Enteric Neural Network Assembly Was Promoted by Basic Fibroblast Growth Factor and Vitamin A but Inhibited by Epidermal Growth Factor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Harvest of Intestine in Fetal Mice
2.3. Culture of Enteric Neurospheres
2.4. Enumeration of Enteric Neurosphere Formation
2.5. Flow Cytometric Analyses for Neurons and ENSCs [21]
2.6. Immunofluorescence Staining for Enteric Neurons and Gliocytes
2.7. Neural Network Assembly from Enteric Neurospheres
2.8. Statistical Analyses
3. Results
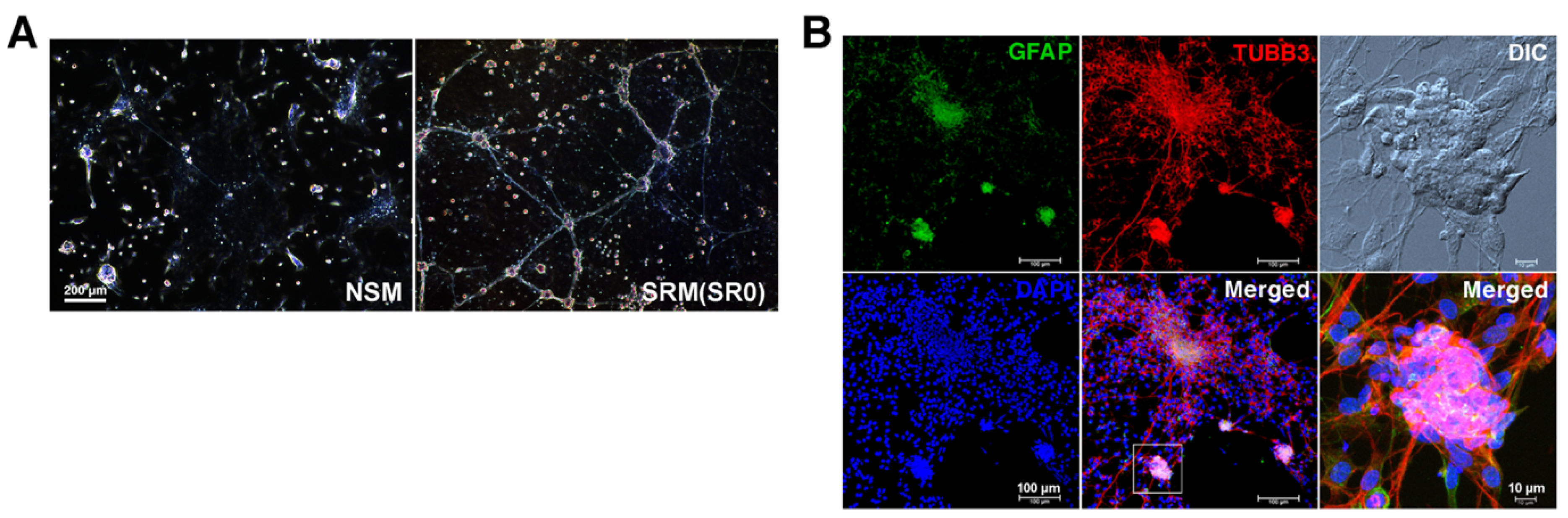
3.1. Formation of Enteric Neurospheres in NSM and SRM
3.2. Assessing Component Effects on Enteric Neurospherogenesis in NSM
3.3. Assessing CEE Effects on Neural Network Assembly in SRM
3.4. Assessing Component Effects on Neural Network Assembly in CEE-Free SRM
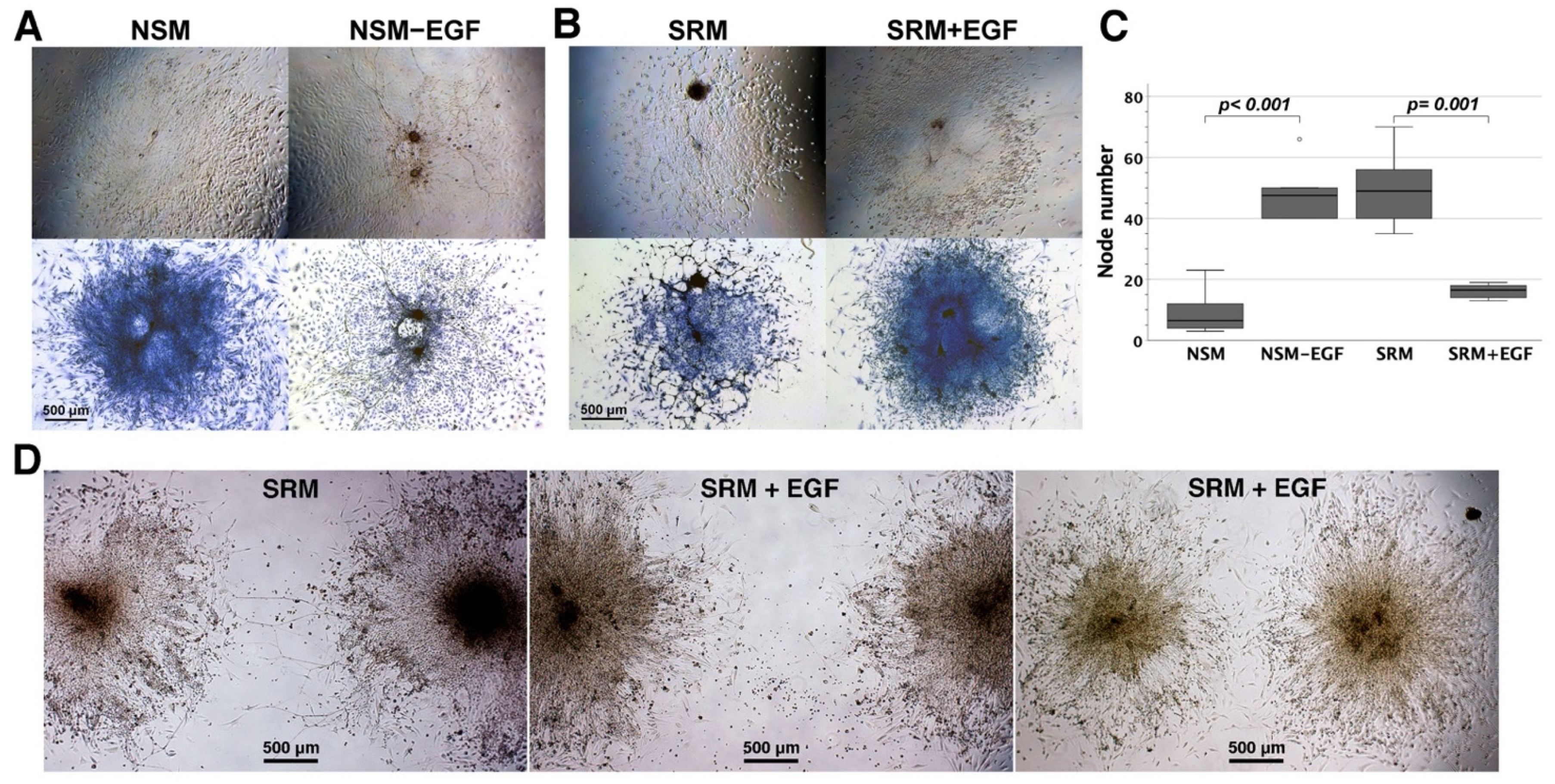
3.5. The Influence of EGF on Neural Network Assembly
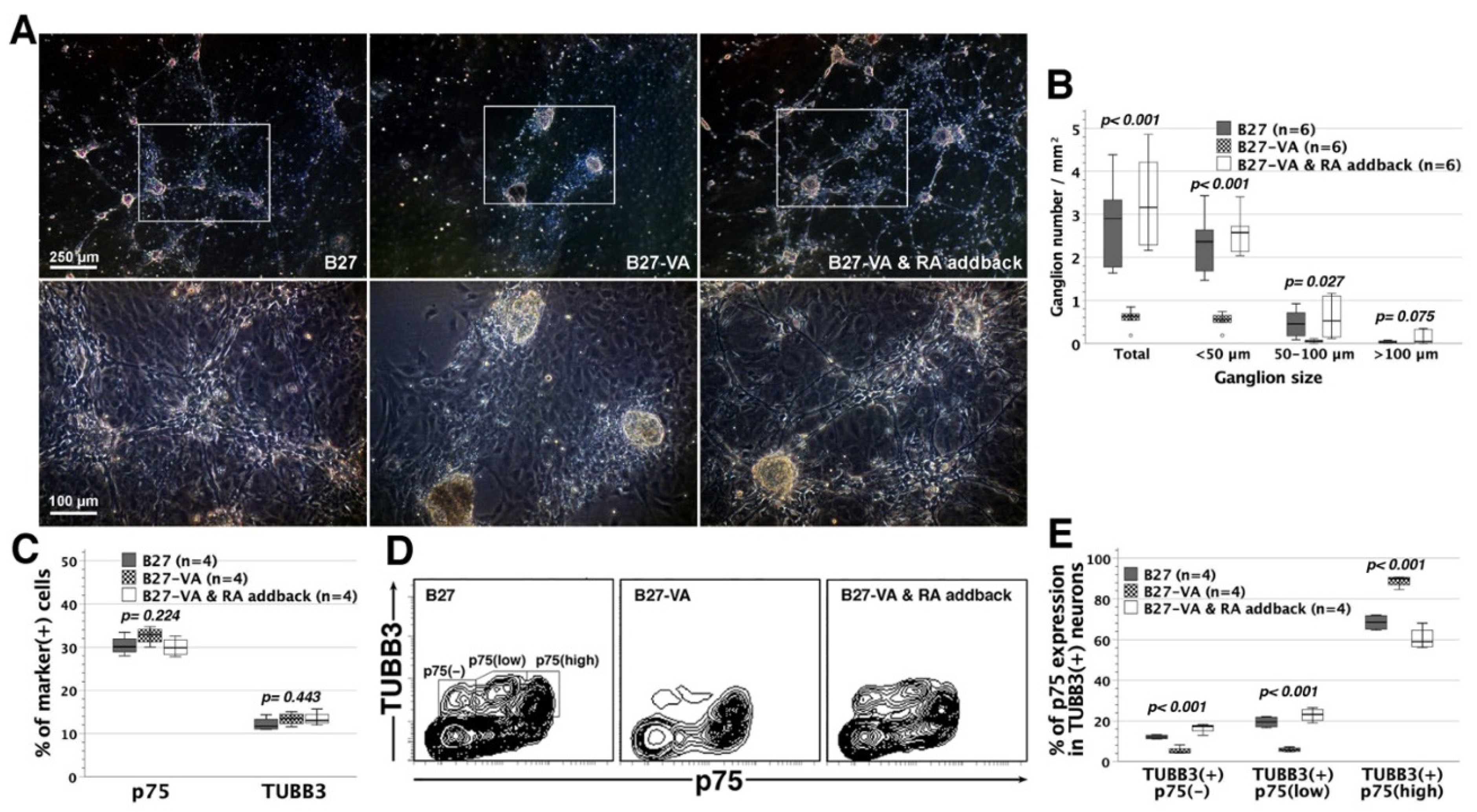
3.6. Reappraisal of RA Effects on Neural Network Assembly
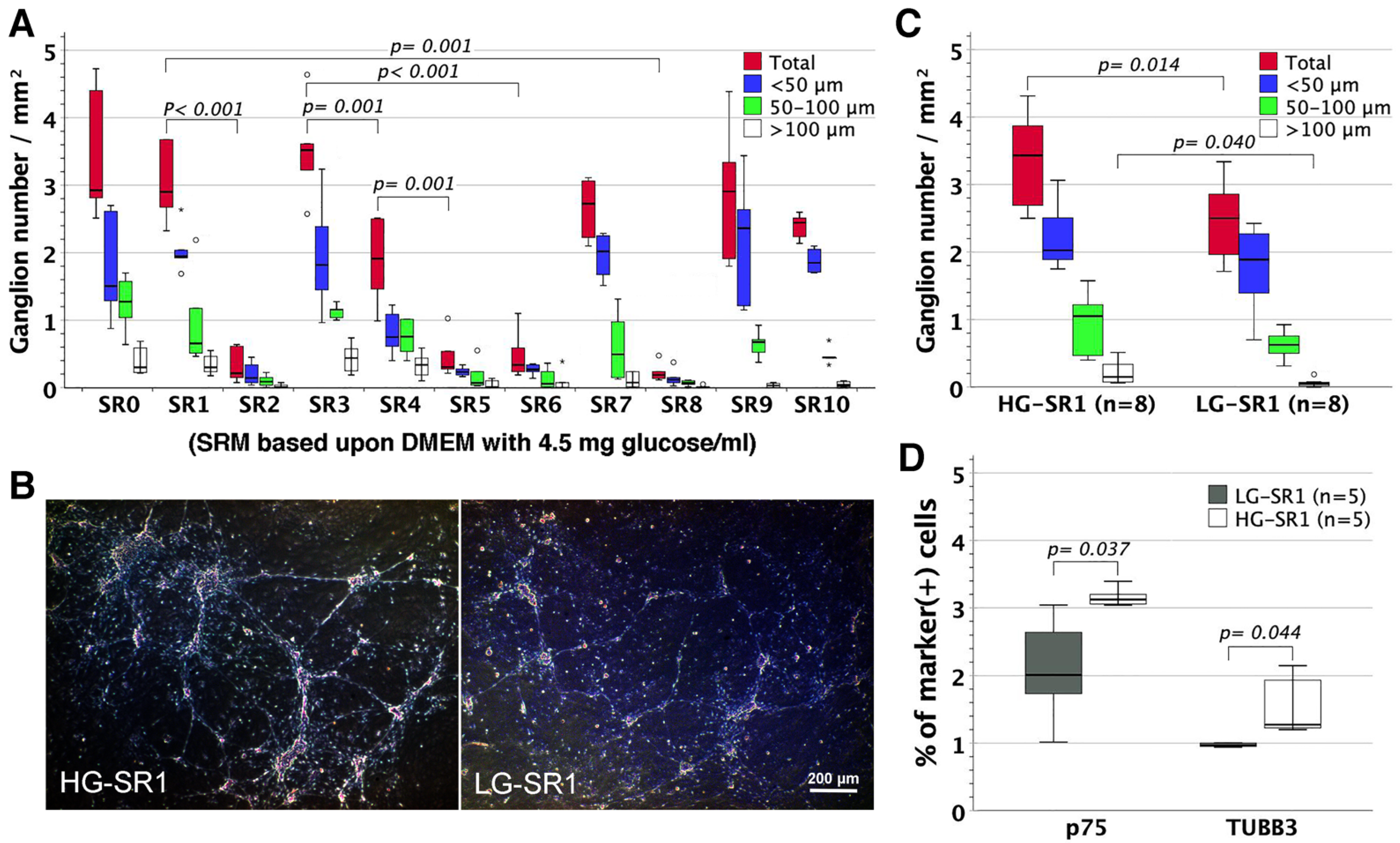
3.7. The Influence of Glucose Concentration on Neural Network Assembly
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, J.E.; McCann, C.J.; Natarajan, D.; Choudhury, S.; Boesmans, W.; Delalande, J.M.; Vanden Berghe, P.; Burns, A.J.; Thapar, N. In Vivo Transplantation of Enteric Neural Crest Cells into Mouse Gut; Engraftment, Functional Integration and Long-Term Safety. PLoS ONE 2016, 11, e0147989. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.M.; He, S.; Quintana, E.; Kim, Y.G.; Nunez, G.; Morrison, S.J. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J. Clin. Investig. 2011, 121, 3398–3411. [Google Scholar] [CrossRef] [PubMed]
