Curcumin Reprograms TAMs from a Protumor Phenotype towards an Antitumor Phenotype via Inhibiting MAO-A/STAT6 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Polarization of Macrophages
2.2. Cell Proliferation Assay
2.3. Co-Culture Experiment
2.4. Transwell Assay
2.5. Wound-Healing Assay
2.6. Quantitative Real-Time PCR
2.7. Western Blot Analysis
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Flow Cytometry
2.10. Reactive Oxygen Species (ROS) Measurement
2.11. Statistical Analysis
3. Results
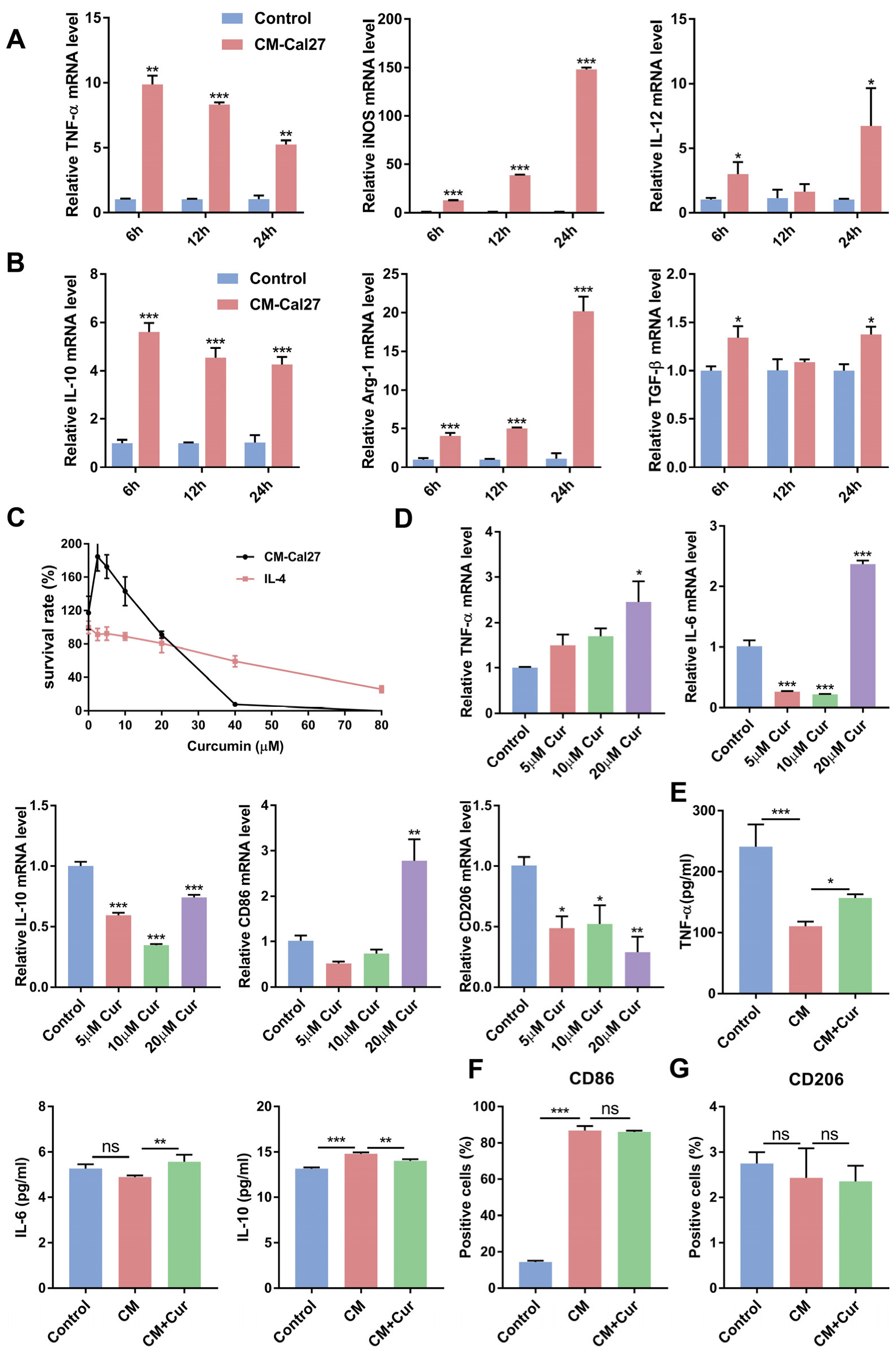
3.1. Curcumin Promoted the Expression of Antitumor Cytokines and Suppressed the Expression of Protumor Cytokines in CM-Cal27-Induced Raw264.7 Cells
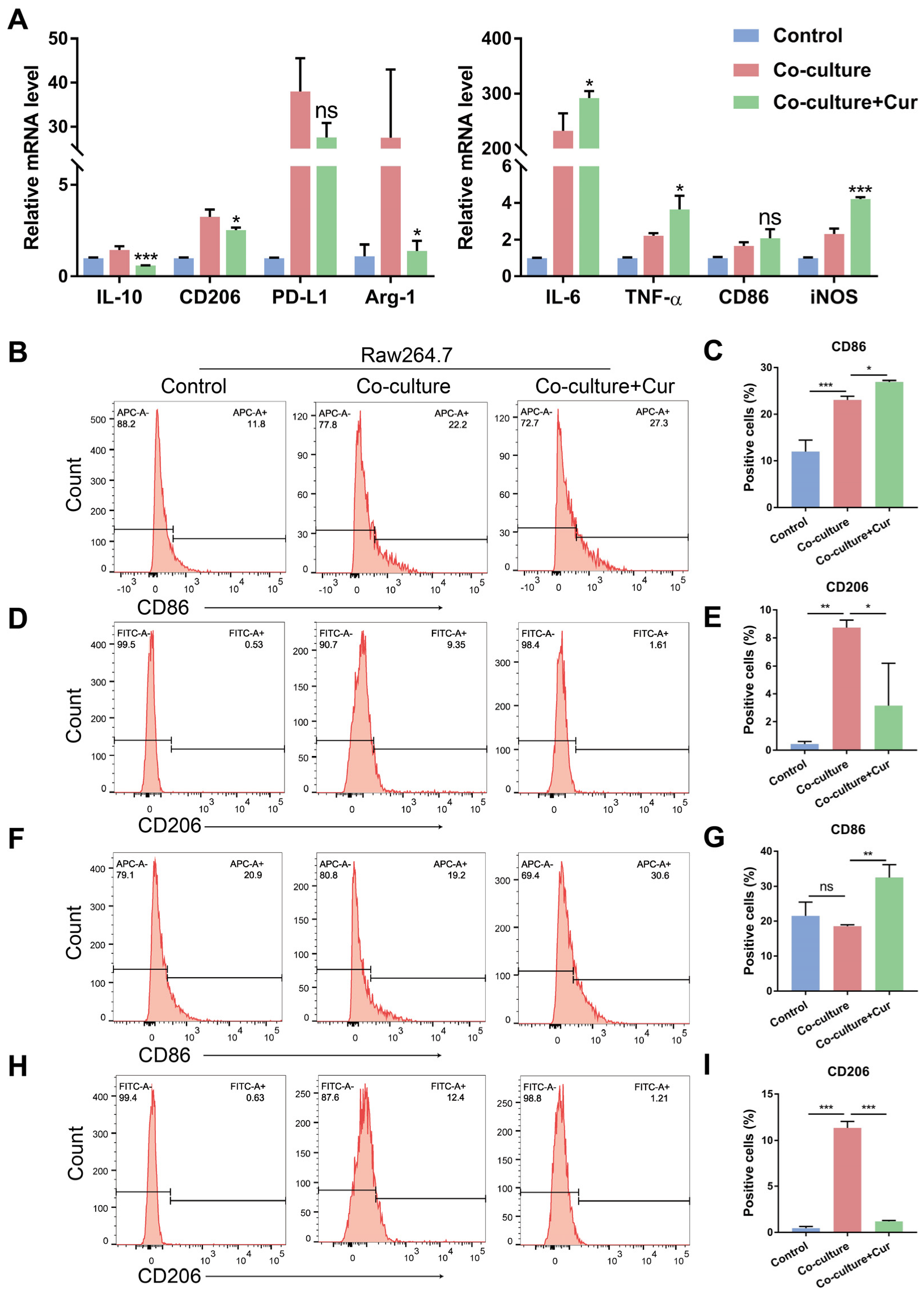
3.2. Curcumin Facilitated TAMs Polarization into an M1-like Population in the Co-Culture System
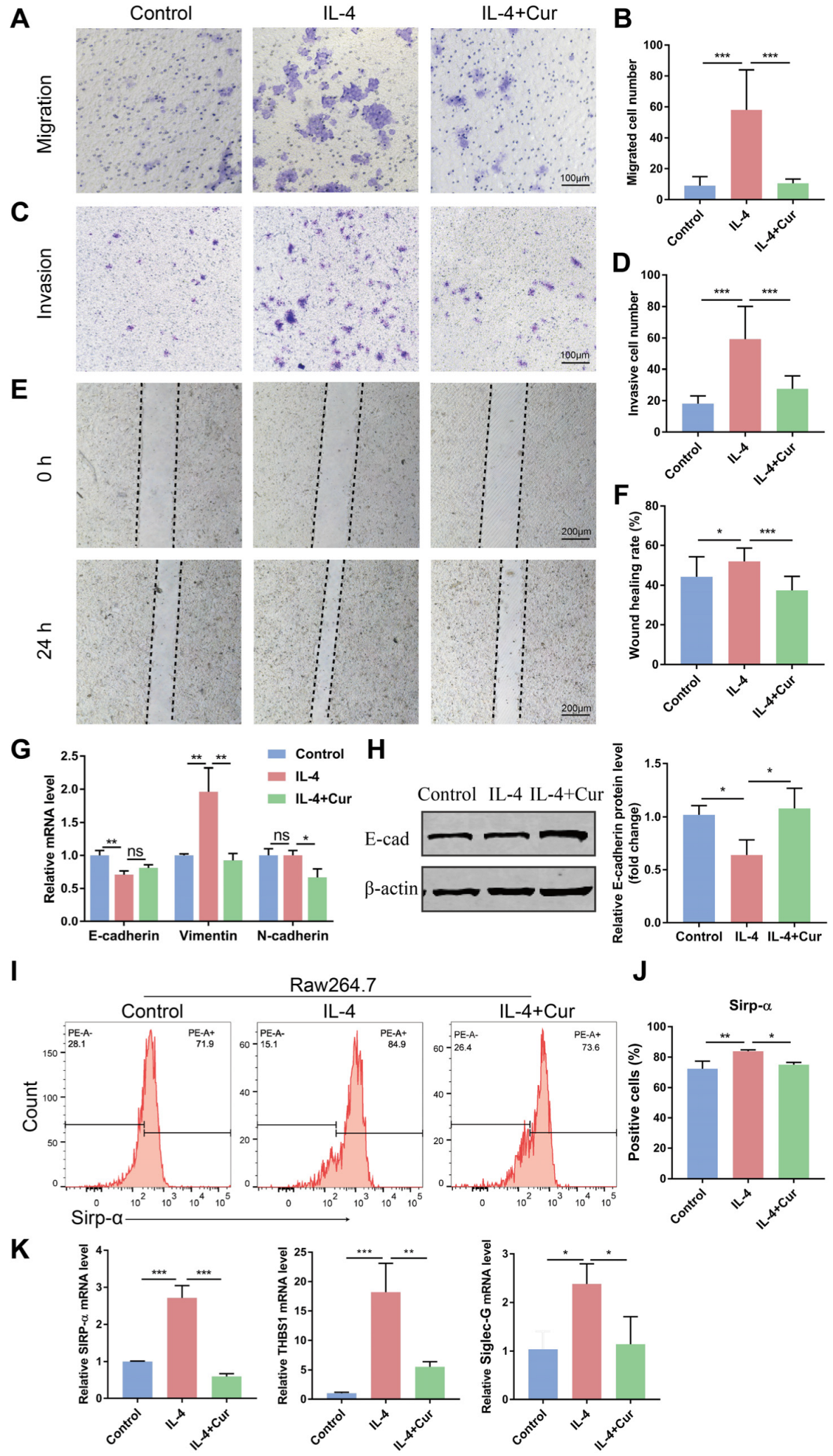
3.3. Curcumin Reprogrammed M2 Macrophages into an M1-like Population in IL-4-Induced Model
3.4. Curcumin Reprogrammed M2 Macrophages from a Protumor Phenotype towards an Antitumor Phenotype
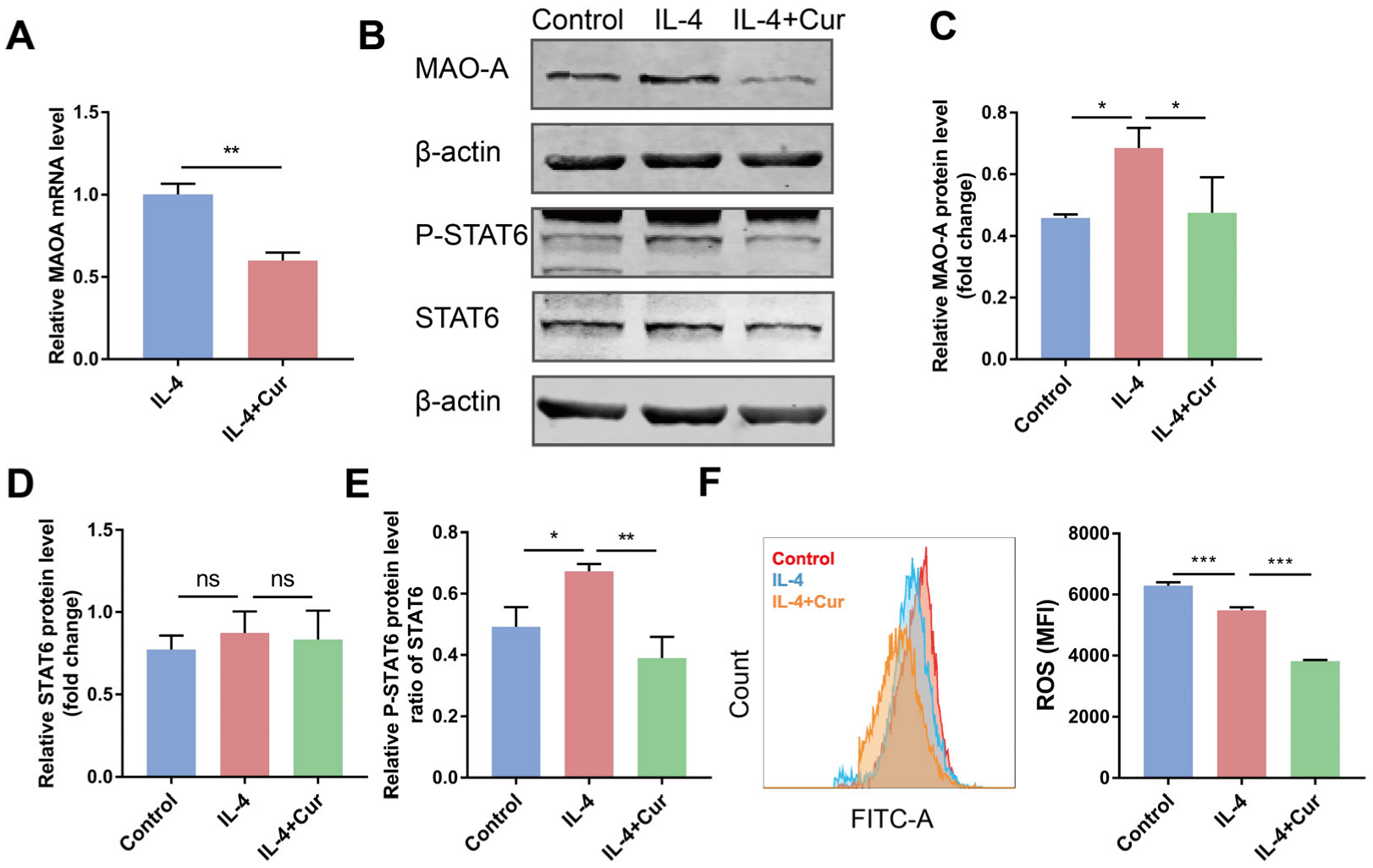
3.5. Curcumin Reprogrammed M2 Macrophages towards M1-like Macrophages by Suppressing MAO-A/STAT6 Signaling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, H.; Wang, Z.; Fu, L.; Xu, T. Macrophage Polarization in the Development and Progression of Ovarian Cancers: An Overview. Front. Oncol. 2019, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, B.; Coussens, L.M. Macrophages and therapeutic resistance in cancer. Cancer Cell 2015, 27, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Bercovici, N.; Guérin, M.V.; Trautmann, A.; Donnadieu, E. The Remarkable Plasticity of Macrophages: A Chance to Fight Cancer. Front. Immunol. 2019, 10, 1563. