Abstract
Introduction: An early and accurate diagnosis of early onset neonatal sepsis (EONS) and late onset neonatal sepsis (LONS) is essential to improve the outcome of this devastating conditions. Especially, preterm infants are at risk. Reliable biomarkers are rare, clinical decision-making depends on clinical appearance and multiple laboratory findings. Markers of NET formation and NET turnover might improve diagnostic precision. Aim of this study was to evaluate the diagnostic value of NETs in sepsis diagnosis in neonatal preterm infants. Methods: Plasma samples of neonatal preterm infants with suspected sepsis were collected. Blood samples were assayed for markers of NET formation and NET turnover: cfDNA, DNase1, nucleosome, NE, and H3Cit. All clinical findings, values of laboratory markers, and epidemiological characteristics were collected retrospectively. Two subpopulations were created to divide EONS from LONS. EMA sepsis criteria for neonatal sepsis were used to generate a sepsis group (EMA positive) and a control group (EMA negative). Results: A total of 31 preterm neonates with suspected sepsis were included. Out of these, nine patients met the criteria for sepsis according to EMA. Regarding early onset neonatal sepsis (3 EONS vs. 10 controls), cfDNA, DNase I, nucleosome, and CRP were elevated significantly. H3Cit and NE did not show any significant elevations. In the late onset sepsis collective (6 LONS vs. 12 controls), cfDNA, DNase I, and CRP differed significantly compared to control group.
1. Introduction
Neonatal sepsis remains a life-threatening medical condition in the postnatal period [1] with an incidence of 2.2/1000 live births in middle- and high-income countries [2]. Neonatal sepsis is subdivided into two entities [3]. One is early onset neonatal sepsis (EONS), which occurs within the first 72 h after birth and usually results from acquisition of maternal microorganisms. The case fatality rate is around 16%, however, in preterm neonates born at 22–24 weeks of gestational age, mortality rate is 54% [1]. In preterm infants, infections with Escherichia coli dominate the microbiological spectrum with 81% [1]. The second entity is late onset neonatal sepsis (LONS), which occurs later than 72 h after birth, and is caused by microorganisms from the environment [3]. Vascular-access catheters, mechanical ventilation and other nosocomial interventions, which are more commonly required during intensive care of preterm infants, are risk factors for LONS [1]. In both entities, empirical antibiotic treatment should be started immediately to improve prognosis, without waiting for pathogen detection and identification [1]. Overall mortality rate of LONS is around 13% and increases with low birth weight and prematurity [4].
Symptoms of neonatal sepsis are non-specific and therefore vital parameters and symptoms of infection must be observed thoroughly to identify sepsis as soon as possible [5]. Established laboratory markers, such as C-reactive protein (CRP), must be considered for diagnosis finding and therapeutical decision-making. European Medical Agency (EMA) developed a scoring system to find an operational sepsis definition in 2010 [6]. Detailed information about the clinical and laboratory parameters included in the EMA sepsis scoring system can be found in Table 1. Nevertheless, there is still a lack of sensitive and reliable biomarkers for sepsis diagnosis, especially in neonates [5,7].

Table 1.
EMA sepsis scoring system, adapted from EMA publication (2010) and Odabasi and Bulbul (2020) [6,8]. The presence of at least two clinical signs and at least two laboratory findings from the table are considered as clinical sepsis in the context of a suspected or proven infection. This scoring system is suitable for EONS and LONS and is applicable up to 44 weeks of age.
In the first days of life, the immune system is faced with a broad spectrum of microbes and pathogens for the first time. As a crucial part of the innate immune system especially in neonates, neutrophils represent the first defense line against them [1,9]. This subpopulation of leucocytes can attack and immobilize pathogens by releasing neutrophil extracellular traps (NETs) [9,10]. NETs consist of DNA filaments, citrullinated histones, and proteolytic enzymes, such as neutrophil elastase (NE) [11]. Degradation of NETs by deoxyribonucleases (DNases) ensures homeostasis of the immune response [10]. The cytokine storm during septic shock may lead to an imbalance of NET formation and NET degradation, resulting in complications like thrombotic events and tissue damage [12,13,14]. Elevated markers of NET formation are described in the context of sepsis and other inflammatory diseases [13]. Aim of this study was to evaluate the value of markers of NET formation and turnover for sepsis diagnosis in a collective of preterm neonates.
