Presynaptic 5-HT2A-mGlu2/3 Receptor–Receptor Crosstalk in the Prefrontal Cortex: Metamodulation of Glutamate Exocytosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Synaptosomes
2.3. Experiments of Transmitter Release
2.4. Western Blot Analysis
2.5. Biotinylation Studies
2.6. Co-Immunoprecipitation
2.7. Calculations and Statistical Analysis
2.8. Chemicals
3. Results
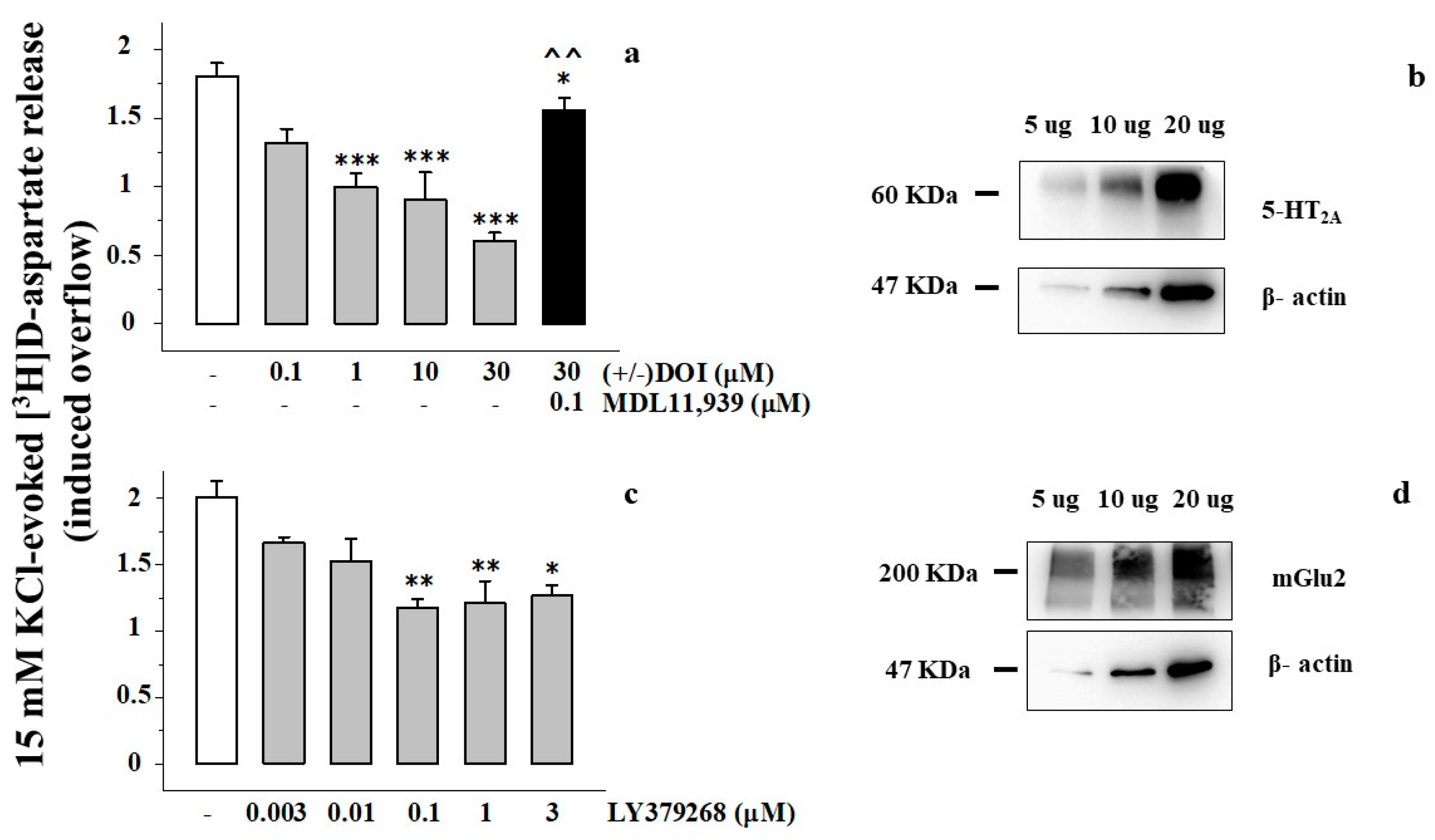
3.1. Presynaptic Release-Regulating mGlu2/3 Autoreceptors and 5-HT2A Heteroreceptors in Rat Prefrontal Cortex Glutamatergic Nerve Endings
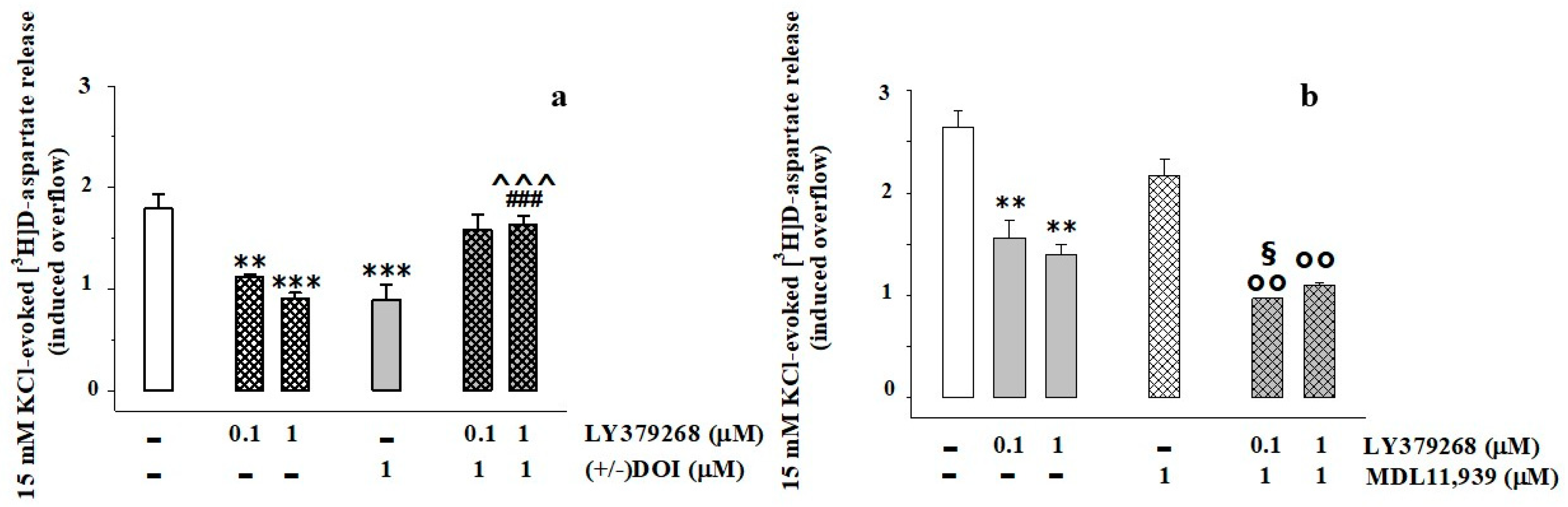
3.2. Presynaptic Release-Regulating mGlu2/3 Autoreceptors and 5-HT2A Heteroreceptors Are Functionally Coupled in Rat Prefrontal Cortex Glutamatergic Nerve Endings
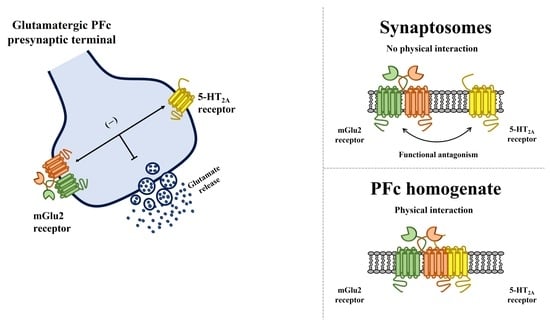
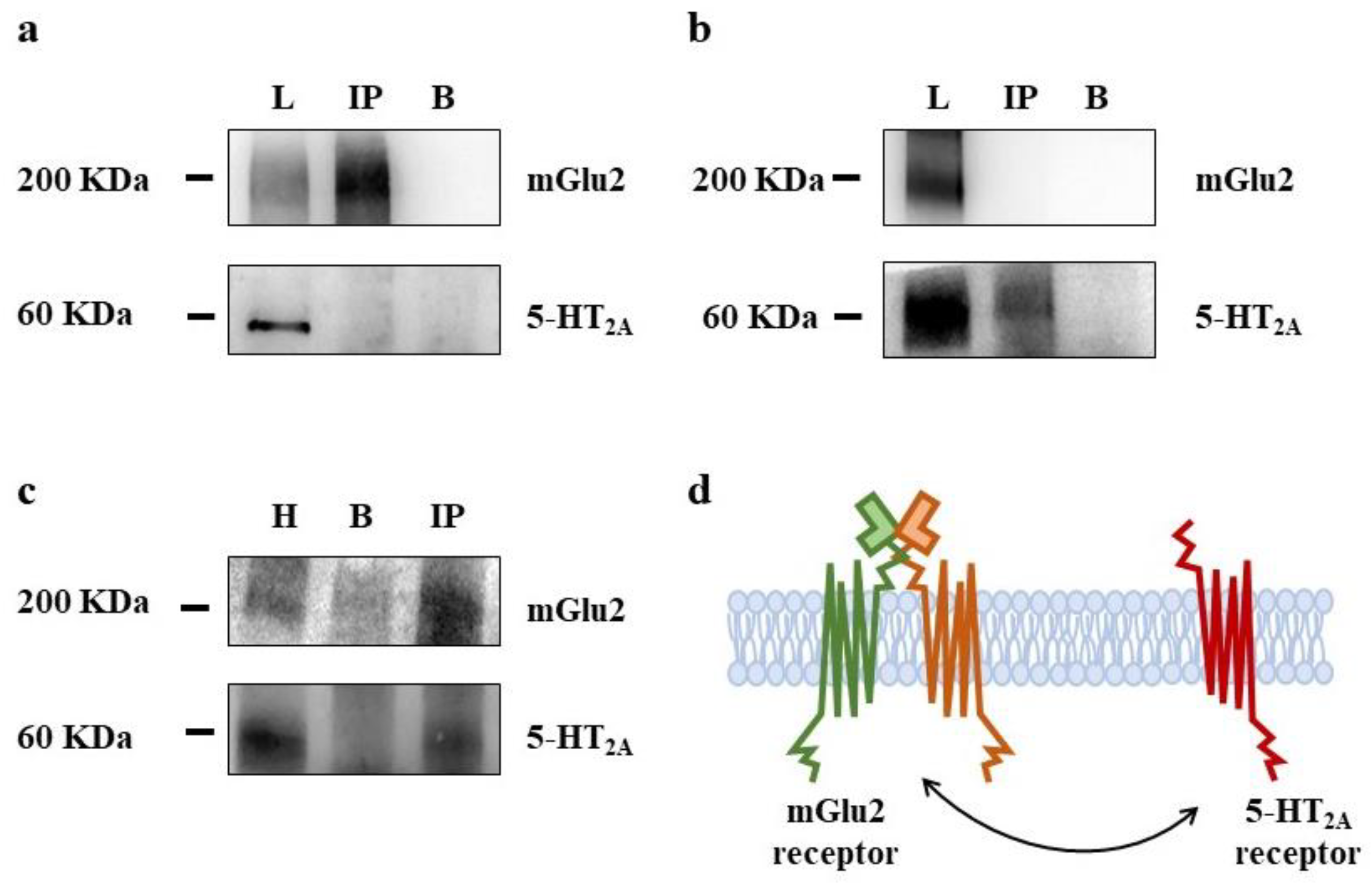
3.3. Presynaptic Release-Regulating mGlu2/3 Autoreceptors and 5-HT2A Heteroreceptors Colocalize but Do Not Physically Associate in Rat Prefrontal Cortex Glutamatergic Nerve Endings
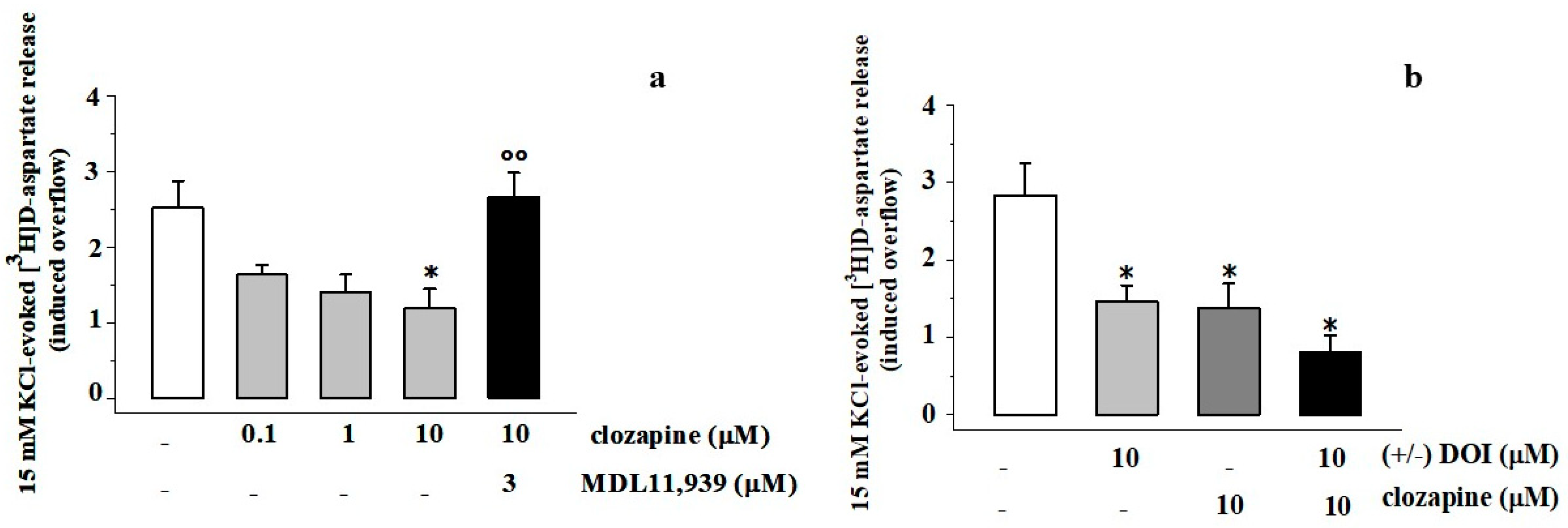
3.4. Clozapine Inhibits the 15 mM KCl-Evoked [3H]D-Aspartate Release in Rat Prefrontal Cortex Glutamatergic Nerve Endings in a MDL11,939-Sensitive Manner
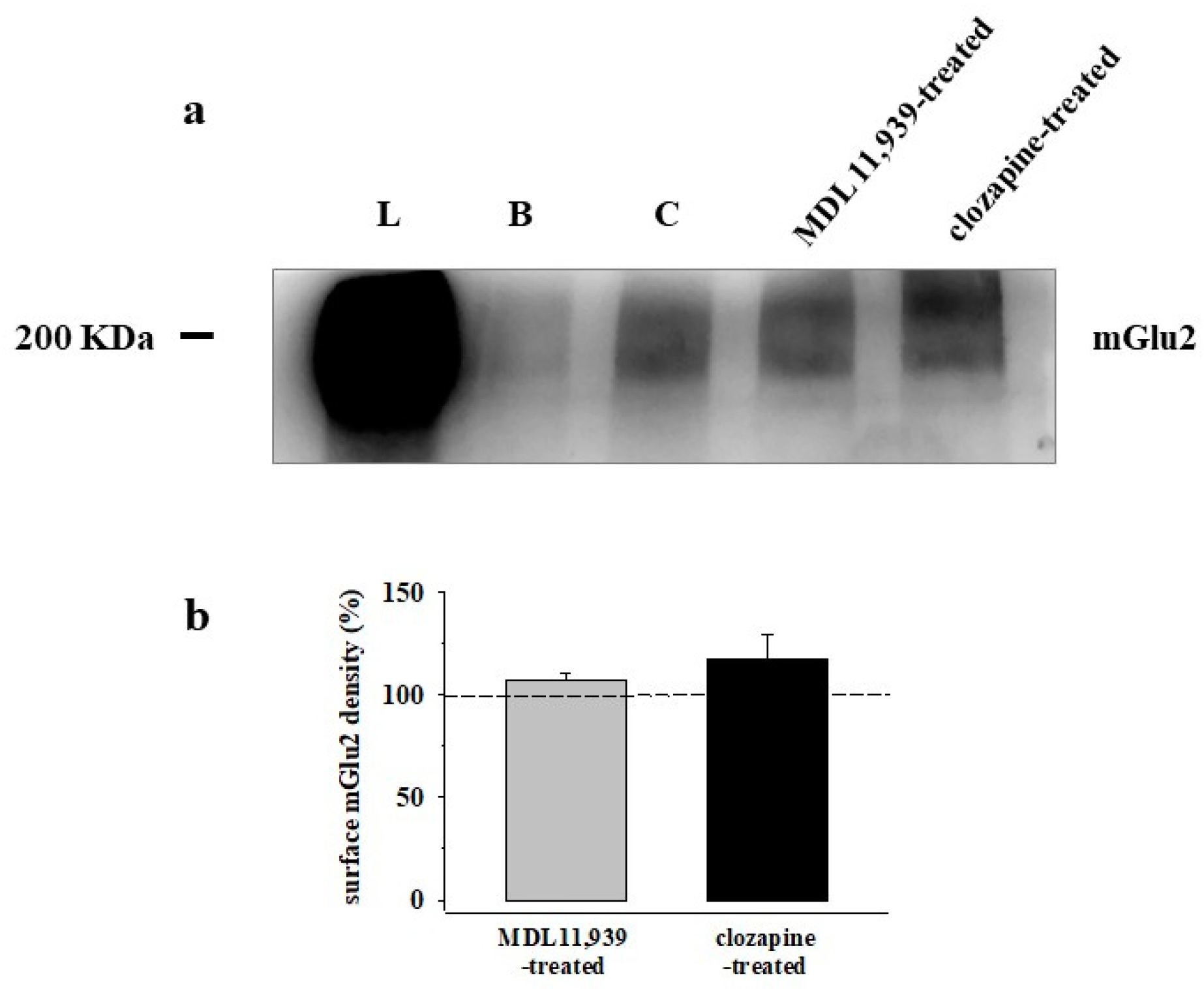
3.5. Preincubation of Prefrontal Cortex Synaptosomes with 5-HT2A Receptor Ligands Does Not Modify the Insertion of mGlu2 Receptor Protein in Synaptosomal Plasma Membranes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eggers, A.E. A Serotonin Hypothesis of Schizophrenia. Med. Hypotheses 2013, 80, 791–794. [Google Scholar] [CrossRef]
- Stahl, S.M. Beyond the Dopamine Hypothesis of Schizophrenia to Three Neural Networks of Psychosis: Dopamine, Serotonin, and Glutamate. CNS Spectr. 2018, 23, 187–191. [Google Scholar] [CrossRef]
- Shah, U.H.; Gonzaíez-Maeso, J. Serotonin and Glutamate Interactions in Preclinical Schizophrenia Models. ACS Chem. Neurosci. 2019, 10, 3068–3077. [Google Scholar] [CrossRef]
- Ebdrup, B.H.; Rasmussen, H.; Arnt, J.; Glenthøj, B. Serotonin 2A Receptor Antagonists for Treatment of Schizophrenia. Expert Opin. Investig. Drugs 2011, 20, 1211–1223. [Google Scholar] [CrossRef]
- Selvaraj, S.; Arnone, D.; Cappai, A.; Howes, O. Alterations in the Serotonin System in Schizophrenia: A Systematic Review and Meta-Analysis of Postmortem and Molecular Imaging Studies. Neurosci. Biobehav. Rev. 2014, 45, 233–245. [Google Scholar] [CrossRef]
- Balu, D.T. The NMDA Receptor and Schizophrenia: From Pathophysiology to Treatment. Adv. Pharm. 2016, 76, 351–382. [Google Scholar] [CrossRef]