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Barreto, J.B.; Andreu, P.; Vasquez, L.; Tawfik, D.; Kolhatkar, N.; Coussens, L.M. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009, 16, 91–102. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, X.; Yu, J.; Ma, F.; Li, Z.; Zhou, Y.; Zeng, S.; Ma, X.; Li, Y.R.; Neal, A.; et al. Targeting monoamine oxidase A-regulated tumor-associated macrophage polarization for cancer immunotherapy. Nat. Commun. 2021, 12, 3530. [Google Scholar] [CrossRef]
- Ou, D.L.; Chen, C.W.; Hsu, C.L.; Chung, C.H.; Feng, Z.R.; Lee, B.S.; Cheng, A.L.; Yang, M.H.; Hsu, C. Regorafenib enhances antitumor immunity via inhibition of p38 kinase/Creb1/Klf4 axis in tumor-associated macrophages. J. Immunother. Cancer 2021, 9, e001657. [Google Scholar] [CrossRef]
- Zhou, Q.; Liang, J.; Yang, T.; Liu, J.; Li, B.; Li, Y.; Fan, Z.; Wang, W.; Chen, W.; Yuan, S.; et al. Carfilzomib modulates tumor microenvironment to potentiate immune checkpoint therapy for cancer. EMBO Mol. Med. 2022, 14, e14502. [Google Scholar] [CrossRef]
- Cao, X.; Chen, J.; Li, B.; Dang, J.; Zhang, W.; Zhong, X.; Wang, C.; Raoof, M.; Sun, Z.; Yu, J.; et al. Promoting antibody-dependent cellular phagocytosis for effective macrophage-based cancer immunotherapy. Sci. Adv. 2022, 8, eabl9171. [Google Scholar]
- Weng, W.; & Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Wang, Y.; Yu, T.; Zhu, C.; Zhang, X.; Guan, J. Curcumin suppresses tumorigenesis by ferroptosis in breast cancer. PLoS ONE 2022, 17, e0261370. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, T.; Lin, J.; Huang, X.; Ke, Q.; Wu, Y.; Fang, C.; Hu, C. Curcumin inhibits the invasion and metastasis of triple negative breast cancer via Hedgehog/Gli1 signaling pathway. J. Ethnopharmacol. 2022, 283, 114689. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, T.; Liu, X.; Liu, Q. Curcumin inhibits migration and invasion of non-small cell lung cancer cells through up-regulation of miR-206 and suppression of PI3K/AKT/mTOR signaling pathway. Acta Pharm. 2020, 70, 399–409. [Google Scholar] [CrossRef]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Amiri Moghadam, S.; ArefNezhad, R.; Sahebkar, A.; Avan, A.; et al. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol. Res. Pract. 2019, 215, 152556. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.Á.; González-Sarrías, A.; Martínez-Díaz, F.; Abellán, B.; Martínez-Torrano, A.J.; Fernández-López, A.J.; Giménez-Bastida, J.A.; Espín, J.C. Disposition of Dietary Polyphenols in Breast Cancer Patients’ Tumors, and Their Associated Anticancer Activity: The Particular Case of Curcumin. Mol. Nutr. Food Res. 2021, 65, e2100163. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Hylind, L.M.; Marrero, J.H.; Zahurak, M.L.; Murray-Stewart, T.; Casero, R.A.; Montgomery, E.A., Jr.; Iacobuzio-Donahue, C.; Brosens, L.A.; Offerhaus, G.J.; et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients with Familial Adenomatous Polyposis. Gastroenterology 2018, 155, 668–673. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.; Irving, G.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Kuang, D.M.; Wu, Y.; Chen, N.; Cheng, J.; Zhuang, S.M.; Zheng, L. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood 2007, 110, 587–595. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, T.; Pan, W.; Zhu, L.Y.; Li, L.; Zheng, L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int. J. Cancer 2009, 125, 1640–1648. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Ding, X.Y.; Li, T.E.; Wang, H.Y.; Gao, Y.H.; Wang, W.J.; Liu, Y.F.; Chen, X.S.; Shen, K.W. Hippo/YAP signaling choreographs the tumor immune microenvironment to promote triple negative breast cancer progression via TAZ/IL-34 axis. Cancer Lett. 2022, 527, 174–190. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, X.; Fujiwara, K.; Jurcak, N.; Muth, S.; Zhou, J.; Xiao, Q.; Li, A.; Che, X.; Li, Z.; et al. Pancreatic cancer cells render tumor-associated macrophages metabolically reprogrammed by a GARP and DNA methylation-mediated mechanism. Signal Transduct. Target. Ther. 2021, 6, 366. [Google Scholar] [CrossRef] [PubMed]
- Junttila, I.S.; Mizukami, K.; Dickensheets, H.; Meier-Schellersheim, M.; Yamane, H.; Donnelly, R.P.; Paul, W.E. Tuning sensitivity to IL-4 and IL-13: Differential expression of IL-4Ralpha, IL-13Ralpha1, and gammac regulates relative cytokine sensitivity. J. Exp. Med. 2008, 205, 2595–2608. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Alves, A.; Diel, L.; Ramos, G.; Pinto, A.; Bernardi, L.; Yates, J., 3rd; Lamers, M. Tumor microenvironment and Oral Squamous Cell Carcinoma: A crosstalk between the inflammatory state and tumor cell migration. Oral. Oncol. 2021, 112, 105038. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Huo, M.; Wang, P.; Liu, X.; Wang, Y.; Li, Y.; Zhou, Z.; Xu, N.; Zhu, H. XBP1 regulates the protumoral function of tumor-associated macrophages in human colorectal cancer. Signal. Transduct. Target Ther. 2021, 6, 357. [Google Scholar] [CrossRef]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D.; van Rooijen, N., Jr.; Weissman, I.L. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef]
- Khatri, D.K.; Juvekar, A.R. Kinetics of Inhibition of Monoamine Oxidase Using Curcumin and Ellagic Acid. Pharmacogn. Mag. 2016, 12 (Suppl. 2), S116–S120. [Google Scholar]
