Multifunctional Role of S100 Protein Family in the Immune System: An Update
Abstract
1. Introduction
2. Function of S100 Protein in Host Defense Mechanism
2.1. Role of S100 Protein in Most Prevalent Innate Immune Cells
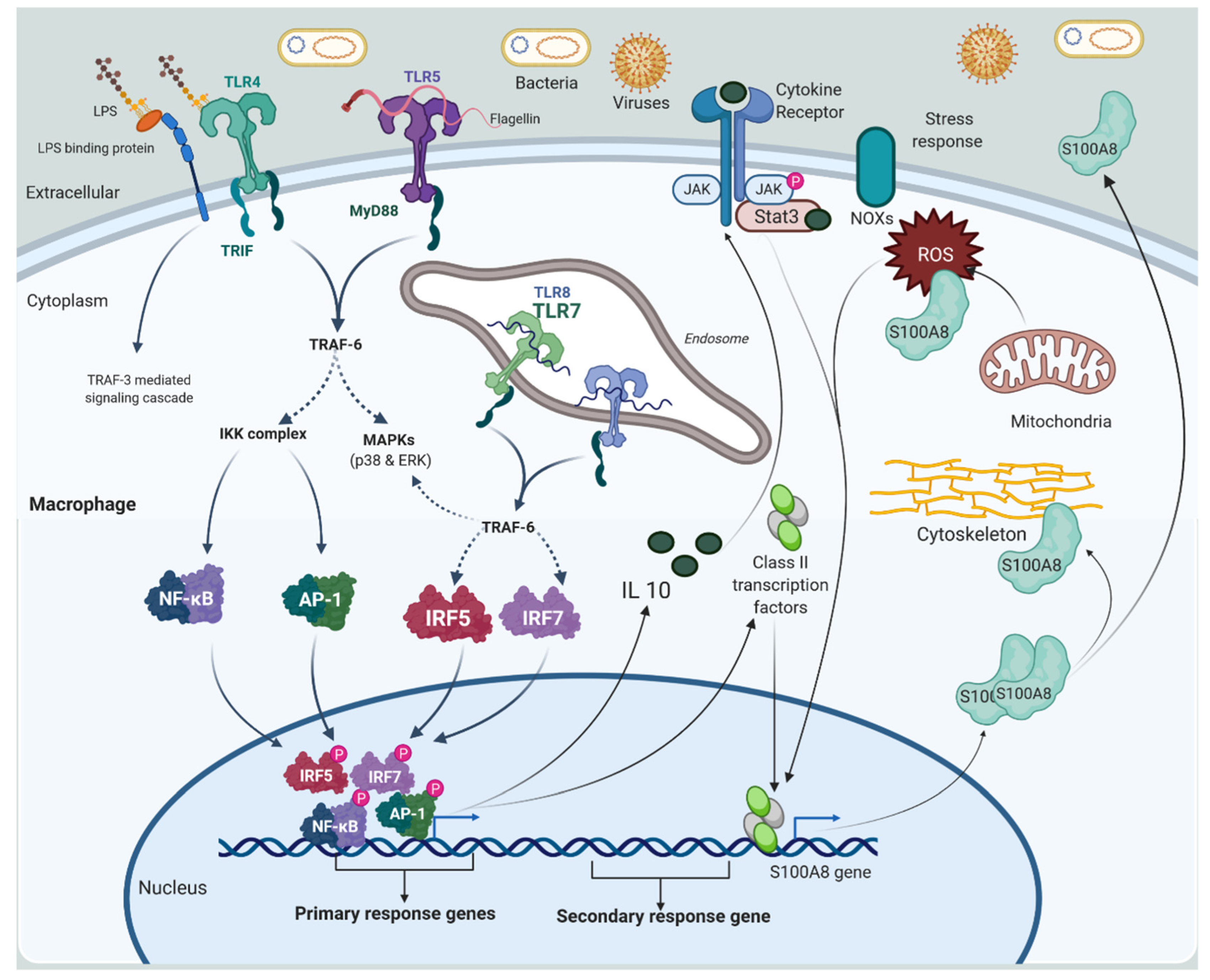
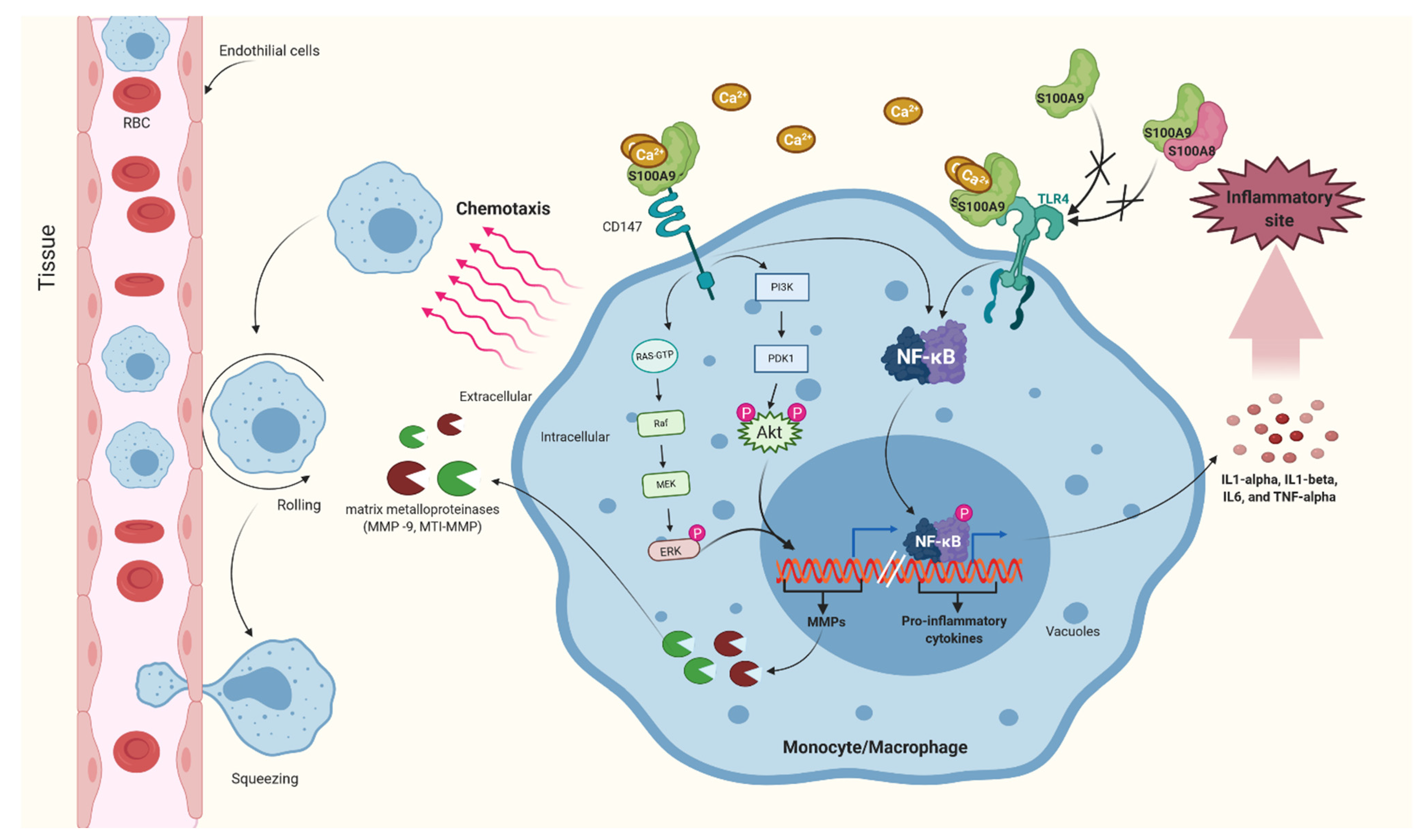
2.1.1. Macrophage and Monocytes
2.1.2. Neutrophil
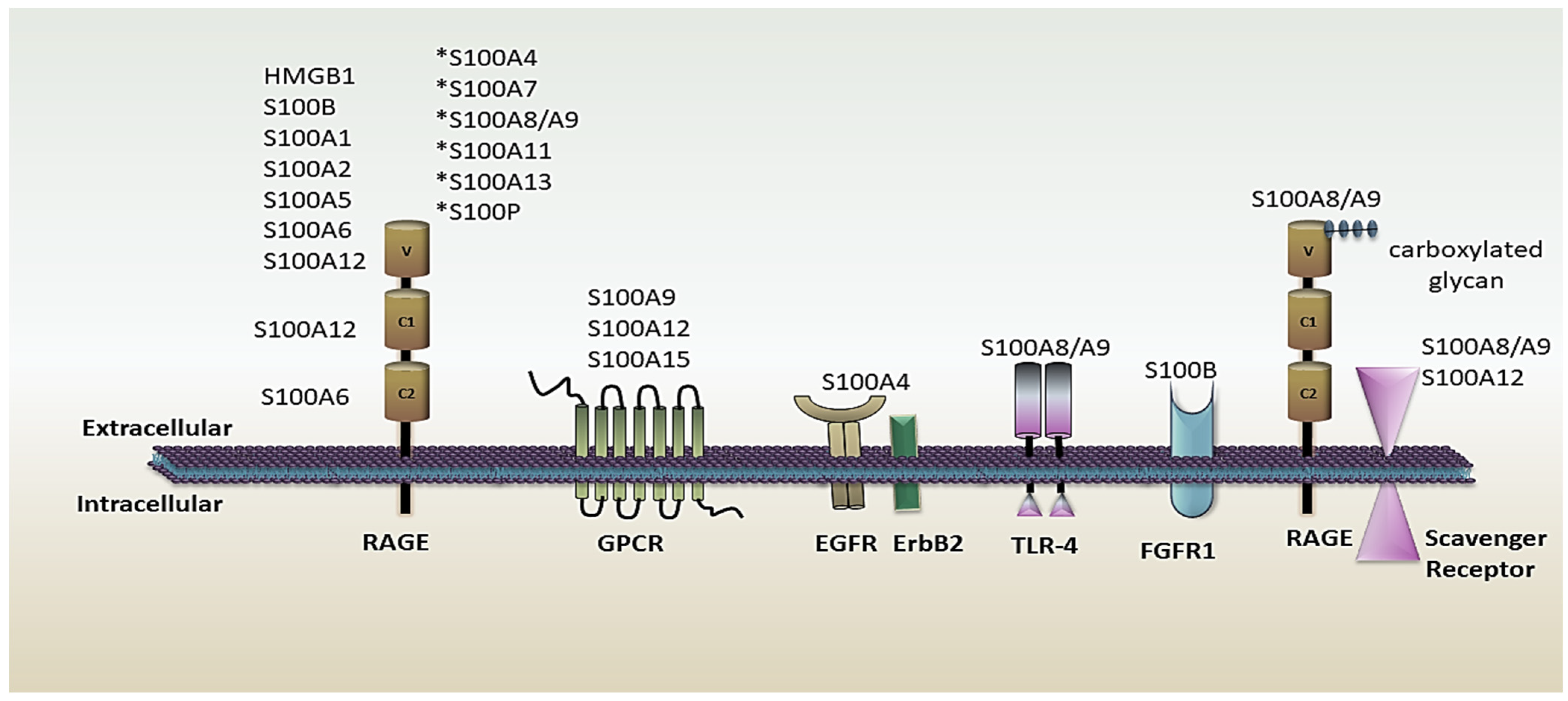
2.2. S100 Protein’s Role in the Immune System as an Active Molecule
2.2.1. Alarmins or DAMPs
