Advances in Biomarkers and Endogenous Regulation of Breast Cancer Stem Cells
Abstract
1. Introduction
2. Identification of BCSCs
3. Endogenous Factors That Regulate BCSCs
3.1. Epigenetic Factors
3.2. Non-coding RNAs
3.3. Transcription Factors and Signal Transduction Pathways
3.3.1. Transcription Factors and Co-Activators
3.3.2. Signal Transduction Pathways
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Feng, R.M.; Zong, Y.N.; Cao, S.M.; Xu, R.H. Current cancer situation in China: Good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019, 39, 22. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Yeo, S.K.; Guan, J.L. Breast Cancer: Multiple Subtypes within a Tumor? Trends Cancer 2017, 3, 753–760. [Google Scholar] [CrossRef]
- Gu, H.F.; Mao, X.Y.; Du, M. Prevention of breast cancer by dietary polyphenols—role of cancer stem cells. Crit. Rev. Food Sci. Nutr. 2019, 60, 810–825. [Google Scholar] [CrossRef]
- Wang, C.; Xu, K.; Wang, R.; Han, X.; Tang, J.; Guan, X. Heterogeneity of BCSCs contributes to the metastatic organotropism of breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 1–14. [Google Scholar] [CrossRef]
- Bai, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat. Rev. 2018, 69, 152–163. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Ponti, D.; Costa, A.; Zaffaroni, N.; Pratesi, G.; Petrangolini, G.; Coradini, D.; Pilotti, S.; Pierotti, M.A.; Daidone, M.G. Isolation and In vitro Propagation of Tumorigenic Breast Cancer Cells with Stem/Progenitor Cell Properties. Cancer Res. 2005, 65, 5506–5511. [Google Scholar] [CrossRef]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Wright, M.H.; Calcagno, A.M.; Salcido, C.D.; Carlson, M.D.; Ambudkar, S.V.; Varticovski, L. Brca1 breast tumors contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008, 10, R10. [Google Scholar] [CrossRef]
- Wang, D.; Cai, C.; Dong, X.; Yu, Q.C.; Zhang, X.-O.; Yang, L.; Zeng, Y.A. Identification of multipotent mammary stem cells by protein C receptor expression. Nature 2014, 517, 81–84. [Google Scholar] [CrossRef]
- Zhu, R.; Gires, O.; Zhu, L.; Liu, J.; Li, J.; Yang, H.; Ju, G.; Huang, J.; Ge, W.; Chen, Y.; et al. TSPAN8 promotes cancer cell stemness via activation of sonic Hedgehog signaling. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.; Deng, Q.; Yang, C.; Wang, D.; Jiang, G.; Yao, X.; He, X.; Ding, J.; Qiang, J.; et al. TEM8 marks neovasculogenic tumor-initiating cells in triple-negative breast cancer. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Zhao, L.; Qiu, T.; Jiang, D.; Xu, H.; Zou, L.; Yang, Q.; Chen, C.; Jiao, B. SGCE Promotes Breast Cancer Stem Cells by Stabilizing EGFR. Adv. Sci. 2020, 7, 1903700. [Google Scholar] [CrossRef]
- Cidado, J.; Wong, H.Y.; Rosen, D.M.; Cimino-Mathews, A.; Garay, J.P.; Fessler, A.G.; Rasheed, Z.A.; Hicks, J.; Cochran, R.L.; Croessmann, S.; et al. Ki-67 is required for maintenance of cancer stem cells but not cell proliferation. Oncotarget 2016, 7, 6281–6293. [Google Scholar] [CrossRef]
- Wang, D.; Liu, C.; Wang, J.; Jia, Y.; Hu, X.; Jiang, H.; Shao, Z.-M.; Zeng, Y.A. Protein C receptor stimulates multiple signaling pathways in breast cancer cells. J. Biol. Chem. 2018, 293, 1413–1424. [Google Scholar] [CrossRef]
- Xue, G.; Wang, K.; Zhou, D.; Zhong, H.; Pan, Z. Light-Induced Protein Degradation with Photocaged PROTACs. J. Am. Chem. Soc. 2019, 141, 18370–18374. [Google Scholar] [CrossRef]
- Sobecki, M.; Mrouj, K.; Camasses, A.; Parisis, N.; Nicolas, E.; Llères, D.; Gerbe, F.; Prieto, S.; Krasinska, L.; David, A.; et al. The cell proliferation antigen Ki-67 organises heterochromatin. eLife 2016, 5, e13722. [Google Scholar] [CrossRef]
- Shimoda, M.; Ota, M.; Okada, Y. Isolation of Cancer Stem Cells by Side Population Method. Methods Mol. Biol. 2017, 1692, 49–59. [Google Scholar] [CrossRef]
- Srinivasan, M.; Bharali, D.J.; Sudha, T.; Khedr, M.; Guest, I.; Sell, S.; Glinsky, G.V.; Mousa, S.A. Downregulation of Bmi1 in breast cancer stem cells suppresses tumor growth and proliferation. Oncotarget 2017, 8, 38731–38742. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chang, W.-W.; Chen, Y.-Y.; Tsai, Y.-H.; Chou, Y.-H.; Tseng, H.-C.; Chen, H.-L.; Wu, C.-C.; Chang-Chien, J.; Lee, H.-T.; et al. Hsp90α Mediates BMI1 Expression in Breast Cancer Stem/Progenitor Cells through Facilitating Nuclear Translocation of c-Myc and EZH2. Int. J. Mol. Sci. 2017, 18, 1986. [Google Scholar] [CrossRef]
- Chen, S.-M.; Wang, B.-Y.; Lee, C.-H.; Lee, H.-T.; Li, J.-J.; Hong, G.-C.; Hung, Y.-C.; Chien, P.-J.; Chang, C.-Y.; Hsu, L.-S.; et al. Hinokitiol up-regulates miR-494-3p to suppress BMI1 expression and inhibits self-renewal of breast cancer stem/progenitor cells. Oncotarget 2017, 8, 76057–76068. [Google Scholar] [CrossRef]
- Zhang, L.; Qiang, J.; Yang, X.; Wang, D.; Rehman, A.U.; He, X.; Chen, W.; Sheng, D.; Zhou, L.; Jiang, Y.; et al. IL1R2 Blockade Suppresses Breast Tumorigenesis and Progression by Impairing USP15-Dependent BMI1 Stability. Adv. Sci. 2019, 7, 1901728. [Google Scholar] [CrossRef]
- van Vlerken, L.E.; Kiefer, C.M.; Morehouse, C.; Li, Y.; Groves, C.; Wilson, S.D.; Yao, Y.; Hollingsworth, R.E.; Hurt, E.M. EZH2 is required for breast and pancreatic cancer stem cell maintenance and can be used as a functional cancer stem cell reporter. Stem Cells Transl. Med. 2013, 2, 43–52. [Google Scholar] [CrossRef]
