Exosomal Plasminogen Activator Inhibitor-1 Induces Ionizing Radiation-Adaptive Glioblastoma Cachexia
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Antibodies, and Reagents
2.2. Cell Lines, Cell Culture, and Irradiation
2.3. A Literature Review to Find the Relationship between GBM, Radiotherapy, and Fatigue
2.4. Animal Care Protocol
2.5. Orthotopic Xenograft Mouse Model
2.6. Gastrocnemius Isolation and Measurement
2.7. Hematoxylin and Eosin (H&E) Staining and Immunohistochemistry (IHC)
2.8. Preparation of Conditioned Media
2.9. Silver Staining
2.10. Exosome Isolation and Characterization
2.11. Transmission Electron Microscopy (TEM)
2.12. Peptide Mass Fingerprinting (PMF)
2.13. CM or Exosome Treatment on C2C12 Cell for the Short-Term or Long-Term
2.14. C2C12 Differentiated Myotube Diameter Measurement
2.15. DiO Vibrant Exosome Labeling
2.16. Total RNA Isolation and qRT-PCR
2.17. Western Blot Analysis
2.18. Transwell Co-Culture System
2.19. Statistical Analysis
3. Results
3.1. Radiotherapy Caused Fatigue, a Symptom of Cachexia in GBM Patients
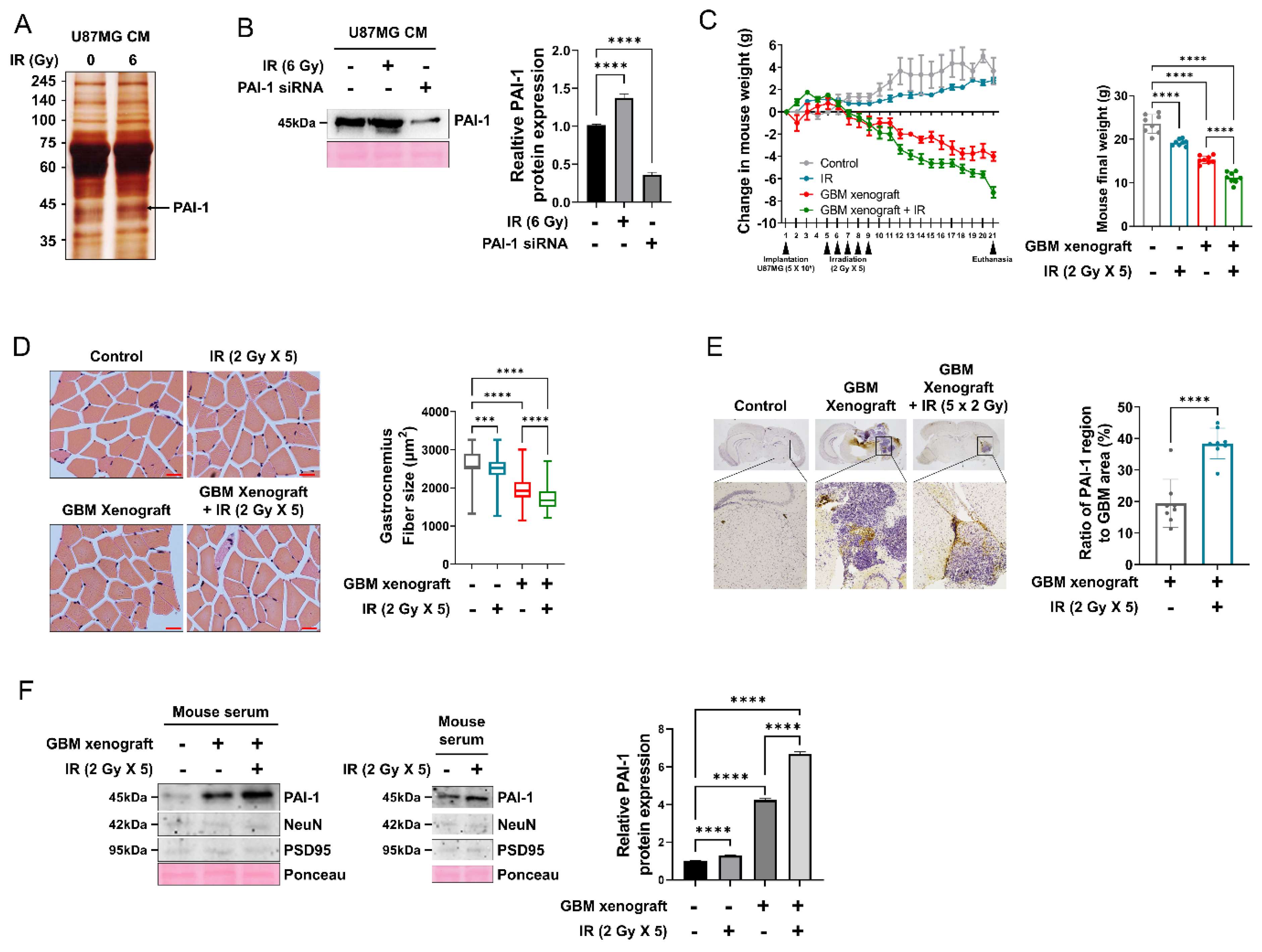
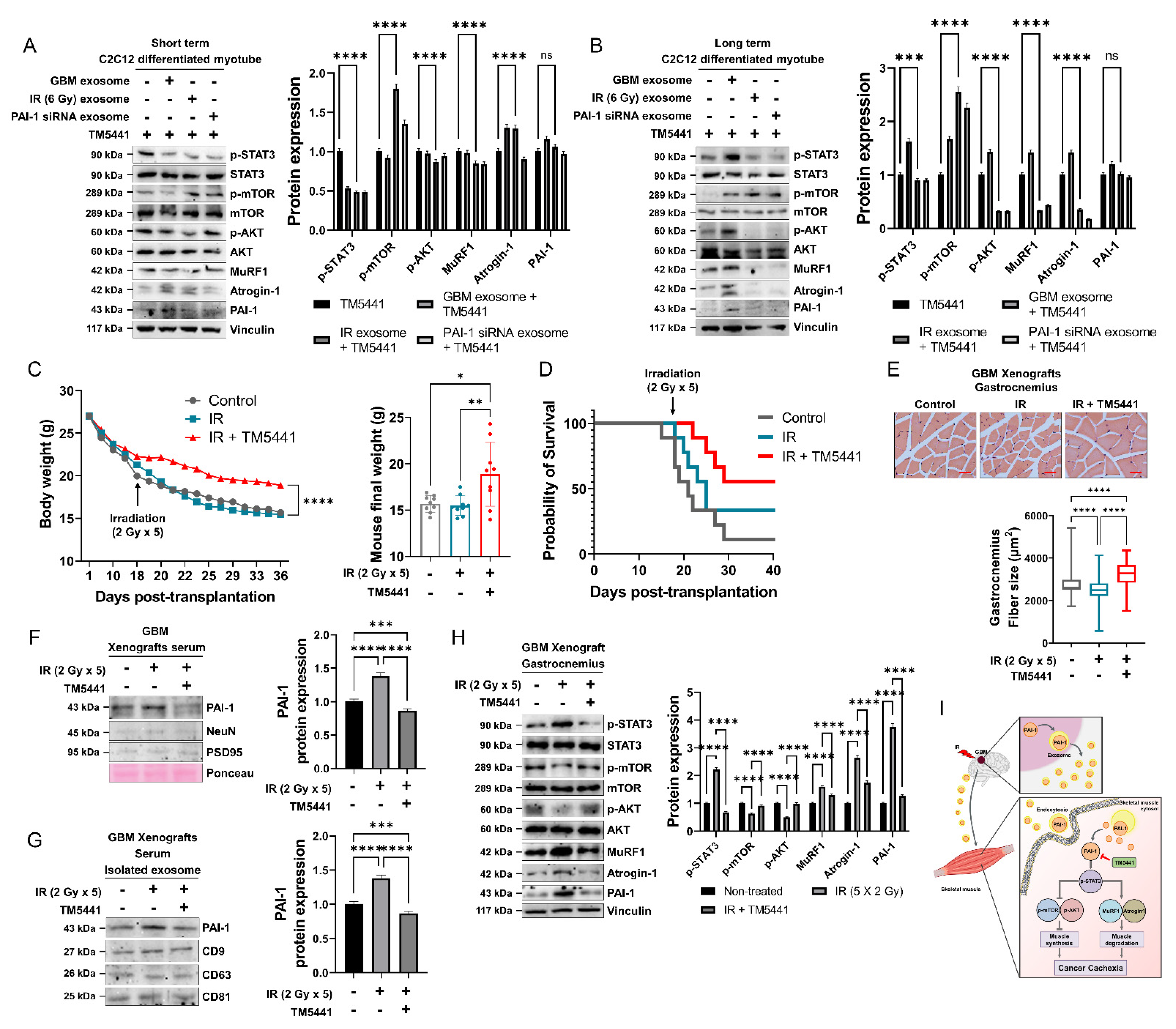
3.2. PAI-1 Expression and Secretion Were Elevated in Irradiated GBM Cells and an IR-Induced GBM Cachexia Mouse Model
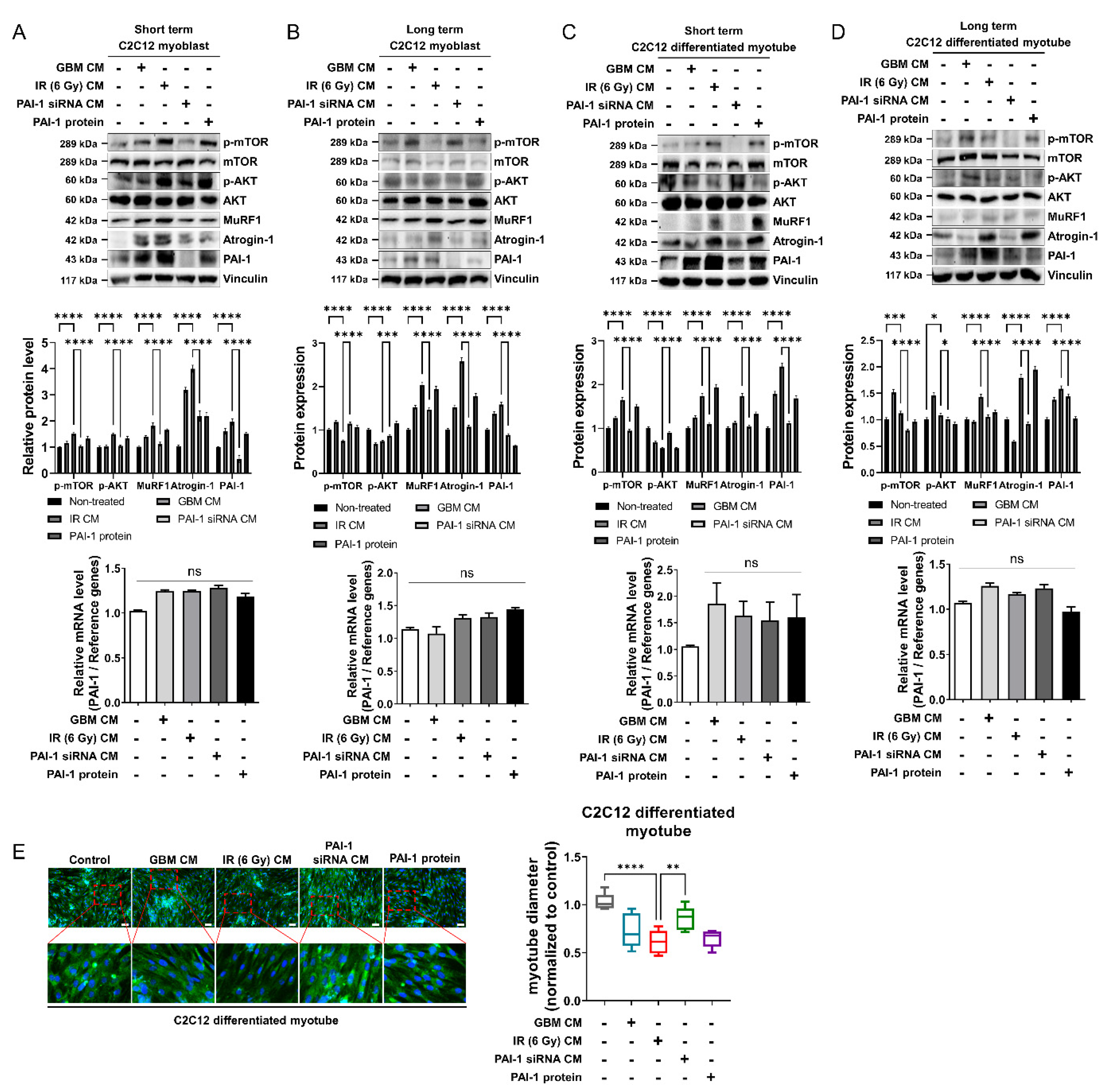
3.3. Muscle Homeostasis Was Disrupted by IR-Induced PAI-1 Secretion from GBM Cells
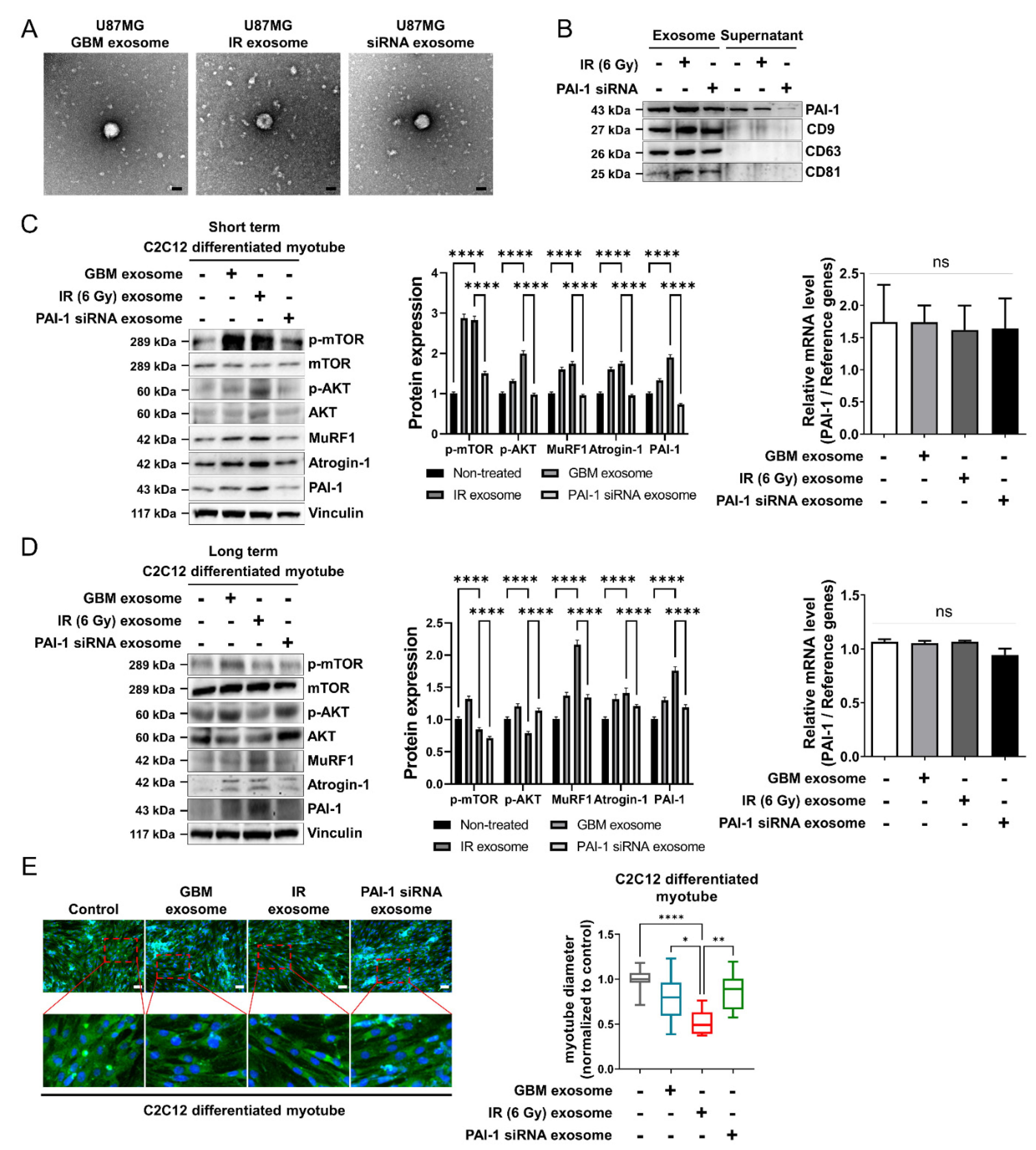
3.4. Ionizing Radiation-Induced PAI-1 from GBM Cells Was Carried by Exosomes
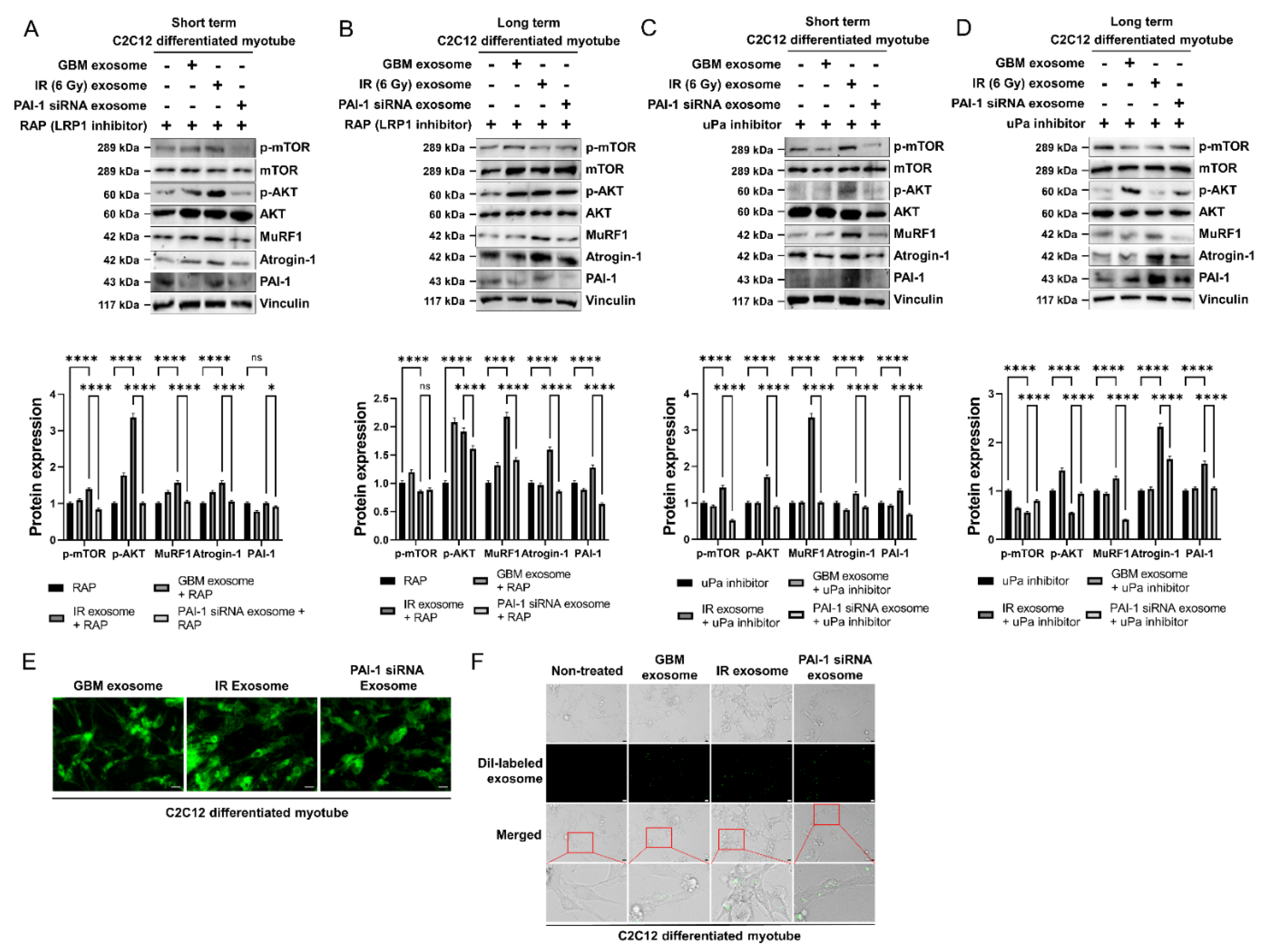
3.5. Exosomal PAI-1 Was Directly Penetrated via Non-Receptor-Mediated Endocytosis in Muscle Cells
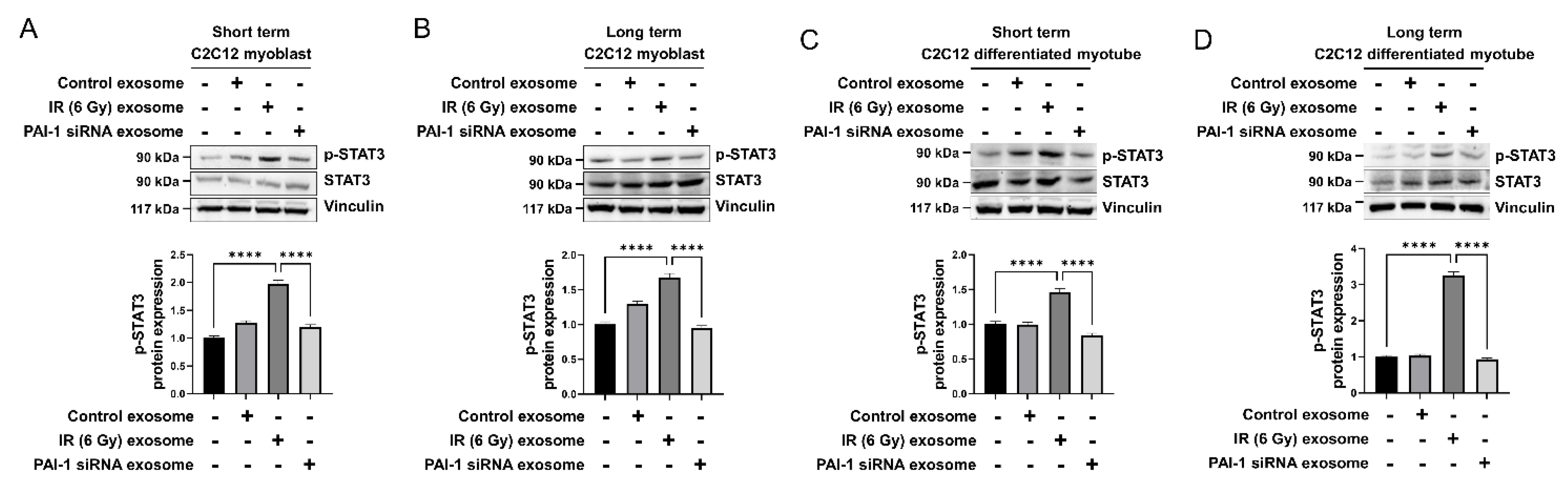
3.6. Exosomal PAI-1 Activated Intracellular STAT3 Pathway in Muscle Cells