- Zhang, Z.; Hamada, H.; Gerk, P.M. Selectivity of Dietary Phenolics for Inhibition of Human Monoamine Oxidases A and B. Biomed. Res. Int. 2019, 2019, 8361858. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, L.; Hao, S.; Liu, Z.; Ding, S.; Zhang, W.; Yang, X.; Li, S. Activation of the IL-4/STAT6 Signaling Pathway Promotes Lung Cancer Progression by Increasing M2 Myeloid Cells. Front. Immunol. 2019, 10, 2638. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, S.J.; Locksley, R.M. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: Roles in homeostasis and disease. Annu. Rev. Immunol. 2013, 31, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Shukla, M.; Yakubenko, V.P.; Mulya, A.; Kundu, S.; Cathcart, M.K. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic. Biol. Med. 2013, 54, 1–16. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Cully, M. Cancer: Re-educating tumour-associated macrophages with nanoparticles. Nat. Rev. Drug Discov. 2018, 17, 468. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; He, X.; Li, C.; He, Y.; Peng, Y.; Zhang, Y.; Lu, Y.; Chen, X.; Zhang, Y.; Chen, Q.; et al. Dandelion-Like Tailorable Nanoparticles for Tumor Microenvironment Modulation. Adv. Sci. 2019, 6, 1901430. [Google Scholar] [CrossRef]
- Teixeira, L.R.; Almeida, L.Y.; Silva, R.N.; Mesquita, A.; Colturato, C.; Silveira, H.A.; Duarte, A.; Ribeiro-Silva, A.; León, J.E. Young and elderly oral squamous cell carcinoma patients present similar angiogenic profile and predominance of M2 macrophages: Comparative immunohistochemical study. Head Neck 2019, 41, 4111–4120. [Google Scholar] [CrossRef]
- Li, X.; Bu, W.; Meng, L.; Liu, X.; Wang, S.; Jiang, L.; Ren, M.; Fan, Y.; Sun, H. CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp. Cell Res. 2019, 378, 131–138. [Google Scholar] [CrossRef]