2.2.2. Functional Implication of S100 Protein as Antimicrobial Peptides and in Nutritional Immunity
2.3. Role of S100 Protein in Various Immunological Process
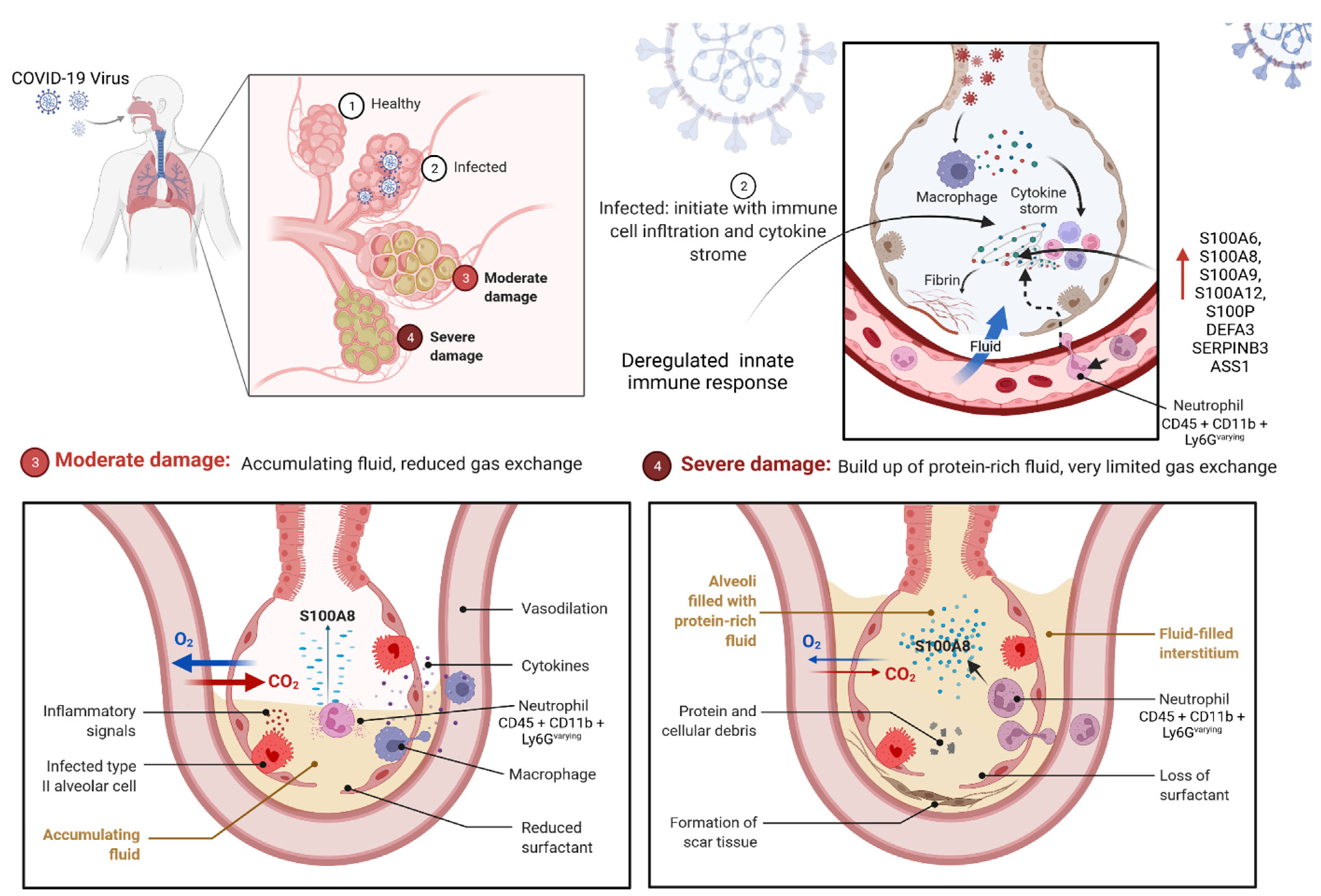
2.3.1. S100 Protein Could Be the Prognostic Marker for COVID-19
2.3.2. Functional Contacts of Nerves with Immune Cells through S100 Protein
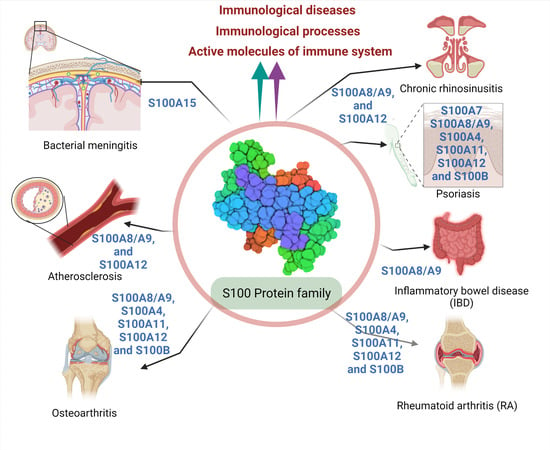
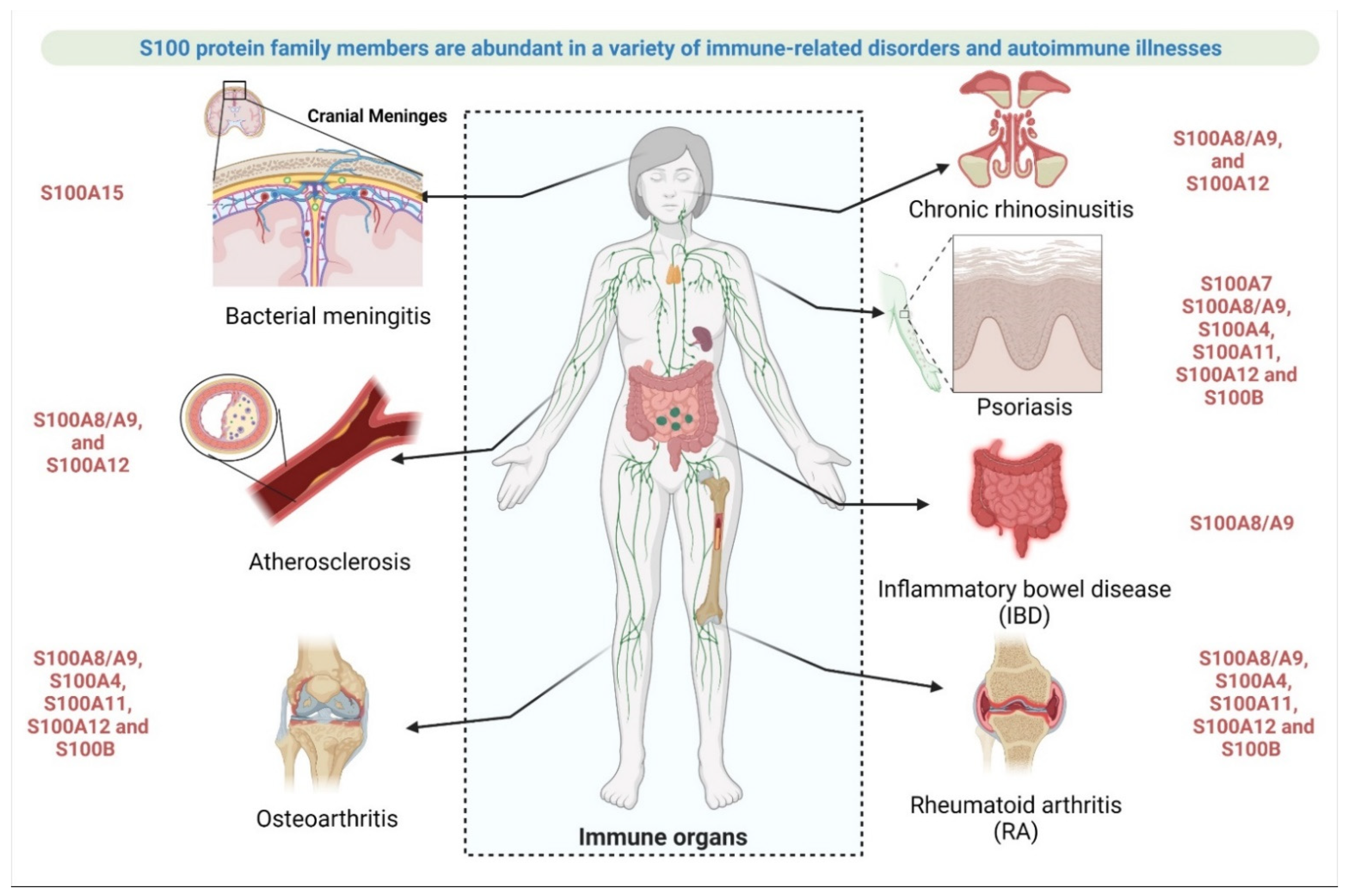
2.3.3. Involvement of S100 Protein in Autoimmune Disease or Immune System-Related Disease
- a.