- Nicoletti, F.; Orlando, R.; di Menna, L.; Cannella, M.; Notartomaso, S.; Mascio, G.; Iacovelli, L.; Matrisciano, F.; Fazio, F.; Caraci, F.; et al. Targeting MGlu Receptors for Optimization of Antipsychotic Activity and Disease-Modifying Effect in Schizophrenia. Front. Psychiatry 2019, 10, 49. [Google Scholar] [CrossRef]
- Fuxe, K.; Agnati, L.F. Receptor-Receptor Interactions in the Central Nervous System. A New Integrative Mechanism in Synapses. Med. Res. Rev. 1985, 5, 441–482. [Google Scholar] [CrossRef]
- Katz, P.S.; Edwards, D.H. Metamodulation: The Control and Modulation of Neuromodulation, 1999th ed.; Oxford Univ. Press: New York, NY, USA, 1999; Volume I. [Google Scholar]
- Pittaluga, A.; Marchi, M. Synaptosomes and Metamodulation of Receptors. Methods Mol. Biol. 2022, 2417, 99–111. [Google Scholar]
- González-Maeso, J.; Ang, R.L.; Yuen, T.; Chan, P.; Weisstaub, N.V.; López-Giménez, J.F.; Zhou, M.; Okawa, Y.; Callado, L.F.; Milligan, G.; et al. Identification of a Serotonin/Glutamate Receptor Complex Implicated in Psychosis. Nature 2008, 452, 93–97. [Google Scholar] [CrossRef]
- Fribourg, M.; Moreno, J.L.; Holloway, T.; Provasi, D.; Baki, L.; Mahajan, R.; Park, G.; Adney, S.K.; Hatcher, C.; Eltit, J.M.; et al. Decoding the Signaling of a GPCR Heteromeric Complex Reveals a Unifying Mechanism of Action of Antipsychotic Drugs. Cell 2011, 147, 1011–1023. [Google Scholar] [CrossRef]
- Delille, H.K.; Becker, J.M.; Burkhardt, S.; Bleher, B.; Terstappen, G.C.; Schmidt, M.; Meyer, A.H.; Unger, L.; Marek, G.J.; Mezler, M. Heterocomplex Formation of 5-HT2A-MGlu2 and Its Relevance for Cellular Signaling Cascades. Neuropharmacology 2012, 62, 2184–2191. [Google Scholar] [CrossRef]
- Moreno, J.L.; Miranda-Azpiazu, P.; García-Bea, A.; Younkin, J.; Cui, M.; Kozlenkov, A.; Ben-Ezra, A.; Voloudakis, G.; Fakira, A.K.; Baki, L.; et al. Allosteric Signaling through an MGlu2 and 5-HT 2A Heteromeric Receptor Complex and Its Potential Contribution to Schizophrenia. Sci. Signal. 2016, 9, ra5. [Google Scholar] [CrossRef]
- Aghajanian, G.K.; Marek, G.J. Serotonin, via 5-HT Receptors, Increases EPSCs in Layer V Pyramidal Cells 2A of Prefrontal Cortex by an Asynchronous Mode of Glutamate Release. Brain Res. 1999, 825, 161–171. [Google Scholar] [CrossRef]
- Marek, G.J.; Wright, R.A.; Schoepp, D.D.; Monn, J.A.; Aghajanian, G.K. Physiological Antagonism between 5-Hydroxytryptamine 2A and Group II Metabotropic Glutamate Receptors in Prefrontal Cortex. J. Pharmacol. Exp. Ther. 2000, 292, 76–87. [Google Scholar]
