Macrophage-Mediated Inflammation in Skin Wound Healing
Abstract
1. Introduction
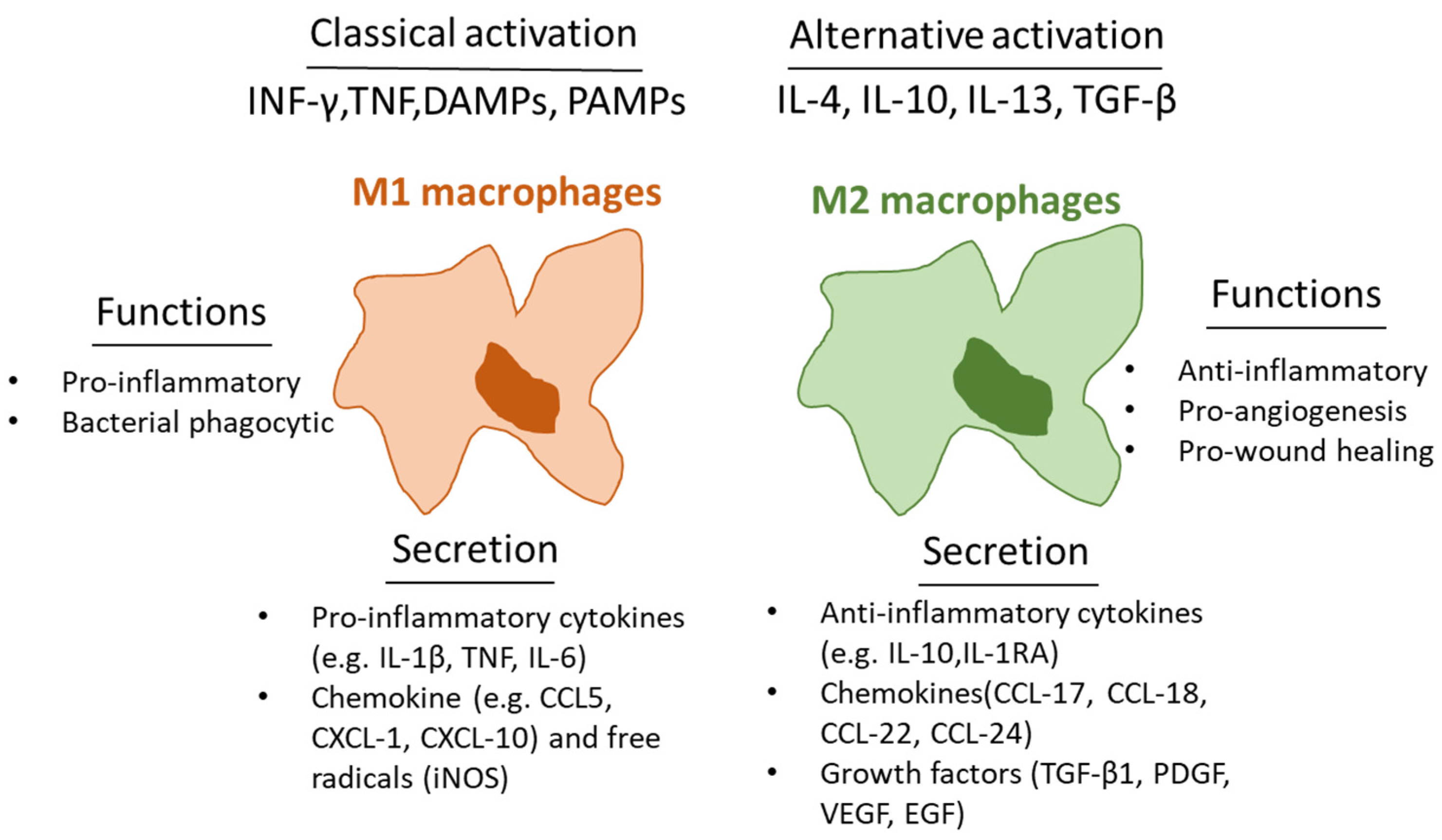
2. Macrophages and Inflammation in Wounds
3. Macrophage Metabolism and Plasticity in Diabetic Wounds
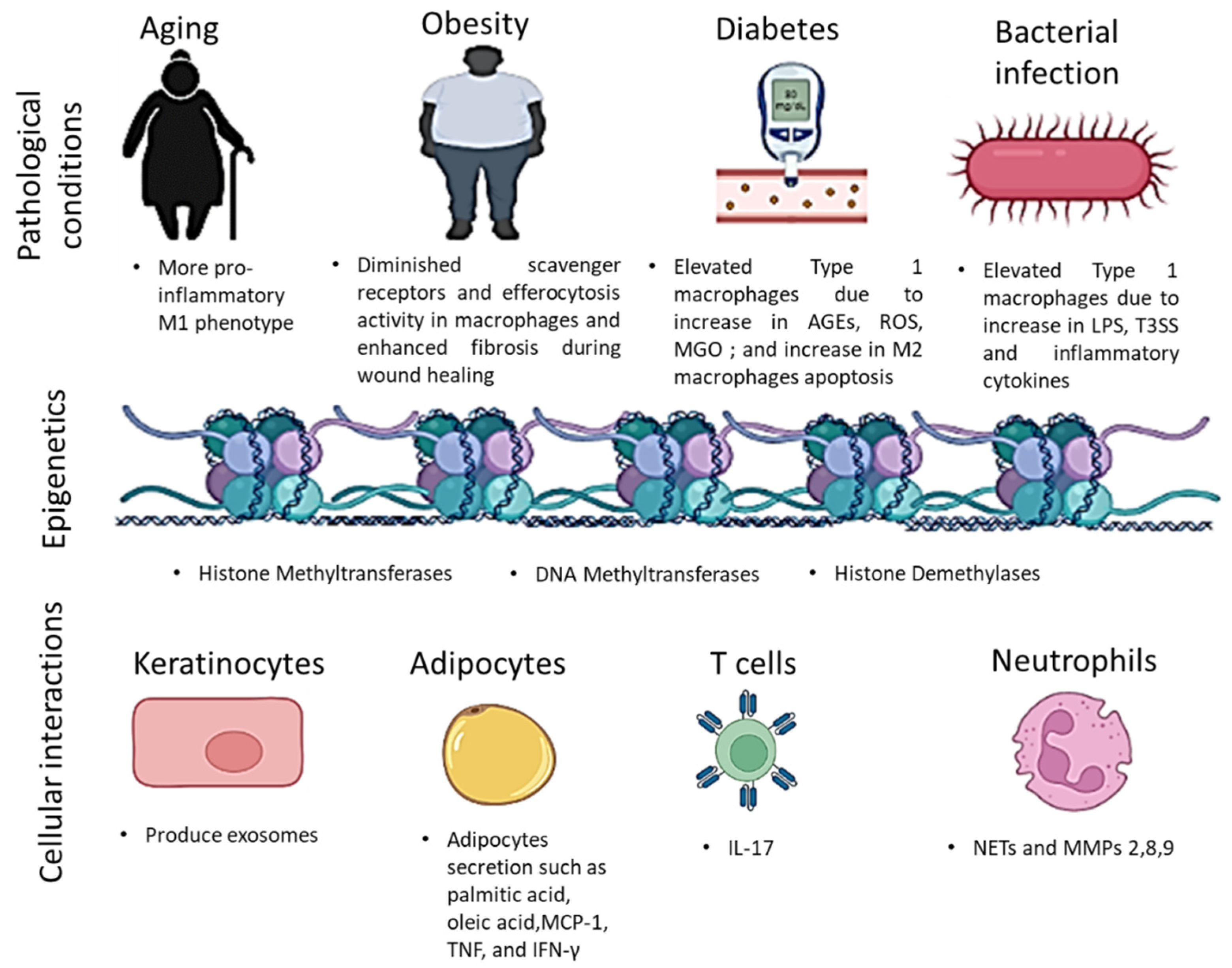
4. Factors Affecting Macrophage Activity
4.1. Epigenetic Modifications
4.1.1. Histone Modification
4.1.2. DNA Methylation
4.2. miRNA Regulation
4.3. ATP-Dependent Remodelling
4.4. Cellular Interaction
4.4.1. Adipocytes
4.4.2. Keratinocytes
4.4.3. Immune Cells
5. Recent Studies and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.-W.; Xian, C.J. Adipose-derived stem cells for wound healing. J. Cell. Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef] [PubMed]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Agren, M.; Eaglstein, W.H.; Ferguson, M.; Harding, K.G.; Moore, K.; Saarialho-Kere, U.; Schultz, G.S. Causes and effects of the chronic inflammation in venous leg ulcers. Acta Derm.-Venereologica. Suppl. 2000, 210, 3–17. [Google Scholar]
- Hassanshahi, M.; Khabbazi, S.; Peymanfar, Y.; Hassanshahi, A.; Hosseini-Khah, Z.; Su, Y.-W.; Xian, C.J. Critical limb ischemia: Current and novel therapeutic strategies. J. Cell Physiol. 2019, 234, 14445–14459. [Google Scholar] [CrossRef] [PubMed]
- Krisp, C.; Jacobsen, F.; McKay, M.J.; Molloy, M.P.; Steinstraesser, L.; Wolters, D.A. Proteome analysis reveals antiangiogenic environments in chronic wounds of diabetes mellitus type 2 patients. Proteomics 2013, 13, 2670–2681. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Cowin, A.J.; Brosnan, M.P.; Holmes, T.M.; Ferguson, M.W. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev. Dyn. 1998, 212, 385–393. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef]
- Novak, M.L.; Koh, T.J. Macrophage phenotypes during tissue repair. J. Leukoc. Biol. 2013, 93, 875–881. [Google Scholar] [CrossRef]
- Brancato, S.K.; Albina, J.E. Wound macrophages as key regulators of repair: Origin, phenotype, and function. Am. J. Pathol. 2011, 178, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Malissen, B.; Tamoutounour, S.; Henri, S. The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol. 2014, 14, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M. Tissue-specific contribution of macrophages to wound healing. Proc. Semin. Cell Dev. Biol. 2017, 6, 3–11. [Google Scholar] [CrossRef]
- Xu, Q.; Choksi, S.; Qu, J.; Jang, J.; Choe, M.; Banfi, B.; Engelhardt, J.F.; Liu, Z.-G. NADPH oxidases are essential for macrophage differentiation. J. Biol. Chem. 2016, 291, 20030–20041. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-mediated inflammation in normal and diabetic wound healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef]
- Ginhoux, F.; Guilliams, M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 2016, 44, 439–449. [Google Scholar] [CrossRef]
- Kolter, J.; Feuerstein, R.; Zeis, P.; Hagemeyer, N.; Paterson, N.; D’Errico, P.; Baasch, S.; Amann, L.; Masuda, T.; Lösslein, A.; et al. A Subset of Skin Macrophages Contributes to the Surveillance and Regeneration of Local Nerves. Immunity 2019, 50, 1482–1497.e1487. [Google Scholar] [CrossRef]
- Hoeffel, G.; Chen, J.; Lavin, Y.; Low, D.; Almeida, F.F.; See, P.; Beaudin, A.E.; Lum, J.; Low, I.; Forsberg, E.C.; et al. C-Myb+ Erythro-Myeloid Progenitor-Derived Fetal Monocytes Give Rise to Adult Tissue-Resident Macrophages. Immunity 2015, 42, 665–678. [Google Scholar] [CrossRef]
