Mast Cell Interaction with Foxp3+ Regulatory T Cells Occur in the Dermis after Initiation of IgE-Mediated Cutaneous Anaphylaxis
Abstract
1. Introduction
2. Materials & Methods
2.1. Mouse Strains
2.2. Passive Cutaneous Anaphylaxis (PCA) Experiments
2.3. Intravital Confocal Microscopy
2.4. Determination of Cell Numbers, Tracking Cellular Motility and Velocity
2.5. Quantification of Cell-Cell Interactions
3. Results
3.1. Mast Cells Are Sessile and, after Conditional Ablation, Undergo Slow Repopulation Kinetics in the Skin
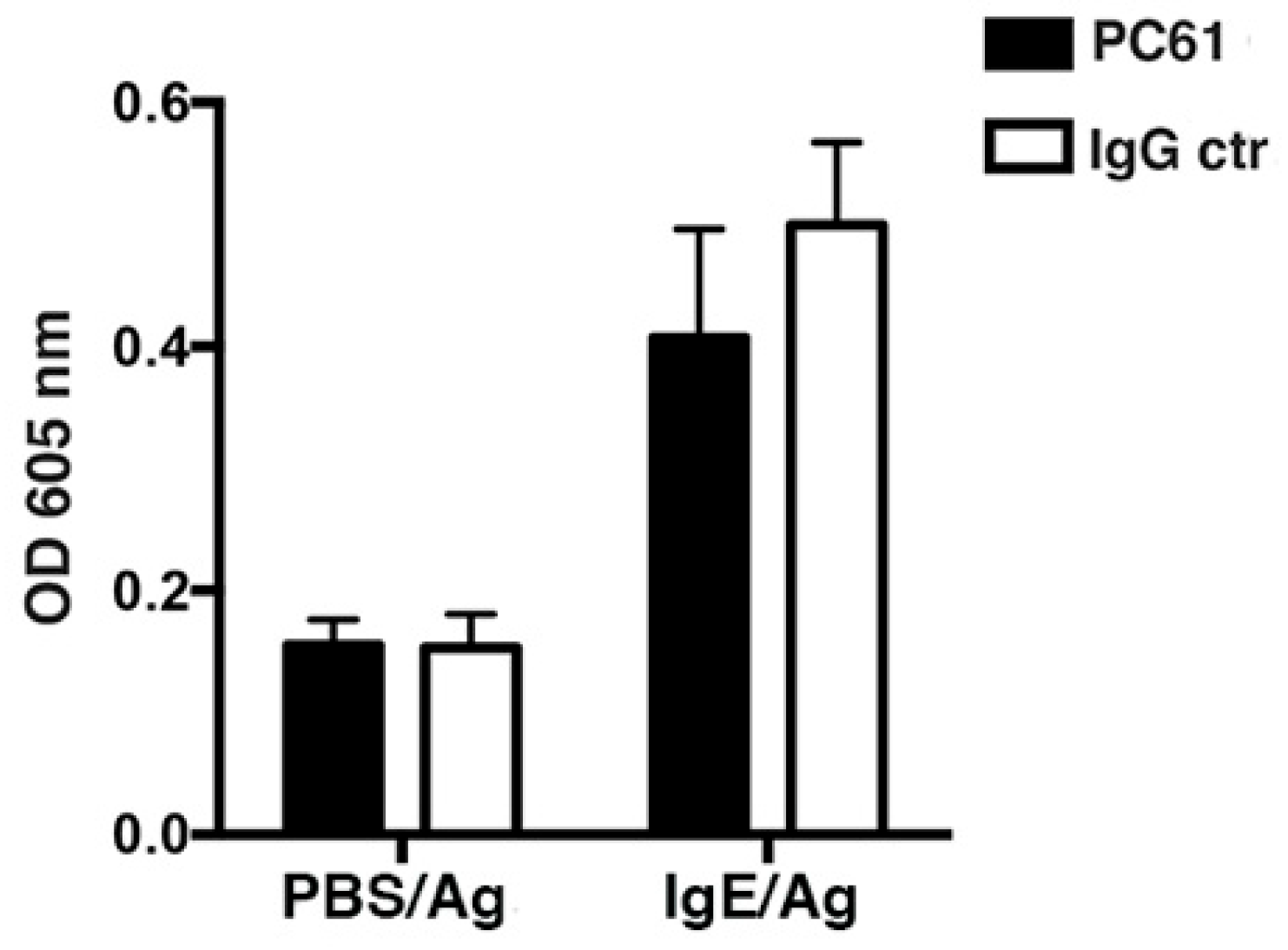
3.2. DT-mediated Depletion of MCs Evidences their Role in Passive Cutaneous Anaphylaxis
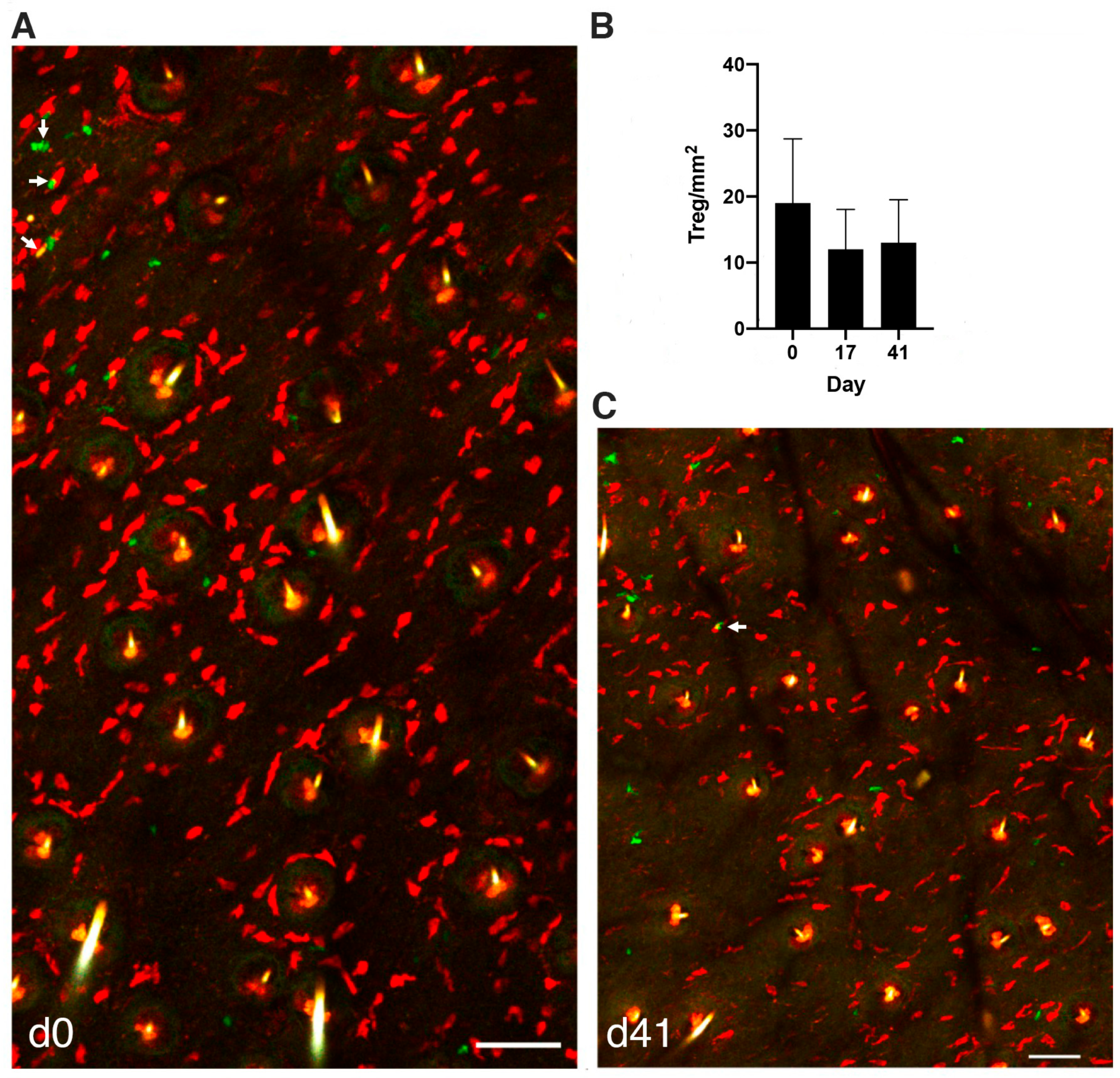
3.3. MCs Interact with Tregs after Initiation of the PCA
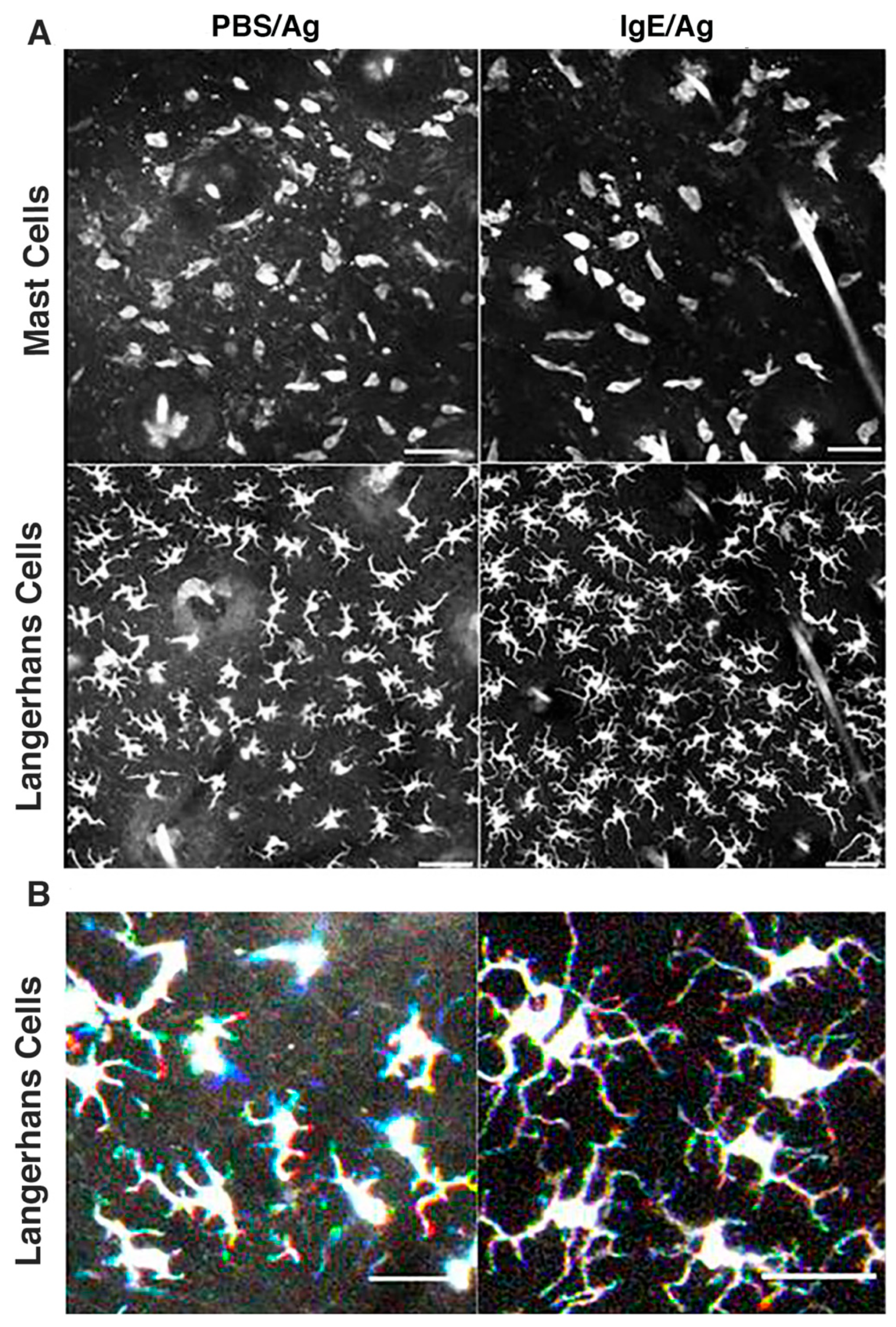
3.4. Langerhans Cells Remain Sessile While Exhibiting Constant Sprouting of Their Dendrites
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nestle, F.O.; Di Meglio, P.; Qin, J.-Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Maurer, M. Innate immunity and allergy in the skin. Curr. Opin. Immunol. 2009, 21, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Levi-Schaffer, F.; Gibbs, B.F.; Hallgren, J.; Pucillo, C.; Redegeld, F.; Siebenhaar, F.; Vitte, J.; Mezouar, S.; Michel, M.; Puzzovio, P.G.; et al. Selected recent advances in understanding the role of human mast cells in health and disease. J. Allergy Clin. Immunol. 2022, 149, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Starkl, P.; Marichal, T.; Galli, S.J. Testing the ‘toxin hypothesis of allergy’: Mast cells, IgE, and innate and acquired immune responses to venoms. Curr. Opin. Immunol. 2015, 36, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.; Cervantes-García, D.; Córdova-Dávalos, L.E.; Pérez-Rodríguez, M.J.; Gonzalez-Espinosa, C.; Salinas, E. Responses of Mast Cells to Pathogens: Beneficial and Detrimental Roles. Front. Immunol. 2021, 12, 685865. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef]
