The Use of Retinal Microvascular Function and Telomere Length in Age and Blood Pressure Prediction in Individuals with Low Cardiovascular Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. General Investigations
2.3. Blood Analyses
2.4. Framingham Risk Score (FRS) Calculation
2.5. Dynamic Retinal Microvascular Function Vessel Analysis
2.6. Relative Telomere Length (RTL) Assessment
2.7. Sample Size and Analysis
2.7.1. Statistical Analysis
2.7.2. Symbolic Regression-Based Analysis
3. Results
3.1. Differences in Retinal Vascular Function
3.2. Correlation Results
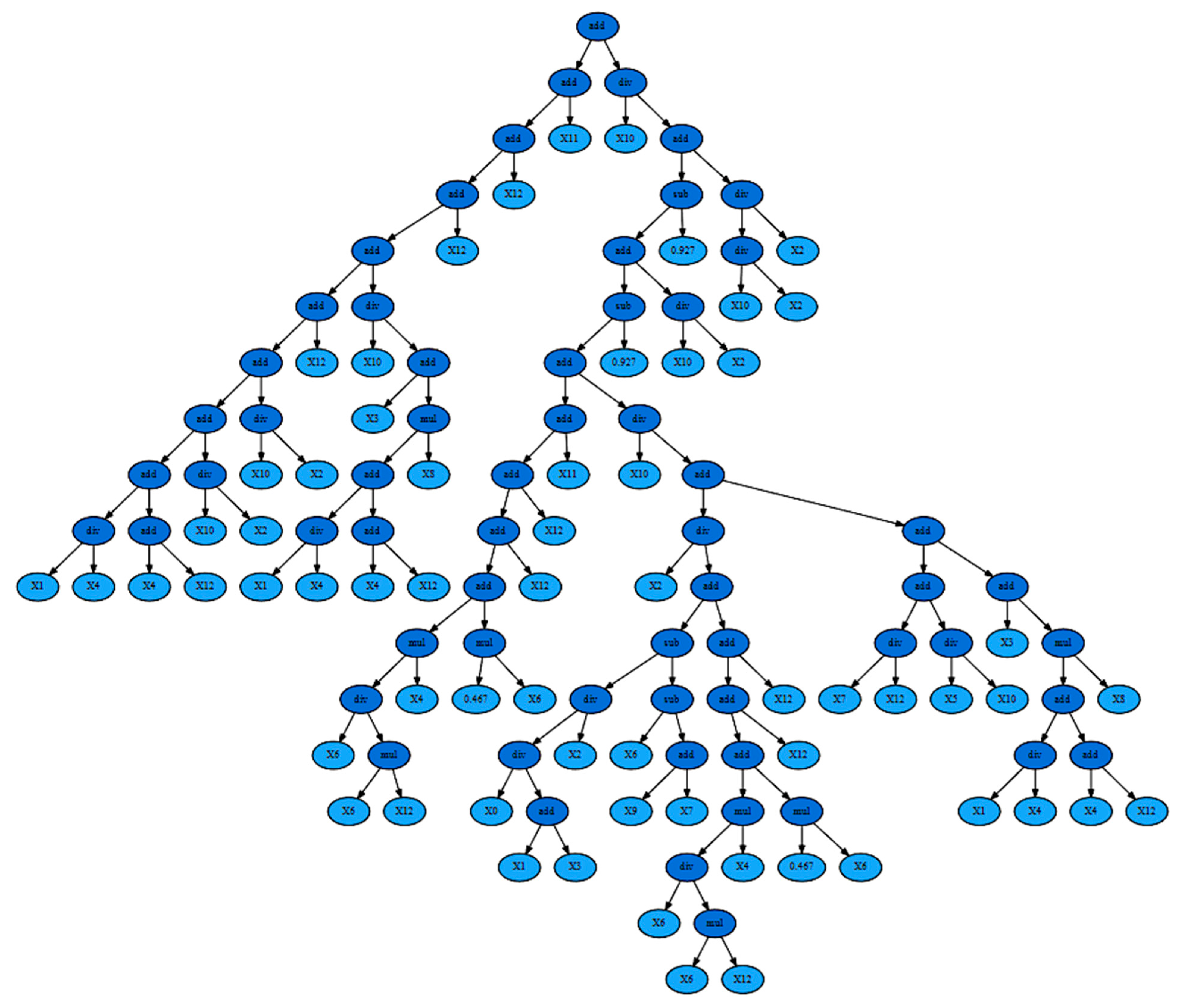
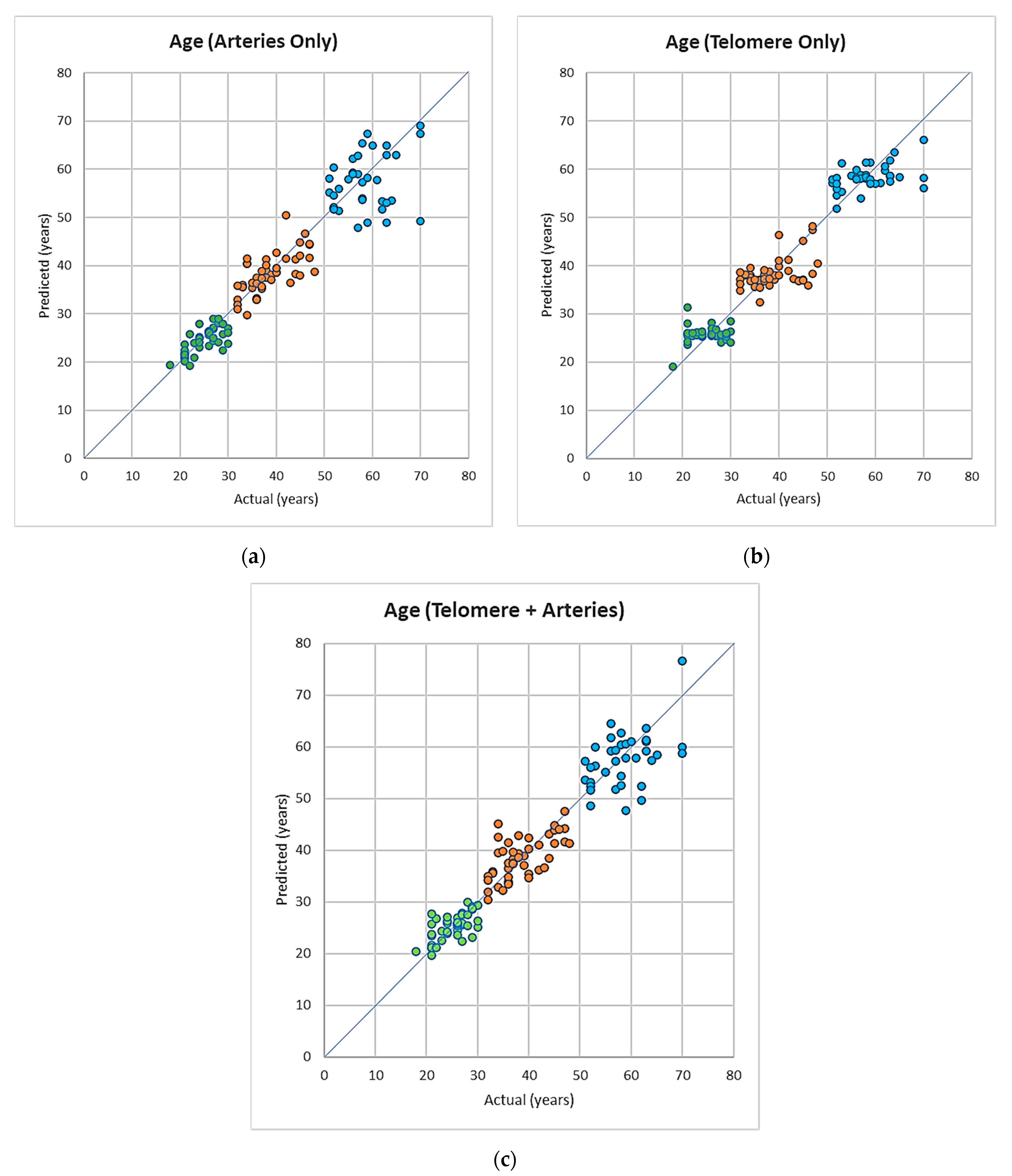
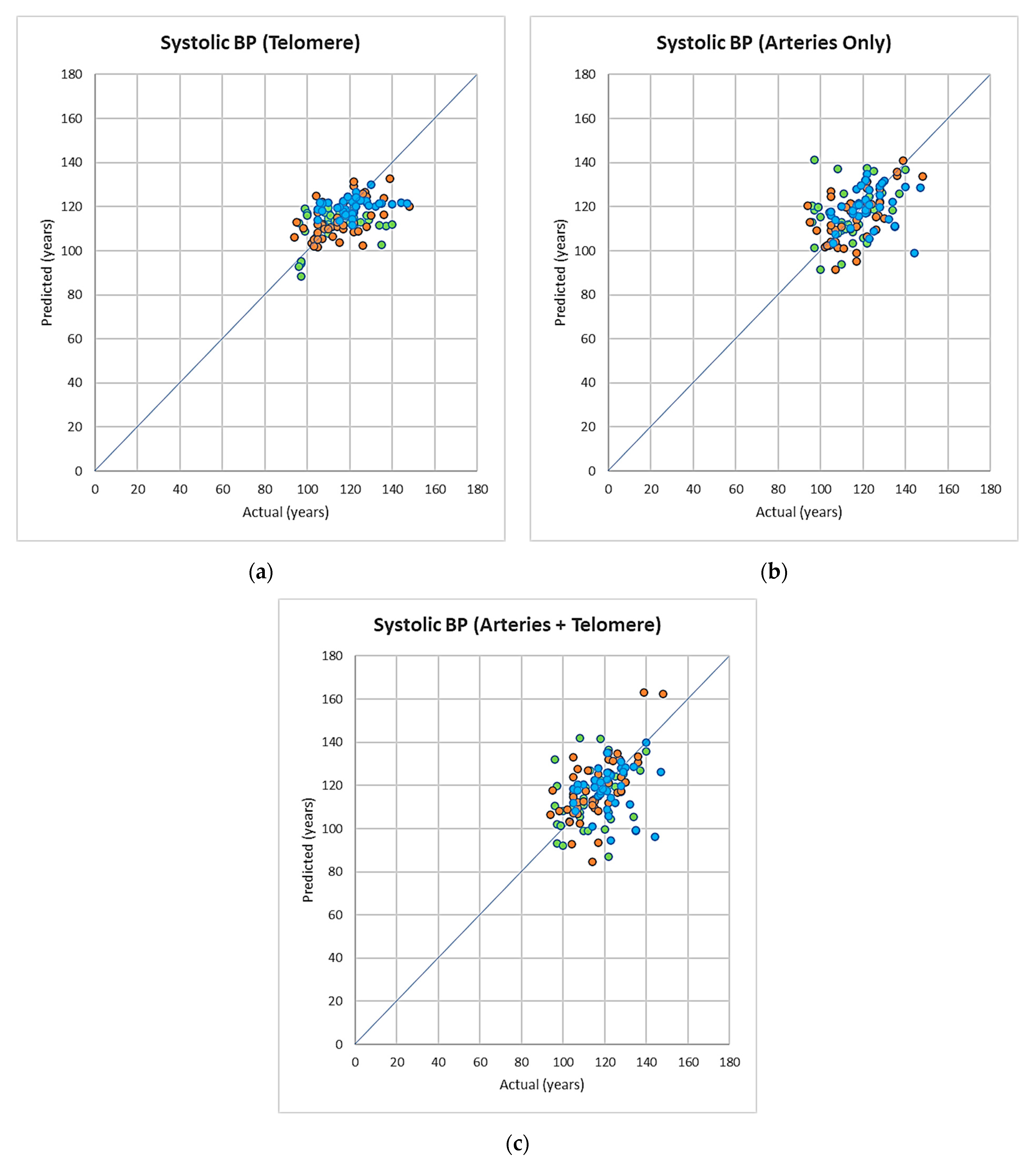
3.3. Symbolic Regression-Based Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDermott, M.M.G. The International Pandemic of Chronic Cardiovascular Disease. J. Am. Med. Assoc. 2007, 297, 1253–1255. [Google Scholar] [CrossRef]
- Rosamond, W.; Flegal, K.; Furie, K.; Go, A.; Greenlund, K.; Haase, N.; Hailpern, S.M.; Ho, M.; Howard, V.; Kissela, B.; et al. Heart Disease and Stroke Statistics-2008 Update: A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008, 117, e25–e146. [Google Scholar] [PubMed]
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. European Cardiovascular Disease Statistics 4th Edition 2012: EuroHeart II. Eur. Heart J. 2013, 34, 3007–3013. [Google Scholar]
- Hamczyk, M.R.; Nevado, R.M.; Barettino, A.; Fuster, V.; Andrés, V. Biological Versus Chronological Aging: JACC Focus Seminar; Elsevier: Amsterdam, The Netherlands, 2020; Volume 75, pp. 919–930. [Google Scholar]
- Franceschi, C.; Valensin, S.; Bonafe, M.; Paolisso, G.; Yashin, A.I.; Monti, D.; De Benedictis, G. The Network and the Remodeling Theories of Aging: Historical Background and New Perspectives. Exp. Gerontol. 2000, 35, 879–896. [Google Scholar] [CrossRef]