- Pfaltzgraff, E.R.; Mundell, N.A.; Labosky, P.A. Isolation and culture of neural crest cells from embryonic murine neural tube. J. Vis. Exp. 2012, 64, e4134. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Pin, J.P.; Sebben, M.; Kemp, D.E.; Sladeczek, F.; Gabrion, J.; Bockaert, J. Synaptogenesis of cultured striatal neurons in serum-free medium: A morphological and biochemical study. Proc. Natl. Acad. Sci. USA 1986, 83, 2238–2242. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Tetzlaff, W.; Weiss, S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 1992, 12, 4565–4574. [Google Scholar] [CrossRef]
- Gritti, A.; Parati, E.A.; Cova, L.; Frolichsthal, P.; Galli, R.; Wanke, E.; Faravelli, L.; Morassutti, D.J.; Roisen, F.; Nickel, D.D.; et al. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J. Neurosci. 1996, 16, 1091–1100. [Google Scholar] [CrossRef]
- Svendsen, C.N.; ter Borg, M.G.; Armstrong, R.J.; Rosser, A.E.; Chandran, S.; Ostenfeld, T.; Caldwell, M.A. A new method for the rapid and long term growth of human neural precursor cells. J. Neurosci. Methods 1998, 85, 141–152. [Google Scholar] [CrossRef]
- Metzger, M.; Conrad, S.; Skutella, T.; Just, L. RGMa inhibits neurite outgrowth of neuronal progenitors from murine enteric nervous system via the neogenin receptor in vitro. J. Neurochem. 2007, 103, 2665–2678. [Google Scholar] [CrossRef]
- Metzger, M.; Bareiss, P.M.; Danker, T.; Wagner, S.; Hennenlotter, J.; Guenther, E.; Obermayr, F.; Stenzl, A.; Koenigsrainer, A.; Skutella, T.; et al. Expansion and differentiation of neural progenitors derived from the human adult enteric nervous system. Gastroenterology 2009, 137, 2063–2073. [Google Scholar] [CrossRef]
- Metzger, M.; Caldwell, C.; Barlow, A.J.; Burns, A.J.; Thapar, N. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology 2009, 136, 2214–2225. [Google Scholar] [CrossRef] [PubMed]
- Hotta, R.; Stamp, L.A.; Foong, J.P.; McConnell, S.N.; Bergner, A.J.; Anderson, R.B.; Enomoto, H.; Newgreen, D.F.; Obermayr, F.; Furness, J.B.; et al. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J. Clin. Investig. 2013, 123, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.J.; Mohsenipour, M.; Bergner, A.J.; Young, H.M.; Stamp, L.A. Exposure to GDNF Enhances the Ability of Enteric Neural Progenitors to Generate an Enteric Nervous System. Stem Cell Rep. 2017, 8, 476–488. [Google Scholar] [CrossRef]