2. Materials and Methods
2.1. Study Design
Leftovers of blood samples for diagnostic purposes from neonatal patients were collected prospectively at the neonatal intensive care unit, Altona Children’s Hospital, Hamburg, Germany from May 2020 to August 2021. A parent’s informed written consent was obtained prior to blood collection. This trial meets the guidelines of the medical research ethics committee of Hamburg (Ethik-Kommission der Ärztekammer Hamburg, PV4991) and the Helsinki Declaration of 1964. The study was preregistered at ClinicalTrials.gov (NCT02567305). All clinical data were collected retrospectively and anonymously by two independent investigators.
According to WHO definition for preterm infants [15], only neonates with a gestational age of less than 37 completed weeks were included. All neonates with suspected sepsis were eligible for participation independent from other secondary diagnosis. However, only blood samples collected before antibiotic therapy were included. The study cohort was divided into two groups based on the age at time of sample collection; 3 days of age was defined as a cut-off value to differentiate EONS and LONS [3]. To evaluate the occurrence of clinical sepsis, European Medical Agency (EMA) sepsis scoring system [6] was used. A positive EMA sepsis score (at least two positive clinical and two positive laboratory categories, and a suspected or proven infection) were needed to assign a patient to the sepsis group. All other individuals were defined as control group. A flow diagram for recruitment and subdivision of the study cohort can be found in Figure 1.
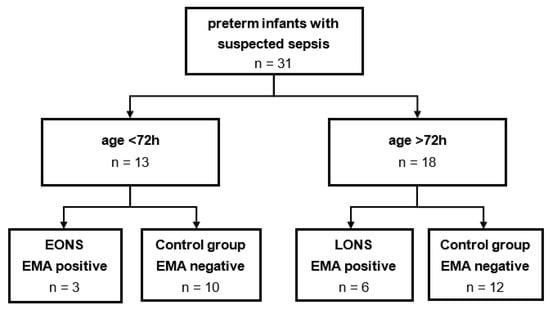
Figure 1.
Flow diagram for study cohort subdivision. A total of 31 samples of preterm infants with suspected sepsis were collected. After the division by age at blood collection, remaining study cohorts were analyzed retrospectively for the occurrence of clinical sepsis according to EMA sepsis scoring system.
Plasma samples were assayed for markers of NET formation and NET degradation, including circulating free deoxyribonucleic acid (cfDNA), nucleosome, deoxyribonuclease I (DNase I), citrullinated Histone 3 (H3Cit), and neutrophil elastase (NE).
2.2. Sample Preparation
Immediately after sample collection, the collected EDTA blood leftovers were centrifuged (2000rcf, 21 °C, 10 min), the resulting plasma was aliquoted and stored at −80 °C until assays were performed.
2.3. Circulating Free Deoxyribonucleic Acid (cfDNA)
Concentration of cfDNA was measured with an established fluorescence-based assay using Sytox Orange Nucleic Acid Stain (Invitrogen, Eugene, OR, USA), as described previously [16]. Briefly, a DNA standard curve (range 0–2000 ng/mL) was generated by serial dilution and placed on a 96-well microtiter plate; 50 µL of each plasma sample (dilution 1:20) were placed four times on the plate. Two wells each were incubated with a Sytox Orange solution. As a blank value, the other two wells were treated with a dilution buffer (0.1% BSA, 2mM EDTA in PBS) only. Fluorescence measurement (Ex: 544 nm, Em: 570 nm) was carried out after 5 min. Results are expressed in ng/mL.