- Li, J.; Xi, Y.; Li, W.; McCarthy, R.L.; A Stratton, S.; Zou, W.; Dent, S.Y.; Jain, A.K.; Barton, M.C. TRIM28 interacts with EZH2 and SWI/SNF to activate genes that promote mammosphere formation. Oncogene 2017, 36, 2991–3001. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, S.; Song, E.; Liu, S. The roles of ncRNAs and histone-modifiers in regulating breast cancer stem cells. Protein Cell 2015, 7, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, D.; Chen, X.; Wang, W.; Wang, P.; Hou, P.; Li, M.; Chu, S.; Qiao, S.; Zheng, J.; et al. PRMT1-mediated EZH2 methylation promotes breast cancer cell proliferation and tumorigenesis. Cell Death Dis. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Lu, J.; Huang, B.; Wang, Y.; Dong, M.; Fan, D.; Li, H.; Gao, Y.; Hou, P.; et al. Methylation of EZH2 by PRMT1 regulates its stability and promotes breast cancer metastasis. Cell Death Differ. 2020, 27, 3226–3242. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Yang, X.H.; Kang, T.; Zhao, Y.; Wang, C.; Evers, B.M.; Zhou, B.P. The Deubiquitinase USP28 Stabilizes LSD1 and Confers Stem-Cell-like Traits to Breast Cancer Cells. Cell Rep. 2013, 5, 224–236. [Google Scholar] [CrossRef]
- Boulding, T.; McCuaig, R.D.; Tan, A.; Hardy, K.; Wu, F.; Dunn, J.; Kalimutho, M.; Sutton, C.R.; Forwood, J.; Bert, A.G.; et al. Author Correction: LSD1 activation promotes inducible EMT programs and modulates the tumour microenvironment in breast cancer. Sci. Rep. 2019, 9, 18771. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.; Zhang, J.; Lu, Y.; Liu, X.; Geng, P.; Huang, B.; Zhang, Y.; Lu, J. The dual function of PRMT1 in modulating epithelial-mesenchymal transition and cellular senescence in breast cancer cells through regulation of ZEB1. Sci. Rep. 2016, 6, 19874. [Google Scholar] [CrossRef]
- Nakai, K.; Xia, W.; Liao, H.-W.; Saito, M.; Hung, M.-C.; Yamaguchi, H. The role of PRMT1 in EGFR methylation and signaling in MDA-MB-468 triple-negative breast cancer cells. Breast Cancer 2017, 25, 74–80. [Google Scholar] [CrossRef]
- Lattouf, H.; Kassem, L.; Jacquemetton, J.; Choucair, A.; Poulard, C.; Tredan, O.; Corbo, L.; Diab-Assaf, M.; Hussein, N.; Treilleux, I.; et al. LKB1 regulates PRMT5 activity in breast cancer. Int. J. Cancer 2019, 144, 595–606. [Google Scholar] [CrossRef]
- Zhou, Z.; Feng, Z.; Hu, D.; Yang, P.; Gur, M.; Bahar, I.; Cristofanilli, M.; Gradishar, W.J.; Xie, X.-Q.; Wan, Y. A novel small-molecule antagonizes PRMT5-mediated KLF4 methylation for targeted therapy. eBioMedicine 2019, 44, 98–111. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, T.; Wu, Y.; Yang, C.; Li, Y.; Du, G.; He, Y.; Liu, W.; Liu, R.; Chen, C.-H.; et al. Arginine methyltransferase PRMT5 methylates and stabilizes KLF5 via decreasing its phosphorylation and ubiquitination to promote basal-like breast cancer. Cell Death Differ. 2021, 28, 2931–2945. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Hsu, C.Y.; Tsai, C.F.; Long, C.Y.; Wu, C.H.; Wu, D.C.; Lee, J.N.; Chang, W.C.; Tsai, E.M. HDAC inhibitors target HDAC5, upregulate microRNA-125a-5p, and induce apoptosis in breast cancer cells. Mol. Ther. 2015, 23, 656–666. [Google Scholar] [CrossRef]
- Witt, A.E.; Lee, C.-W.; Lee, T.I.; Azzam, D.J.; Wang, B.; Caslini, C.; Petrocca, F.; Grosso, J.; Jones, M.; Cohick, E.B.; et al. Identification of a cancer stem cell-specific function for the histone deacetylases, HDAC1 and HDAC7, in breast and ovarian cancer. Oncogene 2017, 36, 1707–1720. [Google Scholar] [CrossRef]
- Kong, Y.; Ren, W.; Fang, H.; Shah, N.A.; Shi, Y.; You, D.; Zhou, C.; Jiang, D.; Yang, C.; Liang, H.; et al. Histone Deacetylase Inhibitors (HDACi) Promote KLF5 Ubiquitination and Degradation in Basal-like Breast Cancer. Int. J. Biol. Sci. 2022, 18, 2104–2115. [Google Scholar] [CrossRef]
- Debeb, B.G.; Lacerda, L.; Xu, W.; Larson, R.; Solley, T.; Atkinson, R.; Sulman, E.P.; Ueno, N.T.; Krishnamurthy, S.; Reuben, J.M.; et al. Histone deacetylase inhibitors stimulate dedifferentiation of human breast cancer cells through WNT/beta-catenin signaling. Stem Cells 2012, 30, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.; Raza, A.; Karedath, T.; Raza, S.; Fathima, H.; Ahmed, E.; Kuttikrishnan, S.; Therachiyil, L.; Kulinski, M.; Dermime, S.; et al. Non-Coding RNAs as Regulators and Markers for Targeting of Breast Cancer and Cancer Stem Cells. Cancers 2020, 12, 351. [Google Scholar] [CrossRef]
- Sun, X.; Xu, C.; Tang, S.-C.; Wang, J.; Wang, H.; Wang, P.; Du, N.; Qin, S.; Li, G.; Xu, S.; et al. Let-7c blocks estrogen-activated Wnt signaling in induction of self-renewal of breast cancer stem cells. Cancer Gene Ther. 2016, 23, 83–89. [Google Scholar] [CrossRef]
- Li, B.; Lu, Y.; Yu, L.; Han, X.; Wang, H.; Mao, J.; Shen, J.; Wang, B.; Tang, J.; Li, C.; et al. miR-221/222 promote cancer stem-like cell properties and tumor growth of breast cancer via targeting PTEN and sustained Akt/NF-kappaB/COX-2 activation. Chem. Biol. Interact 2017, 277, 33–42. [Google Scholar] [CrossRef]
- Li, B.; Lu, Y.; Wang, H.; Han, X.; Mao, J.; Li, J.; Yu, L.; Wang, B.; Fan, S.; Yu, X.; et al. miR-221/222 enhance the tumorigenicity of human breast cancer stem cells via modulation of PTEN/Akt pathway. Biomed. Pharmacother. 2016, 79, 93–101. [Google Scholar] [CrossRef]
- Xia, L.; Li, F.; Qiu, J.; Feng, Z.; Xu, Z.; Chen, Z.; Sun, J. Oncogenic miR-20b-5p contributes to malignant behaviors of breast cancer stem cells by bidirectionally regulating CCND1 and E2F1. BMC Cancer 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Zhang, H.; Li, N.; Zhang, J.; Jin, F.; Shan, M.; Qin, J.; Wang, Y. The influence of miR-34a expression on stemness and cytotoxic susceptibility of breast cancer stem cells. Cancer Biol. Ther. 2016, 17, 614–624. [Google Scholar] [CrossRef]
- Liang, R.; Li, Y.; Wang, M.; Tang, S.-C.; Xiao, G.; Sun, X.; Li, G.; Du, N.; Liu, D.; Ren, H. MiR-146a promotes the asymmetric division and inhibits the self-renewal ability of breast cancer stem-like cells via indirect upregulation of Let-7. Cell Cycle 2018, 17, 1445–1456. [Google Scholar] [CrossRef]
