang, W.; Rankin, S.C.; et al. Global burden of colistin-resistant bacteria: Mobilized colistin resistance genes study (1980–2018). Microorganisms 2019, 7, 461. [Google Scholar] [CrossRef]
- Elbediwi, M.; Beibei, W.; Pan, H.; Jiang, Z.; Biswas, S.; Li, Y.; Yue, M. Genomic characterization of mcr-1-carrying Salmonella enterica Serovar 4,[5],12:i:- ST 34 clone isolated from pigs in China. Front. Bioeng. Biotechnol. 2020, 8, 663. [Google Scholar] [CrossRef]
- He, T.; Wang, R.; Liu, D.; Walsh, T.R.; Zhang, R.; Lv, Y.; Ke, Y.; Ji, Q.; Wei, R.; Liu, Z.; et al. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019, 4, 1450–1456. [Google Scholar] [CrossRef]
- Hussein, N.H.; Al-Kadmy, I.M.S.; Taha, B.M.; Hussein, J.D. Mobilized colistin resistance (mcr) genes from 1 to 10: A comprehensive review. Mol. Biol. Rep. 2021, 48, 2897–2907. [Google Scholar] [CrossRef]
- Portes, A.B.; Rodrigues, G.; Leitão, M.P.; Ferrari, R.; Conte Junior, C.A.; Panzenhagen, P. Global distribution of plasmid-mediated colistin resistance mcr gene in Salmonella: A systematic review. J. Appl. Microbiol. 2022, 132, 872–889. [Google Scholar] [CrossRef]
- Park, J.; Yun, S.J.; Shin, E.; Kim, J.S.; Park, S.H.; Pyeon, H.S.; Kim, M.K.; Joo, S.; Jeong, H.J.; Chun, J.H.; et al. First identification of novel variants of New Delhi metallo-β-lactamase, NDM-30 and NDM-31, in the Republic of Korea. J. Glob. Antimicrob. Resist. 2022, 29, 20–22. [Google Scholar] [CrossRef]
- Umar, Z.; Chen, Q.; Tang, B.; Xu, Y.; Wang, J.; Zhang, H.; Ji, K.; Jia, X.; Feng, Y. The poultry pathogen Riemerella anatipestifer appears as a reservoir for Tet(X) tigecycline resistance. Environ. Microbiol. 2021, 23, 7465–7482. [Google Scholar] [CrossRef]
- Tang, B.; Yang, H.; Jia, X.; Feng, Y. Coexistence and characterization of Tet(X5) and NDM-3 in the MDR-Acinetobacter indicus of duck origin. Microb. Pathog. 2021, 150, 104697. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Feng, Y.; Tang, G.; Qiao, F.; McNally, A.; Zong, Z. NDM Metallo-β-Lactamases and their bacterial producers in health care settings. Clin. Microbiol. Rev. 2019, 32, e00115-18. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Hu, Y.; Sun, Q.; Hu, F.; Zhou, H.; Shu, L.; Ma, T.; Shen, Y.; Wang, Y.; Li, J.; et al. Emerging carriage of NDM-5 and MCR-1 in Escherichia coli from healthy people in multiple regions in China: A cross sectional observational study. EClinicalMedicine 2018, 6, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Ma, Y.; He, X.; Zhou, Q.; Chang, J.; Qian, M.; Xia, X.; Yang, H. Similar antimicrobial resistance of Escherichia coli strains isolated from retail chickens and poultry farms. Foodborne Pathog. Dis. 2021, 18, 489–496. [Google Scholar] [CrossRef]
- Lin, J.; Tang, B.; Zheng, X.; Chang, J.; Ma, J.; He, Y.; Yang, H.; Wu, Y. Emergence of Incl2 plasmid-mediated colistin resistance in avian Escherichia fergusonii. FEMS Microbiol. Lett. 2022, 369, fnac016. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Chang, J.; Cao, L.; Luo, Q.; Xu, H.; Lyu, W.; Qian, M.; Ji, X.; Zhang, Q.; Xia, X.; et al. Characterization of an NDM-5 carbapenemase-producing Escherichia coli ST156 isolate from a poultry farm in Zhejiang, China. BMC Microbiol. 2019, 19, 82. [Google Scholar] [CrossRef]
