Anti-Inflammatory Properties of the SGLT2 Inhibitor Empagliflozin in Activated Primary Microglia
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Primary Microglial Cells
2.2. Cell Stimulation
2.3. Cytotoxicity Test
2.4. Measurement of Nitric Oxide Production
2.5. Quantitative Real Time PCR
2.6. Enzyme-Linked Immunosorbent Assays
2.7. Western Blot
2.8. Immunofluorescence Staining of NFkB
2.9. Statistical Analysis
3. Results
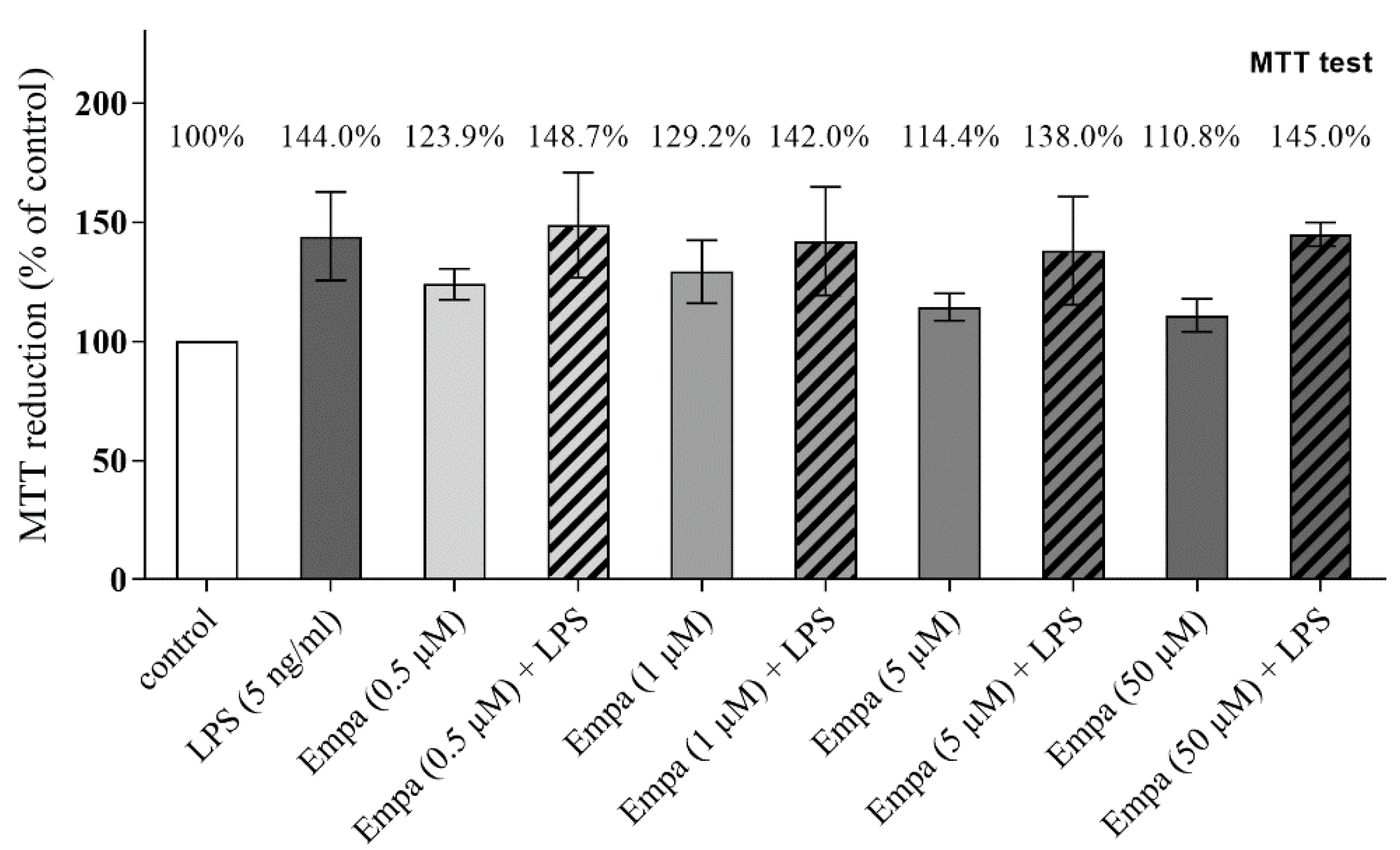
3.1. Empagliflozin Shows No Cytotoxic Effect on Primary Microglia up to a Concentration of 50 µM
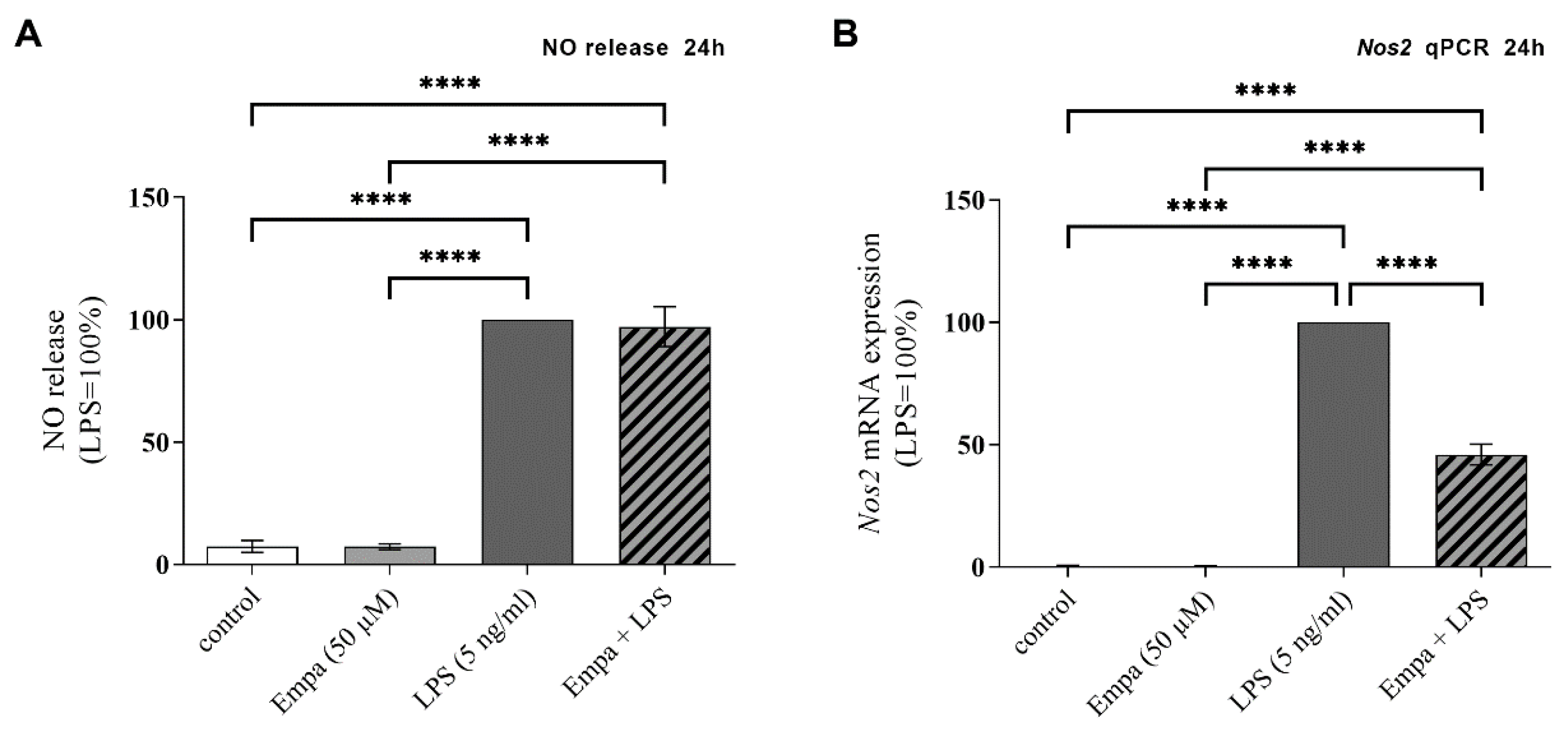
3.2. Empagliflozin Reduces Nos2 mRNA Expression in Activated Primary Microglia but Not NO Release
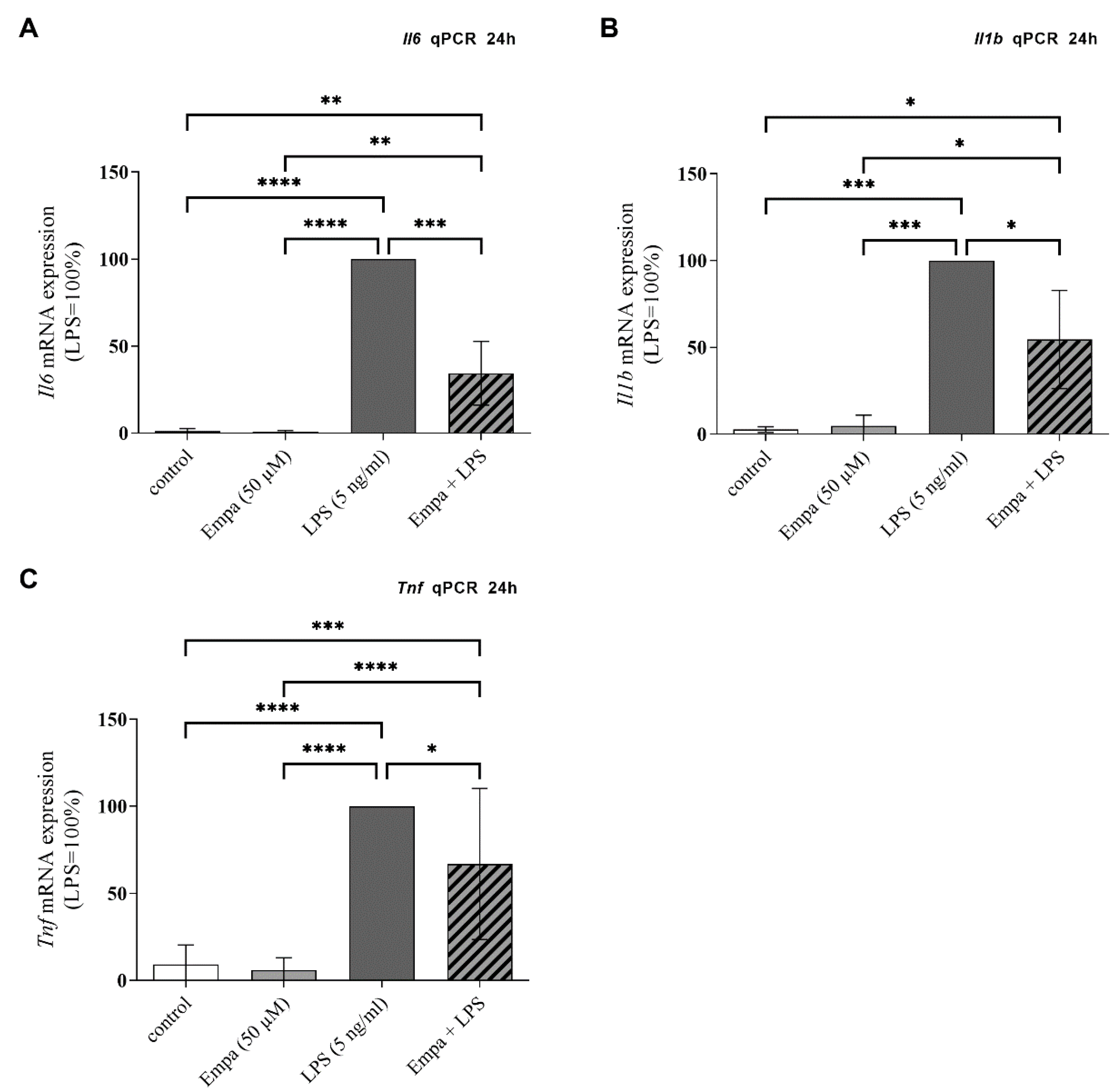
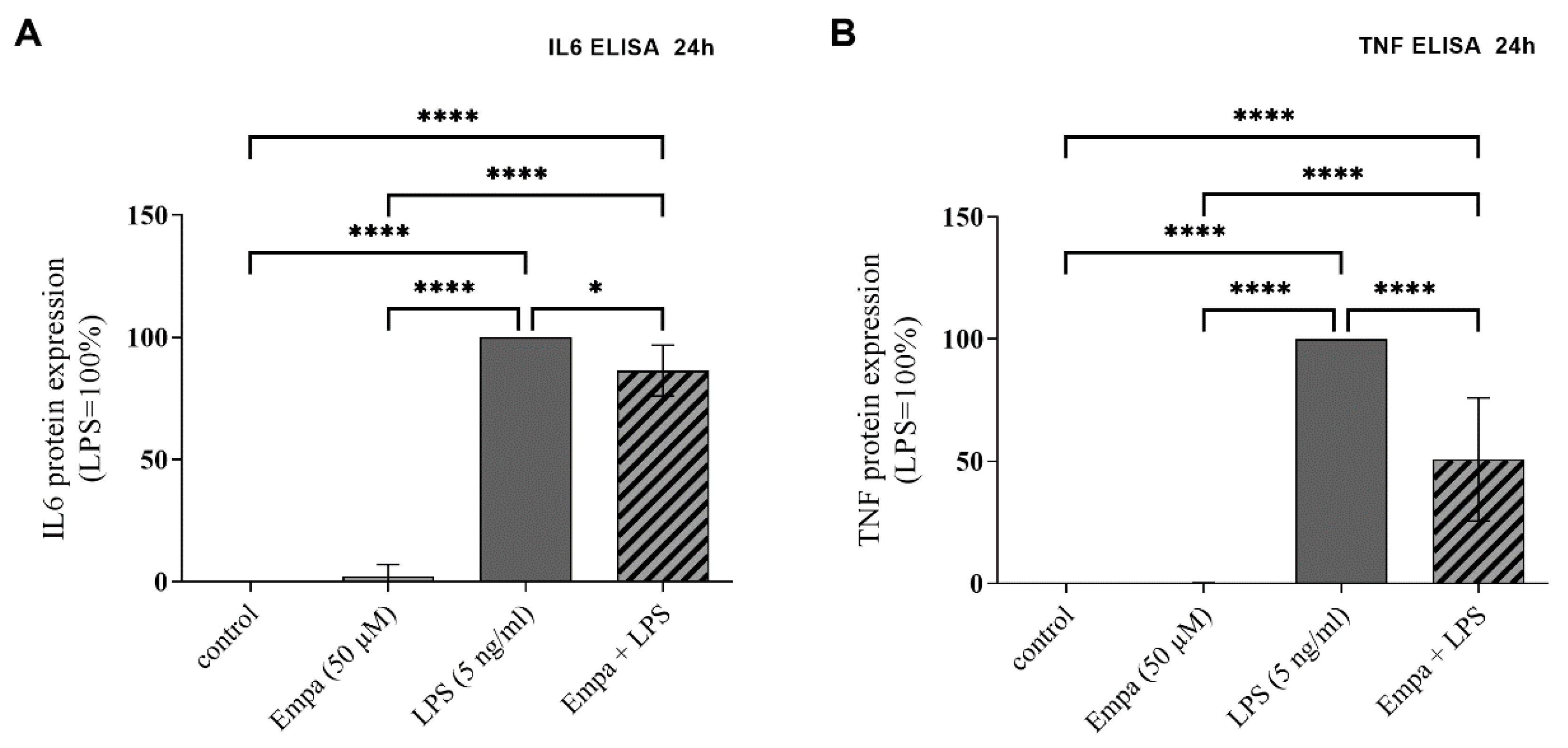
3.3. Empagliflozin Reduces mRNA Synthesis and Protein Production of Pro-Inflammatory Cytokines in Activated Primary Microglia
3.4. Empagliflozin Reduces the IL10 Production in Activated Primary Microglia
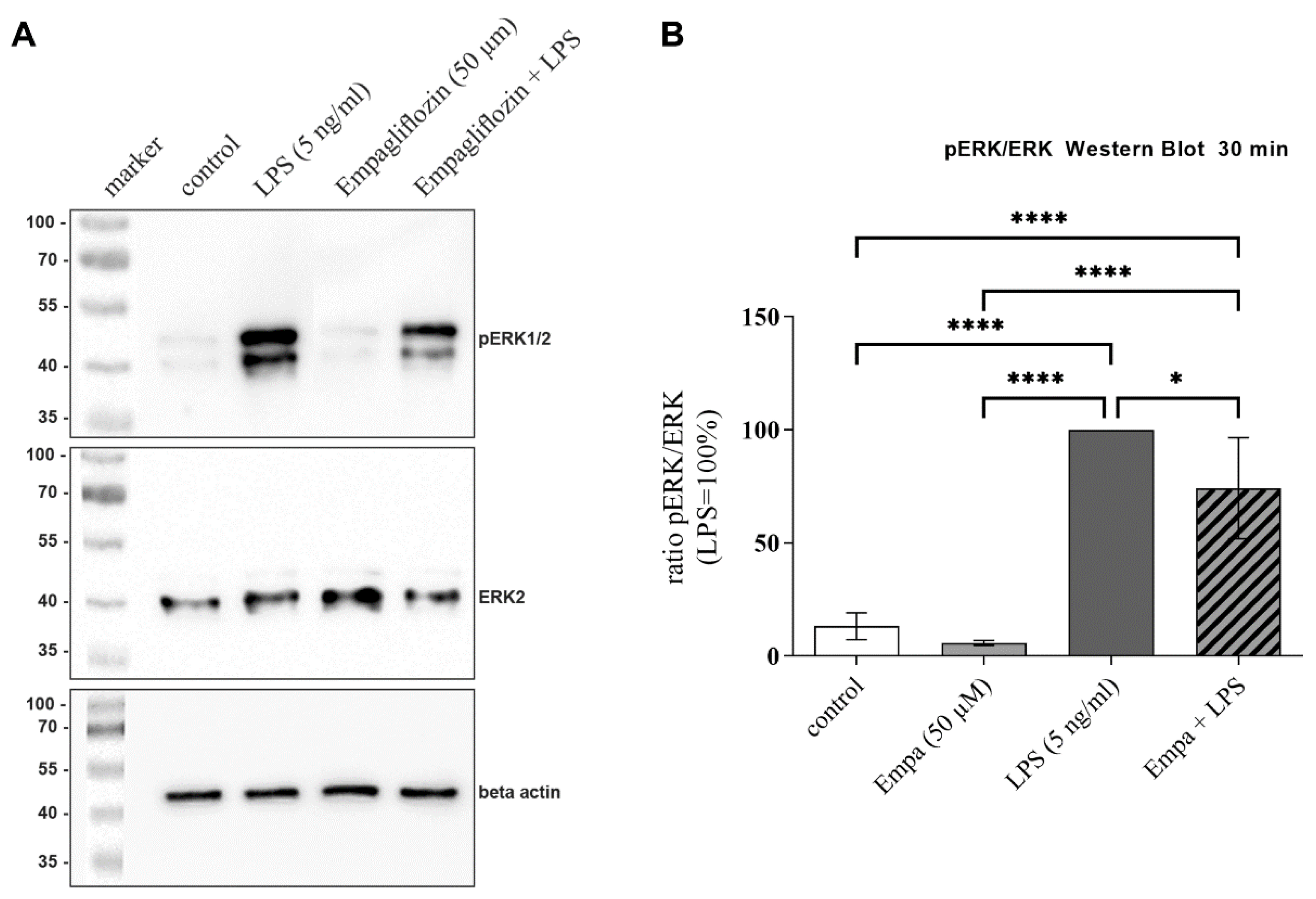
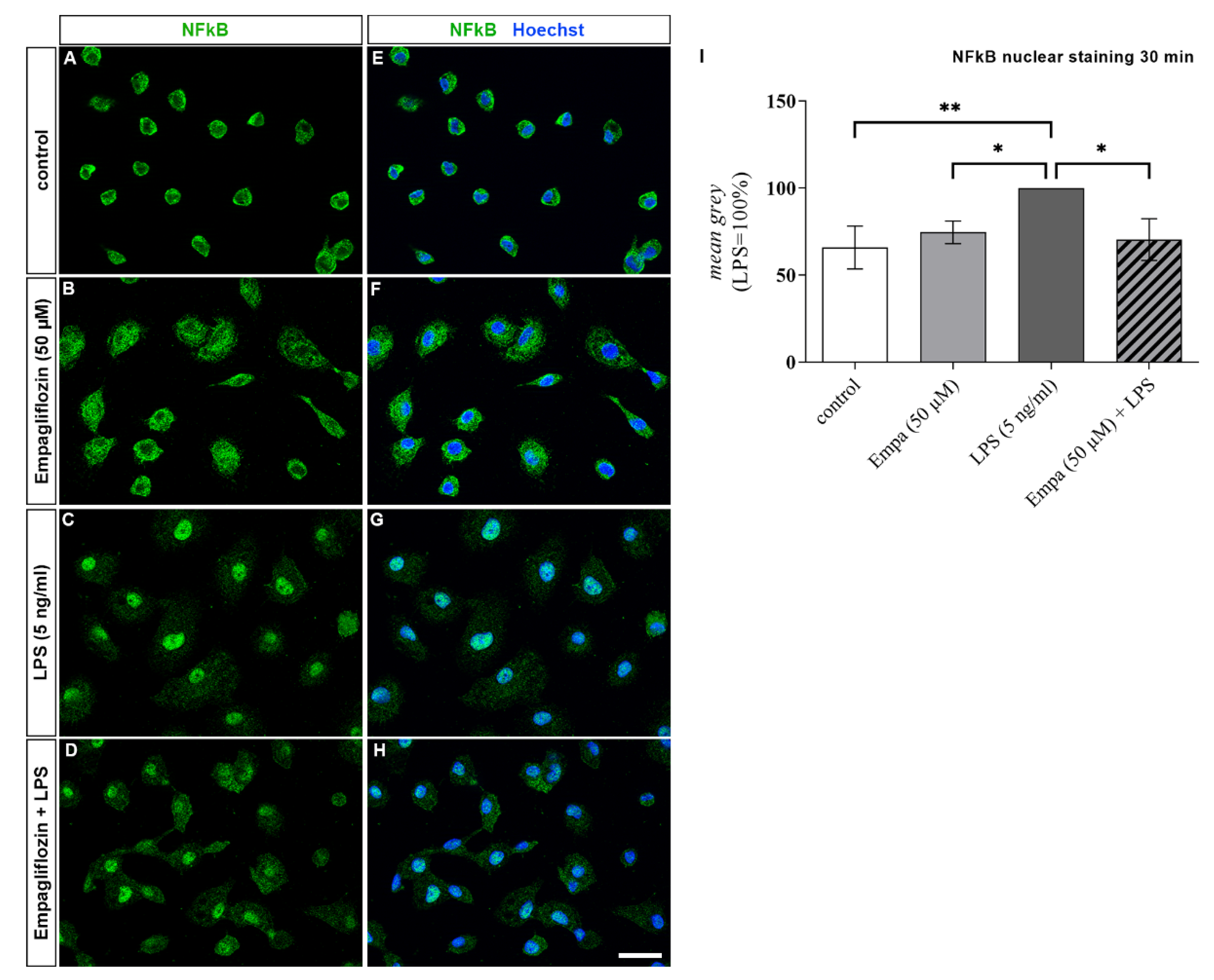
3.5. Empagliflozin Might Mediate Anti-Inflammatory Effects by Inhibiting the ERK1/2 and NFkB Signaling Pathway
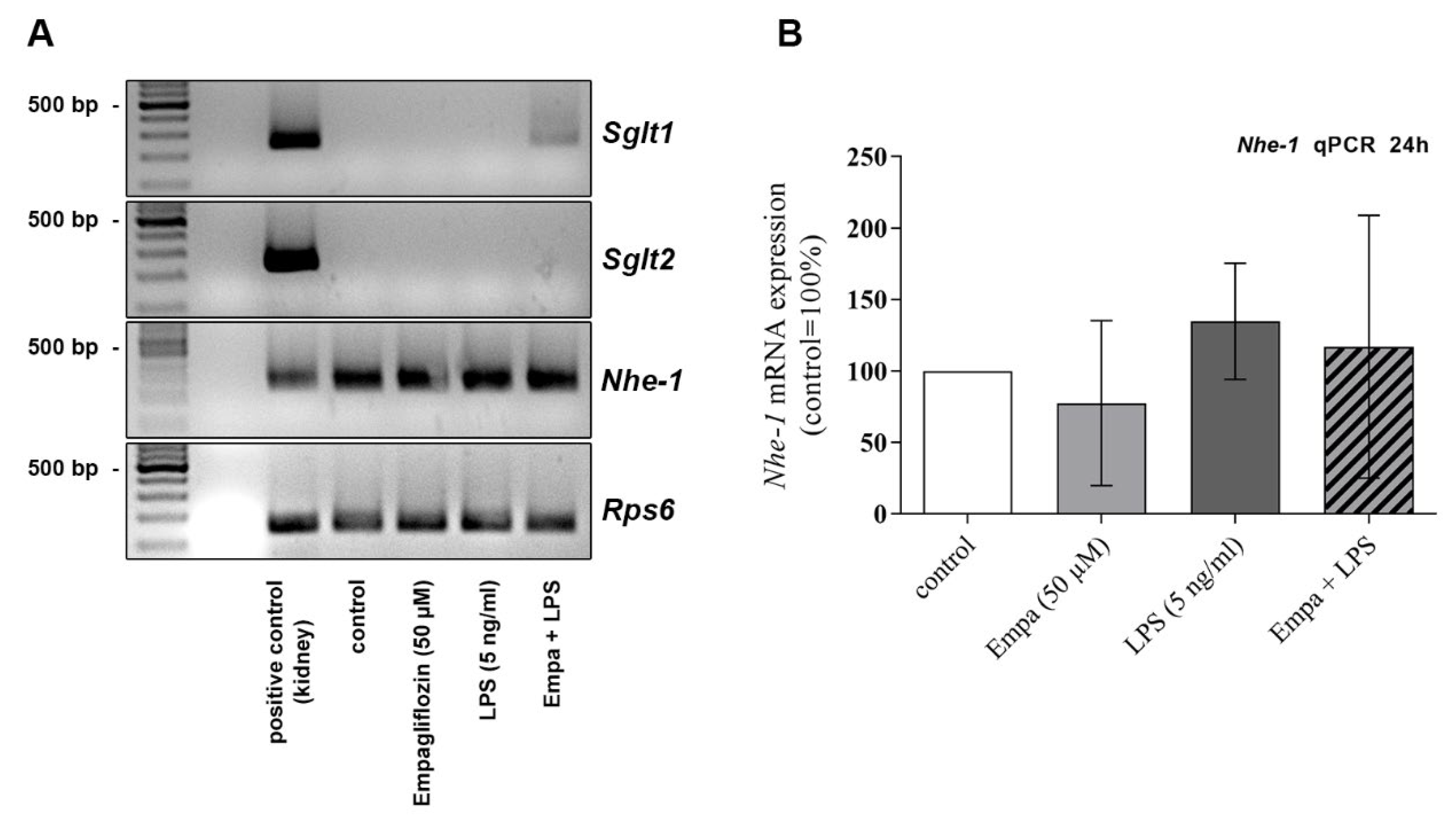
3.6. The Anti-Inflammatory Properties of Empagliflozin Might Be Mediated via the Sodium/Hydrogen Exchanger NHE-1 Rather Than SGLT2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Rickert, U.; Cossais, F.; Heimke, M.; Arnold, P.; Preusse-Prange, A.; Wilms, H.; Lucius, R. Anti-inflammatory properties of Honokiol in activated primary microglia and astrocytes. J. Neuroimmunol. 2018, 323, 78–86. [Google Scholar] [CrossRef]
- Saijo, K.; Crotti, A.; Glass, C.K. Regulation of microglia activation and deactivation by nuclear receptors. Glia 2013, 61, 104–111. [Google Scholar] [CrossRef]
- Trapp, B.D.; Wujek, J.R.; Criste, G.A.; Jalabi, W.; Yin, X.; Kidd, G.J.; Stohlman, S.; Ransohoff, R. Evidence for synaptic stripping by cortical microglia. Glia 2007, 55, 360–368. [Google Scholar] [CrossRef]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Mandrekar-Colucci, S.; Landreth, G.E. Microglia and inflammation in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2010, 9, 156–167. [Google Scholar] [CrossRef]