- Rheumatoid arthritis (RA)
- b.
- Osteoarthritis (OA)
- c.
- Osteoporosis
- d.
- Psoriasis
- e.
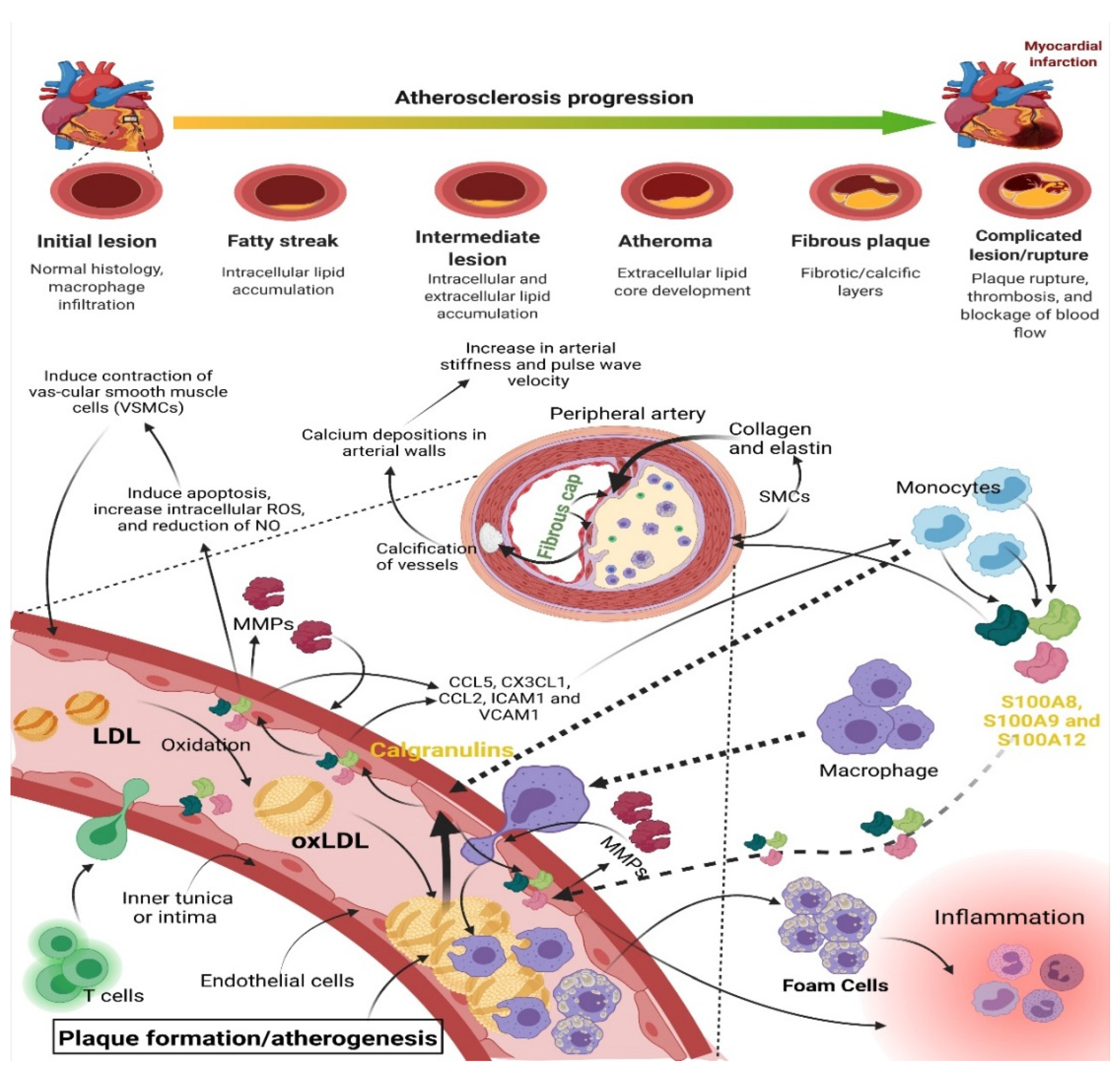
- Atherosclerosis
- f.
- Inflammatory bowel disease (IBD)
- g.
- Chronic rhinosinusitis (CRS)
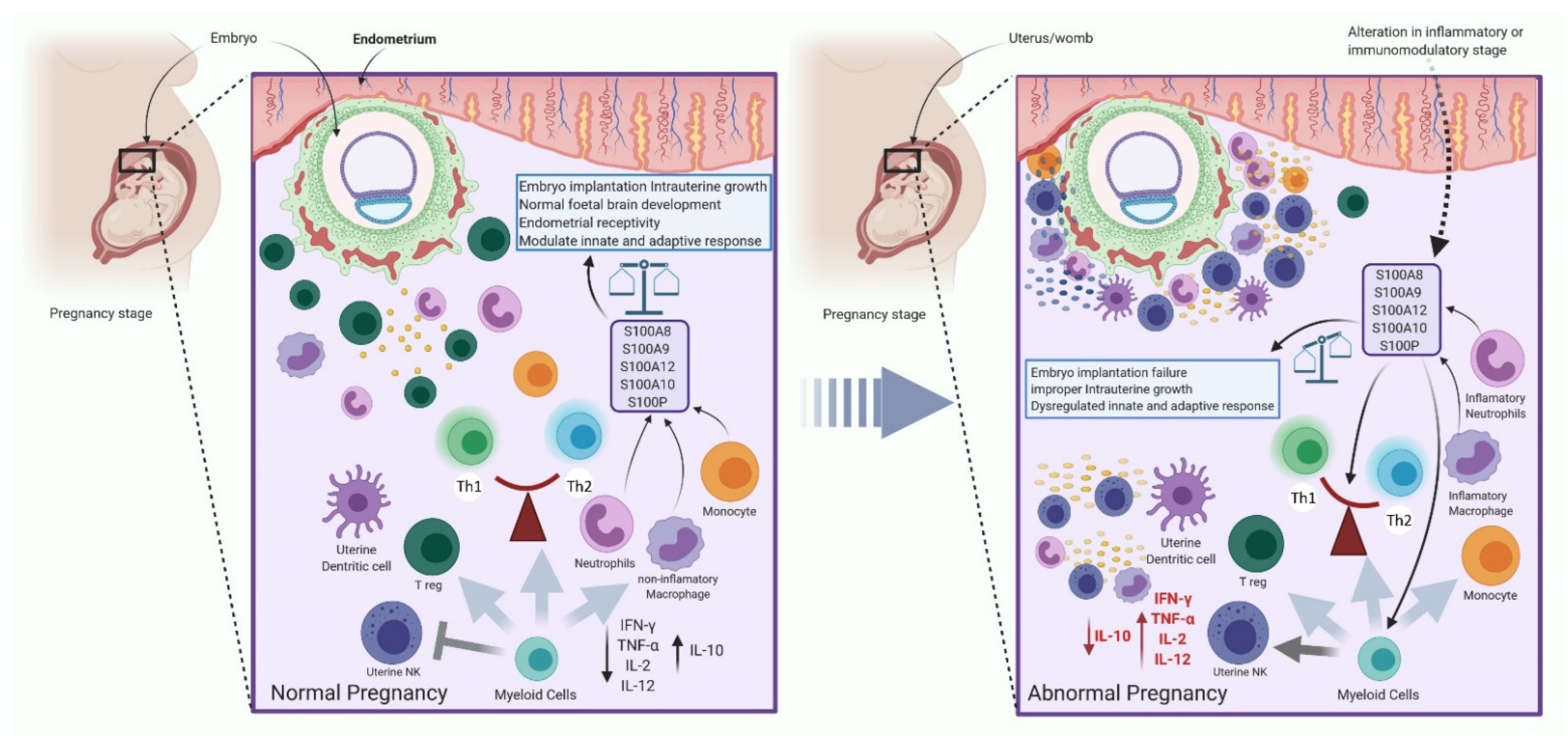
2.3.4. The Immune System Regulates the Expression of S100 Protein during Pregnancy
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Moore, B.W. A soluble protein characteristic of the nervous system. Biochem. Biophys. Res. Commun. 1965, 19, 739–744. [Google Scholar] [CrossRef]
- Hermann, A.; Donato, R.; Weiger, T.M.; Chazin, W.J. S100 Calcium Binding Proteins and Ion Channels. Front. Pharmacol. 2012, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Marenholz, I.; Heizmann, C.W.; Fritz, G. S100 proteins in mouse and man: From evolution to function and pathology (including an update of the nomenclature). Biochem. Biophys. Res. Commun. 2004, 322, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Fritz, G.; Heizmann, C.W. 3D Structures of the Calcium and Zinc Binding S100 Proteins. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 1–12. [Google Scholar] [CrossRef]
- Leśniak, W.; Graczyk-Jarzynka, A. The S100 proteins in epidermis: Topology and function. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 2563–2572. [Google Scholar] [CrossRef]
- Moews, P.; Kretsinger, R. Refinement of the structure of carp muscle calcium-binding parvalbumin by model building and difference fourier analysis. J. Mol. Biol. 1975, 91, 201–225. [Google Scholar] [CrossRef]
- Claus, W.H.; Heizmann, C.W.; Fritz, G.; Schäfer, B.W. S100 proteins: Structure, functions and pathology. Front. Biosci. 2002, 7, d1356. [Google Scholar] [CrossRef]
- Strynadka, N.C.J.; James, M.N.G. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu. Rev. Biochem. 1989, 58, 951–998. [Google Scholar] [CrossRef]
- Kligman, D.; Hilt, D.C. The S100 protein family. Trends Biochem. Sci. 1988, 13, 437–443. [Google Scholar] [CrossRef]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 Proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef]
- Gilston, B.A.; Skaar, E.P.; Chazin, W.J. Binding of transition metals to S100 proteins. Sci. China Life Sci. 2016, 59, 792–801. [Google Scholar] [CrossRef]
- Wheeler, L.C.; Donor, M.T.; Prell, J.S.; Harms, M.J. Multiple Evolutionary Origins of Ubiquitous Cu2+ and Zn2+ Binding in the S100 Protein Family. PLoS ONE 2016, 11, e0164740. [Google Scholar] [CrossRef]
- Fritz, G.; Botelho, H.M.; Morozova-Roche, L.A.; Gomes, C.M. Natural and amyloid self-assembly of S100 proteins: Structural basis of functional diversity. FEBS J. 2010, 277, 4578–4590. [Google Scholar] [CrossRef] [PubMed]
- Boom, A.; Pochet, R.; Authelet, M.; Pradier, L.; Borghgraef, P.; Van Leuven, F.; Heizmann, C.W.; Brion, J.-P. Astrocytic calcium/zinc binding protein S100A6 over expression in Alzheimer’s disease and in PS1/APP transgenic mice models. Biochim. Biophys. Acta 2004, 1742, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Donato, R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 2001, 33, 637–668. [Google Scholar] [CrossRef]
- Leclerc, E.; Heizmann, C.W. The importance of Ca2+/Zn2+ signaling s100 proteins and rage in translational medicine. Front. Biosci. 2011, 3, 1232–1262. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E. HMGB1, IL-1α, IL-33 and S100 proteins: Dual-function alarmins. Cell. Mol. Immunol. 2017, 14, 43–64. [Google Scholar] [CrossRef]
- Sedaghat, F.; Notopoulos, A. S100 protein family and its application in clinical practice. Hippokratia 2008, 12, 198–204. [Google Scholar]
- Pietzsch, J. S100 proteins in health and disease. Amino Acids 2010, 41, 755–760. [Google Scholar] [CrossRef][Green Version]
- Ali, S.A.; Singh, P.; Tomar, S.K.; Mohanty, A.K.; Behare, P. Proteomics fingerprints of systemic mechanisms of adaptation to bile in Lactobacillus fermentum. J. Proteom. 2019, 213, 103600. [Google Scholar] [CrossRef]
- Iacob, S.; Iacob, D.G.; Luminos, L.M. Intestinal Microbiota as a Host Defense Mechanism to Infectious Threats. Front. Microbiol. 2019, 9, 3328. [Google Scholar] [CrossRef]
- Krausgruber, T.; Fortelny, N.; Fife-Gernedl, V.; Senekowitsch, M.; Schuster, L.C.; Lercher, A.; Nemc, A.; Schmidl, C.; Rendeiro, A.F.; Bergthaler, A.; et al. Structural cells are key regulators of organ-specific immune responses. Nature 2020, 583, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Yesudhas, D.; Gosu, V.; Anwar, M.A.; Choi, S. Multiple Roles of Toll-Like Receptor 4 in Colorectal Cancer. Front. Immunol. 2014, 5, 334. [Google Scholar] [CrossRef]
- Hsu, K.; Passey, R.; Endoh, Y.; Rahimi, F.; Youssef, P.; Yen, T.; Geczy, C.L. Regulation of S100A8 by Glucocorticoids. J. Immunol. 2005, 174, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Endoh, Y.; Chung, Y.M.; Clark, I.A.; Geczy, C.L.; Hsu, K. IL-10-Dependent S100A8 Gene Induction in Monocytes/Macrophages by Double-Stranded RNA. J. Immunol. 2009, 182, 2258–2268. [Google Scholar] [CrossRef]
- Geczy, C.L.; Tessier, P.A.; Gomes, L.; Gabrilovich, D. S100 Calgranulins in Inflammation. In The Neutrophils; World Scientific: Singapore, 2013; pp. 310–377. [Google Scholar] [CrossRef]
- Wang, C.; Jinchuan, Y.; Zhu, X.; Yan, J.; Li, G. Function of CD147 in Atherosclerosis and Atherothrombosis. J. Cardiovasc. Transl. Res. 2015, 8, 59–66. [Google Scholar] [CrossRef]
- Schmidt, R.; Bültmann, A.; Fischel, S.; Gillitzer, A.; Cullen, P.; Walch, A.; Jost, P.; Ungerer, M.; Tolley, N.D.; Lindemann, S.; et al. Extracellular Matrix Metalloproteinase Inducer (CD147) Is a Novel Receptor on Platelets, Activates Platelets, and Augments Nuclear Factor κB–Dependent Inflammation in Monocytes. Circ. Res. 2008, 102, 302–309. [Google Scholar] [CrossRef]
- Yuan, W.; Ge, H.; He, B. Pro-inflammatory activities induced by CyPA–EMMPRIN interaction in monocytes. Atherosclerosis 2010, 213, 415–421. [Google Scholar] [CrossRef]
- Alexaki, V.I.; May, A.E.; Fujii, C.; Mund, C.; Gawaz, M.; Ungern-Sternberg, S.N.I.V.; Chavakis, T.; Seizer, P. S100A9 induces monocyte/ macrophage migration via EMMPRIN. Thromb. Haemost. 2017, 117, 636–639. [Google Scholar] [CrossRef]
