Raltegravir Inclusion Decreases CD4 T-Cells Intra-Cellular Viral Load and Increases CD4 and CD28 Positive T-Cells in Selected HIV Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Sample Processing, Cell Separation, and Cell Culture
2.3. Cell Stimulation with CD3/CD28
2.4. Monoclonal Antibodies
2.5. Multiparameter Flow Cytometry
2.6. Viral Load Analysis
2.7. Total Plasma and Cellular Concentration
2.8. Statistical Method
3. Results
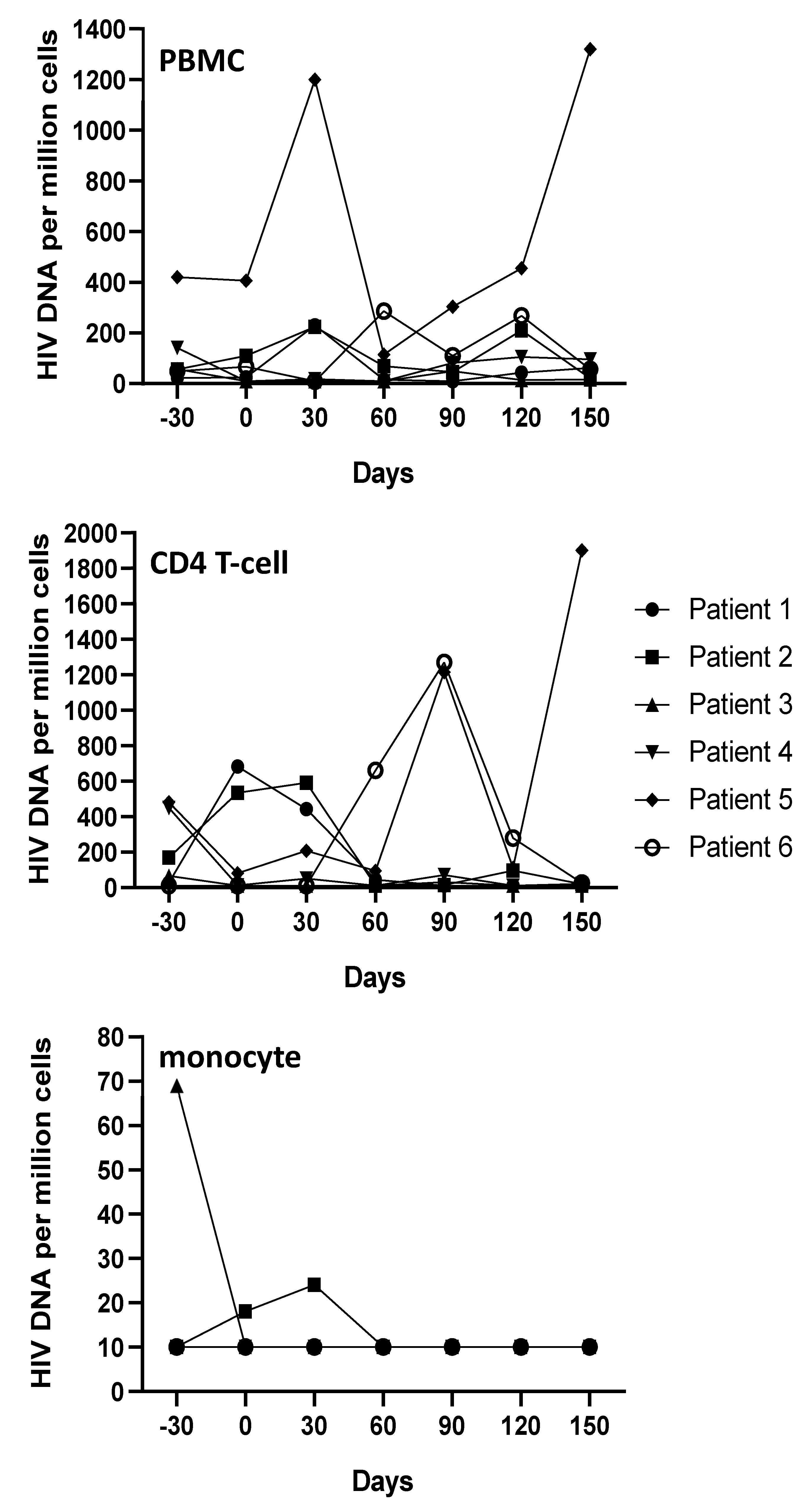
3.1. Plasmatic and Cellular Viral Load
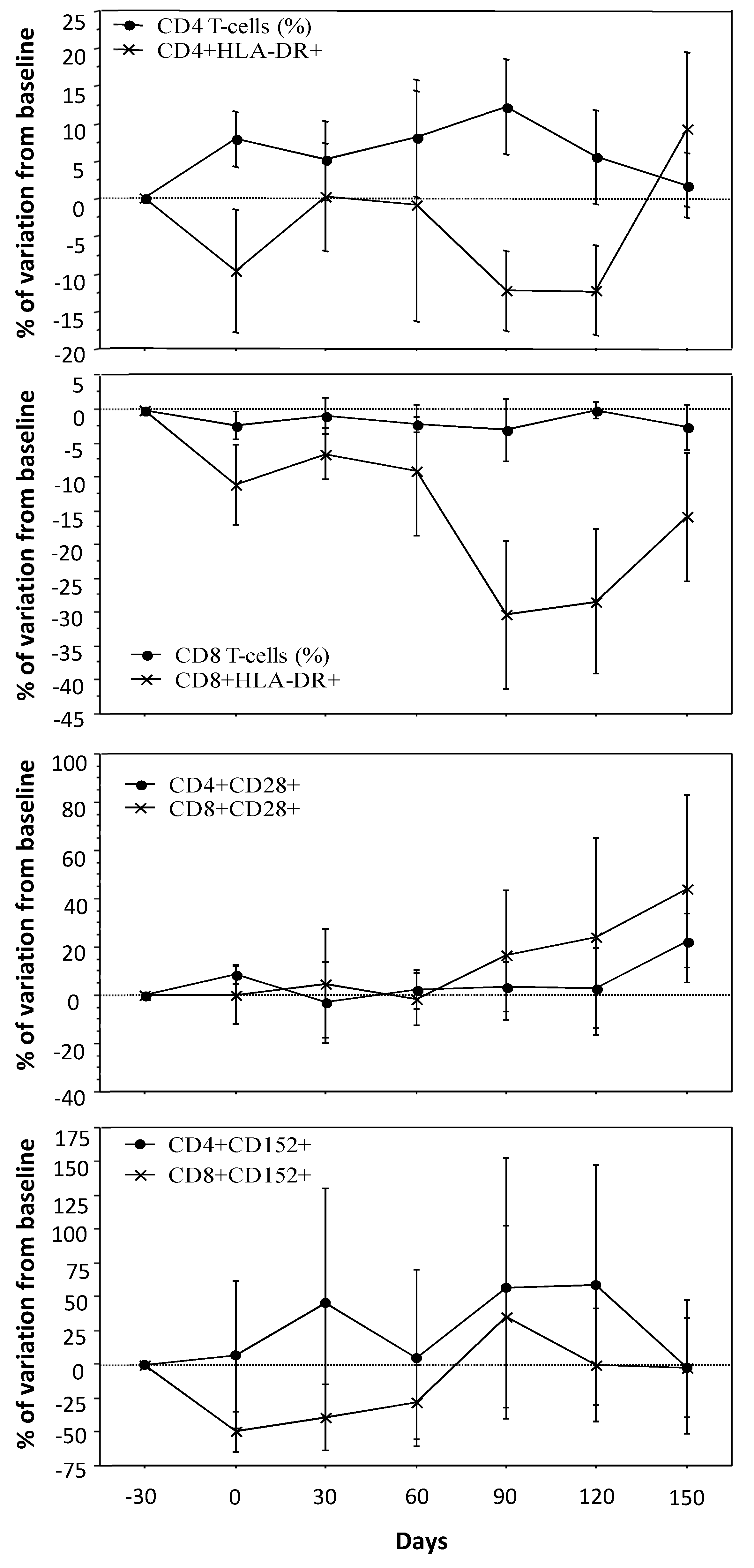
3.2. T-Cell Count, Activation and Costimulatory Molecules
3.3. Proliferation Versus Apoptosis
3.4. Monocyte Phenotype
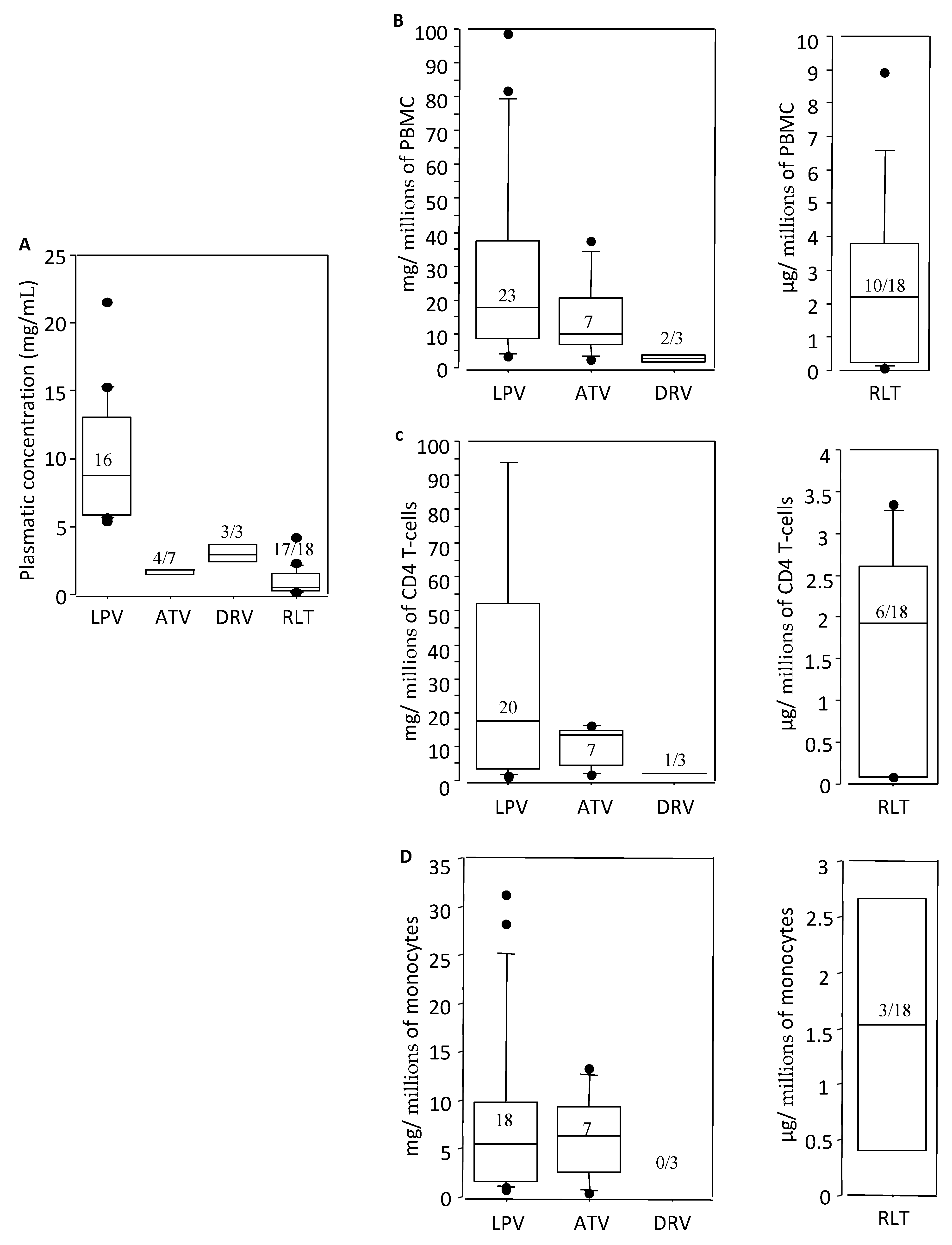
3.5. Intracellular Concentrations of Raltegravir
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hammer, S.M.; Saag, M.S.; Schechter, M.; Montaner, J.S.; Schooley, R.T.; Jacobsen, D.M.; Thompson, M.A.; Carpenter, C.C.; Fischl, M.A.; Gazzard, B.G.; et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA 2006, 296, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Wright, E.J.; Smith, D.M.; He, W.; Catano, G.; Okulicz, J.F.; Young, J.A.; Clark, R.A.; Richman, D.D.; Little, S.J.; et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N. Engl. J. Med. 2013, 368, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Sabin, C.A.; Lampe, F.C.; Kinloch-de-Loes, S.; Gumley, H.; Carroll, A.; Prinz, B.; Youle, M.; Johnson, M.A.; Phillips, A.N. The potential for CD4 cell increases in HIV-positive individuals who control viraemia with highly active antiretroviral therapy. AIDS 2003, 17, 963–969. [Google Scholar] [CrossRef]
- French, M.A.; King, M.S.; Tschampa, J.M.; da Silva, B.A.; Landay, A.L. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J. Infect. Dis. 2009, 200, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Cantero-Perez, J.; Grau-Exposito, J.; Serra-Peinado, C.; Rosero, D.A.; Luque-Ballesteros, L.; Astorga-Gamaza, A.; Castellvi, J.; Sanhueza, T.; Tapia, G.; Lloveras, B.; et al. Resident memory T cells are a cellular reservoir for HIV in the cervical mucosa. Nat. Commun. 2019, 10, 4739. [Google Scholar] [CrossRef]