2.4. Nucleosome
Plasma nucleosome level was assayed using a modified Cell Death Detection ELISA kit (Roche, Rotkreuz, Switzerland). In order to obtain a comparable standard curve, the included positive control was diluted 1:5, 1:10, 1:20, 1:40, 1:80, 1:160, 1:320 and 1:640. All other steps were carried out according to the manufacturer’s protocol. Optical density values were interpolated generating a four-parameter logistic (4-PL) curve-fit. Results are expressed in relative units on a nondimensional scale (0–1000).
2.5. Deoxyribonuclease I (DNase I) ELISA
DNase I, which plays a crucial role in NET turnover [10], was assayed using the Human DNase I ELISA Kit (MyBioSource, San Diego, CA, USA). Producer’s instructions were followed and results are expressed in ng/mL.
2.6. Neutrophile Elastase (NE) ELISA
Neutrophil Elastase, a marker for NETosis, was measured using the Human Polymorphonuclear (PMN)-Elastase ELISA Kit (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). The manufacturer’s protocol was followed, and results are expressed in ng/mL.
2.7. Citrullinated Histone 3 (H3Cit) ELISA
The concentration of H3Cit, which is an integral component of NETs [10], was assayed using the Citrullinated Histone H3 (Clone 11D3) ELISA Kit (Cayman Chemicals, Ann Arbor, MI, USA). All manufacturer’s instructions were followed.
2.8. Statistics
All data were analyzed using SPSS Statistics 26 (IBM, Armonk, NY, USA) and GraphPad Prism 9 (GraphPad, San Diego, CA, USA). As this was a pilot study, power estimation was deduced from previous trials regarding sepsis in neonates [17]. Differences between groups were calculated using Mann–Whitney tests. The data were illustrated with violin plots including markers for mean and interquartile range. The level of significance was set at 0.05. Tables of clinical characteristics were calculated using Mann–Whitney tests and Fisher’s exact tests. Level of significance was set at 0.05.
3. Results
A total of 31 neonates with suspected sepsis were included. Of these, nine patients met the criteria for sepsis according to EMA. The study collective was subdivided into the two entities of EONS and LONS. Clinical characteristics of the two study cohorts are tabularized in Table 2 for EONS and Table 3 for LONS, respectively. In both study groups, personal characteristics like age, gender, and gestational age did not differ significantly. Mortality rate was only significantly elevated in EONS group (p = 0.038). Only one patient from LONS group had a proven infection (via microbiological culture of a peripheral blood sample). Interestingly, we found no significant differences in leucocyte count in both entities, but a significant thrombocytopenia (EONS: p = 0.012; LONS: p = 0.006). Regarding blood gas analysis, lactate was elevated in EONS patients (p = 0.023). EONS individuals showed a significantly elevated rate of hyperglycemia (p = 0.018). Individual clinical information and scoring results of all EMA positive participants can be found in Table 4.

Table 2.
Early onset neonatal sepsis (EONS) group compared to control group. Clinical characteristics compared between the EONS group and control group. p-values for gender, mortality, and positive blood culture were obtained by Fisher’s exact tests. Data are expressed as a percentage (%). For all other categories Mann–Whitney tests were used. These data are expressed as mean (SD). Significance was set at 0.05. n.s.—not significant.

Table 3.
Late onset neonatal sepsis (LONS) group compared to control group. Clinical characteristics compared between the LONS group and control group. p-values for gender, mortality, and positive blood culture were obtained by Fisher’s exact tests. Data are expressed as a percentage (%). For all other categories, Mann–Whitney tests were used. These data are expressed as mean (SD). n.s.—not significant.

Table 4.
Detailed clinical information about all EMA positive participants (n = 9). All positive findings are marked (x). Sum of all positive items for clinical and laboratory findings were calculated. At least two clinical and two laboratory findings were needed to fulfill sepsis definition (EMA positive) [6].