- Hoeffel, G.; Ginhoux, F. Fetal monocytes and the origins of tissue-resident macrophages. Cell Immunol. 2018, 330, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Ruedl, C.; Karjalainen, K. Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity 2015, 43, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Tamoutounour, S.; Guilliams, M.; Sanchis, F.M.; Liu, H.; Terhorst, D.; Malosse, C.; Pollet, E.; Ardouin, L.; Luche, H.; Sanchez, C.; et al. Origins and Functional Specialization of Macrophages and of Conventional and Monocyte-Derived Dendritic Cells in Mouse Skin. Immunity 2013, 39, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V. Wound healing and its impairment in the diabetic foot. The Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Gallagher, K.A.; Joshi, A.; Carson, W.F.; Schaller, M.; Allen, R.; Mukerjee, S.; Kittan, N.; Feldman, E.L.; Henke, P.K.; Hogaboam, C. Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes 2015, 64, 1420–1430. [Google Scholar] [CrossRef]
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011, 472, 476–480. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.J.; Leibovich, S.J. Regulation of macrophage polarization and wound healing. Adv. Wound Care 2012, 1, 10–16. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef]
- Laskin, D.L.; Sunil, V.R.; Gardner, C.R.; Laskin, J.D. Macrophages and tissue injury: Agents of defense or destruction? Annu. Rev. Pharmacol. Toxicol. 2011, 51, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Delavary, B.M.; van der Veer, W.M.; van Egmond, M.; Niessen, F.B.; Beelen, R.H. Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.J.; Melvin, W.J.; Gallagher, K. Macrophage-mediated inflammation in diabetic wound repair. Proc. Semin. Cell Dev. Biol. 2021, 119, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Smigiel, K.S.; Parks, W.C. Macrophages, wound healing, and fibrosis: Recent insights. Curr. Rheumatol. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mirastschijski, U.; Impola, U.; Jahkola, T.; Karlsmark, T.; Ågren, M.S.; Saarialho-Kere, U. Ectopic localization of matrix metalloproteinase-9 in chronic cutaneous wounds. Hum. Pathol. 2002, 33, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.N.; Prazeres, P.H.; Paiva, A.E.; Lousado, L.; Turquetti, A.O.; Barreto, R.S.; de Alvarenga, E.C.; Miglino, M.A.; Gonçalves, R.; Mintz, A. Macrophage-derived GPNMB accelerates skin healing. Exp. Dermatol. 2018, 27, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.S.; Joshi, A.D.; Boniakowski, A.E.; Schaller, M.; Chung, J.; Allen, R.; Bermick, J.; Carson IV, W.F.; Henke, P.K.; Maillard, I. Notch regulates macrophage-mediated inflammation in diabetic wound healing. Front. Immunol. 2017, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Smigiel, K.S.; Parks, W.C. Matrix metalloproteinases and leukocyte activation. Prog. Mol. Biol. Transl. Sci. 2017, 147, 167–195. [Google Scholar] [PubMed]
- Owen, C.A.; Hu, Z.; Barrick, B.; Shapiro, S.D. Inducible expression of tissue inhibitor of metalloproteinases–resistant matrix metalloproteinase-9 on the cell surface of neutrophils. Am. J. Respir. Cell Mol. Biol. 2003, 29, 283–294. [Google Scholar] [CrossRef]
- Vaisar, T.; Kassim, S.Y.; Gomez, I.G.; Green, P.S.; Hargarten, S.; Gough, P.J.; Parks, W.C.; Wilson, C.L.; Raines, E.W.; Heinecke, J.W. MMP-9 Sheds the β2 Integrin Subunit (CD18) from Macrophages* S. Mol. Cell. Proteom. 2009, 8, 1044–1060. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp. Biol. Med. 2016, 241, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Juarez, C.F.; Dedhia, P.H.; Jin, S.; Ruiz-Vega, R.; Ma, D.; Liu, Y.; Yamaga, K.; Shestova, O.; Gay, D.L.; Yang, Z. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.; Schaller, M.; Joshi, A.; Davis, F.M.; denDekker, A.; Boniakowski, A.; Bermick, J.; Obi, A.; Moore, B.; Henke, P.K. Ly6CHi blood monocyte/macrophage drive chronic inflammation and impair wound healing in diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1102–1114. [Google Scholar] [CrossRef]