- Wayne, P. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019; M100-S129; pp. 30–37. [Google Scholar]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ed-Dra, A.; Tang, B.; Kang, X.; Müller, A.; Kehrenberg, C.; Jia, C.; Pan, H.; Yang, H.; Yue, M. Higher tolerance of predominant Salmonella serovars circulating in the antibiotic-free feed farms to environmental stresses. J. Hazard. Mater. 2022, 438, 129476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kang, X.; Ed-Dra, A.; Zhou, X.; Jia, C.; Müller, A.; Liu, Y.; Kehrenberg, C.; Yue, M. Genome-based assessment of antimicrobial resistance and virulence potential of isolates of non-pullorum/gallinarum Salmonella Serovars recovered from dead poultry in China. Microbiol. Spectr. 2022, 10, e0096522. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Ed-Dra, A.; Pan, H.; Dong, C.; Jia, C.; Yue, M. Genomic Investigation of Salmonella isolates recovered from a pig slaughtering process in Hangzhou, China. Front. Microbiol. 2021, 12, 704636. [Google Scholar] [CrossRef]
- Qiu, Y.F.; Nambiar, R.B.; Xu, X.B.; Weng, S.T.; Pan, H.; Zheng, K.C.; Yue, M. Global genomic characterization of Salmonella enterica Serovar Telelkebir. Front. Microbiol. 2021, 12, 704152. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Chang, J.; Zhang, L.; Liu, L.; Xia, X.; Hassan, B.H.; Jia, X.; Yang, H.; Feng, Y. Carriage of distinct mcr-1-harboring plasmids by unusual serotypes of Salmonella. Adv. Biosyst. 2020, 4, e1900219. [Google Scholar] [CrossRef] [PubMed]
- Siguier, P.; Perochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34, D32–D36. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, J.; Feng, J.; Liu, Y.; Yang, B.; Li, R.; Bai, L.; He, T.; Wang, X.; Yang, Z. Characterization of three porcine Acinetobacter towneri strains co-harboring tet(X3) and bla(OXA-58). Front. Cell. Infect. Microbiol. 2020, 10, 586507. [Google Scholar] [CrossRef]
- Tang, B.; Chang, J.; Chen, Y.; Lin, J.; Xiao, X.; Xia, X.; Lin, J.; Yang, H.; Zhao, G. Escherichia fergusonii, an underrated repository for antimicrobial resistance in food animals. Microbiol. Spectr. 2022, 10, e0161721. [Google Scholar] [CrossRef]
- Lei, L.; Rehman, M.U.; Huang, S.; Zhang, L.; Wang, L.; Mehmood, K.; Zhang, H.; Tong, X.; Wang, M.; Li, J. Antimicrobial resistance and prevalence of diarrheagenic Escherichia coli (DEC), in diarrheic yaks of Tibetan Plateau, China. Acta Trop. 2018, 182, 111–114. [Google Scholar] [CrossRef]
- Tobias, J.; Vutukuru, S.R. Simple and rapid multiplex PCR for identification of the main human diarrheagenic Escherichia coli. Microbiol. Res. 2012, 167, 564–570. [Google Scholar] [CrossRef]