- Kurita, M.; Holloway, T.; García-Bea, A.; Kozlenkov, A.; Friedman, A.K.; Moreno, J.L.; Heshmati, M.; Golden, S.A.; Kennedy, P.J.; Takahashi, N.; et al. HDAC2 Regulates Atypical Antipsychotic Responses through the Modulation of MGlu2 Promoter Activity. Nat. Neurosci. 2012, 15, 1245–1254. [Google Scholar] [CrossRef]
- Matrisciano, F.; Dong, E.; Nicoletti, F.; Guidotti, A. Epigenetic Alterations in Prenatal Stress Mice as an Endophenotype Model for Schizophrenia: Role of Metabotropic Glutamate 2/3 Receptors. Front. Mol. Neurosci. 2018, 11, 423. [Google Scholar] [CrossRef]
- Olivero, G.; Grilli, M.; Vergassola, M.; Bonfiglio, T.; Padolecchia, C.; Garrone, B.; di Giorgio, F.P.; Tongiani, S.; Usai, C.; Marchi, M.; et al. 5-HT2A-MGlu2/3 Receptor Complex in Rat Spinal Cord Glutamatergic Nerve Endings: A 5-HT2A to MGlu2/3 Signalling to Amplify Presynaptic Mechanism of Auto-Control of Glutamate Exocytosis. Neuropharmacology 2018, 133, 429–439. [Google Scholar] [CrossRef]
- Cisani, F.; Roggeri, A.; Olivero, G.; Garrone, B.; Tongiani, S.; Paolo, F.; Giorgio, D.; Pittaluga, A.; Corasaniti, M.T.; Ciruela, F. Acute Low Dose of Trazodone Recovers Glutamate Release Efficiency and MGlu2/3 Autoreceptor Impairments in the Spinal Cord of Rats Suffering from Chronic Sciatic Ligation. Front. Pharmacol. 2020, 11, 1108. [Google Scholar] [CrossRef]
- Cartmell, J.; Schoepp, D.D. Regulation of Neurotransmitter Release by Metabotropic Glutamate Receptors. J. Neurochem. 2000, 75, 889–907. [Google Scholar] [CrossRef]
- Olivero, G.; Bonfiglio, T.; Vergassola, M.; Usai, C.; Riozzi, B.; Battaglia, G.; Nicoletti, F.; Pittaluga, A. Immuno-Pharmacological Characterization of Group II Metabotropic Glutamate Receptors Controlling Glutamate Exocytosis in Mouse Cortex and Spinal Cord. Br. J. Pharmacol. 2017, 174, 4785–4796. [Google Scholar] [CrossRef]
- Wang, S.J.; Wang, K.Y.; Wang, W.C.; Sihra, T.S. Unexpected Inhibitory Regulation of Glutamate Release from Rat Cerebrocortical Nerve Terminals by Presynaptic 5-Hydroxytryptamine-2A Receptors. J. Neurosci. Res. 2006, 84, 1528–1542. [Google Scholar] [CrossRef]
- Olivero, G.; Cisani, F.; Marimpietri, D.; di Paolo, D.; Cristina Gagliani, M.; Podestà, M.; Cortese, K.; Pittaluga, A.; Raber, J.; Guescini, M.; et al. The Depolarization-Evoked, Ca2+-Dependent Release of Exosomes From Mouse Cortical Nerve Endings: New Insights Into Synaptic Transmission. Front. Pharmacol. 2021, 12, 670158. [Google Scholar] [CrossRef]
- Raiteri, M.; Angelini, F.; Levi, G. A Simple Apparatus for Studying the Release of Neurotransmitters from Synaptosomes. Eur. J. Pharm. 1974, 25, 411–414. [Google Scholar] [CrossRef]