- Burgess, M.; Wicks, K.; Gardasevic, M.; Mace, K.A. Cx3CR1 expression identifies distinct macrophage populations that contribute differentially to inflammation and repair. Immunohorizons 2019, 3, 262–273. [Google Scholar] [CrossRef]
- Xu, X.; Gu, S.; Huang, X.; Ren, J.; Gu, Y.; Wei, C.; Lian, X.; Li, H.; Gao, Y.; Jin, R. The role of macrophages in the formation of hypertrophic scars and keloids. Burn. Trauma 2020, 8, tkaa006. [Google Scholar] [CrossRef]
- Lurier, E.B.; Dalton, D.; Dampier, W.; Raman, P.; Nassiri, S.; Ferraro, N.M.; Rajagopalan, R.; Sarmady, M.; Spiller, K.L. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology 2017, 222, 847–856. [Google Scholar] [CrossRef]
- Gerber, J.S.; Mosser, D.M. Reversing lipopolysaccharide toxicity by ligating the macrophage Fcγ receptors. J. Immunol. 2001, 166, 6861–6868. [Google Scholar] [CrossRef]
- Edwards, J.P.; Zhang, X.; Frauwirth, K.A.; Mosser, D.M. Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 2006, 80, 1298–1307. [Google Scholar] [CrossRef]
- Melton, D.W.; McManus, L.M.; Gelfond, J.A.; Shireman, P.K. Temporal phenotypic features distinguish polarized macrophages in vitro. Autoimmunity 2015, 48, 161–176. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.; Donners, M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lolmede, K.; Campana, L.; Vezzoli, M.; Bosurgi, L.; Tonlorenzi, R.; Clementi, E.; Bianchi, M.E.; Cossu, G.; Manfredi, A.A.; Brunelli, S. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1-and MMP-9-dependent pathways. J. Leukoc. Biol. 2009, 85, 779–787. [Google Scholar] [CrossRef]
- Hassanshahi, M.; Hassanshahi, A.; Khabbazi, S.; Su, Y.W.; Xian, C.J. Bone marrow sinusoidal endothelium: Damage and potential regeneration following cancer radiotherapy or chemotherapy. Angiogenesis 2017, 20, 427–442. [Google Scholar] [CrossRef]
- Hassanshahi, M.; Hassanshahi, A.; Khabbazi, S.; Su, Y.W.; Xian, C.J. Bone marrow sinusoidal endothelium as a facilitator/regulator of cell egress from the bone marrow. Crit. Rev. Oncol. Hematol. 2019, 137, 43–56. [Google Scholar] [CrossRef]
- Hassanshahi, M.; Su, Y.-W.; Fan, C.-M.; Khabbazi, S.; Hassanshahi, A.; Xian, C.J. Methotrexate chemotherapy–induced damages in bone marrow sinusoids: An in vivo and in vitro study. J. Cell. Biochem. 2019, 120, 3220–3231. [Google Scholar] [CrossRef]
- Hassanshahi, M.; Su, Y.-W.; Khabbazi, S.; Fan, C.-M.; Chen, K.-M.; Wang, J.-F.; Qian, A.; Howe, P.R.; Yan, D.-W.; Zhou, H.-D.; et al. Flavonoid genistein protects bone marrow sinusoidal blood vessels from damage by methotrexate therapy in rats. J. Cell. Physiol. 2019, 234, 11276–11286. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Pearce, E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016, 213, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Caputa, G.; Flachsmann, L.J.; Cameron, A.M. Macrophage metabolism: A wound-healing perspective. Immunol. Cell Biol. 2019, 97, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, Z.; Zhang, K.; Chi, Z.; Xu, T.; Jiang, D.; Chen, S.; Li, W.; Yang, X.; Zhang, X. One-carbon metabolism supports S-adenosylmethionine and histone methylation to drive inflammatory macrophages. Mol. Cell 2019, 75, 1147–1160.e1145. [Google Scholar] [CrossRef]