- Cassol, E.; Alfano, M.; Biswas, P.; Poli, G. Monocyte-derived macrophages and myeloid cell lines as targets of HIV-1 replication and persistence. J. Leukoc. Biol. 2006, 80, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.W.; Finzi, D.; Margolick, J.; Chadwick, K.; Schwartz, D.; Siliciano, R.F. In vivo fate of HIV-1-infected T cells: Quantitative analysis of the transition to stable latency. Nat. Med. 1995, 1, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Lambotte, O.; Deiva, K.; Tardieu, M. HIV-1 persistence, viral reservoir, and the central nervous system in the HAART era. Brain Pathol. 2003, 13, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Finzi, D.; Hermankova, M.; Pierson, T.; Carruth, L.M.; Buck, C.; Chaisson, R.E.; Quinn, T.C.; Chadwick, K.; Margolick, J.; Brookmeyer, R.; et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997, 278, 1295–1300. [Google Scholar] [CrossRef]
- Pomerantz, R.J. Reservoirs of human immunodeficiency virus type 1: The main obstacles to viral eradication. Clin. Infect. Dis. 2002, 34, 91–97. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Siliciano, R.F. In Vivo Dynamics of the Latent Reservoir for HIV-1: New Insights and Implications for Cure. Annu Rev. Pathol. 2021. [Google Scholar] [CrossRef]
- Moar, P.; Sushmita, K.; Kateriya, S.; Tandon, R. Transcriptional profiling indicates cAMP-driven reversal of HIV latency in monocytes occurs via transcription factor SP-1. Virology 2020, 542, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Pakker, N.G.; Notermans, D.W.; de Boer, R.J.; Roos, M.T.; de Wolf, F.; Hill, A.; Leonard, J.M.; Danner, S.A.; Miedema, F.; Schellekens, P.T. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: A composite of redistribution and proliferation. Nat. Med. 1998, 4, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.F.; Price, P.; French, M.A. Dysregulation of CD28 and CTLA-4 expression by CD4 T cells from previously immunodeficient HIV-infected patients with sustained virological responses to highly active antiretroviral therapy. HIV Med. 2005, 6, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Kestens, L.; Vanham, G.; Vereecken, C.; Vandenbruaene, M.; Vercauteren, G.; Colebunders, R.L.; Gigase, P.L. Selective increase of activation antigens HLA-DR and CD38 on CD4 + CD45RO + T lymphocytes during HIV-1 infection. Clin. Exp. Immunol. 1994, 95, 436–441. [Google Scholar] [CrossRef]
- Leng, Q.; Bentwich, Z.; Magen, E.; Kalinkovich, A.; Borkow, G. CTLA-4 upregulation during HIV infection: Association with anergy and possible target for therapeutic intervention. AIDS 2002, 16, 519–529. [Google Scholar] [CrossRef]
- Pakker, N.G.; Kroon, E.D.; Roos, M.T.; Otto, S.A.; Hall, D.; Wit, F.W.; Hamann, D.; van der Ende, M.E.; Claessen, F.A.; Kauffmann, R.H.; et al. Immune restoration does not invariably occur following long-term HIV-1 suppression during antiretroviral therapy. INCAS Study Group. AIDS 1999, 13, 203–212. [Google Scholar] [CrossRef]
- Saukkonen, J.J.; Kornfeld, H.; Berman, J.S. Expansion of a CD8+ CD28-cell population in the blood and lung of HIV-positive patients. J. Acquir. Immune Defic. Syndr. 1993, 6, 1194–1204. [Google Scholar]
- Vingerhoets, J.H.; Vanham, G.L.; Kestens, L.L.; Penne, G.G.; Colebunders, R.L.; Vandenbruaene, M.J.; Goeman, J.; Gigase, P.L.; De Boer, M.; Ceuppens, J.L. Increased cytolytic T lymphocyte activity and decreased B7 responsiveness are associated with CD28 down-regulation on CD8+ T cells from HIV-infected subjects. Clin. Exp. Immunol. 1995, 100, 425–433. [Google Scholar] [CrossRef]
- Borthwick, N.J.; Bofill, M.; Gombert, W.M.; Akbar, A.N.; Medina, E.; Sagawa, K.; Lipman, M.C.; Johnson, M.A.; Janossy, G. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28- T cells. AIDS 1994, 8, 431–441. [Google Scholar] [CrossRef]
- Kammerer, R.; Iten, A.; Frei, P.C.; Burgisser, P. Expansion of T cells negative for CD28 expression in HIV infection. Relation to activation markers and cell adhesion molecules, and correlation with prognostic markers. Med. Microbiol. Immunol. 1996, 185, 19–25. [Google Scholar] [CrossRef]