- Cinti, F.; Moffa, S.; Impronta, F.; Cefalo, C.M.; Sun, V.A.; Sorice, G.P.; Mezza, T.; Giaccari, A. Spotlight on ertugliflozin and its potential in the treatment of type 2 diabetes: Evidence to date. Drug Des. Devel. Ther. 2017, 11, 2905–2919. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J.; Paquot, N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: A review of the clinical evidence. Diabetes Metab. 2014, 40, S4–S11. [Google Scholar] [CrossRef]
- Grempler, R.; Thomas, L.; Eckhardt, M.; Himmelsbach, F.; Sauer, A.; Sharp, D.E.; Bakker, R.A.; Mark, M.; Klein, T.; Eickelmann, P. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: Characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes. Metab. 2012, 14, 83–90. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Kosiborod, M.; Fitchett, D.; Wanner, C.; Hehnke, U.; Kaspers, S.; George, J.T.; Zinman, B. Improvement in Cardiovascular Outcomes With Empagliflozin Is Independent of Glycemic Control. Circulation 2018, 138, 1904–1907. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Oelze, M.; Hanf, A.; Kroller-Schon, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Godtel-Armbrust, U.; Xia, N.; et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef]
- Pawlos, A.; Broncel, M.; Wozniak, E.; Gorzelak-Pabis, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213. [Google Scholar] [CrossRef]
- Hierro-Bujalance, C.; Infante-Garcia, C.; Del Marco, A.; Herrera, M.; Carranza-Naval, M.J.; Suarez, J.; Alves-Martinez, P.; Lubian-Lopez, S.; Garcia-Alloza, M. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Alzheimer’s Res. Ther. 2020, 12, 40. [Google Scholar] [CrossRef]
- Lin, B.; Koibuchi, N.; Hasegawa, Y.; Sueta, D.; Toyama, K.; Uekawa, K.; Ma, M.; Nakagawa, T.; Kusaka, H.; Kim-Mitsuyama, S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 2014, 13, 148. [Google Scholar] [CrossRef]
- Kneusels, J.; Kaehler, M.; Cascorbi, I.; Wedel, T.; Neunlist, M.; Lucius, R.; Cossais, F. Limited Impact of 6-Mercaptopurine on Inflammation-Induced Chemokines Expression Profile in Primary Cultures of Enteric Nervous System. Neurochem. Res. 2021, 46, 1781–1793. [Google Scholar] [CrossRef]
- Arnold, P.; Schmidt, F.; Prox, J.; Zunke, F.; Pietrzik, C.; Lucius, R.; Becker-Pauly, C. Calcium negatively regulates meprin beta activity and attenuates substrate cleavage. FASEB J. 2015, 29, 3549–3557. [Google Scholar] [CrossRef]