- Raiteri, L.; Raiteri, M. Synaptosomes Still Viable after 25 Years of Superfusion. Neurochem. Res. 2000, 25, 1265–1274. [Google Scholar] [CrossRef]
- Pittaluga, A.; di Prisco, S.; Merega, E.; Bonfiglio, T.; Olivero, G.; Cervetto, C.; Grilli, M.; Usai, C.; Marchi, M. Presynaptic, Release-Regulating MGlu 2-Preferring and MGlu 3-Preferring Autoreceptors in CNS: Pharmacological Profiles and Functional Roles in Demyelinating Disease. Br. J. Pharmacol. 2016, 173, 1465–1477. [Google Scholar] [CrossRef]
- Grilli, M.; Raiteri, L.; Pittaluga, A. Somatostatin Inhibits Glutamate Release from Mouse Cerebrocortical Nerve Endings through Presynaptic Sst2 Receptors Linked to the Adenylyl Cyclase-Protein Kinase a Pathway. Neuropharmacology 2004, 46, 388–396. [Google Scholar] [CrossRef]
- Zucchini, S.; Pittaluga, A.; Brocca-Cofano, E.; Summa, M.; Fabris, M.; de Michele, R.; Bonaccorsi, A.; Busatto, G.; Barbanti-Brodano, G.; Altavilla, G.; et al. Increased Excitability in Tat-Transgenic Mice: Role of Tat in HIV-Related Neurological Disorders. Neurobiol. Dis. 2013, 55, 110–119. [Google Scholar] [CrossRef]
- Wischhof, L.; Koch, M. 5-HT2A and mGlu2/3 receptor interactions: On their relevance to cognitive function and psychosis. Behav. Pharmacol. 2016, 27, 1–11. [Google Scholar] [CrossRef]
- Murat, S.; Bigot, M.; Chapron, J.; König, G.M.; Kostenis, E.; Battaglia, G.; Nicoletti, F.; Bourinet, E.; Bockaert, J.; Marin, P.; et al. 5-HT2A Receptor-Dependent Phosphorylation of MGlu2 Receptor at Serine 843 Promotes MGlu2 Receptor-Operated Gi/o Signaling. Mol. Psychiatry 2019, 24, 1610–1626. [Google Scholar] [CrossRef]
- Baki, L.; Fribourg, M.; Younkin, J.; Eltit, J.M.; Moreno, J.L.; Park, G.; Vysotskaya, Z.; Narahari, A.; Sealfon, S.C.; Gonzalez-Maeso, J.; et al. Cross-Signaling in Metabotropic Glutamate 2 and Serotonin 2A Receptor Heteromers in Mammalian Cells. Pflug. Arch. 2016, 468, 775–793. [Google Scholar] [CrossRef]
- Fountoulakis, K.N.; Kelsoe, J.R.; Akiskal, H. Receptor Targets for Antidepressant Therapy in Bipolar Disorder: An Overview. J. Affect. Disord. 2012, 138, 222–238. [Google Scholar] [CrossRef]
- Mestre, T.A.; Zurowski, M.; Fox, S. H 5-Hydroxytryptamine 2A Receptor Antagonists as Potential Treatment for Psychiatric Disorders. Expert Opin. Investig. Drugs 2013, 22, 411–421. [Google Scholar] [CrossRef]
- Marcoli, M.; Rosu, C.; Bonfanti, A.; Raiteri, M.; Maura, G. Inhibitory Presynaptic 5-Hydroxytryptamine 2A Receptors Regulate Evoked Glutamate Release from Rat Cerebellar Mossy Fibers. J. Pharmacol. Exp. Ther. 2001, 299, 1106–1111. [Google Scholar]