- Oweida, A.; Hararah, M.K.; Phan, A.; Binder, D.; Bhatia, S.; Lennon, S.; Bukkapatnam, S.; Van Court, B.; Uyanga, N.; Darragh, L.; et al. Resistance to Radiotherapy and PD-L1 Blockade Is Mediated by TIM-3 Upregulation and Regulatory T-Cell Infiltration. Clin. Cancer Res. 2018, 24, 5368–5380. [Google Scholar] [CrossRef]
- Persa, E.; Balogh, A.; Sáfrány, G.; Lumniczky, K. The effect of ionizing radiation on regulatory T cells in health and disease. Cancer Lett. 2015, 368, 252–261. [Google Scholar] [CrossRef]
- Dan, H.; Liu, S.; Liu, J.; Liu, D.; Yin, F.; Wei, Z.; Wang, J.; Zhou, Y.; Jiang, L.; Ji, N.; et al. RACK1 promotes cancer progression by increasing the M2/M1 macrophage ratio via the NF-κB pathway in oral squamous cell carcinoma. Mol. Oncol. 2020, 14, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Cotechini, T.; Atallah, A.; Grossman, A. Tissue-Resident and Recruited Macrophages in Primary Tumor and Metastatic Microenvironments: Potential Targets in Cancer Therapy. Cells 2021, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Sakamoto, T.; Zhengguang, L.; Yasui, H.; Hamada, H.; Kubo, H.; Nakajima, M. Curcumin inhibits epithelial-mesenchymal transition in oral cancer cells via c-Met blockade. Oncol. Lett. 2020, 19, 4177–4182. [Google Scholar] [CrossRef] [PubMed]
- Zhen, L.; Fan, D.; Yi, X.; Cao, X.; Chen, D.; Wang, L. Curcumin inhibits oral squamous cell carcinoma proliferation and invasion via EGFR signaling pathways. Int. J. Clin. Exp. Pathol. 2014, 7, 6438–6446. [Google Scholar] [PubMed]
- Liu, T.; Long, T.; Li, H. Curcumin suppresses the proliferation of oral squamous cell carcinoma through a specificity protein 1/nuclear factor-κB-dependent pathway. Exp. Ther. Med. 2021, 21, 202. [Google Scholar]
