The Potential of Moringa oleifera to Ameliorate HAART-Induced Pathophysiological Complications
Abstract
1. Introduction
2. HAART-Induced Mitochondrial Toxicity and Oxidative Stress
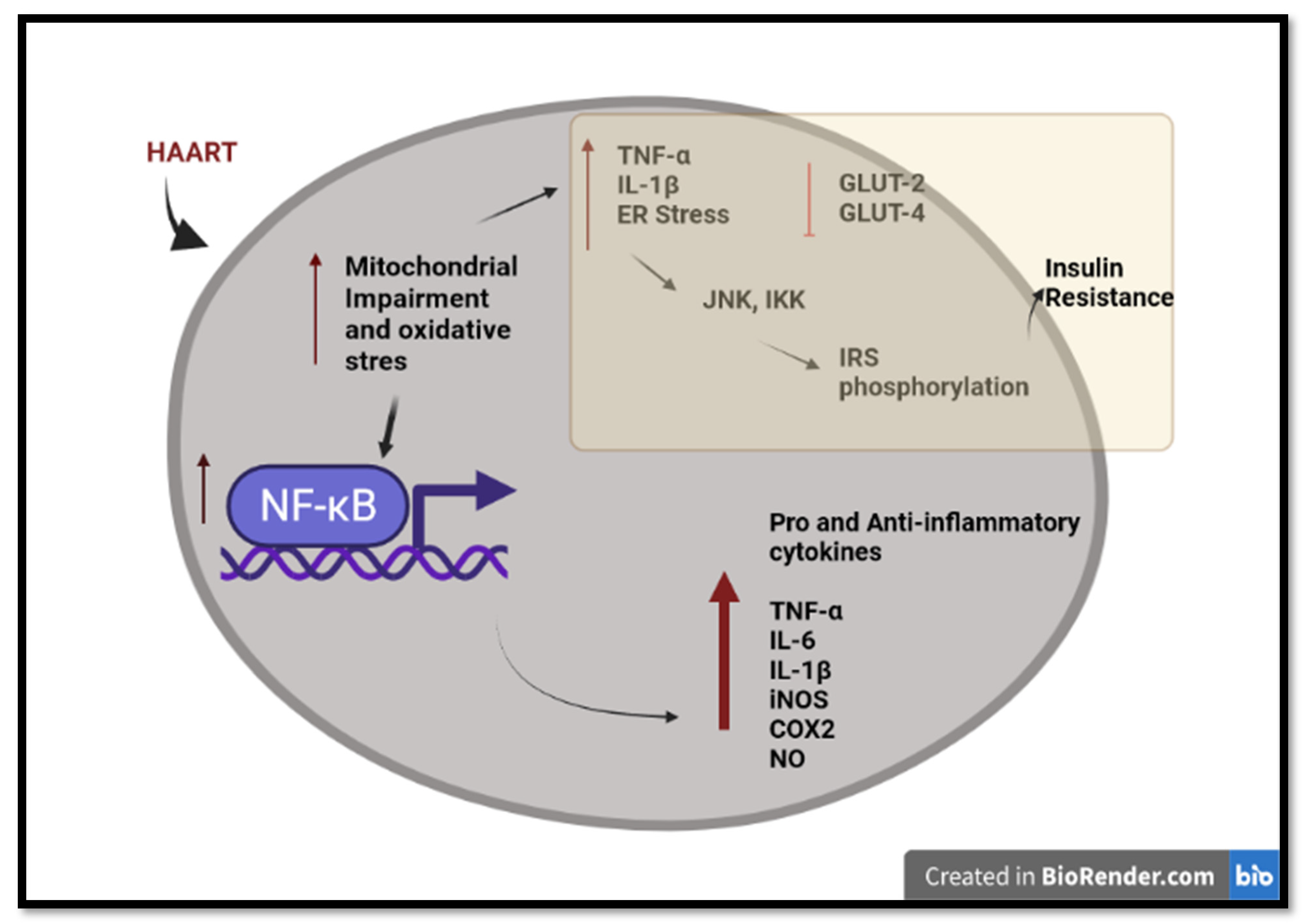
3. HAART-Induced Chronic Inflammation and Insulin Resistance
4. Moringa oleifera (MO) as a Supplementary Medicine
4.1. Anti-Oxidant Properties of MO
4.2. Antiinflammatory Properties of MO
5. HAART-MO
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Summary of the Global HIV Epidemic, 2020. World Heath Organisation. 2021. Available online: https://www.who.int/data/gho/data/themes/hiv-aids (accessed on 14 June 2022).
- Statssa. STATISTICAL RELEASE- P0302 Mid-Year Population Estimates, 2021. Department of statistics South Africa. 2021. Available online: http://www.statssa.gov.za/publications/P0302/P03022021.pdf (accessed on 10 June 2022).
- UNAIDS. Global AIDS Update 2021. UNAIDS Repots. 2021. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 14 June 2022).