- Holzinger, D.; Foell, D.; Kessel, C. The role of S100 proteins in the pathogenesis and monitoring of autoinflammatory diseases. Mol. Cell. Pediatr. 2018, 5, 7. [Google Scholar] [CrossRef]
- Lira-Junior, R.; Holmström, S.B.; Clark, R.; Zwicker, S.; Majster, M.; Johannsen, G.; Axtelius, B.; Åkerman, S.; Svensson, M.; Klinge, B.; et al. S100A12 Expression Is Modulated During Monocyte Differentiation and Reflects Periodontitis Severity. Front. Immunol. 2020, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Wittkowski, H.; Roth, J. Mechanisms of Disease: A ‘DAMP’ view of inflammatory arthritis. Nat. Clin. Pract. Rheumatol. 2007, 3, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Schenten, V.; Plançon, S.; Jung, N.; Hann, J.; Bueb, J.-L.; Bréchard, S.; Tschirhart, E.J.; Tolle, F. Secretion of the Phosphorylated Form of S100A9 from Neutrophils Is Essential for the Proinflammatory Functions of Extracellular S100A8/A9. Front. Immunol. 2018, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.L.; Tablin, F. A Comparative Review of Neutrophil Extracellular Traps in Sepsis. Front. Veter. Sci. 2018, 5, 291. [Google Scholar] [CrossRef]
- Cox, L.E.; Walstein, K.; Völlger, L.; Reuner, F.; Bick, A.; Dötsch, A.; Engler, A.; Peters, J.; von Köckritz-Blickwede, M.; Schäfer, S.T. Neutrophil extracellular trap formation and nuclease activity in septic patients. BMC Anesthesiol. 2020, 20, 15. [Google Scholar] [CrossRef]
- Scheibner, K.A.; Lutz, M.A.; Boodoo, S.; Fenton, M.J.; Powell, J.D.; Horton, M.R. Hyaluronan Fragments Act as an Endogenous Danger Signal by Engaging TLR2. J. Immunol. 2006, 177, 1272–1281. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Erratum: Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2010, 467, 622. [Google Scholar] [CrossRef]
- Panayi, G.S.; Corrigall, V.M.; Henderson, B. Stress cytokines: Pivotal proteins in immune regulatory networks: Opinion. Curr. Opin. Immunol. 2004, 16, 531–534. [Google Scholar] [CrossRef]
- Nefla, M.; Holzinger, D.; Berenbaum, F.; Jacques, C. The danger from within: Alarmins in arthritis. Nat. Rev. Rheumatol. 2016, 12, 669–683. [Google Scholar] [CrossRef]
- Ojcius, D.; Saïd-Sadier, N. Alarmins, inflammasomes and immunity. Biomed. J. 2012, 35, 437–449. [Google Scholar] [CrossRef]
- Oppenheim, J.J.; Yang, D. Alarmins: Chemotactic activators of immune responses. Curr. Opin. Immunol. 2005, 17, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2014, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Danger-Associated Molecular Patterns (DAMPs): The Derivatives and Triggers of Inflammation. Curr. Allergy Asthma Rep. 2018, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Ginaldi, L.; Sirufo, M.M.; Pioggia, G.; Calapai, G.; Gangemi, S.; Mannucci, C. Alarmins in Osteoporosis, RAGE, IL-1, and IL-33 Pathways: A Literature Review. Medicina 2020, 56, 138. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Czura, C.J.; Tracey, K.J. The cytokine activity of HMGB1. J. Leukoc. Biol. 2005, 78, 1–8. [Google Scholar] [CrossRef]
- Crowe, L.A.N.; McLean, M.; Kitson, S.M.; Melchor, E.G.; Patommel, K.; Cao, H.M.; Reilly, J.H.; Leach, W.J.; Rooney, B.P.; Spencer, S.J.; et al. S100A8 & S100A9: Alarmin mediated inflammation in tendinopathy. Sci. Rep. 2019, 9, 1463. [Google Scholar] [CrossRef]
- Mosca, M.J.; Carr, A.J.; Snelling, S.J.; Wheway, K.; Watkins, B.; Dakin, S.G. Differential expression of alarmins—S100A9, IL-33, HMGB1 and HIF-1α in supraspinatus tendinopathy before and after treatment. BMJ Open Sport Exerc. Med. 2017, 3, e000225. [Google Scholar] [CrossRef]
- Safronova, A.; Araujo, A.; Camanzo, E.T.; Moon, T.J.; Elliott, M.; Beiting, D.P.; Yarovinsky, F. Alarmin S100A11 initiates a chemokine response to the human pathogen Toxoplasma gondii. Nat. Immunol. 2018, 20, 64–72. [Google Scholar] [CrossRef]
- Ulas, T.; Pirr, S.; Fehlhaber, B.; Bickes, M.S.; Loof, T.G.; Vogl, T.; Mellinger, L.; Heinemann, A.S.; Burgmann, J.; Schöning, J.; et al. S100-alarmin-induced innate immune programming protects newborn infants from sepsis. Nat. Immunol. 2017, 18, 622–632. [Google Scholar] [CrossRef]
- Pirr, S.; Richter, M.; Fehlhaber, B.; Pagel, J.; Härtel, C.; Roth, J.; Vogl, T.; Viemann, D. High Amounts of S100-Alarmins Confer Antimicrobial Activity on Human Breast Milk Targeting Pathogens Relevant in Neonatal Sepsis. Front. Immunol. 2017, 8, 1822. [Google Scholar] [CrossRef]
- Pisanu, S.; Cacciotto, C.; Pagnozzi, D.; Puggioni, G.M.G.; Uzzau, S.; Ciaramella, P.; Guccione, J.; Penati, M.; Pollera, C.; Moroni, P.; et al. Proteomic changes in the milk of water buffaloes (Bubalus bubalis) with subclinical mastitis due to intramammary infection by Staphylococcus aureus and by non-aureus staphylococci. Sci. Rep. 2019, 9, 15850. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Stratis, A.; Wixler, V.; Völler, T.; Thurainayagam, S.; Jorch, S.K.; Zenker, S.; Dreiling, A.; Chakraborty, D.; Fröhling, M.; et al. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J. Clin. Investig. 2018, 128, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Ovchinnikova, T.V. Immunomodulatory and Allergenic Properties of Antimicrobial Peptides. Int. J. Mol. Sci. 2022, 23, 2499. [Google Scholar] [CrossRef]
- Rademacher, F.; Dreyer, S.; Kopfnagel, V.; Gläser, R.; Werfel, T.; Harder, J. The Antimicrobial and Immunomodulatory Function of RNase 7 in Skin. Front. Immunol. 2019, 10, 2553. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Seeley, R.J. Reg3 Proteins as Gut Hormones? Endocrinology 2019, 160, 1506–1514. [Google Scholar] [CrossRef]
- Parvy, J.-P.; Yu, Y.; Dostalova, A.; Kondo, S.; Kurjan, A.; Bulet, P.; Lemaître, B.; Vidal, M.; Cordero, J.B. The antimicrobial peptide defensin cooperates with tumour necrosis factor to drive tumour cell death in Drosophila. eLife 2019, 8, e45061. [Google Scholar] [CrossRef]
- Bhatt, T.; Bhosale, A.; Bajantri, B.; Mathapathi, M.S.; Rizvi, A.; Scita, G.; Majumdar, A.; Jamora, C. Sustained Secretion of the Antimicrobial Peptide S100A7 Is Dependent on the Downregulation of Caspase-8. Cell Rep. 2019, 29, 2546–2555.e4. [Google Scholar] [CrossRef]
- D’Amico, F.; Skarmoutsou, E.; Granata, M.; Trovato, C.; Rossi, G.A.; Mazzarino, M.C. S100A7: A rAMPing up AMP molecule in psoriasis. Cytokine Growth Factor Rev. 2016, 32, 97–104. [Google Scholar] [CrossRef]
