The Immune and Regenerative Response to Burn Injury
Abstract
1. Introduction
2. Systemic Response to Burns
2.1. Inflammatory Response
2.2. Multi-Organ System Involvement
2.3. Hypermetabolism and Hyperglycemia
2.4. Burn Shock
2.5. Sepsis
3. The Innate Immune Response to Burns
3.1. Neutrophils
3.2. Monocytes and Macrophages
3.3. Mast Cells
3.4. Natural Killer (NK) and Dendritic Cells
4. The Adaptive Immune Response to Burn
4.1. T-Cells
4.2. B-Cells
5. Regenerative Response to Burn Injury
5.1. Keratinocytes
5.2. Epidermal Stem Cells
5.3. Melanocytes
5.4. Fibroblasts
6. Chronic Inflammation following Burn Injury
Hypertrophic Scarring
7. Mitigation of the Post-Burn Inflammatory Response
7.1. Burn Resuscitation Schemes
7.2. Strategies to Decrease Inflammation
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO) Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/burns (accessed on 26 February 2022).
- Greenhalgh, D.G. Management of Burns. New Engl. J. Med. 2019, 380, 2349–2359. [Google Scholar] [CrossRef]
- Peck, M.; Pressman, M.A. The correlation between burn mortality rates from fire and flame and economic status of countries. Burns 2013, 39, 1054. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Kearney, L.; Francis, E.C.; Clover, A.J. New technologies in global burn care—A review of recent advances. Int. J. Burns Trauma 2018, 8, 77–87. [Google Scholar]
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamolz, L.-P. Recent trends in burn epidemiology worldwide: A systematic review. Burns 2017, 43, 249–257. [Google Scholar] [CrossRef]
- Choi, J.; Park, S.; Kim, H.C. Epidemiologic Analysis of Burns in Military Hospital. J. Trauma Inj. 2017, 30, 145–157. [Google Scholar] [CrossRef][Green Version]
- Jackson, D.M. The diagnosis of the depth of burning. Br. J. Surg. 1953, 40, 588–596. [Google Scholar] [CrossRef]
- Tiwari, V.K. Burn wound: How it differs from other wounds? Indian J. Plast. Surg. 2012, 45, 364–373. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Arbuthnot, M.K.; Garcia, A.V. Early resuscitation and management of severe pediatric burns. Semin. Pediatr. Surg. 2019, 28, 73–78. [Google Scholar] [CrossRef]
- Farina, J.A.; Rosique, M.J.; Rosique, R.G. Curbing inflammation in burn patients. Int. J. Inflamm. 2013, 2013, 715645. [Google Scholar] [CrossRef]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef]
- Oosterwijk, A.M.; Mouton, L.J.; Schouten, H.; Disseldorp, L.M.; Van der Schans, C.P.; Nieuwenhuis, M.K. Prevalence of scar contractures after burn: A systematic review. Burns 2017, 43, 41–49. [Google Scholar] [CrossRef]
- Williams, F.N.; Herndon, D.N.; Jeschke, M.G. The hypermetabolic response to burn injury and interventions to modify this response. Clin. Plast. Surg. 2009, 36, 583–596. [Google Scholar] [CrossRef]
- Kaukonen, K.M.; Bailey, M.; Pilcher, D.; Cooper, D.J.; Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015, 372, 1629–1638. [Google Scholar] [CrossRef]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef]
- Lindford, A.; Juteau, S.; Jaks, V.; Klaas, M.; Lagus, H.; Vuola, J.; Kankuri, E. Case Report: Unravelling the Mysterious Lichtenberg Figure Skin Response in a Patient with a High-Voltage Electrical Injury. Front. Med. 2021, 8, 663807. [Google Scholar] [CrossRef]
- Coleman, L.G., Jr.; Maile, R.; Jones, S.W.; Cairns, B.A.; Crews, F.T. HMGB1/IL-1β complexes in plasma microvesicles modulate immune responses to burn injury. PLoS ONE 2018, 13, e0195335. [Google Scholar] [CrossRef]
- Relja, B.; Land, W.G. Damage-associated molecular patterns in trauma. Eur. J. Trauma Emerg. Surg. 2020, 46, 751–775. [Google Scholar] [CrossRef]
- Santos, F.X.; Arroyo, C.; Garcia, I.; Blasco, R.; Obispo, J.M.; Hamann, C.; Espejo, L. Role of mast cells in the pathogenesis of postburn inflammatory response: Reactive oxygen species as mast cell stimulators. Burns 2000, 26, 145–147. [Google Scholar] [CrossRef]
- Nicolete, R.; Lima Kde, M.; Júnior, J.M.; Jose, P.J.; Sanz, M.J.; Faccioli, L.H. Prostaglandin E(2)-loaded microspheres as strategy to inhibit phagocytosis and modulate inflammatory mediators release. Eur. J. Pharm. Biopharm. 2008, 70, 784–790. [Google Scholar] [CrossRef]
- Norbury, W.; Herndon, D.N.; Tanksley, J.; Jeschke, M.G.; Finnerty, C.C. Infection in Burns. Surg. Infect. 2016, 17, 250–255. [Google Scholar] [CrossRef]
- Clark, A.; Imran, J.; Madni, T.; Wolf, S.E. Nutrition and metabolism in burn patients. Burns Trauma 2017, 5, 11. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Gunn, S.W.; Dibo, S.A. Metabolic implications of severe burn injuries and their management: A systematic review of the literature. World J. Surg. 2008, 32, 1857–1869. [Google Scholar] [CrossRef]
