Cerebral Organoids as an Experimental Platform for Human Neurogenomics
Abstract
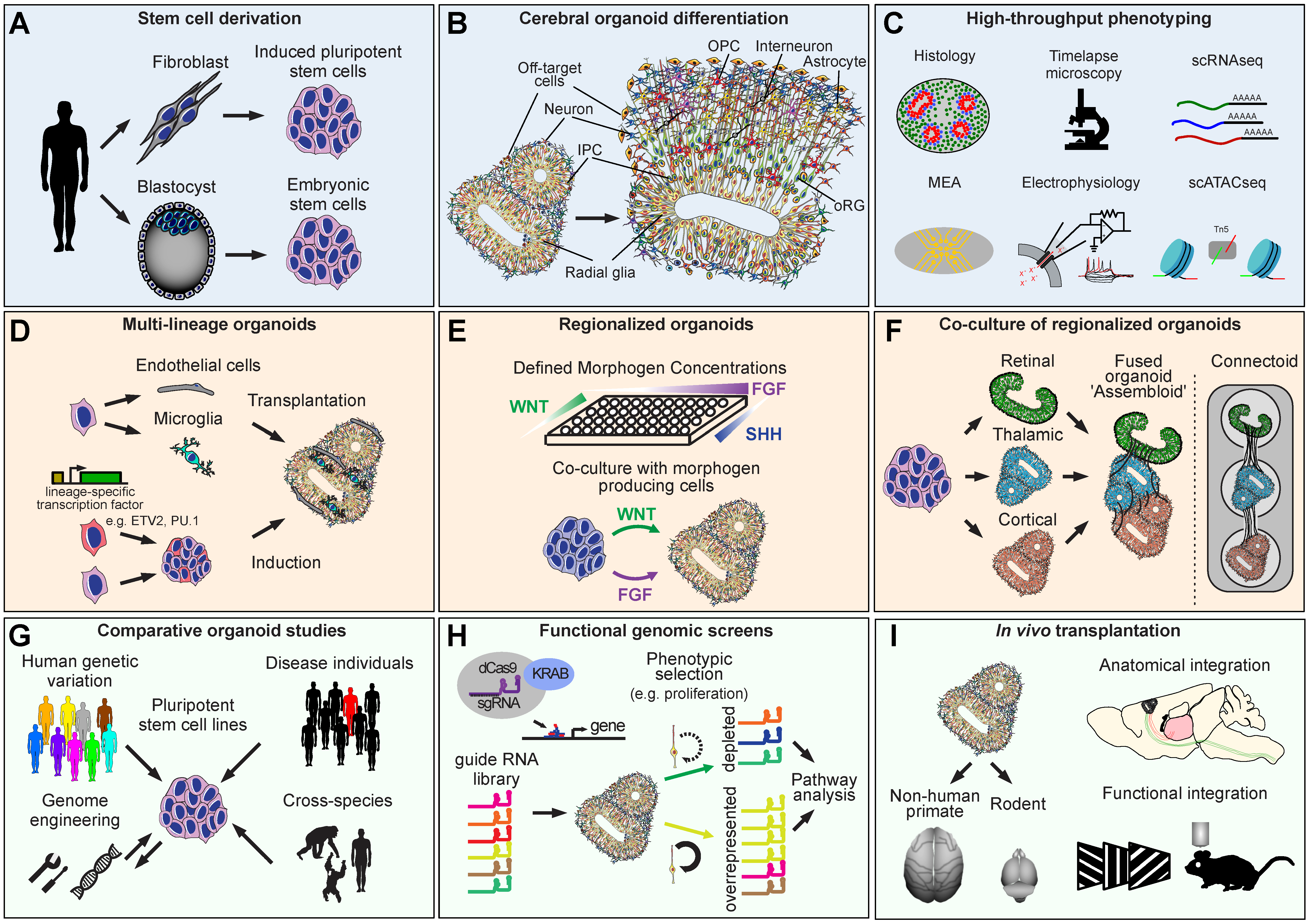
:1. Pluripotent Stem Cells
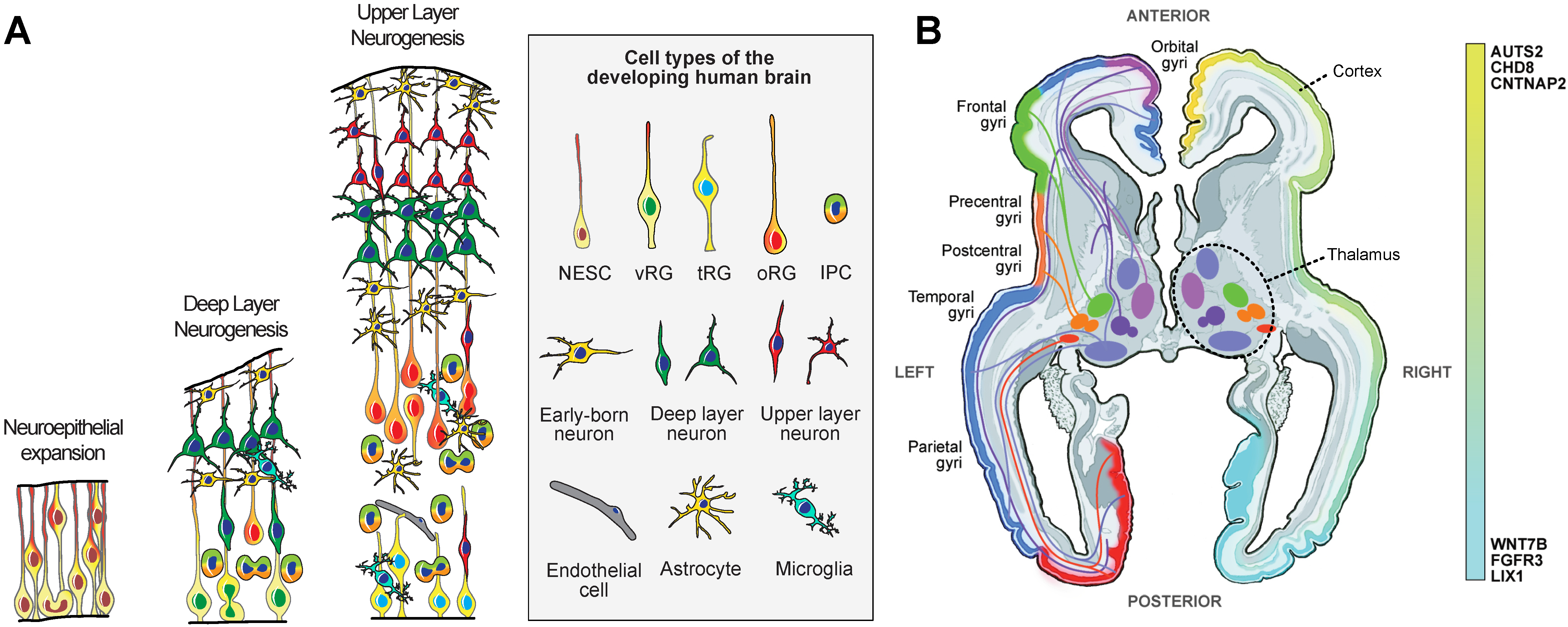
2. Characterization
3. Benchmarking against Primary Tissue
4. Protocol and Data Standards
5. Reducing Stress
6. Multi-Brain Region Organoids
7. Specification of Cortical Neuron Subtypes
8. Transplantation
9. Disease Modeling
9.1. Neurological Disorders
9.2. Psychiatric Conditions
9.3. Neurodegeneration
10. Evolutionary Insights
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, M.J.; Kaufman, M.H. Establishment in Culture of Pluripotential Cells from Mouse Embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Isolation of a Pluripotent Cell Line from Early Mouse Embryos Cultured in Medium Conditioned by Teratocarcinoma Stem Cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Heath, J.K.; Donaldson, D.D.; Wong, G.G.; Moreau, J.; Stahl, M.; Rogers, D. Inhibition of Pluripotential Embryonic Stem Cell Differentiation by Purified Polypeptides. Nature 1988, 336, 688–690. [Google Scholar] [CrossRef]
- Jones-Villeneuve, E.M.; McBurney, M.W.; Rogers, K.A.; Kalnins, V.I. Retinoic Acid Induces Embryonal Carcinoma Cells to Differentiate into Neurons and Glial Cells. J. Cell Biol. 1982, 94, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Park, I.-H.; Zhao, R.; West, J.A.; Yabuuchi, A.; Huo, H.; Ince, T.A.; Lerou, P.H.; Lensch, M.W.; Daley, G.Q. Reprogramming of Human Somatic Cells to Pluripotency with Defined Factors. Nature 2008, 451, 141–146. [Google Scholar] [CrossRef]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly Efficient Neural Conversion of Human ES and iPS Cells by Dual Inhibition of SMAD Signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef]
- Watanabe, K.; Kamiya, D.; Nishiyama, A.; Katayama, T.; Nozaki, S.; Kawasaki, H.; Watanabe, Y.; Mizuseki, K.; Sasai, Y. Directed Differentiation of Telencephalic Precursors from Embryonic Stem Cells. Nat. Neurosci. 2005, 8, 288–296. [Google Scholar] [CrossRef]
- Shi, Y.; Kirwan, P.; Smith, J.; Robinson, H.P.C.; Livesey, F.J. Human Cerebral Cortex Development from Pluripotent Stem Cells to Functional Excitatory Synapses. Nat. Neurosci. 2012, 15, 477–486, S1. [Google Scholar] [CrossRef] [Green Version]
- Eiraku, M.; Watanabe, K.; Matsuo-Takasaki, M.; Kawada, M.; Yonemura, S.; Matsumura, M.; Wataya, T.; Nishiyama, A.; Muguruma, K.; Sasai, Y. Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell 2008, 3, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Paşca, A.M.; Sloan, S.A.; Clarke, L.E.; Tian, Y.; Makinson, C.D.; Huber, N.; Kim, C.H.; Park, J.-Y.; O’Rourke, N.A.; Nguyen, K.D.; et al. Functional Cortical Neurons and Astrocytes from Human Pluripotent Stem Cells in 3D Culture. Nat. Methods 2015, 12, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Kadoshima, T.; Sakaguchi, H.; Nakano, T.; Soen, M.; Ando, S.; Eiraku, M.; Sasai, Y. Self-Organization of Axial Polarity, inside-out Layer Pattern, and Species-Specific Progenitor Dynamics in Human ES Cell-Derived Neocortex. Proc. Natl. Acad. Sci. USA 2013, 110, 20284–20289. [Google Scholar] [CrossRef] [PubMed]
- Sloan, S.A.; Darmanis, S.; Huber, N.; Khan, T.A.; Birey, F.; Caneda, C.; Reimer, R.; Quake, S.R.; Barres, B.A.; Paşca, S.P. Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron 2017, 95, 779–790.e6. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Su, Y.; Adam, C.D.; Deutschmann, A.U.; Pather, S.R.; Goldberg, E.M.; Su, K.; Li, S.; Lu, L.; Jacob, F.; et al. Sliced Human Cortical Organoids for Modeling Distinct Cortical Layer Formation. Cell Stem Cell 2020, 26, 766–781.e9. [Google Scholar] [CrossRef]
- Marton, R.M.; Miura, Y.; Sloan, S.A.; Li, Q.; Revah, O.; Levy, R.J.; Huguenard, J.R.; Pașca, S.P. Differentiation and Maturation of Oligodendrocytes in Human Three-Dimensional Neural Cultures. Nat. Neurosci. 2019, 22, 484–491. [Google Scholar] [CrossRef]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.L.; Min Yang, S.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell Diversity and Network Dynamics in Photosensitive Human Brain Organoids. Nature 2017, 545, 48–53. [Google Scholar] [CrossRef]
- Sharf, T.; van der Molen, T.; Glasauer, S.M.K.; Guzman, E.; Buccino, A.P.; Luna, G.; Cheng, Z.; Audouard, M.; Ranasinghe, K.G.; Kudo, K.; et al. Functional Neuronal Circuitry and Oscillatory Dynamics in Human Brain Organoids. Nat. Commun. 2022, 13, 4403. [Google Scholar] [CrossRef]
- Trujillo, C.A.; Gao, R.; Negraes, P.D.; Gu, J.; Buchanan, J.; Preissl, S.; Wang, A.; Wu, W.; Haddad, G.G.; Chaim, I.A.; et al. Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell 2019, 25, 558–569.e7. [Google Scholar] [CrossRef]
- Park, Y.; Franz, C.K.; Ryu, H.; Luan, H.; Cotton, K.Y.; Kim, J.U.; Chung, T.S.; Zhao, S.; Vazquez-Guardado, A.; Yang, D.S.; et al. Three-Dimensional, Multifunctional Neural Interfaces for Cortical Spheroids and Engineered Assembloids. Sci. Adv. 2021, 7, eabf9153. [Google Scholar] [CrossRef] [PubMed]
- Le Floch, P.; Li, Q.; Lin, Z.; Zhao, S.; Liu, R.; Tasnim, K.; Jiang, H.; Liu, J. Stretchable Mesh Nanoelectronics for 3D Single-Cell Chronic Electrophysiology from Developing Brain Organoids. Adv. Mater. 2022, 34, e2106829. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.; Sebinger, D.; Brauns, L.; Gonzalez-Cano, L.; Menuchin-Lasowski, Y.; Mierzejewski, M.; Psathaki, O.-E.; Stumpf, A.; Wickham, J.; Rauen, T.; et al. A Mesh Microelectrode Array for Non-Invasive Electrophysiology within Neural Organoids. bioRxiv 2021. bioRxiv:2020.09.02.279125. [Google Scholar]
- Voitiuk, K.; Geng, J.; Keefe, M.G.; Parks, D.F.; Sanso, S.E.; Hawthorne, N.; Freeman, D.B.; Currie, R.; Mostajo-Radji, M.A.; Pollen, A.A.; et al. Light-Weight Electrophysiology Hardware and Software Platform for Cloud-Based Neural Recording Experiments. J. Neural Eng. 2021, 18, 066004. [Google Scholar] [CrossRef]
- Camp, J.G.; Badsha, F.; Florio, M.; Kanton, S.; Gerber, T.; Wilsch-Bräuninger, M.; Lewitus, E.; Sykes, A.; Hevers, W.; Lancaster, M.; et al. Human Cerebral Organoids Recapitulate Gene Expression Programs of Fetal Neocortex Development. Proc. Natl. Acad. Sci. USA 2015, 112, 15672–15677. [Google Scholar] [CrossRef]
- Pollen, A.A.; Nowakowski, T.J.; Shuga, J.; Wang, X.; Leyrat, A.A.; Lui, J.H.; Li, N.; Szpankowski, L.; Fowler, B.; Chen, P.; et al. Low-Coverage Single-Cell mRNA Sequencing Reveals Cellular Heterogeneity and Activated Signaling Pathways in Developing Cerebral Cortex. Nat. Biotechnol. 2014, 32, 1053–1058. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Bhaduri, A.; Pollen, A.A.; Alvarado, B.; Mostajo-Radji, M.A.; Di Lullo, E.; Haeussler, M.; Sandoval-Espinosa, C.; Liu, S.J.; Velmeshev, D.; et al. Spatiotemporal Gene Expression Trajectories Reveal Developmental Hierarchies of the Human Cortex. Science 2017, 358, 1318–1323. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, S.; Fan, X.; Wu, Q.; Yan, L.; Dong, J.; Zhang, H.; Li, L.; Sun, L.; Pan, N.; et al. A Single-Cell RNA-Seq Survey of the Developmental Landscape of the Human Prefrontal Cortex. Nature 2018, 555, 524–528. [Google Scholar] [CrossRef]