- Bast, B.O.; Rickert, U.; Schneppenheim, J.; Cossais, F.; Wilms, H.; Arnold, P.; Lucius, R. Aldosterone exerts anti-inflammatory effects on LPS stimulated microglia. Heliyon 2018, 4, e00826. [Google Scholar] [CrossRef] [PubMed]
- De Pablo-Fernandez, E.; Goldacre, R.; Pakpoor, J.; Noyce, A.J.; Warner, T.T. Association between diabetes and subsequent Parkinson disease: A record-linkage cohort study. Neurology 2018, 91, e139–e142. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.J.; Wang, T.J.; Chen, S.D.; Lin, K.L.; Liou, C.W.; Lan, M.Y.; Chuang, Y.C.; Chuang, J.H.; Wang, P.W.; Lee, J.J.; et al. Two Birds One Stone: The Neuroprotective Effect of Antidiabetic Agents on Parkinson Disease-Focus on Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors. Antioxidants 2021, 10, 1935. [Google Scholar] [CrossRef]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Koyani, C.N.; Plastira, I.; Sourij, H.; Hallstrom, S.; Schmidt, A.; Rainer, P.P.; Bugger, H.; Frank, S.; Malle, E.; von Lewinski, D. Empagliflozin protects heart from inflammation and energy depletion via AMPK activation. Pharmacol. Res. 2020, 158, 104870. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Butler, A.E.; Atkin, S.L.; Katsiki, N.; Sahebkar, A. Sodium-glucose cotransporter 2 inhibitors and inflammation in chronic kidney disease: Possible molecular pathways. J. Cell. Physiol. 2018, 234, 223–230. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef]
- Montgomery, S.L.; Bowers, W.J. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J. Neuroimmune Pharmacol. 2012, 7, 42–59. [Google Scholar] [CrossRef]
- Alam, Q.; Alam, M.Z.; Mushtaq, G.; Damanhouri, G.A.; Rasool, M.; Kamal, M.A.; Haque, A. Inflammatory Process in Alzheimer’s and Parkinson’s Diseases: Central Role of Cytokines. Curr. Pharm. Des. 2016, 22, 541–548. [Google Scholar] [CrossRef]
- Konsman, J.P.; Drukarch, B.; Van Dam, A.M. (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clin. Sci. 2007, 112, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Harada, M.; Kondo, T.; Riederer, P.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 1994, 180, 147–150. [Google Scholar] [CrossRef]