- de Vries, V.C.; Noelle, R.J. Mast cell mediators in tolerance. Curr. Opin. Immunol. 2010, 22, 643–648. [Google Scholar] [CrossRef]
- Harvima, I.; Nilsson, G. Mast Cells as Regulators of Skin Inflammation and Immunity. Acta Derm. Venereol. 2011, 91, 644–650. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Ribatti, D. Mast cell-mediated mechanistic pathways in organ transplantation. Eur. J. Pharmacol. 2019, 857, 172458. [Google Scholar] [CrossRef]
- Merad, M.; Manz, M.G. Dendritic cell homeostasis. Blood 2009, 113, 3418–3427. [Google Scholar] [CrossRef]
- Schlitzer, A.; Ginhoux, F. Organization of the mouse and human DC network. Curr. Opin. Immunol. 2014, 26, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Malissen, B.; Tamoutounour, S.; Henri, S. The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol. 2014, 14, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Kashem, S.W.; Haniffa, M.; Kaplan, D.H. Antigen-Presenting Cells in the Skin. Annu. Rev. Immunol. 2017, 35, 469–499. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.H. Ontogeny and function of murine epidermal Langerhans cells. Nat. Immunol. 2017, 18, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Cabeza-Cabrerizo, M.; Cardoso, A.; Minutti, C.M.; da Costa, M.P.; e Sousa, C.R. Dendritic Cells Revisited. Annu. Rev. Immunol. 2021, 39, 131–166. [Google Scholar] [CrossRef]
- Nakamizo, S.; Dutertre, C.-A.; Khalilnezhad, A.; Zhang, X.M.; Lim, S.; Lum, J.; Koh, G.; Foong, C.; Yong, P.J.A.; Tan, K.J.; et al. Single-cell analysis of human skin identifies CD14+ type 3 dendritic cells co-producing IL1B and IL23A in psoriasis. J. Exp. Med. 2021, 218, e20202345. [Google Scholar] [CrossRef]
- Josefowicz, S.Z.; Lu, L.-F.; Rudensky, A.Y. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu. Rev. Immunol. 2012, 30, 531–564. [Google Scholar] [CrossRef]