- Büchau, A.S.; Hassan, M.; Kukova, G.; Lewerenz, V.; Kellermann, S.; Würthner, J.U.; Wolf, R.; Walz, M.; Gallo, R.L.; Ruzicka, T. S100A15, an Antimicrobial Protein of the Skin: Regulation by E. coli through Toll-Like Receptor 4. J. Investig. Dermatol. 2007, 127, 2596–2604. [Google Scholar] [CrossRef]
- Abtin, A.; Eckhart, L.; Gläser, R.; Gmeiner, R.; Mildner, M.; Tschachler, E. The Antimicrobial Heterodimer S100A8/S100A9 (Calprotectin) Is Upregulated by Bacterial Flagellin in Human Epidermal Keratinocytes. J. Investig. Dermatol. 2010, 130, 2423–2430. [Google Scholar] [CrossRef]
- Bei, Y.; Pan, L.-L.; Zhou, Q.; Zhao, C.; Xie, Y.; Wu, C.; Meng, X.; Gu, H.; Xu, J.; Zhou, L.; et al. Cathelicidin-related antimicrobial peptide protects against myocardial ischemia/reperfusion injury. BMC Med. 2019, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kulkarni, N.N.; Lee, E.Y.; Zhang, L.-J.; Wong, G.C.L.; Gallo, R.L. Cathelicidin promotes inflammation by enabling binding of self-RNA to cell surface scavenger receptors. Sci. Rep. 2018, 8, 4032. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Sorenson, B.S.; Ross, K.F.; Herzberg, M.C. Augmentation of Epithelial Resistance to Invading Bacteria by Using mRNA Transfections. Infect. Immun. 2013, 81, 3975–3983. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chessa, C.; Bodet, C.; Jousselin, C.; Wehbe, M.; Lévêque, N.; Garcia, M. Antiviral and Immunomodulatory Properties of Antimicrobial Peptides Produced by Human Keratinocytes. Front. Microbiol. 2020, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Kozlyuk, N.; Monteith, A.J.; Garcia, V.; Damo, S.M.; Skaar, E.P.; Chazin, W.J. S100 Proteins in the Innate Immune Response to Pathogens. Adv. Struct. Saf. Stud. 2019, 1929, 275–290. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Kiatsurayanon, C.; Chieosilapatham, P.; Ogawa, H. Friends or Foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp. Dermatol. 2017, 26, 989–998. [Google Scholar] [CrossRef]
- Hansmann, B.; Schröder, J.-M.; Gerstel, U. Skin-Derived C-Terminal Filaggrin-2 Fragments Are Pseudomonas aeruginosa-Directed Antimicrobials Targeting Bacterial Replication. PLoS Pathog. 2015, 11, e1005159. [Google Scholar] [CrossRef]
- Lutzow, Y.C.S.; Donaldson, L.; Gray, C.P.; Vuocolo, T.; Pearson, R.D.; Reverter, A.; Byrne, K.A.; Sheehy, P.A.; Windon, R.; Tellam, R.L. Identification of immune genes and proteins involved in the response of bovine mammary tissue to Staphylococcus aureus infection. BMC Veter. Res. 2008, 4, 18. [Google Scholar] [CrossRef]
- Smolenski, G.; Haines, S.; Kwan, F.Y.-S.; Bond, J.; Farr, V.; Davis, S.R.; Stelwagen, K.; Wheeler, T.T. Characterisation of Host Defence Proteins in Milk Using a Proteomic Approach. J. Proteome Res. 2006, 6, 207–215. [Google Scholar] [CrossRef]
- Lloren, K.; Ii, R. S100 can be used as a tumor marker in canine mammary tumors. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 928–939. [Google Scholar] [CrossRef]
- Solanki, S.S.; Singh, P.; Kashyap, P.; Sansi, M.S.; Ali, S.A. Promising role of defensins peptides as therapeutics to combat against viral infection. Microb. Pathog. 2021, 155, 104930. [Google Scholar] [CrossRef] [PubMed]
- Regenhard, P.; Petzl, W.; Zerbe, H.; Sauerwein, H. The antibacterial psoriasin is induced by E. coli infection in the bovine udder. Vet. Microbiol. 2010, 143, 293–298. [Google Scholar] [CrossRef]
- Zygiel, E.M.; Nolan, E.M. Transition Metal Sequestration by the Host-Defense Protein Calprotectin. Annu. Rev. Biochem. 2018, 87, 621–643. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, A.T.; Nolan, E.M. Avian MRP126 Restricts Microbial Growth through Ca(II)-Dependent Zn(II) Sequestration. Biochemistry 2019, 59, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.A.; Radin, J.N.; Loh, J.T.; Piazuelo, M.B.; Kehl-Fie, T.E.; Delgado, A.G.; Ilca, F.T.; Peek, R.M.; Cover, T.L.; Chazin, W.J.; et al. The Host Protein Calprotectin Modulates the Helicobacter pylori cag Type IV Secretion System via Zinc Sequestration. PLOS Pathog. 2014, 10, e1004450. [Google Scholar] [CrossRef]
- Zygiel, E.M.; Nelson, C.E.; Brewer, L.K.; Oglesby-Sherrouse, A.G.; Nolan, E.M. The human innate immune protein calprotectin induces iron starvation responses in Pseudomonas aeruginosa. J. Biol. Chem. 2019, 294, 3549–3562. [Google Scholar] [CrossRef]
- Damo, S.M.; Kehl-Fie, T.E.; Sugitani, N.; Holt, M.E.; Rathi, S.; Murphy, W.J.; Zhang, Y.; Betz, C.; Hench, L.; Fritz, G.; et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, 3841–3846. [Google Scholar] [CrossRef]
- Matthijs, S.; Hernalsteens, J.-P.; Roelants, K. An orthologue of the host-defense protein psoriasin (S100A7) is expressed in frog skin. Dev. Comp. Immunol. 2017, 67, 395–403. [Google Scholar] [CrossRef]
- Kasus-Jacobi, A.; Land, C.A.; Stock, A.J.; Washburn, J.L.; Pereira, H.A. Antimicrobial Peptides Derived from the Immune Defense Protein CAP37 Inhibit TLR4 Activation by S100A9. Investig. Opthalmology Vis. Sci. 2020, 61, 16. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.L.; Lacy, D.B.; Ohi, M.D. The Helicobacter pylori Cag Type IV Secretion System. Trends Microbiol. 2020, 28, 682–695. [Google Scholar] [CrossRef]
- Chung, J.M.; Sheedlo, M.J.; Campbell, A.M.; Sawhney, N.; Frick-Cheng, A.E.; Lacy, D.B.; Cover, T.L.; Ohi, M.D. Structure of the Helicobacter pylori Cag type IV secretion system. eLife 2019, 8, e47644. [Google Scholar] [CrossRef] [PubMed]
- Haley, K.P.; Delgado, A.G.; Piazuelo, M.B.; Mortensen, B.L.; Correa, P.; Damo, S.M.; Chazin, W.J.; Skaar, E.P.; Gaddy, J.A. The Human Antimicrobial Protein Calgranulin C Participates in Control of Helicobacter pylori Growth and Regulation of Virulence. Infect. Immun. 2015, 83, 2944–2956. [Google Scholar] [CrossRef] [PubMed]
- Steimle, A.; Autenrieth, I.B.; Frick, J.-S. Structure and function: Lipid a modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef]
- Jackson, E.; Little, S.; Franklin, D.S.; Gaddy, J.A.; Damo, S.M. Expression, Purification, and Antimicrobial Activity of S100A12. J. Vis. Exp. 2017, 123, 55557. [Google Scholar] [CrossRef] [PubMed]