- Qin, X.Y.; Zhang, S.P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Neurol. 2016, 73, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Green, H.F.; Khosousi, S.; Svenningsson, P. Plasma IL-6 and IL-17A Correlate with Severity of Motor and Non-Motor Symptoms in Parkinson’s Disease. J. Park. Dis. 2019, 9, 705–709. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Pott Godoy, M.C.; Tarelli, R.; Chertoff, M.; Depino, A.M.; Pitossi, F.J. Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1beta in the substantia nigra. Neurobiol. Dis. 2006, 24, 183–193. [Google Scholar] [CrossRef]
- Chertoff, M.; Di Paolo, N.; Schoeneberg, A.; Depino, A.; Ferrari, C.; Wurst, W.; Pfizenmaier, K.; Eisel, U.; Pitossi, F. Neuroprotective and neurodegenerative effects of the chronic expression of tumor necrosis factor alpha in the nigrostriatal dopaminergic circuit of adult mice. Exp. Neurol. 2011, 227, 237–251. [Google Scholar] [CrossRef]
- Lee, N.; Heo, Y.J.; Choi, S.E.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J. Anti-inflammatory Effects of Empagliflozin and Gemigliptin on LPS-Stimulated Macrophage via the IKK/NF-kappaB, MKK7/JNK, and JAK2/STAT1 Signalling Pathways. J. Immunol. Res. 2021, 2021, 9944880. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Asensio Lopez, M.D.C.; Lax, A.; Hernandez Vicente, A.; Saura Guillen, E.; Hernandez-Martinez, A.; Fernandez Del Palacio, M.J.; Bayes-Genis, A.; Pascual Figal, D.A. Empagliflozin improves post-infarction cardiac remodeling through GTP enzyme cyclohydrolase 1 and irrespective of diabetes status. Sci. Rep. 2020, 10, 13553. [Google Scholar] [CrossRef]
- Saura, J.; Angulo, E.; Ejarque, A.; Casadó, V.; Tusell, J.M.; Moratalla, R.; Chen, J.F.; Schwarzschild, M.A.; Lluis, C.; Franco, R.; et al. Adenosine A2A receptor stimulation potentiates nitric oxide release by activated microglia. J. Neurochem. 2005, 95, 919–929. [Google Scholar] [CrossRef]
- Brzozowski, M.J.; Jenner, P.; Rose, S. Inhibition of i-NOS but not n-NOS protects rat primary cell cultures against MPP(+)-induced neuronal toxicity. J. Neural. Transm. 2015, 122, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Ledeboer, A.; Breve, J.J.; Wierinckx, A.; van der Jagt, S.; Bristow, A.F.; Leysen, J.E.; Tilders, F.J.; Van Dam, A.M. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur. J. Neurosci. 2002, 16, 1175–1185. [Google Scholar] [CrossRef]
