Elucidating the Anti-Tumorigenic Efficacy of Oltipraz, a Dithiolethione, in Glioblastoma
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture and Treatments
2.2. Cell Viability Assay
2.3. Colony Formation and Cell Migration Assay
2.4. Flow Cytometry Analysis
2.4.1. Cell Cycle Analysis
2.4.2. Measurement of ROS
2.4.3. Changes in Mitochondrial Membrane Potential (MMP: ∆ψm)
2.4.4. GSH Estimation
2.4.5. Assessment of Apoptosis Induction
2.5. Analysis of Stem Cell Markers
2.5.1. ALDH Assay
2.5.2. CD44 Estimation
2.5.3. Flow Cytometry Quantification of Nestin+ and GFAP+ Cells
2.6. Immunocytochemistry
2.7. Formation of Primary and Secondary Oncospheres
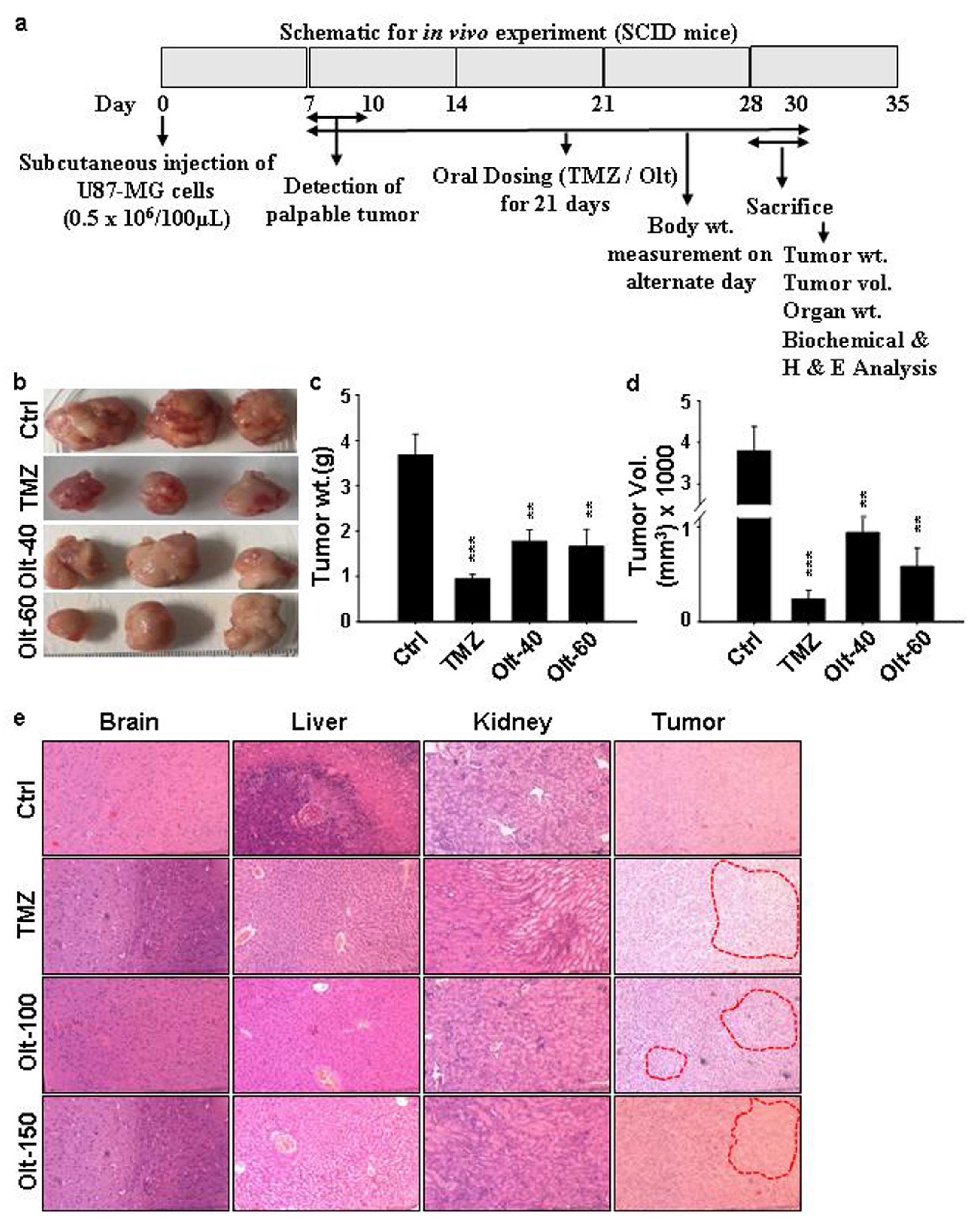
2.8. Ectopic GBM Model In Vivo
2.9. Statistical Data
3. Results
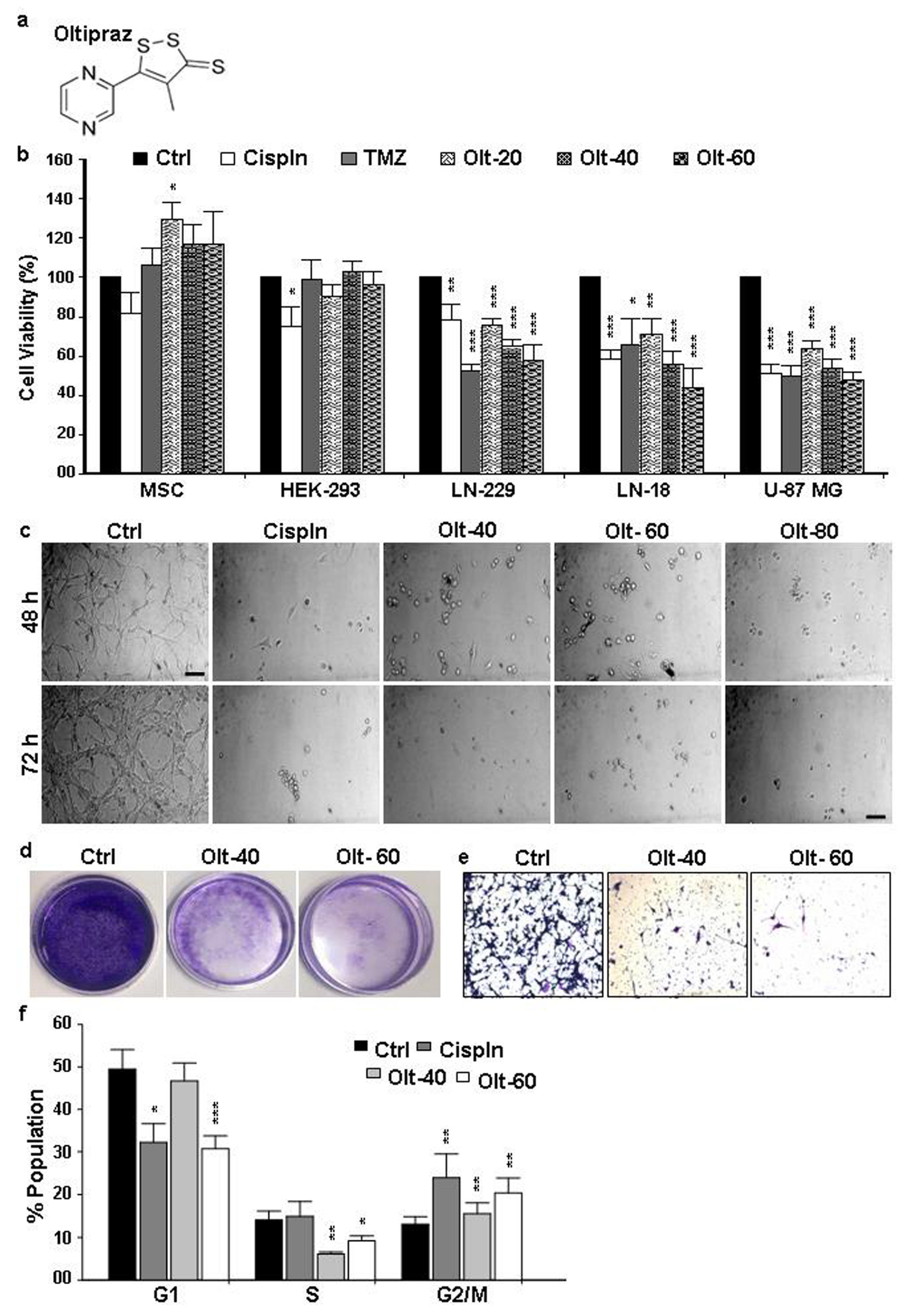
3.1. Olt Alters the Morphology and Growth of GBM Cells
3.2. Olt Impaired Migration and Colony Formation in GBM Cells
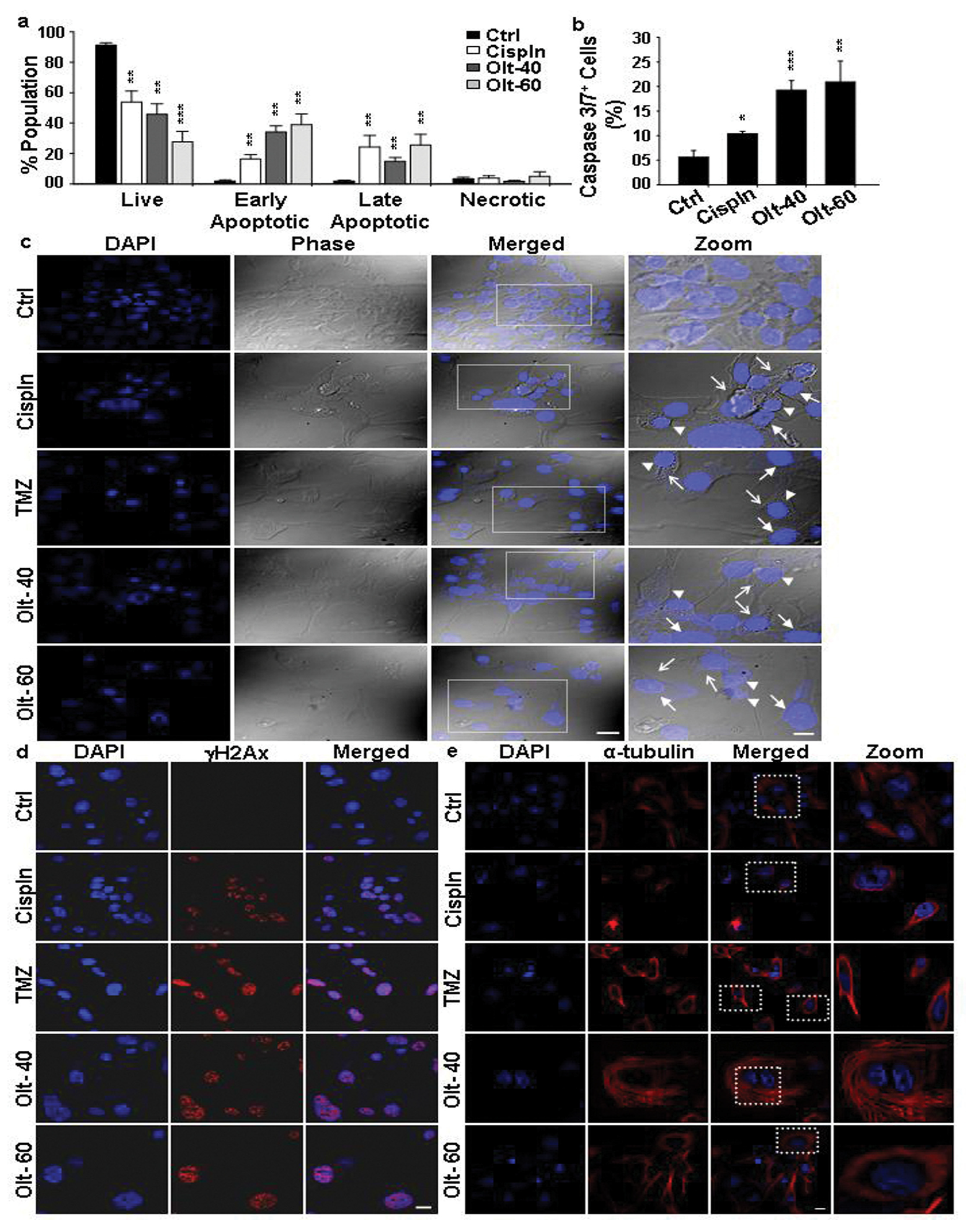
3.3. The Mode of Action of Olt in Rendering Growth Inhibition in GBM Cells
3.4. Olt Renders ROS-Dependent Apoptosis by Reducing Intracellular GSH Content and Disrupting Mitochondrial Membrane Potential
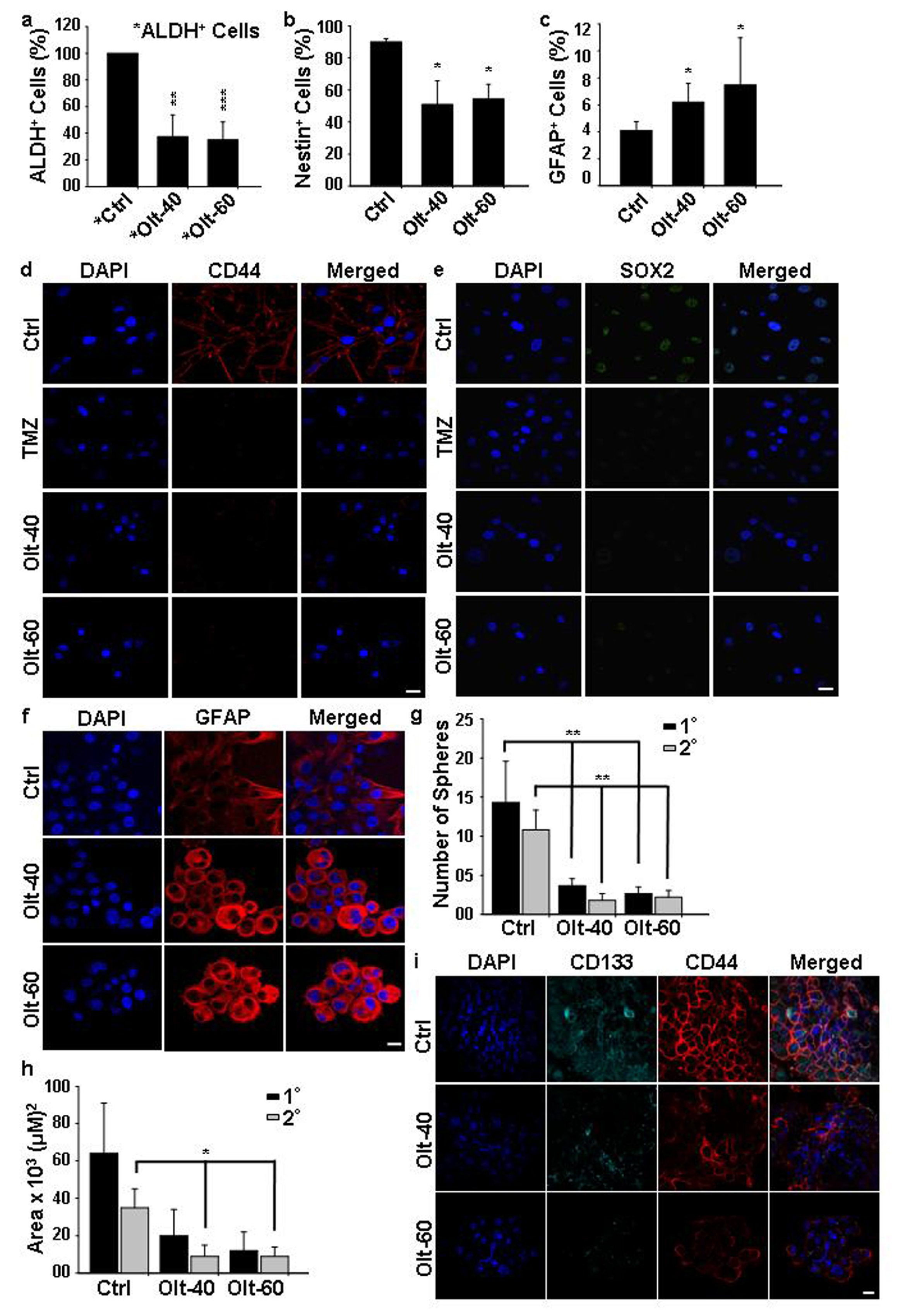
3.5. Olt Attenuates the Growth of GSCs and Induces Their Differentiation In Vitro
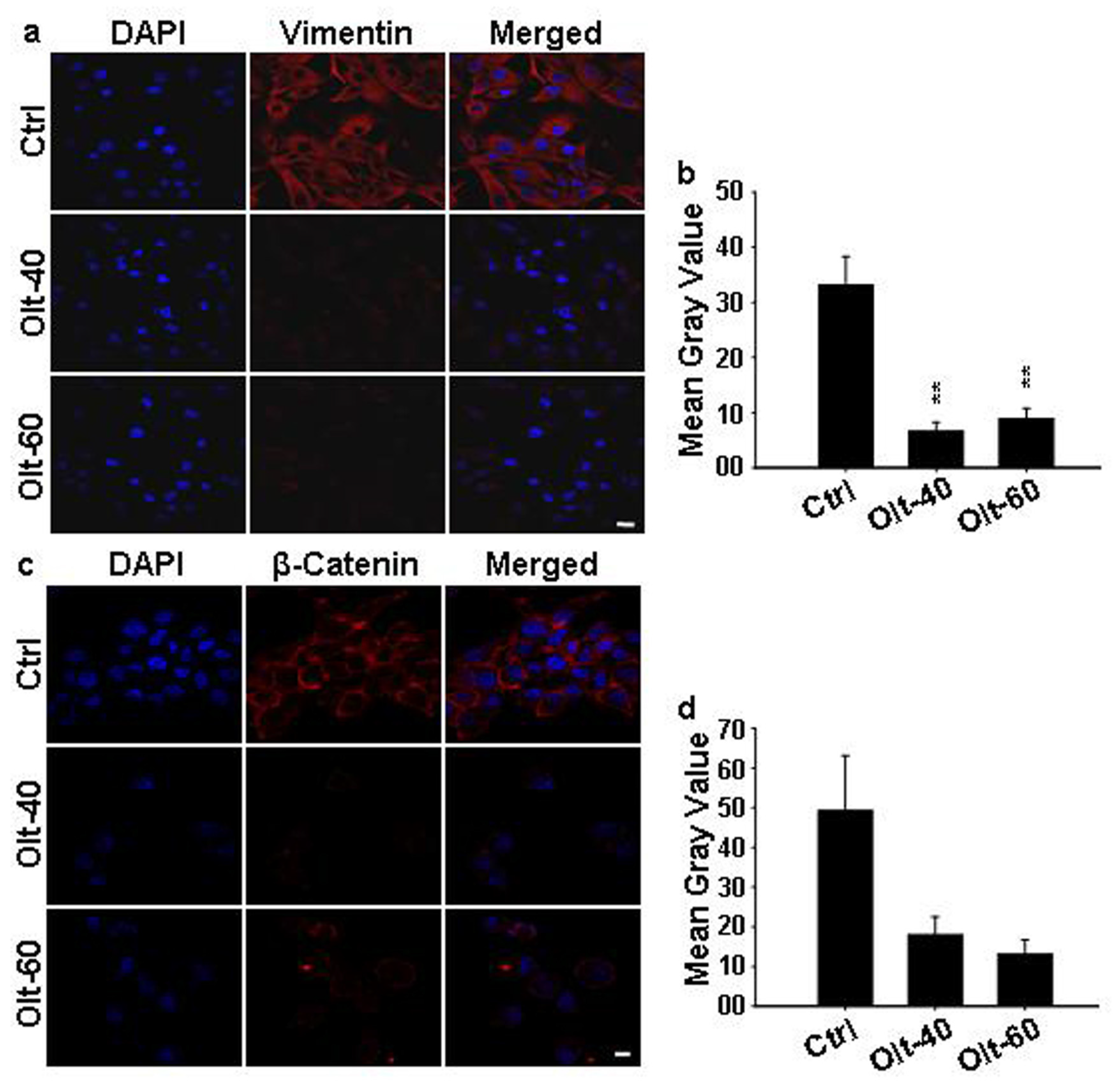
3.6. Olt Attenuates the Epithelial to Mesenchymal Transition
3.7. The Effect of Olt Administration on the Ectopic GBM Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Sathishkumar, K.; Chaturvedi, M.; Das, P.; Sudarshan, K.L.; Santhappan, S.; Nallasamy, V.; John, A.; Narasimhan, S.; Roselind, F.S.; et al. Cancer statistics, 2020: Report from national cancer registry programme, India. JCO Glob. Oncol. 2020, 6, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Bhattacharya, K.; Mandal, C. Nutritional stress reprograms dedifferentiation in glioblastoma multiforme driven by PTEN/Wnt/Hedgehog axis: A stochastic model of cancer stem cells. Cell Death Discov. 2018, 4, 110. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Daniele, S.; Natali, L.; Iofrida, C.; Flamini, G.; Braca, A.; Trincavelli, M.L.; Martini, C. Carnosol controls the human glioblastoma stemness features through the epithelial-mesenchymal transition modulation and the induction of cancer stem cell apoptosis. Sci. Rep. 2017, 7, 15174. [Google Scholar] [CrossRef]