- Volberding, P.A.; Deeks, S.G. Antiretroviral therapy and management of HIV infection. Lancet 2010, 376, 49–62. [Google Scholar] [CrossRef]
- Palella, F.J., Jr.; Delaney, K.M.; Moorman, A.C.; Loveless, M.O.; Fuhrer, J.; Satten, G.A.; Aschman, D.J.; Holmberg, S.D. Declining Morbidity and Mortality among Patients with Advanced Human Immunodeficiency Virus Infection. N. Engl. J. Med. 1998, 338, 553–860. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.C.; Treisman, G.J. Cognitive impairment in patients with AIDS—prevalence and severity. HIV/AIDS- Res. Palliat. Care 2015, 7, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Q.; Lichterfeld, M. Diversity of HIV-1 reservoirs in CD4+ T-cell subpopulations. Curr. Opin. HIV AIDS 2016, 11, 383–387. [Google Scholar] [CrossRef]
- Durand, C.M.; Blankson, J.N.; Siliciano, R.F. Developing strategies for HIV-1 eradication. Trends Immunol. 2012, 33, 554–562. [Google Scholar] [CrossRef]
- Politch, J.; Mayer, K.H.; Welles, S.L.; O’Brien, W.X.; Xu, C.; Bowman, F.; Anderson, D.J. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS 2012, 26, 1535–1543. [Google Scholar] [CrossRef]
- Falasca, K.; Reale, M.; Ucciferri, C.; Di Nicola, M.; Di Martino, G.; D’Angelo, C.; Coladonato, S.; Vecchiet, J. Cytokines, Hepatic Fibrosis, and Antiretroviral Therapy Role in Neurocognitive Disorders HIV Related. AIDS Res. Hum. Retrovir. 2017, 33, 246–253. [Google Scholar] [CrossRef]
- Raposo, M.A.; Armiliato, G.N.D.A.; Guimarães, N.S.; Caram, C.A.; Silveira, R.D.D.S.; Tupinambás, U. Metabolic disorders and cardiovascular risk in people living with HIV/AIDS without the use of antiretroviral therapy. Rev. Soc. Bras. Med. Trop. 2017, 50, 598–606. [Google Scholar] [CrossRef]
- Mohan, J.; Ghazi, T.; Chuturgoon, A.A. A Critical Review of the Biochemical Mechanisms and Epigenetic Modifications in HIV- and Antiretroviral-Induced Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 12020. [Google Scholar] [CrossRef]
- Ghislain, M.; Bastard, J.-P.; Meyer, L.; Capeau, J.; Fellahi, S.; Gérard, L.; May, T.; Simon, A.; Vigouroux, C.; Goujard, C.; et al. Late Antiretroviral Therapy (ART) Initiation Is Associated with Long-Term Persistence of Systemic Inflammation and Metabolic Abnormalities. PLoS ONE 2015, 10, e0144317. [Google Scholar] [CrossRef]
- Hurwitz, B.E.; Klimas, N.G.; Llabre, M.M.; Maher, K.J.; Skyler, J.S.; Bilsker, M.S.; McPherson-Baker, S.; Lawrence, P.J.; LaPerriere, A.R.; Greeson, J.M.; et al. HIV, metabolic syndrome X, inflammation, oxidative stress, and coronary heart disease risk. Cardiovasc. Toxicol. 2004, 4, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Longenecker, C.T.; Jiang, Y.; Yun, C.-H.; Debanne, S.; Funderburg, N.T.; Lederman, M.M.; Storer, N.; Labbato, D.E.; Bezerra, H.G.; McComsey, G.A. Perivascular fat, inflammation, and cardiovascular risk in HIV-infected patients on antiretroviral therapy. Int. J. Cardiol. 2013, 168, 4039–4045. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Roy, D.; Cassol, E. Examining Relationships between Metabolism and Persistent Inflammation in HIV Patients on Antiretroviral Therapy. Mediat. Inflamm. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nzuza, S.; Zondi, S.; Hurchund, R.; Owira, P.M. Highly Active Antiretroviral Therapy-Associated Metabolic Syndrome and Lipodystrophy: Pathophysiology and Current Therapeutic Interventions. J. Endocrinol. Metab. 2017, 7, 103–116. [Google Scholar] [CrossRef]
- Hunt, P.W. HIV and Inflammation: Mechanisms and Consequences. Curr. HIV/AIDS Rep. 2012, 9, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Pérez, P.S.; Romaniuk, M.A.; Duette, G.A.; Zhao, Z.; Huang, Y.; Martin-Jaular, L.; Witwer, K.W.; Théry, C.; Ostrowski, M. Extracellular vesicles and chronic inflammation during HIV infection. J. Extracell. Vesicles 2019, 8, 1687275. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, J.; Chen, X. Bioactive flavonoids in Moringa oleifera and their health-promoting properties. J. Funct. Foods 2018, 47, 469–479. [Google Scholar] [CrossRef]
- Islam, Z.; Islam, S.M.R.; Hossen, F.; Mahtab-Ul-Islam, K.; Hasan, R.; Karim, R. Moringa oleifera is a Prominent Source of Nutrients with Potential Health Benefits. Int. J. Food Sci. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Milla, P.; Peñalver, R.; Nieto, G. Health Benefits of Uses and Applications of Moringa oleifera in Bakery Products. Plants 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Sivanesan, I.; Keum, Y.-S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, S.; Padma, P.R. Antioxidant Activity and Total Phenolic Content of Moringa oleifera Leaves in Two Stages of Maturity. Mater. Veg. 2009, 64, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.J.; Lee, K.S.; Hyacinth, H.I.; Hibbert, J.M.; Reid, M.E.; Wheatley, A.O.; Asemota, H.N. An Investigation of the Antioxidant Capacity in Extracts from Moringa oleifera Plants Grown in Jamaica. Plants 2017, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Vongsak, B.; Sithisarn, P.; Gritsanapan, W. Bioactive contents and free radical scavenging activity of Moringa oleifera leaf extract under different storage conditions. Ind. Crop. Prod. 2013, 49, 419–421. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, G.; Guo, M. Antioxidant and anti-inflammatory activities of the crude extracts of Moringa oleifera from Kenya and their correlations with flavonoids. Antioxidants 2019, 8, 296. [Google Scholar] [CrossRef]