- Rani, M.; Schwacha, M.G. The composition of T-cell subsets are altered in the burn wound early after injury. PLoS ONE 2017, 12, e0179015. [Google Scholar]
- Evers, L.H.; Bhavsar, D.; Mailänder, P. The biology of burn injury. Exp. Dermatol. 2010, 19, 777–783. [Google Scholar] [CrossRef]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef]
- Zhang, P.; Zou, B.; Liou, Y.C.; Huang, C. The pathogenesis and diagnosis of sepsis post burn injury. Burns Trauma 2021, 9, tkaa047. [Google Scholar] [CrossRef]
- Rubio, I.; Osuchowski, M.F.; Shankar-Hari, M.; Skirecki, T.; Winkler, M.S.; Lachmann, G.; La Rosée, P.; Monneret, G.; Venet, F.; Bauer, M.; et al. Current gaps in sepsis immunology: New opportunities for translational research. Lancet Infect. Dis. 2019, 19, e422–e436. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.K.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Branco, L.M.; Franklin, B.S. Regulation of Innate Immune Responses by Platelets. Front. Immunol. 2019, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, E.; Toksoy, A.; Goebeler, M.; Debus, S.; Brocker, E.B.; Gillitzer, R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am. J. Pathol. 1998, 153, 1849–1860. [Google Scholar] [CrossRef]
- Korkmaz, H.I.; Krijnen, P.A.J.; Ulrich, M.M.W.; De Jong, E.; Van Zuijlen, P.P.M.; Niessen, H.W.M. The role of complement in the acute phase response after burns. Burns 2017, 43, 1390–1399. [Google Scholar] [CrossRef]
- Pitchford, S.; Pan, D.; Welch, H.C. Platelets in neutrophil recruitment to sites of inflammation. Curr. Opin. Hematol. 2017, 24, 23–31. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Serra, M.B.; Barroso, W.A.; Da Silva, N.N.; Silva, S.D.N.; Borges, A.C.R.; Abreu, I.C.; Borges, M. From Inflammation to Current and Alternative Therapies Involved in Wound Healing. Int. J. Inflam. 2017, 2017, 3406215. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Morshed, M.; Hlushchuk, R.; Simon, D.; Walls, A.F.; Obata-Ninomiya, K.; Karasuyama, H.; Djonov, V.; Eggel, A.; Kaufmann, T.; Simon, H.U.; et al. NADPH oxidase-independent formation of extracellular DNA traps by basophils. J. Immunol. 2014, 192, 5314–5323. [Google Scholar] [CrossRef]
- Von Köckritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 2008, 111, 3070–3080. [Google Scholar] [CrossRef]
- Granger, V.; Faille, D.; Marani, V.; Noël, B.; Gallais, Y.; Szely, N.; Flament, H.; Pallardy, M.; Chollet-Martin, S.; De Chaisemartin, L. Human blood monocytes are able to form extracellular traps. J. Leukoc. Biol. 2017, 102, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Chow, O.A.; Von Köckritz-Blickwede, M.; Bright, A.T.; Hensler, M.E.; Zinkernagel, A.S.; Cogen, A.L.; Gallo, R.L.; Monestier, M.; Wang, Y.; Glass, C.K.; et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 2010, 8, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.L.; Ambravaneswaran, V.; Agrawal, N.; Bilodeau, M.; Toner, M.; Tompkins, R.G.; Fagan, S.; Irimia, D. Burn injury reduces neutrophil directional migration speed in microfluidic devices. PLoS ONE 2010, 5, e11921. [Google Scholar] [CrossRef] [PubMed]
- Van de Goot, F.; Krijnen, P.A.; Begieneman, M.P.; Ulrich, M.M.; Middelkoop, E.; Niessen, H.W. Acute inflammation is persistent locally in burn wounds: A pivotal role for complement and C-reactive protein. J. Burn Care Res. 2009, 30, 274–280. [Google Scholar] [CrossRef]
- Arturson, G. Neutrophil granulocyte functions in severely burned patients. Burns Incl. Therm. Inj. 1985, 11, 309–319. [Google Scholar] [CrossRef]
- Simpson, D.M.; Ross, R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J. Clin. Investig. 1972, 51, 2009–2023. [Google Scholar] [CrossRef]
- Dovi, J.V.; He, L.K.; DiPietro, L.A. Accelerated wound closure in neutrophil-depleted mice. J. Leukoc. Biol. 2003, 73, 448–455. [Google Scholar] [CrossRef]
- Schwacha, M.G. Macrophages and post-burn immune dysfunction. Burns 2003, 29, 1–14. [Google Scholar] [CrossRef]
- Olingy, C.E.; Emeterio, C.L.S.; Ogle, M.E.; Krieger, J.R.; Bruce, A.C.; Pfau, D.D.; Jordan, B.T.; Peirce, S.M.; Botchwey, E.A. Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Sci. Rep. 2017, 7, 447. [Google Scholar] [CrossRef]
- Leibovich, S.J.; Ross, R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am. J. Pathol. 1975, 78, 71–100. [Google Scholar]