- Polioudakis, D.; de la Torre-Ubieta, L.; Langerman, J.; Elkins, A.G.; Shi, X.; Stein, J.L.; Vuong, C.K.; Nichterwitz, S.; Gevorgian, M.; Opland, C.K.; et al. A Single-Cell Transcriptomic Atlas of Human Neocortical Development during Mid-Gestation. Neuron 2019, 103, 785–801.e8. [Google Scholar] [CrossRef]
- Bhaduri, A.; Sandoval-Espinosa, C.; Otero-Garcia, M.; Oh, I.; Yin, R.; Eze, U.C.; Nowakowski, T.J.; Kriegstein, A.R. An Atlas of Cortical Arealization Identifies Dynamic Molecular Signatures. Nature 2021, 598, 200–204. [Google Scholar] [CrossRef]
- La Manno, G.; Gyllborg, D.; Codeluppi, S.; Nishimura, K.; Salto, C.; Zeisel, A.; Borm, L.E.; Stott, S.R.W.; Toledo, E.M.; Villaescusa, J.C.; et al. Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell 2016, 167, 566–580.e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollen, A.A.; Bhaduri, A.; Andrews, M.G.; Nowakowski, T.J.; Meyerson, O.S.; Mostajo-Radji, M.A.; Di Lullo, E.; Alvarado, B.; Bedolli, M.; Dougherty, M.L.; et al. Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution. Cell 2019, 176, 743–756.e17. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, A.; Andrews, M.G.; Mancia Leon, W.; Jung, D.; Shin, D.; Allen, D.; Jung, D.; Schmunk, G.; Haeussler, M.; Salma, J.; et al. Cell Stress in Cortical Organoids Impairs Molecular Subtype Specification. Nature 2020, 578, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Vértesy, Á.; Eichmüller, O.L.; Naas, J.; Novatchkova, M.; Esk, C.; Balmaña, M.; Ladstaetter, S.; Bock, C.; von Haeseler, A.; Knoblich, J.A. Gruffi: An Algorithm for Computational Removal of Stressed Cells from Brain Organoid Transcriptomic Datasets. EMBO J. 2022, e111118. [Google Scholar] [CrossRef]
- Speir, M.L.; Bhaduri, A.; Markov, N.S.; Moreno, P.; Nowakowski, T.J.; Papatheodorou, I.; Pollen, A.A.; Raney, B.J.; Seninge, L.; Kent, W.J.; et al. UCSC Cell Browser: Visualize Your Single-Cell Data. Bioinformatics 2021, 37, 4578–4580. [Google Scholar] [CrossRef]
- Gordon, A.; Yoon, S.-J.; Tran, S.S.; Makinson, C.D.; Park, J.Y.; Andersen, J.; Valencia, A.M.; Horvath, S.; Xiao, X.; Huguenard, J.R.; et al. Long-Term Maturation of Human Cortical Organoids Matches Key Early Postnatal Transitions. Nat. Neurosci. 2021, 24, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Lancaster, M.A.; Castanon, R.; Nery, J.R.; Knoblich, J.A.; Ecker, J.R. Cerebral Organoids Recapitulate Epigenomic Signatures of the Human Fetal Brain. Cell Rep. 2016, 17, 3369–3384. [Google Scholar] [CrossRef]
- Yoon, K.-J.; Ringeling, F.R.; Vissers, C.; Jacob, F.; Pokrass, M.; Jimenez-Cyrus, D.; Su, Y.; Kim, N.-S.; Zhu, Y.; Zheng, L.; et al. Temporal Control of Mammalian Cortical Neurogenesis by m6A Methylation. Cell 2017, 171, 877–889.e17. [Google Scholar] [CrossRef]
- Amiri, A.; Coppola, G.; Scuderi, S.; Wu, F.; Roychowdhury, T.; Liu, F.; Pochareddy, S.; Shin, Y.; Safi, A.; Song, L.; et al. Transcriptome and Epigenome Landscape of Human Cortical Development Modeled in Organoids. Science 2018, 362, eaat6720. [Google Scholar] [CrossRef]
- Trevino, A.E.; Müller, F.; Andersen, J.; Sundaram, L.; Kathiria, A.; Shcherbina, A.; Farh, K.; Chang, H.Y.; Pașca, A.M.; Kundaje, A.; et al. Chromatin and Gene-Regulatory Dynamics of the Developing Human Cerebral Cortex at Single-Cell Resolution. Cell 2021, 184, 5053–5069.e23. [Google Scholar] [CrossRef]
- Ziffra, R.S.; Kim, C.N.; Ross, J.M.; Wilfert, A.; Turner, T.N.; Haeussler, M.; Casella, A.M.; Przytycki, P.F.; Keough, K.C.; Shin, D.; et al. Single-Cell Epigenomics Reveals Mechanisms of Human Cortical Development. Nature 2021, 598, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, P.; Hartfuss, E.; Götz, M. Isolation of Radial Glial Cells by Fluorescent-Activated Cell Sorting Reveals a Neuronal Lineage. Development 2000, 127, 5253–5263. [Google Scholar] [CrossRef] [PubMed]
- Hartfuss, E.; Galli, R.; Heins, N.; Götz, M. Characterization of CNS Precursor Subtypes and Radial Glia. Dev. Biol. 2001, 229, 15–30. [Google Scholar] [CrossRef]
- Temple, S. The Development of Neural Stem Cells. Nature 2001, 414, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P. Mode of Cell Migration to the Superficial Layers of Fetal Monkey Neocortex. J. Comp. Neurol. 1972, 145, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Noctor, S.C.; Flint, A.C.; Weissman, T.A.; Dammerman, R.S.; Kriegstein, A.R. Neurons Derived from Radial Glial Cells Establish Radial Units in Neocortex. Nature 2001, 409, 714–720. [Google Scholar] [CrossRef]
- Miyata, T.; Kawaguchi, A.; Okano, H.; Ogawa, M. Asymmetric Inheritance of Radial Glial Fibers by Cortical Neurons. Neuron 2001, 31, 727–741. [Google Scholar] [CrossRef]
- Haubensak, W.; Attardo, A.; Denk, W.; Huttner, W.B. Neurons Arise in the Basal Neuroepithelium of the Early Mammalian Telencephalon: A Major Site of Neurogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 3196–3201. [Google Scholar] [CrossRef]
- Noctor, S.C.; Martínez-Cerdeño, V.; Ivic, L.; Kriegstein, A.R. Cortical Neurons Arise in Symmetric and Asymmetric Division Zones and Migrate through Specific Phases. Nat. Neurosci. 2004, 7, 136–144. [Google Scholar] [CrossRef]
- Englund, C.; Fink, A.; Lau, C.; Pham, D.; Daza, R.A.M.; Bulfone, A.; Kowalczyk, T.; Hevner, R.F. Pax6, Tbr2, and Tbr1 Are Expressed Sequentially by Radial Glia, Intermediate Progenitor Cells, and Postmitotic Neurons in Developing Neocortex. J. Neurosci. 2005, 25, 247–251. [Google Scholar] [CrossRef]
- Takahashi, T.; Nowakowski, R.S.; Caviness, V.S., Jr. Cell Cycle Parameters and Patterns of Nuclear Movement in the Neocortical Proliferative Zone of the Fetal Mouse. J. Neurosci. 1993, 13, 820–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angevine, J.B., Jr.; Sidman, R.L. Autoradiographic Study of Cell Migration during Histogenesis of Cerebral Cortex in the Mouse. Nature 1961, 192, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Temple, S. Cell Types to Order: Temporal Specification of CNS Stem Cells. Curr. Opin. Neurobiol. 2009, 19, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Smart, I.H.M.; Dehay, C.; Giroud, P.; Berland, M.; Kennedy, H. Unique Morphological Features of the Proliferative Zones and Postmitotic Compartments of the Neural Epithelium Giving Rise to Striate and Extrastriate Cortex in the Monkey. Cereb. Cortex 2002, 12, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Betizeau, M.; Cortay, V.; Patti, D.; Pfister, S.; Gautier, E.; Bellemin-Ménard, A.; Afanassieff, M.; Huissoud, C.; Douglas, R.J.; Kennedy, H.; et al. Precursor Diversity and Complexity of Lineage Relationships in the Outer Subventricular Zone of the Primate. Neuron 2013, 80, 442–457. [Google Scholar] [CrossRef]