- Biji, A.; Khatun, O.; Swaraj, S.; Narayan, R.; Rajmani, R.S.; Sardar, R.; Satish, D.; Mehta, S.; Bindhu, H.; Jeevan, M.; et al. Identification of COVID-19 prognostic markers and therapeutic targets through meta-analysis and validation of Omics data from nasopharyngeal samples. eBioMedicine 2021, 70, 103525. [Google Scholar] [CrossRef] [PubMed]
- Velavan, T.P.; Meyer, C.G. Mild versus severe COVID-19: Laboratory markers. Int. J. Infect. Dis. 2020, 95, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Yang, L.; Li, Y.; Liang, B.; Li, L.; Ye, T.; Li, L.; Liu, D.; Gui, S.; Hu, Y.; et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): A case-control study. Int. J. Med Sci. 2020, 17, 1281–1292. [Google Scholar] [CrossRef]
- Chen, L.; Long, X.; Xu, Q.; Tan, J.; Wang, G.; Cao, Y.; Wei, J.; Luo, H.; Zhu, H.; Huang, L.; et al. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 992–994. [Google Scholar] [CrossRef]
- Chan, J.K.; Roth, J.; Oppenheim, J.J.; Tracey, K.J.; Vogl, T.; Feldmann, M.; Horwood, N.; Nanchahal, J. Alarmins: Awaiting a clinical response. J. Clin. Investig. 2012, 122, 2711–2719. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Xia, H.; Wang, C.; Tan, C.Y.; Cai, X.; Liu, Y.; Ji, F.; Xiong, P.; Liu, R.; et al. Transcriptional and proteomic insights into the host response in fatal COVID-19 cases. Proc. Natl. Acad. Sci. USA 2020, 117, 28336–28343. [Google Scholar] [CrossRef]
- Mete, E.; Sabirli, R.; Goren, T.; Turkcuer, I.; Kurt, Ö.; Koseler, A. Association Between S100b Levels and COVID-19 Pneumonia: A Case Control Study. In Vivo 2021, 35, 2923–2928. [Google Scholar] [CrossRef] [PubMed]
- Aceti, A.; Margarucci, L.M.; Scaramucci, E.; Orsini, M.; Salerno, G.; Di Sante, G.; Gianfranceschi, G.; Di Liddo, R.; Valeriani, F.; Ria, F.; et al. Serum S100B protein as a marker of severity in COVID-19 patients. Sci. Rep. 2020, 10, 18665. [Google Scholar] [CrossRef] [PubMed]
- Piazza, O.; Leggiero, E.; De Benedictis, G.; Pastore, L.; Salvatore, F.; Tufano, R.; De Robertis, E. S100B Induces the Release of Pro-Inflammatory Cytokines in Alveolar Type I-like Cells. Int. J. Immunopathol. Pharmacol. 2013, 26, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Sorci, G.; Giovannini, G.; Riuzzi, F.; Bonifazi, P.; Zelante, T.; Zagarella, S.; Bistoni, F.; Donato, R.; Romani, L. The Danger Signal S100B Integrates Pathogen– and Danger–Sensing Pathways to Restrain Inflammation. PLoS Pathog. 2011, 7, e1001315. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Hosseinabadi, Z.; Abbasi, M.; Kahnooji, M.; Ghorbani, Z.; Abbasifard, M. The prognostic value of S100A calcium binding protein family members in predicting severe forms of COVID-19. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2022, 71, 369–376. [Google Scholar] [CrossRef]
- Deguchi, A.; Yamamoto, T.; Shibata, N.; Maru, Y. S100A8 may govern hyper-inflammation in severe COVID-19. FASEB J. 2021, 35, e21798. [Google Scholar] [CrossRef]
- Nagareddy, P.R.; Murphy, A.J.; Stirzaker, R.A.; Hu, Y.; Yu, S.; Miller, R.G.; Ramkhelawon, B.; Distel, E.; Westerterp, M.; Huang, L.S.; et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of athero-sclerosis. Cell Metab. 2013, 17, 695–708. [Google Scholar] [CrossRef]
- Foell, D.; Frosch, M.; Sorg, C.; Roth, J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin. Chim. Acta 2004, 344, 37–51. [Google Scholar] [CrossRef]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, Y.; Li, J.; Liu, J.; Yang, X.; Guo, X.; Kuang, M.; Xia, H.; Zhang, Z.; Cao, L.; et al. Induction of alarmin S100A8/A9 mediates activation of aberrant neutrophils in the pathogenesis of COVID-19. Cell Host Microbe 2021, 29, 222–235. [Google Scholar] [CrossRef]
- Schulte-Schrepping, J.; Reusch, N.; Paclik, D.; Baßler, K.; Schlickeiser, S.; Zhang, B.; Krämer, B.; Krammer, T.; Brumhard, S.; Bonaguro, L.; et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell 2020, 182, 1419–1440.e23. [Google Scholar] [CrossRef] [PubMed]
- Silvin, A.; Chapuis, N.; Dunsmore, G.; Goubet, A.G.; Dubuisson, A.; Derosa, L.; Almire, C.; Hénon, C.; Kosmider, O.; Droin, N.; et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell 2020, 182, 1401–1418.e18. [Google Scholar] [CrossRef] [PubMed]
- Wilk, A.J.; Rustagi, A.; Zhao, N.Q.; Roque, J.; Martínez-Colón, G.J.; McKechnie, J.L.; Ivison, G.T.; Ranganath, T.; Vergara, R.; Hollis, T.; et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020, 26, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Noristani, R.; Kuehn, S.; Stute, G.; Reinehr, S.; Stellbogen, M.; Dick, H.B.; Joachim, S.C. Retinal and Optic Nerve Damage is Associated with Early Glial Responses in an Experimental Autoimmune Glaucoma Model. J. Mol. Neurosci. 2016, 58, 470–482. [Google Scholar] [CrossRef]
- Reinehr, S.; Reinhard, J.; Gandej, M.; Gottschalk, I.; Stute, G.; Faissner, A.; Dick, H.B.; Joachim, S.C. S100B immunization triggers NFκB and complement activation in an autoimmune glaucoma model. Sci. Rep. 2018, 8, 9821. [Google Scholar] [CrossRef]
- Kuehn, S.; Meißner, W.; Grotegut, P.; Theiss, C.; Dick, H.B.; Joachim, S.C. Intravitreal S100B Injection Leads to Progressive Glaucoma Like Damage in Retina and Optic Nerve. Front. Cell. Neurosci. 2018, 12, 312. [Google Scholar] [CrossRef]
- Kohm, A.P.; Sanders, V.M. Norepinephrine: A messenger from the brain to the immune system. Immunol. Today 2000, 21, 539–542. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, C.; Zhang, P.; Zhu, Q.; Liu, Y.; Zhu, Z.; Wang, M.; Li, W.; Yang, G.; Dong, N.; et al. S100+ cells: A new neuro-immune cross-talkers in lymph organs. Sci. Rep. 2013, 3, 1114. [Google Scholar] [CrossRef]
- Tian, Z.-Y.; Wang, C.-Y.; Wang, T.; Li, Y.-C.; Wang, Z.-Y. Glial S100A6 Degrades β-amyloid Aggregation through Targeting Competition with Zinc Ions. Aging Dis. 2019, 10, 756–769. [Google Scholar] [CrossRef]
- Hagmeyer, S.; Cristóvão, J.S.; Mulvihill, J.J.E.; Boeckers, T.M.; Gomes, C.M.; Grabrucker, A.M. Zinc Binding to S100B Affords Regulation of Trace Metal Homeostasis and Excitotoxicity in the Brain. Front. Mol. Neurosci. 2018, 10, 456. [Google Scholar] [CrossRef]