- Mori, K.; Hiroi, M.; Shimada, J.; Ohmori, Y. Infiltration of M2 tumor-associated macrophages in oral squamous cell carcinoma correlates with tumor malignancy. Cancers 2011, 3, 3726–3739. [Google Scholar] [CrossRef]
- Weber, M.; Wehrhan, F.; Baran, C.; Agaimy, A.; Büttner-Herold, M.; Öztürk, H.; Neubauer, K.; Wickenhauser, C.; Kesting, M.; Ries, J. Malignant transformation of oral leukoplakia is associated with macrophage polarization. J. Transl. Med. 2020, 18, 11. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Z.; Chen, M.; Jing, Y.; Shu, W.; Xie, Z.; Li, Z.; Xu, J.; He, F.; Jiao, P.; et al. Macrophage-Derived Exosomal miR-31-5p Promotes Oral Squamous Cell Carcinoma Tumourigenesis Through the Large Tumor Suppressor 2-Mediated Hippo Signalling Pathway. J. Biomed. Nanotechnol. 2021, 17, 822–837. [Google Scholar] [CrossRef]
- Yang, C.; Dou, R.; Wei, C.; Liu, K.; Shi, D.; Zhang, C.; Liu, Q.; Wang, S.; Xiong, B. Tumor-derived exosomal microRNA-106b-5p activates EMT-cancer cell and M2-subtype TAM interaction to facilitate CRC metastasis. Mol. Ther. 2021, 29, 2088–2107. [Google Scholar] [CrossRef]
- Cai, J.; Qiao, B.; Gao, N.; Lin, N.; He, W. Oral squamous cell carcinoma-derived exosomes promote M2 subtype macrophage polarization mediated by exosome-enclosed miR-29a-3p. Am. J. Physiol. Cell Physiol. 2019, 316, C731–C740. [Google Scholar] [CrossRef]

| Gene | Forward Primer 5′-3′ | Reverse Primer 5′-3′ |
|---|---|---|
| IL-6 | ACTTCCATCCAGTTGCCTTCTTGG | TTAAGCCTCCGACTTGTGAAGTGG |
| IL-10 | CTGCTATGCTGCCTGCTCTTACTG | ATGTGGCTCTGGCCGACTGG |
| IL-12 | GAGGACTTGAAGATGTACCAG | TTCTATCTGTGTGAGGAGGGC |
| CD86 | ACGGAGTCAATGAAGATTTCCT | GATTCGGCTTCTTGTGACATAC |
| CD206 | CTCTGTTCAGCTATTGGACGC | CGGAATTTCTGGGATTCAGCTTC |
| PD-L1 | CAGAAGCTGAGGTAATCTGGA | TGAGTCCTGTTCTGTGGAGG |
| iNOS | GAGACAGGGAAGTCTGAAGCAC | CCAGCAGTAGTTGCTCCTCTTC |