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp. Biol. Med. 2016, 241, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Liang, B.; Liu, G.; Liu, K.; Ding, Z. Low dose of glucocorticoid decreases the incidence of complications in severely burned patients by attenuating systemic inflammation. J. Crit. Care 2015, 30, 436.e7–436.e11. [Google Scholar] [CrossRef] [PubMed]
- Caputa, G.; Flachsmann, L.J.; Cameron, A.M. Macrophage metabolism: A wound-healing perspective. Immunol. Cell Biol. 2019, 97, 268–278. [Google Scholar] [CrossRef]
- Seim, G.L.; Fan, J. A matter of time: Temporal structure and functional relevance of macrophage metabolic rewiring. Trends Endocrinol. Metab. 2022, 33, 345–358. [Google Scholar] [CrossRef]
- Lis-López, L.; Bauset, C.; Seco-Cervera, M.; Cosín-Roger, J. Is the Macrophage Phenotype Determinant for Fibrosis Development? Biomedicines 2021, 9, 1747. [Google Scholar] [CrossRef] [PubMed]
- Ud-Din, S.; Wilgus, T.A.; Bayat, A. Mast Cells in Skin Scarring: A Review of Animal and Human Research. Front. Immunol. 2020, 11, 552205. [Google Scholar] [CrossRef] [PubMed]
- Bankova, L.G.; Lezcano, C.; Pejler, G.; Stevens, R.L.; Murphy, G.F.; Austen, K.F.; Gurish, M.F. Mouse mast cell proteases 4 and 5 mediate epidermal injury through disruption of tight junctions. J. Immunol. 2014, 192, 2812–2820. [Google Scholar] [CrossRef]
- Douaiher, J.; Succar, J.; Lancerotto, L.; Gurish, M.F.; Orgill, D.P.; Hamilton, M.J.; Krilis, S.A.; Stevens, R.L. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv. Immunol. 2014, 122, 211–252. [Google Scholar]
- Wilgus, T.A.; Wulff, B.C. The Importance of Mast Cells in Dermal Scarring. Adv. Wound Care 2014, 3, 356–365. [Google Scholar] [CrossRef]
- Miteva, K.T.; Pedicini, L.; Wilson, L.A.; Jayasinghe, I.; Slip, R.G.; Marszalek, K.; Gaunt, H.J.; Bartoli, F.; Deivasigamani, S.; Sobradillo, D.; et al. Rab46 integrates Ca(2+) and histamine signaling to regulate selective cargo release from Weibel-Palade bodies. J. Cell Biol. 2019, 218, 2232–2246. [Google Scholar] [CrossRef]
- Yukami, T.; Hasegawa, M.; Matsushita, Y.; Fujita, T.; Matsushita, T.; Horikawa, M.; Komura, K.; Yanaba, K.; Hamaguchi, Y.; Nagaoka, T.; et al. Endothelial selectins regulate skin wound healing in cooperation with L-selectin and ICAM-1. J. Leukoc. Biol. 2007, 82, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Oskeritzian, C.A. Mast Cells and Wound Healing. Adv. Wound Care 2012, 1, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. Mast cells: Versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J. Dermatol. Sci. 2008, 49, 7–19. [Google Scholar] [CrossRef]
- Moretta, A. Natural killer cells and dendritic cells: Rendezvous in abused tissues. Nat. Rev. Immunol. 2002, 2, 957–964. [Google Scholar] [CrossRef]
- Stetson, D.B.; Medzhitov, R. Type I interferons in host defense. Immunity 2006, 25, 373–381. [Google Scholar] [CrossRef]
- Vidal, S.M.; Khakoo, S.I.; Biron, C.A. Natural killer cell responses during viral infections: Flexibility and conditioning of innate immunity by experience. Curr. Opin. Virol. 2001, 1, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kobayashi, M.; Herndon, D.N.; Suzuki, F. Role of natural killer dendritic cells in host resistance against Pseudomonas aeruginosa infection after thermal injury in mice. Shock 2010, 34, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, N.C.; Lozier, A.; Flament, C.; Ricciardi-Castagnoli, P.; Bellet, D.; Suter, M.; Perricaudet, M.; Tursz, T.; Maraskovsky, E.; Zitvogel, L. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 1999, 5, 405–411. [Google Scholar] [CrossRef]
- Takahashi, H.; Tashiro, T.; Miyazaki, M.; Kobayashi, M.; Pollard, R.B.; Suzuki, F. An essential role of macrophage inflammatory protein 1alpha/CCL3 on the expression of host’s innate immunities against infectious complications. J. Leukoc. Biol. 2002, 72, 1190–1197. [Google Scholar] [CrossRef]
- Haniffa, M.; Gunawan, M.; Jardine, L. Human skin dendritic cells in health and disease. J. Dermatol. Sci. 2015, 77, 85–92. [Google Scholar] [CrossRef]
- Gregorio, J.; Meller, S.; Conrad, C.; Di Nardo, A.; Homey, B.; Lauerma, A.; Arai, N.; Gallo, R.L.; Digiovanni, J.; Gilliet, M. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J. Exp. Med. 2010, 207, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Vinish, M.; Cui, W.; Stafford, E.; Bae, L.; Hawkins, H.; Cox, R.; Toliver-Kinsky, T. Dendritic cells modulate burn wound healing by enhancing early proliferation. Wound Repair Regen. 2016, 24, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Chapter 24—The Adaptive Immune System. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York City, NY, USA, 2002. [Google Scholar]