- Ito, Y.; Aoki, K.; Ishii, Y.; Nakayama, H.; Otsuka, M.; Kaneko, N.; Yoshida, M.; Tateda, K.; Matsuse, H. Whole-genome sequencing analysis of bla(NDM-5)/IncX3 plasmid estimated to be conjugative-transferred in the gut. Microb. Drug Resist. 2022, 28, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, M.; Dai, X.; Zhou, Y.; Zhang, Z.; Qiu, Y.; Li, C.; Zhang, L. Whole-genomic analysis of NDM-5-producing Enterobacteriaceae recovered from an urban river in China. Infect. Drug Resist. 2021, 14, 4427–4440. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.T.; Song, F.J. Emergence of two Escherichia coli strains co-harboring mcr-1 and bla(NDM) in fresh vegetables from China. Infect. Drug Resist. 2019, 12, 2627–2635. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Duan, R.; Fan, R.; Mu, H.; Liang, J.; Xiao, M.; He, Z.; Qin, S.; Yang, J.; Jing, H.; et al. bla(NDM) and mcr-1 to mcr-5 gene distribution characteristics in gut specimens from different regions of China. Antibiotics 2021, 10, 233. [Google Scholar] [CrossRef]
- Dong, N.; Zeng, Y.; Cai, C.; Sun, C.; Lu, J.; Liu, C.; Zhou, H.; Sun, Q.; Shu, L.; Wang, H.; et al. Prevalence, transmission, and molecular epidemiology of tet(X)-positive bacteria among humans, animals, and environmental niches in China: An epidemiological, and genomic-based study. Sci. Total Environ. 2022, 818, 151767. [Google Scholar] [CrossRef]
- Feng, J.; Su, M.; Li, K.; Ma, J.; Li, R.; Bai, L.; Wang, X.; Wang, J.; Yang, Z. Extensive spread of tet(X4) in multidrug-resistant Escherichia coli of animal origin in western China. Vet. Microbiol. 2022, 269, 109420. [Google Scholar] [CrossRef]
- Fan, R.; Li, C.; Duan, R.; Qin, S.; Liang, J.; Xiao, M.; Lv, D.; Jing, H.; Wang, X. Retrospective screening and analysis of mcr-1 and bla(NDM) in gram-negative bacteria in China, 2010–2019. Front. Microbiol. 2020, 11, 121. [Google Scholar] [CrossRef]
- Anyanwu, M.U.; Jaja, I.F.; Nwobi, O.C. Occurrence and characteristics of mobile colistin resistance (mcr) gene-containing isolates from the environment: A review. Int. J. Environ. Res. Public Health 2020, 17, 1028. [Google Scholar] [CrossRef]
- Tartor, Y.H.; Gharieb, R.M.A.; Abd El-Aziz, N.K.; El Damaty, H.M.; Enany, S.; Khalifa, E.; Attia, A.S.A.; Abdellatif, S.S.; Ramadan, H. Virulence determinants and plasmid-mediated colistin resistance mcr genes in gram-negative bacteria isolated from bovine milk. Front. Cell. Infect. Microbiol. 2021, 11, 761417. [Google Scholar] [CrossRef]
- Pérez-Vázquez, M.; Sola Campoy, P.J.; Ortega, A.; Bautista, V.; Monzón, S.; Ruiz-Carrascoso, G.; Mingorance, J.; González-Barberá, E.M.; Gimeno, C.; Aracil, B.; et al. Emergence of NDM-producing Klebsiella pneumoniae and Escherichia coli in Spain: Phylogeny, resistome, virulence and plasmids encoding blaNDM-like genes as determined by WGS. J. Antimicrob. Chemother. 2019, 74, 3489–3496. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Wang, Y.L.; Sun, Q.L.; Huang, Z.X.; Wang, H.Y.; Zhang, R.; Chen, G.X. Colistin resistance gene mcr-1 in gut flora of children. Int. J. Antimicrob. Agents 2017, 50, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Ćwiek, K.; Woźniak-Biel, A.; Karwańska, M.; Siedlecka, M.; Lammens, C.; Rebelo, A.R.; Hendriksen, R.S.; Kuczkowski, M.; Chmielewska-Władyka, M.; Wieliczko, A. Phenotypic and genotypic characterization of mcr-1-positive multidrug-resistant Escherichia coli ST93, ST117, ST156, ST10, and ST744 isolated from poultry in Poland. Braz. J. Microbiol. 