3.7. Pharmacological Inhibition of PAI-1 Rescued IR-Induced GBM Cachexia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Reviews. Dis. Prim. 2018, 4, 17105. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Shao, W.; Huang, C.; Wu, C.J.; Jiang, B.; Lin, D. Metabolic derangements of skeletal muscle from a murine model of glioma cachexia. Skelet. Muscle 2019, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Komori, T. The 2021 WHO classification of tumors, 5th edition, central nervous system tumors: The 10 basic principles. Brain Tumor Pathol. 2022, 39, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Barani, I.J.; Larson, D.A. Radiation therapy of glioblastoma. Cancer Treat. Res. 2015, 163, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.L.; Hochberg, F.H. Anorexia and weight loss in glioma patients. Psychosomatics 1988, 29, 335–337. [Google Scholar] [CrossRef]
- van Coevorden-van Loon, E.M.P.; Coomans, M.B.; Heijenbrok-Kal, M.H.; Ribbers, G.M.; van den Bent, M.J. Fatigue in patients with low grade glioma: Systematic evaluation of assessment and prevalence. J. Neuro-Oncol. 2017, 133, 237–246. [Google Scholar] [CrossRef]
- Wu, B.; Liu, W.; Zhu, H.; Feng, H.; Liu, J. Primary glioblastoma of the cerebellopontine angle in adults. J. Neurosurg. 2011, 114, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Wende, T.; Kasper, J.; Prasse, G.; Glass, Ä.; Kriesen, T.; Freiman, T.M.; Meixensberger, J.; Henker, C. Newly Diagnosed IDH-Wildtype Glioblastoma and Temporal Muscle Thickness: A Multicenter Analysis. Cancers 2021, 13, 5610. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, A.J.; Mohamed, A.S.; Fuller, C.D. Cachexia in Radiotherapy-Treated Patients With Head and Neck Cancer—Reply. JAMA Oncol. 2016, 2, 831–832. [Google Scholar] [CrossRef] [PubMed]
- Lees, J. Incidence of weight loss in head and neck cancer patients on commencing radiotherapy treatment at a regional oncology centre. Eur. J. Cancer Care 1999, 8, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.B., II. Cancer cachexia: Definitions, outcomes, and treatments. Ann. Palliat. Med. 2019, 8, E1–E3. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lin, J.B.; Chen, T.C.; Jan, Y.T.; Sun, F.J.; Chen, Y.J.; Wu, M.H. Progressive Skeletal Muscle Loss After Surgery and Adjuvant Radiotherapy Impact Survival Outcomes in Patients With Early Stage Cervical Cancer. Front. Nutr. 2021, 8, 773506. [Google Scholar] [CrossRef] [PubMed]