- de Almeida, J.; Mengod, G. Quantitative Analysis of Glutamatergic and GABAergic Neurons Expressing 5-HT(2A) Receptors in Human and Monkey Prefrontal Cortex. J. Neurochem. 2007, 103, 475–486. [Google Scholar] [CrossRef]
- Santana, N.; Bortolozzi, A.; Serrats, J.; Mengod, G.; Artigas, F. Expression of Serotonin 1A and Serotonin 2A Receptors in Pyramidal and GABAergic Neurons of the Rat Prefrontal Cortex. Cereb. Cortex 2004, 14, 1100–1109. [Google Scholar] [CrossRef]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, Pharmacological and Functional Diversity of 5-HT Receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Mocci, G.; Jiménez-Sánchez, L.; Adell, A.; Cortés, R.; Artigas, F. Expression of 5-HT2A Receptors in Prefrontal Cortex Pyramidal Neurons Projecting to Nucleus Accumbens. Potential Relevance for Atypical Antipsychotic Action. Neuropharmacology 2014, 79, 49–58. [Google Scholar] [CrossRef]
- Martín-Ruiz, R.; Victoria Puig, M.; Celada, P.; Shapiro, D.A.; Roth, B.L.; Mengod, G.; Artigas, F. Control of Serotonergic Function in Medial Prefrontal Cortex by Serotonin-2A Receptors through a Glutamate-Dependent Mechanism. J. Neurosci. 2001, 21, 9856–9866. [Google Scholar] [CrossRef]
- Marek, G.J.; Wright, R.A.; Gewirtz, J.C.; Schoepp, D.D. A Major Role for Thalamocortical Afferents in Serotonergic Hallucinogen Receptor Function in the Rat Neocortex. Neuroscience 2001, 105, 379–392. [Google Scholar] [CrossRef]
- Beique, J.-C.; Mladenovic, L.; Gingrich, J.A.; Andrade, R. Mechanism of the 5-Hydroxytryptamine 2A Receptor-Mediated Facilitation of Synaptic Activity in Prefrontal Cortex. Proc. Natl. Acad. Sci. USA 2007, 104, 9870–9875. [Google Scholar] [CrossRef] [PubMed]
- Scruggs, J.L.; Schmidt, D.; Deutch, A.Y. The Hallucinogen 1-[2,5-Dimethoxy-4-Iodophenyl]-2-Aminopropane (DOI) Increases Cortical Extracellular Glutamate Levels in Rats. Neurosci. Lett. 2003, 346, 137–140. [Google Scholar] [CrossRef]
- Zhou, F.-M.; Hablitz, J.J. Activation of Serotonin Receptors Modulates Synaptic Transmission in Rat Cerebral Cortex. J. Neurophysiol. 1999, 82, 2989–2999. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Berthoux, C.; de Bundel, D.; Valjent, E.; Bockaert, J.; Marin, P.; Bécamel, C. Presynaptic Serotonin 2A Receptors Modulate Thalamocortical Plasticity and Associative Learning. Proc. Natl. Acad. Sci. USA 2016, 113, E1382–E1391. [Google Scholar] [CrossRef] [PubMed]