- Coz-Bolaños, X.; Campos-Vega, R.; Reynoso-Camacho, R.; Ramos-Gómez, M.; Loarca-Piña, G.F.; Guzmán-Maldonado, S.H. Moringa infusion (Moringa oleifera) rich in phenolic compounds and high antioxidant capacity attenuate nitric oxide pro-inflammatory mediator in vitro. Ind. Crops Prod. 2018, 118, 95–101. [Google Scholar] [CrossRef]
- Khor, K.Z.; Lim, V.; Moses, E.J.; Abdul Samad, N. The In Vitro and In Vivo Anticancer Properties of Moringa oleifera. Evid. -Based Complement. Altern. Med. 2018, 2018, 1071243. [Google Scholar] [CrossRef]
- Sodvadiya, M.; Patel, H.; Mishra, A.; Nair, S. Emerging Insights into Anticancer Chemopreventive Activities of Nutraceutical Moringa oleifera: Molecular Mechanisms, Signal Transduction and In Vivo Efficacy. Curr. Pharmacol. Rep. 2020, 6, 38–51. [Google Scholar] [CrossRef]
- Toppo, R.; Roy, B.K.; Gora, R.H.; Baxla, S.L.; Kumar, P. Hepatoprotective activity of Moringa oleifera against cadmium toxicity in rats. Veter- World 2015, 8, 537–540. [Google Scholar] [CrossRef]
- Nova, E.; Redondo-Useros, N.; Martínez-García, R.M.; Gómez-Martínez, S.; Díaz-Prieto, L.E.; Marcos, A. Potential of Moringa oleifera to Improve Glucose Control for the Prevention of Diabetes and Related Metabolic Alterations: A Systematic Review of Animal and Human Studies. Nutrients 2020, 12, 2050. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wang, C.; Li, S.; Chu, X.; Sun, K. Nutritional compositions of Indian Moringa oleifera seed and antioxidant activity of its polypeptides. Food Sci. Nutr. 2019, 7, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Adewumi, O.O.; Felix-Minnaar, J.V.; Jideani, V.A. Functional Properties and Amino Acid Profile of Bambara Groundnut and Moringa oleifera Leaf Protein Complex. Processes 2022, 10, 205. [Google Scholar] [CrossRef]
- White, A.J. Mitochondrial toxicity and HIV therapy. Sex. Transm. Infect. 2001, 77, 158–173. [Google Scholar] [CrossRef]
- Feeney, E.R.; Mallon, P.W. Impact of mitochondrial toxicity of HIV-1 antiretroviral drugs on lipodystrophy and metabolic dysregulation. Curr. Pharm. Des. 2010, 16, 3339–3351. [Google Scholar]
- Nooka, S.; Ghorpade, A. HIV-1-associated inflammation and antiretroviral therapy regulate astrocyte endoplasmic reticulum stress responses. Cell Death Discov. 2017, 3, 17061. [Google Scholar] [CrossRef]
- Ganta, K.K.; Chaubey, B. Mitochondrial dysfunctions in HIV infection and antiviral drug treatment. Expert Opin. Drug Metab. Toxicol. 2019, 15, 1043–1052. [Google Scholar] [CrossRef]
- del MGutierrez, M.; GMateo, M.; Vidal, F.; Domingo, P. The toxicogenetics of antirretroviral therapy: The evil inside. J Curr. Med. Chem. 2011, 18, 209–219. [Google Scholar] [CrossRef]
- Kolakowska, A.; Maresca, A.F.; Collins, I.J.; Cailhol, J. Update on Adverse Effects of HIV Integrase Inhibitors. Curr. Treat. Options Infect. Dis. 2019, 11, 372–387. [Google Scholar] [CrossRef]
- Wang, Y.; De Clercq, E.; Li, G. Current and emerging non-nucleoside reverse transcriptase inhibitors (NNRTIs) for HIV-1 treatment. Expert Opin. Drug Metab. Toxicol. 2019, 15, 813–829. [Google Scholar] [CrossRef]
- Post, F.A.; Sinxadi, P. Tenofovir disoproxil and renal mitochondrial toxicity: More studies in Africans are needed. AIDS 2022, 36, 1047–1048. [Google Scholar] [CrossRef] [PubMed]
- Manda, K.R.; Banerjee, A.; Banks, W.A.; Ercal, N. Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood–brain barrier endothelial cells. Free Radic. Biol. Med. 2011, 50, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Chiao, S.K.; Romero, D.L.; Johnson, D.E. Current HIV therapeutics: Mechanistic and chemical determinants of toxicity. Curr. Opin. Drug Discov. Dev. 2009, 12, 53–60. [Google Scholar]
- Caron, M.; Auclair, M.; Vissian, A.; Vigouroux, C.; Capeau, J. Contribution of Mitochondrial Dysfunction and Oxidative Stress to Cellular Premature Senescence Induced by Antiretroviral Thymidine Analogues. Antivir. Ther. 2008, 13, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Nadezda, A.; Ana, B.-G.; Juan, V.E. Mitochondrial Toxicity in HAART: An Overview of In Vitro Evidence. Curr. Pharm. Des. 2011, 17, 2130–2144. [Google Scholar]
- Williams, A.A.; Sitole, L.J.; Meyer, D. HIV/HAART-associated oxidative stress is detectable by metabonomics. Mol. BioSyst. 2017, 13, 2202–2217. [Google Scholar] [CrossRef]