- Zhou, H.M.; Zhang, J.G.; Zhang, X.; Li, Q. Targeting cancer stem cells for reversing therapy resistance: Mechanism, signaling, and prospective agents. Signal Transduct. Target Ther. 2021, 6, 62. [Google Scholar] [CrossRef]
- Qazi, M.A.; Vora, P.; Venugopal, C.; Sidhu, S.S.; Moffat, J.; Swanton, C.; Singh, S.K. Intratumoral heterogeneity: Pathways to treatment resistance and relapse in human glioblastoma. Ann. Oncol. 2017, 28, 1448–1456. [Google Scholar] [CrossRef]
- Sawant, M.A.; Dasgupta, A.; Lavhale, M.S.; Sitasawad, S.L. Novel triterpenoid AECHL-1 induces apoptosis in breast cancer cells by perturbing the mitochondria–endoplasmic reticulum interactions and targeting diverse apoptotic pathways. Biochim. Biophysic. Acta. 2016, 1860, 1056–1070. [Google Scholar] [CrossRef]
- Sorribes, I.C.; Handelman, S.K.; Jain, H.V. Mitigating temozolomide resistance in glioblastoma via DNA damage-repair inhibition. J. R. Soc. Interface 2020, 17, 20190722. [Google Scholar] [CrossRef]
- Lan, F.; Pan, Q.; Yu, H.; Yue, X. Sulforaphane enhances temozolomide-induced apoptosis because of down-regulation of miR-21 via Wnt/β-catenin signaling in glioblastoma. J. Neurochem. 2015, 134, 811–818. [Google Scholar] [CrossRef]
- Moskwa, J.; Naliwajko, S.K.; Markiewicz-Żukowskam, R.; Gromkowska-Kępka, K.J.; Nowakowski, P.; Strawa, J.W.; Borawska, M.H.; Tomczyk, M.; Socha, K. Chemical composition of Polish propolis and its antiproliferative effect in combination with Bacopa monnieri on glioblastoma cell lines. Sci. Rep. 2020, 10, 21127. [Google Scholar] [CrossRef]
- Fernando, W.; Rupasinghe, H.P.V.; Hoskin, D.W. Dietary phytochemicals with antioxidant and prooxidant activities: A double-edged sword in relation to adjuvant chemotherapy and radiotherapy? Cancer Lett. 2019, 452, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Haponska, M.; Balik, K.; Modrakowska, P.; Matulewicz, K.; Kazmierski, L.; Lis, A.; Kozlowska, J.; Garcia-Valls, R.; Giamberini, M.; et al. Stability and anti-proliferative properties of biologically active compounds extracted from Cistus L. after sterilization treatments. Sci. Rep. 2020, 10, 6521. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.F.; Polo, S.; Gallardo, N.; Leánez, S.; Pol, O. Analgesic and antidepressant effects of oltipraz on neuropathic pain in mice by modulating microglial activation. J. Clin. Med. 2019, 8, 890. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Bian, M.; Wu, J.; Li, D.; Ding, L.; Zeng, Q. Oltipraz prevents high glucose-induced oxidative stress and apoptosis in RSC96 cells through the Nrf2/NQO1 signaling pathway. BioMed Res. Int. 2020, 23, 2020. [Google Scholar]