- Binder, E.; Natarajan, D.; Cooper, J.; Kronfli, R.; Cananzi, M.; Delalande, J.M.; McCann, C.; Burns, A.J.; Thapar, N. Enteric neurospheres are not specific to neural crest cultures: Implications for neural stem cell therapies. PLoS ONE 2015, 10, e0119467. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; White, P.M.; Zock, C.; Anderson, D.J. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 1999, 96, 737–749. [Google Scholar] [CrossRef]
- Bixby, S.; Kruger, G.M.; Mosher, J.T.; Joseph, N.M.; Morrison, S.J. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron 2002, 35, 643–656. [Google Scholar] [CrossRef]
- Bondurand, N.; Natarajan, D.; Thapar, N.; Atkins, C.; Pachnis, V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development 2003, 130, 6387–6400. [Google Scholar] [CrossRef]
- Kruger, G.M.; Mosher, J.T.; Bixby, S.; Joseph, N.; Iwashita, T.; Morrison, S.J. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron 2002, 35, 657–669. [Google Scholar] [CrossRef]
- Iwashita, T.; Kruger, G.M.; Pardal, R.; Kiel, M.J.; Morrison, S.J. Hirschsprung disease is linked to defects in neural crest stem cell function. Science 2003, 301, 972–976. [Google Scholar] [CrossRef]
- Heanue, T.A.; Pachnis, V. Enteric nervous system development and Hirschsprung’s disease: Advances in genetic and stem cell studies. Nat. Rev. Neurosci. 2007, 8, 466–479. [Google Scholar] [CrossRef]
- Menon, V.; Thomas, R.; Ghale, A.R.; Reinhard, C.; Pruszak, J. Flow cytometry protocols for surface and intracellular antigen analyses of neural cell types. J. Vis. Exp. 2014, 94, e52241. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.H.; Qin, C.H.; Xu, W.Y.; Pan, W.K.; Zhao, Y.Y.; Zheng, B.J.; Chen, X.L.; Liu, Y.; Gao, Y.; Yu, H. Phenotypic and functional comparison of rat enteric neural crest-derived cells during fetal and early-postnatal stages. Neural Regen Res. 2021, 16, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Torricelli, J.R.; Evege, E.K.; Price, P.J. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci Res. 1993, 35, 567–576. [Google Scholar] [CrossRef]