- Collino, S.; Montoliu, I.; Martin, F.-P.J.; Scherer, M.; Mari, D.; Salvioli, S.; Bucci, L.; Ostan, R.; Monti, D.; Biagi, E.; et al. Correction: Metabolic Signatures of Extreme Longevity in Northern Italian Centenarians Reveal a Complex Remodeling of Lipids, Amino Acids, and Gut Microbiota Metabolism. PLoS ONE 2013, 8, e56564. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Matam, B.R.; Bellary, S.; Ghosh, G.; Chattopadhyay, A.K. CHD Risk Minimization through Lifestyle Control: Machine Learning Gateway. Sci. Rep. 2020, 10, 4090. [Google Scholar] [CrossRef]
- Koenig, W. Cardiovascular Biomarkers: Added Value with an Integrated Approach? Circulation 2007, 116, 3–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cohn, J.N. Identifying the Risk and Preventing the Consequences of Cardiovascular Disease. Heart Lung Circ. 2013, 22, 512–516. [Google Scholar] [CrossRef]
- Ferrucci, L.; Gonzalez-Freire, M.; Fabbri, E.; Simonsick, E.; Tanaka, T.; Moore, Z.; Salimi, S.; Sierra, F.; de Cabo, R. Measuring Biological Aging in Humans: A Quest. Aging Cell 2020, 19, e13080. [Google Scholar] [CrossRef]
- Shokr, H.; Dias, I.H.K.; Gherghel, D. Oxysterols and Retinal Microvascular Dysfunction as Early Risk Markers for Cardiovascular Disease in Normal, Ageing Individuals. Antioxidants 2021, 10, 1756. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Q.; Liu, L.; Lo, K.; Huang, J.-Y.; Zhang, B.; Feng, Y.-Q. The Relationship between Mean Telomere Length and Blood Pressure: Results from the National Health and Nutrition Examination Surveys. Ann. Transl. Med. 2020, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, A.; Spigoni, V.; Franzini, L.; Preti, M.; Ardigò, D.; Derlindati, E.; Metra, M.; Monti, L.D.; Dell’Era, P.; Gnudi, L.; et al. Lower Endothelial Progenitor Cell Number, Family History of Cardiovascular Disease and Reduced HDL-Cholesterol Levels Are Associated with Shorter Leukocyte Telomere Length in Healthy Young Adults. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Voghel, G.; Thorin-Trescases, N.; Farhat, N.; Nguyen, A.; Villeneuve, L.; Mamarbachi, A.M.; Fortier, A.; Perrault, L.P.; Carrier, M.; Thorin, E. Cellular Senescence in Endothelial Cells from Atherosclerotic Patients Is Accelerated by Oxidative Stress Associated with Cardiovascular Risk Factors. Mech. Ageing Dev. 2007, 128, 662–671. [Google Scholar] [CrossRef]