- Kwilasz, A.J.; Grace, P.M.; Serbedzija, P.; Maier, S.F.; Watkins, L.R. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology 2015, 96, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, T.; Choi, D.Y.; Lu, X.; Liu, M.; Nguyen, X.V.; Zheng, N.; Stewart, C.A.; Kim, H.C.; Bing, G. Interleukin-10 protects against inflammation-mediated degeneration of dopaminergic neurons in substantia nigra. Neurobiol. Aging 2007, 28, 894–906. [Google Scholar] [CrossRef]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the immune response in the brain: IL-10 and its regulation. J Neuroinflammation 2016, 13, 297. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Wu, M.; Xu, B.; Kang, L. Empagliflozin protects against atherosclerosis progression by modulating lipid profiles and sympathetic activity. Lipids Health Dis. 2021, 20, 5. [Google Scholar] [CrossRef]
- Iannantuoni, F.; A, M.d.M.; Diaz-Morales, N.; Falcon, R.; Banuls, C.; Abad-Jimenez, Z.; Victor, V.M.; Hernandez-Mijares, A.; Rovira-Llopis, S. The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes. J. Clin. Med. 2019, 8, 1814. [Google Scholar] [CrossRef]
- Sun, J.; Nan, G. The extracellular signal-regulated kinase 1/2 pathway in neurological diseases: A potential therapeutic target (Review). Int. J. Mol. Med. 2017, 39, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Singh, S.P. NF-kappaB-Mediated Neuroinflammation in Parkinson’s Disease and Potential Therapeutic Effect of Polyphenols. Neurotox. Res. 2020, 37, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Panchapakesan, U.; Pegg, K.; Gross, S.; Komala, M.G.; Mudaliar, H.; Forbes, J.; Pollock, C.; Mather, A. Effects of SGLT2 inhibition in human kidney proximal tubular cells--renoprotection in diabetic nephropathy? PLoS ONE 2013, 8, e54442. [Google Scholar] [CrossRef]
- Zhang, H.; Uthman, L.; Bakker, D.; Sari, S.; Chen, S.; Hollmann, M.W.; Coronel, R.; Weber, N.C.; Houten, S.M.; van Weeghel, M.; et al. Empagliflozin Decreases Lactate Generation in an NHE-1 Dependent Fashion and Increases alpha-Ketoglutarate Synthesis From Palmitate in Type II Diabetic Mouse Hearts. Front. Cardiovasc. Med. 2020, 7, 592233. [Google Scholar] [CrossRef]