- George, J.W.; Mattingly, J.E.; Roland, N.J.; Small, C.M.; Lamberty, B.G.; Fox, H.S.; Stauch, K.L. Physiologically Relevant Concentrations of Dolutegravir, Emtricitabine, and Efavirenz Induce Distinct Metabolic Alterations in HeLa Epithelial and BV2 Microglial Cells. Front. Immunol. 2021, 12, 639378. [Google Scholar] [CrossRef]
- Maagaard, A.; Kvale, D. Long term adverse effects related to nucleoside reverse transcriptase inhibitors: Clinical impact of mitochondrial toxicity. Scand. J. Infect. Dis. 2009, 41, 808–817. [Google Scholar] [CrossRef]
- Hargreaves, I.P.; Al Shahrani, M.; Wainwright, L.; Heales, S.J.R. Drug-Induced Mitochondrial Toxicity. Drug Saf. 2016, 39, 661–674. [Google Scholar] [CrossRef]
- Birkus, G.; Hitchcock, M.; Cihlar, T. Assessment of mitochondrial toxicity in human cells treated with tenofovir: Comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 2002, 46, 716–723. [Google Scholar] [CrossRef]
- Margolis, A.M.; Heverling, H.; Pham, P.A.; Stolbach, A. A review of the toxicity of HIV medications. J Med Toxicol 2014, 10, 26–39. [Google Scholar] [CrossRef]
- Dagan, T.; Sable, C.; Bray, J.; Gerschenson, M. Mitochondrial dysfunction and antiretroviral nucleoside analog toxicities: What is the evidence? Mitochondrion 2002, 1, 397–412. [Google Scholar] [CrossRef]
- Gerschenson, M.; Nguyen, V.; Ewings, E.L.; Ceresa, A.; Shaw, J.A.; St. Claire, M.C.; Nagashima, K.; Harbaugh, S.W.; Harbaugh, J.W.; Olivero, O.A.; et al. Mitochondrial Toxicity in Fetal Erythrocebus patas Monkeys Exposed Transplacentally to Zidovudine Plus Lamivudine. AIDS Res. Hum. Retrovir. 2004, 20, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Samuels, R.; Bayerri, C.R.; Sayer, J.A.; Price, D.A.; Payne, B.A.I. Tenofovir disoproxil fumarate-associated renal tubular dysfunction: Noninvasive assessment of mitochondrial injury. AIDS 2017, 31, 1297–1301. [Google Scholar] [CrossRef]
- Mccomsey, G.A.; Daar, E.S.; O’Riordan, M.; Collier, A.C.; Kosmiski, L.; Santana, J.L.; Fichtenbaum, C.J.; Fink, H.; Sax, P.E.; Libutti, D.E.; et al. Changes in Fat Mitochondrial DNA and Function in Subjects Randomized to Abacavir-Lamivudine or Tenofovir DF–Emtricitabine With Atazanavir-Ritonavir or Efavirenz: AIDS Clinical Trials Group Study A5224s, Substudy of A5202. J. Infect. Dis. 2012, 207, 604–611. [Google Scholar] [CrossRef]
- Kohler, J.J.; Hosseini, S.H.; Hoying-Brandt, A.; Green, E.; Johnson, D.M.; Russ, R.; Tran, D.; Raper, C.M.; Santoianni, R.; Lewis, W. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab. Investig. 2009, 89, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Khalili, H.; Dashti-Khavidaki, S. Tenofovir-induced nephrotoxicity: Incidence, mechanism, risk factors, prognosis and proposed agents for prevention. Eur. J. Clin. Pharmacol. 2014, 70, 1029–1040. [Google Scholar] [CrossRef]
- Ikekpeazu, J.E.; Orji, O.C.; Uchendu, I.K.; Ezeanyika, L.U. Mitochondrial and Oxidative Impacts of Short and Long-term Administration of HAART on HIV Patients. Curr. Clin. Pharmacol. 2020, 15, 110–124. [Google Scholar] [CrossRef]
- Neukam, K.; Mira, J.A.; Ruiz-Morales, J.; Rivero, A.; Collado, A.; Torres-Cornejo, A.; Merino, D.; de Los Santos-Gil, I.; Macías, J.; González-Serrano, M.; et al. Liver toxicity associated with antiretroviral therapy including efavirenz or ritonavir-boosted protease inhibitors in a cohort of HIV/hepatitis C virus co-infected patients. J. Antimicrob. Chemother. 2011, 66, 2605–2614. [Google Scholar] [CrossRef]
- Li, M.; Sopeyin, A.; Paintsil, E. Combination of Tenofovir and Emtricitabine with Efavirenz Does Not Moderate Inhibitory Effect of Efavirenz on Mitochondrial Function and Cholesterol Biosynthesis in Human T Lymphoblastoid Cell Line. Antimicrob. Agents Chemother. 2018, 62, e00691-18. [Google Scholar] [CrossRef]
- Funes, H.A.; Blas-Garcia, A.; Esplugues, J.V.; Apostolova, N. Efavirenz alters mitochondrial respiratory function in cultured neuron and glial cell lines. J. Antimicrob. Chemother. 2015, 70, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Blas-Garcia, A.; Galindo, M.J.; Esplugues, J.V. Efavirenz: What is known about the cellular mechanisms responsible for its adverse effects. Eur. J. Pharmacol. 2017, 812, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Schank, M.; Zhao, J.; Moorman, J.; Yao, Z. The Impact of HIV- and ART-Induced Mitochondrial Dysfunction in Cellular Senescence and Aging. Cells 2021, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Nagiah, S.; Phulukdaree, A.; Chuturgoon, A. Mitochondrial and Oxidative Stress Response in HepG2 Cells Following Acute and Prolonged Exposure to Antiretroviral Drugs. J. Cell. Biochem. 2015, 116, 1939–1946. [Google Scholar] [CrossRef]
- Tripathi, A.; Thangaraj, A.; Chivero, E.T.; Periyasamy, P.; Callen, S.; Burkovetskaya, M.E.; Guo, M.-L.; Buch, S. Antiretroviral-Mediated Microglial Activation Involves Dysregulated Autophagy and Lysosomal Dysfunction. Cells 2019, 8, 1168. [Google Scholar] [CrossRef]