- Brierley, D.I.; Harman, J.R.; Giallourou, N.; Leishman, E.; Roashan, A.E.; Mellows, B.A.D.; Bradshaw, H.B.; Swann, J.R.; Patel, K.; Whalley, B.J.; et al. Chemotherapy-induced cachexia dysregulates hypothalamic and systemic lipoamines and is attenuated by cannabigerol. J. Cachexia Sarcopenia Muscle 2019, 10, 844–859. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Stemmler, B.; Busquets, S. Therapeutic strategies against cancer cachexia. Eur. J. Transl. Myol. 2019, 29, 7960. [Google Scholar] [CrossRef]
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; Del Fabbro, E.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of Cancer Cachexia: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2438–2453. [Google Scholar] [CrossRef]
- Sulima, S.O.; De Keersmaecker, K. Ribosomal proteins: A novel class of oncogenic drivers. Oncotarget 2017, 8, 89427–89428. [Google Scholar] [CrossRef] [PubMed]
- Werle, B.; Kotzsch, M.; Lah, T.T.; Kos, J.; Gabrijelcic-Geiger, D.; Spiess, E.; Schirren, J.; Ebert, W.; Fiehn, W.; Luther, T.; et al. Cathepsin B, plasminogenactivator-inhibitor (PAI-1) and plasminogenactivator-receptor (uPAR) are prognostic factors for patients with non-small cell lung cancer. Anticancer. Res. 2004, 24, 4147–4161. [Google Scholar]
- Naderi, J.; Bernreuther, C.; Grabinski, N.; Putman, C.T.; Henkel, B.; Bell, G.; Glatzel, M.; Sultan, K.R. Plasminogen activator inhibitor type 1 up-regulation is associated with skeletal muscle atrophy and associated fibrosis. Am. J. Pathol. 2009, 175, 763–771. [Google Scholar] [CrossRef]
- Koh, T.J.; Bryer, S.C.; Pucci, A.M.; Sisson, T.H. Mice deficient in plasminogen activator inhibitor-1 have improved skeletal muscle regeneration. Am. J. Physiology. Cell Physiol. 2005, 289, C217–C223. [Google Scholar] [CrossRef]