- Baardman, J.; Licht, I.; De Winther, M.P.; Van den Bossche, J. Metabolic–epigenetic crosstalk in macrophage activation. Epigenomics 2015, 7, 1155–1164. [Google Scholar] [CrossRef]
- Mullarky, E.; Cantley, L.C. Diverting glycolysis to combat oxidative stress. In Innovative Medicine: Basic Research and Development; Springer: Tokyo, Japan, 2015; pp. 3–23. [Google Scholar]
- Pavlou, S.; Lindsay, J.; Ingram, R.; Xu, H.; Chen, M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 2018, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, J.I.; Chawla, A. Alternative macrophage activation and metabolism. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Boscá, L.; González-Ramos, S.; Prieto, P.; Fernández-Velasco, M.; Mojena, M.; Martín-Sanz, P.; Alemany, S. Metabolic signatures linked to macrophage polarization: From glucose metabolism to oxidative phosphorylation. Biochem. Soc. Trans. 2015, 43, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; O’neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.; Madureira, P.; Leal, E.; Fonseca, A.; Carvalho, E. Immune aging in diabetes and its implications in wound healing. Clin. Immunol. 2019, 200, 43–54. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, J.; Li, L.; Chen, H.; Chai, Y. NLRP3 inflammasome expression and signaling in human diabetic wounds and in high glucose induced macrophages. J. Diabetes Res. 2017, 2017, 5281358. [Google Scholar] [CrossRef]
- Sakamoto, T.; Seiki, M. A membrane protease regulates energy production in macrophages by activating hypoxia-inducible factor-1 via a non-proteolytic mechanism. J. Biol. Chem. 2010, 285, 29951–29964. [Google Scholar] [CrossRef] [PubMed]
- Dehne, N.; Brüne, B. HIF-1 in the inflammatory microenvironment. Exp. Cell Res. 2009, 315, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Ruthenborg, R.J.; Ban, J.-J.; Wazir, A.; Takeda, N.; Kim, J.-W. Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol. Cells 2014, 37, 637. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Kimball, A.; Joshi, A.D.; El Azzouny, M.; Wolf, S.J.; Obi, A.T.; Lipinski, J.; Gudjonsson, J.E.; Xing, X.; Plazyo, O. Epigenetic regulation of TLR4 in diabetic macrophages modulates immunometabolism and wound repair. J. Immunol. 2020, 204, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. J. Lab. Clin. Med. 2021, 236, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Tsoi, L.C.; Wasikowski, R. Epigenetic regulation of the PGE2 pathway modulates macrophage phenotype in normal and pathologic wound repair. JCI Insight 2020, 5, e138443. [Google Scholar] [CrossRef]
- Arbibe, L.; Mira, J.-P.; Teusch, N.; Kline, L.; Guha, M.; Mackman, N.; Godowski, P.J.; Ulevitch, R.J.; Knaus, U.G. Toll-like receptor 2–mediated NF-κB activation requires a Rac1-dependent pathway. Nat. Immunol. 2000, 1, 533–540. [Google Scholar] [CrossRef]
- Yuan, Y.; Das, S.K.; Li, M. Vitamin D ameliorates impaired wound healing in streptozotocin-induced diabetic mice by suppressing NF-κB-mediated inflammatory genes. Biosci. Rep. 2018, 38, BSR20171294. [Google Scholar] [CrossRef]
- Wood, S.; Jayaraman, V.; Huelsmann, E.J.; Bonish, B.; Burgad, D.; Sivaramakrishnan, G.; Qin, S.; DiPietro, L.A.; Zloza, A.; Zhang, C. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS ONE 2014, 9, e91574. [Google Scholar] [CrossRef]
- Nio, Y.; Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Funata, M.; Yamaguchi, M.; Ueki, K.; Kadowaki, T. Monocyte chemoattractant protein-1 (MCP-1) deficiency enhances alternatively activated M2 macrophages and ameliorates insulin resistance and fatty liver in lipoatrophic diabetic A-ZIP transgenic mice. Diabetologia 2012, 55, 3350–3358. [Google Scholar] [CrossRef]