- Abraham, P.; Ramamoorthy, H.; Isaac, B. Depletion of the cellular antioxidant system contributes to tenofovir disoproxil fumarate—Induced mitochondrial damage and increased oxido-nitrosative stress in the kidney. J. Biomed. Sci. 2013, 20, 61. [Google Scholar] [CrossRef]
- Wang, B.; Abbott, L.; Childs, K.; Taylor, C.; Agarwal, K.; Cormack, I.; Miquel, R.; Suddle, A. Dolutegravir-induced liver injury leading to sub-acute liver failure requiring transplantation: A case report and review of literature. Int. J. STD AIDS 2018, 29, 414–417. [Google Scholar] [CrossRef]
- Christensen, E.S.; Jain, R.; Roxby, A.C. Abacavir/Dolutegravir/Lamivudine (Triumeq)–Induced Liver Toxicity in a Human Immunodeficiency Virus–Infected Patient. Open Forum Infect. Dis. 2017, 4, ofx122. [Google Scholar] [CrossRef]
- Olaniyan, L.W.B.; Maduagwu, E.N.; Akintunde, O.W.; Oluwayelu, O.O.; Brai, B.I.C. Lamivudine-Induced Liver Injury. Open Access Maced. J. Med. Sci. 2015, 3, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Jin, J.; Ferrell, D.; Sadic, E.; Obregon, D.; Smith, A.J.; Tan, J.; Giunta, B. Efavirenz Promotes β-Secretase Expression and Increased Aβ1-40,42 via Oxidative Stress and Reduced Microglial Phagocytosis: Implications for HIV Associated Neurocognitive Disorders (HAND). PLoS ONE 2014, 9, e95500. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Aremu, A.; Akhigbe, R. Concomitant administration of HAART aggravates anti-Koch-induced oxidative hepatorenal damage via dysregulation of glutathione and elevation of uric acid production. Biomed. Pharmacother. 2021, 137, 111309. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B. Oxidative Stress in HIV Patients Receiving Antiretroviral Therapy. Curr. HIV Res. 2014, 12, 13–21. [Google Scholar] [CrossRef]
- Mak, I.T.; Chmielinska, J.J.; Spurney, C.F.; Weglicki, W.B.; Kramer, J.H. Combination ART-Induced Oxidative/Nitrosative Stress, Neurogenic Inflammation and Cardiac Dysfunction in HIV-1 Transgenic (Tg) Rats: Protection by Mg. Int. J. Mol. Sci. 2018, 19, 2409. [Google Scholar] [CrossRef]
- Deeks, S.G.; Tracy, R.; Douek, D.C. Systemic Effects of Inflammation on Health during Chronic HIV Infection. Immunity 2013, 39, 633–645. [Google Scholar] [CrossRef]
- Funderburg, N. Markers of coagulation and inflammation often remain elevated in ART-treated HIV-infected patients. Curr. Opin. HIV AIDS 2014, 9, 80–86. [Google Scholar] [CrossRef]
- Gutierrez, M.d.M.; Mateo, M.G.; Vidal, F.; Domingo, P. Does choice of antiretroviral drugs matter for inflammation? Expert Rev. Clin. Pharmacol. 2019, 12, 389–396. [Google Scholar] [CrossRef]
- Kalayjian, R.C.; Machekano, R.N.; Rizk, N.; Robbins, G.K.; Gandhi, R.T.; Rodriguez, B.A.; Pollard, R.B.; Lederman, M.M.; Landay, A. Pretreatment Levels of Soluble Cellular Receptors and Interleukin-6 Are Associated with HIV Disease Progression in Subjects Treated with Highly Active Antiretroviral Therapy. J. Infect. Dis. 2010, 201, 1796–1805. [Google Scholar] [CrossRef]
- Hunt, P.W.; Sinclair, E.; Rodriguez, B.; Shive, C.; Clagett, B.; Funderburg, N.; Robinson, J.; Huang, Y.; Epling, L.; Martin, J.N.; et al. Gut Epithelial Barrier Dysfunction and Innate Immune Activation Predict Mortality in Treated HIV Infection. J. Infect. Dis. 2014, 210, 1228–1238. [Google Scholar] [CrossRef]
- Tenorio, A.R.; Chan, E.S.; Bosch, R.J.; Macatangay, B.J.C.; Read, S.W.; Yesmin, S.; Taiwo, B.; Margolis, D.M.; Jacobson, J.M.; Landay, A.L.; et al. Rifaximin has a marginal impact on microbial translocation, T-cell activation and inflammation in HIV-positive immune non-responders to antiretroviral therapy—ACTG A5286. J. Infect. Dis. 2015, 211, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N. Innate immune activation in obesity. Mol. Asp. Med. 2013, 34, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Hirabara, S.M.; Gorjão, R.; Vinolo, M.A.; Rodrigues, A.C.; Nachbar, R.T.; Curi, R. Molecular Targets Related to Inflammation and Insulin Resistance and Potential Interventions. J. Biomed. Biotechnol. 2012, 2012, 379024. [Google Scholar] [CrossRef]
- Fields, J.A.; Swinton, M.K.; Carson, A.; Soontornniyomkij, B.; Lindsay, C.; Han, M.M.; Frizzi, K.; Sambhwani, S.; Murphy, A.; Achim, C.L.; et al. Tenofovir disoproxil fumarate induces peripheral neuropathy and alters inflammation and mitochondrial biogenesis in the brains of mice. Sci. Rep. 2019, 9, 17158. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, H.; Abraham, P.; Isaac, B.; Selvakumar, D. Role for NF-κB inflammatory signalling pathway in tenofovir disoproxil fumarate (TDF) induced renal damage in rats. Food Chem. Toxicol. 2017, 99, 103–118. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4+T Cells in Neurodegenerative Diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef] [PubMed]