- Krause, M.P.; Moradi, J.; Nissar, A.A.; Riddell, M.C.; Hawke, T.J. Inhibition of plasminogen activator inhibitor-1 restores skeletal muscle regeneration in untreated type 1 diabetic mice. Diabetes 2011, 60, 1964–1972. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. CCS 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet. Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, C.; Zhu, H.; Li, J.; Xu, W.; Sun, L.; Pan, L.; Dai, J.; Wang, Y.; Wang, E.; et al. Hypofractionated Radiosurgery Plus Bevacizumab for Locally Recurrent Brain Metastasis with Previously High-Dose Irradiation. World Neurosurg. 2020, 133, e252–e258. [Google Scholar] [CrossRef]
- Korshoej, A.R.; Lukacova, S.; Lassen-Ramshad, Y.; Rahbek, C.; Severinsen, K.E.; Guldberg, T.L.; Mikic, N.; Jensen, M.H.; Cortnum, S.O.S.; von Oettingen, G.; et al. OptimalTTF-1: Enhancing tumor treating fields therapy with skull remodeling surgery. A clinical phase I trial in adult recurrent glioblastoma. Neuro-Oncol. Adv. 2020, 2, vdaa121. [Google Scholar] [CrossRef]
- Møller, S.; Munck Af Rosenschöld, P.; Costa, J.; Law, I.; Poulsen, H.S.; Engelholm, S.A.; Engelholm, S. Toxicity and efficacy of re-irradiation of high-grade glioma in a phase I dose- and volume escalation trial. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2017, 125, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Sahebjam, S.; Forsyth, P.A.; Tran, N.D.; Arrington, J.A.; Macaulay, R.; Etame, A.B.; Walko, C.M.; Boyle, T.; Peguero, E.N.; Jaglal, M.; et al. Hypofractionated stereotactic re-irradiation with pembrolizumab and bevacizumab in patients with recurrent high-grade gliomas: Results from a phase I study. Neuro-Oncology 2021, 23, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Mantica, M.; Pritchard, A.; Lieberman, F.; Drappatz, J. Retrospective study of nivolumab for patients with recurrent high grade gliomas. J. Neuro-Oncol. 2018, 139, 625–631. [Google Scholar] [CrossRef]