- Fyhrquist, F.; Saijonmaa, O.; Strandberg, T. The Roles of Senescence and Telomere Shortening in Cardiovascular Disease. Nat. Rev. Cardiol. 2013, 10, 274–283. [Google Scholar] [CrossRef]
- Reichert, S.; Stier, A. Does Oxidative Stress Shorten Telomeres in Vivo? A Review. Biol. Lett. 2017, 13, 20170463. [Google Scholar] [CrossRef]
- Chang, E.; Harley, C.B. Telomere Length and Replicative Aging in Human Vascular Tissues. Proc. Natl. Acad. Sci. USA 1995, 92, 11190–11194. [Google Scholar] [CrossRef]
- Savji, N.; Rockman, C.B.; Skolnick, A.H.; Guo, Y.; Adelman, M.A.; Riles, T.; Berger, J.S. Association between Advanced Age and Vascular Disease in Different Arterial Territories: A Population Database of over 3.6 Million Subjects. J. Am. Coll. Cardiol. 2013, 61, 1736–1743. [Google Scholar] [CrossRef]
- Shokr, H.; Gherghel, D. European Society of Cardiology/European Society of Hypertension versus the American College of Cardiology/American Heart Association Guidelines on the Cut-off Values for Early Hypertension: A Microvascular Perspective. Sci. Rep. 2021, 11, 3473. [Google Scholar] [CrossRef]
- Shokr, H.; Dias, I.H.K.; Gherghel, D. Microvascular Function and Oxidative Stress in Adult Individuals with Early Onset of Cardiovascular Disease. Sci. Rep. 2020, 10, 4881. [Google Scholar] [CrossRef]
- Seshadri, S.; Karimzad, S.E.; Shokr, H.; Gherghel, D. Retinal Vascular Function in Asymptomatic Individuals with a Positive Family History of Cardiovascular Disease. Acta Ophthalmol. 2018, 96, e956–e962. [Google Scholar] [CrossRef] [PubMed]