- Ortega, R.; Collado, A.; Selles, F.; Gonzalez-Navarro, H.; Sanz, M.J.; Real, J.T.; Piqueras, L. SGLT-2 (Sodium-Glucose Cotransporter 2) Inhibition Reduces Ang II (Angiotensin II)-Induced Dissecting Abdominal Aortic Aneurysm in ApoE (Apolipoprotein E) Knockout Mice. Arter. Thromb. Vasc. Biol. 2019, 39, 1614–1628. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi Jigheh, Z.; Ghorbani Haghjo, A.; Argani, H.; Roshangar, L.; Rashtchizadeh, N.; Sanajou, D.; Nazari Soltan Ahmad, S.; Rashedi, J.; Dastmalchi, S.; Mesgari Abbasi, M. Empagliflozin alleviates renal inflammation and oxidative stress in streptozotocin-induced diabetic rats partly by repressing HMGB1-TLR4 receptor axis. Iran J. Basic Med. Sci. 2019, 22, 384–390. [Google Scholar] [CrossRef]
- Abdelhamid, A.M.; Elsheakh, A.R.; Abdelaziz, R.R.; Suddek, G.M. Empagliflozin ameliorates ethanol-induced liver injury by modulating NF-kappaB/Nrf-2/PPAR-gamma interplay in mice. Life Sci. 2020, 256, 117908. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; De Rosa, S.; Tamme, L.; Iaconetti, C.; Sorrentino, S.; Polimeni, A.; Mignogna, C.; Amorosi, A.; Spaccarotella, C.; Yasuda, M.; et al. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc. Diabetol. 2020, 19, 66. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Chen, G.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Sakai, Y.; Kaneko, S.; Ota, T. Empagliflozin reverses obesity and insulin resistance through fat browning and alternative macrophage activation in mice fed a high-fat diet. BMJ Open Diabetes Res. Care 2019, 7, e000783. [Google Scholar] [CrossRef]
- Liu, Y.; Kintner, D.B.; Chanana, V.; Algharabli, J.; Chen, X.; Gao, Y.; Chen, J.; Ferrazzano, P.; Olson, J.K.; Sun, D. Activation of microglia depends on Na+/H+ exchange-mediated H+ homeostasis. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 15210–15220. [Google Scholar] [CrossRef]
- Chen, S.; Coronel, R.; Hollmann, M.W.; Weber, N.C.; Zuurbier, C.J. Direct cardiac effects of SGLT2 inhibitors. Cardiovasc. Diabetol. 2022, 21, 45. [Google Scholar] [CrossRef]
- Trum, M.; Riechel, J.; Wagner, S. Cardioprotection by SGLT2 Inhibitors-Does It All Come Down to Na(+)? Int. J. Mol. Sci. 2021, 22, 7976. [Google Scholar] [CrossRef]