- Kam, R.K.; Deng, Y.; Chen, Y.; Zhao, H. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci. 2012, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Young, H.M.; Ciampoli, D.; Hsuan, J.; Canty, A.J. Expression of Ret-, p75(NTR)-, Phox2a-, Phox2b-, and tyrosine hydroxylase-immunoreactivity by undifferentiated neural crest-derived cells and different classes of enteric neurons in the embryonic mouse gut. Dev. Dyn. 1999, 216, 137–152. [Google Scholar] [CrossRef]
- Burns, A.J.; Thapar, N. Neural stem cell therapies for enteric nervous system disorders. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Romijn, H.J. Development and advantages of serum-free, chemically defined nutrient media for culturing of nerve tissue. Biol. Cell 1988, 63, 263–268. [Google Scholar] [CrossRef]
- Romijn, H.J.; van Huizen, F.; Wolters, P.S. Towards an improved serum-free, chemically defined medium for long-term culturing of cerebral cortex tissue. Neurosci. Biobehav. Rev. 1984, 8, 301–334. [Google Scholar] [CrossRef]
- Luduena, M.A. The growth of spinal ganglion neurons in serum-free medium. Dev. Biol. 1973, 33, 470–476. [Google Scholar] [CrossRef]
- Snyder, E.Y.; Kim, S.U. Hormonal requirements for neuronal survival in culture. Neurosci. Lett. 1979, 13, 225–230. [Google Scholar] [CrossRef]
- Honegger, P.; Lenoir, D.; Favrod, P. Growth and differentiation of aggregating fetal brain cells in a serum-free defined medium. Nature 1979, 282, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Bottenstein, J.E.; Sato, G.H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc. Natl. Acad. Sci. USA 1979, 76, 514–517. [Google Scholar] [CrossRef] [PubMed]