- Adeberg, S.; Harrabi, S.B.; Bougatf, N.; Bernhardt, D.; Rieber, J.; Koerber, S.A.; Syed, M.; Sprave, T.; Mohr, A.; Abdollahi, A.; et al. Intensity-modulated proton therapy, volumetric-modulated arc therapy, and 3D conformal radiotherapy in anaplastic astrocytoma and glioblastoma: A dosimetric comparison. Strahlenther. Onkol. 2016, 192, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Soffietti, R.; Trevisan, E.; Bertero, L.; Cassoni, P.; Morra, I.; Fabrini, M.G.; Pasqualetti, F.; Lolli, I.; Castiglione, A.; Ciccone, G.; et al. Bevacizumab and fotemustine for recurrent glioblastoma: A phase II study of AINO (Italian Association of Neuro-Oncology). J. Neuro-Oncol. 2014, 116, 533–541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vredenburgh, J.J.; Desjardins, A.; Kirkpatrick, J.P.; Reardon, D.A.; Peters, K.B.; Herndon, J.E., 2nd; Marcello, J.; Bailey, L.; Threatt, S.; Sampson, J.; et al. Addition of bevacizumab to standard radiation therapy and daily temozolomide is associated with minimal toxicity in newly diagnosed glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, T.J.; Thornton, A.F.; Diaz, R.F.; Chandler, W.F.; Ensminger, W.D.; Junck, L.; Page, M.A.; Gebarski, S.S.; Hood, T.W.; Stetson, P.L.; et al. Intra-arterial bromodeoxyuridine radiosensitization of malignant gliomas. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 421–428. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Sampson, J.H.; Kunwar, S.; Chang, S.M.; Shaffrey, M.; Asher, A.L.; Lang, F.F.; Croteau, D.; Parker, K.; Grahn, A.Y.; et al. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: Phase 1 study of final safety results. Neurosurgery 2007, 61, 1031–1037; discussion 1037–1038. [Google Scholar] [CrossRef] [PubMed]
- Penna, F.; Ballarò, R.; Beltrà, M.; De Lucia, S.; García Castillo, L.; Costelli, P. The Skeletal Muscle as an Active Player Against Cancer Cachexia. Front. Physiol. 2019, 10, 41. [Google Scholar] [CrossRef]