- Han, M.-L.; Wang, F.; Gu, Y.-T.; Pei, X.-H.; Ge, X.; Guo, G.-C.; Li, L.; Duan, X.; Zhu, M.-Z.; Wang, Y.-M. MicroR-760 suppresses cancer stem cell subpopulation and breast cancer cell proliferation and metastasis: By down-regulating NANOG. Biomed. Pharmacother. 2016, 80, 304–310. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, Y.; Yao, S.; Deng, G.; Liu, D.; Yuan, X.; Liu, S.; Rao, J.; Xiong, H.; Yuan, X.; et al. MiR-422a weakened breast cancer stem cells properties by targeting PLP2. Cancer Biol. Ther. 2018, 19, 436–444. [Google Scholar] [CrossRef]
- Troschel, F.M.; Böhly, N.; Borrmann, K.; Braun, T.; Schwickert, A.; Kiesel, L.; Eich, H.T.; Götte, M.; Greve, B. miR-142-3p attenuates breast cancer stem cell characteristics and decreases radioresistance in vitro. Tumor Biol. 2018, 40, 1010428318791887. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, T.; He, D.; Li, X.; Jiang, Y. miR-1 inhibits the proliferation of breast cancer stem cells by targeting EVI-1. OncoTargets Ther. 2018, 11, 8773–8781. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, N.; Liu, L.; Dong, H.; Liu, X. microRNA-128-3p overexpression inhibits breast cancer stem cell characteristics through suppression of Wnt signalling pathway by down-regulating NEK2. J. Cell. Mol. Med. 2020, 24, 7353–7369. [Google Scholar] [CrossRef]
- Lin, Q.-Y.; Wang, J.-Q.; Wu, L.-L.; Zheng, W.-E.; Chen, P.-R. miR-638 represses the stem cell characteristics of breast cancer cells by targeting E2F2. Breast Cancer 2019, 27, 147–158. [Google Scholar] [CrossRef]
- Zhao, F.; Zhong, M.; Pei, W.; Tian, B.; Cai, Y. miR-376c-3p modulates the properties of breast cancer stem cells by targeting RAB2A. Exp. Ther. Med. 2020, 20, 68. [Google Scholar] [CrossRef]
- Chen, J.H.; Huang, W.C.; Bamodu, O.A.; Chang, P.M.; Chao, T.Y.; Huang, T.H. Monospecific antibody targeting of CDH11 inhibits epithelial-to-mesenchymal transition and represses cancer stem cell-like phenotype by up-regulating miR-335 in metastatic breast cancer, in vitro and in vivo. BMC Cancer 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Liang, H.; Xiao, J.; Zhou, Z.; Wu, J.; Ge, F.; Li, Z.; Zhang, H.; Sun, J.; Li, F.; Liu, R.; et al. Hypoxia induces miR-153 through the IRE1alpha-XBP1 pathway to fine tune the HIF1alpha/VEGFA axis in breast cancer angiogenesis. Oncogene 2018, 37, 1961–1975. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Shi, P.; Nie, Z.; Liang, H.; Zhou, Z.; Chen, W.; Chen, H.; Dong, C.; Yang, R.; Liu, S.; et al. Mifepristone Suppresses Basal Triple-Negative Breast Cancer Stem Cells by Down-regulating KLF5 Expression. Theranostics 2016, 6, 533–544. [Google Scholar] [CrossRef]
- Polytarchou, C.; Iliopoulos, D.; Struhl, K. An integrated transcriptional regulatory circuit that reinforces the breast cancer stem cell state. Proc. Natl. Acad. Sci. 2012, 109, 14470–14475. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Wang, T.; Yang, Y.; Ni, H.; Liu, H.; Guo, Q.; Xi, T.; Zheng, L. MiR-375 inhibits the stemness of breast cancer cells by blocking the JAK2/STAT3 signaling. Eur. J. Pharmacol. 2020, 884, 173359. [Google Scholar] [CrossRef]
- Deng, J.; Yang, M.; Jiang, R.; An, N.; Wang, X.; Liu, B. Long Non-Coding RNA HOTAIR Regulates the Proliferation, Self-Renewal Capacity, Tumor Formation and Migration of the Cancer Stem-Like Cell (CSC) Subpopulation Enriched from Breast Cancer Cells. PLoS ONE 2017, 12, e0170860. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Meng, D.; Wang, R. Long non-coding RNA SOX21-AS1 enhances the stemness of breast cancer cells via the Hippo pathway. FEBS Open Bio 2020, 11, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Liu, X.; Zhou, S.; Li, W.; Liu, C.; Chadwick, M.; Qian, C. Long non-coding RNA FGF13-AS1 inhibits glycolysis and stemness properties of breast cancer cells through FGF13-AS1/IGF2BPs/Myc feedback loop. Cancer Lett. 2019, 450, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Guo, C.; Xia, T.; Zhang, R.; Zen, K.; Pan, Y.; Jin, L. LncCCAT1 Promotes Breast Cancer Stem Cell Function through Activating WNT/beta-catenin Signaling. Theranostics 2019, 9, 7384–7402. [Google Scholar] [CrossRef]
- Peng, F.; Li, T.T.; Wang, K.L.; Xiao, G.Q.; Wang, J.H.; Zhao, H.D.; Kang, Z.J.; Fan, W.J.; Zhu, L.L.; Li, M.; et al. H19/let-7/LIN28 reciprocal negative regulatory circuit promotes breast cancer stem cell maintenance. Cell Death Dis. 2017, 8, e2569. [Google Scholar] [CrossRef]
- Song, X.; Zhang, X.; Wang, X.; Chen, L.; Jiang, L.; Zheng, A.; Zhang, M.; Zhao, L.; Wei, M. LncRNA SPRY4-IT1 regulates breast cancer cell stemness through competitively binding miR-6882-3p with TCF7L2. J. Cell Mol. Med. 2020, 24, 772–784. [Google Scholar] [CrossRef]
- Lu, G.; Li, Y.; Ma, Y.; Lu, J.; Chen, Y.; Jiang, Q.; Qin, Q.; Zhao, L.; Huang, Q.; Luo, Z.; et al. Long noncoding RNA LINC00511 contributes to breast cancer tumourigenesis and stemness by inducing the miR-185-3p/E2F1/Nanog axis. J. Exp. Clin. Cancer Res. 2018, 37, 289. [Google Scholar] [CrossRef]
- Han, L.; Yan, Y.; Zhao, L.; Liu, Y.; Lv, X.; Zhang, L.; Zhao, Y.; Zhao, H.; He, M.; Wei, M. LncRNA HOTTIP facilitates the stemness of breast cancer via regulation of miR-148a-3p/WNT1 pathway. J. Cell. Mol. Med. 2020, 24, 6242–6252. [Google Scholar] [CrossRef]
- Zheng, A.; Song, X.; Zhang, L.; Lu, G.; Zhao, L.; Mao, X.; Wei, M.; Jin, F. Long non-coding RNA LUCAT1/miR-5582-3p/TCF7L2 axis regulates breast cancer stemness via Wnt/beta-catenin pathway. J. Exp. Clin. Cancer Res. 2019, 38, 305. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, L.; Zhang, Y.; Lu, G.; Li, Y.; Wei, Z. Long non-coding RNA FEZF1-AS1 promotes breast cancer stemness and tumorigenesis via targeting miR-30a/Nanog axis. J. Cell. Physiol. 2018, 233, 8630–8638. [Google Scholar] [CrossRef]