- Hansen, D.V.; Lui, J.H.; Parker, P.R.L.; Kriegstein, A.R. Neurogenic Radial Glia in the Outer Subventricular Zone of Human Neocortex. Nature 2010, 464, 554–561. [Google Scholar] [CrossRef]
- Kelava, I.; Reillo, I.; Murayama, A.Y.; Kalinka, A.T.; Stenzel, D.; Tomancak, P.; Matsuzaki, F.; Lebrand, C.; Sasaki, E.; Schwamborn, J.C.; et al. Abundant Occurrence of Basal Radial Glia in the Subventricular Zone of Embryonic Neocortex of a Lissencephalic Primate, the Common Marmoset Callithrix Jacchus. Cereb. Cortex 2012, 22, 469–481. [Google Scholar] [CrossRef]
- Fietz, S.A.; Kelava, I.; Vogt, J.; Wilsch-Bräuninger, M.; Stenzel, D.; Fish, J.L.; Corbeil, D.; Riehn, A.; Distler, W.; Nitsch, R.; et al. OSVZ Progenitors of Human and Ferret Neocortex Are Epithelial-like and Expand by Integrin Signaling. Nat. Neurosci. 2010, 13, 690–699. [Google Scholar] [CrossRef]
- Reillo, I.; de Juan Romero, C.; García-Cabezas, M.Á.; Borrell, V. A Role for Intermediate Radial Glia in the Tangential Expansion of the Mammalian Cerebral Cortex. Cereb. Cortex 2011, 21, 1674–1694. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Pollen, A.A.; Sandoval-Espinosa, C.; Kriegstein, A.R. Transformation of the Radial Glia Scaffold Demarcates Two Stages of Human Cerebral Cortex Development. Neuron 2016, 91, 1219–1227. [Google Scholar] [CrossRef]
- Bilgic, M.; Wu, Q.; Suetsugu, T.; Tsunekawa, Y.; Sitamukai, A.; Kadota, M.; Nishimura, O.; Kuraku, S.; Matsuzaki, F. Single Cell Transcriptomics of Ferrets Reveal a Common Temporal Pattern of Progenitors in Brain Development of Gyrencephalic Mammals. bioRxiv 2022. bioRxiv:2022.05.05.490846. [Google Scholar]
- Pollen, A.A.; Nowakowski, T.J.; Chen, J.; Retallack, H.; Sandoval-Espinosa, C.; Nicholas, C.R.; Shuga, J.; Liu, S.J.; Oldham, M.C.; Diaz, A.; et al. Molecular Identity of Human Outer Radial Glia during Cortical Development. Cell 2015, 163, 55–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, E.R.; Mich, J.K.; Yao, Z.; Hodge, R.D.; Doyle, A.M.; Jang, S.; Shehata, S.I.; Nelson, A.M.; Shapovalova, N.V.; Levi, B.P.; et al. Fixed Single-Cell Transcriptomic Characterization of Human Radial Glial Diversity. Nat. Methods 2016, 13, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Buth, J.E.; Vishlaghi, N.; de la Torre-Ubieta, L.; Taxidis, J.; Khakh, B.S.; Coppola, G.; Pearson, C.A.; Yamauchi, K.; Gong, D.; et al. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep. 2017, 21, 517–532. [Google Scholar] [CrossRef]
- Bonni, A.; Sun, Y.; Nadal-Vicens, M.; Bhatt, A.; Frank, D.A.; Rozovsky, I.; Stahl, N.; Yancopoulos, G.D.; Greenberg, M.E. Regulation of Gliogenesis in the Central Nervous System by the JAK-STAT Signaling Pathway. Science 1997, 278, 477–483. [Google Scholar] [CrossRef]
- Huang, W.; Bhaduri, A.; Velmeshev, D.; Wang, S.; Wang, L.; Rottkamp, C.A.; Alvarez-Buylla, A.; Rowitch, D.H.; Kriegstein, A.R. Origins and Proliferative States of Human Oligodendrocyte Precursor Cells. Cell 2020, 182, 594–608.e11. [Google Scholar] [CrossRef]
- van Bruggen, D.; Pohl, F.; Langseth, C.M.; Kukanja, P.; Lee, H.; Kabbe, M.; Meijer, M.; Hilscher, M.M.; Nilsson, M.; Sundström, E.; et al. Developmental Landscape of Human Forebrain at a Single-Cell Level Unveils Early Waves of Oligodendrogenesis. bioRxiv 2021. bioRxiv:2021.07.22.453317. [Google Scholar]
- deAzevedo, L.C.; Fallet, C.; Moura-Neto, V.; Daumas-Duport, C.; Hedin-Pereira, C.; Lent, R. Cortical Radial Glial Cells in Human Fetuses: Depth-Correlated Transformation into Astrocytes. J. Neurobiol. 2003, 55, 288–298. [Google Scholar] [CrossRef]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.B.; Li, G.; et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef]
- Bayraktar, O.A.; Bartels, T.; Holmqvist, S.; Kleshchevnikov, V.; Martirosyan, A.; Polioudakis, D.; Ben Haim, L.; Young, A.M.H.; Batiuk, M.Y.; Prakash, K.; et al. Astrocyte Layers in the Mammalian Cerebral Cortex Revealed by a Single-Cell in Situ Transcriptomic Map. Nat. Neurosci. 2020, 23, 500–509. [Google Scholar] [CrossRef]
- Allen, D.E.; Donohue, K.C.; Cadwell, C.R.; Shin, D.; Keefe, M.G.; Sohal, V.S.; Nowakowski, T.J. Fate Mapping of Neural Stem Cell Niches Reveals Distinct Origins of Human Cortical Astrocytes. Science 2022, 376, eabm5224. [Google Scholar] [CrossRef] [PubMed]
- Falcone, C.; McBride, E.L.; Hopkins, W.D.; Hof, P.R.; Manger, P.R.; Sherwood, C.C.; Noctor, S.C.; Martínez-Cerdeño, V. Redefining Varicose Projection Astrocytes in Primates. Glia 2022, 70, 145–154. [Google Scholar] [CrossRef]
- Falcone, C.; Penna, E.; Hong, T.; Tarantal, A.F.; Hof, P.R.; Hopkins, W.D.; Sherwood, C.C.; Noctor, S.C.; Martínez-Cerdeño, V. Cortical Interlaminar Astrocytes Are Generated Prenatally, Mature Postnatally, and Express Unique Markers in Human and Nonhuman Primates. Cereb. Cortex 2021, 31, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Raff, M. Oligodendrocyte Precursor Cells Reprogrammed to Become Multipotential CNS Stem Cells. Science 2000, 289, 1754–1757. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Z.; Liu, G.; Li, X.; Yang, Z. Developmental Origins of Human Cortical Oligodendrocytes and Astrocytes. Neurosci. Bull. 2022, 38, 47–68. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, M.; Yu, H.; Wang, Y.; Wu, X.; Yong, J.; Mao, Y.; Cui, Y.; Fan, X.; Wen, L.; et al. Heterogeneity of Glial Progenitor Cells during the Neurogenesis-to-Gliogenesis Switch in the Developing Human Cerebral Cortex. Cell Rep. 2021, 34, 108788. [Google Scholar] [CrossRef]
- Hansen, D.V.; Lui, J.H.; Flandin, P.; Yoshikawa, K.; Rubenstein, J.L.; Alvarez-Buylla, A.; Kriegstein, A.R. Non-Epithelial Stem Cells and Cortical Interneuron Production in the Human Ganglionic Eminences. Nat. Neurosci. 2013, 16, 1576–1587. [Google Scholar] [CrossRef]
- Ma, T.; Wang, C.; Wang, L.; Zhou, X.; Tian, M.; Zhang, Q.; Zhang, Y.; Li, J.; Liu, Z.; Cai, Y.; et al. Subcortical Origins of Human and Monkey Neocortical Interneurons. Nat. Neurosci. 2013, 16, 1588–1597. [Google Scholar] [CrossRef]
- Delgado, R.N.; Allen, D.E.; Keefe, M.G.; Mancia Leon, W.R.; Ziffra, R.S.; Crouch, E.E.; Alvarez-Buylla, A.; Nowakowski, T.J. Individual Human Cortical Progenitors Can Produce Excitatory and Inhibitory Neurons. Nature 2022, 601, 397–403. [Google Scholar] [CrossRef]
- Schmitz, M.T.; Sandoval, K.; Chen, C.P.; Mostajo-Radji, M.A.; Seeley, W.W.; Nowakowski, T.J.; Ye, C.J.; Paredes, M.F.; Pollen, A.A. The Development and Evolution of Inhibitory Neurons in Primate Cerebrum. Nature 2022, 603, 871–877. [Google Scholar] [CrossRef]