- Weisstaub, N.V.; Zhou, M.; Lira, A.; Lambe, E.; González-Maeso, J.; Hornung, J.-P.; Sibille, E.; Underwood, M.; Itohara, S.; Dauer, W.T.; et al. Cortical 5-HT2A Receptor Signaling Modulates Anxiety-like Behaviors in Mice. Science 2006, 313, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Herrero, I.; Miras-Portugal, T.; Sá Nchez-Prieto, J. Functional Switch from Facilitation to Inhibition in the Control of Glutamate Release by Metabotropic Glutamate Receptors. J. Biol. Chem. 1998, 273, 1951–1958. [Google Scholar] [CrossRef]
- Pittaluga, A. CCL5-Glutamate Cross-Talk in Astrocyte-Neuron Communication in Multiple Sclerosis. Front. Immunol. 2017, 8, 1079. [Google Scholar] [CrossRef]
- di Prisco, S.; Olivero, G.; Merega, E.; Bonfiglio, T.; Marchi, M.; Pittaluga, A. CXCR4 and NMDA Receptors Are Functionally Coupled in Rat Hippocampal Noradrenergic and Glutamatergic Nerve Endings. J. Neuroimmune Pharmacol. 2016, 11, 645–656. [Google Scholar] [CrossRef]
- Bockaert, J.; Bécamel, C.; Chaumont-Dubel, S.; Claeysen, S.; Vandermoere, F.; Marin, P. Faculty Opinions Novel and Atypical Pathways for Serotonin Signaling. Fac. Rev. 2021, 10, 52. [Google Scholar] [CrossRef]
- Pittaluga, A. Presynaptic Release-Regulating NMDA Receptors in Isolated Nerve Terminals: A Narrative Review. Br. J. Pharmacol. 2020, 178, 1001–1017. [Google Scholar] [CrossRef]
- Nicoletti, F.; Bockaert, J.; Collingridge, G.L.; Conn, P.J.; Ferraguti, F.; Schoepp, D.D.; Wroblewski, J.T.; Pin, J.P. Metabotropic Glutamate Receptors: From the Workbench to the Bedside. Neuropharmacology 2011, 60, 1017–1041. [Google Scholar] [CrossRef] [PubMed]
- Olivero, G.; Vergassola, M.; Cisani, F.; Roggeri, A.; Pittaluga, A. Send Orders for Reprints to Reprints@benthamscience.Net Presynaptic Release-Regulating Metabotropic Glutamate Receptors: An Update. Curr. Neuropharmacol. 2020, 18, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D. Expert Opinion on Therapeutic Targets G-Protein-Coupled Receptor Type A Heteromers as an Emerging Therapeutic Target. Expert Opin. Ther. Targets 2014, 19, 265–283. [Google Scholar] [CrossRef] [PubMed]
- de La Fuente Revenga, M.; Ibi, D.; Cuddy, T.; Toneatti, R.; Kurita, M.; Ijaz, M.K.; Miles, M.F.; Wolstenholme, J.T.; González-Maeso, J. Chronic Clozapine Treatment Restrains via HDAC2 the Performance of MGlu2 Receptor Agonism in a Rodent Model of Antipsychotic Activity. Neuropsychopharmacology 2018, 44, 443–454. [Google Scholar] [CrossRef]
- Nucifora, F.C.; Mihaljevic, M.; Lee, B.J.; Sawa, A. Clozapine as a Model for Antipsychotic Development. Neurotherapeutics 2017, 14, 750–761. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Massey, B.W. The Role of Serotonin Receptors in the Action of Atypical Antipsychotic Drugs. Curr. Opin. Pharm. 2011, 11, 59–67. [Google Scholar] [CrossRef]