As shown in Figure 2, markers of neutrophil extracellular traps formation (cfDNA) and NET degradation (nucleosome and DNase I) were significantly elevated in EONS; as well as CRP which is one of the possible laboratory signs of sepsis according to the EMA criteria. However, NE, which is a marker of neutrophil activation, and the surrogate marker of NET formation H3Cit, did not show any significant differences.
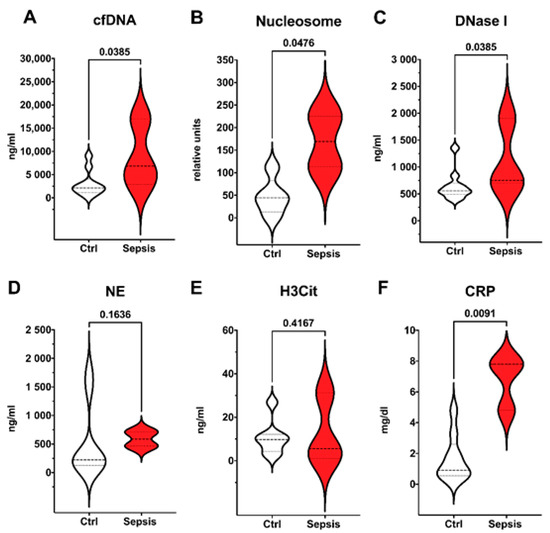
Figure 2.
Early onset neonatal sepsis (EONS) in preterm infants. Preterm infants meeting EMA sepsis criteria and definition of EONS (red, n = 3) compared to control group (white, n = 10). Plasma levels of surrogate parameters of NET formation and degradation (A–E) and CRP (F) are shown. Significant differences between preterm infants were found for cfDNA (A); Nucleosome (B); DNase I (C), and CRP (F). Data are visualized with violin plots including markers for median and interquartile range. Mann–Whitney tests were used. Significance was set at 0.05.
In premature born neonates with LONS, cfDNA, and DNase I, as well as CRP were significantly elevated compared to controls as shown in Figure 3. No differences were found for nucleosome, NE, and H3Cit.
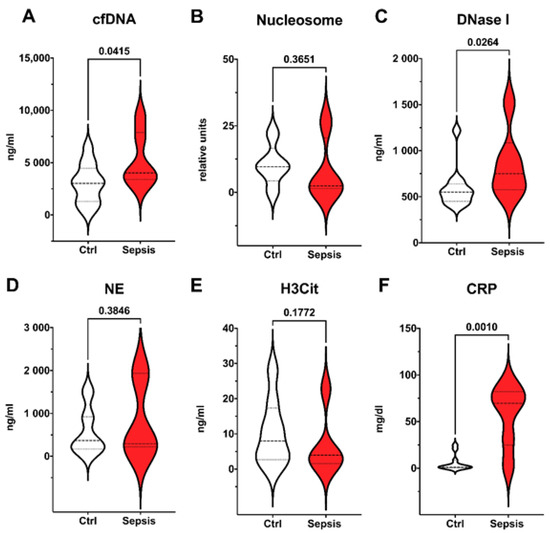
Figure 3.
Late onset neonatal sepsis (LONS) in preterm infants. Preterm infants meeting EMA sepsis criteria and definition of LONS (red, n = 6) compared to control group (white, n = 12). Plasma levels of surrogate parameters of NETosis and NET degradation (A–E) and CRP (F) are shown. Significant differences were found for cfDNA (A); DNase I (C), and CRP (F). Data are visualized with violin plots including markers for median and interquartile range. Mann–Whitney tests were used. Significance was set at 0.05.
4. Discussion
Early diagnosis and adequate treatment of sepsis in preterm infants remains a challenge in neonatal intensive care. Established biomarkers for neonatal sepsis are helpful for clinical decision-making but have limitations in terms of their reliability and predictive power [18]. Hence, complex scoring systems, like the sepsis criteria for neonates developed by the Pediatric Committee (PDCO) of the EMA used in this study [6], which contains clinical symptoms and laboratory signs, are necessary for diagnosis. Elevated levels of NET-associated markers have been found in septic adult patients [16], and especially cfDNA has been discussed as a biomarker for posttraumatic sepsis [19]. The current study suggests that markers of NET formation and degradation may also help to diagnose sepsis in preterm infants.