- Mayer, C.; Jaglin, X.H.; Cobbs, L.V.; Bandler, R.C.; Streicher, C.; Cepko, C.L.; Hippenmeyer, S.; Fishell, G. Clonally Related Forebrain Interneurons Disperse Broadly across Both Functional Areas and Structural Boundaries. Neuron 2015, 87, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Pebworth, M.-P.; Ross, J.; Andrews, M.; Bhaduri, A.; Kriegstein, A.R. Human Intermediate Progenitor Diversity during Cortical Development. Proc. Natl. Acad. Sci. USA 2021, 118, e2019415118. [Google Scholar] [CrossRef] [PubMed]
- Letinic, K.; Zoncu, R.; Rakic, P. Origin of GABAergic Neurons in the Human Neocortex. Nature 2002, 417, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaberi, N.; Lindsay, S.; Sarma, S.; Bayatti, N.; Clowry, G.J. The Early Fetal Development of Human Neocortical GABAergic Interneurons. Cereb. Cortex 2015, 25, 631–645. [Google Scholar] [CrossRef]
- Velasco, S.; Kedaigle, A.J.; Simmons, S.K.; Nash, A.; Rocha, M.; Quadrato, G.; Paulsen, B.; Nguyen, L.; Adiconis, X.; Regev, A.; et al. Individual Brain Organoids Reproducibly Form Cell Diversity of the Human Cerebral Cortex. Nature 2019, 570, 523–527. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-Bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Wang, R.; Sharma, R.; Shen, X.; Laughney, A.M.; Funato, K.; Clark, P.J.; Shpokayte, M.; Morgenstern, P.; Navare, M.; Xu, Y.; et al. Adult Human Glioblastomas Harbor Radial Glia-like Cells. Stem Cell Reports 2020, 14, 338–350. [Google Scholar] [CrossRef]
- Tirosh, I.; Venteicher, A.S.; Hebert, C.; Escalante, L.E.; Patel, A.P.; Yizhak, K.; Fisher, J.M.; Rodman, C.; Mount, C.; Filbin, M.G.; et al. Single-Cell RNA-Seq Supports a Developmental Hierarchy in Human Oligodendroglioma. Nature 2016, 539, 309–313. [Google Scholar] [CrossRef]
- Bhaduri, A.; Di Lullo, E.; Jung, D.; Müller, S.; Crouch, E.E.; Espinosa, C.S.; Ozawa, T.; Alvarado, B.; Spatazza, J.; Cadwell, C.R.; et al. Outer Radial Glia-like Cancer Stem Cells Contribute to Heterogeneity of Glioblastoma. Cell Stem Cell 2020, 26, 48–63.e6. [Google Scholar] [CrossRef]
- La Manno, G.; Soldatov, R.; Zeisel, A.; Braun, E.; Hochgerner, H.; Petukhov, V.; Lidschreiber, K.; Kastriti, M.E.; Lönnerberg, P.; Furlan, A.; et al. RNA Velocity of Single Cells. Nature 2018, 560, 494–498. [Google Scholar] [CrossRef]
- Trapnell, C.; Cacchiarelli, D.; Grimsby, J.; Pokharel, P.; Li, S.; Morse, M.; Lennon, N.J.; Livak, K.J.; Mikkelsen, T.S.; Rinn, J.L. The Dynamics and Regulators of Cell Fate Decisions Are Revealed by Pseudotemporal Ordering of Single Cells. Nat. Biotechnol. 2014, 32, 381–386. [Google Scholar] [CrossRef]
- Wagner, D.E.; Klein, A.M. Lineage Tracing Meets Single-Cell Omics: Opportunities and Challenges. Nat. Rev. Genet. 2020, 21, 410–427. [Google Scholar] [CrossRef] [PubMed]
- Gertz, C.C.; Lui, J.H.; LaMonica, B.E.; Wang, X.; Kriegstein, A.R. Diverse Behaviors of Outer Radial Glia in Developing Ferret and Human Cortex. J. Neurosci. 2014, 34, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- LaMonica, B.E.; Lui, J.H.; Hansen, D.V.; Kriegstein, A.R. Mitotic Spindle Orientation Predicts Outer Radial Glial Cell Generation in Human Neocortex. Nat. Commun. 2013, 4, 1665. [Google Scholar] [CrossRef] [PubMed]
- Bershteyn, M.; Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Nene, A.; Wynshaw-Boris, A.; Kriegstein, A.R. Human iPSC-Derived Cerebral Organoids Model Cellular Features of Lissencephaly and Reveal Prolonged Mitosis of Outer Radial Glia. Cell Stem Cell 2017, 20, 435–449.e4. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, L.; Bershteyn, M.; Paredes, M.F.; Kriegstein, A.R. Dynamic Behaviour of Human Neuroepithelial Cells in the Developing Forebrain. Nat. Commun. 2017, 8, 14167. [Google Scholar] [CrossRef]
- Coquand, L.; Macé, A.-S.; Farcy, S.; Avalos, C.B.; Di Cicco, A.; Lampic, M.; Bessières, B.; Attie-Bitach, T.; Fraisier, V.; Guimiot, F.; et al. A Cell Fate Decision Map Reveals Abundant Direct Neurogenesis in the Human Developing Neocortex. bioRxiv 2022. bioRxiv:2022.02.01.478661. [Google Scholar]
- Esk, C.; Lindenhofer, D.; Haendeler, S.; Wester, R.A.; Pflug, F.; Schroeder, B.; Bagley, J.A.; Elling, U.; Zuber, J.; von Haeseler, A.; et al. A Human Tissue Screen Identifies a Regulator of ER Secretion as a Brain-Size Determinant. Science 2020, 370, 935–941. [Google Scholar] [CrossRef]
- He, Z.; Maynard, A.; Jain, A.; Gerber, T.; Petri, R.; Lin, H.-C.; Santel, M.; Ly, K.; Dupré, J.-S.; Sidow, L.; et al. Lineage Recording in Human Cerebral Organoids. Nat. Methods 2022, 19, 90–99. [Google Scholar] [CrossRef]
- Bandler, R.C.; Vitali, I.; Delgado, R.N.; Ho, M.C.; Dvoretskova, E.; Ibarra Molinas, J.S.; Frazel, P.W.; Mohammadkhani, M.; Machold, R.; Maedler, S.; et al. Single-Cell Delineation of Lineage and Genetic Identity in the Mouse Brain. Nature 2021, 601, 404–409. [Google Scholar] [CrossRef]
- Ratz, M.; von Berlin, L.; Larsson, L.; Martin, M.; Westholm, J.O.; La Manno, G.; Lundeberg, J.; Frisén, J. Clonal Relations in the Mouse Brain Revealed by Single-Cell and Spatial Transcriptomics. Nat. Neurosci. 2022, 25, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-J.; Elahi, L.S.; Pașca, A.M.; Marton, R.M.; Gordon, A.; Revah, O.; Miura, Y.; Walczak, E.M.; Holdgate, G.M.; Fan, H.C.; et al. Reliability of Human Cortical Organoid Generation. Nat. Methods 2019, 16, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for Scientific Data Management and Stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef] [PubMed]
- Puigdevall, P.; Jerber, J.; Danecek, P.; Castellano, S.; Kilpinen, H. Effects of Somatic Mutations on Cellular Differentiation in iPSC Models of Neurodevelopment. bioRxiv 2022. bioRxiv:2022.03.04.482992. [Google Scholar]
- Giandomenico, S.L.; Sutcliffe, M.; Lancaster, M.A. Generation and Long-Term Culture of Advanced Cerebral Organoids for Studying Later Stages of Neural Development. Nat. Protoc. 2021, 16, 579–602. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Popova, G.; Soliman, S.S.; Kim, C.N.; Keefe, M.G.; Hennick, K.M.; Jain, S.; Li, T.; Tejera, D.; Shin, D.; Chhun, B.B.; et al. Human Microglia States Are Conserved across Experimental Models and Regulate Neural Stem Cell Responses in Chimeric Organoids. Cell Stem Cell 2021, 28, 2153–2166.e6. [Google Scholar] [CrossRef]
- Cakir, B.; Tanaka, Y.; Kiral, F.R.; Xiang, Y.; Dagliyan, O.; Wang, J.; Lee, M.; Greaney, A.M.; Yang, W.S.; duBoulay, C.; et al. Expression of the Transcription Factor PU.1 Induces the Generation of Microglia-like Cells in Human Cortical Organoids. Nat. Commun. 2022, 13, 430. [Google Scholar] [CrossRef]