- Yuan, L.; Han, J.; Meng, Q.; Xi, Q.; Zhuang, Q.; Jiang, Y.; Han, Y.; Zhang, B.; Fang, J.; Wu, G. Muscle-specific E3 ubiquitin ligases are involved in muscle atrophy of cancer cachexia: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Castillero, E.; Alamdari, N.; Lecker, S.H.; Hasselgren, P.O. Suppression of atrogin-1 and MuRF1 prevents dexamethasone-induced atrophy of cultured myotubes. Metab. Clin. Exp. 2013, 62, 1495–1502. [Google Scholar] [CrossRef]
- White, J.P.; Puppa, M.J.; Gao, S.; Sato, S.; Welle, S.L.; Carson, J.A. Muscle mTORC1 suppression by IL-6 during cancer cachexia: A role for AMPK. Am. J. Physiology. Endocrinol. Metab. 2013, 304, E1042–E1052. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Bres, E.E.; Faissner, A. Low Density Receptor-Related Protein 1 Interactions With the Extracellular Matrix: More Than Meets the Eye. Front. Cell Dev. Biol. 2019, 7, 31. [Google Scholar] [CrossRef]
- Hu, Q.; Su, H.; Li, J.; Lyon, C.; Tang, W.; Wan, M.; Hu, T.Y. Clinical applications of exosome membrane proteins. Precis. Clin. Med. 2020, 3, 54–66. [Google Scholar] [CrossRef]
- Sala, D.; Sacco, A. Signal transducer and activator of transcription 3 signaling as a potential target to treat muscle wasting diseases. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 171–176. [Google Scholar] [CrossRef]
- Kawao, N.; Ishida, M.; Kaji, H. Roles of leptin in the recovery of muscle and bone by reloading after mechanical unloading in high fat diet-fed obese mice. PLoS ONE 2019, 14, e0224403. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.D. Inhibition of Stat3 signaling ameliorates atrophy of the soleus muscles in mice lacking the vitamin D receptor. Skelet. Muscle 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.A.; Dong, J.; Dong, Y.; Dong, Y.; Schor, N.; Tweardy, D.J.; Zhang, L.; Mitch, W.E. Inhibition of Stat3 activation suppresses caspase-3 and the ubiquitin-proteasome system, leading to preservation of muscle mass in cancer cachexia. J. Biol. Chem. 2015, 290, 11177–11187. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.Q.; Lang, W.H.; Yu, H.Y.; Wang, L. Metabolic activation of TM5441 in vitro and in vivo: Formation of reactive metabolites and human enzymes involved. Eur. J. Pharm. Sci. 2020, 143, 105195. [Google Scholar] [CrossRef] [PubMed]