- Wen, S.; Qin, Y.; Wang, R.; Yang, L.; Zeng, H.; Zhu, P.; Li, Q.; Qiu, Y.; Chen, S.; Liu, Y.; et al. A novel Lnc408 maintains breast cancer stem cell stemness by recruiting SP3 to suppress CBY1 transcription and increasing nuclear beta-catenin levels. Cell Death Dis. 2021, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, C.; Zhao, Q.; Lü, J.; Ding, X.; Luo, A.; He, J.; Wang, G.; Li, Y.; Cai, Z.; et al. Long non-coding RNA CCAT2 promotes oncogenesis in triple-negative breast cancer by regulating stemness of cancer cells. Pharmacol. Res. 2020, 152, 104628. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Hou, Y.; Yang, G.; Zhang, H.; Tu, G.; Du, Y.E.; Wen, S.; Xu, L.; Tang, X.; Tang, S.; et al. LncRNA-Hh Strengthen Cancer Stem Cells Generation in Twist-Positive Breast Cancer via Activation of Hedgehog Signaling Pathway. Stem Cells 2016, 34, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Hou, Y.; Liu, S.; Zhu, P.; Wan, X.; Zhao, M.; Peng, M.; Zeng, H.; Li, Q.; Jin, T.; et al. A Novel Long Non-Coding RNA lnc030 Maintains Breast Cancer Stem Cell Stemness by Stabilizing SQLE mRNA and Increasing Cholesterol Synthesis. Adv. Sci. 2020, 8, 2002232. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Cen, Y.; Chen, J. Long non-coding RNA MALAT-1 contributes to maintenance of stem cell-like phenotypes in breast cancer cells. Oncol. Lett. 2018, 15, 2117–2122. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Hou, Y.; Yang, L.; Wan, X.; Qin, Y.; Liu, Y.; Wang, R.; Zhu, P.; Teng, Y.; et al. A novel lncRNA ROPM-mediated lipid metabolism governs breast cancer stem cell properties. J. Hematol. Oncol. 2021, 14, 1–23. [Google Scholar] [CrossRef]
- Kim, R.-J.; Nam, J.-S. OCT4 Expression Enhances Features of Cancer Stem Cells in a Mouse Model of Breast Cancer. Lab. Anim. Res. 2011, 27, 147–152. [Google Scholar] [CrossRef]
- Hu, T.; Liu, S.; Breiter, D.R.; Wang, F.; Tang, Y.; Sun, S. Octamer 4 Small Interfering RNA Results in Cancer Stem Cell–Like Cell Apoptosis. Cancer Res. 2008, 68, 6533–6540. [Google Scholar] [CrossRef]
- Bak, M.J.; Furmanski, P.; Shan, N.L.; Lee, H.J.; Bao, C.; Lin, Y.; Shih, W.J.; Yang, C.S.; Suh, N. Tocopherols inhibit estrogen-induced cancer stemness and OCT4 signaling in breast cancer. Carcinogenesis 2018, 39, 1045–1055. [Google Scholar] [CrossRef]
- Almozyan, S.; Colak, D.; Mansour, F.; Alaiya, A.; Al-Harazi, O.; Qattan, A.; Al-Mohanna, F.; Al-Alwan, M.; Ghebeh, H. PD-L1 promotes OCT4 and Nanog expression in breast cancer stem cells by sustaining PI3K/AKT pathway activation. Int. J. Cancer 2017, 141, 1402–1412. [Google Scholar] [CrossRef]
- Xun, J.; Wang, D.; Shen, L.; Gong, J.; Gao, R.; Du, L.; Chang, A.; Song, X.; Xiang, R.; Tan, X. JMJD3 suppresses stem cell-like characteristics in breast cancer cells by downregulation of Oct4 independently of its demethylase activity. Oncotarget 2017, 8, 21918–21929. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, J.; Chen, H.; Fu, J.; Ray, S.; Huang, S.; Zheng, H.; Ai, W. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene 2011, 30, 2161–2172. [Google Scholar] [CrossRef] [PubMed]
- Okuda, H.; Xing, F.; Pandey, P.R.; Sharma, S.; Watabe, M.; Pai, S.K.; Mo, Y.-Y.; Iiizumi-Gairani, M.; Hirota, S.; Liu, Y.; et al. miR-7 Suppresses Brain Metastasis of Breast Cancer Stem-Like Cells By Modulating KLF4. Cancer Res. 2013, 73, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Liu, Y.; Wang, J.; Fan, H.; Fang, H.; Li, S.; Yuan, L.; Liu, C.; Peng, Y.; Zhao, W.; et al. Histone demethylase KDM7A is required for stem cell maintenance and apoptosis inhibition in breast cancer. J. Cell. Physiol. 2019, 235, 932–943. [Google Scholar] [CrossRef]
- Mimoto, R.; Imawari, Y.; Hirooka, S.; Takeyama, H.; Yoshida, K. Impairment of DYRK2 augments stem-like traits by promoting KLF4 expression in breast cancer. Oncogene 2016, 36, 1862–1872. [Google Scholar] [CrossRef]
- Liu, R.; Shi, P.; Zhou, Z.; Zhang, H.; Li, W.; Zhang, H.; Chen, C. Krüpple-like factor 5 is essential for mammary gland development and tumorigenesis. J. Pathol. 2018, 246, 497–507. [Google Scholar] [CrossRef]
- Shi, P.; Liu, W.; Tala; Wang, H.; Li, F.; Zhang, H.; Wu, Y.; Kong, Y.; Zhou, Z.; Wang, C.; et al. Metformin suppresses triple-negative breast cancer stem cells by targeting KLF5 for degradation. Cell Discov. 2017, 3, 17010. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.R.; Sun, H.; Goel, H.L.; Pursell, B.M.; Chang, C.; Khan, A.Q.; Greiner, D.L.; Cao, S.; Lim, E.; Shultz, L.D.; et al. IMP3 promotes stem-like properties in triple-negative breast cancer by regulating SLUG. Oncogene 2015, 35, 1111–1121. [Google Scholar] [CrossRef]
- Hu, C.; Xu, L.; Liang, S.; Zhang, Z.; Zhang, Y.; Zhang, F. Lentivirus-mediated shRNA targeting Nanog inhibits cell proliferation and attenuates cancer stem cell activities in breast cancer. J. Drug Target. 2015, 24, 422–432. [Google Scholar] [CrossRef]
- Zhang, C.; Samanta, D.; Lu, H.; Bullen, J.W.; Zhang, H.; Chen, I.; He, X.; Semenza, G.L. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m 6 A-demethylation of NANOG mRNA. Proc. Natl. Acad. Sci. 2016, 113, E2047–E2056. [Google Scholar] [CrossRef]
- Yin, S.; Cheryan, V.T.; Xu, L.; Rishi, A.K.; Reddy, K.B. Myc mediates cancer stem-like cells and EMT changes in triple negative breast cancers cells. PLoS ONE 2017, 12, e0183578. [Google Scholar] [CrossRef]