- Sabate-Soler, S.; Nickels, S.L.; Saraiva, C.; Berger, E.; Dubonyte, U.; Barmpa, K.; Lan, Y.J.; Kouno, T.; Jarazo, J.; Robertson, G.; et al. Microglia Integration into Human Midbrain Organoids Leads to Increased Neuronal Maturation and Functionality. Glia 2022, 70, 1267–1288. [Google Scholar] [CrossRef]
- Fagerlund, I.; Dougalis, A.; Shakirzyanova, A.; Gómez-Budia, M.; Pelkonen, A.; Konttinen, H.; Ohtonen, S.; Fazaludeen, M.F.; Koskuvi, M.; Kuusisto, J.; et al. Microglia-like Cells Promote Neuronal Functions in Cerebral Organoids. Cells 2021, 11, 124. [Google Scholar] [CrossRef]
- Ross, J.M.; Kim, C.; Allen, D.; Crouch, E.E.; Narsinh, K.; Cooke, D.L.; Abla, A.A.; Nowakowski, T.J.; Winkler, E.A. The Expanding Cell Diversity of the Brain Vasculature. Front. Physiol. 2020, 11, 600767. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, L.; Wang, M.; Liu, J.; Zhong, S.; Li, R.; Li, P.; Guo, L.; Fang, A.; Chen, R.; et al. Vascularized Human Cortical Organoids (vOrganoids) Model Cortical Development in Vivo. PLoS Biol. 2020, 18, e3000705. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Ju, X.-C.; Li, Y.; Zeng, P.-M.; Wu, J.; Zhou, Y.-Y.; Shen, L.-B.; Dong, J.; Chen, Y.-J.; Luo, Z.-G. Generation of Vascularized Brain Organoids to Study Neurovascular Interactions. Elife 2022, 11, e76707. [Google Scholar] [CrossRef]
- Kook, M.G.; Lee, S.-E.; Shin, N.; Kong, D.; Kim, D.-H.; Kim, M.-S.; Kang, H.K.; Choi, S.W.; Kang, K.-S. Generation of Cortical Brain Organoid with Vascularization by Assembling with Vascular Spheroid. Int J Stem Cells 2022, 15, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.-J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.-S.; et al. Engineering of Human Brain Organoids with a Functional Vascular-like System. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef]
- Ang, L.T.; Nguyen, A.T.; Liu, K.J.; Chen, A.; Xiong, X.; Curtis, M.; Martin, R.M.; Raftry, B.C.; Ng, C.Y.; Vogel, U.; et al. Generating Human Artery and Vein Cells from Pluripotent Stem Cells Highlights the Arterial Tropism of Nipah and Hendra Viruses. Cell 2022, 185, 2523–2541.e30. [Google Scholar] [CrossRef]
- Nikolova, M.T.; He, Z.; Wimmer, R.A.; Seimiya, M.; Nikoloff, J.M.; Penninger, J.M.; Gray Camp, J.; Treutlein, B. Fate and State Transitions during Human Blood Vessel Organoid Development. bioRxiv 2022. bioRxiv:2022.03.23.485329. [Google Scholar]
- Winkler, E.A.; Kim, C.N.; Ross, J.M.; Garcia, J.H.; Gil, E.; Oh, I.; Chen, L.Q.; Wu, D.; Catapano, J.S.; Raygor, K.; et al. A Single-Cell Atlas of the Normal and Malformed Human Brain Vasculature. Science 2022, 375, eabi7377. [Google Scholar] [CrossRef]
- Yang, A.C.; Vest, R.T.; Kern, F.; Lee, D.P.; Agam, M.; Maat, C.A.; Losada, P.M.; Chen, M.B.; Schaum, N.; Khoury, N.; et al. A Human Brain Vascular Atlas Reveals Diverse Mediators of Alzheimer’s Risk. Nature 2022, 603, 885–892. [Google Scholar] [CrossRef]
- Birey, F.; Andersen, J.; Makinson, C.D.; Islam, S.; Wei, W.; Huber, N.; Fan, H.C.; Metzler, K.R.C.; Panagiotakos, G.; Thom, N.; et al. Assembly of Functionally Integrated Human Forebrain Spheroids. Nature 2017, 545, 54–59. [Google Scholar] [CrossRef]
- Xiang, Y.; Tanaka, Y.; Patterson, B.; Kang, Y.-J.; Govindaiah, G.; Roselaar, N.; Cakir, B.; Kim, K.-Y.; Lombroso, A.P.; Hwang, S.-M.; et al. Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration. Cell Stem Cell 2017, 21, 383–398.e7. [Google Scholar] [CrossRef] [PubMed]
- Bagley, J.A.; Reumann, D.; Bian, S.; Lévi-Strauss, J.; Knoblich, J.A. Fused Cerebral Organoids Model Interactions between Brain Regions. Nat. Methods 2017, 14, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Li, M.-Y.; Birey, F.; Ikeda, K.; Revah, O.; Thete, M.V.; Park, J.-Y.; Puno, A.; Lee, S.H.; Porteus, M.H.; et al. Generation of Human Striatal Organoids and Cortico-Striatal Assembloids from Human Pluripotent Stem Cells. Nat. Biotechnol. 2020, 38, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, H.; Kadoshima, T.; Soen, M.; Narii, N.; Ishida, Y.; Ohgushi, M.; Takahashi, J.; Eiraku, M.; Sasai, Y. Generation of Functional Hippocampal Neurons from Self-Organizing Human Embryonic Stem Cell-Derived Dorsomedial Telencephalic Tissue. Nat. Commun. 2015, 6, 8896. [Google Scholar] [CrossRef]
- Suga, H.; Kadoshima, T.; Minaguchi, M.; Ohgushi, M.; Soen, M.; Nakano, T.; Takata, N.; Wataya, T.; Muguruma, K.; Miyoshi, H.; et al. Self-Formation of Functional Adenohypophysis in Three-Dimensional Culture. Nature 2011, 480, 57–62. [Google Scholar] [CrossRef]
- Ozone, C.; Suga, H.; Eiraku, M.; Kadoshima, T.; Yonemura, S.; Takata, N.; Oiso, Y.; Tsuji, T.; Sasai, Y. Functional Anterior Pituitary Generated in Self-Organizing Culture of Human Embryonic Stem Cells. Nat. Commun. 2016, 7, 10351. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, A.; Muguruma, K.; Sasai, Y. Generation of Thalamic Neurons from Mouse Embryonic Stem Cells. Development 2017, 144, 1211–1220. [Google Scholar] [CrossRef]
- Xiang, Y.; Tanaka, Y.; Cakir, B.; Patterson, B.; Kim, K.-Y.; Sun, P.; Kang, Y.-J.; Zhong, M.; Liu, X.; Patra, P.; et al. hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell Stem Cell 2019, 24, 487–497.e7. [Google Scholar] [CrossRef]
- Jo, J.; Xiao, Y.; Sun, A.X.; Cukuroglu, E.; Tran, H.-D.; Göke, J.; Tan, Z.Y.; Saw, T.Y.; Tan, C.-P.; Lokman, H.; et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 2016, 19, 248–257. [Google Scholar] [CrossRef]
- Zagare, A.; Gobin, M.; Monzel, A.S.; Schwamborn, J.C. A Robust Protocol for the Generation of Human Midbrain Organoids. STAR Protoc 2021, 2, 100524. [Google Scholar] [CrossRef]
- Fiorenzano, A.; Sozzi, E.; Birtele, M.; Kajtez, J.; Giacomoni, J.; Nilsson, F.; Bruzelius, A.; Sharma, Y.; Zhang, Y.; Mattsson, B.; et al. Single-Cell Transcriptomics Captures Features of Human Midbrain Development and Dopamine Neuron Diversity in Brain Organoids. Nat. Commun. 2021, 12, 7302. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, K.; Nishiyama, A.; Kawakami, H.; Hashimoto, K.; Sasai, Y. Self-Organization of Polarized Cerebellar Tissue in 3D Culture of Human Pluripotent Stem Cells. Cell Rep. 2015, 10, 537–550. [Google Scholar] [CrossRef]
- Fleck, J.S.; Sanchís-Calleja, F.; He, Z.; Santel, M.; Boyle, M.J.; Camp, J.G.; Treutlein, B. Resolving Organoid Brain Region Identities by Mapping Single-Cell Genomic Data to Reference Atlases. Cell Stem Cell 2021, 28, 1148–1159.e8. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.; Revah, O.; Miura, Y.; Thom, N.; Amin, N.D.; Kelley, K.W.; Singh, M.; Chen, X.; Thete, M.V.; Walczak, E.M.; et al. Generation of Functional Human 3D Cortico-Motor Assembloids. Cell 2020, 183, 1913–1929.e26. [Google Scholar] [CrossRef] [PubMed]