- Shevyrev, D.; Tereshchenko, V. Treg Heterogeneity, Function, and Homeostasis. Front. Immunol. 2020, 10, 3100. [Google Scholar] [CrossRef]
- Bennett, C.; Christie, J.; Ramsdell, F.; Brunkow, M.E.; Ferguson, P.J.; Whitesell, L.; Kelly, T.E.; Saulsbury, F.T.; Chance, P.F.; Ochs, H.D. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001, 27, 20–21. [Google Scholar] [CrossRef]
- Frossi, B.; D’Incà, F.; Crivellato, E.; Sibilano, R.; Gri, G.; Mongillo, M.; Danelli, L.; Maggi, L.; Pucillo, C.E. Single-cell dynamics of mast cell-CD4+CD25+ regulatory T cell interactions. Eur. J. Immunol. 2011, 41, 1872–1882. [Google Scholar] [CrossRef]
- Katsoulis-Dimitriou, K.; Kotrba, J.; Voss, M.; Dudeck, J.; Dudeck, A. Mast Cell Functions Linking Innate Sensing to Adaptive Immunity. Cells 2020, 9, 2538. [Google Scholar] [CrossRef] [PubMed]
- Roediger, B.; Kyle, R.; Yip, K.H.; Sumaria, N.; Guy, T.V.; Kim, B.S.; Mitchell, A.J.; Tay, S.S.; Jain, R.; Forbes-Blom, E.; et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat. Immunol. 2013, 14, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Rafei-Shamsabadi, D.A.; Klose, C.S.N.; Halim, T.Y.F.; Tanriver, Y.; Jakob, T. Context Dependent Role of Type 2 Innate Lymphoid Cells in Allergic Skin Inflammation. Front. Immunol. 2019, 10, 2591. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-F.; Lind, E.F.; Gondek, D.C.; Bennett, K.A.; Gleeson, M.W.; Pino-Lagos, K.; Scott, Z.A.; Coyle, A.J.; Reed, J.L.; Van Snick, J.; et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 2006, 442, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- de Vries, V.C.; Pino-Lagos, K.; Nowak, E.C.; Bennett, K.A.; Oliva, C.; Noelle, R.J. Mast Cells Condition Dendritic Cells to Mediate Allograft Tolerance. Immunity 2011, 35, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Gri, G.; Piconese, S.; Frossi, B.; Manfroi, V.; Merluzzi, S.; Tripodo, C.; Viola, A.; Odom, S.; Rivera, J.; Colombo, M.P.; et