- Wang, S.; Wang, N.; Zheng, Y.; Yang, B.; Liu, P.; Zhang, F.; Li, M.; Song, J.; Chang, X.; Wang, Z. Caveolin-1 inhibits breast cancer stem cells via c-Myc-mediated metabolic reprogramming. Cell Death Dis. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Z.; Li, S.S.; Zhou, W.; Kang, Z.J.; Zhang, Q.X.; Kamran, M.; Xu, J.; Liang, D.P.; Wang, C.L.; Hou, Z.J.; et al. p62/SQSTM1 enhances breast cancer stem-like properties by stabilizing MYC mRNA. Oncogene 2017, 36, 304–317. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, L.-C.; Jiang, N.; Wang, X.-L.; Zhou, X.-N.; Luo, X.-L.; Ren, J. MicroRNA miR-590-5p inhibits breast cancer cell stemness and metastasis by targeting SOX2. Eur. Rev. Med Pharmacol. Sci. 2017, 21, 87–94. [Google Scholar]
- Gong, X.; Liu, W.; Wu, L.; Ma, Z.; Wang, Y.; Yu, S.; Zhang, J.; Xie, H.; Wei, G.; Ma, F.; et al. Transcriptional repressor GATA binding 1–mediated repression of SRY-box 2 expression suppresses cancer stem cell functions and tumor initiation. J. Biol. Chem. 2018, 293, 18646–18654. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, Y.; Qiu, H.; Liu, Y.; Luo, K.; Yi, Y.; Jiang, G.; Lu, M.; Zhang, Z.; Yin, J.; et al. Downregulation of FOXO3a by DNMT1 promotes breast cancer stem cell properties and tumorigenesis. Cell Death Differ. 2019, 27, 966–983. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Keckesova, Z.; Donaher, J.L.; Shibue, T.; Tischler, V.; Reinhardt, F.; Itzkovitz, S.; Noske, A.; Zürrer-Härdi, U.; Bell, G.; et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 2012, 148, 1015–1028. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Du, X.; Wang, X.; Guan, S.; Cao, Y.; Jin, F.; Li, F. HES1 promotes breast cancer stem cells by elevating Slug in triple-negative breast cancer. Int. J. Biol. Sci. 2021, 17, 247–258. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, D.; Sheng, D.; Xu, J.; Chen, W.; Qin, Y.; Du, R.; Yang, X.; He, X.; Xie, N.; et al. NOTCH4 maintains quiescent mesenchymal-like breast cancer stem cells via transcriptionally activating SLUG and GAS1 in triple-negative breast cancer. Theranostics 2020, 10, 2405–2421. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, B.; Deng, Q.; Sheng, D.; Xu, J.; He, X.; Zhang, L.; Liu, S. UCP1 regulates ALDH-positive breast cancer stem cells through releasing the suppression of Snail on FBP1. Cell Biol. Toxicol. 2020, 37, 277–291. [Google Scholar] [CrossRef]
- Doherty, M.R.; Parvani, J.G.; Tamagno, I.; Junk, D.J.; Bryson, B.L.; Cheon, H.J.; Stark, G.R.; Jackson, M.W. The opposing effects of interferon-beta and oncostatin-M as regulators of cancer stem cell plasticity in triple-negative breast cancer. Breast Cancer Res. 2019, 21, 1–12. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, X.; Tang, J.; Zhang, Z.; Du, R.; Luo, D.; Liu, X.; Xia, Y.; Li, Y.; Wang, S.; et al. CCL16 maintains stem cell-like properties in breast cancer by activating CCR2/GSK3beta/beta-catenin/OCT4 axis. Theranostics 2021, 11, 2297–2317. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Cao, J.; Tian, J.H.; Yu, C.Y.; Huang, Q.; Yu, J.J.; Ma, R.; Wang, J.; Xu, F.; Wang, L.B. Mortalin maintains breast cancer stem cells stemness via activation of Wnt/GSK3beta/beta-catenin signaling pathway. Am. J. Cancer Res. 2021, 11, 2696–2716. [Google Scholar] [PubMed]
- Zhang, F.; Li, P.; Liu, S.; Yang, M.; Zeng, S.; Deng, J.; Chen, D.; Yi, Y.; Liu, H. Beta-Catenin-CCL2 feedback loop mediates crosstalk between cancer cells and macrophages that regulates breast cancer stem cells. Oncogene 2021, 40, 5854–5865. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Xu, T.; Tian, T.; Fu, X.; Wang, W.; Li, S.; Shi, T.; Suo, A.; Ruan, Z.; Guo, H.; et al. Tripartite motif 16 suppresses breast cancer stem cell properties through regulation of Gli-1 degradation via the ubiquitin-proteasome pathway. Oncol. Rep. 2015, 35, 1204–1212. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Fan, C.; Gao, P.; Wang, X.; Wei, G.; Wei, J. Estrogen promotes stemness and invasiveness of ER-positive breast cancer cells through Gli1 activation. Mol. Cancer 2014, 13, 137. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, J.H.; Kim, S.L.; Lee, D.S. Disruption of the NF-kappaB/IL-8 Signaling Axis by Sulconazole Inhibits Human Breast Cancer Stem Cell Formation. Cells 2019, 8, 1007. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhang, X.-T.; Wang, M.-L.; Zheng, H.-Y.; Liu, L.-J.; Wang, Z.-Y. ER-α36-Mediated Rapid Estrogen Signaling Positively Regulates ER-Positive Breast Cancer Stem/Progenitor Cells. PLoS ONE 2014, 9, e88034. [Google Scholar] [CrossRef]
- Bartucci, M.; Dattilo, R.; Moriconi, C.; Pagliuca, A.; Mottolese, M.; Federici, G.; Di Benedetto, A.; Todaro, M.; Stassi, G.; Sperati, F.; et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene 2014, 34, 681–690. [Google Scholar] [CrossRef]
- Xiang, L.; Gilkes, D.M.; Hu, H.; Takano, N.; Luo, W.; Lu, H.; Bullen, J.W.; Samanta, D.; Liang, H.; Semenza, G.L. Hypoxia-inducible factor 1 mediates TAZ expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget 2014, 5, 12509–12527. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Mao, X.; Jiang, Y.; Liu, J.; Li, J.; Wang, J.; Wang, R.; She, J.; Zhang, J.; et al. CRB3 downregulation confers breast cancer stem cell traits through TAZ/beta-catenin. Oncogenesis 2017, 6, e322. [Google Scholar] [CrossRef]
- Ko, Y.-C.; Choi, H.S.; Liu, R.; Lee, D.-S. Physalin A, 13,14-Seco-16, 24-Cyclo-Steroid, Inhibits Stemness of Breast Cancer Cells by Regulation of Hedgehog Signaling Pathway and Yes-Associated Protein 1 (YAP1). Int. J. Mol. Sci. 2021, 22, 8718. [Google Scholar] [CrossRef] [PubMed]