- Gougeon, M.L.; Lecoeur, H.; Dulioust, A.; Enouf, M.G.; Crouvoiser, M.; Goujard, C.; Debord, T.; Montagnier, L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: Increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J. Immunol. 1996, 156, 3509–3520. [Google Scholar]
- Cavert, W.; Notermans, D.W.; Staskus, K.; Wietgrefe, S.W.; Zupancic, M.; Gebhard, K.; Henry, K.; Zhang, Z.Q.; Mills, R.; McDade, H.; et al. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science 1997, 276, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Demeter, L.M.; Bosch, R.J.; Coombs, R.W.; Fiscus, S.; Bremer, J.; Johnson, V.A.; Erice, A.; Jackson, J.B.; Spector, S.A.; Squires, K.M.; et al. Detection of replication-competent human immunodeficiency virus type 1 (HIV-1) in cultures from patients with levels of HIV-1 RNA in plasma suppressed to less than 500 or 50 copies per milliliter. J. Clin. Microbiol. 2002, 40, 2089–2094. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharkey, M.E.; Teo, I.; Greenough, T.; Sharova, N.; Luzuriaga, K.; Sullivan, J.L.; Bucy, R.P.; Kostrikis, L.G.; Haase, A.; Veryard, C.; et al. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat. Med. 2000, 6, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Eron, J.J.; Young, B.; Cooper, D.A.; Youle, M.; Dejesus, E.; Andrade-Villanueva, J.; Workman, C.; Zajdenverg, R.; Fatkenheuer, G.; Berger, D.S.; et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): Two multicentre, double-blind, randomised controlled trials. Lancet 2010, 375, 396–407. [Google Scholar] [CrossRef]
- Hicks, C.; Gulick, R.M. Raltegravir: The first HIV type 1 integrase inhibitor. Clin. Infect. Dis. 2009, 48, 931–939. [Google Scholar] [CrossRef]
- Lennox, J.L.; DeJesus, E.; Lazzarin, A.; Pollard, R.B.; Madruga, J.V.; Berger, D.S.; Zhao, J.; Xu, X.; Williams-Diaz, A.; Rodgers, A.J.; et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: A multicentre, double-blind randomised controlled trial. Lancet 2009, 374, 796–806. [Google Scholar] [CrossRef]