- Yagishita, Y.; Gatbonton-Schwager, T.N.; McCallum, M.L.; Kensler, T.W. Current Landscape of NRF2 Biomarkers in Clinical Trials. Antioxidants 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Sirohi, V.K.; Gupta, K.; Dwivedi, A. Naringenin ameliorates progression of endometriosis by modulating Nrf2/Keap1/HO1 axis and inducing apoptosis in rats. J. Nutri. Biochem. 2019, 70, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, L.; Wang, Z.; Guan, Q.; Bao, K.; Wu, L. G2/M cell cycle arrest and apoptosis induced by COH-203 in human promyelocytic leukemia HL-60 cells. Oncol. Lett. 2021, 22, 815. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Zamzami, N.; de La Motte Rouge, T.; Lemaire, C.; Brenner, C.; Kroemer, G. Methods for the assessment of mitochondrial membrane permeabilization in apoptosis. Apoptosis 2007, 12, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Tripathi, M.; Chaudhari, B.P.; Pandey, P.K.; Kakkar, P. Natural terpenes prevent mitochondrial dysfunction, oxidative stress and release of apoptotic proteins during nimesulide-hepatotoxicity in rats. PLoS ONE 2012, 7, e34200. [Google Scholar] [CrossRef]
- Nguyen, P.; Doan, P.; Murugesan, A.; Ramesh, T.; Rimpilainen, T.; Candeias, N.R.; Yli-Harja, O.; Kandhavelu, M. GPR17 signaling activation by CHBC agonist induced cell death via modulation of MAPK pathway in glioblastoma. Life Sci. 2022, 291, 120307. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.H.; Lee, K.H.; Lee, B.S.; Alishir, A.; Ko, Y.J.; Kang, K.S.; Kim, K.H. Aviculin isolated from Lespedeza cuneata induce apoptosis in breast cancer cells through mitochondria-mediated caspase activation pathway. Molecules 2020, 25, 1708. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, Y.; Li, Z.; Wang, Y.; Liu, Y.; Cui, R.; Wang, Y. miR-504 suppresses mesenchymal phenotype of glioblastoma by directly targeting the FZD7-mediated Wnt–β-catenin pathway. J. Exp. Clin. Cancer Res. 2019, 38, 358. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.K.; Lenka, N. Notch exhibits ligand bias and maneuvers stage-specific steering of neural differentiation in embryonic stem cells. Mol. Cell. Biol. 2010, 30, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.K.; Lenka, N. Temporal and contextual orchestration of cardiac fate by WNT-BMP synergy and threshold. J. Cell. Mol. Med. 2010, 8, 2094–2108. [Google Scholar] [CrossRef]