- Boe, A.E.; Eren, M.; Murphy, S.B.; Kamide, C.E.; Ichimura, A.; Terry, D.; McAnally, D.; Smith, L.H.; Miyata, T.; Vaughan, D.E. Plasminogen activator inhibitor-1 antagonist TM5441 attenuates Nω-nitro-L-arginine methyl ester-induced hypertension and vascular senescence. Circulation 2013, 128, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Dhanapal, R.; Saraswathi, T.; Govind, R.N. Cancer cachexia. J. Oral Maxillofac. Pathol. JOMFP 2011, 15, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Ramakrishna, R.; Magge, R.; Wernicke, A.G. Advances in Radiotherapy for Glioblastoma. Front. Neurol. 2017, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Elmaci, İ.; Altinoz, M.A. A Metabolic Inhibitory Cocktail for Grave Cancers: Metformin, Pioglitazone and Lithium Combination in Treatment of Pancreatic Cancer and Glioblastoma Multiforme. Biochem. Genet. 2016, 54, 573–618. [Google Scholar] [CrossRef] [PubMed]
- Yee, P.P.; Wei, Y.; Kim, S.Y.; Lu, T.; Chih, S.Y.; Lawson, C.; Tang, M.; Liu, Z.; Anderson, B.; Thamburaj, K.; et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat. Commun. 2020, 11, 5424. [Google Scholar] [CrossRef]
- Mazzola, R.; Fiorentino, A.; Alongi, F. Cachexia in Radiotherapy-Treated Patients With Head and Neck Cancer: A Phenomenon That Should Be Investigated. JAMA Oncol. 2016, 2, 831. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Xu, B.; Akers, J.; Nguyen, T.; Ma, J.; Dhawan, S.; Ning, J.; Mao, Y.; Hua, W.; Kokkoli, E.; et al. Radiation-induced extracellular vesicle (EV) release of miR-603 promotes IGF1-mediated stem cell state in glioblastomas. EBioMedicine 2020, 55, 102736. [Google Scholar] [CrossRef]
- Shin, E.; Lee, S.; Kang, H.; Kim, J.; Kim, K.; Youn, H.; Jin, Y.W.; Seo, S.; Youn, B. Organ-Specific Effects of Low Dose Radiation Exposure: A Comprehensive Review. Front. Genet. 2020, 11, 566244. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, J.; Dong, Y.; Tweardy, D.J.; Dong, Y.; Garibotto, G.; Mitch, W.E. Stat3 activation links a C/EBPδ to myostatin pathway to stimulate loss of muscle mass. Cell Metab. 2013, 18, 368–379. [Google Scholar] [CrossRef]