- Matsubara, S.; Meltzer, H.Y. Effect of Typical and Atypical Antipsychotic Drugs on 5-HT2 Receptor Density in Rat Cerebral Cortex. Life Sci. 1989, 45, 1397–1406. [Google Scholar] [CrossRef]
- Moreno, J.L.; Holloway, T.; Umali, A.; Rayannavar, V.; Sealfon, S.C.; González-Maeso, J. Persistent Effects of Chronic Clozapine on the Cellular and Behavioral Responses to LSD in Mice. Psychopharmacology 2013, 225, 217–226. [Google Scholar] [CrossRef]
- Peroutka, S.; Snyder, S. Long-Term Antidepressant Treatment Decreases Spiroperidol-Labeled Serotonin Receptor Binding; Springer: Berlin/Heidelberg, Germany, 1980; Volume 40. [Google Scholar]
- Yadav, P.N.; Abbas, A.I.; Farrell, M.S.; Setola, V.; Sciaky, N.; Huang, X.-P.; Kroeze, W.K.; Crawford, L.K.; Piel, D.A.; Keiser, M.J.; et al. The Presynaptic Component of the Serotonergic System Is Required for Clozapine’s Efficacy. Neuropsychopharmacology 2011, 36, 638–651. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Willins, D.L.; Gray, J.A.; Woods, J.; Benovic, J.L.; Roth, B.L. The Dynamin-Dependent, Arrestin-Independent Internalization of 5-Hydroxytryptamine 2A (5-HT2A) Serotonin Receptors Reveals Differential Sorting of Arrestins and 5-HT2A Receptors during Endocytosis. J. Biol. Chem. 2001, 276, 8269–8277. [Google Scholar] [CrossRef]
- Willins, D.L.; Alsayegh, L.; Berry, S.A.; Backstrom, J.R.; Sanders-Bush, E.; Friedman, L.; Khan, N.; Roth, B.L. Serotonergic Antagonist Effects on Trafficking of Serotonin 5-HT2A Receptors in Vitro and in Vivo. Ann. N. Y. Acad. Sci. 1998, 861, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.S.; Donohoe, D. Induction of Hyperglycemia in Mice with Atypical Antipsychotic Drugs That Inhibit Glucose Uptake. Pharm. Biochem. Behav. 2003, 75, 255–260. [Google Scholar] [CrossRef]
- Sutton, L.P.; Rushlow, W.J. The Effects of Neuropsychiatric Drugs on Glycogen Synthase Kinase-3 Signaling. Neuroscience 2011, 199, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.; Li, Y.-C.; Snyder, M.A.; Gao, R.Y.; Adelman, A.E.; Zhang, W.; Shumsky, J.S.; Gao, W.-J. Group II Metabotropic Glutamate Receptor Agonist Ameliorates MK801-Induced Dysfunction of NMDA Receptors via the Akt/GSK-3β Pathway in Adult Rat Prefrontal Cortex. Neuropsychopharmacology 2011, 36, 1260–1274. [Google Scholar] [CrossRef] [PubMed]
- Corne, S.J.; Pickering, R.W. A Possible Correlation between Drug-Induced Hallucinations in Man and a Behavioural Response in Mice. Psychopharmacologia 1967, 11, 65–78. [Google Scholar] [CrossRef] [PubMed]
