Dual Immune Regulatory Roles of Interleukin-33 in Pathological Conditions
Abstract
1. Introduction
2. Expression and Release of IL-33
3. Nuclear IL-33 Regulates Gene Expression
4. ST2 Expression in Immune Cells
5. Negative Regulation of the IL-33/ST2 Axis
6. Effects of IL-33/ST2 on Immune Cells
6.1. Effects on DCs
6.2. Effects on Macrophages
6.3. Effects on Mast Cells
6.4. Effects on Granulocytes and MDSCs
6.5. Effects on NKs and ILC2s
6.6. Effects on T Cells
6.7. Effects on B Cells
7. Extracellular IL-33-Induced Signaling Pathways
8. IL-33 and Immune-Related Diseases
8.1. Inflammatory and Autoimmune Diseases
8.2. Infectious Diseases
8.3. Cardiovascular Diseases
8.4. Neurological Diseases
8.5. Tumors
8.6. Transplantation
8.7. Other Diseases
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | β-amyloid |
| AP-1 | activator protein 1 |
| AREG | amphiregulin |
| BTK | Bruton’s tyrosine kinase |
| CCL | C-C motif chemokine ligand |
| CRC | colorectal cancer |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| CXCL | C-X-C motif chemokine ligand |
| CXCR | C-X-C motif chemokine receptor |
| DAMP | damage-associated molecular pattern |
| DCs | dendritic cells |
| EAE | experimental autoimmune encephalomyelitis |
| ERK | extracellular signal-regulated kinase |
| GC | gastric cancer |
| GM-CSF | granulocyte macrophage colony-stimulating factor |
| GVHD | graft-versus-host disease |
| icIL-33 | intracellular IL-33 |
| IFN-γ | interferon-γ |
| IL | interleukin |
| IR | ischemia/reperfusion |
| IL-1F | IL-1 family |
| IL-1R | IL-1 receptor |
| IL-1Racp | IL-1 receptor accessory protein |
| ILC2s | group 2 innate lymphoid cells |
| iNKT cell | invariant natural killer T cell |
| IRAK | IL-1 receptor-associated kinase |
| JAK2 | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LCMV | lymphocyte choriomeningitis virus |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MD2 | myeloid differentiation protein 2 |
| MDSCs | myeloid-derived suppressor cells |
| MMP | matrix metallopeptidase |
| MyD88 | myeloid differentiation primary-response protein-88 |
| NETs | neutrophil extracellular traps |
| NFAT | nuclear factor of activated T cells |
| NF-κB | nuclear factor-κB |
| NK cells | natural killer cells |
| NSCLC | non-small cell lung cancer |
| PD-1 | programmed cell death protein 1 |
| SIGIRR | single immunoglobulin IL–1R-related molecule |
| SIK | salt-inducible kinase |
| SLE | systemic lupus erythematosus |
| sST2 | soluble ST2 |
| ST2 | suppression of tumorigenicity 2 |
| STAT | signal transducer and activator of transcription |
| TGF-β | transforming growth factor-β |
| TLRs | Toll-like receptors |
| TNF-α | tumor necrosis factor-α |
| TRAF6 | TNF receptor-associated factor-6 |
| TSLP | thymic stromal lymphopoietin |
References
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.L.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.Z.; Li, X.X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Baekkevold, E.S.; Roussigne, M.; Yamanaka, T.; Johansen, F.E.; Jahnsen, F.L.; Amalric, F.; Brandtzaeg, P.; Erard, M.; Haraldsen, G.; Girard, J.P. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am. J. Pathol. 2003, 163, 69–79. [Google Scholar] [CrossRef]
- Carriere, V.; Roussel, L.; Ortega, N.; Lacorre, D.A.; Americh, L.; Aguilar, L.; Bouche, G.; Girard, J.P. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 282–287. [Google Scholar] [CrossRef]
- Roussel, L.; Erard, M.; Cayrol, C.; Girard, J.P. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep. 2008, 9, 1006–1012. [Google Scholar] [CrossRef]
- Liew, F.Y.; Girard, J.P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef]
- Ali, S.; Mohs, A.; Thomas, M.; Klare, J.; Ross, R.; Schmitz, M.L.; Martin, M.U. The Dual Function Cytokine IL-33 Interacts with the Transcription Factor NF-kappa B To Dampen NF-kappa B-Stimulated Gene Transcription. J. Immunol. 2011, 187, 1609–1616. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. IL-33: An alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 2014, 31, 31–37. [Google Scholar] [CrossRef]
- Drake, L.Y.; Kita, H. IL-33: Biological properties, functions, and roles in airway disease. Immunol. Rev. 2017, 278, 173–184. [Google Scholar] [CrossRef]
- Martin, N.T.; Martin, M.U. Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol. 2016, 17, 122–131. [Google Scholar] [CrossRef]
- Molofsky, A.B.; Savage, A.K.; Locksley, R.M. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity 2015, 42, 1005–1019. [Google Scholar] [CrossRef]
- Vainchtein, I.D.; Chin, G.; Cho, F.S.; Kelley, K.W.; Miller, J.G.; Chien, E.C.; Liddelow, S.A.; Nguyen, P.T.; Nakao-Inoue, H.; Dorman, L.C.; et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 2018, 359, 1269–1273. [Google Scholar] [CrossRef]
- Takeda, T.; Unno, H.; Morita, H.; Futamura, K.; Emi-Sugie, M.; Arae, K.; Shoda, T.; Okada, N.; Igarashi, A.; Inoue, E.; et al. Platelets constitutively express IL-33 protein and modulate eosinophilic airway inflammation. J. Allergy Clin. Immunol. 2016, 138, 1395–1403. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine 2022, 156, 155891. [Google Scholar] [CrossRef]
- Moussion, C.; Ortega, N.; Girard, J.P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: A novel ‘alarmin’? PLoS ONE 2008, 3, e3331. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. USA 2009, 106, 9021–9026. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef]
- Seltmann, J.; Werfel, T.; Wittmann, M. Evidence for a regulatory loop between IFN-γ and IL-33 in skin inflammation. Exp. Dermatol. 2013, 22, 102–107. [Google Scholar] [CrossRef]
- Dahlgren, M.W.; Jones, S.W.; Cautivo, K.M.; Dubinin, A.; Ortiz-Carpena, J.F.; Farhat, S.; Yu, K.S.; Lee, K.; Wang, C.; Molofsky, A.V.; et al. Adventitial Stromal Cells Define Group 2 Innate Lymphoid Cell Tissue Niches. Immunity 2019, 50, 707–722.e706. [Google Scholar] [CrossRef]
- Sundlisaeter, E.; Edelmann, R.J.; Hol, J.; Sponheim, J.; Küchler, A.M.; Weiss, M.; Udalova, I.A.; Midwood, K.S.; Kasprzycka, M.; Haraldsen, G. The alarmin IL-33 is a notch target in quiescent endothelial cells. Am. J. Pathol. 2012, 181, 1099–1111. [Google Scholar] [CrossRef]
- Kuchler, A.M.; Pollheimer, J.; Balogh, J.; Sponheim, J.; Manley, L.; Sorensen, D.R.; De Angelis, P.M.; Scott, H.; Haraldsen, G. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am. J. Pathol. 2008, 173, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Pichery, M.; Mirey, E.; Mercier, P.; Lefrancais, E.; Dujardin, A.; Ortega, N.; Girard, J.P. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: In situ analysis using a novel Il-33-LacZ gene trap reporter strain. J. Immunol. 2012, 188, 3488–3495. [Google Scholar] [CrossRef]
- Sundnes, O.; Pietka, W.; Loos, T.; Sponheim, J.; Rankin, A.L.; Pflanz, S.; Bertelsen, V.; Sitek, J.C.; Hol, J.; Haraldsen, G.; et al. Epidermal Expression and Regulation of Interleukin-33 during Homeostasis and Inflammation: Strong Species Differences. J. Investig. Dermatol. 2015, 135, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Prefontaine, D.; Nadigel, J.; Chouiali, F.; Audusseau, S.; Semlali, A.; Chakir, J.; Martin, J.G.; Hamid, Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J. Allergy Clin. Immunol. 2010, 125, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Louten, J.; Rankin, A.L.; Li, Y.; Murphy, E.E.; Beaumont, M.; Moon, C.; Bourne, P.; McClanahan, T.K.; Pflanz, S.; Malefyt, R.D. Endogenous IL-33 enhances T(h)2 cytokine production and T-cell responses during allergic airway inflammation. Int. Immunol. 2011, 23, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Hardman, C.S.; Panova, V.; McKenzie, A.N. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur. J. Immunol. 2013, 43, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.R.; Shokri, H.; Hassan Al-Heidary, S.; Ghafarifar, F. Evaluation of murine lung epithelial cells (TC-1 JHU-1) line to develop Th2-promoting cytokines IL-25/IL-33/TSLP and genes Tlr2/Tlr4 in response to Aspergillus fumigatus. J. Mycol. Med. 2018, 28, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Lüthi, A.U.; Cullen, S.P.; McNeela, E.A.; Duriez, P.J.; Afonina, I.S.; Sheridan, C.; Brumatti, G.; Taylor, R.C.; Kersse, K.; Vandenabeele, P.; et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 2009, 31, 84–98. [Google Scholar] [CrossRef]
- Ali, S.; Nguyen, D.Q.; Falk, W.; Martin, M.U. Caspase 3 inactivates biologically active full length interleukin-33 as a classical cytokine but does not prohibit nuclear translocation. Biochem. Biophys. Res. Commun. 2010, 391, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Che, D.N.; Cho, B.O.; Kim, J.S.; Shin, J.Y.; Kang, H.J.; Jang, S.I. Effect of Luteolin and Apigenin on the Production of Il-31 and Il-33 in Lipopolysaccharides-Activated Microglia Cells and Their Mechanism of Action. Nutrients 2020, 12, 811. [Google Scholar] [CrossRef]