- Gharaibeh, L.F.; Elmadany, N.; Alwosaibai, K.; Alshaer, W. Notch1 in Cancer Therapy: Possible Clinical Implications and Challenges. Mol. Pharmacol. 2020, 98, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Giuli, M.V.; Giuliani, E.; Screpanti, I.; Bellavia, D.; Checquolo, S. Notch Signaling Activation as a Hallmark for Triple-Negative Breast Cancer Subtype. J. Oncol. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Baker, A.; Wyatt, D.; Bocchetta, M.; Li, J.; Filipovic, A.; Green, A.; Peiffer, D.S.; Fuqua, S.; Miele, L.; Albain, K.S.; et al. Notch-1-PTEN-ERK1/2 signaling axis promotes HER2+ breast cancer cell proliferation and stem cell survival. Oncogene 2018, 37, 4489–4504. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, J. Breast cancer stem cells: Features, key drivers and treatment options. Semin. Cancer Biol. 2018, 53, 59–74. [Google Scholar] [CrossRef]
- Wang, D.; Xu, J.; Liu, B.; He, X.; Zhou, L.; Hu, X.; Qiao, F.; Zhang, A.; Xu, X.; Zhang, H.; et al. IL6 blockade potentiates the anti-tumor effects of gamma-secretase inhibitors in Notch3-expressing breast cancer. Cell Death Differ. 2018, 25, 330–339. [Google Scholar] [CrossRef]
- Yue, Z.; Yuan, Z.; Zeng, L.; Wang, Y.; Lai, L.; Li, J.; Sun, P.; Xue, X.; Qi, J.; Yang, Z.; et al. LGR4 modulates breast cancer initiation, metastasis, and cancer stem cells. FASEB J. 2017, 32, 2422–2437. [Google Scholar] [CrossRef]
- Satriyo, P.B.; Bamodu, O.A.; Chen, J.-H.; Aryandono, T.; Haryana, S.M.; Yeh, C.-T.; Chao, T.-Y. Cadherin 11 Inhibition Downregulates β-catenin, Deactivates the Canonical WNT Signalling Pathway and Suppresses the Cancer Stem Cell-Like Phenotype of Triple Negative Breast Cancer. J. Clin. Med. 2019, 8, 148. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lo, M.-C.; Moody, R.; Jiang, H.; Harouaka, R.; Stevers, N.; Tinsley, S.; Gasparyan, M.; Wicha, M.; Sun, D. Targeting LRP8 inhibits breast cancer stem cells in triple-negative breast cancer. Cancer Lett. 2018, 438, 165–173. [Google Scholar] [CrossRef]
- Tang, W.; Li, M.; Qi, X.; Li, J. Beta1,4-Galactosyltransferase V Modulates Breast Cancer Stem Cells through Wnt/beta-catenin Signaling Pathway. Cancer Res. Treat. 2020, 52, 1084–1102. [Google Scholar]
- Qin, T.; Li, B.; Feng, X.; Fan, S.; Liu, L.; Liu, D.; Mao, J.; Lu, Y.; Yang, J.; Yu, X.; et al. Abnormally elevated USP37 expression in breast cancer stem cells regulates stemness, epithelial-mesenchymal transition and cisplatin sensitivity. J. Exp. Clin. Cancer Res. 2018, 37, 1–18. [Google Scholar] [CrossRef]
- Wellner, U.; Schubert, J.; Burk, U.C.; Schmalhofer, O.; Zhu, F.; Sonntag, A.; Waldvogel, B.; Vannier, C.; Darling, D.; zur Hausen, A.; et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 2009, 11, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.S.; Miele, L. Breast Cancer Stem Cells. Biomedicines 2018, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Elaimy, A.L.; Guru, S.; Chang, C.; Ou, J.; Amante, J.J.; Zhu, L.J.; Goel, H.L.; Mercurio, A.M. VEGF-neuropilin-2 signaling promotes stem-like traits in breast cancer cells by TAZ-mediated repression of the Rac GAP beta2-chimaerin. Sci. Signal 2018, 11, eaao6897. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Li, P.; Jiang, Y.; Chen, H.; Wang, R.; Cao, F.; Liu, P. DLG5 suppresses breast cancer stem cell-like characteristics to restore tamoxifen sensitivity by inhibiting TAZ expression. J. Cell. Mol. Med. 2018, 23, 512–521. [Google Scholar] [CrossRef]
- Zhao, D.; Zhi, X.; Zhou, Z.; Chen, C. TAZ antagonizes the WWP1-mediated KLF5 degradation and promotes breast cell proliferation and tumorigenesis. Carcinogenesis 2011, 33, 59–67. [Google Scholar] [CrossRef]
- Liu, W.; Lu, X.; Shi, P.; Yang, G.; Zhou, Z.; Li, W.; Mao, X.; Jiang, D.; Chen, C. TNF-alpha increases breast cancer stem-like cells through up-regulating TAZ expression via the non-canonical NF-kappaB pathway. Sci. Rep. 2020, 10, 1804. [Google Scholar] [CrossRef]
- Zhi, X.; Zhao, D.; Zhou, Z.; Liu, R.; Chen, C. YAP Promotes Breast Cell Proliferation and Survival Partially through Stabilizing the KLF5 Transcription Factor. Am. J. Pathol. 2012, 180, 2452–2461. [Google Scholar] [CrossRef]
- Zheng, L.; Xiang, C.; Li, X.; Guo, Q.; Gao, L.; Ni, H.; Xia, Y.; Xi, T. STARD13-correlated ceRNA network-directed inhibition on YAP/TAZ activity suppresses stemness of breast cancer via co-regulating Hippo and Rho-GTPase/F-actin signaling. J. Hematol. Oncol. 2018, 11, 1–18. [Google Scholar] [CrossRef]
- Quinn, H.M.; Vogel, R.; Popp, O.; Mertins, P.; Lan, L.; Messerschmidt, C.; Landshammer, A.; Lisek, K.; Château-Joubert, S.; Marangoni, E.; et al. YAP and beta-Catenin Cooperate to Drive Oncogenesis in Basal Breast Cancer. Cancer Res. 2021, 81, 2116–2127. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-W.; Lin, R.-J.; Yu, J.; Fu, C.-H.; Lai, A.C.-Y.; Yu, J.-C.; Yu, A.L. The expression and significance of insulin-like growth factor-1 receptor and its pathway on breast cancer stem/progenitors. Breast Cancer Res. 2013, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Chen, W.-B.; Zhang, X.-Y.; Kang, X.-N.; Jin, L.-J.; Zhang, H.; Wang, Z.-Y. HIF-2α regulates CD44 to promote cancer stem cell activation in triple-negative breast cancer via PI3K/AKT/mTOR signaling. World J. Stem Cells 2020, 12, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, W.; Phillips, J.B.; Arora, R.; McClellan, S.; Li, J.; Kim, J.-H.; Sobol, R.W.; Tan, M. Immunoregulatory protein B7-H3 regulates cancer stem cell enrichment and drug resistance through MVP-mediated MEK activation. Oncogene 2018, 38, 88–102. [Google Scholar] [CrossRef]
- Wang, X.; Reyes, M.E.; Zhang, D.; Funakoshi, Y.; Trape, A.P.; Gong, Y.; Kogawa, T.; Eckhardt, B.L.; Masuda, H.; Jr, D.A.P.; et al. EGFR signaling promotes inflammation and cancer stem-like activity in inflammatory breast cancer. Oncotarget 2017, 8, 67904–67917. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fahrmann, J.F.; Lee, H.; Li, Y.J.; Tripathi, S.C.; Yue, C.; Zhang, C.; Lifshitz, V.; Song, J.; Yuan, Y.; et al. JAK/STAT3-Regulated Fatty Acid beta-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018, 27, 1357. [Google Scholar] [CrossRef]