- Wu, D.; Molofsky, A.B.; Liang, H.-E.; Ricardo-Gonzalez, R.R.; Jouihan, H.A.; Bando, J.K.; Chawla, A.; Locksley, R.M. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011, 332, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Yussof, S.J.M.; Omar, E.; Pai, D.R.; Sood, S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012, 45, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, Inflammation, and Insulin Resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.J.; Mardaryev, A.N.; Sharov, A.A.; Fessing, M.Y.; Botchkarev, V.A. The Epigenetic Regulation of Wound Healing. Adv. Wound Care (New Rochelle) 2014, 3, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.S.; Davis, F.M.; Joshi, A.D.; Schaller, M.A.; Bermick, J.; Xing, X.; Burant, C.F.; Obi, A.T.; Nysz, D.; Robinson, S. The histone methyltransferase Setdb2 modulates macrophage phenotype and uric acid production in diabetic wound repair. Immunity 2019, 51, 258–271.e255. [Google Scholar] [CrossRef]
- Ishii, M.; Wen, H.; Corsa, C.A.; Liu, T.; Coelho, A.L.; Allen, R.M.; Carson IV, W.F.; Cavassani, K.A.; Li, X.; Lukacs, N.W. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood J. Am. Soc. Hematol. 2009, 114, 3244–3254. [Google Scholar] [CrossRef]
- Kimball, A.S.; Joshi, A.; Carson, W.F.; Boniakowski, A.E.; Schaller, M.; Allen, R.; Bermick, J.; Davis, F.M.; Henke, P.K.; Burant, C.F. The histone methyltransferase MLL1 directs macrophage-mediated inflammation in wound healing and is altered in a murine model of obesity and type 2 diabetes. Diabetes 2017, 66, 2459–2471. [Google Scholar] [CrossRef]
- Grunstein, M. Histone acetylation in chromatin structure and transcription. Nature 1997, 389, 349–352. [Google Scholar] [CrossRef]
- Davis, F.M.; denDekker, A.; Joshi, A.D.; Wolf, S.J.; Audu, C.; Melvin, W.J.; Mangum, K.; Riordan, M.O.; Kunkel, S.L.; Gallagher, K.A. Palmitate-TLR4 signaling regulates the histone demethylase, JMJD3, in macrophages and impairs diabetic wound healing. Eur. J. Immunol. 2020, 50, 1929–1940. [Google Scholar] [CrossRef]
- Jia, Y.; Han, S.; Li, J.; Wang, H.; Liu, J.; Li, N.; Yang, X.; Shi, J.; Han, J.; Li, Y. IRF8 is the target of SIRT1 for the inflammation response in macrophages. Innate Immun. 2017, 23, 188–195. [Google Scholar] [CrossRef]
- Boniakowski, A.; Kimball, A.; Joshi, A.; Kunkel, S.; Gallagher, K. Loss of a mitochondrial sirtuin protein, SIRT3, alters the inflammatory phase of wound healing. J. Am. Coll. Surg. 2016, 223, S167. [Google Scholar] [CrossRef]
- Ahmed, M.; de Winther, M.P.; Van den Bossche, J. Epigenetic mechanisms of macrophage activation in type 2 diabetes. Immunobiology 2017, 222, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Gao, M.; Yang, P.; Liu, D.; Wang, D.; Song, F.; Zhang, X.; Liu, Y. Insulin promotes macrophage phenotype transition through PI3K/Akt and PPAR-γ signaling during diabetic wound healing. J. Cell. Physiol. 2019, 234, 4217–4231. [Google Scholar] [CrossRef] [PubMed]
- Mallik, S.B.; Jayashree, B.; Shenoy, R.R. Epigenetic modulation of macrophage polarization-perspectives in diabetic wounds. J. Diabetes Its Complicat. 2018, 32, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Takeuchi, O.; Vandenbon, A.; Yasuda, K.; Tanaka, Y.; Kumagai, Y.; Miyake, T.; Matsushita, K.; Okazaki, T.; Saitoh, T. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 2010, 11, 936–944. [Google Scholar] [CrossRef]