- Liu, L.; Mao, L.; Wu, X.; Wu, T.; Liu, W.; Yang, Y.; Zhang, T.; Xu, Y. BRG1 regulates endothelial-derived IL-33 to promote ischemia-reperfusion induced renal injury and fibrosis in mice. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Talabot-Ayer, D.; Calo, N.; Vigne, S.; Lamacchia, C.; Gabay, C.; Palmer, G. The mouse interleukin (Il)33 gene is expressed in a cell type- and stimulus-dependent manner from two alternative promoters. J. Leukoc. Biol. 2012, 91, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Polumuri, S.K.; Jayakar, G.G.; Shirey, K.A.; Roberts, Z.J.; Perkins, D.J.; Pitha, P.M.; Vogel, S.N. Transcriptional Regulation of Murine IL-33 by TLR and Non-TLR Agonists. J. Immunol. 2012, 189, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Kopach, P.; Lockatell, V.; Pickering, E.M.; Haskell, R.E.; Anderson, R.D.; Hasday, J.D.; Todd, N.W.; Luzina, I.G.; Atamas, S.P. IFN-γ directly controls IL-33 protein level through a STAT1- and LMP2-dependent mechanism. J. Biol. Chem. 2014, 289, 11829–11843. [Google Scholar] [CrossRef]
- Griffith, B.G.C.; Upstill-Goddard, R.; Brunton, H.; Grimes, G.R.; Biankin, A.V.; Serrels, B.; Byron, A.; Frame, M.C. FAK regulates IL-33 expression by controlling chromatin accessibility at c-Jun motifs. Sci. Rep. 2021, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Palma, M.; Buechel, B.; Moore, J.; Davra, V.; Chu, N.; Millman, A.; Hallab, N.J.; Kanneganti, T.D.; Birge, R.B.; et al. Sterile particle-induced inflammation is mediated by macrophages releasing IL-33 through a Bruton’s tyrosine kinase-dependent pathway. Nat. Mater. 2019, 18, 289–297. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.Y.; Tanaka, Y.; Herbine, K.; Pastore, C.; Singh, B.; Ferguson, A.; Vora, N.; Douglas, B.; Zullo, K.; Behrens, E.M.; et al. Cellular context of IL-33 expression dictates impact on anti-helminth immunity. Sci. Immunol. 2020, 5, eabc6259. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, S.; Yan, C.; Zhang, Y.; Zhang, R.; Chen, M.; Zhong, S.; Fan, W.; Zhu, S.; Zhang, D.; et al. Allergen protease-activated stress granule assembly and gasdermin D fragmentation control interleukin-33 secretion. Nat. Immunol. 2022, 23, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Lefrancais, E.; Duval, A.; Mirey, E.; Roga, S.; Espinosa, E.; Cayrol, C.; Girard, J.P. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc. Natl. Acad. Sci. USA 2014, 111, 15502–15507. [Google Scholar] [CrossRef] [PubMed]
- Lefrancais, E.; Roga, S.; Gautier, V.; Gonzalez-de-Peredo, A.; Monsarrat, B.; Girard, J.P.; Cayrol, C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc. Natl. Acad. Sci. USA 2012, 109, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Duval, A.; Schmitt, P.; Roga, S.; Camus, M.; Stella, A.; Burlet-Schiltz, O.; Gonzalez-de-Peredo, A.; Girard, J.P. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat. Immunol. 2018, 19, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Brusilovsky, M.; Rochman, M.; Rochman, Y.; Caldwell, J.M.; Mack, L.E.; Felton, J.M.; Habel, J.E.; Porollo, A.; Pasare, C.; Rothenberg, M.E. Environmental allergens trigger type 2 inflammation through ripoptosome activation. Nat. Immunol. 2021, 22, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Helgason, H.; Sulem, P.; Bjornsdottir, U.S.; Lim, A.C.; Sveinbjornsson, G.; Hasegawa, H.; Brown, M.; Ketchem, R.R.; Gavala, M.; et al. A rare IL33 loss-of-function mutation reduces blood eosinophil counts and protects from asthma. PLoS Genet. 2017, 13, e1006659. [Google Scholar] [CrossRef] [PubMed]
- Mousas, A.; Ntritsos, G.; Chen, M.H.; Song, C.; Huffman, J.E.; Tzoulaki, I.; Elliott, P.; Psaty, B.M.; Blood-Cell, C.; Auer, P.L.; et al. Rare coding variants pinpoint genes that control human hematological traits. PLoS Genet. 2017, 13, e1006925. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.M.; Perros, F.; Caramori, G.; Meng, C.; Dormuller, P.; Chou, P.C.; Church, C.; Papi, A.; Casolari, P.; Welsh, D.; et al. Nuclear IL-33 regulates soluble ST2 receptor and IL-6 expression in primary human arterial endothelial cells and is decreased in idiopathic pulmonary arterial hypertension. Biochem. Biophys. Res. Commun. 2014, 451, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Park, J.A.; Kim, J.; Rho, S.S.; Park, H.; Kim, Y.M.; Kwon, Y.G. Nuclear IL-33 is a transcriptional regulator of NF-kappa B p65 and induces endothelial cell activation. Biochem. Biophys. Res. Commun. 2012, 421, 305–311. [Google Scholar] [CrossRef]
- Zhang, F.L.; Tossberg, J.T.; Spurlock, C.F.; Yao, S.Y.; Aune, T.M.; Sriram, S. Expression of IL-33 and its epigenetic regulation in multiple sclerosis. Ann. Clin. Transl. Neurol. 2014, 1, 307–318. [Google Scholar] [CrossRef]
- Yangngam, S.; Thongchot, S.; Vaeteewoottacharn, K.; Thuwajit, P.; Hermoso, M.A.; Okada, S.; Thuwajit, C. Intracellular IL-33 Attenuates Extracellular IL-33-induced Cholangiocarcinoma Cell Proliferation and Invasion via NF-kappaB and GSK-3beta Pathways. Anticancer Res. 2021, 41, 4917–4928. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Tago, K.; Funakoshi-Tago, M.; Matsugi, J.; Yanagisawa, K. Intracellular NF-HEV/IL-33 harbors essential roles in Ras-induced cellular transformation by contributing to cyclin D1 protein synthesis. Cell. Signal. 2016, 28, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Bisen, S.; Kaur, G.; Van Buren, E.C.; Rao, G.N.; Singh, N.K. IL-33 enhances Jagged1 mediated NOTCH1 intracellular domain (NICD) deubiquitination and pathological angiogenesis in proliferative retinopathy. Commun. Biol. 2022, 5, 479. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ameri, A.H.; Dempsey, K.E.; Conrad, D.N.; Kem, M.; Mino-Kenudson, M.; Demehri, S. Nuclear IL-33/SMAD signaling axis promotes cancer development in chronic inflammation. EMBO J. 2021, 40, e106151. [Google Scholar] [CrossRef] [PubMed]
- Gautier, V.; Cayrol, C.; Farache, D.; Roga, S.; Monsarrat, B.; Burlet-Schiltz, O.; Gonzalez de Peredo, A.; Girard, J.P. Extracellular IL-33 cytokine, but not endogenous nuclear IL-33, regulates protein expression in endothelial cells. Sci. Rep. 2016, 6, 34255. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.; Rochman, M.; Miracle, C.E.; Habel, J.E.; Brusilovsky, M.; Caldwell, J.M.; Rymer, J.K.; Rothenberg, M.E. Chromatin regulates IL-33 release and extracellular cytokine activity. Nat. Commun. 2018, 9, 3244. [Google Scholar] [CrossRef] [PubMed]
- Mirchandani, A.S.; Salmond, R.J.; Liew, F.Y. Interleukin-33 and the function of innate lymphoid cells. Trends Immunol. 2012, 33, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Lunderius-Andersson, C.; Enoksson, M.; Nilsson, G. Mast cells respond to cell injury through the recognition of IL-33. Front. Immunol. 2012, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.; Bonilla, W.V.; Frohlich, A.; Helmstetter, C.; Peine, M.; Hegazy, A.N.; Pinschewer, D.D.; Lohning, M. T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc. Natl. Acad. Sci. USA 2015, 112, 4056–4061. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.T.; Li, G.; Zhu, Y.B.; Liu, L.; Chen, E.; Turnquist, H.; Zhang, X.G.; Finn, O.J.; Chen, X.C.; Lu, B.F. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8(+) T cells. Eur. J. Immunol. 2011, 41, 3351–3360. [Google Scholar] [CrossRef]
- Hayakawa, H.; Hayakawa, M.; Kume, A.; Tominaga, S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J. Biol. Chem. 2007, 282, 26369–26380. [Google Scholar] [CrossRef]
- Bulek, K.; Swaidani, S.; Qin, J.; Lu, Y.; Gulen, M.F.; Herjan, T.; Min, B.; Kastelein, R.A.; Aronica, M.; Kosz-Vnenchak, M.; et al. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J. Immunol. 2009, 182, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.S.; Scott, I.C.; Majithiya, J.B.; Rapley, L.; Kemp, B.P.; England, E.; Rees, D.G.; Overed-Sayer, C.L.; Woods, J.; Bond, N.J.; et al. Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation. Nat. Commun. 2015, 6, 8327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Duffen, J.L.; Nocka, K.H.; Kasaian, M.T. IL-13 Controls IL-33 Activity through Modulation of ST2. J. Immunol. 2021, 207, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wei, J.; Bowser, R.K.; Traister, R.S.; Fan, M.H.; Zhao, Y. Focal adhesion kinase-mediated activation of glycogen synthase kinase 3β regulates IL-33 receptor internalization and IL-33 signaling. J. Immunol. 2015, 194, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Lin, J.; Lu, F.; Zhang, X.; Zhang, L.; Gandhi, N.B.; de Paiva, C.S.; Pflugfelder, S.C.; Li, D.Q. Potential autocrine regulation of interleukin-33/ST2 signaling of dendritic cells in allergic inflammation. Mucosal Immunol. 2013, 6, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Besnard, A.G.; Togbe, D.; Guillou, N.; Erard, F.; Quesniaux, V.; Ryffel, B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur. J. Immunol. 2011, 41, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Matta, B.M.; Lott, J.M.; Mathews, L.R.; Liu, Q.; Rosborough, B.R.; Blazar, B.R.; Turnquist, H.R. IL-33 Is an Unconventional Alarmin That Stimulates IL-2 Secretion by Dendritic Cells To Selectively Expand IL-33R/ST2(+) Regulatory T Cells. J. Immunol. 2014, 193, 4010–4020. [Google Scholar] [CrossRef]
- Espinassous, Q.; Garcia-de-Paco, E.; Garcia-Verdugo, I.; Synguelakis, M.; von Aulock, S.; Sallenave, J.M.; McKenzie, A.N.J.; Kanellopoulos, J. IL-33 Enhances Lipopolysaccharide-Induced Inflammatory Cytokine Production from Mouse Macrophages by Regulating Lipopolysaccharide Receptor Complex. J. Immunol. 2009, 183, 1446–1455. [Google Scholar] [CrossRef]