- Chang, R.; Song, L.; Xu, Y.; Wu, Y.; Dai, C.; Wang, X.; Sun, X.; Hou, Y.; Li, W.; Zhan, X.; et al. Loss of Wwox drives metastasis in triple-negative breast cancer by JAK2/STAT3 axis. Nat Commun. 2018, 9, 3486. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Chen, W.; Yang, R.; Zhou, Z.; Chang, N.; Chen, C.; Zou, T.; Liu, R.; Tan, J.; Ren, G. WWOX suppresses KLF5 expression and breast cancer cell growth. Chin. J. Cancer Res. 2014, 26, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, Z.; Yu, Z. EGFR Promotes the Development of Triple Negative Breast Cancer Through JAK/STAT3 Signaling. Cancer Manag. Res. 2020, 12, 703–717. [Google Scholar] [CrossRef]
- Woosley, A.N.; Dalton, A.C.; Hussey, G.S.; Howley, B.V.; Mohanty, B.K.; Grelet, S.; Dincman, T.; Bloos, S.; Olsen, S.K.; Howe, P.H. TGFbeta promotes breast cancer stem cell self-renewal through an ILEI/LIFR signaling axis. Oncogene 2019, 38, 3794–3811. [Google Scholar] [CrossRef]
- Tian, J.; Hachim, M.Y.; Hachim, I.Y.; Dai, M.; Lo, C.; Raffa, F.A.; Ali, S.; Lebrun, J.J. Cyclooxygenase-2 regulates TGFbeta-induced cancer stemness in triple-negative breast cancer. Sci. Rep. 2017, 7, 40258. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Santillan, K.; Melendez-Zajgla, J.; Jimenez-Hernandez, L.E.; Gaytan-Cervantes, J.; Muñoz-Galindo, L.; Piña-Sanchez, P.; Martinez-Ruiz, G.; Torres, J.; Garcia-Lopez, P.; Gonzalez-Torres, C.; et al. NF-kappaBeta-inducing kinase regulates stem cell phenotype in breast cancer. Sci. Rep. 2016, 6, 37340. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lee, J.S.; Jie, C.; Park, M.H.; Iwakura, Y.; Patel, Y.; Soni, M.; Reisman, D.; Chen, H. HER2 Overexpression Triggers an IL1α Proinflammatory Circuit to Drive Tumorigenesis and Promote Chemotherapy Resistance. Cancer Res. 2018, 78, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Torres, S.J.; Benight, N.M.; Karns, R.A.; Lower, E.E.; Guan, J.L.; Waltz, S.E. HGFL-mediated RON signaling supports breast cancer stem cell phenotypes via activation of non-canonical beta-catenin signaling. Oncotarget 2017, 8, 58918–58933. [Google Scholar] [CrossRef][Green Version]
- Hinohara, K.; Kobayashi, S.; Kanauchi, H.; Shimizu, S.; Nishioka, K.; Tsuji, E.; Tada, K.; Umezawa, K.; Mori, M.; Ogawa, T.; et al. ErbB receptor tyrosine kinase/NF-kappaB signaling controls mammosphere formation in human breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 6584–6589. [Google Scholar] [CrossRef]
- Wei, L.; Liu, T.T.; Wang, H.H.; Hong, H.M.; Yu, A.L.; Feng, H.P.; Chang, W.W. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-kappaB. Breast Cancer Res. 2011, 13, R101. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Lian, C.; Cao, X.; Wang, Y.; Li, X.; Cong, M.; Tian, P.; Zhang, X.; Wei, G.; et al. SH3RF3 promotes breast cancer stem-like properties via JNK activation and PTX3 upregulation. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Barker, A.D.; Sigman, C.C.; Kelloff, G.J.; Hylton, N.M.; A Berry, D.; Esserman, L.J. I-SPY 2: An Adaptive Breast Cancer Trial Design in the Setting of Neoadjuvant Chemotherapy. Clin. Pharmacol. Ther. 2009, 86, 97–100. [Google Scholar] [CrossRef]
- Wang, H.; Yee, D. I-SPY 2: A Neoadjuvant Adaptive Clinical Trial Designed to Improve Outcomes in High-Risk Breast Cancer. Curr. Breast Cancer Rep. 2019, 11, 303–310. [Google Scholar] [CrossRef]
| Names | Mechanism | References |
|---|---|---|
| miRNA-200c | inhibits the expression of Pin1, BMI1 and Suz12. | [42] |
| Let-7 family | inhibits the Wnt signaling pathway. | [43] |
| miR-34 family | targets Notch1. | [47] |
| miRNA-146a | promotes the asymmetric division of BCSCs. | [48] |
| miRNA-760 | inhibits the expression of NANOG. | [49] |
| miR-422a | reduces the expression of Proteolipid Protein 2 (PLP2). | [50] |
| miRNA-142-3p | targets β-catenin. | [51] |
| miRNA-1 | targets ecotropic virus integration site 1 (EVI1). | [52] |
| miRNA-128-3p | downregulates NIMA related kinase 2 (NEK2) to inhibit the Wnt signaling pathway. | [53] |
| miRNA-638 | reduces the expression of E2F2. | [54] |
| miR-376c-3p | reduces the expression of RAB2A. | [55] |
| miRNA-221/222 | inhibits PTEN expression. | [44] |
| miR-20b-5p | bidirectionally regulates cyclin D1 and E2F1. | [46] |
| miR-335 | inhibits cadherin 11 (CDH11), β-catenin, and vimentin. | [56] |
| miR-153 | downregulates hypoxia-inducible factor 1 subunit alpha (HIF1ɑ) and KLF5. | [57,58] |
| miR-145 | suppresses BCSCs growth by inhibiting KLF4. | [59] |
| miR-375 | decreases BCSCs by interrupting the JAK2-STAT3 pathway. | [60] |
| Names | Mechanism | References |
|---|---|---|
| HOTAIR | regulates miR-34a to upregulate the expression of SOX2 in BCSCs. | [61] |
| SOX21-AS1 | inhibits the Hippo signaling pathway. | [62] |
| CCAT1 | enhances the expression of T-cell factor 4 (TCF4) to activate the Wnt signaling pathway. | [64] |
| H19 | forms a two-way negative feedback loop with miRNA let-7 and LIN28. | [65] |
| SPRY4-IT1 | sponges miR-6882-3p. | [66] |
| LINC00511 | regulates the miR-185-3p/E2F1/NANOG axis. | [67] |
| HOTTIP | acts as an miR-148a-3p sponge and regulates Wnt 1. | [68] |
| LUCAT1 | competitively binds to miR-5582-3p and transcription factor 7 like 2 (TCF7L2) to enhance the Wnt/β-catenin pathway. | [69] |
| FEZF1-AS1 | regulates the miR-30a/NANOG signal pathway. | [70] |
| Lnc408 | recruit specificity protein 3 (Sp3) to inhibit chibby family member 1 (CBY1) and β-catenin expression. | [71] |