In the present cohort of preterm neonates with EONS, blood levels of cfDNA, nucleosome, and DNase1 were elevated but no difference for the surrogate markers H3Cit and NE were found. These findings must be interpreted with caution, because of the low sample size included in the study. In a previous study, NET markers in umbilical cord blood drawn immediately after birth were unable to predict future EONS in neonates [17]. The main reason may be that prenatal NET formation is limited by the neonatal NET inhibitory factor (nNIF), which can be found in cord blood samples of neonates [20]. In neonates, nNIF seems to block pivotal steps of NET formation, such as PAD4 activity or nuclear decondensation [20]. It is assumed that this mechanism may contribute to the limited ability of very young neonates to kill bacteria, rendering them more susceptible to sepsis during the first days of life [21]. Presumably, perinatal NET formation is regulated tightly to prevent negative repercussions during perinatal adaptation, such as hyperinflammation, NET-mediated vascular injury, and thrombosis [20]. It has been reported that the effects of nNIF subside rapidly after delivery [20] and it appears that NET markers may be used to diagnose early onset sepsis in preterm neonates. However, further investigations are needed to understand the clinical relevance of NET inhibition in the first days of life of preterm infants.
In neonates with LONS, also cfDNA, DNase1, and CRP were significantly elevated, but no differences were found for NE, nucleosome and H3Cit. It stands out, that for both entities of sepsis, cfDNA and DNase1 were elevated significantly. The combinations of these two markers cover the whole process of NETosis and NET degradation [22,23].
Circulating free DNA is released into peripheral blood during apoptosis, necrosis, and NET formation [24,25]. CfDNA appears particularly interesting as a diagnostic biomarker because healthy individuals show minimal levels only [26,27]. High levels of cfDNA have been suggested as a predictor of mortality in adult sepsis patients [28] DNase1 regulates NETs by degradation of DNA fragments [29]. An elevated enzyme activity indicates an increased degradation of cfDNA, before cfDNA level effectively rises [22]. This effect might be an early marker for the beginning of immune-mediated dysregulation during sepsis. Interestingly, we found elevated plasma levels of DNase1 in EONS as well as in LONS group. Combined with the finding of elevated levels of cfDNA in both groups, it is possible that we found first hints for a significant influence of NETosis in sepsis evolvement in preterm infants. These impressions should be evaluated in future studies because other pathophysiological explanations are still conceivable. CfDNA and its counterpart DNase I are involved in several immunological mechanisms, like necrosis [25]. Furthermore, it would be interesting to examine the enzyme activity of DNAse1 in combination with DNase1 level to discover the protein regulation during septic conditions in preterm infants.
As this was a pilot study with a limited number of subjects, some limitations apply. Sample size allows only a careful analysis of the role of NETs in EONS and LONS in preterm infants. Surrogate markers of NET formation like H3Cit [30] or NE [23] did not differ between sepsis and non-sepsis.
EMA negative control group might still include sepsis patients, because EMA sepsis scoring system is only an approximation for a reliable sepsis score. However, it is also possible that other conditions like respiratory disorders resulted in elevated levels of the NET markers in the non-sepsis group. This might be a significant confounding factors since various respiratory syndromes have been associated with NETs in neonates [31]. A control group with healthy individuals only would be desirable, but hard to realize in the context of a clinical study. Recruitment on an intensive care unit is not ideal, because all patients have several medical conditions. Ethically, blood sampling in preterm neonates should be reduced to a minimum. As we used leftovers from diagnostic procedures to meet these ethnical claims, we were faced with a longer period from collection to storage at −80 °C, which might affect the quality of blood samples. To minimize this effect, we followed a strict protocol for all blood samples to make all samples comparable.