- Méndez-García, L.A.; Trejo-Millán, F.; Martínez-Reyes, C.P.; Manjarrez-Reyna, A.N.; Esquivel-Velázquez, M.; Melendez-Mier, G.; Islas-Andrade, S.; Rojas-Bernabé, A.; Kzhyshkowska, J.; Escobedo, G. Infliximab ameliorates tumor necrosis factor-alpha-induced insulin resistance by attenuating PTP1B activation in 3T3L1 adipocytes in vitro. Scand. J. Immunol. 2018, 88, e12716. [Google Scholar] [CrossRef]
- Pedro, M.N.; Rocha, G.Z.; Guadagnini, D.; Santos, A.; Magro, D.O.; Assalin, H.B.; Oliveira, A.G.; Pedro, R.D.J.; Saad, M.J.A. Insulin Resistance in HIV-Patients: Causes and Consequences. Front. Endocrinol. 2018, 9, 514. [Google Scholar] [CrossRef] [PubMed]
- Hruz, P.W. Molecular Mechanisms for Altered Glucose Homeostasis in HIV Infection. Am. J. Infect. Dis. 2006, 2, 187–192. [Google Scholar] [CrossRef]
- Avari, P.; Devendra, S. Human immunodeficiency virus and type 2 diabetes. Lond. J. Prim. Care 2017, 9, 38–42. [Google Scholar] [CrossRef]
- Gorwood, J.; Bourgeois, C.; Pourcher, V.; Pourcher, G.; Charlotte, F.; Mantecon, M.; Rose, C.; Morichon, R.; Atlan, M.; Le Grand, R.; et al. The Integrase Inhibitors Dolutegravir and Raltegravir Exert Proadipogenic and Profibrotic Effects and Induce Insulin Resistance in Human/Simian Adipose Tissue and Human Adipocytes. Clin. Infect. Dis. 2020, 71, e549–e560. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; CABI: Wallingford, UK, 2018. [Google Scholar]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Bartlett, J.; Shanmugam, K.; Münch, G.; Wu, M.J. Antioxidant and Anti-inflammatory Activities of Selected Medicinal Plants Containing Phenolic and Flavonoid Compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. Biological, nutritional, and therapeutic significance of Moringa oleifera Lam. Phytother Res. 2019, 33, 2870–2903. [Google Scholar] [CrossRef]
- Oyeyinka, A.T.; Oyeyinka, S.A. Moringa oleifera as a food fortificant: Recent trends and prospects. J. Saudi Soc. Agric. Sci. 2018, 17, 127–136. [Google Scholar] [CrossRef]
- Chhikara, N.; Kaur, A.; Mann, S.; Garg, M.K.; Sofi, S.A.; Panghal, A. Bioactive compounds, associated health benefits and safety considerations of Moringa oleifera L.: An updated review. Nutr. Food Sci. 2021, 51, 255–277. [Google Scholar] [CrossRef]
- Aderinola, T.A.; Fagbemi, T.N.; Enujiugha, V.N.; Alashi, A.M.; Aluko, R.E. Amino acid composition and antioxidant properties of Moringa oleifera seed protein isolate and enzymatic hydrolysates. Heliyon 2018, 4, e00877. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Mukumbo, F.E.; Idamokoro, E.M.; Lorenzo, J.M.; Afolayan, A.J.; Muchenje, V. Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products: A review. Food Res. Int. 2018, 106, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, M.; Kim, S.-H.; Sasireka, A.; Chandrasekaran, M.; Chung, I.-M. Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera. Food Biosci. 2018, 26, 23–29. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Thurber, M.D.; Fahey, J.W. Adoption of Moringa oleifera to Combat Under-Nutrition Viewed Through the Lens of the “Diffusion of Innovations” Theory. Ecol. Food Nutr. 2009, 48, 212–225. [Google Scholar] [CrossRef]
- Santos, A.F.S.; Argolo, A.C.C.; Paiva, P.M.G.; Coelho, L.C.B.B. Antioxidant Activity of Moringa oleifera Tissue Extracts. Phytother. Res. 2012, 26, 1366–1370. [Google Scholar] [CrossRef]
- Aju, B.; Rajalakshmi, R.; Mini, S. Protective role of Moringa oleifera leaf extract on cardiac antioxidant status and lipid peroxidation in streptozotocin induced diabetic rats. Heliyon 2019, 5, e02935. [Google Scholar] [CrossRef]
- Sinha, M.; Das, D.K.; Bhattacharjee, S.; Majumdar, S.; Dey, S. Leaf Extract of Moringa oleifera Prevents Ionizing Radiation-Induced Oxidative Stress in Mice. J. Med. Food 2011, 14, 1167–1172. [Google Scholar] [CrossRef]
- Luqman, S. Ferric reducing antioxidant power and free radical scavenging activity of Moringa oleifera: Relevance in oxidative stress. Nat. Preced. 2012, 7, 1. [Google Scholar] [CrossRef]
- Ju, W.-T.; Kwon, O.-C.; Kim, H.-B.; Sung, G.-B.; Kim, H.-W.; Kim, Y.-S. Qualitative and quantitative analysis of flavonoids from 12 species of Korean mulberry leaves. J. Food Sci. Technol. 2018, 55, 1789–1796. [Google Scholar] [CrossRef]
- Zou, X.-Y.; He, Y.-J.; Yang, Y.-H.; Yan, X.-P.; Li, Z.-B.; Yang, H. Systematic Identification of Bioactive Compositions in Leaves of Morus Cultivars Using UHPLC-ESI-QTOF-MS/MS and Comprehensive Screening of High-Quality Resources. Separations 2022, 9, 76. [Google Scholar] [CrossRef]
- Uma, N.; Fakurazi, S.; Hairuszah, I. Moringa oleifera Enhances Liver Antioxidant Status via Elevation of Antioxidant Enzymes Activity and Counteracts Paracetamol-induced Hepatotoxicity. Malays. J. Nutr. 2010, 16, 293–307. [Google Scholar] [PubMed]
- Duranti, G.; Maldini, M.; Crognale, D.; Horner, K.; Dimauro, I.; Sabatini, S.; Ceci, R. Moringa oleifera Leaf Extract Upregulates Nrf2/HO-1 Expression and Ameliorates Redox Status in C2C12 Skeletal Muscle Cells. Molecules 2021, 26, 5041. [Google Scholar] [CrossRef]