- McCann, C.J.; Cooper, J.E.; Natarajan, D.; Jevans, B.; Burnett, L.E.; Burns, A.J.; Thapar, N. Transplantation of enteric nervous system stem cells rescues nitric oxide synthase deficient mouse colon. Nat. Commun. 2017, 8, 15937. [Google Scholar] [CrossRef] [PubMed]
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef]
- Niederreither, K.; Vermot, J.; Le Roux, I.; Schuhbaur, B.; Chambon, P.; Dolle, P. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development 2003, 130, 2525–2534. [Google Scholar] [CrossRef]
- Frith, T.J.R.; Gogolou, A.; Hackland, J.O.S.; Hewitt, Z.A.; Moore, H.D.; Barbaric, I.; Thapar, N.; Burns, A.J.; Andrews, P.W.; Tsakiridis, A.; et al. Retinoic Acid Accelerates the Specification of Enteric Neural Progenitors from In-Vitro-Derived Neural Crest. Stem Cell Rep. 2020, 15, 557–565. [Google Scholar] [CrossRef]
- Fu, M.; Sato, Y.; Lyons-Warren, A.; Zhang, B.; Kane, M.A.; Napoli, J.L.; Heuckeroth, R.O. Vitamin A facilitates enteric nervous system precursor migration by reducing Pten accumulation. Development 2010, 137, 631–640. [Google Scholar] [CrossRef]
- Uribe, R.A.; Hong, S.S.; Bronner, M.E. Retinoic acid temporally orchestrates colonization of the gut by vagal neural crest cells. Dev. Biol. 2018, 433, 17–32. [Google Scholar] [CrossRef]
- Brewer, G.J.; Torricelli, J.R. Isolation and culture of adult neurons and neurospheres. Nat. Protoc. 2007, 2, 1490–1498. [Google Scholar] [CrossRef]
- Dmetrichuk, J.M.; Carlone, R.L.; Spencer, G.E. Retinoic acid induces neurite outgrowth and growth cone turning in invertebrate neurons. Dev. Biol. 2006, 294, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Clagett-Dame, M.; McNeill, E.M.; Muley, P.D. Role of all-trans retinoic acid in neurite outgrowth and axonal elongation. J. Neurobiol. 2006, 66, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Heuckeroth, R.O. Retinoic acid regulates murine enteric nervous system precursor proliferation, enhances neuronal precursor differentiation, and reduces neurite growth in vitro. Dev. Biol. 2008, 320, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.W.; Bargmann, C.I. Dynamic regulation of axon guidance. Nat. Neurosci. 2001, 4, 1169–1176. [Google Scholar] [CrossRef]