- Yang, Y.; Andersson, P.; Hosaka, K.; Zhang, Y.; Cao, R.; Iwamoto, H.; Yang, X.; Nakamura, M.; Wang, J.; Zhuang, R.; et al. The PDGF-BB-SOX7 axis-modulated IL-33 in pericytes and stromal cells promotes metastasis through tumour-associated macrophages. Nat. Commun. 2016, 7, 11385. [Google Scholar] [CrossRef]
- Ariyoshi, W.; Okinaga, T.; Chaweewannakorn, W.; Akifusa, S.; Nisihara, T. Mechanisms involved in enhancement of matrix metalloproteinase-9 expression in macrophages by interleukin-33. J. Cell. Physiol. 2017, 232, 3481–3495. [Google Scholar] [CrossRef]
- Mai, S.; Liu, L.; Jiang, J.; Ren, P.; Diao, D.; Wang, H.; Cai, K. Oesophageal squamous cell carcinoma-associated IL-33 rewires macrophage polarization towards M2 via activating ornithine decarboxylase. Cell Prolif. 2021, 54, e12960. [Google Scholar] [CrossRef]
- Xu, H.; Li, D.; Ma, J.; Zhao, Y.; Xu, L.; Tian, R.; Liu, Y.; Sun, L.; Su, J. The IL-33/ST2 axis affects tumor growth by regulating mitophagy in macrophages and reprogramming their polarization. Cancer Biol. Med. 2021, 18, 172–183. [Google Scholar] [CrossRef]
- Kurowska-Stolarska, M.; Stolarski, B.; Kewin, P.; Murphy, G.; Corrigan, C.J.; Ying, S.; Pitman, N.; Mirchandani, A.; Rana, B.; van Rooijen, N.; et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 2009, 183, 6469–6477. [Google Scholar] [CrossRef]
- Faas, M.; Ipseiz, N.; Ackermann, J.; Culemann, S.; Grüneboom, A.; Schröder, F.; Rothe, T.; Scholtysek, C.; Eberhardt, M.; Böttcher, M.; et al. IL-33-induced metabolic reprogramming controls the differentiation of alternatively activated macrophages and the resolution of inflammation. Immunity 2021, 54, 2531–2546.e2535. [Google Scholar] [CrossRef]
- Ke, J.; Cai, G. Effect of IL-33 on pyroptosis of macrophages in mice with sepsis via NF-kappaB/p38 MAPK signaling pathway. Acta Cirúrgica Bras. 2021, 36, e360501. [Google Scholar] [CrossRef]
- Wu, J.; Carlock, C.; Zhou, C.; Nakae, S.; Hicks, J.; Adams, H.P.; Lou, Y. IL-33 is required for disposal of unnecessary cells during ovarian atresia through regulation of autophagy and macrophage migration. J. Immunol. 2015, 194, 2140–2147. [Google Scholar] [CrossRef]
- Joulia, R.; L’Faqihi, F.E.; Valitutti, S.; Espinosa, E. IL-33 fine tunes mast cell degranulation and chemokine production at the single-cell level. J. Allergy Clin. Immunol. 2017, 140, 497–509. [Google Scholar] [CrossRef]
- Sesti-Costa, R.; Silva, G.K.; Proenca-Modena, J.L.; Carlos, D.; Silva, M.L.; Alves, J.C.; Arruda, E.; Liew, F.Y.; Silva, J.S. The IL-33/ST2 Pathway Controls Coxsackievirus B5-Induced Experimental Pancreatitis. J. Immunol. 2013, 191, 283–292. [Google Scholar] [CrossRef]
- Morita, H.; Arae, K.; Unno, H.; Miyauchi, K.; Toyama, S.; Nambu, A.; Oboki, K.; Ohno, T.; Motomura, K.; Matsuda, A.; et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity 2015, 43, 175–186. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Zhang, B.; Kempuraj, D.; Tagen, M.; Vasiadi, M.; Angelidou, A.; Alysandratos, K.D.; Kalogeromitros, D.; Asadi, S.; Stavrianeas, N.; et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc. Natl. Acad. Sci. USA 2010, 107, 4448–4453. [Google Scholar] [CrossRef]
- Sun, B.; Zhu, L.; Tao, Y.; Sun, H.X.; Li, Y.; Wang, P.; Hou, Y.; Zhao, Y.; Zhang, X.; Zhang, L.; et al. Characterization and allergic role of IL-33-induced neutrophil polarization. Cell. Mol. Immunol. 2018, 15, 782–793. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Guo, H.; Zhang, Z.; Zhang, J.; Dong, X.; Liu, Y.; Zhuang, Y.; Zhao, Y. The Effects of Adoptively Transferred IL-23/IL-18-Polarized Neutrophils on Tumor and Collagen-Induced Arthritis in Mice. J. Inflamm. Res. 2021, 14, 4669–4686. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Chen, L.; Yuan, B.; Liu, T.; Dong, Q.; Liu, Y.; Yin, H. Interleukin-33 facilitates cutaneous defense against Staphylococcus aureus by promoting the development of neutrophil extracellular trap. Int. Immunopharmacol. 2020, 81, 106256. [Google Scholar] [CrossRef]
- Yazdani, H.O.; Chen, H.W.; Tohme, S.; Tai, S.; van der Windt, D.J.; Loughran, P.; Rosborough, B.R.; Sud, V.; Beer-Stolz, D.; Turnquist, H.R.; et al. IL-33 exacerbates liver sterile inflammation by amplifying neutrophil extracellular trap formation. J. Hepatol. 2017, 68, 130–139. [Google Scholar] [CrossRef]
- Willebrand, R.; Voehringer, D. IL-33-Induced Cytokine Secretion and Survival of Mouse Eosinophils Is Promoted by Autocrine GM-CSF. PLoS ONE 2016, 11, e0163751. [Google Scholar] [CrossRef] [PubMed]
- Hollande, C.; Boussier, J.; Ziai, J.; Nozawa, T.; Bondet, V.; Phung, W.; Lu, B.; Duffy, D.; Paradis, V.; Mallet, V.; et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat. Immunol. 2019, 20, 257–264. [Google Scholar] [CrossRef]
- Kienzl, M.; Hasenoehrl, C.; Valadez-Cosmes, P.; Maitz, K.; Sarsembayeva, A.; Sturm, E.; Heinemann, A.; Kargl, J.; Schicho, R. IL-33 reduces tumor growth in models of colorectal cancer with the help of eosinophils. Oncoimmunology 2020, 9, 1776059. [Google Scholar] [CrossRef]
- Andreone, S.; Spadaro, F.; Buccione, C.; Mancini, J.; Tinari, A.; Sestili, P.; Gambardella, A.R.; Lucarini, V.; Ziccheddu, G.; Parolini, I.; et al. IL-33 Promotes CD11b/CD18-Mediated Adhesion of Eosinophils to Cancer Cells and Synapse-Polarized Degranulation Leading to Tumor Cell Killing. Cancers 2019, 11, 1664. [Google Scholar] [CrossRef]
- Legrand, F.; Driss, V.; Delbeke, M.; Loiseau, S.; Hermann, E.; Dombrowicz, D.; Capron, M. Human eosinophils exert TNF-alpha and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J. Immunol. 2010, 185, 7443–7451. [Google Scholar] [CrossRef]
- Suzukawa, M.; Iikura, M.; Koketsu, R.; Nagase, H.; Tamura, C.; Komiya, A.; Nakae, S.; Matsushima, K.; Ohta, K.; Yamamoto, K.; et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J. Immunol. 2008, 181, 5981–5989. [Google Scholar] [CrossRef]
- Smithgall, M.D.; Comeau, M.R.; Yoon, B.R.; Kaufman, D.; Armitage, R.; Smith, D.E. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 2008, 20, 1019–1030. [Google Scholar] [CrossRef]
- Marone, G.; Gambardella, A.R.; Mattei, F.; Mancini, J.; Schiavoni, G.; Varricchi, G. Basophils in Tumor Microenvironment and Surroundings. Adv. Exp. Med. Biol. 2020, 1224, 21–34. [Google Scholar]
- Blom, L.; Poulsen, B.C.; Jensen, B.M.; Hansen, A.; Poulsen, L.K. IL-33 induces IL-9 production in human CD4+ T cells and basophils. PLoS ONE 2011, 6, e21695. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, I.P.; Pejnovic, N.N.; Radosavljevic, G.D.; Pantic, J.M.; Milovanovic, M.Z.; Arsenijevic, N.N.; Lukic, M.L. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int. J. Cancer 2014, 134, 1669–1682. [Google Scholar] [CrossRef]
- Xiao, P.; Wan, X.; Cui, B.; Liu, Y.; Qiu, C.; Rong, J.; Zheng, M.; Song, Y.; Chen, L.; He, J.; et al. Interleukin 33 in tumor microenvironment is crucial for the accumulation and function of myeloid-derived suppressor cells. Oncoimmunology 2016, 5, e1063772. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, V.; Ziccheddu, G.; Macchia, I.; La Sorsa, V.; Peschiaroli, F.; Buccione, C.; Sistigu, A.; Sanchez, M.; Andreone, S.; D’Urso, M.T.; et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 2017, 6, e1317420. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.X.; Choi, S.; Cho, D.; Kim, T.S. IL-33 inhibits the differentiation and immunosuppressive activity of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Immunol. Cell Biol. 2017, 95, 99–107. [Google Scholar] [CrossRef]
- Nabekura, T.; Girard, J.P.; Lanier, L.L. IL-33 Receptor ST2 Amplifies the Expansion of NK Cells and Enhances Host Defense during Mouse Cytomegalovirus Infection. J. Immunol. 2015, 194, 5948–5952. [Google Scholar] [CrossRef]
- Ochayon, D.E.; Ali, A.; Alarcon, P.C.; Krishnamurthy, D.; Kottyan, L.C.; Borchers, M.T.; Waggoner, S.N. IL-33 promotes type 1 cytokine expression via p38 MAPK in human NK cells. J. Leukoc. Biol. 2020, 107, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Flamar, A.L.; Klose, C.S.N.; Moeller, J.B.; Mahlakõiv, T.; Bessman, N.J.; Zhang, W.; Moriyama, S.; Stokic-Trtica, V.; Rankin, L.C.; Putzel, G.G.; et al. Interleukin-33 Induces the Enzyme Tryptophan Hydroxylase 1 to Promote Inflammatory Group 2 Innate Lymphoid Cell-Mediated Immunity. Immunity 2020, 52, 606–619.e606. [Google Scholar] [CrossRef]
- Neill, D.R.; Wong, S.H.; Bellosi, A.; Flynn, R.J.; Daly, M.; Langford, T.K.A.; Bucks, C.; Kane, C.M.; Fallon, P.G.; Pannell, R.; et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Yagi, R.J.; Zhong, C.; Northrup, D.L.; Yu, F.; Bouladoux, N.; Spencer, S.; Hu, G.Q.; Barron, L.; Sharma, S.; Nakayama, T.; et al. The Transcription Factor GATA3 Is Critical for the Development of All IL-7R alpha-Expressing Innate Lymphoid Cells. Immunity 2014, 40, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef]
- Furusawa, J.; Moro, K.; Motomura, Y.; Okamoto, K.; Zhu, J.; Takayanagi, H.; Kubo, M.; Koyasu, S. Critical role of p38 and GATA3 in natural helper cell function. J. Immunol. 2013, 191, 1818–1826. [Google Scholar] [CrossRef]