- Giandomenico, S.L.; Mierau, S.B.; Gibbons, G.M.; Wenger, L.M.D.; Masullo, L.; Sit, T.; Sutcliffe, M.; Boulanger, J.; Tripodi, M.; Derivery, E.; et al. Cerebral Organoids at the Air-Liquid Interface Generate Diverse Nerve Tracts with Functional Output. Nat. Neurosci. 2019, 22, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Takata, N.; Sakakura, E.; Eiraku, M.; Kasukawa, T.; Sasai, Y. Self-Patterning of Rostral-Caudal Neuroectoderm Requires Dual Role of Fgf Signaling for Localized Wnt Antagonism. Nat. Commun. 2017, 8, 1339. [Google Scholar] [CrossRef] [Green Version]
- Cederquist, G.Y.; Asciolla, J.J.; Tchieu, J.; Walsh, R.M.; Cornacchia, D.; Resh, M.D.; Studer, L. Specification of Positional Identity in Forebrain Organoids. Nat. Biotechnol. 2019, 37, 436–444. [Google Scholar] [CrossRef]
- Osaki, T.; Ikeuchi, Y. Advanced Complexity and Plasticity of Neural Activity in Reciprocally Connected Human Cerebral Organoids. bioRxiv 2021. bioRxiv:2021.02.16.431387. [Google Scholar]
- Molyneaux, B.J.; Arlotta, P.; Menezes, J.R.L.; Macklis, J.D. Neuronal Subtype Specification in the Cerebral Cortex. Nat. Rev. Neurosci. 2007, 8, 427–437. [Google Scholar] [CrossRef]
- Greig, L.C.; Woodworth, M.B.; Galazo, M.J.; Padmanabhan, H.; Macklis, J.D. Molecular Logic of Neocortical Projection Neuron Specification, Development and Diversity. Nat. Rev. Neurosci. 2013, 14, 755–769. [Google Scholar] [CrossRef]
- Anderson, S.; Vanderhaeghen, P. Cortical Neurogenesis from Pluripotent Stem Cells: Complexity Emerging from Simplicity. Curr. Opin. Neurobiol. 2014, 27, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Cadwell, C.R.; Bhaduri, A.; Mostajo-Radji, M.A.; Keefe, M.G.; Nowakowski, T.J. Development and Arealization of the Cerebral Cortex. Neuron 2019, 103, 980–1004. [Google Scholar] [CrossRef] [PubMed]
- Cederquist, G.Y.; Tchieu, J.; Callahan, S.J.; Ramnarine, K.; Ryan, S.; Zhang, C.; Rittenhouse, C.; Zeltner, N.; Chung, S.Y.; Zhou, T.; et al. A Multiplex Human Pluripotent Stem Cell Platform Defines Molecular and Functional Subclasses of Autism-Related Genes. Cell Stem Cell 2020, 27, 35–49.e6. [Google Scholar] [CrossRef]
- Hasenpusch-Theil, K.; Watson, J.A.; Theil, T. Direct Interactions Between Gli3, Wnt8b, and Fgfs Underlie Patterning of the Dorsal Telencephalon. Cereb. Cortex 2017, 27, 1137–1148. [Google Scholar] [PubMed]
- Ypsilanti, A.R.; Pattabiraman, K.; Catta-Preta, R.; Golonzhka, O.; Lindtner, S.; Tang, K.; Jones, I.R.; Abnousi, A.; Juric, I.; Hu, M.; et al. Transcriptional Network Orchestrating Regional Patterning of Cortical Progenitors. Proc. Natl. Acad. Sci. USA 2021, 118, e2024795118. [Google Scholar] [CrossRef] [PubMed]
- Markenscoff-Papadimitriou, E.; Whalen, S.; Przytycki, P.; Thomas, R.; Binyameen, F.; Nowakowski, T.J.; Kriegstein, A.R.; Sanders, S.J.; State, M.W.; Pollard, K.S.; et al. A Chromatin Accessibility Atlas of the Developing Human Telencephalon. Cell 2020, 182, 754–769.e18. [Google Scholar] [CrossRef]
- Shibata, M.; Pattabiraman, K.; Lorente-Galdos, B.; Andrijevic, D.; Kim, S.-K.; Kaur, N.; Muchnik, S.K.; Xing, X.; Santpere, G.; Sousa, A.M.M.; et al. Regulation of Prefrontal Patterning and Connectivity by Retinoic Acid. Nature 2021, 598, 483–488. [Google Scholar] [CrossRef]
- Espuny-Camacho, I.; Michelsen, K.A.; Gall, D.; Linaro, D.; Hasche, A.; Bonnefont, J.; Bali, C.; Orduz, D.; Bilheu, A.; Herpoel, A.; et al. Pyramidal Neurons Derived from Human Pluripotent Stem Cells Integrate Efficiently into Mouse Brain Circuits in Vivo. Neuron 2013, 77, 440–456. [Google Scholar] [CrossRef]
- Linaro, D.; Vermaercke, B.; Iwata, R.; Ramaswamy, A.; Libé-Philippot, B.; Boubakar, L.; Davis, B.A.; Wierda, K.; Davie, K.; Poovathingal, S.; et al. Xenotransplanted Human Cortical Neurons Reveal Species-Specific Development and Functional Integration into Mouse Visual Circuits. Neuron 2019, 104, 972–986.e6. [Google Scholar] [CrossRef]
- Mansour, A.A.; Gonçalves, J.T.; Bloyd, C.W.; Li, H.; Fernandes, S.; Quang, D.; Johnston, S.; Parylak, S.L.; Jin, X.; Gage, F.H. An in Vivo Model of Functional and Vascularized Human Brain Organoids. Nat. Biotechnol. 2018, 36, 432–441. [Google Scholar] [CrossRef]
- Daviaud, N.; Friedel, R.H.; Zou, H. Vascularization and Engraftment of Transplanted Human Cerebral Organoids in Mouse Cortex. eNeuro 2018, 5, ENEURO.0219-18.2018. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, T.; Sakaguchi, H.; Morizane, A.; Kikuchi, T.; Miyamoto, S.; Takahashi, J. Axonal Extensions along Corticospinal Tracts from Transplanted Human Cerebral Organoids. Stem Cell Reports 2020, 15, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.I.; Song, H.; Ming, G.-L. Applications of Human Brain Organoids to Clinical Problems. Dev. Dyn. 2019, 248, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Britanova, O.; de Juan Romero, C.; Cheung, A.; Kwan, K.Y.; Schwark, M.; Gyorgy, A.; Vogel, T.; Akopov, S.; Mitkovski, M.; Agoston, D.; et al. Satb2 Is a Postmitotic Determinant for Upper-Layer Neuron Specification in the Neocortex. Neuron 2008, 57, 378–392. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Policy and Global Affairs; Committee on Science, Technology, and Law; Committee on Ethical, Legal, and Regulatory Issues Associated with Neural Chimeras and Organoids. The Emerging Field of Human Neural Organoids, Transplants, and Chimeras: Science, Ethics, and Governance; National Academies Press (US): Washington, DC, USA, 2021; ISBN 9780309303361. [Google Scholar]
- Powell, K. Hybrid Brains: The Ethics of Transplanting Human Neurons into Animals. Nature 2022, 608, 22–25. [Google Scholar] [CrossRef]
- Brennand, K.J.; Simone, A.; Jou, J.; Gelboin-Burkhart, C.; Tran, N.; Sangar, S.; Li, Y.; Mu, Y.; Chen, G.; Yu, D.; et al. Modelling Schizophrenia Using Human Induced Pluripotent Stem Cells. Nature 2011, 473, 221–225. [Google Scholar] [CrossRef]
- Paşca, S.P.; Portmann, T.; Voineagu, I.; Yazawa, M.; Shcheglovitov, A.; Paşca, A.M.; Cord, B.; Palmer, T.D.; Chikahisa, S.; Nishino, S.; et al. Using iPSC-Derived Neurons to Uncover Cellular Phenotypes Associated with Timothy Syndrome. Nat. Med. 2011, 17, 1657–1662. [Google Scholar] [CrossRef]
- Dolmetsch, R.; Geschwind, D.H. The Human Brain in a Dish: The Promise of iPSC-Derived Neurons. Cell 2011, 145, 831–834. [Google Scholar] [CrossRef]
- Rowe, R.G.; Daley, G.Q. Induced Pluripotent Stem Cells in Disease Modelling and Drug Discovery. Nat. Rev. Genet. 2019, 20, 377–388. [Google Scholar] [CrossRef]
- Panda, A.; Suvakov, M.; Mariani, J.; Drucker, K.L.; Park, Y.; Jang, Y.; Kollmeyer, T.M.; Sarkar, G.; Bae, T.; Kim, J.J.; et al. Clonally Selected Lines after CRISPR/Cas Editing Are Not Isogenic. bioRxiv 2022. bioRxiv:2022.05.17.492193. [Google Scholar]
- Kato, M.; Dobyns, W.B. Lissencephaly and the Molecular Basis of Neuronal Migration. Hum. Mol. Genet. 2003, 12, R89–R96. [Google Scholar] [CrossRef] [PubMed]