- Cianciolo, G.; De Pascalis, A.; Gasperoni, L.; Tondolo, F.; Zappulo, F.; Capelli, I.; Cappuccilli, M.; La Manna, G. The Off-Target Effects, Electrolyte and Mineral Disorders of SGLT2i. Molecules 2020, 25, 2757. [Google Scholar] [CrossRef] [PubMed]
- De Pascalis, A.; Cianciolo, G.; Capelli, I.; Brunori, G.; La Manna, G. SGLT2 inhibitors, sodium and off-target effects: An overview. J. Nephrol. 2021, 34, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Wen, S.; Gong, M.; Yuan, X.; Xu, D.; Wang, C.; Jin, J.; Zhou, L. Dapagliflozin Activates Neurons in the Central Nervous System and Regulates Cardiovascular Activity by Inhibiting SGLT-2 in Mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2781–2799. [Google Scholar] [CrossRef] [PubMed]
- Jayarathne, H.S.M.; Debarba, L.K.; Jaboro, J.J.; Ginsburg, B.C.; Miller, R.A.; Sadagurski, M. Neuroprotective effects of Canagliflozin: Lessons from aged genetically diverse UM-HET3 mice. Aging Cell 2022, 21, e13653. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.W.; Que, J.Q.; Liu, S.; Huang, K.Y.; Qian, L.; Weng, Y.B.; Rong, F.N.; Wang, L.; Zhou, Y.Y.; Xue, Y.J.; et al. Sodium-Glucose Co-transporter-2 Inhibitor of Dapagliflozin Attenuates Myocardial Ischemia/Reperfusion Injury by Limiting NLRP3 Inflammasome Activation and Modulating Autophagy. Front. Cardiovasc. Med. 2021, 8, 768214. [Google Scholar] [CrossRef]
- Lin, K.; Yang, N.; Luo, W.; Qian, J.F.; Zhu, W.W.; Ye, S.J.; Yuan, C.X.; Xu, D.Y.; Liang, G.; Huang, W.J.; et al. Direct cardio-protection of Dapagliflozin against obesity-related cardiomyopathy via NHE1/MAPK signaling. Acta Pharmacol. Sin. 2022, 43, 2624–2635. [Google Scholar] [CrossRef]
- Amin, E.F.; Rifaai, R.A.; Abdel-Latif, R.G. Empagliflozin attenuates transient cerebral ischemia/reperfusion injury in hyperglycemic rats via repressing oxidative-inflammatory-apoptotic pathway. Fundam. Clin. Pharmacol. 2020, 34, 548–558. [Google Scholar] [CrossRef]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; DeMarco, V.G. Empagliflozin Ameliorates Type 2 Diabetes-Induced Ultrastructural Remodeling of the Neurovascular Unit and Neuroglia in the Female db/db Mouse. Brain Sci. 2019, 9, 57. [Google Scholar] [CrossRef]
- Wicinski, M.; Wodkiewicz, E.; Gorski, K.; Walczak, M.; Malinowski, B. Perspective of SGLT2 Inhibition in Treatment of Conditions Connected to Neuronal Loss: Focus on Alzheimer’s Disease and Ischemia-Related Brain Injury. Pharmaceuticals 2020, 13, 379. [Google Scholar] [CrossRef]