- Massanella, M.; Negredo, E.; Puig, J.; Puertas, M.C.; Buzon, M.J.; Perez-Alvarez, N.; Carrillo, J.; Clotet, B.; Martinez-Picado, J.; Blanco, J. Raltegravir intensification shows differing effects on CD8 and CD4 T cells in HIV-infected HAART-suppressed individuals with poor CD4 T-cell recovery. AIDS 2012, 26, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Santinelli, L.; Ceccarelli, G.; Borrazzo, C.; Celani, L.; Pavone, P.; Innocenti, G.P.; Spagnolello, O.; Fimiani, C.; Ceci, F.; Di Sora, F.; et al. Real word outcomes associated with use of raltegravir in older people living with HIV: Results from the 60 months follow-up of the RAL-age cohort. Expert Rev. Anti Infect. Ther. 2020, 18, 485–492. [Google Scholar] [CrossRef]
- Badley, A.D.; McElhinny, J.A.; Leibson, P.J.; Lynch, D.H.; Alderson, M.R.; Paya, C.V. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J. Virol. 1996, 70, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.H.; Tudor-Williams, G.; Banda, N.K.; Cotton, M.F.; Curiel, T.; Monks, C.; Baba, T.W.; Ruprecht, R.M.; Kupfer, A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1995, 1, 129–134. [Google Scholar] [CrossRef]
- Garaci, E.; Aquaro, S.; Lapenta, C.; Amendola, A.; Spada, M.; Covaceuszach, S.; Perno, C.F.; Belardelli, F. Anti-nerve growth factor Ab abrogates macrophage-mediated HIV-1 infection and depletion of CD4+ T lymphocytes in hu-SCID mice. Proc. Natl. Acad. Sci. USA 2003, 100, 8927–8932. [Google Scholar] [CrossRef]
- Roger, P.M.; Breittmayer, J.P.; Durant, J.; Sanderson, F.; Ceppi, C.; Brignone, C.; Cua, E.; Clevenbergh, P.; Fuzibet, J.G.; Pesce, A.; et al. Early CD4(+) T cell recovery in human immunodeficiency virus-infected patients receiving effective therapy is related to a down-regulation of apoptosis and not to proliferation. J. Infect. Dis. 2002, 185, 463–470. [Google Scholar] [CrossRef]