al. CD4+CD25+ Regulatory T Cells Suppress Mast Cell Degranulation and Allergic Responses through OX40-OX40L Interaction. Immunity 2008, 29, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Piconese, S.; Gri, G.; Tripodo, C.; Musio, S.; Gorzanelli, A.; Frossi, B.; Pedotti, R.; Pucillo, C.E.; Colombo, M.P. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood 2009, 114, 2639–2648. [Google Scholar] [CrossRef]
- Sibilano, R.; Gri, G.; Frossi, B.; Tripodo, C.; Suzuki, R.; Rivera, J.; MacDonald, A.S.; Pucillo, C.E. Technical Advance: Soluble OX40 molecule mimics regulatory T cell modulatory activity on FcεRI-dependent mast cell degranulation. J. Leukoc. Biol. 2011, 90, 831–838. [Google Scholar] [CrossRef]
- Kanjarawi, R.; Dy, M.; Bardel, E.; Sparwasser, T.; Dubois, B.; Mecheri, S.; Kaiserlian, D. Regulatory CD4+Foxp3+ T Cells Control the Severity of Anaphylaxis. PLoS ONE 2013, 8, e69183. [Google Scholar] [CrossRef]
- Ganeshan, K.; Bryce, P.J. Regulatory T Cells Enhance Mast Cell Production of IL-6 via Surface-Bound TGF-β. J. Immunol. 2011, 188, 594–603. [Google Scholar] [CrossRef]
- Dahdah, A.; Gautier, G.; Attout, T.; Fiore, F.; Lebourdais, E.; Msallam, R.; Daëron, M.; Monteiro, R.; Benhamou, M.; Charles, N.; et al. Mast cells aggravate sepsis by inhibiting peritoneal macrophage phagocytosis. J. Clin. Investig. 2014, 124, 4577–4589. [Google Scholar] [CrossRef] [PubMed]
- Kissenpfennig, A.; Henri, S.; Dubois, B.; Laplace-Builhé, C.; Perrin, P.; Romani, N.; Tripp, C.H.; Douillard, P.; Leserman, L.; Kaiserlian, D.; et al. Dynamics and Function of Langerhans Cells In Vivo: Dermal Dendritic Cells Colonize Lymph Node AreasDistinct from Slower Migrating Langerhans Cells. Immunity 2005, 22, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kissenpfennig, A.; Mingueneau, M.; Richelme, S.; Perrin, P.; Chevrier, S.; Genton, C.; Lucas, B.; DiSanto, J.P.; Acha-Orbea, H.; et al. The Lymphoproliferative Disorder of LatY136F Mutant Mice Unfolds Independently of TCR-MHC Engagement and Is Insensitive to the Action of Foxp3+ Regulatory T Cells. J. Immunol. 