- Haas, W.; Pereira, P.; Tonegawa, S. Gamma/delta cells. Annu. Rev. Immunol. 1993, 11, 637–685. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.; Ugarte, K.; Chen, N.; Yachi, P.; Fuchs, E.; Boismenu, R.; Havran, W.L. A role for skin gammadelta T cells in wound repair. Science 2002, 296, 747–749. [Google Scholar] [CrossRef]
- Matsushima, A.; Ogura, H.; Fujita, K.; Koh, T.; Tanaka, H.; Sumi, Y.; Yoshiya, K.; Hosotsubo, H.; Kuwagata, Y.; Shimazu, T.; et al. Early activation of gammadelta T lymphocytes in patients with severe systemic inflammatory response syndrome. Shock 2004, 22, 11–15. [Google Scholar] [CrossRef]
- Ramirez, K.; Witherden, D.A.; Havran, W.L. All hands on DE(T)C: Epithelial-resident gammadelta T cells respond to tissue injury. Cell. Immunol. 2015, 296, 57–61. [Google Scholar] [CrossRef]
- Toth, B.; Alexander, M.; Daniel, T.; Chaudry, I.H.; Hubbard, W.J.; Schwacha, M.G. The role of gammadelta T cells in the regulation of neutrophil-mediated tissue damage after thermal injury. J. Leukoc. Biol. 2004, 76, 545–552. [Google Scholar] [CrossRef]
- Schwacha, M.G.; Ayala, A.; Chaudry, I.H. Insights into the role of gammadelta T lymphocytes in the immunopathogenic response to thermal injury. J. Leukoc. Biol. 2000, 67, 644–650. [Google Scholar] [CrossRef]
- Rani, M.; Zhang, Q.; Schwacha, M.G. Gamma delta T cells regulate wound myeloid cell activity after burn. Shock 2014, 42, 133–141. [Google Scholar] [CrossRef]
- Trottein, F.; Paget, C. Natural Killer T Cells and Mucosal-Associated Invariant T Cells in Lung Infections. Front. Immunol. 2018, 9, 1750. [Google Scholar] [CrossRef]
- Constantinides, M.G.; Link, V.M.; Tamoutounour, S.; Wong, A.C.; Perez-Chaparro, P.J.; Han, S.-J.; Chen, Y.E.; Li, K.; Farhat, S.; Weckel, A.; et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 2019, 366, eaax6624. [Google Scholar] [CrossRef] [PubMed]
- Tanno, H.; Kawakami, K.; Kanno, E.; Suzuki, A.; Takagi, N.; Yamamoto, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Invariant NKT cells promote skin wound healing by preventing a prolonged neutrophilic inflammatory response. Wound Repair Regen. 2017, 25, 805–815. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, F.R.; Kurzhagen, J.T.; Rabb, H. Reparative T lymphocytes in organ injury. J. Clin. Investig. 2019, 129, 2608–2618. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.K.; Luan, L.; Bohannon, J.K.; Guo, Y.; Hernandez, A.; Fensterheim, B.; Sherwood, E.R. IL-15 Superagonist Expands mCD8+ T, NK and NKT Cells after Burn Injury but Fails to Improve Outcome during Burn Wound Infection. PLoS ONE 2016, 11, e0148452. [Google Scholar] [CrossRef] [PubMed]
- Arturson, G.; Hogman, C.F.; Johansson, S.G.; Killander, J. Changes in immunoglobulin levels in severely burned patients. Lancet 1969, 1, 546–548. [Google Scholar] [CrossRef]
- Munster, A.M.; Hoagland, H.C.; Pruitt, B.A., Jr. The effect of thermal injury on serum immunoglobulins. Ann. Surg. 1970, 172, 965–969. [Google Scholar] [CrossRef]
- Singh, M.; Nuutila, K.; Kruse, C.; Robson, M.C.; Caterson, E.; Eriksson, E. Challenging the Conventional Therapy: Emerging Skin Graft Techniques for Wound Healing. Plast. Reconstr. Surg. 2015, 136, 524e–530e. [Google Scholar] [CrossRef]
- Johnson, R.M.; Richard, R. Partial-thickness burns: Identification and management. Adv. Skin Wound Care 2003, 16, 178–187. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. New Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Heng, M.C. Wound healing in adult skin: Aiming for perfect regeneration. Int. J. Dermatol. 2011, 50, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, R.M.; Sloan, D.K.; Heck, J.M.; Murray, A.R.; O’Dell, S.J. Interleukin 6 indirectly induces keratinocyte migration. J. Investig. Dermatol. 2004, 122, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Stone Ii, R.; Natesan, S.; Kowalczewski, C.J.; Mangum, L.H.; Clay, N.E.; Clohessy, R.M.; Carlsson, A.H.; Tassin, D.H.; Chan, R.K.; Rizzo, J.A.; et al. Advancements in Regenerative Strategies Through the Continuum of Burn Care. Front. Pharmacol. 2018, 9, 672. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Yang, R.; Liu, F.; Wang, J.; Chen, X.; Xie, J.; Xiong, K. Epidermal stem cells in wound healing and their clinical applications. Stem Cell Res. Ther. 2019, 10, 229. [Google Scholar] [CrossRef]