- Ranjan, A.; Kaushik, I.; Srivastava, S.K. Pimozide suppresses the growth of brain tumors by targeting STAT3-mediated autophagy. Cells 2020, 9, 2141. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Hueng, D.Y.; Huang, H.Y.; Chen, J.Y.; Chen, Y. Magnolol and honokiol exert a synergistic anti-tumor effect through autophagy and apoptosis in human glioblastomas. Oncotarget 2016, 7, 29116–29130. [Google Scholar] [CrossRef]
- Yang, H.L.; Tsai, C.H.; Shrestha, S.; Lee, C.C.; Liao, J.W.; Hseu, Y.C. Coenzyme Q0, a novel quinone derivative of Antrodia camphorata, induces ROS-mediated cytotoxic autophagy and apoptosis against human glioblastoma cells in vitro and in vivo. Food Chem. Toxicol. 2021, 155, 112384. [Google Scholar] [CrossRef]
- Moody, T.W.; Switzer, C.; Santana-Flores, W.; Ridnour, L.A.; Berna, M.; Thill, M.; Jensen, R.T.; Sparatore, A.; DelSoldato, P.; Yeh, G.C.; et al. Dithiolethione modified valproate and diclofenac increase E-cadherin expression and decrease proliferation of non-small cell lung cancer cells. Lung Cancer 2010, 68, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Switzer, C.H.; Cheng, R.Y.; Ridnour, L.A.; Murray, M.C.; Tazzari, V.; Sparatore, A.; Del Soldato, P.; Hines, H.B.; Glynn, S.A.; Ambs, S.; et al. Dithiolethiones inhibit NF-κB activity via covalent modification in human estrogen receptor–negative breast cancer. Cancer Res. 2012, 72, 2394–2404. [Google Scholar]
- Nho, C.W.; O’Dwyer, P.J. NF-κB activation by the chemopreventive dithiolethione oltipraz is exerted through stimulation of MEKK3 signaling. J. Biol. Chem. 2004, 279, 26019–26027. [Google Scholar] [CrossRef]
- Tang, Q.; Ren, L.; Liu, J.; Li, W.; Zheng, X.; Wang, J.; Du, G. Withaferin A triggers G2/M arrest and intrinsic apoptosis in glioblastoma cells via ATF4-ATF3-CHOP axis. Cell Prolif. 2020, 53, e12706. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.Z.; Jan, C.R.; Hsu, S.S. Cytotoxic effects of gastrodin extracted from the rhizome of Gastrodia elata Blume in glioblastoma cells, but not in normal astrocytes, via the induction of oxidative stress-associated apoptosis that involved cell cycle arrest and p53 activation. Food Chem. Toxicol. 