- Cahana, A.; Escamez, T.; Nowakowski, R.S.; Hayes, N.L.; Giacobini, M.; von Holst, A.; Shmueli, O.; Sapir, T.; McConnell, S.K.; Wurst, W.; et al. Targeted Mutagenesis of Lis1 Disrupts Cortical Development and LIS1 Homodimerization. Proc. Natl. Acad. Sci. USA 2001, 98, 6429–6434. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.H.; Pramparo, T.; Hirotsune, S.; Wynshaw-Boris, A. Distinct Dose-Dependent Cortical Neuronal Migration and Neurite Extension Defects in Lis1 and Ndel1 Mutant Mice. J. Neurosci. 2009, 29, 15520–15530. [Google Scholar] [CrossRef] [PubMed]
- Karzbrun, E.; Kshirsagar, A.; Cohen, S.R.; Hanna, J.H.; Reiner, O. Human Brain Organoids on a Chip Reveal the Physics of Folding. Nat. Phys. 2018, 14, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Thiele, E.A. Managing and Understanding Epilepsy in Tuberous Sclerosis Complex. Epilepsia 2010, 51 (Suppl. S1), 90–91. [Google Scholar] [CrossRef]
- European Chromosome 16 Tuberous Sclerosis Consortium Identification and Characterization of the Tuberous Sclerosis Gene on Chromosome 16. Cell 1993, 75, 1305–1315. [CrossRef]
- van Slegtenhorst, M.; de Hoogt, R.; Hermans, C.; Nellist, M.; Janssen, B.; Verhoef, S.; Lindhout, D.; van den Ouweland, A.; Halley, D.; Young, J.; et al. Identification of the Tuberous Sclerosis Gene TSC1 on Chromosome 9q34. Science 1997, 277, 805–808. [Google Scholar] [CrossRef]
- Tee, A.R.; Fingar, D.C.; Manning, B.D.; Kwiatkowski, D.J.; Cantley, L.C.; Blenis, J. Tuberous Sclerosis Complex-1 and -2 Gene Products Function Together to Inhibit Mammalian Target of Rapamycin (mTOR)-Mediated Downstream Signaling. Proc. Natl. Acad. Sci. USA 2002, 99, 13571–13576. [Google Scholar] [CrossRef] [Green Version]
- Costa, V.; Aigner, S.; Vukcevic, M.; Sauter, E.; Behr, K.; Ebeling, M.; Dunkley, T.; Friedlein, A.; Zoffmann, S.; Meyer, C.A.; et al. mTORC1 Inhibition Corrects Neurodevelopmental and Synaptic Alterations in a Human Stem Cell Model of Tuberous Sclerosis. Cell Rep. 2016, 15, 86–95. [Google Scholar] [CrossRef]
- Andrews, M.G.; Subramanian, L.; Kriegstein, A.R. mTOR Signaling Regulates the Morphology and Migration of Outer Radial Glia in Developing Human Cortex. Elife 2020, 9, e58737. [Google Scholar] [CrossRef]
- Onda, H.; Crino, P.B.; Zhang, H.; Murphey, R.D.; Rastelli, L.; Gould Rothberg, B.E.; Kwiatkowski, D.J. Tsc2 Null Murine Neuroepithelial Cells Are a Model for Human Tuber Giant Cells, and Show Activation of an mTOR Pathway. Mol. Cell. Neurosci. 2002, 21, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.D.; Hockemeyer, D.; Bateup, H.S. Genetically Engineered Human Cortical Spheroid Models of Tuberous Sclerosis. Nat. Med. 2018, 24, 1568–1578. [Google Scholar] [CrossRef]
- Klaus, J.; Kanton, S.; Kyrousi, C.; Ayo-Martin, A.C.; Di Giaimo, R.; Riesenberg, S.; O’Neill, A.C.; Camp, J.G.; Tocco, C.; Santel, M.; et al. Altered Neuronal Migratory Trajectories in Human Cerebral Organoids Derived from Individuals with Neuronal Heterotopia. Nat. Med. 2019, 25, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Birey, F.; Li, M.-Y.; Gordon, A.; Thete, M.V.; Valencia, A.M.; Revah, O.; Paşca, A.M.; Geschwind, D.H.; Paşca, S.P. Dissecting the Molecular Basis of Human Interneuron Migration in Forebrain Assembloids from Timothy Syndrome. Cell Stem Cell 2022, 29, 248–264.e7. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, M.; Akamatsu, W.; Okada, Y.; Ohnishi, T.; Balan, S.; Hisano, Y.; Iwayama, Y.; Toyota, T.; Matsumoto, T.; Itasaka, N.; et al. Analysis of Induced Pluripotent Stem Cells Carrying 22q11.2 Deletion. Transl. Psychiatry 2016, 6, e934. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.; Marinaro, F.; De Pietri Tonelli, D.; Berdondini, L. Developmental Excitatory-to-Inhibitory GABA-Polarity Switch Is Disrupted in 22q11.2 Deletion Syndrome: A Potential Target for Clinical Therapeutics. Sci. Rep. 2017, 7, 15752. [Google Scholar] [CrossRef]
- Li, J.; Ryan, S.K.; Deboer, E.; Cook, K.; Fitzgerald, S.; Lachman, H.M.; Wallace, D.C.; Goldberg, E.M.; Anderson, S.A. Mitochondrial Deficits in Human iPSC-Derived Neurons from Patients with 22q11.2 Deletion Syndrome and Schizophrenia. Transl. Psychiatry 2019, 9, 302. [Google Scholar] [CrossRef]
- Khan, T.A.; Revah, O.; Gordon, A.; Yoon, S.-J.; Krawisz, A.K.; Goold, C.; Sun, Y.; Kim, C.H.; Tian, Y.; Li, M.-Y.; et al. Neuronal Defects in a Human Cellular Model of 22q11.2 Deletion Syndrome. Nat. Med. 2020, 26, 1888–1898. [Google Scholar] [CrossRef]
- Landthaler, M.; Yalcin, A.; Tuschl, T. The Human DiGeorge Syndrome Critical Region Gene 8 and Its D. Melanogaster Homolog Are Required for miRNA Biogenesis. Curr. Biol. 2004, 14, 2162–2167. [Google Scholar] [CrossRef]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett Syndrome Is Caused by Mutations in X-Linked MECP2, Encoding Methyl-CpG-Binding Protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- Lewis, J.D.; Meehan, R.R.; Henzel, W.J.; Maurer-Fogy, I.; Jeppesen, P.; Klein, F.; Bird, A. Purification, Sequence, and Cellular Localization of a Novel Chromosomal Protein That Binds to Methylated DNA. Cell 1992, 69, 905–914. [Google Scholar] [CrossRef]
- Tropea, D.; Giacometti, E.; Wilson, N.R.; Beard, C.; McCurry, C.; Fu, D.D.; Flannery, R.; Jaenisch, R.; Sur, M. Partial Reversal of Rett Syndrome-like Symptoms in MeCP2 Mutant Mice. Proc. Natl. Acad. Sci. USA 2009, 106, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Marchetto, M.C.N.; Carromeu, C.; Acab, A.; Yu, D.; Yeo, G.W.; Mu, Y.; Chen, G.; Gage, F.H.; Muotri, A.R. A Model for Neural Development and Treatment of Rett Syndrome Using Human Induced Pluripotent Stem Cells. Cell 2010, 143, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, C.A.; Adams, J.W.; Negraes, P.D.; Carromeu, C.; Tejwani, L.; Acab, A.; Tsuda, B.; Thomas, C.A.; Sodhi, N.; Fichter, K.M.; et al. Pharmacological Reversal of Synaptic and Network Pathology in Human MECP2-KO Neurons and Cortical Organoids. EMBO Mol. Med. 2021, 13, e12523. [Google Scholar] [CrossRef]
- Samarasinghe, R.A.; Miranda, O.A.; Buth, J.E.; Mitchell, S.; Ferando, I.; Watanabe, M.; Allison, T.F.; Kurdian, A.; Fotion, N.N.; Gandal, M.J.; et al. Identification of Neural Oscillations and Epileptiform Changes in Human Brain Organoids. Nat. Neurosci. 2021, 24, 1488–1500. [Google Scholar] [CrossRef]
- Ohashi, M.; Korsakova, E.; Allen, D.; Lee, P.; Fu, K.; Vargas, B.S.; Cinkornpumin, J.; Salas, C.; Park, J.C.; Germanguz, I.; et al. Loss of MECP2 Leads to Activation of P53 and Neuronal Senescence. Stem Cell Reports 2018, 10, 1453–1463. [Google Scholar] [CrossRef]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.-Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e23. [Google Scholar] [CrossRef]