| CCAT2 | upregulates OCT4-PG1 and the miR-205-Notch1 pathway. | [72] |
| Hh | stimulates hedgehog signaling. | [73] |
| Lnc030 | interacts with poly (RC) binding protein 2 (PCBP2) to stabilize squalene epoxidase (SQLE) and activate the PI3K/Akt signaling pathway. | [74] |
| MALAT1 | positively regulates SOX2. | [75] |
| ROPM | maintains group XVI phospholipase A2 (PLA2G16) to facilitate lipid metabolism, thereby activating the Wnt/β-catenin pathway. | [76] |
| FGF13-AS1 | regulates the IGF2BPs/Myc feedback loop. | [63] |
| Names | Hallmarks | References |
|---|---|---|
| OCT4 | OCT4 promotes sphere formation of BCSCs in vitro, while inhibition of OCT4 induces apoptosis, reduces BCSC characteristics, and inhibits tumor growth. | [77,78,79] |
| KLF4 | KDM7A and DYRK2 increase BCSCs by upregulating KLF4 expression, and miR-7 inhibits KLF4 to inhibit BCSCs self-renewal and invasion. | [83,84,85] |
| KLF5 | Mifepristone and metformin inhibit KLF5 and BCSC. PRMT5 increases stemness of BCSC by stabilizing KLF5. | [37,58,87] |
| C-MYC | Caveoli-1 inhibits C-MYC-mediated BSCS metabolic reprogramming and p62 stabilizes C-MYC to enhance BCSC properties. | [92,93] |
| SOX2 | Knockdown of SOX2 attenuates stemness of BCSC. TRPS1 and FOXO3a inhibit SOX2 the expression and tumorigenesis of BCSCs. | [94,95,96] |
| SOX9 | Knockdown of SOX9 significantly inhibits the tumorigenicity of MDA-MB-231 cells. | [97] |
| SLUG | Hes family BHLH transcription factor 1 (HES1) increases SLUG transcription and BCSC stemness. Interestingly, the Notch4/SLUG/Gas1 axis maintains mesenchymal-like BCSCs. | [98,99] |
| SNAIL | Uncoupling Protein 1 (UCP1)-mediated fructose-bisphosphatase 1 (FBP1) expression promotes BCSC properties, which can be reversed by SNAIL. Interferon beta (IFN-β restrains SNAIL-induced tumor initiation. | [100,101] |
| β-catenin | β-catenin facilitates BCSC properties through CCL2-mediated macrophage polarization and infiltration. CCL16 and mortalin maintain the stemness of BCSCs by promoting the translocation of β-catenin. | [102,103,104] |
| GLI1 | Tripartite motif 16 (TRIM16) inhibits BCSCs partially via Glioma-related homologue 1 (GLI1). In contrast, estrogen promotes BCSCs by activating GLI1. | [105,106] |
| p65 | p65 is important for BCSC survival. | [107] |
| ERα | Reduction in expression of ER inhibits CSC tumor-seeding efficiency. | [108] |
| FOXO3a | FOXO3a inhibits the characteristics and tumorigenesis of BCSCs by negatively regulating SOX2. | [96] |
| TAZ | Overexpression of transcription activator with PDZ-binding motif (TAZ) in BCSCs enhances tumorigenicity and cell migration. The ability of HIF1 and Crumbs homolog 3 (CRB3) to maintain or induce BCSC properties is partially achieved by activating TAZ. | [109,110,111] |
| YAP | Downregulation of YAP1 has a negative effect on BCSC tumorigenicity and stemness markers. | [112] |
| Related Signaling Pathway Factors | Mechanism | References | |
|---|---|---|---|
| NOTCH | NOTCH-1 | inhibits PTEN and activates ERK1/2 to maintain BCSCs. | [115] |
| NOTCH-2 | promotes BCSC survival. | [116] | |
| NOTCH-3 | inhibits BCSC self-renewal by IL6/STAT3. | [117] | |
| NOTCH-4 | promotes mammosphere formation. | [114] | |
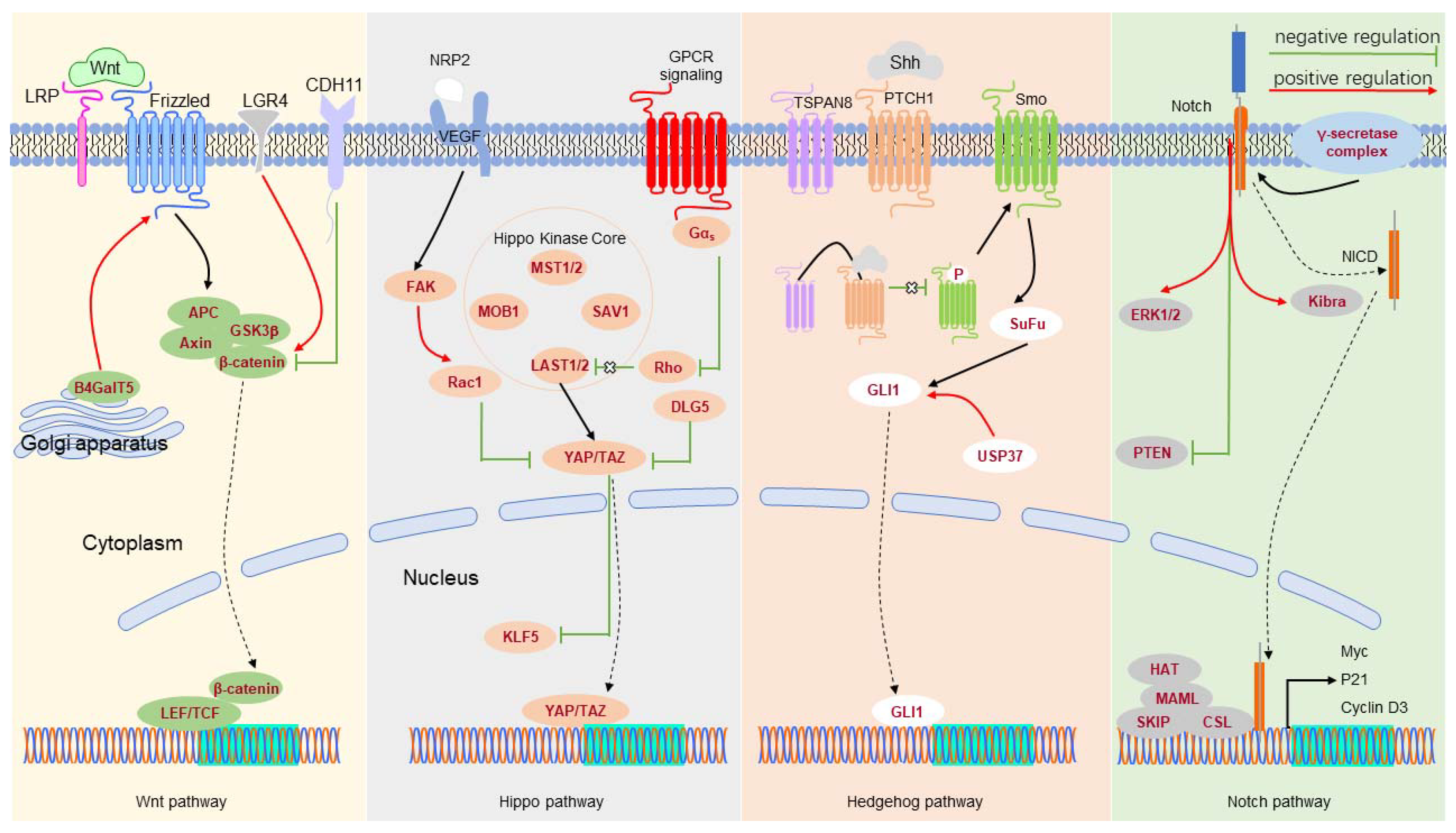
| WNT | LGR4 | promotes BCSCs. | [118] |
| CDH11 | inhibits TNBC cell stemness. | [119] | |
| LRP8 | decreases the percentage of BCSCs. | [120] | |
| B4GalT5 | maintains BCSCs by stabilizing Frizzled. | [121] | |
| HH | TSPAN8 | interacts with the SHH-PTCH1 complex to promote stemness of breast cancer. | [14] |
| USP37 | increases Smo and GLI1 expression to enhance BCSC characteristics. | [122] | |
| HIPPO | VEGF/NRP2 | activates TAZ to enhance BCSC sphere-forming ability. | [125] |
| DLG5 | enhances TAZ activity to maintain BCSC self-renewal. | [126] | |
| TNF-ɑ | induces TAZ expression to increase BCSC stemness. | [127,128] | |
| STARD13 | reduces YAP/YAZ activity, thereby inhibiting the formation of BCSCs. | [130] | |
| RTK | IGF-1R | maintains BCSCs by activating the PI3K/Akt/mTOR pathway. | [132] |
| HIF-2ɑ | inhibits BCSCs by inhibiting the PI3K/Akt/mTOR pathway. | [133] | |
| B7-H3 | activates MEK and increases BCSC proportions. | [134] | |
| SGCE | stabilizes EGFR levels, thereby fostering breast cell stemness. | [16] | |
| JAK/STAT3 | WWOX | hinders STAT3 activation to block breast cancer cell proliferation and metastasis. | [137] |
| EGFR | promotes STAT3 phosphorylation to facilitate tumor cell proliferation and invasion. | [139] | |
| TGF-β | LIFR | drives the formation of BCSCs. | [140] |
| Fibronectin, COX2 | enhances the self-renewal capacity of BCSCs. | [141] | |
| NF-κB | IL-1α | maintains BCSCs. | [143] |
| HGFL-RON | supports the self-renewal capacity of BCSCs. | [144] | |
| HRG | stimulates mammosphere formation. | [145] | |
| Hsp27 | degrades IκBα to maintain BCSCs. | [146] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhang, L.; Liu, S.; Chen, C. Advances in Biomarkers and Endogenous Regulation of Breast Cancer Stem Cells. Cells 2022, 11, 2941. https://doi.org/10.3390/cells11192941
Chen W, Zhang L, Liu S, Chen C. Advances in Biomarkers and Endogenous Regulation of Breast Cancer Stem Cells. Cells. 2022; 11(19):2941. https://doi.org/10.3390/cells11192941
Chicago/Turabian StyleChen, Wenmin, Lu Zhang, Suling Liu, and Ceshi Chen. 2022. "Advances in Biomarkers and Endogenous Regulation of Breast Cancer Stem Cells" Cells 11, no. 19: 2941. https://doi.org/10.3390/cells11192941
APA StyleChen, W., Zhang, L., Liu, S., & Chen, C. (2022). Advances in Biomarkers and Endogenous Regulation of Breast Cancer Stem Cells. Cells, 11(19), 2941. https://doi.org/10.3390/cells11192941