2008, 180, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Longé, C.; Bratti, M.; Kurowska, M.; Vibhushan, S.; David, P.; Desmeure, V.; Huang, J.-D.; Fischer, A.; Basile, G.D.S.; Sepulveda, F.E.; et al. Rab44 regulates murine mast cell–driven anaphylaxis through kinesin-1–dependent secretory granule translocation. J. Allergy Clin. Immunol. 2022, 150, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Kohm, A.P.; McMahon, J.S.; Podojil, J.R.; Begolka, W.S.; DeGutes, M.; Kasprowicz, D.J.; Ziegler, S.F.; Miller, S.D. Cutting Edge: Anti-CD25 Monoclonal Antibody Injection Results in the Functional Inactivation, Not Depletion, of CD4+CD25+ T Regulatory Cells. J. Immunol. 2006, 176, 3301–3305. [Google Scholar] [CrossRef]
- Stephens, L.A.; Anderton, S.M. Comment on “Cutting Edge: Anti-CD25 Monoclonal Antibody Injection Results in the Functional Inactivation, not Depletion, of CD4+CD25+ T Regulatory Cells”. J. Immunol. 2006, 177, 2036. [Google Scholar] [CrossRef]
- Zelenay, S.; Demengeot, J. Comment on “Cutting Edge: Anti-CD25 Monoclonal Antibody Injection Results in the Functional Inactivation, not Depletion, of CD4+CD25+ T Regulatory Cells”. J. Immunol. 2006, 177, 2036–2037. [Google Scholar] [CrossRef]
- Demandolx, D.; Davoust, J. Multicolour analysis and local image correlation in confocal microscopy. J. Microsc. 1997, 185, 21–36. [Google Scholar] [CrossRef]
- Chappert, P.; Leboeuf, M.; Rameau, P.; Stockholm, D.; Liblau, R.; Danos, O.; Davoust, J.; Gross, D. Antigen-Driven Interactions with Dendritic Cells and Expansion of Foxp3+ Regulatory T Cells Occur in the Absence of Inflammatory Signals. J. Immunol. 2007, 180, 327–334. [Google Scholar] [CrossRef]