- Koelsch, K.K.; Boesecke, C.; McBride, K.; Gelgor, L.; Fahey, P.; Natarajan, V.; Baker, D.; Bloch, M.; Murray, J.M.; Zaunders, J.; et al. Impact of treatment with raltegravir during primary or chronic HIV infection on RNA decay characteristics and the HIV viral reservoir. AIDS 2011, 25, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, A.; Gutierrez, C.; Hernandez-Novoa, B.; Diaz, L.; Madrid, N.; Abad-Fernandez, M.; Dronda, F.; Perez-Elias, M.J.; Zamora, J.; Munoz, E.; et al. The effect of intensification with raltegravir on the HIV-1 reservoir of latently infected memory CD4 T cells in suppressed patients. AIDS 2012, 26, 1885–1894. [Google Scholar] [CrossRef]
- Delaugerre, C.; Charreau, I.; Braun, J.; Nere, M.L.; de Castro, N.; Yeni, P.; Ghosn, J.; Aboulker, J.P.; Molina, J.M.; Simon, F.; et al. Time course of total HIV-1 DNA and 2-long-terminal repeat circles in patients with controlled plasma viremia switching to a raltegravir-containing regimen. AIDS 2010, 24, 2391–2395. [Google Scholar] [CrossRef]
- Hatano, H.; Hayes, T.L.; Dahl, V.; Sinclair, E.; Lee, T.H.; Hoh, R.; Lampiris, H.; Hunt, P.W.; Palmer, S.; McCune, J.M.; et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J. Infect. Dis. 2011, 203, 960–968. [Google Scholar] [CrossRef]
- Lam, Y.M.; McBride, K.L.; Amin, J.; Cordery, D.V.; Kelleher, A.D.; Cooper, D.A.; Koelsch, K.K. Switching virally suppressed, treatment-experienced patients to a raltegravir-containing regimen does not alter levels of HIV-1 DNA. PLoS ONE 2012, 7, e31990. [Google Scholar] [CrossRef] [PubMed]
- Buzon, M.J.; Massanella, M.; Llibre, J.M.; Esteve, A.; Dahl, V.; Puertas, M.C.; Gatell, J.M.; Domingo, P.; Paredes, R.; Sharkey, M.; et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat. Med. 2010, 16, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Catalfamo, M.; Di Mascio, M.; Hu, Z.; Srinivasula, S.; Thaker, V.; Adelsberger, J.; Rupert, A.; Baseler, M.; Tagaya, Y.; Roby, G.; et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc. Natl. Acad. Sci. USA 2008, 105, 19851–19856. [Google Scholar] [CrossRef] [PubMed]