| TNF-α | CCCCAAAGGGATGAGAAGTT | CACTTGGTGGTTTGCTACGA |
| TGF-β | CTAATGGTGGAAACCCACAACG | TATCGCCAGGAATTGTTGCTG |
| Arg-1 | CATTGGCTTGCGAGACGTAGAC | GCTGAAGGTCTCTTCCATCACC |
| E-Cadherin | CCTGGGACTCCACCTACAGAA | AGGAGTTGGGAAATGTGAGC |
| N-Cadherin | AACAGCAACGACGGGTTAGT | CAGACACGGTTGCAGTTGAC |
| Vimentin | AGGCGAGGAGAGCAGGATTT | AGTGGGTATCAACCAGAGGGA |
| PD-1 | CGGTTTCAAGGCATGGTCATTGG | TCAGAGTGTCGTCCTTGCTTCC |
| Siglec-G | GAGGAGTTCAGGCTACAAGTGG | GGCATTGGTTGAAGGTCCAGGA |
| THBS1 | GTCCACTCAGACCAGGGAGA | AAAGGTGTCCTGTCCCATCA |
| SIRP-α | GGCAACAAGGAGGTCACAGT | TCCGCGTCCTGTTTCTGTA |
| MAO-A | CCTGGTATCATGACTCTGTATGG | CTTGGACTCAGGCTCTTGAAC |
| GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGT |
| β-actin | GTGCTATGTTGCTCTAGACTTCG | ATGCCACAGGATTCCATACC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Qi, Y.; Huang, W.; Lin, Y.; Li, B. Curcumin Reprograms TAMs from a Protumor Phenotype towards an Antitumor Phenotype via Inhibiting MAO-A/STAT6 Pathway. Cells 2022, 11, 3473. https://doi.org/10.3390/cells11213473
Jiang M, Qi Y, Huang W, Lin Y, Li B. Curcumin Reprograms TAMs from a Protumor Phenotype towards an Antitumor Phenotype via Inhibiting MAO-A/STAT6 Pathway. Cells. 2022; 11(21):3473. https://doi.org/10.3390/cells11213473
Chicago/Turabian StyleJiang, Mingjing, Ying Qi, Wei Huang, Ying Lin, and Bo Li. 2022. "Curcumin Reprograms TAMs from a Protumor Phenotype towards an Antitumor Phenotype via Inhibiting MAO-A/STAT6 Pathway" Cells 11, no. 21: 3473. https://doi.org/10.3390/cells11213473
APA StyleJiang, M., Qi, Y., Huang, W., Lin, Y., & Li, B. (2022). Curcumin Reprograms TAMs from a Protumor Phenotype towards an Antitumor Phenotype via Inhibiting MAO-A/STAT6 Pathway. Cells, 11(21), 3473. https://doi.org/10.3390/cells11213473