- Qi, L.; Zhou, Y.; Li, W.; Zheng, M.; Zhong, R.; Jin, X.; Lin, Y. Effect of Moringa oleifera stem extract on hydrogen peroxide-induced opacity of cultured mouse lens. BMC Complement. Altern. Med. 2019, 19, 144. [Google Scholar] [CrossRef] [PubMed]
- Akter, T.; Rahman, M.A.; Moni, A.; Apu, M.A.I.; Fariha, A.; Hannan, M.A.; Uddin, M.J. Prospects for Protective Potential of Moringa oleifera against Kidney Diseases. Plants 2021, 10, 2818. [Google Scholar] [CrossRef]
- Paolicchi, A.; Dominici, S.; Pieri, L.; Maellaro, E.; Pompella, A. Glutathione catabolism as a signaling mechanism. Biochem. Pharmacol. 2002, 64, 1027–1035. [Google Scholar] [CrossRef]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial Glutathione, a Key Survival Antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef]
- Gaucher, C.; Boudier, A.; Bonetti, J.; Clarot, I.; Leroy, P.; Parent, M. Glutathione: Antioxidant Properties Dedicated to Nanotechnologies. Antioxidants 2018, 7, 62. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.-L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef]
- Cheng, D.; Gao, L.; Su, S.; Sargsyan, D.; Wu, R.; Raskin, I.; Kong, A.-N. Moringa Isothiocyanate Activates Nrf2: Potential Role in Diabetic Nephropathy. AAPS J. 2019, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Abdou, K.H.; Moselhy, W.A.; Mohamed, H.M.; El-Nahass, E.-S.; Khalifa, A.G. Moringa oleifera Leaves Extract Protects Titanium Dioxide Nanoparticles-Induced Nephrotoxicity via Nrf2/HO-1 Signaling and Amelioration of Oxidative Stress. Biol. Trace Elem. Res. 2019, 187, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Aldhahrani, A.; Alkhedaide, A.; Nassan, M.A.; Althobaiti, F.; Mohamed, W.A. The ameliorative impacts of Moringa oleifera leaf extract against oxidative stress and methotrexate-induced hepato-renal dysfunction. Biomed. Pharmacother. 2020, 128, 110259. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ahmad, J.; Zhang, H.; Khan, I.; Muhammad, S. Evaluation of phytochemical and medicinal properties of Moringa (Moringa oleifera) as a potential functional food. South Afr. J. Bot. 2020, 129, 40–46. [Google Scholar] [CrossRef]
- Sun, B.; Karin, M. NF-κB signaling, liver disease and hepatoprotective agents. Oncogene 2008, 27, 6228–6244. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Schwabe, R.F. NF-κB in the liver—Linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef]
- Khafaga, A.F.; Noreldin, A.E.; Taha, A.E. The adaptogenic anti-ageing potential of resveratrol against heat stress-mediated liver injury in aged rats: Role of HSP70 and NF-kB signalling. J. Therm. Biol. 2019, 83, 8–21. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Murakami, M.; Hirano, T. The molecular mechanisms of chronic inflammation development. Front. Immunol. 2012, 3, 323. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Park, E.-J.; Yoshida, W.Y.; Barit, C.; Wall, M.; Pezzuto, J.M.; Chang, L.C. Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleifera fruits. Bioorg. Med. Chem. 2010, 18, 6598–6602. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Tan, W.S.; Gothai, S.; Muniandy, K.; Fakurazi, S.; Esa, N.M.; Alarfaj, A.A.; Kumar, S.S. Anti-Inflammatory Potential of Ethyl Acetate Fraction of Moringa oleifera in Downregulating the NF-κB Signaling Pathway in Lipopolysaccharide-Stimulated Macrophages. Molecules 2016, 21, 1452. [Google Scholar] [CrossRef] [PubMed]
- Uetragoon, T.; Pankla Sranujit, R.; Noysang, C.; Thongsri, Y.; Potup, P.; Suphrom, N.; Nuengchamnong, N.; Usuwanthim, K. Bioactive Compounds in Moringa oleifera Lam. Leaves Inhibit the Pro-Inflammatory Mediators in Lipopolysaccharide-Induced Human Monocyte-Derived Macrophages. Molecules 2020, 25, 191. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.H.; Oza, M.J. Comprehensive Review of Bioactive and Molecular Aspects of Moringa Oleifera Lam. Food Rev. Int. 2020, 38, 1427–1460. [Google Scholar] [CrossRef]
- Park, E.-J.; Cheenpracha, S.; Chang, L.C.; Kondratyuk, T.P.; Pezzuto, J.M. Inhibition of Lipopolysaccharide-Induced Cyclooxygenase-2 and Inducible Nitric Oxide Synthase Expression by 4-[(2′-O-acetyl-α-L-Rhamnosyloxy)Benzyl]Isothiocyanate from Moringa oleifera. Nutr. Cancer 2011, 63, 971–982. [Google Scholar] [CrossRef]
- Jaja-Chimedza, A.; Graf, B.L.; Simmler, C.; Kim, Y.; Kuhn, P.; Pauli, G.F.; Raskin, I. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS ONE 2017, 12, e0182658. [Google Scholar] [CrossRef]
- Sailaja, B.S.; Aita, R.; Maledatu, S.; Ribnicky, D.; Verzi, M.P.; Raskin, I. Moringa isothiocyanate-1 regulates Nrf2 and NF-κB pathway in response to LPS-driven sepsis and inflammation. PLoS ONE 2021, 16, e0248691. [Google Scholar] [CrossRef]