- Hagmeyer, S.; Romão, M.A.; Cristóvão, J.S.; Vilella, A.; Zoli, M.; Gomes, C.M.; Grabrucker, A.M. Distribution and Relative Abundance of S100 Proteins in the Brain of the APP23 Alzheimer’s Disease Model Mice. Front. Neurosci. 2019, 13, 640. [Google Scholar] [CrossRef] [PubMed]
- Cristóvão, J.S.; Gomes, C.M. S100 Proteins in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- García-González, L.; Pilat, D.; Baranger, K.; Rivera, S. Emerging Alternative Proteinases in APP Metabolism and Alzheimer’s Disease Pathogenesis: A Focus on MT1-MMP and MT5-MMP. Front. Aging Neurosci. 2019, 11, 244. [Google Scholar] [CrossRef]
- Jansen, S.; Podschun, R.; Leib, S.L.; Grötzinger, J.; Oestern, S.; Michalek, M.; Pufe, T.; Brandenburg, L.-O. Expression and Function of Psoriasin (S100A7) and Koebnerisin (S100A15) in the Brain. Infect. Immun. 2013, 81, 1788–1797. [Google Scholar] [CrossRef][Green Version]
- Serrano, A.; Apolloni, S.; Rossi, S.; Lattante, S.; Sabatelli, M.; Peric, M.; Andjus, P.; Michetti, F.; Carrì, M.T.; Cozzolino, M.; et al. The S100A4 Transcriptional Inhibitor Niclosamide Reduces Pro-Inflammatory and Migratory Phenotypes of Microglia: Implications for Amyotrophic Lateral Sclerosis. Cells 2019, 8, 1261. [Google Scholar] [CrossRef]
- Wang, J.; Huang, A.; Xu, W.; Su, L. Insights into IL-29: Emerging role in inflammatory autoimmune diseases. J. Cell. Mol. Med. 2019, 23, 7926–7932. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Rao, X.; Sigdel, K.R. Regulation of Inflammation in Autoimmune Disease. J. Immunol. Res. 2019, 2019, 7403796. [Google Scholar] [CrossRef]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef]
- Sherwood, R.; Walsham, N. Fecal calprotectin in inflammatory bowel disease. Clin. Exp. Gastroenterol. 2016, 9, 21–29. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Alunno, A.; Carubbi, F.; Giacomelli, R.; Gerli, R. Cytokines in the pathogenesis of rheumatoid arthritis: New players and therapeutic targets. BMC Rheumatol. 2017, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Wittkowski, H.; Vogl, T.; Roth, J. S100 proteins expressed in phagocytes: A novel group of damage-associated molecular pattern molecules. J. Leukoc. Biol. 2007, 81, 28–37. [Google Scholar] [CrossRef]
- Austermann, J.; Spiekermann, C.; Roth, J. S100 proteins in rheumatic diseases. Nat. Rev. Rheumatol. 2018, 14, 528–541. [Google Scholar] [CrossRef]
- van Lent, P.L.E.M.; Blom, A.B.; Schelbergen, R.F.P.; Slöetjes, A.; Lafeber, F.P.J.G.; Lems, W.F.; Cats, H.; Vogl, T.; Roth, J.; Berg, W.B.V.D. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Care Res. 2011, 64, 1466–1476. [Google Scholar] [CrossRef]
- Schelbergen, R.F.P.; De Munter, W.; Bosch, M.H.J.V.D.; Lafeber, F.P.J.G.; Sloetjes, A.; Vogl, T.; Roth, J.; Berg, W.B.V.D.; Van Der Kraan, P.M.; Blom, A.B.; et al. Alarmins S100A8/S100A9 aggravate osteophyte formation in experimental osteoarthritis and predict osteophyte progression in early human symptomatic osteoarthritis. Ann. Rheum. Dis. 2014, 75, 218–225. [Google Scholar] [CrossRef]
- Gohar, F.; Anink, J.; Moncrieffe, H.; Van Suijlekom-Smit, L.W.; Prince, F.H.; van Rossum, M.A.; Dolman, K.M.; Hoppenreijs, E.P.; Cate, R.T.; Ursu, S.; et al. S100A12 Is Associated with Response to Therapy in Juvenile Idiopathic Arthritis. J. Rheumatol. 2018, 45, 547–554. [Google Scholar] [CrossRef]
- Holzinger, D.; Tenbrock, K.; Roth, J. Alarmins of the S100-Family in Juvenile Autoimmune and Auto-Inflammatory Diseases. Front. Immunol. 2019, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Hinze, C.H.; Foell, D.; Johnson, A.L.; Spalding, S.J.; Gottlieb, B.S.; Morris, P.W.; Kimura, Y.; Onel, K.; Li, S.C.; Grom, A.A.; et al. Serum S100A8/A9 and S100A12 Levels in Children with Polyarticular Forms of Juvenile Idiopathic Arthritis: Relationship to Maintenance of Clinically Inactive Disease During Anti–Tumor Necrosis Factor Therapy and Occurrence of Disease Flare After Discontinuation of Therapy. Arthritis Rheumatol. 2018, 71, 451–459. [Google Scholar] [CrossRef]
- Tomizawa, T.; Ito, H.; Murata, K.; Hashimoto, M.; Tanaka, M.; Murakami, K.; Nishitani, K.; Azukizawa, M.; Okahata, A.; Doi, K.; et al. Distinct biomarkers for different bones in osteoporosis with rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 174. [Google Scholar] [CrossRef]
- Li, D.; Zhang, R.; Zhu, W.; Xue, Y.; Zhang, Y.; Huang, Q.; Liu, M.; Liu, Y. S100A16 inhibits osteogenesis but stimulates adipogenesis. Mol. Biol. Rep. 2013, 40, 3465–3473. [Google Scholar] [CrossRef] [PubMed]
- Wilsmanntheis, D.; Wagenpfeil, J.; Holzinger, D.; Roth, J.; Koch, S.; Schnautz, S.; Bieber, T.; Wenzel, J. Among the S100 proteins, S100A12 is the most significant marker for psoriasis disease activity. J. Eur. Acad. Dermatol. Venereol. 2015, 30, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Duvetorp, A.; Söderman, J.; Assarsson, M.; Skarstedt, M.; Svensson, Å.; Seifert, O. Observational study on Swedish plaque psoriasis patients receiving narrowband-UVB treatment show decreased S100A8/A9 protein and gene expression levels in lesional psoriasis skin but no effect on S100A8/A9 protein levels in serum. PLoS ONE 2019, 14, e0213344. [Google Scholar] [CrossRef] [PubMed]
- Zibert, J.R.; Skov, L.; Thyssen, J.P.; Jacobsen, G.K.; Grigorian, M. Significance of the S100A4 Protein in Psoriasis. J. Investig. Dermatol. 2010, 130, 150–160. [Google Scholar] [CrossRef]
- Salem, S.A.M.; El-Khateeb, E.A.; Harvy, M.; Emam, H.M.E.-S.; Abdelaal, W.; El Nemr, R.; El-Hagry, O.O. Study of serum levels and skin expression of S100B protein in psoriasis. An. Bras. Dermatol. 2017, 92, 323–328. [Google Scholar] [CrossRef]
- Borsky, P.; Fiala, Z.; Andrys, C.; Beranek, M.; Hamakova, K.; Malkova, A.; Svadlakova, T.; Krejsek, J.; Palicka, V.; Borska, L.; et al. Alarmins HMGB1, IL-33, S100A7, and S100A12 in Psoriasis Vulgaris. Mediators Inflamm. 2020, 2020, 8465083. [Google Scholar] [CrossRef]
- Boteanu, R.M.; Suica, V.I.; Uyy, E.; Ivan, L.; Dima, S.O.; Popescu, I.; Simionescu, M.; Antohe, F. Alarmins in chronic noncommunicable diseases: Atherosclerosis, diabetes and cancer. J. Proteom. 2016, 153, 21–29. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Z.; Fang, A.; Jin, Q.; Fang, D.; Liu, Y.; Wu, J.; Tan, X.; Wei, Y.; Jiang, C.; et al. Macrophage Foam Cell-Targeting Immunization Attenuates Atherosclerosis. Front. Immunol. 2019, 9, 3127. [Google Scholar] [CrossRef]