- Short, C.A.; Onesto, M.M.; Rempel, S.K.; Catlett, T.S.; Gomez, T.M. Familiar growth factors have diverse roles in neural network assembly. Curr. Opin. Neurobiol. 2021, 66, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Onesto, M.M.; Short, C.A.; Rempel, S.K.; Catlett, T.S.; Gomez, T.M. Growth Factors as Axon Guidance Molecules: Lessons From in vitro Studies. Front. Neurosci. 2021, 15, 678454. [Google Scholar] [CrossRef] [PubMed]
- Temple, S.; Qian, X. bFGF, neurotrophins, and the control or cortical neurogenesis. Neuron 1995, 15, 249–252. [Google Scholar] [CrossRef]
- Morrison, R.S.; Sharma, A.; de Vellis, J.; Bradshaw, R.A. Basic fibroblast growth factor supports the survival of cerebral cortical neurons in primary culture. Proc. Natl. Acad. Sci. USA 1986, 83, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Estevez, V.; Defterali, C.; Vicario-Abejon, C. IGF-I: A Key Growth Factor that Regulates Neurogenesis and Synaptogenesis from Embryonic to Adult Stages of the Brain. Front. Neurosci. 2016, 10, 52. [Google Scholar] [CrossRef]
- Nieto Guil, A.F.; Oksdath, M.; Weiss, L.A.; Grassi, D.J.; Sosa, L.J.; Nieto, M.; Quiroga, S. IGF-1 receptor regulates dynamic changes in neuronal polarity during cerebral cortical migration. Sci. Rep. 2017, 7, 7703. [Google Scholar] [CrossRef]
- Stemple, D.L.; Mahanthappa, N.K.; Anderson, D.J. Basic FGF induces neuronal differentiation, cell division, and NGF dependence in chromaffin cells: A sequence of events in sympathetic development. Neuron 1988, 1, 517–525. [Google Scholar] [CrossRef]