- Tahara, A.; Takasu, T.; Yokono, M.; Imamura, M.; Kurosaki, E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors in pharmacokinetics, pharmacodynamics, and pharmacologic effects. J. Pharmacol. Sci. 2016, 130, 159–169. [Google Scholar] [CrossRef]

| Gene | Sequence (5′-3′) or Reference | |
|---|---|---|
| Nos2 | Thermo Fisher Scientific | Rn00561646_m1 |
| Il6 | Thermo Fisher Scientific | Rn00561420_m1 |
| Il1b | Thermo Fisher Scientific | Rn00580432_m1 |
| Tnf | Thermo Fisher Scientific | Rn99999017_m1 |
| 18s | Thermo Fisher Scientific | Hs99999901_s1 |
| Gapdh sense | Eurogentec, Köln, Germany | CAGCAAGGATACTGAGAGCAAGAGA |
| Gapdh antisense | Eurogentec | CGATGGAATTGTGAGGGAGATG |
| Gapdh probe | Eurogentec | AGGAGTCCCCATCCCAACTCAGCCC |
| Nhe-1 sense | Sigma-Aldrich | GTACGCACACCCTTCGAGAT |
| Nhe-1 antisense | Sigma-Aldrich | CAGAGGCAGGAAGTAGCCTG |
| Sglt1 sense | Sigma-Aldrich | CCAGTGGGCTGTACCAACAT |
| Sglt1 antisense | Sigma-Aldrich | ATGCCAATCAGCACGAGGAT |
| Sglt 2 sense | Sigma-Aldrich | GCGTATTTCCTGCTGGTCATT |
| Sglt 2 antisense | Sigma-Aldrich | GAGGAGCAACACCACAAAGAG |
| Rps6 sense | Sigma-Aldrich | CCAAGCTTATTCAGCGTCTTGTTACTCC |
| Rps6 antisense | Sigma-Aldrich | CCCTCGAGTCCTTCATTCTCTTGGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heimke, M.; Lenz, F.; Rickert, U.; Lucius, R.; Cossais, F. Anti-Inflammatory Properties of the SGLT2 Inhibitor Empagliflozin in Activated Primary Microglia. Cells 2022, 11, 3107. https://doi.org/10.3390/cells11193107
Heimke M, Lenz F, Rickert U, Lucius R, Cossais F. Anti-Inflammatory Properties of the SGLT2 Inhibitor Empagliflozin in Activated Primary Microglia. Cells. 2022; 11(19):3107. https://doi.org/10.3390/cells11193107
Chicago/Turabian StyleHeimke, Marvin, Florian Lenz, Uta Rickert, Ralph Lucius, and François Cossais. 2022. "Anti-Inflammatory Properties of the SGLT2 Inhibitor Empagliflozin in Activated Primary Microglia" Cells 11, no. 19: 3107. https://doi.org/10.3390/cells11193107
APA StyleHeimke, M., Lenz, F., Rickert, U., Lucius, R., & Cossais, F. (2022). Anti-Inflammatory Properties of the SGLT2 Inhibitor Empagliflozin in Activated Primary Microglia. Cells, 11(19), 3107. https://doi.org/10.3390/cells11193107