- Shokr, H.; Wolffsohn, J.S.; Trave Huarte, S.; Scarpello, E.; Gherghel, D.; Ophthalmol, A. Dry Eye Disease Is Associated with Retinal Microvascular Dysfunction and Possible Risk for Cardiovascular Disease. Acta Ophthalmol. 2021, 99, e1236–e1242. [Google Scholar] [CrossRef] [PubMed]
- Karimzad, S.E.; Shokr, H.; Gherghel, D. Retinal and Peripheral Vascular Function in Healthy Individuals with Low Cardiovascular Risk. Microvasc. Res. 2019, 126, 103908. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimationof the Concentrationof Low-Density LipoproteinCholesterolin Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.F.; Castelli, W.P.; Kannel, W.B. Coronary Risk Prediction in Adults (The Framingham Heart Study). Am. J. Cardiol. 1987, 59, G91–G94. [Google Scholar] [CrossRef]
- Ford, E.S.; Giles, W.H.; Mokdad, A.H. The Distribution of 10-Year Risk for Coronary Heart Disease among U.S. Adults: Findings from the National Health and Nutrition Examination Survey III. J. Am. Coll. Cardiol. 2004, 43, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Gherghel, D.; Mroczkowska, S.; Qin, L. Reduction in Blood Glutathione Levels Occurs Similarly in Patients with Primary-Open Angle or Normal Tension Glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3333–3339. [Google Scholar] [CrossRef]
- Jones, D.P.; Carlson, J.L.; Samiec, P.S.; Sternberg, P.; Mody, V.C.; Reed, R.L.; Brown, L.A.S. Glutathione Measurement in Human Plasma. Evaluation of Sample Collection, Storage and Derivatization Conditions for Analysis of Dansyl Derivatives by HPLC. Clin. Chim. Acta 1998, 275, 175–184. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic Method for Quantitative Determination of Nanogram Amounts of Total and Oxidized Glutathione: Applications to Mammalian Blood and Other Tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Anderson, M.E. Glutathione: An Overview of Biosynthesis and Modulation. Chem.-Biol. Interact. 1998, 111, 1–14. [Google Scholar] [CrossRef]
- Framingham Risk Score—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/framingham-risk-score (accessed on 2 January 2022).