- Carney, B.C.; Travis, T.E.; Moffatt, L.T.; Johnson, L.S.; McLawhorn, M.M.; Simbulan-Rosenthal, C.M.; Rosenthal, D.S.; Shupp, J.W. Hypopigmented burn hypertrophic scar contains melanocytes that can be signaled to re-pigment by synthetic alpha-melanocyte stimulating hormone in vitro. PLoS ONE 2021, 16, e0248985. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Nuutila, K.; Laukkanen, A.; Lindford, A.; Juteau, S.; Nuopponen, M.; Vuola, J.; Kankuri, E. Inhibition of Skin Wound Contraction by Nanofibrillar Cellulose Hydrogel. Plast. Reconstr. Surg. 2018, 141, 357e–366e. [Google Scholar] [CrossRef]
- Sarrazy, V.; Billet, F.; Micallef, L.; Coulomb, B.; Desmouliere, A. Mechanisms of pathological scarring: Role of myofibroblasts and current developments. Wound Repair Regen. 2011, 19 (Suppl. 1), s10–s15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jinnin, M.; Nakamura, K.; Harada, M.; Kudo, H.; Nakayama, W.; Inoue, K.; Nakashima, T.; Honda, N.; Fukushima, S.; et al. Long non-coding RNA TSIX is upregulated in scleroderma dermal fibroblasts and controls collagen mRNA stabilization. Exp. Dermatol. 2016, 25, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yu, A. EZH2-mediated suppression of lncRNA-LET promotes cell apoptosis and inhibits the proliferation of post-burn skin fibroblasts. Int. J. Mol. Med. 2018, 4, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Bai, W.D.; Li, C.; Zheng, Z.; Guan, H.; Liu, J.Q.; Yang, X.K.; Han, S.C.; Gao, J.X.; Wang, H.T.; et al. Knockdown of lncRNA-ATB suppresses autocrine secretion of TGF-beta2 by targeting ZNF217 via miR-200c in keloid fibroblasts. Sci. Rep. 2016, 6, 24728. [Google Scholar] [CrossRef] [PubMed]
- Rabello, F.B.; Souza, C.D.; Farina Júnior, J.A. Update on hypertrophic scar treatment. Clinics 2014, 69, 565–573. [Google Scholar] [CrossRef]
- Finnerty, C.C.; Jeschke, M.G.; Branski, L.K.; Barret, J.P.; Dziewulski, P.; Herndon, D.N. Hypertrophic scarring: The greatest unmet challenge after burn injury. Lancet 2016, 388, 1427–1436. [Google Scholar] [CrossRef]
- Gomes, A.; Teixeira, C.; Ferraz, R.; Prudêncio, C.; Gomes, P. Wound-healing peptides for treatment of chronic diabetic foot ulcers and other infected skin injuries. Molecules 2017, 22, 1743. [Google Scholar] [CrossRef]
- Slemp, A.E.; Kirschner, R.E. Keloids and scars: A review of keloids and scars, their pathogenesis, risk factors, and management. Curr. Opin. Pediatr. 2006, 18, 396–402. [Google Scholar] [CrossRef]
- Armour, A.; Scott, P.G.; Tredget, E.E. Cellular and molecular pathology of HTS: Basis for treatment. Wound Repair Regen. 2007, 15 (Suppl. 1), S6. [Google Scholar] [CrossRef]
- Verhaegen, P.D.; Van Zuijlen, P.P.; Pennings, N.M.; Van Marle, J.; Niessen, F.B.; Van der Horst, C.M.; Middelkoop, E. Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: An objective histopathological analysis. Wound Repair Regen. 2009, 17, 649. [Google Scholar] [CrossRef]
- Wolfram, D.; Tzankov, A.; Pulzl, P.; Piza-Katzer, H. Hypertrophic scars and keloids—A review of their pathophysiology, risk factors, and therapeutic management. Dermatol. Surg. 2009, 35, 171. [Google Scholar] [CrossRef]
- Colwell, A.S.; Phan, T.T.; Kong, W.; Longaker, M.T.; Lorenz, P.H. Hypertrophic scar fibroblasts have increased connective tissue growth factor expression after transforming growth factor-beta stimulation. Plast. Reconstr. Surg. 2005, 116, 1387–1390. [Google Scholar] [CrossRef]
- Baghy, K.; Iozzo, R.V.; Kovalszky, I. Decorin-TGFβ axis in hepatic fibrosis and cirrhosis. J. Histochem. Cytochem. 2012, 60, 262–268. [Google Scholar] [CrossRef]
- Tredget, E.E.; Levi, B.; Donelan, M.B. Biology and principles of scar management and burn reconstruction. Surg. Clin. N. Am. 2014, 94, 793–815. [Google Scholar] [CrossRef]
- Berman, B.; Viera, M.H.; Amini, S.; Huo, R.; Jones, I.S. Prevention and management of hypertrophic scars and keloids after burns in children. J. Craniofac. Surg. 2008, 19, 989–1006. [Google Scholar] [CrossRef]
- Bloemen, M.C.; Van der Veer, W.M.; Ulrich, M.M.; Van Zuijlen, P.P.; Niessen, F.B.; Middelkoop, E. Prevention and curative management of hypertrophic scar formation. Burns 2009, 35, 463–475. [Google Scholar] [CrossRef]
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef]
- Gurney, J.M.; Kozar, R.A.; Cancio, L.C. Plasma for burn shock resuscitation: Is it time to go back to the future? Transfusion 2019, 59, 1578–1586. [Google Scholar] [CrossRef]
- Cotton, B.A.; Guy, J.S.; Morris, J.A., Jr.; Abumrad, N.N. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock 2006, 26, 115–121. [Google Scholar] [CrossRef]