- González-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens Recruit Specific Cortical 5-HT2A Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef]
| Stimulus | Induced Overflow (% of Total Tritium Content) | % Changes (vs. KCl-Induced Tritium Overflow) |
|---|---|---|
| 15 mM KCl | 1.73 ± 0.14 | |
| 15 mM KCl/100 nM ketanserin | 1.65 ± 0.09 | −4.62 ± 5.73% |
| 15 mM KCl/10 μM (±) DOI | 1.17 ± 0.09 a | −32.36 ± 4.74% |
| 15 mM KCl/10 μM (±) DOI/100 nM ketanserin | 1.45 ± 0.11 b | −17.04 ± 5.71% |
| Stimulus | Induced Overflow (% of Total Tritium Content) | % Changes (vs. KCl-Induced Tritium Overflow) |
|---|---|---|
| 15 mM KCl | 1.77 ± 0.15 (n = 4) | |
| 15 mM KCl/LY379268 (3 nM) | 1.57 ± 0.03 (n = 4) | −11.29 ± 9.73% |
| 15 mM KCl/MDL11,939 (1 µM) | 1.70 ± 0.09 (n = 4) | −3.76 ± 5.09% |
| 15 mM KCl/LY379268 (3 nM)/MDL11,939 (1 µM) | 1.14 ± 0.08 (n = 4) | −35.59 ± 6.32% a,b |
| 15 mM KCl/ketanserin (0.1 µM) | 1.69 ± 0.07 (n = 4) | −4.15 ± 4.38% |
| 15 mM KCl/LY379268 (3 nM)/ketanserin (0.1 µM) | 1.12 ± 0.06 (n = 4) | −36.72 ± 5.21% a,b |
| 15 mM KCl/trazodone (0.1 µM) | 1.74 ± 0.06 (n = 4) | −1.50 ± 3.78% a,b |
| 15 mM KCl/LY379268 (3 nM)/trazodone (0.1 µM) | 1.18 ± 0.09 (n = 4) | −33.33 ± 4.35% a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taddeucci, A.; Olivero, G.; Roggeri, A.; Milanese, C.; Giorgio, F.P.D.; Grilli, M.; Marchi, M.; Garrone, B.; Pittaluga, A. Presynaptic 5-HT2A-mGlu2/3 Receptor–Receptor Crosstalk in the Prefrontal Cortex: Metamodulation of Glutamate Exocytosis. Cells 2022, 11, 3035. https://doi.org/10.3390/cells11193035
Taddeucci A, Olivero G, Roggeri A, Milanese C, Giorgio FPD, Grilli M, Marchi M, Garrone B, Pittaluga A. Presynaptic 5-HT2A-mGlu2/3 Receptor–Receptor Crosstalk in the Prefrontal Cortex: Metamodulation of Glutamate Exocytosis. Cells. 2022; 11(19):3035. https://doi.org/10.3390/cells11193035
Chicago/Turabian StyleTaddeucci, Alice, Guendalina Olivero, Alessandra Roggeri, Claudio Milanese, Francesco Paolo Di Giorgio, Massimo Grilli, Mario Marchi, Beatrice Garrone, and Anna Pittaluga. 2022. "Presynaptic 5-HT2A-mGlu2/3 Receptor–Receptor Crosstalk in the Prefrontal Cortex: Metamodulation of Glutamate Exocytosis" Cells 11, no. 19: 3035. https://doi.org/10.3390/cells11193035
APA StyleTaddeucci, A., Olivero, G., Roggeri, A., Milanese, C., Giorgio, F. P. D., Grilli, M., Marchi, M., Garrone, B., & Pittaluga, A. (2022). Presynaptic 5-HT2A-mGlu2/3 Receptor–Receptor Crosstalk in the Prefrontal Cortex: Metamodulation of Glutamate Exocytosis. Cells, 11(19), 3035. https://doi.org/10.3390/cells11193035