- Hagl, C.I.; Holland-Cunz, S.; Schafer, K.H. What do knockout models teach us about the enteric nervous system? Eur. J. Pediatr. Surg. 2003, 13, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.A.; Weiss, S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol. 1996, 175, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.S.; Kornblum, H.I.; Leslie, F.M.; Bradshaw, R.A. Trophic stimulation of cultured neurons from neonatal rat brain by epidermal growth factor. Science 1987, 238, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Garcez, R.C.; Teixeira, B.L.; Schmitt Sdos, S.; Alvarez-Silva, M.; Trentin, A.G. Epidermal growth factor (EGF) promotes the in vitro differentiation of neural crest cells to neurons and melanocytes. Cell. Mol. Neurobiol. 2009, 29, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Maklad, A.; Nicolai, J.R.; Bichsel, K.J.; Evenson, J.E.; Lee, T.C.; Threadgill, D.W.; Hansen, L.A. The EGFR is required for proper innervation to the skin. J. Investig. Dermatol. 2009, 129, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Szebenyi, G.; Dent, E.W.; Callaway, J.L.; Seys, C.; Lueth, H.; Kalil, K. Fibroblast growth factor-2 promotes axon branching of cortical neurons by influencing morphology and behavior of the primary growth cone. J. Neurosci. 2001, 21, 3932–3941. [Google Scholar] [CrossRef]
- Santiago-Medina, M.; Gregus, K.A.; Nichol, R.H.; O’Toole, S.M.; Gomez, T.M. Regulation of ECM degradation and axon guidance by growth cone invadosomes. Development 2015, 142, 486–496. [Google Scholar] [CrossRef]
- Liu, B.; Neufeld, A.H. Activation of epidermal growth factor receptors directs astrocytes to organize in a network surrounding axons in the developing rat optic nerve. Dev. Biol. 2004, 273, 297–307. [Google Scholar] [CrossRef]
- Rigby, M.J.; Gomez, T.M.; Puglielli, L. Glial Cell-Axonal Growth Cone Interactions in Neurodevelopment and Regeneration. Front. Neurosci. 2020, 14, 203. [Google Scholar] [CrossRef]
- Povlsen, G.K.; Berezin, V.; Bock, E. Neural cell adhesion molecule-180-mediated homophilic binding induces epidermal growth factor receptor (EGFR) down-regulation and uncouples the inhibitory function of EGFR in neurite outgrowth. J. Neurochem. 2008, 104, 624–639. [Google Scholar] [CrossRef]