- Boberg, E.; Johansson, K.; Malmhall, C.; Calven, J.; Weidner, J.; Radinger, M. Interplay Between the IL-33/ST2 Axis and Bone Marrow ILC2s in Protease Allergen-Induced IL-5-Dependent Eosinophilia. Front. Immunol. 2020, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- De Kleer, I.M.; Kool, M.; de Bruijn, M.J.; Willart, M.; van Moorleghem, J.; Schuijs, M.J.; Plantinga, M.; Beyaert, R.; Hams, E.; Fallon, P.G.; et al. Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung. Immunity 2016, 45, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Steer, C.A.; Matha, L.; Shim, H.; Takei, F. Lung group 2 innate lymphoid cells are trained by endogenous IL-33 in the neonatal period. JCI Insight 2020, 5, e135961. [Google Scholar] [CrossRef]
- Kurowska-Stolarska, M.; Kewin, P.; Murphy, G.; Russo, R.C.; Stolarski, B.; Garcia, C.C.; Komai-Koma, M.; Pitman, N.; Li, Y.B.; McKenzie, A.N.J.; et al. IL-33 induces antigen-specific IL-5(+) T cells and promotes allergic-induced airway inflammation independent of IL-4. J. Immunol. 2008, 181, 4780–4790. [Google Scholar] [CrossRef]
- Guo, L.Y.; Wei, G.; Zhu, J.F.; Liao, W.; Leonard, W.J.; Zhao, K.J.; Paul, W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl. Acad. Sci. USA 2009, 106, 13463–13468. [Google Scholar] [CrossRef]
- Uyttenhove, C.; Brombacher, F.; Van Snick, J. TGF-beta interactions with IL-1 family members trigger IL-4-independent IL-9 production by mouse CD4(+) T cells. Eur. J. Immunol. 2010, 40, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Schiering, C.; Krausgruber, T.; Chomka, A.; Fröhlich, A.; Adelmann, K.; Wohlfert, E.A.; Pott, J.; Griseri, T.; Bollrath, J.; Hegazy, A.N.; et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 2014, 513, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Green, J.A.; Moltedo, B.; Arvey, A.; Hemmers, S.; Yuan, S.P.; Treuting, P.M.; Rudensky, A.Y. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 2015, 162, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Burzyn, D.; Kuswanto, W.; Kolodin, D.; Shadrach, J.L.; Cerletti, M.; Jang, Y.; Sefik, E.; Tan, T.G.; Wagers, A.J.; Benoist, C.; et al. A special population of regulatory T cells potentiates muscle repair. Cell 2013, 155, 1282–1295. [Google Scholar] [CrossRef]
- Hatzioannou, A.; Banos, A.; Sakelaropoulos, T.; Fedonidis, C.; Vidali, M.S.; Kohne, M.; Handler, K.; Boon, L.; Henriques, A.; Koliaraki, V.; et al. An intrinsic role of IL-33 in Treg cell-mediated tumor immunoevasion. Nat. Immunol. 2020, 21, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Lian, J.; Wang, T.; Luo, C.; Yuan, Y.; Qin, G.; Zhang, B.; Zhang, Y. Interleukin-33-nuclear factor-kappaB-CCL2 signaling pathway promotes progression of esophageal squamous cell carcinoma by directing regulatory T cells. Cancer Sci. 2020, 111, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Herbst, R.H.; Canner, D.; Schenkel, J.M.; Smith, O.C.; Kim, J.Y.; Hillman, M.; Bhutkar, A.; Cuoco, M.S.; Rappazzo, C.G.; et al. IL-33 Signaling Alters Regulatory T Cell Diversity in Support of Tumor Development. Cell Rep. 2019, 29, 2998–3008.e2998. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cheng, K.; Wang, T.; Xie, Q.; Chen, M.; Chen, Q.; Wen, Q. miR-217 inhibits proliferation, migration, and invasion via targeting AKT3 in thyroid cancer. Biomed. Pharmacother. 2017, 95, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, W.V.; Frohlich, A.; Senn, K.; Kallert, S.; Fernandez, M.; Johnson, S.; Kreutzfeldt, M.; Hegazy, A.N.; Schrick, C.; Fallon, P.G.; et al. The Alarmin Interleukin-33 Drives Protective Antiviral CD8(+) T Cell Responses. Science 2012, 335, 984–989. [Google Scholar] [CrossRef]
- Peine, M.; Marek, R.M.; Lohning, M. IL-33 in T Cell Differentiation, Function, and Immune Homeostasis. Trends Immunol. 2016, 37, 321–333. [Google Scholar] [CrossRef]
- Moral, J.A.; Leung, J.; Rojas, L.A.; Ruan, J.; Zhao, J.; Sethna, Z.; Ramnarain, A.; Gasmi, B.; Gururajan, M.; Redmond, D.; et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 2020, 579, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, Y.; Jiang, Y.; Gao, S.; Wang, Y.; Wang, D.; Wang, A.; Yi, H.; Gu, R.; Yi, Q.; et al. Interleukin-33 Contributes to the Induction of Th9 Cells and Antitumor Efficacy by Dectin-1-Activated Dendritic Cells. Front. Immunol. 2018, 9, 1787. [Google Scholar] [CrossRef] [PubMed]
- Jevtovic, A.; Pantic, J.; Jovanovic, I.; Milovanovic, M.; Stanojevic, I.; Vojvodic, D.; Arsenijevic, N.; Lukic, M.L.; Radosavljevic, G.D. Interleukin-33 pretreatment promotes metastatic growth of murine melanoma by reducing the cytotoxic capacity of CD8(+) T cells and enhancing regulatory T cells. Cancer Immunol. Immunother. 2020, 69, 1461–1475. [Google Scholar] [CrossRef] [PubMed]
- Stier, M.T.; Mitra, R.; Nyhoff, L.E.; Goleniewska, K.; Zhang, J.; Puccetti, M.V.; Casanova, H.C.; Seegmiller, A.C.; Newcomb, D.C.; Kendall, P.L.; et al. IL-33 Is a Cell-Intrinsic Regulator of Fitness during Early B Cell Development. J. Immunol. 2019, 203, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Komai-Koma, M.; Gilchrist, D.S.; McKenzie, A.N.; Goodyear, C.S.; Xu, D.; Liew, F.Y. IL-33 activates B1 cells and exacerbates contact sensitivity. J. Immunol. 2011, 186, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.; Ling, G.S.; Xu, D.M.; Hussaarts, L.; Romaine, A.; Zhao, H.Z.; Fossati-Jimack, L.; Malik, T.; Cook, H.T.; Botto, M.; et al. IL-10-producing regulatory B cells induced by IL-33 (Breg(IL-33)) effectively attenuate mucosal inflammatory responses in the gut. J. Autoimmun. 2014, 50, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Iwahana, H.; Yanagisawa, K.; Ito-Kosaka, A.; Kuroiwa, K.; Tago, K.; Komatsu, N.; Katashima, R.; Itakura, M.; Tominaga, S. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UF7 and TM12 cells. Eur. J. Biochem. 1999, 264, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.J.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and card ioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef]
- Ali, S.; Huber, M.; Kollewe, C.; Bischoff, S.C.; Falk, W.; Martin, M.U. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc. Natl. Acad. Sci. USA 2007, 104, 18660–18665. [Google Scholar] [CrossRef]
- Palmer, G.; Lipsky, B.P.; Smithgall, M.D.; Meininger, D.; Siu, S.; Talabot-Ayer, D.; Gabay, C.; Smith, D.E. The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33. Cytokine 2008, 42, 358–364. [Google Scholar] [CrossRef]
- Lingel, A.; Weiss, T.M.; Niebuhr, M.; Pan, B.; Appleton, B.A.; Wiesmann, C.; Bazan, J.F.; Fairbrother, W.J. Structure of IL-33 and Its Interaction with the ST2 and IL-1RAcP Receptors-Insight into Heterotrimeric IL-1 Signaling Complexes. Structure 2009, 17, 1398–1410. [Google Scholar] [CrossRef]
- Liu, X.; Hammel, M.; He, Y.F.; Tainer, J.A.; Jeng, U.S.; Zhang, L.Q.; Wang, S.Y.; Wang, X.Q. Structural insights into the interaction of IL-33 with its receptors. Proc. Natl. Acad. Sci. USA 2013, 110, 14918–14923. [Google Scholar] [CrossRef]
- Kabata, H.; Moro, K.; Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol. Rev. 2018, 286, 37–52. [Google Scholar] [CrossRef]
- Drube, S.; Heink, S.; Walter, S.; Löhn, T.; Grusser, M.; Gerbaulet, A.; Berod, L.; Schons, J.; Dudeck, A.; Freitag, J.; et al. The receptor tyrosine kinase c-Kit controls IL-33 receptor signaling in mast cells. Blood 2010, 115, 3899–3906. [Google Scholar] [CrossRef] [PubMed]
- Minutti, C.M.; Drube, S.; Blair, N.; Schwartz, C.; McCrae, J.C.; McKenzie, A.N.; Kamradt, T.; Mokry, M.; Coffer, P.J.; Sibilia, M.; et al. Epidermal Growth Factor Receptor Expression Licenses Type-2 Helper T Cells to Function in a T Cell Receptor-Independent Fashion. Immunity 2017, 47, 710–722.e716. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi-Tago, M.; Tago, K.; Sato, Y.; Tominaga, S.; Kasahara, T. JAK2 is an important signal transducer in IL-33-induced NF-kappa B activation. Cell. Signal. 2011, 23, 363–370. [Google Scholar] [CrossRef]
- De la Fuente, M.; MacDonald, T.T.; Hermoso, M.A. The IL-33/ST2 axis: Role in health and disease. Cytokine Growth Factor Rev. 2015, 26, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lin, Y.C.; Tsai, M.L.; Tsai, Y.G.; Kuo, C.H.; Hung, C.H. IL-33 regulates M1/M2 chemokine expression via mitochondrial redox-related mitophagy in human monocytes. Chem. Biol. Interact. 2022, 359, 109915. [Google Scholar] [CrossRef]
- Nechama, M.; Kwon, J.; Wei, S.; Kyi, A.T.; Welner, R.S.; Ben-Dov, I.Z.; Arredouani, M.S.; Asara, J.M.; Chen, C.H.; Tsai, C.Y.; et al. The IL-33-PIN1-IRAK-M axis is critical for type 2 immunity in IL-33-induced allergic airway inflammation. Nat. Commun. 2018, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Darling, N.J.; Arthur, J.S.C.; Cohen, P. Salt-inducible kinases are required for the IL-33-dependent secretion of cytokines and chemokines in mast cells. J. Biol. Chem. 2021, 296, 100428. [Google Scholar] [CrossRef]
- Pinto, S.M.; Nirujogi, R.S.; Rojas, P.L.; Patil, A.H.; Manda, S.S.; Subbannayya, Y.; Roa, J.C.; Chatterjee, A.; Prasad, T.S.; Pandey, A. Quantitative phosphoproteomic analysis of IL-33-mediated signaling. Proteomics 2015, 15, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.A.B.; Subbannayya, Y.; Modi, P.K.; Palollathil, A.; Gopalakrishnan, L.; Bhandary, Y.P.; Prasad, T.S.K.; Pinto, S.M. Temporal Quantitative Phosphoproteomics Profiling of Interleukin-33 Signaling Network Reveals Unique Modulators of Monocyte Activation. Cells 2022, 11, 138. [Google Scholar] [CrossRef]
- Oboki, K.; Ohno, T.; Kajiwara, N.; Arae, K.; Morita, H.; Ishii, A.; Nambu, A.; Abe, T.; Kiyonari, H.; Matsumoto, K.; et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 18581–18586. [Google Scholar] [CrossRef]