- Hemann, B.A.; Bimson, W.F.; Taylor, A.J. The Framingham Risk Score: An Appraisal of Its Benefits and Limitations. Am. Heart Hosp. J. 2007, 5, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Nagel, E.; Vilser, W.; Lanzl, I. Age, Blood Pressure, and Vessel Diameter as Factors Influencing the Arterial Retinal Flicker Response. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Mroczkowska, S.; Benavente-Perez, A.; Negi, A.; Sung, V.; Patel, S.R.; Gherghel, D. Primary Open-Angle Glaucoma vs Normal-Tension Glaucoma: The Vascular Perspective. Arch. Ophthalmol. 2013, 131, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Karimzad, S.; Bilkhu, P.S.; Wolffsohn, J.S.; Bellary, S.; Shokr, H.; Singhal, R.; Gherghel, D. Impact of Bariatric Surgery-Induced Weight Loss on Anterior Eye Health in Patients with Obesity. Nutrients 2022, 14, 2462. [Google Scholar] [CrossRef]
- Seifert, B.-U.; Vilser, W. Retinal Vessel Analyzer (RVA)—Design and Function. Biomed. Tech. 2002, 47, 678–681. [Google Scholar] [CrossRef]
- Nagel, E.; Vilser, W.; Fink, A.; Riemer, T. Variance of retinal vessel diameter response to flicker light. A methodical clinical study. Ophthalmologe 2006, 103, 114–119. [Google Scholar] [CrossRef]
- Resources—QIAGEN. Available online: https://www.qiagen.com/us/resources/ (accessed on 9 February 2021).
- Cawthon, R.M. Telomere Length Measurement by a Novel Monochrome Multiplex Quantitative PCR Method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere Measurement by Quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Garhöfer, G.; Zawinka, C.; Resch, H.; Kothy, P.; Schmetterer, L.; Dorner, G.T. Reduced Response of Retinal Vessel Diameters to Flicker Stimulation in Patients with Diabetes. Br. J. Ophthalmol. 2004, 88, 887–891. [Google Scholar] [CrossRef]
- Patel, S.R.; Bellary, S.; Qin, L.; Balanos, G.M.; McIntyre, D.; Gherghel, D. Abnormal Retinal Vascular Reactivity in Individuals with Impaired Glucose Tolerance: A Preliminary Study. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5102–5108. [Google Scholar] [CrossRef]
- Patel, S.R.; Bellary, S.; Qin, L.; Gill, P.S.; Taheri, S.; Heitmar, R.; Gibson, J.M.; Gherghel, D. Abnormal Retinal Vascular Function and Lipid Levels in a Sample of Healthy UK South Asians. Br. J. Ophthalmol. 2011, 95, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Ekart, A.; Gherghel, D. Ageing Effect on Flicker-Induced Diameter Changes in Retinal Microvessels of Healthy Individuals. Acta Ophthalmol. 2016, 94, e35–e42. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Mroczkowska, S.; Qin, L.; Patel, S.; Ekart, A.; Gherghel, D. Systemic Circulatory Influences on Retinal Microvascular Function in Middle-Age Individuals with Low to Moderate Cardiovascular Risk. Acta Ophthalmol. 2015, 93, e266–e274. [Google Scholar] [CrossRef] [PubMed]
- McKay, B.; Willis, M.J.; Barton, G.W. Using a Tree Structured Genetic Algorithm to Perform Symbolic Regression. In Proceedings of the First International Conference on Genetic Algorithms in Engineering Systems: Innovations and Applications, Sheffield, UK, 12–14 September 1995; pp. 487–492. [Google Scholar]
- Orzechowski, P.; La Cava, W.; Moore, J.H. Where Are We Now? A Large Benchmark Study of Recent Symbolic Regression Methods. In Proceedings of the Genetic and Evolutionary Computation Conference, Kyoto, Japan, 15–19 July 2018; Association for Computing Machinery: New York, NY, USA, 2018; pp. 1183–1190. [Google Scholar]
- Udrescu, S.-M.; Tegmark, M. AI Feynman: A Physics-Inspired Method for Symbolic Regression. Sci. Adv. 2020, 6, eaay2631. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Song, Z.; Zhu, R.; Yan, Q.; Sun, Q.; Grice, C.G.; Yan, Y.; Yin, W.-J. Simple Descriptor Derived from Symbolic Regression Accelerating the Discovery of New Perovskite Catalysts. Nat. Commun. 2020, 11, 3513. [Google Scholar] [CrossRef]
- Symbolic Regression for Interpretable Scientific Discovery|SpringerLink. Available online: https://link.springer.com/chapter/10.1007/978-3-030-96600-3_3 (accessed on 24 September 2022).