- Rockstroh, J.K.; Teppler, H.; Zhao, J.; Sklar, P.; Miller, M.D.; Harvey, C.M.; Strohmaier, K.M.; Leavitt, R.Y.; Nguyen, B.Y. Clinical efficacy of raltegravir against B and non-B subtype HIV-1 in phase III clinical studies. AIDS 2011, 25, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Chege, D.; Kovacs, C.; la Porte, C.; Ostrowski, M.; Raboud, J.; Su, D.; Kandel, G.; Brunetta, J.; Kim, C.J.; Sheth, P.M.; et al. Effect of raltegravir intensification on HIV proviral DNA in the blood and gut mucosa of men on long-term therapy: A randomized controlled trial. AIDS 2012, 26, 167–174. [Google Scholar] [CrossRef]
- Molto, J.; Valle, M.; Back, D.; Cedeno, S.; Watson, V.; Liptrott, N.; Egan, D.; Miranda, C.; Barbanoj, M.J.; Clotet, B. Plasma and intracellular (peripheral blood mononuclear cells) pharmacokinetics of once-daily raltegravir (800 milligrams) in HIV-infected patients. Antimicrob. Agents Chemother. 2011, 55, 72–75. [Google Scholar] [CrossRef][Green Version]
- Fayet Mello, A.; Buclin, T.; Franc, C.; Colombo, S.; Cruchon, S.; Guignard, N.; Biollaz, J.; Telenti, A.; Decosterd, L.A.; Cavassini, M. Cell disposition of raltegravir and newer antiretrovirals in HIV-infected patients: High inter-individual variability in raltegravir cellular penetration. J. Antimicrob. Chemother. 2011, 66, 1573–1581. [Google Scholar] [CrossRef][Green Version]
- Jackson, A.; Watson, V.; Back, D.; Khoo, S.; Liptrott, N.; Egan, D.; Gedela, K.; Higgs, C.; Abbas, R.; Gazzard, B.; et al. Plasma and intracellular pharmacokinetics of darunavir/ritonavir once daily and raltegravir once and twice daily in HIV-infected individuals. J. Acquir. Immune Defic. Syndr. 2011, 58, 450–457. [Google Scholar] [CrossRef]
- Asahchop, E.L.; Meziane, O.; Mamik, M.K.; Chan, W.F.; Branton, W.G.; Resch, L.; Gill, M.J.; Haddad, E.; Guimond, J.V.; Wainberg, M.A.; et al. Reduced antiretroviral drug efficacy and concentration in HIV-infected microglia contributes to viral persistence in brain. Retrovirology 2017, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.H.; Hearps, A.C.; Martin, G.E.; Williams, K.C.; Crowe, S.M. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS 2014, 28, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, F.; Pollicita, M.; Ceccherini-Silberstein, F.; Di Santo, F.; Surdo, M.; Aquaro, S.; Perno, C.F. Comparative antiviral activity of integrase inhibitors in human monocyte-derived macrophages and lymphocytes. Antivir. Res. 2011, 92, 255–261. [Google Scholar] [CrossRef]