- Zaiss, D.M.W.; Gause, W.C.; Osborne, L.C.; Artis, D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 2015, 42, 216–226. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, F.; Liu, O. Interleukin (IL)-33: An orchestrator of immunity from host defence to tissue homeostasis. Clin. Transl. Immunol. 2020, 9, e1146. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Spallanzani, R.G.; Mathis, D. Visceral adipose tissue Tregs and the cells that nurture them. Immunol. Rev. 2020, 295, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Donovan, C.; Bourke, J.E.; Vlahos, R. Targeting the IL-33/IL-13 Axis for Respiratory Viral Infections. Trends Pharmacol. Sci. 2016, 37, 252–261. [Google Scholar] [CrossRef]
- Portelli, M.A.; Dijk, F.N.; Ketelaar, M.E.; Shrine, N.; Hankinson, J.; Bhaker, S.; Grotenboer, N.S.; Obeidat, M.; Henry, A.P.; Billington, C.K.; et al. Phenotypic and functional translation of IL1RL1 locus polymorphisms in lung tissue and asthmatic airway epithelium. JCI Insight 2020, 5, e132446. [Google Scholar] [CrossRef] [PubMed]
- Préfontaine, D.; Lajoie-Kadoch, S.; Foley, S.; Audusseau, S.; Olivenstein, R.; Halayko, A.J.; Lemière, C.; Martin, J.G.; Hamid, Q. Increased expression of IL-33 in severe asthma: Evidence of expression by airway smooth muscle cells. J. Immunol. 2009, 183, 5094–5103. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.L.; Peel, S.; Fox, J.; Panova, V.; Hardman, C.S.; Camelo, A.; Bucks, C.; Wu, X.Y.; Kane, C.M.; Neill, D.R.; et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J. Allergy Clin. Immunol. 2013, 132, 933–941. [Google Scholar] [CrossRef]
- Chang, Y.J.; Kim, H.Y.; Albacker, L.A.; Baumgarth, N.; McKenzie, A.N.J.; Smith, D.E.; DeKruyff, R.H.; Umetsu, D.T. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 2011, 12, 631–638. [Google Scholar] [CrossRef]
- Bartemes, K.R.; Iijima, K.; Kobayashi, T.; Kephart, G.M.; McKenzie, A.N.; Kita, H. IL-33-Responsive Lineage(-)CD25(+)CD44(hi) Lymphoid Cells Mediate Innate Type 2 Immunity and Allergic Inflammation in the Lungs. J. Immunol. 2012, 188, 1503–1513. [Google Scholar] [CrossRef]
- Stolarski, B.; Kurowska-Stolarska, M.; Kewin, P.; Xu, D.; Liew, F.Y. IL-33 Exacerbates Eosinophil-Mediated Airway Inflammation. J. Immunol. 2010, 185, 3472–3480. [Google Scholar] [CrossRef]
- Rank, M.A.; Kobayashi, T.; Kozaki, H.; Bartemes, K.R.; Squillace, D.L.; Kita, H. IL-33-activated dendritic cells induce an atypical TH2-type response. J. Allergy Clin. Immunol. 2009, 123, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.E. IL-33 meets allergens at the gate. Nat. Immunol. 2018, 19, 318–320. [Google Scholar] [CrossRef]
- Teufelberger, A.R.; Nordengrun, M.; Braun, H.; Maes, T.; De Grove, K.; Holtappels, G.; O’Brien, C.; Provoost, S.; Hammad, H.; Goncalves, A.; et al. The IL-33/ST2 axis is crucial in type 2 airway responses induced by Staphylococcus aureus-derived serine protease-like protein D. J. Allergy Clin. Immunol. 2018, 141, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Van Nevel, S.; van Ovost, J.; Holtappels, G.; De Ruyck, N.; Zhang, N.; Braun, H.; Maes, T.; Bachert, C.; Krysko, O. Neutrophils Affect IL-33 Processing in Response to the Respiratory Allergen Alternaria alternata. Front. Immunol. 2021, 12, 677848. [Google Scholar] [CrossRef] [PubMed]
- Toki, S.; Goleniewska, K.; Reiss, S.; Zhang, J.; Bloodworth, M.H.; Stier, M.T.; Zhou, W.; Newcomb, D.C.; Ware, L.B.; Stanwood, G.D.; et al. Glucagon-like peptide 1 signaling inhibits allergen-induced lung IL-33 release and reduces group 2 innate lymphoid cell cytokine production in vivo. J. Allergy Clin. Immunol. 2018, 142, 1515–1528.e1518. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, M.; Soares, D.C.; Vacca, F.; Cohen, E.S.; Scott, I.C.; Gregory, W.F.; Smyth, D.J.; Toivakka, M.; Kemter, A.M.; le Bihan, T.; et al. HpARI Protein Secreted by a Helminth Parasite Suppresses Interleukin-33. Immunity 2017, 47, 739–751.e735. [Google Scholar] [CrossRef] [PubMed]
- Khodoun, M.V.; Tomar, S.; Tocker, J.E.; Wang, Y.H.; Finkelman, F.D. Prevention of food allergy development and suppression of established food allergy by neutralization of thymic stromal lymphopoietin, IL-25, and IL-33. J. Allergy Clin. Immunol. 2018, 141, 171–179.e171. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Ruddy, M.K.; Pavord, I.D.; Israel, E.; Rabe, K.F.; Ford, L.B.; Maspero, J.F.; Abdulai, R.M.; Hu, C.C.; Martincova, R.; et al. Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma. N. Engl. J. Med. 2021, 385, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Kosloski, M.P.; Kalliolias, G.D.; Xu, C.R.; Harel, S.; Lai, C.H.; Zheng, W.; Davis, J.D.; Kamal, M.A. Pharmacokinetics and pharmacodynamics of itepekimab in healthy adults and patients with asthma: Phase I first-in-human and first-in-patient trials. Clin. Transl. Sci. 2022, 15, 384–395. [Google Scholar] [CrossRef]
- Rabe, K.F.; Celli, B.R.; Wechsler, M.E.; Abdulai, R.M.; Luo, X.; Boomsma, M.M.; Staudinger, H.; Horowitz, J.E.; Baras, A.; Ferreira, M.A.; et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: A genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir. Med. 2021, 9, 1288–1298. [Google Scholar] [CrossRef]
- Glück, J.; Rymarczyk, B.; Rogala, B. Serum IL-33 but not ST2 level is elevated in intermittent allergic rhinitis and is a marker of the disease severity. Inflamm. Res. 2012, 61, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Haenuki, Y.; Matsushita, K.; Futatsugi-Yumikura, S.; Ishii, K.J.; Kawagoe, T.; Imoto, Y.; Fujieda, S.; Yasuda, M.; Hisa, Y.; Akira, S.; et al. A critical role of IL-33 in experimental allergic rhinitis. J. Allergy. Clin. Immunol. 2012, 130, 184–194.e111. [Google Scholar] [CrossRef]
- Vocca, L.; Di Sano, C.; Uasuf, C.G.; Sala, A.; Riccobono, L.; Gangemi, S.; Albano, G.D.; Bonanno, A.; Gagliardo, R.; Profita, M. IL-33/ST2 axis controls Th2/IL-31 and Th17 immune response in allergic airway diseases. Immunobiology 2015, 220, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.; et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Iikura, M.; Suto, H.; Kajiwara, N.; Oboki, K.; Ohno, T.; Okayama, Y.; Saito, H.; Galli, S.J.; Nakae, S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab. Investig. 2007, 87, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lai, L.; Chen, H.; Shi, J.; Zeng, F.; Li, J.; Feng, H.; Mao, J.; Zhang, F.; Wu, N.; et al. Interleukin-33 deficiency exacerbated experimental autoimmune encephalomyelitis with an influence on immune cells and glia cells. Mol. Immunol. 2018, 101, 550–563. [Google Scholar] [CrossRef]
- Kearley, J.; Silver, J.S.; Sanden, C.; Liu, Z.; Berlin, A.A.; White, N.; Mori, M.; Pham, T.H.; Ward, C.K.; Criner, G.J.; et al. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity 2015, 42, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Iwaszko, M.; Wielinska, J.; Swierkot, J.; Kolossa, K.; Sokolik, R.; Bugaj, B.; Chaszczewska-Markowska, M.; Jeka, S.; Bogunia-Kubik, K. IL-33 Gene Polymorphisms as Potential Biomarkers of Disease Susceptibility and Response to TNF Inhibitors in Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis Patients. Front. Immunol. 2021, 12, 631603. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Huang, H.; Hu, F.; Zhou, W.; Guo, J.; Jiang, H.; Mu, R.; Li, Z. Increased IL-33 in synovial fluid and paired serum is associated with disease activity and autoantibodies in rheumatoid arthritis. Clin. Dev. Immunol. 2013, 2013, 985301. [Google Scholar] [CrossRef]
- Manetti, M.; Guiducci, S.; Ceccarelli, C.; Romano, E.; Bellando-Randone, S.; Conforti, M.L.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Increased circulating levels of interleukin 33 in systemic sclerosis correlate with early disease stage and microvascular involvement. Ann. Rheum. Dis. 2011, 70, 1876–1878. [Google Scholar] [CrossRef]
- Mok, M.Y.; Huang, F.P.; Ip, W.K.; Lo, Y.; Wong, F.Y.; Chan, E.Y.; Lam, K.F.; Xu, D. Serum levels of IL-33 and soluble ST2 and their association with disease activity in systemic lupus erythematosus. Rheumatology 2010, 49, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Leung, B.P.; Xu, D.; Culshaw, S.; McInnes, I.B.; Liew, F.Y. A novel therapy of murine collagen-induced arthritis with soluble T1/ST2. J. Immunol. 2004, 173, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, H.R.; Kewin, P.; Li, Y.; Mu, R.; Fraser, A.R.; Pitman, N.; Kurowska-Stolarska, M.; McKenzie, A.N.J.; McInnes, I.B.; et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc. Natl. Acad. Sci. USA 2008, 105, 10913–10918. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, L.; Chu, Z.; Yang, T.; Sun, H.X.; Yang, F.; Wang, W.; Hou, Y.; Wang, P.; Zhao, Q.; et al. Characterization and biological significance of IL-23-induced neutrophil polarization. Cell. Mol. Immunol. 2017, 15, 518–530. [Google Scholar] [CrossRef]
- Pastorelli, L.; Garg, R.R.; Hoang, S.B.; Spina, L.; Mattioli, B.; Scarpa, M.; Fiocchi, C.; Vecchi, M.; Pizarro, T.T. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc. Natl. Acad. Sci. USA 2010, 107, 8017–8022. [Google Scholar] [CrossRef] [PubMed]