- Den Dekker, A.; Davis, F.M.; Kunkel, S.L.; Gallagher, K.A. Targeting epigenetic mechanisms in diabetic wound healing. Transl. Res. 2019, 204, 39–50. [Google Scholar] [CrossRef]
- Yan, J.; Tie, G.; Wang, S.; Tutto, A.; DeMarco, N.; Khair, L.; Fazzio, T.G.; Messina, L.M. Diabetes impairs wound healing by Dnmt1-dependent dysregulation of hematopoietic stem cells differentiation towards macrophages. Nat. Commun. 2018, 9, 1–13. [Google Scholar]
- Wang, X.; Cao, Q.; Yu, L.; Shi, H.; Xue, B.; Shi, H. Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight 2016, 1, e87748. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Liu, D.; Yu, L.; Xue, B.; Shi, H. Epigenetic regulation of macrophage polarization by DNA methyltransferase 3b. Mol. Endocrinol. 2014, 28, 565–574. [Google Scholar] [CrossRef]
- Petkovic, M.; Sørensen, A.E.; Leal, E.C.; Carvalho, E.; Dalgaard, L.T. Mechanistic Actions of microRNAs in Diabetic Wound Healing. Cells 2020, 9, 2228. [Google Scholar] [CrossRef] [PubMed]
- Ban, E.; Jeong, S.; Park, M.; Kwon, H.; Park, J.; Song, E.J.; Kim, A. Accelerated wound healing in diabetic mice by miRNA-497 and its anti-inflammatory activity. Biomed. Pharmacother. 2020, 121, 109613. [Google Scholar] [CrossRef] [PubMed]
- Madhyastha, R.; Madhyastha, H.; Nurrahmah, Q.I.; Purbasari, B.; Maruyama, M.; Nakajima, Y. MicroRNa 21 elicits a pro-inflammatory response in macrophages, with exosomes functioning as delivery vehicles. Inflammation 2021, 44, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liechty, C.; Hu, J.; Zhang, L.; Liechty, K.W.; Xu, J. Role of microRNA-21 and its underlying mechanisms in inflammatory responses in diabetic wounds. Int. J. Mol. Sci. 2020, 21, 3328. [Google Scholar] [CrossRef] [PubMed]
- Kölling, M.; Kaucsar, T.; Schauerte, C.; Hübner, A.; Dettling, A.; Park, J.-K.; Busch, M.; Wulff, X.; Meier, M.; Scherf, K. Therapeutic miR-21 silencing ameliorates diabetic kidney disease in mice. Mol. Ther. 2017, 25, 165–180. [Google Scholar] [CrossRef]
- Wang, Z.; Brandt, S.; Medeiros, A.; Wang, S.; Wu, H.; Dent, A.; Serezani, C.H. MicroRNA 21 is a homeostatic regulator of macrophage polarization and prevents prostaglandin E2-mediated M2 generation. PLoS ONE 2015, 10, e0115855. [Google Scholar] [CrossRef]
- Self-Fordham, J.B.; Naqvi, A.R.; Uttamani, J.R.; Kulkarni, V.; Nares, S. MicroRNA: Dynamic regulators of macrophage polarization and plasticity. Front. Immunol. 2017, 8, 1062. [Google Scholar] [CrossRef]
- Huang, C.; Liu, X.-J.; Xie, J.; Ma, T.-T.; Meng, X.-m.; Li, J. MiR-146a modulates macrophage polarization by inhibiting Notch1 pathway in RAW264. 7 macrophages. Int. Immunopharmacol. 2016, 32, 46–54. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Taganov, K.D.; Boldin, M.P.; Cheng, G.; Baltimore, D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA 2007, 104, 1604–1609. [Google Scholar] [CrossRef]
- Kotwal, G.J.; Chien, S. Macrophage differentiation in normal and accelerated wound healing. Macrophages 2017, 62, 353–364. [Google Scholar]
- Wang, J.; Zhang, Q.; Wan, R.; Mo, Y.; Li, M.; Tseng, M.T.; Chien, S. Intracellular adenosine triphosphate delivery enhanced skin wound healing in rabbits. Ann. Plast. Surg. 2009, 62, 180. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sarojini, H.; Chien, S. Chromatin remodeling complex activation results in rapid in situ macrophage proliferation in wound healing. Wound Repair Regen. 2015, 23, A46. [Google Scholar]
- Wang, D.; Huang, N.-N.; Heppel, L.A. Extracellular ATP shows synergistic enhancement of DNA synthesis when combined with agents that are active in wound healing or as neurotransmitters. Biochem. Biophys. Res. Commun. 1990, 166, 251–258. [Google Scholar] [CrossRef]