- Müezzinler, A.; Zaineddin, A.K.; Brenner, H. A Systematic Review of Leukocyte Telomere Length and Age in Adults. Ageing Res. Rev. 2013, 12, 509–519. [Google Scholar] [CrossRef]
- Chen, W.; Kimura, M.; Kim, S.; Cao, X.; Srinivasan, S.R.; Berenson, G.S.; Kark, J.D.; Aviv, A. Longitudinal versus Cross-Sectional Evaluations of Leukocyte Telomere Length Dynamics: Age-Dependent Telomere Shortening Is the Rule. J. Gerontol. Ser. A 2011, 66A, 312–319. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Mahdi, F. Telomere Length Variations in Aging and Age-Related Diseases. Curr. Aging Sci. 2014, 7, 161–167. [Google Scholar] [CrossRef]
- Koliada, A.K.; Krasnenkov, D.S.; Vaiserman, A.M. Telomeric Aging: Mitotic Clock or Stress Indicator? Front. Genet. 2015, 6, 82. [Google Scholar] [CrossRef]
- Notterman, D.A.; Schneper, L. Telomere Time—Why We Should Treat Biological Age Cautiously. JAMA Netw. Open 2020, 3, e204352. [Google Scholar] [CrossRef]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2021, 11, 1816. [Google Scholar] [CrossRef] [PubMed]
- Rim, T.H.; Lee, G.; Kim, Y.; Tham, Y.C.; Lee, C.J.; Baik, S.J.; Kim, Y.A.; Yu, M.; Deshmukh, M.; Lee, B.K.; et al. Prediction of Systemic Biomarkers from Retinal Photographs: Development and Validation of Deep-Learning Algorithms. Lancet Digit. Health 2020, 2, e526–e536. [Google Scholar] [CrossRef]
- Streese, L.; Lona, G.; Wagner, J.; Knaier, R.; Burri, A.; Nève, G.; Infanger, D.; Vilser, W.; Schmidt-Trucksäss, A.; Hanssen, H. Normative Data and Standard Operating Procedures for Static and Dynamic Retinal Vessel Analysis as Biomarker for Cardiovascular Risk. Sci. Rep. 2021, 11, 14136. [Google Scholar] [CrossRef] [PubMed]

| Variables | Age Group (1) (19–30 Year) | Age Group (2) (31–50 Year) | Age Group (3) (>50 Year) | p-Value | Post Hoc Analysis |
|---|---|---|---|---|---|
| Number | 40 | 47 | 36 | >0.05 | - |
| Gender | 20M:20F | 23M:24F | 19M:17F | >0.05 | - |
| Age (years) | 24.95 (0.72) | 38.28 (0.67) | 58.53 (0.76) | 0.0000 * | 1 < 2 < 3 |
| SBP | 114.9 (1.84) | 114.96 (1.70) | 121.86 (1.94) | 0.0129 * | 1 = 2 < 3 |
| DBP | 66 (1.35) | 70.64 (1.25) | 72.83 (1.43) | 0.0022 * | 1 = 2 < 3 |
| MAP | 82.3 (1.87) | 84.6 (1.92) | 89.1 (1.98) | 0.004 * | 1 = 2 < 3 |
| HR (bpm) | 66.05 (1.32) | 64.94 (1.22) | 62.11 (1.39) | 0.1133 | - |
| BMI (kg/m2) | 24.92 (0.71) | 26.07 (0.65) | 26.53 (0.76) | 0.2727 | - |
| Glucose | 4.43 (0.11) | 4.68 (0.12) | 5.00 (0.12) | 0.0034 * | 1 = 2 < 3 |
| TG (mmol/L) | 0.83 (0.050) | 0.92 (0.047) | 0.98 (0.05) | 0.1269 | - |
| T-CHOL | 3.94 (0.12) | 4.63 (0.12) | 4.51 (0.14) | <0.001 * | 2 = 3 > 1 |
| HDL-C (mmol/L) | 1.33 (0.06) | 1.26 (0.06) | 1.16 (0.06) | 0.051 | - |
| LDL-C (mmol/L) | 2.21 (0.13) | 3.13 (0.12) | 2.92 (0.14) | 0.001 * | 2 = 3 > 1 |
| GSH | 348.79 (47.91) | 412.13 (41.03) | 410.09 (46.52) | 0.549 | - |
| GSSG | 31.19 (3.43) | 36.80 (2.93) | 28.70 (3.33) | 0.169 | - |
| RTL | 0.64 (0.22) | 0.09 (0.21) | −0.36 (0.24) | 0.010 * | 1 > 2 > 3 |
| Mean (SD) | |||||
|---|---|---|---|---|---|
| Parameter | Age Group (1) (19–30 Years) | Age Group (2) (31–50 Years) | Age Group (3) (>50 Years) | p-Value | Post Hoc Analysis |