- Masterson, J.C.; Capocelli, K.E.; Hosford, L.; Biette, K.; McNamee, E.N.; de Zoeten, E.F.; Harris, R.; Fernando, S.D.; Jedlicka, P.; Protheroe, C.; et al. Eosinophils and IL-33 Perpetuate Chronic Inflammation and Fibrosis in a Pediatric Population with Stricturing Crohn’s Ileitis. Inflamm. Bowel Dis. 2015, 21, 2429–2440. [Google Scholar]
- Kayamuro, H.; Yoshioka, Y.; Abe, Y.; Arita, S.; Katayama, K.; Nomura, T.; Yoshikawa, T.; Kubota-Koketsu, R.; Ikuta, K.; Okamoto, S.; et al. Interleukin-1 Family Cytokines as Mucosal Vaccine Adjuvants for Induction of Protective Immunity against Influenza Virus. J. Virol. 2010, 84, 12703–12712. [Google Scholar] [CrossRef] [PubMed]
- De Salvo, C.; Buela, K.A.; Creyns, B.; Corridoni, D.; Rana, N.; Wargo, H.L.; Cominelli, C.L.; Delaney, P.G.; Rodriguez-Palacios, A.; Cominelli, F.; et al. NOD2 drives early IL-33-dependent expansion of group 2 innate lymphoid cells during Crohn’s disease-like ileitis. J. Clin. Investig. 2021, 131, e140624. [Google Scholar] [CrossRef] [PubMed]
- Tahaghoghi-Hajghorbani, S.; Ajami, A.; Ghorbanalipoor, S.; Hosseini-Khah, Z.; Taghiloo, S.; Khaje-Enayati, P.; Hosseini, V. Protective effect of TSLP and IL-33 cytokines in ulcerative colitis. Autoimmun. Highlights 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.H.; Chen, J.; Zhang, H.W.; Yang, H.; Zhu, P.; Xiong, A.; Xia, Q.S.; Zheng, F.; Tan, Z.; Gong, F.L.; et al. Interleukin-33 Ameliorates Experimental Colitis through Promoting Th2/Foxp3(+) Regulatory T-Cell Responses in Mice. Mol. Med. 2012, 18, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.A.; Osborne, L.C.; Noti, M.; Tran, S.V.; Zaiss, D.M.W.; Artis, D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc. Natl. Acad. Sci. USA 2015, 112, 10762–10767. [Google Scholar] [CrossRef]
- Sarrand, J.; Soyfoo, M. Involvement of IL-33 in the Pathophysiology of Systemic Lupus Erythematosus: Review. Int. J. Mol. Sci. 2022, 23, 3138. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lin, W.; Zheng, X. IL-33 neutralization suppresses lupus disease in lupus-prone mice. Inflammation 2014, 37, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Oyoshi, M.K.; Venturelli, N.; Geha, R.S. Thymic stromal lymphopoietin and IL-33 promote skin inflammation and vaccinia virus replication in a mouse model of atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 283–286. [Google Scholar] [CrossRef]
- Dong, Y.; Ming, B.; Gao, R.; Mo, Q.; Wu, X.; Zheng, F.; Zhong, J.; Dong, L. The IL-33/ST2 Axis Promotes Primary Sjogren’s Syndrome by Enhancing Salivary Epithelial Cell Activation and Type 1 Immune Response. J. Immunol. 2022, 208, 2652–2662. [Google Scholar] [CrossRef]
- Wilson, S.; Jones, F.M.; Fofana, H.K.M.; Landoure, A.; Kimani, G.; Mwatha, J.K.; Sacko, M.; Vennervald, B.J.; Dunne, D.W. A late IL-33 response after exposure to Schistosoma haematobium antigen is associated with an up-regulation of IL-13 in human eosinophils. Parasite Immunol. 2013, 35, 224–228. [Google Scholar] [CrossRef]
- Ayimba, E.; Hegewald, J.; Segbena, A.Y.; Gantin, R.G.; Lechner, C.J.; Agosssou, A.; Banla, M.; Soboslay, P.T. Proinflammatory and regulatory cytokines and chemokines in infants with uncomplicated and severe Plasmodium falciparum malaria. Clin. Exp. Immunol. 2011, 166, 218–226. [Google Scholar] [CrossRef]
- Besnard, A.G.; Guabiraba, R.; Niedbala, W.; Palomo, J.; Reverchon, F.; Shaw, T.N.; Couper, K.N.; Ryffel, B.; Liew, F.Y. IL-33-Mediated Protection against Experimental Cerebral Malaria Is Linked to Induction of Type 2 Innate Lymphoid Cells, M2 Macrophages and Regulatory T Cells. PLoS Pathog. 2015, 11, e100460. [Google Scholar] [CrossRef]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef]
- Humphreys, N.E.; Xu, D.; Hepworth, M.R.; Liew, F.Y.; Grencis, R.K. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J. Immunol. 2008, 180, 2443–2449. [Google Scholar] [CrossRef]
- Nelson, M.P.; Christmann, B.S.; Werner, J.L.; Metz, A.E.; Trevor, J.L.; Lowell, C.A.; Steele, C. IL-33 and M2a Alveolar Macrophages Promote Lung Defense against the Atypical Fungal Pathogen Pneumocystis murina. J. Immunol. 2011, 186, 2372–2381. [Google Scholar] [CrossRef] [PubMed]
- Piehler, D.; Grahnert, A.; Eschke, M.; Richter, T.; Köhler, G.; Stenzel, W.; Alber, G. T1/ST2 promotes T helper 2 cell activation and polyfunctionality in bronchopulmonary mycosis. Mucosal Immunol. 2013, 6, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Matsuyama, T.; Nishioka, T.; Hagiwara, M.; Kiyoura, Y.; Shimauchi, H.; Matsushita, K. Porphyromonas gingivalis Gingipain-Dependently Enhances IL-33 Production in Human Gingival Epithelial Cells. PLoS ONE 2016, 11, e0152794. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, J.; Awang, R.A.; Oliver-Bell, J.; Butcher, J.P.; Campbell, L.; Planell, A.A.; Lappin, D.F.; Fukada, S.Y.; Nile, C.J.; Liew, F.Y.; et al. IL-33 Exacerbates Periodontal Disease through Induction of RANKL. J. Dent. Res. 2015, 94, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, L.D.; McClellan, S.A.; Barrett, R.P.; Huang, X.; Zhang, Y.; Wu, M.; van Rooijen, N.; Szliter, E. IL-33 shifts macrophage polarization, promoting resistance against Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1524–1532. [Google Scholar] [CrossRef]
- Kuo, C.F.; Chen, W.Y.; Yu, H.H.; Tsai, Y.H.; Chang, Y.C.; Chang, C.P.; Tsao, N. IL-33/ST2 Axis Plays a Protective Effect in Streptococcus pyogenes Infection through Strengthening of the Innate Immunity. Int. J. Mol. Sci. 2021, 22, 10566. [Google Scholar] [CrossRef]
- Becerra, A.; Warke, R.V.; de Bosch, N.; Rothman, A.L.; Bosch, I. Elevated levels of soluble ST2 protein in dengue virus infected patients. Cytokine 2008, 41, 114–120. [Google Scholar] [CrossRef]
- Miyagaki, T.; Sugaya, M.; Yokobayashi, H.; Kato, T.; Ohmatsu, H.; Fujita, H.; Saeki, H.; Kikuchi, Y.; Tamaki, T.; Sato, S. High Levels of Soluble ST2 and Low Levels of IL-33 in Sera of Patients with HIV Infection. J. Investig. Dermatol. 2011, 131, 794–796. [Google Scholar] [CrossRef]
- Walzl, G.; Matthews, S.; Kendall, S.; Gutierrez-Ramos, J.C.; Coyle, A.J.; Openshaw, P.J.M.; Hussell, T. Inhibition of T1/ST2 during respiratory syncytial virus infection prevents T helper cell type 2 (Th2)- but not Th1-driven immunopathology. J. Exp. Med. 2001, 193, 785–792. [Google Scholar] [CrossRef]
- Ravanetti, L.; Dijkhuis, A.; Dekker, T.; Sabogal Pineros, Y.S.; Ravi, A.; Dierdorp, B.S.; Erjefält, J.S.; Mori, M.; Pavlidis, S.; Adcock, I.M.; et al. IL-33 drives influenza-induced asthma exacerbations by halting innate and adaptive antiviral immunity. J. Allergy Clin. Immunol. 2019, 143, 1355–1370.e1316. [Google Scholar] [CrossRef]
- Liang, Y.; Jie, Z.; Hou, L.; Yi, P.; Wang, W.; Kwota, Z.; Salvato, M.; de Waal Malefyt, R.; Soong, L.; Sun, J. IL-33 promotes innate IFN-gamma production and modulates dendritic cell response in LCMV-induced hepatitis in mice. Eur. J. Immunol. 2015, 45, 3052–3063. [Google Scholar] [CrossRef] [PubMed]
- Shimpo, M.; Morrow, D.A.; Weinberg, E.O.; Sabatine, M.S.; Murphy, S.A.; Antman, E.M.; Lee, R.T. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation 2004, 109, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.; Luk, K.S.; Yuen, V.L.C.; Chiang, L.; Chan, C.K.; Ho, K.; Gong, M.; Lee, T.T.L.; Leung, K.S.K.; Roever, L.; et al. Soluble suppression of tumorigenicity 2 (sST2) for predicting disease severity or mortality outcomes in cardiovascular diseases: A systematic review and meta-analysis. IJC Heart Vasc. 2021, 37, 100887. [Google Scholar] [CrossRef]
- Xiang, N.; Liao, H.; Zhai, Z.; Gong, J. Expression and significance of inflammatory reactions mediated by the IL-33/ST2 signaling pathway in the serum of heart failure patients. Am. J. Transl. Res. 2021, 13, 8247–8252. [Google Scholar] [PubMed]
- Miller, A.M.; Asquith, D.L.; Hueber, A.J.; Anderson, L.A.; Holmes, W.M.; McKenzie, A.N.; Xu, D.M.; Sattar, N.; McInnes, I.B.; Liew, F.Y. Interleukin-33 Induces Protective Effects in Adipose Tissue Inflammation During Obesity in Mice. Circ. Res. 2010, 107, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Kolodin, D.; van Panhuys, N.; Li, C.R.; Magnuson, A.M.; Cipolletta, D.; Miller, C.M.; Wagers, A.; Germain, R.N.; Benoist, C.; Mathis, D. Antigen- and Cytokine-Driven Accumulation of Regulatory T Cells in Visceral Adipose Tissue of Lean Mice. Cell Metab. 2015, 21, 543–557. [Google Scholar] [CrossRef]
- Miller, A.M.; Xu, D.; Asquith, D.L.; Denby, L.; Li, Y.B.; Sattar, N.; Baker, A.H.; McInnes, I.B.; Liew, F.Y. IL-33 reduces the development of atherosclerosis. J. Exp. Med. 2008, 205, 339–346. [Google Scholar] [CrossRef]
- McLaren, J.E.; Michael, D.R.; Salter, R.C.; Ashlin, T.G.; Calder, C.J.; Miller, A.M.; Liew, F.Y.; Ramji, D.P. IL-33 Reduces Macrophage Foam Cell Formation. J. Immunol. 2010, 185, 1222–1229. [Google Scholar] [CrossRef]
- Seki, K.; Sanada, S.; Kudinova, A.Y.; Steinhauser, M.L.; Handa, V.; Gannon, J.; Lee, R.T. Interleukin-33 Prevents Apoptosis and Improves Survival After Experimental Myocardial Infarction Through ST2 Signaling. Circ. Heart Fail. 2009, 2, 684–691. [Google Scholar] [CrossRef]
- Du, L.X.; Wang, Y.Q.; Hua, G.Q.; Mi, W.L. IL-33/ST2 Pathway as a Rational Therapeutic Target for CNS Diseases. Neuroscience 2018, 369, 222–230. [Google Scholar] [CrossRef]