2017, 107, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Ma, Y.L.; Thakur, K.; Hussain, S.S.; Wang, J.; Zhang, Q.; Zhang, J.G.; Wei, Z.J. Molecular mechanism and inhibitory targets of dioscin in HepG2 cells. Food Chem. Toxicol. 2018, 120, 143–154. [Google Scholar] [CrossRef]
- Sahai, E. Mechanisms of cancer cell invasion. Curr. Opin. Gen. Dev. 2005, 15, 87–96. [Google Scholar] [CrossRef]
- Hirtz, A.; Lebourdais, N.; Rech, F.; Bailly, Y.; Vaginay, A.; Smaïl-Tabbone, M.; Dubois-Pot-Schneider, H.; Dumond, H. GPER agonist G-1 disrupts tubulin dynamics and potentiates temozolomide to impair glioblastoma cell proliferation. Cells 2021, 10, 3438. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Khandelwal, S. Role of oxidative stress and apoptosis in cadmium induced thymic atrophy and splenomegaly in mice. Toxicol. Lett. 2007, 169, 95–108. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Tsai, T.J.; Korivi, M.; Liu, J.Y.; Chen, H.J.; Lin, C.M.; Shen, Y.C.; Yang, H.L. Antitumor properties of Coenzyme Q0 against human ovarian carcinoma cells via induction of ROS-mediated apoptosis and cytoprotective autophagy. Sci. Rep. 2017, 7, 8062. [Google Scholar] [CrossRef]
- Queiroz, R.M.; Takiya, C.M.; Guimarães, L.P.; Rocha, G.D.; Alviano, D.S.; Blank, A.F.; Alviano, C.S.; Gattass, C.R. Apoptosis-inducing effects of Melissa officinalis L. essential oil in glioblastoma multiforme cells. Cancer Investig. 2014, 32, 226–235. [Google Scholar] [CrossRef]
- Czarnik-Kwaśniak, J.; Kwaśniak, K.; Kwasek, P.; Świerzowska, E.; Strojewska, A.; Tabarkiewicz, J. The influence of lycopene, [6]-gingerol, and silymarin on the apoptosis on U-118MG glioblastoma cells in vitro model. Nutrients 2019, 12, 96. [Google Scholar] [CrossRef]
- Suzuka, J.; Tsuda, M.; Wang, L.; Kohsaka, S.; Kishida, K.; Semba, S.; Sugino, H.; Aburatani, S.; Frauenlob, M.; Kurokawa, T.; et al. Rapid reprogramming of tumour cells into cancer stem cells on double-network hydrogels. Nat. Biomed. Eng. 2021, 5, 914–925. [Google Scholar] [CrossRef]
- Brescia, P.; Richichi, C.; Pelicci, G. Current strategies for identification of glioma stem cells: Adequate or unsatisfactory? J. Oncol. 2012, 2012, 376894. [Google Scholar] [CrossRef] [PubMed]