- Mirza, R.E.; Fang, M.M.; Ennis, W.J.; Koh, T.J. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013, 62, 2579–2587. [Google Scholar] [CrossRef]
- Mirza, R.E.; Fang, M.M.; Weinheimer-Haus, E.M.; Ennis, W.J.; Koh, T.J. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes 2014, 63, 1103–1114. [Google Scholar] [CrossRef]
- Spravchikov, N.; Sizyakov, G.; Gartsbein, M.; Accili, D.; Tennenbaum, T.; Wertheimer, E. Glucose effects on skin keratinocytes: Implications for diabetes skin complications. Diabetes 2001, 50, 1627–1635. [Google Scholar] [CrossRef]
- Hirota, T.; Levy, J.H.; Iba, T. The influence of hyperglycemia on neutrophil extracellular trap formation and endothelial glycocalyx damage in a mouse model of type 2 diabetes. Microcirculation 2020, 27, e12617. [Google Scholar] [CrossRef]
- Martinez, N.; Vallerskog, T.; West, K.; Nunes-Alves, C.; Lee, J.; Martens, G.W.; Behar, S.M.; Kornfeld, H. Chromatin decondensation and T cell hyperresponsiveness in diabetes-associated hyperglycemia. J. Immunol. 2014, 193, 4457–4468. [Google Scholar] [CrossRef]
- Shook, B.A.; Wasko, R.R.; Mano, O.; Rutenberg-Schoenberg, M.; Rudolph, M.C.; Zirak, B.; Rivera-Gonzalez, G.C.; López-Giráldez, F.; Zarini, S.; Rezza, A.; et al. Dermal Adipocyte Lipolysis and Myofibroblast Conversion Are Required for Efficient Skin Repair. Cell Stem Cell 2020, 26, 880–895.e886. [Google Scholar] [CrossRef]
- Johnson, A.R.; Qin, Y.; Cozzo, A.J.; Freemerman, A.J.; Huang, M.J.; Zhao, L.; Sampey, B.P.; Milner, J.J.; Beck, M.A.; Damania, B.; et al. Metabolic reprogramming through fatty acid transport protein 1 (FATP1) regulates macrophage inflammatory potential and adipose inflammation. Mol. Metab. 2016, 5, 506–526. [Google Scholar] [CrossRef]
- Alvarez-Curto, E.; Milligan, G. Metabolism meets immunity: The role of free fatty acid receptors in the immune system. Biochem. Pharmacol. 2016, 114, 3–13. [Google Scholar] [CrossRef]
- Sohn, J.H.; Lee, Y.K.; Han, J.S.; Jeon, Y.G.; Kim, J.I.; Choe, S.S.; Kim, S.J.; Yoo, H.J.; Kim, J.B. Perilipin 1 (Plin1) deficiency promotes inflammatory responses in lean adipose tissue through lipid dysregulation. J. Biol. Chem. 2018, 293, 13974–13988. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Apovian, C. Macrophage functions in lean and obese adipose tissue. Metabolism 2017, 72, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, A.; Naffah de Souza, C.; Câmara, N.O.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2015, 6, 637. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Kahlenberg, J.M.; Gudjonsson, J.E. Cytokinocytes: The diverse contribution of keratinocytes to immune responses in skin. JCI Insight 2020, 5, e142067. [Google Scholar] [CrossRef]
- Usui, M.L.; Mansbridge, J.N.; Carter, W.G.; Fujita, M.; Olerud, J.E. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J. Histochem. Cytochem. 2008, 56, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Ponce, A.; Tiruneh, M.W.; Lee, J.; Guerrero-Juarez, C.F.; Kuhn, J.; David, J.A.; Dammeyer, K.; Mc Kell, R.; Kwong, J.; Rabbani, P.S.; et al. Keratinocyte-Macrophage Crosstalk by the Nrf2/Ccl2/EGF Signaling Axis Orchestrates Tissue Repair. Cell Rep. 2020, 33, 108417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Brown, B.A.; Siegel, A.P.; El Masry, M.S.; Zeng, X.; Song, W.; Das, A.; Khandelwal, P.; Clark, A.; Singh, K.; et al. Exosome-Mediated Crosstalk between Keratinocytes and Macrophages in Cutaneous Wound Healing. ACS Nano 2020, 14, 12732–12748. [Google Scholar] [CrossRef]