- Nyekel, F.N.; Pacreau, E.; Benadda, S.; Msallam, R.; Åbrink, M.; Pejler, G.; Davoust, J.; Benhamou, M.; Charles, N.; Launay, P.; et al. Mast Cell Degranulation Exacerbates Skin Rejection by Enhancing Neutrophil Recruitment. Front. Immunol. 2018, 9, 2690. [Google Scholar] [CrossRef]
- Reber, L.L.; Marichal, T.; Galli, S.J. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012, 33, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Henri, S.; Poulin, L.F.; Tamoutounour, S.; Ardouin, L.; Guilliams, M.; De Bovis, B.; Devilard, E.; Viret, C.; Azukizawa, H.; Kissenpfennig, A.; et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J. Exp. Med. 2010, 207, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Grimbaldeston, M.A.; Tsai, M. Immunomodulatory mast cells: Negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008, 8, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Beghdadi, W.; Madjène, L.C.; Benhamou, M.; Charles, N.; Gautier, G.; Launay, P.; Blank, U. Mast cells as cellular sensors in inflammation and immunity. Front. Immunol. 2011, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Blank, U.; Huang, H.; Kawakami, T. The high affinity IgE receptor: A signaling update. Curr. Opin. Immunol. 2021, 72, 51–58. [Google Scholar] [CrossRef]
- Ménasché, G.; Longé, C.; Bratti, M.; Blank, U. Cytoskeletal Transport, Reorganization, and Fusion Regulation in Mast Cell-Stimulus Secretion Coupling. Front. Cell Dev. Biol. 2021, 9, 652077. [Google Scholar] [CrossRef]
- Grimbaldeston, M.A.; Chen, C.-C.; Piliponsky, A.M.; Tsai, M.; Tam, S.-Y.; Galli, S.J. Mast Cell-Deficient W-sash c-kit Mutant KitW-sh/W-sh Mice as a Model for Investigating Mast Cell Biology in Vivo. Am. J. Pathol. 2005, 167, 835–848. [Google Scholar] [CrossRef]