- Colapietro, A.; Mancini, A.; Vitale, F.; Martellucci, S.; Angelucci, A.; Llorens, S.; Mattei, V.; Gravina, G.L.; Alonso, G.L.; Festuccia, C. Crocetin extracted from saffron shows antitumor effects in models of human glioblastoma. Int. J. Mol. Sci. 2020, 21, 423. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Xu, X.; Lin, S.; Zhang, Y.; Liu, H.; Zhang, C.; Mo, R. A nanotherapeutic strategy to overcome chemotherapeutic resistance of cancer stem-like cells. Nat. Nanotech. 2021, 16, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.; Wang, Y.Y.; Yoo, J.Y.; Oh, C.; Otani, Y.; Kang, J.M.; Park, E.S.; Kim, E.; Chung, S.; Jeon, Y.J.; et al. MicroRNA-138 suppresses glioblastoma proliferation through downregulation of CD44. Sci. Rep. 2021, 11, 9219. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Inoue, A.; Ohnishi, T.; Yano, H.; Ozaki, S.; Kanemura, Y.; Suehiro, S.; Ohtsuka, Y.; Kohno, S.; Ohue, S.; et al. Hypoxia-induced phenotypic transition from highly invasive to less invasive tumors in glioma stem-like cells: Significance of CD44 and osteopontin as therapeutic targets in glioblastoma. Transl. Oncol. 2021, 14, 101137. [Google Scholar] [CrossRef]
- Rasper, M.; Schäfer, A.; Piontek, G.; Teufel, J.; Brockhoff, G.; Ringel, F.; Heindl, S.; Zimmer, C.; Schlegel, J. Aldehyde dehydrogenase 1 positive glioblastoma cells show brain tumor stem cell capacity. Neuro. Oncol. 2010, 12, 1024–1033. [Google Scholar] [CrossRef]
- van den Hoogen, C.; vander Horst, G.; Cheung, H.; Buijs, J.T.; Lippitt, J.M.; Guzmán-Ramírez, N.; Hamdy, F.C.; Eaton, C.L.; Thalmann, G.N.; Cecchini, M.G.; et al. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010, 70, 5163–5173. [Google Scholar] [CrossRef]
- MacDonagh, L.; Santiago, R.M.; Gray, S.G.; Breen, E.; Cuffe, S.; Finn, S.P.; O’Byrne, K.J.; Barr, M.P. Exploitation of the vitamin A/retinoic acid axis depletes ALDH1-positive cancer stem cells and re-sensitises resistant non-small cell lung cancer cells to cisplatin. Transl. Oncol. 2021, 14, 101025. [Google Scholar] [CrossRef]
- de Thé, H. Differentiation therapy revisited. Nat. Rev. Cancer 2018, 18, 117–127. [Google Scholar] [CrossRef]
- Enane, F.O.; Saunthararajah, Y.; Korc, M. Differentiation therapy and the mechanisms that terminate cancer cell proliferation without harming normal cells. Cell Death Dis. 2018, 9, 912. [Google Scholar] [CrossRef]