| Artery baseline | 125.71 (5.02) | 114.86 (2.22) | 106.46 (6.07) | 0.107 | |
| Artery-BDF | 6.34 (0.41) | 5.22 (0.38) | 4.80 (0.44) | 0.029 | |
| Artery-DA a | 10.80 (0.70) | 9.94 (0.66) | 8.18 (0.76) | 0.041 | |
| Artery-BCFR b | 4.48 (0.40) | 4.69 (0.37) | 3.26 (0.43) | 0.034 | |
| Artery-MD | 124.11 (2.04) | 118.36 (1.90) | 116.11 (2.15) | 0.021 | |
| Artery-tMD | 17.44 (0.58) | 17.55 (0.53) | 19.89 (0.61) | 0.005 * | 1 = 2 < 3 |
| Artery-MD% | 5.31 (0.32) | 4.66 (0.29) | 4.30 (0.34) | 0.104 | - |
| Artery-MC | 113.17 (2.30) | 109.92 (2.12) | 112.80 (2.43) | 0.521 | - |
| Artery-tMC | 24.65 (2.49) | 23.94 (1.13) | 30.75 (3.11) | 0.007 * | 1 = 2 < 3 |
| Artery-MC% | −3.40 (0.30) | −3.55 (0.27) | −2.70 (0.30) | 0.010 * | 1 = 2 > 3 |
| Artery-SlopeAD c | 0.45 (0.04) | 0.39 (0.04) | 0.38 (0.05) | 0.567 | - |
| Artery-SlopeAC c | −0.56 (0.04) | −0.44 (0.04) | −0.36 (0.05) | 0.126 | - |
| Feature Representation | Corresponding Measurements |
|---|---|
| X0 | RTL |
| X1 | Artery baseline |
| X2 | Artery Baseline Diameter Fluctuation |
| X3 | Artery Maximum Dilation |
| X4 | Artery Time to Maximum Dilation |
| X5 | Artery Maximum Dilation Percentage |
| X6 | Artery Maximum Constriction |
| X7 | Artery Time to Maximum Constriction |
| X8 | Artery Maximum Constriction Percentage |
| X9 | Artery Dilation Amplitude |
| X10 | Artery Baseline Corrected Flicker Response |
| X11 | Artery Dilation Slope |
| X12 | Artery Constriction Slope |
| Arteries + Telomere | Arteries Only | Telomere Only | |||
|---|---|---|---|---|---|
| (a) | |||||
| Fold | MAE | Fold | MAE | Fold | MAE |
| 1 | 1.488355 | 1 | 1.457137 | 1 | 2.150139 |
| 2 | 2.03976 | 2 | 1.628655 | 2 | 2.466321 |
| 3 | 1.634165 | 3 | 1.580925 | 3 | 3.263452 |
| 4 | 2.157625 | 4 | 2.038026 | 4 | 3.874383 |
| 5 | 1.157639 | 5 | 1.40857 | 5 | 1.776776 |
| Average | 1.695509 | Average | 1.622663 | Average | 2.706214 |
| (b) | |||||
| Fold | MAE | Fold | MAE | Fold | MAE |
| 1 | 2.812663 | 1 | 2.527317 | 1 | 3.204149 |
| 2 | 2.107845 | 2 | 2.245985 | 2 | 1.518323 |
| 3 | 2.790105 | 3 | 2.620622 | 3 | 3.189129 |
| 4 | 3.892265 | 4 | 3.516084 | 4 | 4.30067 |
| 5 | 2.632783 | 5 | 2.965419 | 5 | 3.701804 |
| Average | 2.847132 | Average | 2.775085 | Average | 3.182815 |
| (c) | |||||
| Fold | MAE | Fold | MAE | Fold | MAE |
| 1 | 4.902158 | 1 | 6.013113 | 1 | 2.392696 |
| 2 | 2.922922 | 2 | 5.230758 | 2 | 4.52436 |
| 3 | 4.003612 | 3 | 3.691483 | 3 | 3.489574 |
| 4 | 4.052891 | 4 | 5.85124 | 4 | 4.656914 |
| 5 | 6.184841 | 5 | 4.847482 | 5 | 3.400724 |
| Average | 4.413285 | Average | 5.126815 | Average | 3.692854 |
| Arteries and Telomere | Arteries Only | Telomere Only | |||
|---|---|---|---|---|---|
| (a) | |||||
| Fold | MAE | Fold | MAE | Fold | MAE |
| 1 | 7.60824 | 1 | 9.781193 | 1 | 9.214715 |
| 2 | 14.67967 | 2 | 15.0163 | 2 | 8.569096 |
| 3 | 8.132338 | 3 | 6.466947 | 3 | 9.16523 |
| 4 | 8.570601 | 4 | 14.36258 | 4 | 6.129705 |
| 5 | 13.69032 | 5 | 7.829224 | 5 | 9.043941 |
| Average | 10.53623 | Average | 10.69125 | Average | 8.424537 |