- Raghunathan, K.; Murray, P.T.; Beattie, W.S.; Lobo, D.N.; Myburgh, J.; Sladen, R.; Kellum, J.A.; Mythen, M.G.; Shaw, A.D.; ADQI XII Investigators Group. Choice offluid inacute illness: What should be given? An international consen-sus. Br. J. Anaesth. 2014, 113, 772–783. [Google Scholar] [CrossRef][Green Version]
- Toshiaki, I.; Saitoh, D. Efficacy of antithrombin in preclinical and clinical applications for sepsis-associated disseminated intravascular coagulation. J. Intensive Care 2014, 2, 66. [Google Scholar]
- Kowal-Vern, A.; Orkin, B.A. Antithrombin in the treatment of burn trauma. World J. Crit. Care Med. 2016, 5, 17–26. [Google Scholar] [CrossRef]
- Salibian, A.A.; Rosario, A.T.D.; Severo, L.D.A.M.; Nguyen, L.; Banyard, D.A.; Toranto, J.D.; Evans, G.R.D.; Widgerow, A. Current concepts on burn wound conversion—A review of recent advances in understanding the secondary progressions of burns. Burns 2016, 42, 1025–1035. [Google Scholar] [CrossRef]
- Singh, V.; Devgan, L.; Bhat, S.; Milner, S.M. The pathogenesis of burn wound conversion. Ann. Plast. Surg. 2007, 59, 109–115. [Google Scholar] [CrossRef]
- Schmauss, D.; Rezaeian, F.; Finck, T.; Machens, H.G.; Wettstein, R.; Harder, Y. Treatment of secondary burn wound progression in contact burns—A systematic review of experimental approaches. J. Burn Care Res. 2015, 36, e176–e189. [Google Scholar] [CrossRef]
- Battal, M.N.; Hata, Y.; Matsuka, K.; Ito, O.; Matsuda, H.; Yoshida, Y.; Kawazoe, T. Reduction of progressive burn injury by using a new nonselective endothelin-A and endothelin-B receptor antagonist, TAK-044: An experimental study in rats. Plast. Reconstr. Surg. 1997, 99, 1610–1619. [Google Scholar] [CrossRef]
- Eski, M.; Ozer, F.; Firat, C.; Alhan, D.; Arslan, N.; Senturk, T.; Işik, S. Cerium nitrate treatment pre-vents progressive tissue necrosis in the zone of stasis following burn. Burns 2012, 38, 283–289. [Google Scholar] [CrossRef]
- Yuhua, S.; Ligen, L.; Jiake, C.; Tongzhu, S. Effect of Poloxamer 188 on deepening of deep second-degree burn wounds in the early stage. Burns 2012, 38, 95–101. [Google Scholar] [CrossRef]
- Taira, B.R.; Singer, A.J.; McClain, S.A.; Lin, F.; Rooney, J.; Zimmerman, T.; Clark, R.A.F. Rosiglitazone, a PPAR-gamma ligand, reduces burn progression in rats. J. Burn Care Res. 2009, 30, 499–504. [Google Scholar] [CrossRef]
- Bey, E.; Prat, M.; Duhamel, P.; Benderitter, M.; Brachet, M.; Trompier, F.; Battaglini, P.; Ernou, I.; Boutin, L.; Gourven, M.; et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen. 2010, 18, 50–58. [Google Scholar] [CrossRef]
- Stubhaug, A.; Romundstad, L.; Kaasa, T.; Breivik, H. Methylprednisolone and ketorolac rapidly reduce hyperalgesia around a skin burn injury and increase pressure pain thresholds. Acta Anaesthesiol. Scand. 2007, 51, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.F.; Yao, Y.M.; Dong, N.; Yu, Y.; He, L.X.; Sheng, Z.Y. Association of high mobility group box-1 protein levels with sepsis and outcome of severely burned patients. Cytokine 2011, 53, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Rook, J.M.; Hasan, W.; McCarson, K.E. Morphine-induced early delays in wound closure: Involvement of sensory neuropeptides and modification of neurokinin receptor expression. Biochem. Pharmacol. 2009, 77, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Rook, J.M.; McCarson, K.E. Delay of cutaneous wound closure by morphine via local blockade of peripheral tachykinin release. Biochem. Pharmacol. 2007, 74, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Buchner, S.; Rufli, T.; Bigliardi-Qi, M. Specific stimulation of migration of human keratinocytes by mu-opiate receptor agonists. J. Recept. Signal. Transduct. Res. 2002, 22, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Kuchler, S. Targeting inflammation and wound healing by opioids. Trends Pharmacol. Sci. 2013, 34, 303–312. [Google Scholar] [CrossRef]
- Kim, D.E.; Pruskowski, K.A.; Ainsworth, C.R.; Linsenbardt, H.R.; Rizzo, J.A.; Cancio, L.C. A Review of Adjunctive Therapies for Burn Injury Pain during the Opioid Crisis. J. Burn Care Res. 2019, 40, 983–995. [Google Scholar] [CrossRef]
- Liu, H.F.; Zhang, F.; Lineaweaver, W.C. History and Advancement of Burn Treatments. Ann. Plast. Surg. 2017, 78, S2–S8. [Google Scholar] [CrossRef]
- Coban, Y.K. Infection control in severely burned patients. World J. Crit. Care Med. 2012, 1, 94–101. [Google Scholar] [CrossRef]