- Lee, M.K.; Sreejit, G.; Nagareddy, P.R.; Murphy, A.J. Attack of the NETs! NETosis primes IL-1β-mediated inflammation in diabetic foot ulcers. Clin. Sci. 2020, 134, 1399–1401. [Google Scholar] [CrossRef]
- Nabzdyk, L.P.; Kuchibhotla, S.; Guthrie, P.; Chun, M.; Auster, M.E.; Nabzdyk, C.; Deso, S.; Andersen, N.; Gnardellis, C.; LoGerfo, F.W. Expression of neuropeptides and cytokines in a rabbit model of diabetic neuroischemic wound healing. J. Vasc. Surg. 2013, 58, 766–775.e712. [Google Scholar] [CrossRef]
- Seraphim, P.M.; Leal, E.C.; Moura, J.; Gonçalves, P.; Gonçalves, J.P.; Carvalho, E. Lack of lymphocytes impairs macrophage polarization and angiogenesis in diabetic wound healing. Life Sci. 2020, 254, 117813. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rodero, M.P.; Patel, J.; Moi, D.; Mazzieri, R.; Khosrotehrani, K. Interleukin-23 regulates interleukin-17 expression in wounds, and its inhibition accelerates diabetic wound healing through the alteration of macrophage polarization. FASEB J. 2018, 32, 2086–2094. [Google Scholar] [CrossRef]
- Rodero, M.P.; Hodgson, S.S.; Hollier, B.; Combadiere, C.; Khosrotehrani, K. Reduced Il17a expression distinguishes a Ly6cloMHCIIhi macrophage population promoting wound healing. J. Investig. Dermatol. 2013, 133, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Theocharidis, G.; Rahmani, S.; Lee, S.; Li, Z.; Lobao, A.; Kounas, K.; Katopodi, X.-L.; Wang, P.; Moon, S.; Vlachos, I.S.; et al. Murine macrophages or their secretome delivered in alginate dressings enhance impaired wound healing in diabetic mice. Biomaterials 2022, 288, 121692. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Chen, L.; Cai, Z.; Qian, S.; Liu, Z.; Zhao, B.; Zhang, Y.; Sun, X.; Cui, W. Advanced Biomaterials for Regulating Polarization of Macrophages in Wound Healing. Adv. Funct. Mater. 2022, 32, 2111003. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Rahman, M.; Matin, M.A.; Hossen, M.J.; Sikder, M.H. Chapter 16-Role of macrophages in systemic inflammation: Wound healing. In Recent Advancements in Microbial Diversity; Cho, J.Y., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 335–360. [Google Scholar]
- Wang, K.; Dong, R.; Tang, J.; Li, H.; Dang, J.; Zhang, Z.; Yu, Z.; Guo, B.; Yi, C. Exosomes laden self-healing injectable hydrogel enhances diabetic wound healing via regulating macrophage polarization to accelerate angiogenesis. Chem. Eng. J. 2022, 430, 132664. [Google Scholar] [CrossRef]
- Xu, C.; Yu, D.; Zhu, H. Research progress on the regulation of macrophage polarization by mechanical stimulation in wound healing. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2022, 36, 1041–1046. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-Mediated Inflammation in Skin Wound Healing. Cells 2022, 11, 2953. https://doi.org/10.3390/cells11192953
Hassanshahi A, Moradzad M, Ghalamkari S, Fadaei M, Cowin AJ, Hassanshahi M. Macrophage-Mediated Inflammation in Skin Wound Healing. Cells. 2022; 11(19):2953. https://doi.org/10.3390/cells11192953
Chicago/Turabian StyleHassanshahi, Alireza, Mohammad Moradzad, Saman Ghalamkari, Moosa Fadaei, Allison J. Cowin, and Mohammadhossein Hassanshahi. 2022. "Macrophage-Mediated Inflammation in Skin Wound Healing" Cells 11, no. 19: 2953. https://doi.org/10.3390/cells11192953
APA StyleHassanshahi, A., Moradzad, M., Ghalamkari, S., Fadaei, M., Cowin, A. J., & Hassanshahi, M. (2022). Macrophage-Mediated Inflammation in Skin Wound Healing. Cells, 11(19), 2953. https://doi.org/10.3390/cells11192953