| Patient No. | Sex | Age | HIV Follow-Up (Months) | ARV Duration (Months) | Antiretroviral Regimen | CD4 Nadir | CD4 Max. |
|---|---|---|---|---|---|---|---|
| 1 | M | 50 | 18 | 12 | LO + FTC + TDF | 357 | 675 |
| 2 | M | 34 | 17 | 10 | LO + FTC + TDF | 252 | 576 |
| 3 | M | 31 | 15 | 12 | LO + FTC + TDF | 221 | 897 |
| 4 | M | 38 | 34 | 7 | AT + FTC + TDF | 235 | 528 |
| 5 | F | 65 | 13 | 12 | LO + FTC + TDF | 205 | 672 |
| 6 | M | 34 | 26 | 8 | DA + FTC + TDF | 188 | 501 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, G.; Cottalorda-Dufayard, J.; Garraffo, R.; De Salvador-Guillouët, F.; Cua, E.; Roger, P.-M. Raltegravir Inclusion Decreases CD4 T-Cells Intra-Cellular Viral Load and Increases CD4 and CD28 Positive T-Cells in Selected HIV Patients. Cells 2022, 11, 208. https://doi.org/10.3390/cells11020208
Kumar G, Cottalorda-Dufayard J, Garraffo R, De Salvador-Guillouët F, Cua E, Roger P-M. Raltegravir Inclusion Decreases CD4 T-Cells Intra-Cellular Viral Load and Increases CD4 and CD28 Positive T-Cells in Selected HIV Patients. Cells. 2022; 11(2):208. https://doi.org/10.3390/cells11020208
Chicago/Turabian StyleKumar, Gaurav, Jacqueline Cottalorda-Dufayard, Rodolphe Garraffo, Francine De Salvador-Guillouët, Eric Cua, and Pierre-Marie Roger. 2022. "Raltegravir Inclusion Decreases CD4 T-Cells Intra-Cellular Viral Load and Increases CD4 and CD28 Positive T-Cells in Selected HIV Patients" Cells 11, no. 2: 208. https://doi.org/10.3390/cells11020208
APA StyleKumar, G., Cottalorda-Dufayard, J., Garraffo, R., De Salvador-Guillouët, F., Cua, E., & Roger, P.-M. (2022). Raltegravir Inclusion Decreases CD4 T-Cells Intra-Cellular Viral Load and Increases CD4 and CD28 Positive T-Cells in Selected HIV Patients. Cells, 11(2), 208. https://doi.org/10.3390/cells11020208