- Friedman, M.D.; Jeevan, D.S.; Tobias, M.; Murali, R.; Jhanwar-Uniyal, M. Targeting cancer stem cells in glioblastomamultiforme using mTOR inhibitors and the differentiating agent all-trans retinoic acid. Oncol. Rep. 2013, 30, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Gersey, Z.C.; Rodriguez, G.A.; Barbarite, E.; Sanchez, A.; Walters, W.M.; Ohaeto, K.C.; Komotar, R.J.; Graham, R.M. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer 2017, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Long, L.; Zheng, B.; Ji, W.; Yang, N.; Zhang, Q.; Liang, Z. Curcumin promotes differentiation of glioma-initiating cells by inducing autophagy. Cancer Sci. 2012, 103, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Gilles, C.; Polette, M.; Mestdagt, M.; Nawrocki-Raby, B.; Ruggeri, P.; Birembaut, P.; Foidart, J.M. Transactivation of vimentin by β-catenin in human breast cancer cells. Cancer Res. 2003, 63, 2658–2664. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, J.; Dai, X.; Ye, L.; Wang, B.; Cheng, H.; Wang, W. Raddeanin A inhibited epithelial-mesenchymal transition (EMT) and angiogenesis in glioblastoma by downregulating β-catenin expression. Int. J. Med. Sci. 2021, 18, 1609. [Google Scholar] [CrossRef]
- Schmitt, M.; Metzger, M.; Gradl, D.; Davidson, G.; Orian-Rousseau, V. CD44 functions in Wnt signaling by regulating LRP6 localization and activation. Cell Death Differ. 2015, 22, 677–689. [Google Scholar] [CrossRef]
- Baek, S.; Lee, W.R. Down-regulation of CD44 inhibits Wnt/β-catenin mediated cancer cell migration and invasion in gastric cancer. J. Emerg. Investig. 2021, 4, 1–6. [Google Scholar]
- Zanotto-Filho, A.; Braganhol, E.; Klafke, K.; Figueiro, F.; Terra, S.R.; Paludo, F.J.; Morrone, M.; Bristot, I.J.; Battastini, A.M.; Forcelini, C.M.; et al. Autophagy inhibition improves the efficacy of curcumin/temozolomide combination therapy in glioblastomas. Cancer Lett. 2015, 358, 220–231. [Google Scholar] [CrossRef]
- Ghosh, S.; Dutta, N.; Banerjee, P.; Gajbhiye, R.L.; Sareng, H.R.; Kapse, P.; Pal, S.; Burdelya, L.; Mandal, N.C.; Ravichandiran, V.; et al. Induction of monoamine oxidase A-mediated oxidative stress and impairment of NRF2-antioxidant defence response by polyphenol-rich fraction of Bergenialigulata sensitizes prostate cancer cells in vitro and in vivo. Free Radic. Biol. Med. 2021, 172, 136–151. [Google Scholar] [CrossRef]

| Groups | Initial/Day 0 | Week-I | Week-II | Week-III |