- Garcia-Alonso, L.; Romani, S.; Jimenez, F. The EGF and FGF receptors mediate neuroglian function to control growth cone decisions during sensory axon guidance in Drosophila. Neuron 2000, 28, 741–752. [Google Scholar] [CrossRef]
- Romano, R.; Bucci, C. Role of EGFR in the Nervous System. Cells 2020, 9, 1887. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, F.; Zimmer, C. From cradle to grave: The multiple roles of fibroblast growth factors in neural development. Neuron 2011, 71, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Rafalski, V.A.; Brunet, A. Energy metabolism in adult neural stem cell fate. Prog. Neurobiol. 2011, 93, 182–203. [Google Scholar] [CrossRef]
- Suarez-Rodriguez, R.; Belkind-Gerson, J. Cultured nestin-positive cells from postnatal mouse small bowel differentiate ex vivo into neurons, glia, and smooth muscle. Stem Cells 2004, 22, 1373–1385. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-C.; Yang, W.; Tseng, L.-Y.; Chang, H.-L. Enteric Neural Network Assembly Was Promoted by Basic Fibroblast Growth Factor and Vitamin A but Inhibited by Epidermal Growth Factor. Cells 2022, 11, 2841. https://doi.org/10.3390/cells11182841
Chen J-C, Yang W, Tseng L-Y, Chang H-L. Enteric Neural Network Assembly Was Promoted by Basic Fibroblast Growth Factor and Vitamin A but Inhibited by Epidermal Growth Factor. Cells. 2022; 11(18):2841. https://doi.org/10.3390/cells11182841
Chicago/Turabian StyleChen, Jeng-Chang, Wendy Yang, Li-Yun Tseng, and Hsueh-Ling Chang. 2022. "Enteric Neural Network Assembly Was Promoted by Basic Fibroblast Growth Factor and Vitamin A but Inhibited by Epidermal Growth Factor" Cells 11, no. 18: 2841. https://doi.org/10.3390/cells11182841
APA StyleChen, J.-C., Yang, W., Tseng, L.-Y., & Chang, H.-L. (2022). Enteric Neural Network Assembly Was Promoted by Basic Fibroblast Growth Factor and Vitamin A but Inhibited by Epidermal Growth Factor. Cells, 11(18), 2841. https://doi.org/10.3390/cells11182841