2021, 52, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Moon, J.S.; Oh, D.H.; Chon, J.W.; Song, B.R.; Lim, J.S.; Heo, E.J.; Park, H.J.; Wee, S.H.; Sung, K. Genotypic characterization of ESBL-producing E. coli from imported meat in South Korea. Food Res. Int. 2018, 107, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Vingino, A.; Roberts, M.C.; Wainstein, M.; West, J.; Norman, S.A.; Lambourn, D.; Lahti, J.; Ruiz, R.; D’Angeli, M.; Weissman, S.J.; et al. Surveillance for antibiotic-resistant E. coli in the Salish Sea Ecosystem. Antibiotics 2021, 10, 1201. [Google Scholar] [CrossRef]
- Xia, F.; Jiang, M.; Wen, Z.; Wang, Z.; Wang, M.; Xu, Y.; Zhuge, X.; Dai, J. Complete genomic analysis of ST117 lineage extraintestinal pathogenic Escherichia coli (ExPEC) to reveal multiple genetic determinants to drive its global transmission: ST117 E. coli as an emerging multidrug-resistant foodborne ExPEC with zoonotic potential. Transbound. Emerg. Dis. 2022. [Google Scholar] [CrossRef]
- Hornsey, M.; Phee, L.; Wareham, D.W. A novel variant, NDM-5, of the New Delhi metallo-β-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob. Agents Chemother. 2011, 55, 5952–5954. [Google Scholar] [CrossRef]
- Li, F.; Ye, K.; Li, X.; Ye, L.; Guo, L.; Wang, L.; Yang, J. Genetic characterization of carbapenem-resistant Escherichia coli from China, 2015–2017. BMC Microbiol. 2021, 21, 248. [Google Scholar] [CrossRef]
- Tang, B.; Ni, J.; Lin, J.; Sun, Y.; Lin, H.; Wu, Y.; Yang, H.; Yue, M. Genomic characterization of multidrug-resistance gene cfr in Escherichia coli recovered from food animals in Eastern China. Front. Microbiol. 2022, 13, 999778. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Zhu, J.H.; Cai, R.M.; Zheng, X.R.; Zhang, L.J.; Chang, M.X.; Lu, Y.W.; Fang, L.X.; Sun, J.; Jiang, H.X. IS26 is responsible for the evolution and transmission of bla(NDM)-harboring plasmids in Escherichia coli of poultry origin in China. mSystems 2021, 6, e0064621. [Google Scholar] [CrossRef]
- Farzana, R.; Jones, L.S.; Barratt, A.; Rahman, M.A.; Sands, K.; Portal, E.; Boostrom, I.; Espina, L.; Pervin, M.; Uddin, A.; et al. Emergence of mobile colistin resistance (mcr-8) in a highly successful Klebsiella pneumoniae sequence type 15 clone from clinical infections in Bangladesh. mSphere 2020, 5, e00023-20. [Google Scholar] [CrossRef]
- Foley, S.L.; Kaldhone, P.R.; Ricke, S.C.; Han, J. Incompatibility group I1 (IncI1) plasmids: Their genetics, biology, and public health relevance. Microbiol. Mol. Biol. Rev. 2021, 85, e00031-20. [Google Scholar] [CrossRef] [PubMed]
- Stosic, M.S.; Sunde, M.; Mo, S.S.; Telke, A.A.; Rudi, K. Interference of ISEcp1-bla(CTX-M-1) with the shufflon rearrangement in IncI1 plasmids. Plasmid 2021, 116, 102578. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.S.M.; Jurburg, S.D.; Harders, F.; Kant, A.; Mevius, D.J.; Roberts, A.P.; Bossers, A. The shufflon of IncI1 plasmids is rearranged constantly during different growth conditions. Plasmid 2019, 102, 51–55. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).