- Gerhardt, R.T.; Matthews, J.M.; Sullivan, S.G. The effect of systemic antibiotic prophylaxis and wound irrigation on penetrating combat wounds in a return-to-duty population. Prehosp. Emerg. Care. 2009, 13, 500–504. [Google Scholar] [CrossRef]
- Garau, J.; Bouza, E.; Chastre, J.; Gudiol, F.; Harbarth, S. Management of methicillin-resistant Staphylococcus aureus infections. Clin. Microbiol. Infect. 2009, 15, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Klugman, K.P.; Pneumococcal Advisory Council of Experts (PACE); Garau, J.; European Society of Clinical Microbiology and Infection (ESCMID). A preventable killer: Pneumonia. Clin. Microbiol. Infect. 2009, 15, 989–990. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lentino, J.R.; Narita, M.; Yu, V.L. New antimicrobial agents as therapy for resistant gram-positive cocci. Eur. J. Clin. Microbiol. Infect Dis. 2008, 27, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Dacso, C.C.; Luterman, A.; Curreri, P.W. Systemic antibiotic treatment in burned patients. Surg. Clin. N. Am. 1987, 67, 57–68. [Google Scholar] [CrossRef]
- Gera, S.; Kankuri, E.; Kogermann, K. Antimicrobial peptides—Unleashing their therapeutic potential using nanotechnology. Pharmacol. Ther. 2022, 232, 107990. [Google Scholar] [CrossRef]
- Schini-Kerth, V.B. Vascular biosynthesis of nitric oxide: Effect on hemostasis and fibrinolysis. Transfus. Clin. Biol. 1999, 6, 355–363. [Google Scholar] [CrossRef]
- Man, M.Q.; Wakefield, J.S.; Mauro, T.M.; Elias, P.M. Regulatory Role of Nitric Oxide in Cutaneous Inflammation. Inflammation 2022, 45, 949–964. [Google Scholar] [CrossRef]
- Man, M.Q.; Wakefield, J.S.; Mauro, T.M.; Elias, P.M. Role of nitric oxide in regulating epidermal permeability barrier function. Exp. Dermatol. 2022, 31, 290–298. [Google Scholar] [CrossRef]
- Wu, M.; Lu, Z.; Wu, K.; Nam, C.; Zhang, L.; Guo, J. Recent advances in the development of nitric oxide-releasing biomaterials and their application potentials in chronic wound healing. J. Mater. Chem. B 2021, 9, 7063–7075. [Google Scholar] [CrossRef]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef]
- Guilabert, P.; Usúa, G.; Martín, N.; Abarca, L.; Barret, J.P.; Colomina, M.J. Fluid resuscitation management in patients with burns: Update. Br. J. Anaesth. 2016, 117, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Guillory, A.N.; Clayton, R.P.; Herndon, D.N.; Finnerty, C.C. Cardiovascular Dysfunction Following Burn Injury: What We Have Learned from Rat and Mouse Models. Int. J. Mol. Sci. 2016, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ge, S.; Peng, Y.; Chen, X. Inflammation and cardiac dysfunction during sepsis, muscular dystrophy, and myocarditis. Burns Trauma 2013, 1, 109–121. [Google Scholar] [PubMed]
- Jeschke, M.G.; Chinkes, D.L.; Finnerty, C.C.; Kulp, G.; Suman, O.E.; Norbury, W.B.; Branski, L.K.; Gauglitz, G.G.; Mlcak, R.P.; Herndon, D.N. Pathophysiologic response to severe burn injury. Ann. Surg. 2008, 248, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.N.; Branski, L.K.; Jeschke, M.G.; Herndon, D.N. What, how, and how much should patients with burns be fed? Surg. Clin. N. Am. 2011, 91, 609–629. [Google Scholar] [CrossRef]
- Demling, R.H.; Orgill, D.P. The anticatabolic and wound healing effects of the testosterone analog oxandrolone after severe burn injury. J. Crit. Care 2000, 15, 12–17. [Google Scholar] [CrossRef]
- Herndon, D.N.; Barrow, R.E.; Rutan, T.C.; Minifee, P.; Jahoor, F.; Wolfe, R.R. Effect of Propranolol Administration on Hemodynamic and Metabolic Responses of Burned Pediatric-Patients. Ann. Surg. 1988, 208, 484–492. [Google Scholar] [CrossRef]
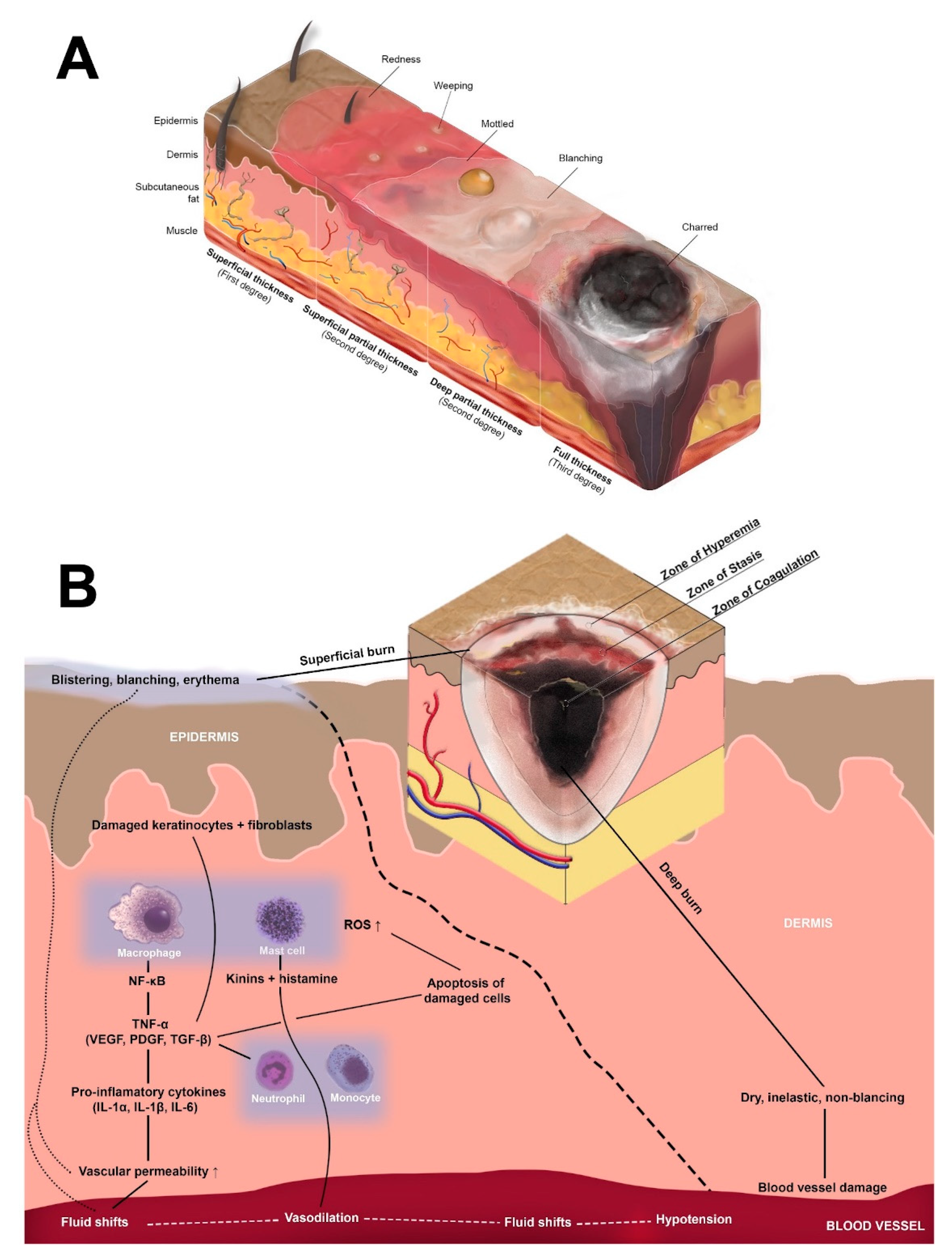
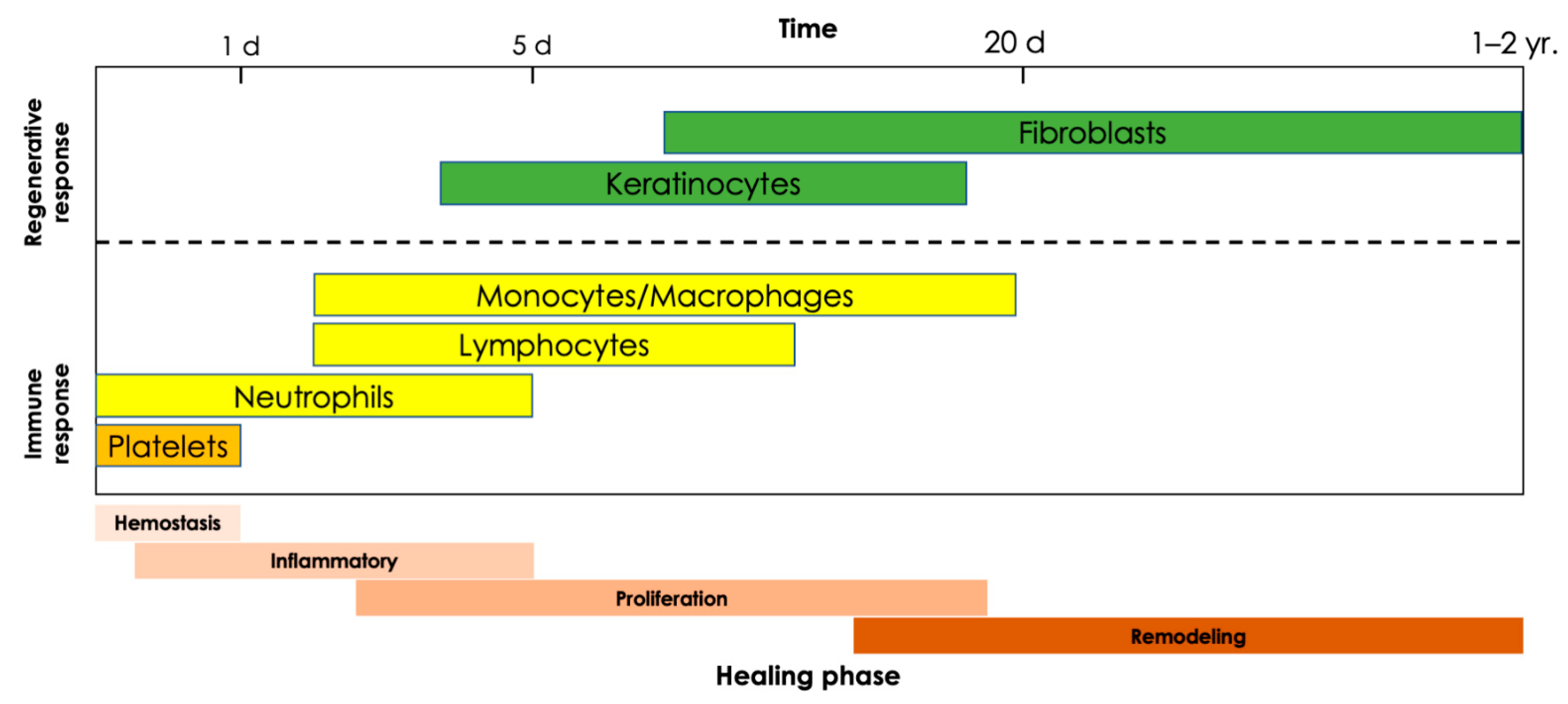
| Cell Type | Function | Recruited or Activated by | Releases | Targets | |
|---|---|---|---|---|---|
| Innate immune cells | Neutrophils | Microbe Elimination | P-Selectin (cell migration) | ROS | Pathogens |
| Extracellular Matrix (ECM) Clearance | Extruded nets of histones and DNA | Lymphocytes | |||
| Recruitment of Additional Inflammatory Cells | MMPs | Macrophages | |||
| Elastase | Dendritic Cells | ||||
| Collagenase | Endothelial Cells | ||||
| TNF-α/β | Epithelial Cells | ||||
| IL-1β, IL-6 | |||||
| Monocytes | Immune Function Regulation | TLR-2, TLR-4 | IL-1β, IL-8 | Damaged Matrix and Cellular Debris | |
| Tissue Repair | TNF-α | ||||
| M1 macrophages | Phagocytosis | Growth factors, cytokines (such as ILs) | Prostaglandin E2 | Microbes | |
| ROS/RNS | Necrotic Cells | ||||
| TNF-α | Activated Lymphocytes/Th1 Cells | ||||