- Danelli, L.; Madjene, L.C.; Madera-Salcedo, I.K.; Gautier, G.; Pacreau, E.; Ben Mkaddem, S.; Charles, N.; Daugas, E.; Launay, P.; Blank, U. Early Phase Mast Cell Activation Determines the Chronic Outcome of Renal Ischemia–Reperfusion Injury. J. Immunol. 2017, 198, 2374–2382. [Google Scholar] [CrossRef]
- Morgan, R.K.; McAllister, B.; Cross, L.; Green, D.S.; Kornfeld, H.; Center, D.M.; Cruikshank, W.W. Histamine 4 Receptor Activation Induces Recruitment of FoxP3+ T Cells and Inhibits Allergic Asthma in a Murine Model. J. Immunol. 2007, 178, 8081–8089. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gao, T.-Y.; Huang, F.-F.; Xie, Y.-Y.; Wang, W.-Q.; Wang, L.-D.; Mu, D.; Cui, Y. Dynamic changes in the systemic immune responses of spinal cord injury model mice. Neural Regen. Res. 2021, 16, 382–387. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msallam, R.; Malissen, B.; Launay, P.; Blank, U.; Gautier, G.; Davoust, J. Mast Cell Interaction with Foxp3+ Regulatory T Cells Occur in the Dermis after Initiation of IgE-Mediated Cutaneous Anaphylaxis. Cells 2022, 11, 3055. https://doi.org/10.3390/cells11193055
Msallam R, Malissen B, Launay P, Blank U, Gautier G, Davoust J. Mast Cell Interaction with Foxp3+ Regulatory T Cells Occur in the Dermis after Initiation of IgE-Mediated Cutaneous Anaphylaxis. Cells. 2022; 11(19):3055. https://doi.org/10.3390/cells11193055
Chicago/Turabian StyleMsallam, Rasha, Bernard Malissen, Pierre Launay, Ulrich Blank, Gregory Gautier, and Jean Davoust. 2022. "Mast Cell Interaction with Foxp3+ Regulatory T Cells Occur in the Dermis after Initiation of IgE-Mediated Cutaneous Anaphylaxis" Cells 11, no. 19: 3055. https://doi.org/10.3390/cells11193055
APA StyleMsallam, R., Malissen, B., Launay, P., Blank, U., Gautier, G., & Davoust, J. (2022). Mast Cell Interaction with Foxp3+ Regulatory T Cells Occur in the Dermis after Initiation of IgE-Mediated Cutaneous Anaphylaxis. Cells, 11(19), 3055. https://doi.org/10.3390/cells11193055