- Delmas, C.; Dalmas, E. IL-33 Deals with the Gray Matter. Immunity 2018, 48, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Gadani, S.P.; Walsh, J.T.; Smirnov, I.; Zheng, J.J.; Kipnis, J. The Glia-Derived Alarmin IL-33 Orchestrates the Immune Response and Promotes Recovery following CNS Injury. Neuron 2015, 85, 703–709. [Google Scholar] [CrossRef]
- Jiang, H.R.; Milovanovic, M.; Allan, D.; Niedbala, W.; Besnard, A.G.; Fukada, S.Y.; Alves, J.C.; Togbe, D.; Goodyear, C.S.; Linington, C.; et al. IL-33 attenuates EAE by suppressing IL-17 and IFN-gamma production and inducing alternatively activated macrophages. Eur. J. Immunol. 2012, 42, 3084. [Google Scholar] [CrossRef] [PubMed]
- Russi, A.E.; Ebel, M.E.; Yang, Y.C.; Brown, M.A. Male-specific IL-33 expression regulates sex-dimorphic EAE susceptibility. Proc. Natl. Acad. Sci. USA 2018, 115, E1520–E1529. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Roberts, F.; Nickdel, M.B.; Brombacher, F.; McKenzie, A.N.; Henriquez, F.L.; Alexander, J.; Roberts, C.W. IL-33 receptor (T1/ST2) signalling is necessary to prevent the development of encephalitis in mice infected with Toxoplasma gondii. Eur. J. Immunol. 2010, 40, 426–436. [Google Scholar] [CrossRef]
- Fu, A.K.Y.; Hung, K.W.; Yuen, M.Y.F.; Zhou, X.P.; Mak, D.S.Y.; Chan, I.C.W.; Cheung, T.H.; Zhang, B.R.; Fu, W.Y.; Liew, F.Y.; et al. IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc. Natl. Acad. Sci. USA 2016, 113, E2705–E2713. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.F.; Chen, C.; Fu, W.Y.; Qu, J.Y.; Cheung, T.H.; Fu, A.K.Y.; Ip, N.Y. IL-33-PU.1 Transcriptome Reprogramming Drives Functional State Transition and Clearance Activity of Microglia in Alzheimer’s Disease. Cell Rep. 2020, 31, 107530. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Miao, W.; Xu, F.; Yuan, C.; Li, S.; Wang, C.; Junagade, A.; Hu, X. IL-33/ST2 Axis Protects Against Traumatic Brain Injury Through Enhancing the Function of Regulatory T Cells. Front. Immunol. 2022, 13, 860772. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; O’Grady, K.; Lavelle, E.C.; Fallon, P.G. Interleukin 33: An innate alarm for adaptive responses beyond Th2 immunity-emerging roles in obesity, intestinal inflammation, and cancer. Eur. J. Immunol. 2016, 46, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Shan, S.; Yang, Z.J.; Gu, X.; Wang, Y.Y.; Wang, C.H.; Ren, T. IL-33 blockade suppresses tumor growth of human lung cancer through direct and indirect pathways in a preclinical model. Oncotarget 2017, 8, 68571–68582. [Google Scholar] [CrossRef] [PubMed]
- Wasmer, M.H.; Krebs, P. The Role of IL-33-Dependent Inflammation in the Tumor Microenvironment. Front. Immunol. 2017, 7, 682. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.R.; Sosman, J.A.; Zhang, B. The Janus Face of IL-33 Signaling in Tumor Development and Immune Escape. Cancers 2021, 13, 3281. [Google Scholar] [CrossRef]
- Yang, K.; Tian, C.; Zhang, C.; Xiang, M. The Controversial Role of IL-33 in Lung Cancer. Front. Immunol. 2022, 13, 897356. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, G.; Liang, T. Pancreatic tumor initiation: The potential role of IL-33. Signal Transduct Target Ther. 2021, 6, 204. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, M.; Huang, L.; Mo, D.; Liang, Y.; Huang, Z.; Zhu, B.; Fang, M. CD44, IL-33, and ST2 Gene Polymorphisms on Hepatocellular Carcinoma Susceptibility in the Chinese Population. BioMed Res. Int. 2020, 2020, 2918517. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, E.; Heo, J.S.; Bae, D.J.; Lee, J.U.W.; Lee, T.H.; Lee, H.J.; Chang, H.S.; Park, J.S.; Jang, A.S.; et al. Circulating IL-33 level is associated with the progression of lung cancer. Lung Cancer 2015, 90, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.A.; Fu, Y.; Zhang, D.N.; Zhang, J. Serum IL-33 as a diagnostic and prognostic marker in non- small cell lung cancer. Asian Pac. J. Cancer Prev. 2013, 14, 2563–2566. [Google Scholar] [CrossRef]
- Wang, C.H.; Chen, Z.S.; Bu, X.M.; Han, Y.; Shan, S.; Ren, T.; Song, W.Q. IL-33 signaling fuels outgrowth and metastasis of human lung cancer. Biochem. Biophys. Res. Commun. 2016, 479, 461–468. [Google Scholar] [CrossRef]
- Akimoto, M.; Takenaga, K. Role of the IL-33/ST2L axis in colorectal cancer progression. Cell. Immunol. 2018, 343, 103740. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.L.; Qi, H.L.; Gundersen, M.D.; Yang, H.; Christiansen, I.; Sorbye, S.W.; Goll, R.; Florholmen, J. Dynamics of the IL-33/ST2 network in the progression of human colorectal adenoma to sporadic colorectal cancer. Cancer Immunol. Immunother. 2015, 64, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Davis, C.; Shah, S.; Hughes, D.; Ryan, J.C.; Altomare, D.; Peña, M.M. IL-33 promotes growth and liver metastasis of colorectal cancer in mice by remodeling the tumor microenvironment and inducing angiogenesis. Mol. Carcinog. 2017, 56, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Zhu, L.L.; Lu, X.; Bian, H.R.; Wu, X.; Yang, W.C.; Qin, Q.L. IL-33/ST2 pathway contributes to metastasis of human colorectal. Biochem. Biophys. Res. Commun. 2014, 453, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Kudo-Saito, C.; Miyamoto, T.; Imazeki, H.; Shoji, H.; Aoki, K.; Boku, N. IL33 Is a Key Driver of Treatment Resistance of Cancer. Cancer Res. 2020, 80, 1981–1990. [Google Scholar] [CrossRef]
- Van der Jeught, K.; Sun, Y.; Fang, Y.; Zhou, Z.; Jiang, H.; Yu, T.; Yang, J.; Kamocka, M.M.; So, K.M.; Li, Y.; et al. ST2 as checkpoint target for colorectal cancer immunotherapy. JCI Insight 2020, 5, e136073. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Lian, J.; Yue, Y.; Zhang, Y. IL-33/ST2 as a potential target for tumor immunotherapy. Eur. J. Immunol. 2021, 51, 1943–1955. [Google Scholar] [CrossRef]
- Akimoto, M.; Maruyama, R.; Takamaru, H.; Ochiya, T.; Takenaga, K. Soluble IL-33 receptor sST2 inhibits colorectal cancer malignant growth by modifying the tumour microenvironment. Nat. Commun. 2016, 7, 13589. [Google Scholar] [CrossRef] [PubMed]
- Shani, O.; Vorobyov, T.; Monteran, L.; Lavie, D.; Cohen, N.; Raz, Y.; Tsarfaty, G.; Avivi, C.; Barshack, I.; Erez, N. Fibroblast-Derived IL33 Facilitates Breast Cancer Metastasis by Modifying the Immune Microenvironment and Driving Type 2 Immunity. Cancer Res. 2020, 80, 5317–5329. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, X.F.; Yang, Q.T.; Zhao, X.; Wen, W.; Li, G.; Lu, J.F.; Qin, W.X.; Qi, Y.; Xie, F.; et al. Tumoral Expression of IL-33 Inhibits Tumor Growth and Modifies the Tumor Microenvironment through CD8(+) T and NK Cells. J. Immunol. 2015, 194, 438–445. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, A.; Ahn, B.Y.; D’Mello, C.; Lun, X.; Menon, S.V.; Alshehri, M.M.; Szulzewsky, F.; Shen, Y.; Khan, L.; Dang, N.H.; et al. Glioma-derived IL-33 orchestrates an inflammatory brain tumor microenvironment that accelerates glioma progression. Nat. Commun. 2020, 11, 4997. [Google Scholar] [CrossRef]
- Taniguchi, S.; Elhance, A.; Van Duzer, A.; Kumar, S.; Leitenberger, J.J.; Oshimori, N. Tumor-initiating cells establish an IL-33-TGF-beta niche signaling loop to promote cancer progression. Science 2020, 369, eaay1813. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.X.; Hu, Z.; Shen, X.; Dong, L.Y.; Zhou, W.Z.; Hu, W.H. IL-33 Promotes Gastric Cancer Cell Invasion and Migration Via ST2-ERK1/2 Pathway. Dig. Dis. Sci. 2015, 60, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.L.; Zhao, Y.R.; Weng, G.B.; Chen, Y.C.; Wei, X.N.; Shao, J.P.; Ji, H. IL-33-induced JNK pathway activation confers gastric cancer chemotherapy resistance. Oncol. Rep. 2015, 33, 2746–2752. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.Y.; Wang, H.; Shan, T.; Chen, Y.G.; Zhou, H.; Zhao, Q.; Xia, J.Z. Tristetraprolin inhibits gastric cancer progression through suppression of IL-33. Sci. Rep. 2016, 6, 24505. [Google Scholar] [CrossRef]
- Luo, P.; Deng, S.; Ye, H.; Yu, X.; Deng, Q.; Zhang, Y.; Jiang, L.; Li, J.; Yu, Y.; Han, W. The IL-33/ST2 pathway suppresses murine colon cancer growth and metastasis by upregulating CD40 L signaling. Biomed. Pharmacother. 2020, 127, 110232. [Google Scholar] [CrossRef]
- Jin, Z.; Lei, L.; Lin, D.; Liu, Y.; Song, Y.; Gong, H.; Zhu, Y.; Mei, Y.; Hu, B.; Wu, Y.; et al. IL-33 Released in the Liver Inhibits Tumor Growth via Promotion of CD4(+) and CD8(+) T Cell Responses in Hepatocellular Carcinoma. J. Immunol. 2018, 201, 3770–3779. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ohno, T.; Nishii, N.; Bhingare, A.; Tachinami, H.; Kashima, Y.; Nagai, S.; Saito, H.; Nakae, S.; Azuma, M. Endogenous IL-33 exerts CD8(+) T cell antitumor responses overcoming pro-tumor effects by regulatory T cells in a colon carcinoma model. Biochem. Biophys. Res. Commun. 2019, 518, 331–336. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Yang, F.; Zhang, X.; Ren, X.; Wei, F. Relationship and prognostic significance of IL-33, PD-1/PD-L1, and tertiary lymphoid structures in cervical cancer. J. Leukoc. Biol. 2022, 2. [Google Scholar] [CrossRef]
- Suga, Y.; Nagatomo, I.; Kinehara, Y.; Koyama, S.; Okuzaki, D.; Osa, A.; Naito, Y.; Takamatsu, H.; Nishide, M.; Nojima, S.; et al. IL-33 Induces Sema4A Expression in Dendritic Cells and Exerts Antitumor Immunity. J. Immunol. 2021, 207, 1456–1467. [Google Scholar] [CrossRef]