| (b) | |||||
| Fold | MAE | Fold | MAE | Fold | MAE |
| 1 | 8.581326 | 1 | 8.418168 | 1 | 7.495257 |
| 2 | 7.755753 | 2 | 7.490878 | 2 | 7.130104 |
| 3 | 10.37444 | 3 | 7.814303 | 3 | 5.463688 |
| 4 | 10.96121 | 4 | 8.422463 | 4 | 10.27759 |
| 5 | 8.381083 | 5 | 6.844803 | 5 | 8.355181 |
| Average | 9.210761 | Average | 7.798123 | Average | 7.744364 |
| (c) | |||||
| Fold | MAE | Fold | MAE | Fold | MAE |
| 1 | 17.1292 | 1 | 16.13368 | 1 | 12.3724 |
| 2 | 6.913672 | 2 | 5.783934 | 2 | 5.320442 |
| 3 | 8.221233 | 3 | 8.26078 | 3 | 8.073649 |
| 4 | 8.246475 | 4 | 6.949804 | 4 | 5.237078 |
| 5 | 7.398514 | 5 | 5.531346 | 5 | 6.151203 |
| Average | 9.581819 | Average | 8.531909 | Average | 7.430954 |
| Arteries and Telomere | Arteries Only | Telomere Only | |||
|---|---|---|---|---|---|
| (a) | |||||
| Fold | MAE | Fold | MAE | Fold | MAE |
| 1 | 5.110043 | 1 | 6.43493 | 1 | 6.644792 |
| 2 | 6.032743 | 2 | 7.535935 | 2 | 5.476441 |
| 3 | 4.814686 | 3 | 4.070671 | 3 | 5.058709 |
| 4 | 4.77134 | 4 | 4.221734 | 4 | 5.449827 |
| 5 | 4.172079 | 5 | 3.580786 | 5 | 4.426252 |
| Average | 4.980178 | Average | 5.168811 | Average | 5.411204 |
| (b) | |||||
| Fold | MAE | Fold | MAE | Fold | MAE |
| 1 | 4.733007 | 1 | 5.99453 | 1 | 4.215411 |
| 2 | 4.618492 | 2 | 6.014625 | 2 | 5.779917 |
| 3 | 7.849373 | 3 | 8.224371 | 3 | 5.561008 |
| 4 | 6.65106 | 4 | 8.225318 | 4 | 5.896184 |
| 5 | 2.782652 | 5 | 3.812176 | 5 | 8.340123 |
| Average | 5.326917 | Average | 6.454204 | Average | 5.958529 |
| (c) | |||||
| Fold | MAE | Fold | MAE | Fold | MAE |
| 1 | 8.175021 | 1 | 7.292121 | 1 | 7.451285 |
| 2 | 6.597758 | 2 | 5.685044 | 2 | 6.589668 |
| 3 | 4.907752 | 3 | 5.379021 | 3 | 7.05904 |
| 4 | 3.739657 | 4 | 5.28883 | 4 | 4.040507 |
| 5 | 5.058974 | 5 | 6.01973 | 5 | 3.199624 |
| Average | 5.695832 | Average | 5.932949 | Average | 5.668025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokr, H.; Lush, V.; Dias, I.H.; Ekárt, A.; De Moraes, G.; Gherghel, D. The Use of Retinal Microvascular Function and Telomere Length in Age and Blood Pressure Prediction in Individuals with Low Cardiovascular Risk. Cells 2022, 11, 3037. https://doi.org/10.3390/cells11193037
Shokr H, Lush V, Dias IH, Ekárt A, De Moraes G, Gherghel D. The Use of Retinal Microvascular Function and Telomere Length in Age and Blood Pressure Prediction in Individuals with Low Cardiovascular Risk. Cells. 2022; 11(19):3037. https://doi.org/10.3390/cells11193037
Chicago/Turabian StyleShokr, Hala, Victoria Lush, Irundika HK Dias, Anikó Ekárt, Gustavo De Moraes, and Doina Gherghel. 2022. "The Use of Retinal Microvascular Function and Telomere Length in Age and Blood Pressure Prediction in Individuals with Low Cardiovascular Risk" Cells 11, no. 19: 3037. https://doi.org/10.3390/cells11193037
APA StyleShokr, H., Lush, V., Dias, I. H., Ekárt, A., De Moraes, G., & Gherghel, D. (2022). The Use of Retinal Microvascular Function and Telomere Length in Age and Blood Pressure Prediction in Individuals with Low Cardiovascular Risk. Cells, 11(19), 3037. https://doi.org/10.3390/cells11193037