|---|---|---|---|---|
| Ctrl | 21.5 ± 1.73 | 21.9 ± 1.21 | 21.3 ± 1.24 | 22.5 ± 1.58 |
| TMZ-50 * | 21.5 ± 1.96 | 21.9 ± 1.36 | 20.5 ± 1.41 | 21.8 ± 1.09 |
| Olt-100 * | 21.3 ± 2.31 | 20.0 ± 1.02 | 20.3 ± 0.74 | 20.5 ± 0.86 |
| Olt-150 * | 20.4 ± 1.56 | 20.8 ± 1.07 | 20.9 ± 0.82 | 21.1 ± 1.40 |
| Organs | Ctrl | TMZ-50 * | Olt-100 * | Olt-150 * |
|---|---|---|---|---|
| Brain | 1.52 ± 0.30 | 1.89 ± 0.11 | 2.16 ± 0.14 | 2.06 ± 0.22 |
| Liver | 4.27 ± 0.93 | 5.78 ± 0.54 | 5.38 ± 0.40 | 5.86 ± 1.54 |
| Kidney | 1.15 ± 0.27 | 1.39 ± 0.13 | 1.35 ± 0.07 | 1.34 ± 0.20 |
| Heart | 0.44 ± 0.10 | 0.68 ± 0.09 | 0.57 ± 0.09 | 0.52 ± 0.07 |
| Lungs | 0.93 ± 0.24 | 0.95 ± 0.16 | 1.28 ± 0.19 | 1.17 ± 0.19 |
| Spleen | 0.80 ± 0.24 | 0.45 ± 0.03 * | 0.76 ± 0.12 | 0.56 ± 0.21 |
| Biochemical Parameters | Ctrl | TMZ-50 * | Olt-100 * | Olt-150 * |
|---|---|---|---|---|
| Total Bilirubin (mg/dL) | 0.42 ± 0.04 | 0.47 ± 0.05 | 0.60 ± 0.20 | 0.57 ± 0.08 |
| Bilirubin Direct (mg/dL) | 0.25 ± 0.03 | 0.28 ± 0.04 | 0.30 ± 0.05 | 0.26 ± 0.03 |
| Bilirubin Indirect (mg/dL) | 0.17 ± 0.02 | 0.15 ± 0.02 | 0.30 ± 0.10 | 0.23 ± 0.03 |
| SGPT (IU/L) | 34.67 ± 17.33 | 43.08 ± 5.84 | 55.63 ± 6.71 | 44.66 ± 8.17 |
| SGOT (IU/L) | 108.32 ± 15.2 | 122.28 ± 22.91 | 93.00 ± 21.0 | 131 ± 77.7 |
| Blood Urea (mg/dL) | 39.77 ± 6.14 | 44.87 ± 3.48 | 38.33 ± 4.40 | 38.5 ± 3.06 |
| Blood Urea nitrogen (mg/dL) | 18.58 ± 2.87 | 20.96 ± 1.62 | 17.91 ± 2.06 | 17.99 ± 1.43 |
| Uric Acid (mg/dL) | 1.3 ± 0.33 | 1.75 ± 0.09 | 1.60 ± 0.20 | 1.60 ± 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapoor-Narula, U.; Lenka, N. Elucidating the Anti-Tumorigenic Efficacy of Oltipraz, a Dithiolethione, in Glioblastoma. Cells 2022, 11, 3057. https://doi.org/10.3390/cells11193057
Kapoor-Narula U, Lenka N. Elucidating the Anti-Tumorigenic Efficacy of Oltipraz, a Dithiolethione, in Glioblastoma. Cells. 2022; 11(19):3057. https://doi.org/10.3390/cells11193057
Chicago/Turabian StyleKapoor-Narula, Upasana, and Nibedita Lenka. 2022. "Elucidating the Anti-Tumorigenic Efficacy of Oltipraz, a Dithiolethione, in Glioblastoma" Cells 11, no. 19: 3057. https://doi.org/10.3390/cells11193057
APA StyleKapoor-Narula, U., & Lenka, N. (2022). Elucidating the Anti-Tumorigenic Efficacy of Oltipraz, a Dithiolethione, in Glioblastoma. Cells, 11(19), 3057. https://doi.org/10.3390/cells11193057