- Dominguez, D.; Ye, C.; Geng, Z.; Chen, S.; Fan, J.; Qin, L.; Long, A.; Wang, L.; Zhang, Z.; Zhang, Y.; et al. Exogenous IL-33 Restores Dendritic Cell Activation and Maturation in Established Cancer. J. Immunol. 2017, 198, 1365–1375. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, Y.; Wang, J.; Xu, Y.; Xie, Y.; Yang, Z.P. IL33 activates CD8+T and NK cells through MyD88 pathway to suppress the lung cancer cell growth in mice. Biotechnol. Lett. 2020, 42, 1113–1121. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, Y.; Chen, J.; Nan, H.; Zhao, Y.; Chu, X.; Wang, A.; Wang, D.; Qin, T.; Gao, S.; et al. IL-33 drives the antitumor effects of dendritic cells via the induction of Tc9 cells. Cell. Mol. Immunol. 2019, 16, 644–651. [Google Scholar] [CrossRef]
- Ikutani, M.; Yanagibashi, T.; Ogasawara, M.; Tsuneyama, K.; Yamamoto, S.; Hattori, Y.; Kouro, T.; Itakura, A.; Nagai, Y.; Takaki, S.; et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J. Immunol. 2012, 188, 703–713. [Google Scholar] [CrossRef]
- Qin, L.; Dominguez, D.; Chen, S.; Fan, J.; Long, A.; Zhang, M.; Fang, D.; Zhang, Y.; Kuzel, T.M.; Zhang, B. Exogenous IL-33 overcomes T cell tolerance in murine acute myeloid leukemia. Oncotarget 2016, 7, 61069–61080. [Google Scholar] [CrossRef]
- Matta, B.M.; Reichenbach, D.K.; Blazar, B.R.; Turnquist, H.R. Alarmins and Their Receptors as Modulators and Indicators of Alloimmune Responses. Am. J. Transplant. 2017, 17, 320–327. [Google Scholar] [CrossRef]
- Yu, M.Y.; Kwon, S.; Moon, J.J.; Kim, Y.C.; Song, E.Y.; Lee, H.; Moon, K.C.; Ha, J.; Kim, D.K.; Han, S.W.; et al. Role of the IL-33/ST2 pathway in renal allograft rejection. Exp. Cell Res. 2021, 405, 112705. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, C.; Wang, Z.; Tao, J.; Han, Z.; Zhang, W.; Tan, R.; Gu, M. Interleukin-33 levels are elevated in chronic allograft dysfunction of kidney transplant recipients and promotes epithelial to mesenchymal transition of human kidney (HK-2) cells. Gene 2018, 644, 113–121. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wang, Z.J.; Xu, Z.; Han, Z.J.; Tao, J.; Lu, P.; Huang, Z.K.; Zhou, W.L.; Zhao, C.C.; Tan, R.Y.; et al. The Potential Role of IL-33 in Renal Transplant Recipients with Chronic Allograft Dysfunction. Ann. Transpl. 2016, 21, 611–618. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Garrido, I.P.; Blanco, R.; Minguela, A.; Lax, A.; Ordoñez-Llanos, J.; Bayes-Genis, A.; Valdés, M.; Moore, S.A.; Januzzi, J.L. Soluble ST2 is a marker for acute cardiac allograft rejection. Ann. Thorac. Surg. 2011, 92, 2118–2124. [Google Scholar] [CrossRef]
- Kim, M.S.; Jeong, T.D.; Han, S.B.; Min, W.K.; Kim, J.J. Role of Soluble ST2 as a Prognostic Marker in Patients with Acute Heart Failure and Renal Insufficiency. J. Korean Med. Sci. 2015, 30, 569–575. [Google Scholar] [CrossRef]
- Lee, G.Y.; Choi, J.O.; Ju, E.S.; Lee, Y.J.; Jeon, E.S. Role of Soluble ST2 as a Marker for Rejection after Heart Transplant. Korean Circ. J. 2016, 46, 811–820. [Google Scholar] [CrossRef][Green Version]
- Mathews, L.R.; Lott, J.M.; Isse, K.; Lesniak, A.; Landsittel, D.; Demetris, A.J.; Sun, Y.; Mercer, D.F.; Webber, S.A.; Zeevi, A.; et al. Elevated ST2 Distinguishes Incidences of Pediatric Heart and Small Bowel Transplant Rejection. Am. J. Transplant. 2016, 16, 938–950. [Google Scholar] [CrossRef]
- Grupper, A.; AbouEzzeddine, O.F.; Maleszewski, J.J.; Grupper, A.; Geske, J.R.; Kremers, W.K.; Kushwaha, S.S.; Pereira, N.L. Elevated ST2 levels are associated with antibody-mediated rejection in heart transplant recipients. Clin. Transplant. 2018, 32, e13349. [Google Scholar] [CrossRef]
- Ruisong, M.; Xiaorong, H.; Gangying, H.; Chunfeng, Y.; Changjiang, Z.; Xuefei, L.; Yuanhong, L.; Hong, J. The Protective Role of Interleukin-33 in Myocardial Ischemia and Reperfusion Is Associated with Decreased HMGB1 Expression and Up-Regulation of the P38 MAPK Signaling Pathway. PLoS ONE 2015, 10, e0143064. [Google Scholar] [CrossRef]
- Chen, J.; He, Y.; Xie, Z.; Wei, Y.; Duan, L. The Role of IL-33 in Experimental Heart Transplantation. Cardiol. Res. Pract. 2020, 2020, 6108362. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Z.; Bartolacci, J.G.; Dwyer, G.K.; Liu, Q.; Mathews, L.R.; Velayutham, M.; Roessing, A.S.; Lee, Y.C.; Dai, H.; et al. Graft IL-33 regulates infiltrating macrophages to protect against chronic rejection. J. Clin. Investig. 2020, 130, 5397–5412. [Google Scholar] [CrossRef]
- Yin, H.; Li, X.Y.; Jin, X.B.; Zhang, B.B.; Gong, Q.; Yang, H.; Zheng, F.; Gong, F.L.; Zhu, J.Y. IL-33 prolongs murine cardiac allograft survival through induction of TH2-type immune deviation. Transplantation 2010, 89, 1189–1197. [Google Scholar] [CrossRef]
- Brunner, S.M.; Schiechl, G.; Falk, W.; Schlitt, H.J.; Geissler, E.K.; Fichtner-Feigl, S. Interleukin-33 prolongs allograft survival during chronic cardiac rejection. Transpl. Int. 2011, 24, 1027–1039. [Google Scholar] [CrossRef]
- Turnquist, H.R.; Zhao, Z.L.; Rosborough, B.R.; Liu, Q.; Castellaneta, A.; Isse, K.; Wang, Z.L.; Lang, M.G.; Stolz, D.B.; Zheng, X.X.; et al. IL-33 Expands Suppressive CD11b(+) Gr-1(int) and Regulatory T Cells, including ST2L(+) Foxp3(+) Cells, and Mediates Regulatory T Cell-Dependent Promotion of Cardiac Allograft Survival. J. Immunol. 2011, 187, 4598–4610. [Google Scholar] [CrossRef]
- Guo, Y.; Mei, Z.; Li, D.; Banerjee, A.; Khalil, M.A.; Burke, A.; Ritter, J.; Lau, C.; Kreisel, D.; Gelman, A.E.; et al. Ischemia reperfusion injury facilitates lung allograft acceptance through IL-33-mediated activation of donor-derived IL-5 producing group 2 innate lymphoid cells. Am. J. Transplant. 2022, 22, 1963–1975. [Google Scholar] [CrossRef]
- Kawai, K.; Uchiyama, M.; Hester, J.; Issa, F. IL-33 drives the production of mouse regulatory T cells with enhanced in vivo suppressive activity in skin transplantation. Am. J. Transplant. 2021, 21, 978–992. [Google Scholar] [CrossRef]
- Matta, B.M.; Reichenbach, D.K.; Zhang, X.L.; Mathews, L.; Koehn, B.H.; Dwyer, G.K.; Lott, J.M.; Uhl, F.M.; Pfeifer, D.; Feser, C.J.; et al. Peri-alloHCT IL-33 administration expands recipient T-regulatory cells that protect mice against acute GVHD. Blood 2016, 128, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, G.K.; Mathews, L.R.; Villegas, J.A.; Lucas, A.; Gonzalez de Peredo, A.; Blazar, B.R.; Girard, J.P.; Poholek, A.C.; Luther, S.A.; Shlomchik, W.; et al. IL-33 acts as a costimulatory signal to generate alloreactive Th1 cells in graft-versus-host disease. J. Clin. Investig. 2022, 132, e150927. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, D.K.; Schwarze, V.; Matta, B.M.; Tkachev, V.; Lieberknecht, E.; Liu, Q.; Koehn, B.H.; Pfeifer, D.; Taylor, P.A.; Prinz, G.; et al. The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood 2015, 125, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Gilchrist, D.S.; Akbar, M.; Reilly, J.H.; Kerr, S.C.; Campbell, A.L.; Murrell, G.A.C.; Liew, F.Y.; Kurowska-Stolarska, M.; McInnes, I.B. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat. Commun. 2015, 6, 6774. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Kurowska-Stolarska, M.; Bohm, C.; Gary, R.; Scholtysek, C.; Stolarski, B.; Reilly, J.; Kerr, S.; Millar, N.L.; Kamradt, T.; et al. IL-33 Shifts the Balance from Osteoclast to Alternatively Activated Macrophage Differentiation and Protects from TNF-alpha-Mediated Bone Loss. J. Immunol. 2011, 186, 6097–6105. [Google Scholar] [CrossRef]
- Rak, G.D.; Osborne, L.C.; Siracusa, M.C.; Kim, B.S.; Wang, K.; Bayat, A.; Artis, D.; Volk, S.W. IL-33-Dependent Group 2 Innate Lymphoid Cells Promote Cutaneous Wound Healing. J. Investig. Dermatol. 2016, 136, 487–496. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H. Upregulation of the IL-33/ST2 pathway in dry eye. Mol. Vis. 2019, 25, 583–592. [Google Scholar]
- Hu, J.; Gao, N.; Zhang, Y.; Chen, X.; Li, J.; Bian, F.; Chi, W.; Liu, Z.; de Paiva, C.S.; Pflugfelder, S.C.; et al. IL-33/ST2/IL-9/IL-9R signaling disrupts ocular surface barrier in allergic inflammation. Mucosal Immunol. 2020, 13, 919–930. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, M. The Functional Roles of IL-33/ST2 Axis in Ocular Diseases. Mediators Inflamm. 2020, 2020, 5230716. [Google Scholar] [CrossRef]
- Tu, L.; Yang, L. IL-33 at the Crossroads of Metabolic Disorders and Immunity. Front. Endocrinol 2019, 10, 26. [Google Scholar] [CrossRef]
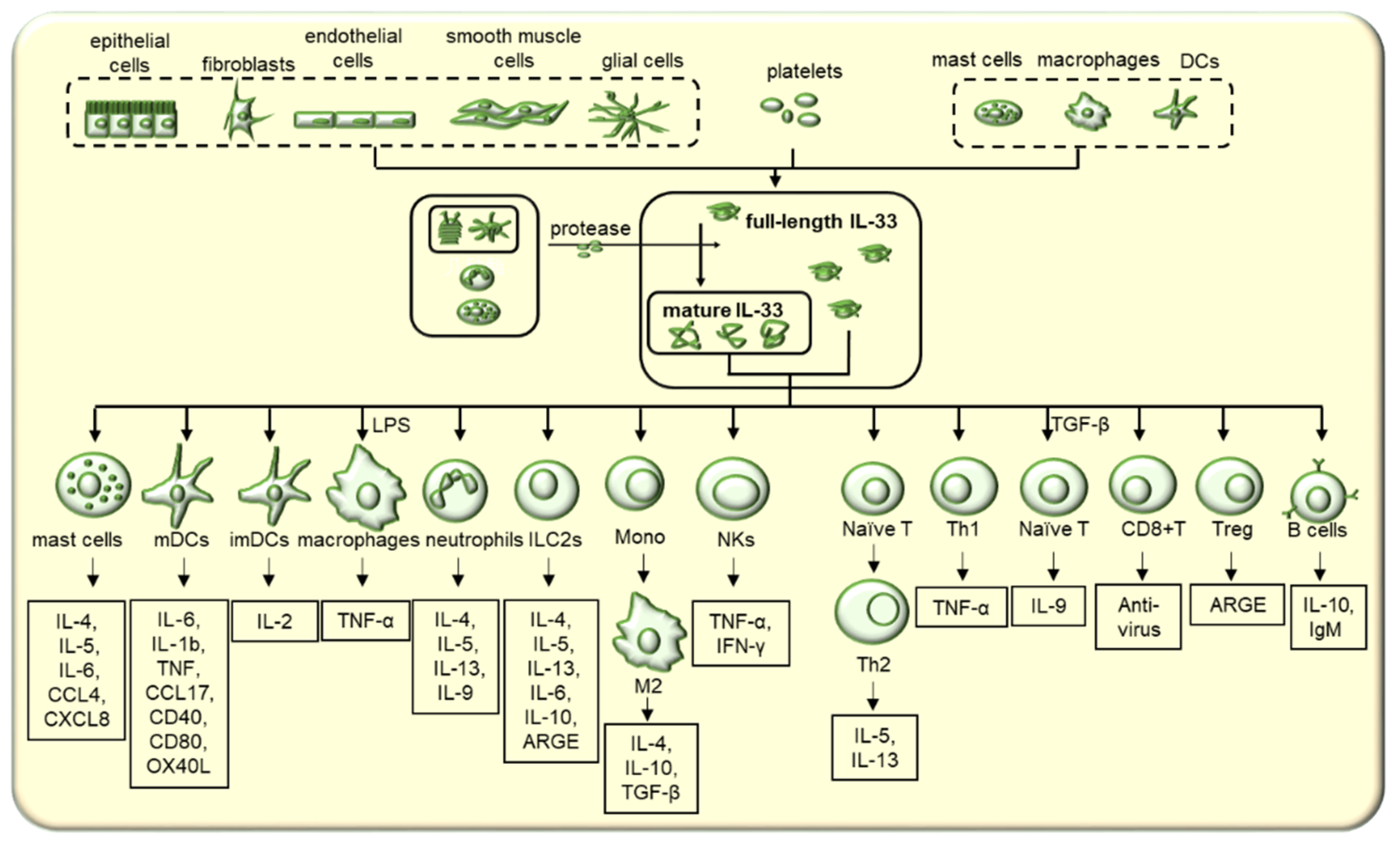
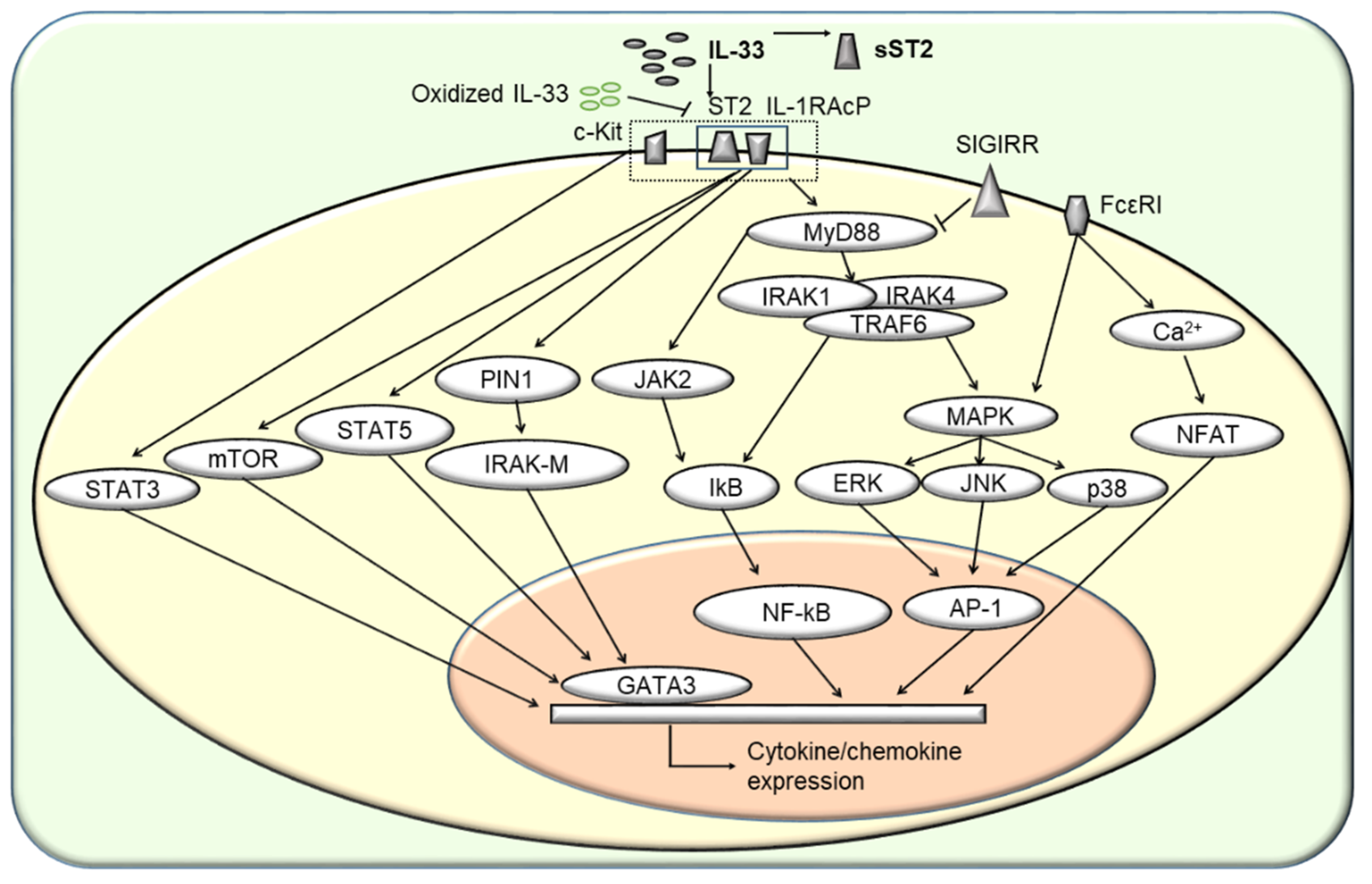
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Bossila, E.A.; Ma, X.; Zhao, C.; Zhao, Y. Dual Immune Regulatory Roles of Interleukin-33 in Pathological Conditions. Cells 2022, 11, 3237. https://doi.org/10.3390/cells11203237
Guo H, Bossila EA, Ma X, Zhao C, Zhao Y. Dual Immune Regulatory Roles of Interleukin-33 in Pathological Conditions. Cells. 2022; 11(20):3237. https://doi.org/10.3390/cells11203237
Chicago/Turabian StyleGuo, Han, Elhusseny A. Bossila, Xinran Ma, Chenxu Zhao, and Yong Zhao. 2022. "Dual Immune Regulatory Roles of Interleukin-33 in Pathological Conditions" Cells 11, no. 20: 3237. https://doi.org/10.3390/cells11203237
APA StyleGuo, H., Bossila, E. A., Ma, X., Zhao, C., & Zhao, Y. (2022). Dual Immune Regulatory Roles of Interleukin-33 in Pathological Conditions. Cells, 11(20), 3237. https://doi.org/10.3390/cells11203237