- Otsuka, K.; Villiger, M.; Van Zandvoort, L.; Neleman, T.; Karanasos, A.; Dijkstra, J.; Nadkarni, S.; Regar, E.; Daemen, J.; Bouma, B. Fibrous cap composition in patients with acute or chronic coronary syndromes: Insights from intravascular polarimetry. J. Am. Coll. Cardiol. 2020, 75, 40. [Google Scholar] [CrossRef]
- Anlamlert, W.; Lenbury, Y.; Bell, J. Modeling fibrous cap formation in atherosclerotic plaque development: Stability and oscillatory behavior. Adv. Differ. Equ. 2017, 2017, 195. [Google Scholar] [CrossRef]
- Mani, A.J.; Edep, M.E.; Brown, D.L. Pathophysiology of Acute Coronary Syndromes: Plaque Rupture and Atherothrombosis. In Cardiac Intensive Care, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 73–86. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, C.; Qu, S.-L.; Shao, Y.-D.; Zhou, C.-Y.; Chao, R.; Huang, L.; Zhang, C. S100 proteins in atherosclerosis. Clin. Chim. Acta 2019, 502, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Bowman, M.A.H. S100A12 and the S100/Calgranulins: Emerging Biomarkers for Atherosclerosis and Possibly Therapeutic Targets. Arter. Thromb. Vasc. Biol. 2015, 35, 2496–2507. [Google Scholar] [CrossRef]
- Mumolo, M.G.; Bertani, L.; Ceccarelli, L.; Laino, G.; Di Fluri, G.; Albano, E.; Tapete, G.; Costa, F. From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World J. Gastroenterol. 2018, 24, 3681–3694. [Google Scholar] [CrossRef]
- Freeman, K.; Willis, B.; Fraser, H.; Taylor-Phillips, S.; Clarke, A. Faecal calprotectin to detect inflammatory bowel disease: A systematic review and exploratory meta-analysis of test accuracy. BMJ Open 2019, 9, e027428. [Google Scholar] [CrossRef] [PubMed]
- Suhail, A.; Rizvi, Z.A.; Mujagond, P.; Ali, S.A.; Gaur, P.; Singh, M.; Ahuja, V.; Awasthi, A.; Srikanth, C.V. DeSUMOylase SENP7-Mediated Epithelial Signaling Triggers Intestinal Inflammation via Expansion of Gamma-Delta T Cells. Cell Rep. 2019, 29, 3522–3538.e7. [Google Scholar] [CrossRef]
- Kim, D.K.; Wi, Y.C.; Shin, S.J.; Kim, K.R.; Kim, D.W.; Cho, S.H. Diverse phenotypes and endotypes of fungus balls caused by mixed bacterial colonization in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2019, 9, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Sumsion, J.S.; Pulsipher, A.; Alt, J.A. Differential expression and role of S100 proteins in chronic rhinosinusitis. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 14–22. [Google Scholar] [CrossRef]
- Pulsipher, A.; Davis, B.M.; Smith, K.A.; Ashby, S.; Qin, X.; Firpo, M.; Orlandi, R.R.; Alt, J.A. Calgranulin C (S100A12) Is Differentially Expressed in Subtypes of Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2018, 32, 380–387. [Google Scholar] [CrossRef]
- Boruk, M.; Railwah, C.; Lora, A.; Nath, S.; Wu, D.; Chow, L.; Borhanjoo, P.; Dabo, A.J.; Chowdhury, S.; Kaiser, R.; et al. Elevated S100A9 expression in chronic rhinosinusitis coincides with elevated MMP production and proliferation in vitro. Sci. Rep. 2020, 10, 16350. [Google Scholar] [CrossRef]
- Morelli, S.; Mandal, M.; Goldsmith, L.T.; Kashani, B.N.; Ponzio, N.M. The maternal immune system during pregnancy and its influence on fetal development. Res. Rep. Biol. 2015, 6, 171–189. [Google Scholar] [CrossRef]
- Aghaeepour, N.; Ganio, E.A.; Mcilwain, D.; Tsai, A.S.; Tingle, M.; Van Gassen, S.; Gaudilliere, D.K.; Baca, Q.; McNeil, L.; Okada, R.; et al. An immune clock of human pregnancy. Sci. Immunol. 2017, 2, eaan2946. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2011, 63, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Yang, W.; Wei, X.; Wu, K.; Huang, D. The Unique Microbiome and Innate Immunity during Pregnancy. Front. Immunol. 2019, 10, 2886. [Google Scholar] [CrossRef]
- Golubinskaya, V.; Puttonen, H.; Fyhr, I.-M.; Rydbeck, H.; Hellström, A.; Jacobsson, B.; Nilsson, H.; Mallard, C.; Sävman, K. Expression of S100A Alarmins in Cord Blood Monocytes Is Highly Associated with Chorioamnionitis and Fetal Inflammation in Preterm Infants. Front. Immunol. 2020, 11, 1194. [Google Scholar] [CrossRef]
- Sadigh, A.R.; Mihanfar, A.; Fattahi, A.; Latifi, Z.; Akbarzadeh, M.; Hajipour, H.; Bahrami-Asl, Z.; Ghasemzadeh, A.; Hamdi, K.; Nejabati, H.R.; et al. S100 protein family and embryo implantation. J. Cell. Biochem. 2019, 120, 19229–19244. [Google Scholar] [CrossRef]
- Bissonnette, L.; Drissennek, L.; Antoine, Y.; Tiers, L.; Hirtz, C.; Lehmann, S.; Perrochia, H.; Bissonnette, F.; Kadoch, I.-J.; Haouzi, D.; et al. Human S100A10 plays a crucial role in the acquisition of the endometrial receptivity phenotype. Cell Adhes. Migr. 2016, 10, 282–298. [Google Scholar] [CrossRef]
- Nair, R.R.; Khanna, A.; Singh, K. Association of Increased S100A8 Serum Protein with Early Pregnancy Loss. Am. J. Reprod. Immunol. 2014, 73, 91–94. [Google Scholar] [CrossRef]
- Nair, R.R.; Khanna, A.; Singh, K. Role of Inflammatory Proteins S100A8 and S100A9 in Pathophysiology of Recurrent Early Pregnancy Loss. Placenta 2013, 34, 824–827. [Google Scholar] [CrossRef]
- Verma, R.; Verma, P.; Budhwar, S.; Singh, K. S100 Proteins: An Emerging Cynosure in Pregnancy & Adverse Reproductive Outcome. J. Dent. Educ. 2018, 76, 100–106. [Google Scholar] [CrossRef]
- Li, X.; Cheng, F.; Cao, G. Expression of S100 Calcium-Binding Protein A8 in Peripheral Blood of Patients with Preeclampsia during Pregnancy. Eur. J. Inflamm. 2019, 17, 2058739219858527. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, P.; Ali, S.A. Multifunctional Role of S100 Protein Family in the Immune System: An Update. Cells 2022, 11, 2274. https://doi.org/10.3390/cells11152274
Singh P, Ali SA. Multifunctional Role of S100 Protein Family in the Immune System: An Update. Cells. 2022; 11(15):2274. https://doi.org/10.3390/cells11152274
Chicago/Turabian StyleSingh, Parul, and Syed Azmal Ali. 2022. "Multifunctional Role of S100 Protein Family in the Immune System: An Update" Cells 11, no. 15: 2274. https://doi.org/10.3390/cells11152274
APA StyleSingh, P., & Ali, S. A. (2022). Multifunctional Role of S100 Protein Family in the Immune System: An Update. Cells, 11(15), 2274. https://doi.org/10.3390/cells11152274