In conclusion, surrogate markers of NET formation and degradation, especially cfDNA and DNase appear to be potential biomarkers for EONS and LONS in preterm infants. Further studies with large cohorts of preterm infants are needed to examine the real diagnostic potential of NETs. Ultimately, a combination of markers for NET formation and degradation might be promising to identify immune dysregulation in children with sepsis.
5. Conclusions
CfDNA and DNase appear to be potential biomarkers for diagnosis of early and late onset neonatal sepsis in preterm infants. Further studies should validate the diagnostic value of NET-associated markers in larger study collectives.
Author Contributions
Conceptualization, M.B.; methodology, J.E., M.B.; software, J.E.; validation, M.L., K.R.; formal analysis, J.E., M.B.; investigation, M.L., T.M., L.A., A.K.; resources, K.R., M.B.; data curation, M.L., J.E.; writing—original draft preparation, M.L., M.B.; writing—review and editing, L.A., A.K., A.V.d.W.; visualization, M.L..; supervision, J.E., M.B.; project administration, J.E., M.B.; funding acquisition, K.R., M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Medical Chamber of Hamburg (Ethikkommission der Ärztekammer Hamburg–PV4991).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is available upon request.
Conflicts of Interest
The authors declare no conflict of interest. The authors have no financial relationships relevant to this article to disclose.
References
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef]
- Fleischmann-Struzek, C.; Goldfarb, D.M.; Schlattmann, P.; Schlapbach, L.J.; Reinhart, K.; Kissoon, N. The global burden of paediatric and neonatal sepsis: A systematic review. Lancet Respir. Med. 2018, 6, 223–230. [Google Scholar] [CrossRef]
- Procianoy, R.S.; Silveira, R.C. The challenges of neonatal sepsis management. J. Pediatr. 2020, 96, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-H.; Hsu, J.-F.; Chu, S.-M.; Lien, R.; Huang, H.-R.; Chiang, M.-C.; Fu, R.-H.; Lee, C.-W.; Huang, Y.-C. Incidence, Clinical Characteristics and Risk Factors for Adverse Outcome in Neonates with Late-onset Sepsis. Pediatr. Infect. Dis. J. 2014, 33, e7–e13. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, K.A.; Anderson-Berry, A.L.; Delair, S.F.; Dele Davies, H. Early-Onset Neonatal Sepsis. Clin. Microbiol. Rev. 2014, 27, 21–47. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency (EMA). Report on the Expert Meeting on Neonatal and Paediatric Sepsis London; European Medicines Agency (EMA): Amsterdam, The Netherlands, 2010.
- Wynn, J.L. Defining neonatal sepsis. Curr. Opin. Pediatr. 2016, 28, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odabasi, I.O.; Bulbul, A. Neonatal Sepsis. Sisli. Etfal. Hastan. Tip. Bul. 2020, 54, 142–158. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Manda, A.; Pruchniak, M.P.; Arazna, M.; Demkow, U.A. Neutrophil extracellular traps in physiology and pathology. Centr. Eur. J. Immunol. 2014, 39, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Altrichter, J.; Zedler, S.; Kraft, R.; Faist, E.; Mitzner, S.R.; Sauer, M.; Windolf, J.; Scholz, M.; Lögters, T. Neutrophil-derived circulating free DNA (cf-DNA/NETs), a potential prognostic marker for mortality in patients with severe burn injury. Eur. J. Trauma Emerg. Surg. 2010, 36, 551–557. [Google Scholar] [CrossRef]