- Minaiyan, M.; Asghari, G.; Taheri, D.; Saeidi, M.; Nasr-Esfahani, S. Anti-inflammatory effect of Moringa oleifera Lam. seeds on acetic acid-induced acute colitis in rats. Avicenna J. Phytomed. 2014, 4, 127–136. [Google Scholar]
- Adedapo, A.A.; Ogunmiluyi, I.O.; Falayi, O.O.; Ogunpolu, B.S.; Oyagbemi, A.A.; Orishadipe, A.; Omobowale, T.O.; Yakubu, M.A.; Oguntibeju, O.O. The lyophilized aqueous leaf extract of Moringa oleifera blunts streptozocin-induced diabetes in rats through upregulation of GLUT 4 signaling pathway and anti-oxidant effect. Sci. Afr. 2020, 10, e00619. [Google Scholar] [CrossRef]
- López, M.; Ríos-Silva, M.; Huerta, M.; Cárdenas, Y.; Bricio-Barrios, J.A.; Díaz-Reval, M.I.; Urzúa, Z.; Huerta-Trujillo, M.; López-Quezada, K.; Trujillo, X. Effects of Moringa oleifera leaf powder on metabolic syndrome induced in male Wistar rats: A preliminary study. J. Int. Med Res. 2018, 46, 3327–3336. [Google Scholar] [CrossRef]
- Haddad, P.S.; Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Sánchez, K.; Garay-Jaramillo, E.; González-Reyes, R.E. Effects of Moringa oleifera on Glycaemia and Insulin Levels: A Review of Animal and Human Studies. Nutrients 2019, 11, 2907. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Ona, M.A.; Papafragkakis, H.; Carey, J.; Moshenyat, Y.; Alhaddad, A.; Anand, S. Acute Liver Toxicity due to Efavirenz/Emtricitabine/Tenofovir. Case Rep. Hepatol. 2015, 2015, 280353. [Google Scholar] [CrossRef] [PubMed]
- Chiwandire, N.; Zungu, N.; Mabaso, M.; Chasela, C. Trends, prevalence and factors associated with hypertension and diabetes among South African adults living with HIV, 2005–2017. BMC Public Health 2021, 21, 462. [Google Scholar] [CrossRef] [PubMed]
- Tamuhla, T.; Dave, J.A.; Raubenheimer, P.; Tiffin, N. Diabetes in a TB and HIV-endemic South African population: Analysis of a virtual cohort using routine health data. PLoS ONE 2021, 16, e0251303. [Google Scholar] [CrossRef]
- Ma, K.S.-K. Metabolic complications of highly active antiretroviral therapy in adult HIV-infected patients with heart failure: A 7-year prospective cohort study. Metabolism 2022, 128, 154985. [Google Scholar] [CrossRef]
- Sandoval, M.A.; Jimeno, C.A. Effect of Malunggay (Moringa oleifera) Capsules on Lipid and Glucose Levels. Acta Med. Philipp. 2013, 47. [Google Scholar] [CrossRef]
- Taweerutchana, R.; Lumlerdkij, N.; Vannasaeng, S.; Akarasereenont, P.; Sriwijitkamol, A. Effect of Moringa oleifera Leaf Capsules on Glycemic Control in Therapy-Naïve Type 2 Diabetes Patients: A Randomized Placebo Controlled Study. Evid. -Based Complement. Altern. Med. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Fungtammasan, S.; Phupong, V. The effect of Moringa oleifera capsule in increasing breastmilk volume in early postpartum patients: A double-blind, randomized controlled trial. PLoS ONE 2021, 16, e0248950. [Google Scholar] [CrossRef]
- Mehta, A.; Agrawal, B. Antiasthmatic activity of Moringa oleifera Lam: A clinical study. Indian J. Pharmacol. 2008, 40, 28–31. [Google Scholar] [CrossRef]
- Tshingani, K.; Donnen, P.; Mukumbi, H.; Duez, P.; Dramaix-Wilmet, M. Impact of Moringa oleifera lam. Leaf powder supplementation versus nutritional counseling on the body mass index and immune response of HIV patients on antiretroviral therapy: A single-blind randomized control trial. BMC Complement. Altern. Med. 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gambo, A.; Moodley, I.; Babashani, M.; Babalola, T.K.; Gqaleni, N. A double-blind, randomized controlled trial to examine the effect of Moringa oleifera leaf powder supplementation on the immune status and anthropometric parameters of adult HIV patients on antiretroviral therapy in a resource-limited setting. PLoS ONE 2021, 16, e0261935. [Google Scholar] [CrossRef] [PubMed]
- Ogunlade, B.; Jeje, S.O.; Adelakun, S.A.; Akingbade, G.T. Moringa oleifera restored semen quality, hormonal profile, and testicular morphology against Highly Active Antiretroviral Therapy-induced toxicity in adult male Wistar rats. JBRA Assist. Reprod. 2022, 26, 3–12. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndlovu, S.S.; Ghazi, T.; Chuturgoon, A.A. The Potential of Moringa oleifera to Ameliorate HAART-Induced Pathophysiological Complications. Cells 2022, 11, 2981. https://doi.org/10.3390/cells11192981
Ndlovu SS, Ghazi T, Chuturgoon AA. The Potential of Moringa oleifera to Ameliorate HAART-Induced Pathophysiological Complications. Cells. 2022; 11(19):2981. https://doi.org/10.3390/cells11192981
Chicago/Turabian StyleNdlovu, Siqiniseko S., Terisha Ghazi, and Anil A. Chuturgoon. 2022. "The Potential of Moringa oleifera to Ameliorate HAART-Induced Pathophysiological Complications" Cells 11, no. 19: 2981. https://doi.org/10.3390/cells11192981
APA StyleNdlovu, S. S., Ghazi, T., & Chuturgoon, A. A. (2022). The Potential of Moringa oleifera to Ameliorate HAART-Induced Pathophysiological Complications. Cells, 11(19), 2981. https://doi.org/10.3390/cells11192981