| Article References | GBM Patients (n) | Age Mean | Type of Treatment | Fatigue (Outcome) |
|---|---|---|---|---|
| [26] | 24 | 58 (36–73) | RT, CT | 1st–2nd 8 (33%), 3rd 1 (4%) |
| [27] | 15 | 57 (39–67) | RT, CT, S | 1st–2nd 8 (53%), 3rd 1 (6.7%) |
| [27] | 33 | 56 | RT, CT | 1st–2nd 6 (20%) |
| [28] | 25 | 54 (30–74) | RT, CT, S | Side effect |
| [29] | 36 | 53.5 (34–68) | RT, CT | 1st–2nd 15 (41.6%), 3rd 1 (2.7%) |
| [30] | 48 | 55 (25–75) | RT, CT, S | 1st–2nd 8 (12.9%) |
| [31] | 12 | 36.5 (26–63) | RT | 1st–2nd 11 (91.7%) |
| [32] | 54 | 57.1 | RT, CT, S | 1st–2nd 23 (42.6%), 3rd 2 (3.7%) |
| [33] | 125 | 56.2 (19–80) | RT, CT | 4th 2 (1.6%) |
| [34] | 23 | 48 (20–71) | RT, CT | Side effect |
| [35] | 21 | 55 (30–76) | RT, CT | 1–2nd 16 (73%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, E.; Kang, H.; Lee, H.; Lee, S.; Jeon, J.; Seong, K.; Youn, H.; Youn, B. Exosomal Plasminogen Activator Inhibitor-1 Induces Ionizing Radiation-Adaptive Glioblastoma Cachexia. Cells 2022, 11, 3102. https://doi.org/10.3390/cells11193102
Shin E, Kang H, Lee H, Lee S, Jeon J, Seong K, Youn H, Youn B. Exosomal Plasminogen Activator Inhibitor-1 Induces Ionizing Radiation-Adaptive Glioblastoma Cachexia. Cells. 2022; 11(19):3102. https://doi.org/10.3390/cells11193102
Chicago/Turabian StyleShin, Eunguk, Hyunkoo Kang, Haksoo Lee, Sungmin Lee, Jaewan Jeon, Kimoon Seong, Hyesook Youn, and Buhyun Youn. 2022. "Exosomal Plasminogen Activator Inhibitor-1 Induces Ionizing Radiation-Adaptive Glioblastoma Cachexia" Cells 11, no. 19: 3102. https://doi.org/10.3390/cells11193102
APA StyleShin, E., Kang, H., Lee, H., Lee, S., Jeon, J., Seong, K., Youn, H., & Youn, B. (2022). Exosomal Plasminogen Activator Inhibitor-1 Induces Ionizing Radiation-Adaptive Glioblastoma Cachexia. Cells, 11(19), 3102. https://doi.org/10.3390/cells11193102