- Shen, X.-F.; Cao, K.; Jiang, J.-P.; Guan, W.-X.; Du, J.-F. Neutrophil dysregulation during sepsis: An overview and update. J. Cell. Mol. Med. 2017, 21, 1687–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, L.; Pithon-Curi, T.C.; Curi, R.; Hatanaka, E. COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps. Mediat. Inflamm. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- World Health Organisation. WHO: Recommended definitions, terminology and format for statistical tables related to the per-inatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet. Gynecol. Scand. 1977, 56, 247–253. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Kremer Hovinga, J.A..; Schatzberg, D.; Wagner, D.D.; Lämmle, B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood 2012, 120, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Stiel, C.U.; Ebenebe, C.U.; Trochimiuk, M.; Raluy, L.P.; Vincent, D.; Singer, D.; Reinshagen, K.; Boettcher, M. Markers of NETosis Do Not Predict Neonatal Early Onset Sepsis: A Pilot Study. Front. Pediatr. 2020, 7, 555. [Google Scholar] [CrossRef] [Green Version]
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E. Management of neonates born at ≥35 0/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics 2018, 142, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margraf, S.; Lögters, T.; Reipen, J.; Altrichter, J.; Scholz, M.; Windolf, J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): A potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock 2008, 30, 352–358. [Google Scholar] [CrossRef]
- Yost, C.C.; Schwertz, H.; Cody, M.J.; Wallace, J.A.; Campbell, R.A.; Vieira-De-Abreu, A.; Araujo, C.V.; Schubert, S.; Harris, E.S.; Rowley, J.W.; et al. Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. J. Clin. Investig. 2016, 126, 3783–3798. [Google Scholar] [CrossRef]
- Melvan, J.N.; Bagby, G.J.; Welsh, D.A.; Nelson, S.; Zhang, P. Neonatal Sepsis and Neutrophil Insufficiencies. Int. Rev. Immunol. 2010, 29, 315–348. [Google Scholar] [CrossRef] [Green Version]
- Golonka, R.M.; Yeoh, B.S.; Petrick, J.L.; Weinstein, S.J.; Albanes, D.; Gewirtz, A.T.; McGlynn, K.A.; Vijay-Kumar, M. Deoxyribonuclease I Activity, Cell-Free DNA, and Risk of Liver Cancer in a Prospective Cohort. JNCI Cancer Spectr. 2018, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Alcázar, M.; Kim, N.; Fuchs, T.A. Circulating Extracellular DNA: Cause or Consequence of Thrombosis? Semin. Thromb. Hemost. 2017, 43, 553–561. [Google Scholar] [CrossRef]
- Cheng, Z.; Abrams, S.T.; Toh, J.; Wang, S.S.; Wang, Z.; Yu, Q.; Yu, W.; Toh, C.-H.; Wang, G. The Critical Roles and Mechanisms of Immune Cell Death in Sepsis. Front. Immunol. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Hashiba, M.; Huq, A.; Tomino, A.; Hirakawa, A.; Hattori, T.; Miyabe, H.; Tsuda, M.; Takeyama, N. Neutrophil extracellular traps in patients with sepsis. J. Surg. Res. 2015, 194, 248–254. [Google Scholar] [CrossRef]
- Sandquist, M.; Wong, H.R. Biomarkers of sepsis and their potential value in diagnosis, prognosis and treatment. Expert Rev. Clin. Immunol. 2014, 10, 1349–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, A.; Wort, S.J.; Thomas, H.; Collinson, P.; David, E.D. Plasma DNA concentration as a predictor of mortality and sepsis in critically ill patients. Crit. Care 2006, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Keyel, P.A. Dnases in health and disease. Dev. Biol. 2017, 429, 1–11. [Google Scholar] [CrossRef]
- Lee, K.H.; Cavanaugh, L.; Leung, H.; Yan, F.; Ahmadi, Z.; Chong, B.H.; Passam, F. Quantification of NETs-associated markers by flow cytometry and serum assays in patients with thrombosis and sepsis. Int. J. Lab. Hematol. 2018, 40, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Grunwell, J.R.; Stephenson, S.T.; Mohammad, A.F.; Jones, K.; Mason, C.; Opolka, C.; Fitzpatrick, A.M. Differential type I interferon response and primary airway neutrophil extracellular trap release in children with acute respiratory distress syndrome. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).