| IL-1, IL-6, and IL-8 | |||||
| M2 macrophages | IL-1 Receptor antagonist | M1 Macrophages | |||
| PDGF | Polarized Th2 Cells | ||||
| TGF-β1 | |||||
| FGF | |||||
| Mast cells | Immune Cell Recruitment and Migration | Resident Dermal Cells | Histamine | Endothelial Cells | |
| Dampens Excessive Immune Responses | TNF-α/β | Nerve endings | |||
| Fibroblast Recruitment via Proteases | Cytokines (IL-1, IL-3, IL-5, IL-6, and IL-8) | Smooth Muscle Cells | |||
| Growth Factors (VEGF, FGF, and PDGF) | |||||
| Prostaglandins | |||||
| GM-CSF | |||||
| MIP-1β | |||||
| Natural killer cells | Bacterial Infection Control via Cytolytic Activity | Type I + III IFN | Type II IFN + IFNγ | Neutrophils | |
| IL-12 | TNF-α | Macrophages | |||
| GM-CSF | GM-CSF | ||||
| Cytokines (IL-2, IL-13, and IL-17) | |||||
| Dendritic cells | Pathogen Recognition | TLR-7 + TLR-9 | Type I IFN | Pathogens | |
| Induces Early Inflammatory Response | T-Cells (Activation) | ||||
| Re-epithelialization | NK Cells (Activation) | ||||
| Keratinocyte Proliferation | Keratinocyte (Proliferation) | ||||
| Enhances Antimicrobial Function of NK Cells | |||||
| Activate T-Cells | |||||
| Adaptive immune cells | Helper T-Cells | Augmentation of the Innate Immune System | Cells Presenting MHC-I + -II | IL-2, IL-4, IL-5, IL-10, IL-17 | B-Cells |
| Cytotoxic T-Cells | IFNγ | Cytotoxic T-Cells | |||
| Macrophages | |||||
| Killer T-Cells | Direct Defense Against Foreign Antigens | Cells Presenting MHC-I + -II | IL-2 | Pathogens | |
| IFNγ | |||||
| Unconventional T-Cells (MAIT, iNKT, γδ) | Modulation of the inflammatory response | Nonpeptidic antigens | Cytokines (such as IL-17) | Pathogens | |
| MHC molecules | Chemokines | Dendritic cells | |||
| αß T-Cells | |||||
| B-Cells | |||||
| B-Cells | Immunoglobulin/Antibody Production | IL-4, IL-5, IL-15 | Immunoglobulins/Antibodies | Pathogens | |
| Regenerative cells | Keratinocytes | Formation of epithelium | Growth Factors (KGF, EGF, TGFβ, VEGF, and FGF) | Membrane Proteins (Collagen IV + VII) | Neutrophils |
| Restoration of Barrier Function | Cytokines (IL-1, IL-6, IL-8, and TNF-a) | Growth Factors (MSF, NGF, VEGF, GM-CSF) | Macrophages | ||
| Hair Follicle and Sweat Gland Regeneration | Integrins | Cytokines (TNF-α + IL-1α/β) | Fibroblasts | ||
| Promotes Remodeling and Angiogenesis | Keratins | Melanocytes | |||
| MMPs | Endothelial Cells | ||||
| Epidermal Stem Cells | Promote tissue regeneration | IL-1 and TNF-α | Cytokeratins | Epithelial Cells | |
| Growth Factors (EGF, TGF-β, VEGF, IGF) | Fibroblasts | ||||
| Basement Membrane Proteins | Endothelial Cells | ||||
| Smooth Muscle Cells | |||||
| Innate immune cells | |||||
| Melanocytes | Barrier Function | Tyrosine | Melanin | Keratinocytes | |
| Pigmentation | α-MSH | ||||
| Prevention of UV Damage to the Integument | |||||
| Fibroblasts | Promote connective Tissue Formation andvdermal Remodeling | TGF-β | Collagen | Endothelial Cells | |
| Fibrillin | Epithelial Cells | ||||
| Elastin | Immune Cells | ||||
| MMPs | Adipocytes | ||||
| Growth Factors (FGF, TGF-β, KGF, GM-CSF) |
| Major Burn (>20% TBSA) | |
|---|---|
| Local Treatment | Systemic Treatment |
| Prevention of burn conversion | Burn shock |
| Topical treatment | Fluid resuscitation |
| Systemic pharmacological agents | |
| Infection control | Hypermetabolic state |
| Topical antimicrobials | Nutrition support |
| Systemic antibiotics | |
| Removal of necrotic tissue | Sepsis |
| Surgical debridement | Systemic antibiotics |
| Enzymatic debridement | |
| Barrier function restoration | Pain control |
| STSG | Opiates |
| Minced skin transplantation | NSAID |
| Cell therapy | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgess, M.; Valdera, F.; Varon, D.; Kankuri, E.; Nuutila, K. The Immune and Regenerative Response to Burn Injury. Cells 2022, 11, 3073. https://doi.org/10.3390/cells11193073
Burgess M, Valdera F, Varon D, Kankuri E, Nuutila K. The Immune and Regenerative Response to Burn Injury. Cells. 2022; 11(19):3073. https://doi.org/10.3390/cells11193073
Chicago/Turabian StyleBurgess, Matthew, Franklin Valdera, David Varon, Esko Kankuri, and Kristo Nuutila. 2022. "The Immune and Regenerative Response to Burn Injury" Cells 11, no. 19: 3073. https://doi.org/10.3390/cells11193073
APA StyleBurgess, M., Valdera, F., Varon, D., Kankuri, E., & Nuutila, K. (2022). The Immune and Regenerative Response to Burn Injury. Cells, 11(19), 3073. https://doi.org/10.3390/cells11193073