- Sanders, S.J.; He, X.; Willsey, A.J.; Ercan-Sencicek, A.G.; Samocha, K.E.; Cicek, A.E.; Murtha, M.T.; Bal, V.H.; Bishop, S.L.; Dong, S.; et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron 2015, 87, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Kim, C.; Bakken, T.E.; Gillentine, M.A.; Henning, B.; Mao, Y.; Gilissen, C.; The SPARK Consortium; Nowakowski, T.J.; Eichler, E.E. Integrated Gene Analyses of de Novo Mutations from 46,612 Trios with Autism and Developmental Disorders. bioRxiv 2021. bioRxiv:2021.09.15.460398. [Google Scholar]
- Willsey, H.R.; Willsey, A.J.; Wang, B.; State, M.W. Genomics, Convergent Neuroscience and Progress in Understanding Autism Spectrum Disorder. Nat. Rev. Neurosci. 2022, 23, 323–341. [Google Scholar] [CrossRef]
- Weiss, L.A.; Shen, Y.; Korn, J.M.; Arking, D.E.; Miller, D.T.; Fossdal, R.; Saemundsen, E.; Stefansson, H.; Ferreira, M.A.R.; Green, T.; et al. Association between Microdeletion and Microduplication at 16p11.2 and Autism. N. Engl. J. Med. 2008, 358, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Yadav, S.; Dao, D.Q.; Wu, Z.-Y.; Hokanson, K.C.; Cahill, M.K.; Wiita, A.P.; Jan, Y.-N.; Ullian, E.M.; Weiss, L.A. Cellular Phenotypes in Human iPSC-Derived Neurons from a Genetic Model of Autism Spectrum Disorder. Cell Rep. 2017, 21, 2678–2687. [Google Scholar] [CrossRef] [PubMed]
- Urresti, J.; Zhang, P.; Moran-Losada, P.; Yu, N.-K.; Negraes, P.D.; Trujillo, C.A.; Antaki, D.; Amar, M.; Chau, K.; Pramod, A.B.; et al. Cortical Organoids Model Early Brain Development Disrupted by 16p11.2 Copy Number Variants in Autism. Mol. Psychiatry 2021, 26, 7560–7580. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.T.; Chan, Y.; Dawes, P.; Guo, X.; Erdin, S.; Tai, D.J.C.; Liu, S.; Reichert, J.M.; Burns, M.J.; Chan, Y.K.; et al. Orgo-Seq Integrates Single-Cell and Bulk Transcriptomic Data to Identify Cell Type Specific-Driver Genes Associated with Autism Spectrum Disorder. Nat. Commun. 2022, 13, 3243. [Google Scholar] [CrossRef]
- Kang, H.J.; Kawasawa, Y.I.; Cheng, F.; Zhu, Y.; Xu, X.; Li, M.; Sousa, A.M.M.; Pletikos, M.; Meyer, K.A.; Sedmak, G.; et al. Spatio-Temporal Transcriptome of the Human Brain. Nature 2011, 478, 483–489. [Google Scholar] [CrossRef]
- Willsey, A.J.; Sanders, S.J.; Li, M.; Dong, S.; Tebbenkamp, A.T.; Muhle, R.A.; Reilly, S.K.; Lin, L.; Fertuzinhos, S.; Miller, J.A.; et al. Coexpression Networks Implicate Human Midfetal Deep Cortical Projection Neurons in the Pathogenesis of Autism. Cell 2013, 155, 997–1007. [Google Scholar] [CrossRef]
- Parikshak, N.N.; Luo, R.; Zhang, A.; Won, H.; Lowe, J.K.; Chandran, V.; Horvath, S.; Geschwind, D.H. Integrative Functional Genomic Analyses Implicate Specific Molecular Pathways and Circuits in Autism. Cell 2013, 155, 1008–1021. [Google Scholar] [CrossRef]
- Gamache, T.R.; Araki, Y.; Huganir, R.L. Twenty Years of SynGAP Research: From Synapses to Cognition. J. Neurosci. 2020, 40, 1596–1605. [Google Scholar] [CrossRef]
- Birtele, M.; Del Dosso, A.; Xu, T.; Nguyen, T.; Wilkinson, B.; Urenda, J.-P.; Knight, G.; Moore, R.; Sharma, R.; Pirrotte, P.; et al. The Autism-Associated Gene SYNGAP1 Regulates Human Cortical Neurogenesis. bioRxiv 2022. bioRxiv:2022.05.10.491244. [Google Scholar]
- Lalli, M.A.; Avey, D.; Dougherty, J.D.; Milbrandt, J.; Mitra, R.D. High-Throughput Single-Cell Functional Elucidation of Neurodevelopmental Disease-Associated Genes Reveals Convergent Mechanisms Altering Neuronal Differentiation. Genome Res. 2020, 30, 1317–1331. [Google Scholar] [CrossRef]
- Paulsen, B.; Velasco, S.; Kedaigle, A.J.; Pigoni, M.; Quadrato, G.; Deo, A.J.; Adiconis, X.; Uzquiano, A.; Sartore, R.; Yang, S.M.; et al. Autism Genes Converge on Asynchronous Development of Shared Neuron Classes. Nature 2022, 602, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.E.; Cheroni, C.; Dotter, C.P.; López-Tóbon, A.; Oliveira, B.; Sacco, R.; Yahya, A.Ç.; Morandell, J.; Gabriele, M.; Tavakoli, M.R.; et al. CHD8 Haploinsufficiency Links Autism to Transient Alterations in Excitatory and Inhibitory Trajectories. Cell Rep. 2022, 39, 110615. [Google Scholar] [CrossRef]
- Crucitti, J.; Hyde, C.; Enticott, P.G.; Stokes, M.A. Head Circumference Trends in Autism between 0 and 100 Months. Autism 2020, 24, 1726–1739. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Coppola, G.; Zhang, P.; Abyzov, A.; Provini, L.; Tomasini, L.; Amenduni, M.; Szekely, A.; Palejev, D.; Wilson, M.; et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell 2015, 162, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Adhya, D.; Swarup, V.; Nagy, R.; Dutan, L.; Shum, C.; Valencia-Alarcón, E.P.; Jozwik, K.M.; Mendez, M.A.; Horder, J.; Loth, E.; et al. Atypical Neurogenesis in Induced Pluripotent Stem Cells from Autistic Individuals. Biol. Psychiatry 2021, 89, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Jourdon, A.; Wu, F.; Mariani, J.; Capauto, D.; Norton, S.; Tomasini, L.; Amiri, A.; Suvakov, M.; Schreiner, J.D.; Jang, Y.; et al. ASD Modelling in Organoids Reveals Imbalance of Excitatory Cortical Neuron Subtypes during Early Neurogenesis. bioRxiv 2022. bioRxiv:2022.03.19.484988. [Google Scholar]
- Hergenreder, E.; Zorina, Y.; Zhao, Z.; Munguba, H.; Calder, E.L.; Baggiolini, A.; Minotti, A.P.; Walsh, R.M.; Liston, C.; Levitz, J.; et al. Combined Small Molecule Treatment Accelerates Timing of Maturation in Human Pluripotent Stem Cell-Derived Neurons. bioRxiv 2022. bioRxiv:2022.06.02.494616. [Google Scholar]
- Ciceri, G.; Cho, H.; Kshirsagar, M.; Baggiolini, A.; Aromolaran, K.A.; Walsh, R.M.; Goldstein, P.A.; Koche, R.P.; Leslie, C.S.; Studer, L. An Epigenetic Barrier Sets the Timing of Human Neuronal Maturation. bioRxiv 2022. bioRxiv:2022.06.02.490114. [Google Scholar]
- Feldman, D.; Singh, A.; Schmid-Burgk, J.L.; Carlson, R.J.; Mezger, A.; Garrity, A.J.; Zhang, F.; Blainey, P.C. Optical Pooled Screens in Human Cells. Cell 2019, 179, 787–799.e17. [Google Scholar] [CrossRef]
- Rubin, A.J.; Parker, K.R.; Satpathy, A.T.; Qi, Y.; Wu, B.; Ong, A.J.; Mumbach, M.R.; Ji, A.L.; Kim, D.S.; Cho, S.W.; et al. Coupled Single-Cell CRISPR Screening and Epigenomic Profiling Reveals Causal Gene Regulatory Networks. Cell 2019, 176, 361–376.e17. [Google Scholar] [CrossRef]
- Cleary, B.; Regev, A. The Necessity and Power of Random, under-Sampled Experiments in Biology. arXiv 2020, arXiv:2012.12961. [Google Scholar]
- Wu, Z.; Chhun, B.B.; Popova, G.; Guo, S.-M.; Kim, C.N.; Yeh, L.-H.; Nowakowski, T.; Zou, J.; Mehta, S.B. DynaMorph: Self-Supervised Learning of Morphodynamic States of Live Cells. Mol. Biol. Cell 2022, 33, ar59. [Google Scholar] [CrossRef]
- Moreau, C.A.; Kumar, K.; Harvey, A.; Huguet, G.; Urchs, S.; Douard, E.A.; Schultz, L.M.; Sharmarke, H.; Jizi, K.; Martin, C.-O.; et al. Atlas of Functional Connectivity Relationships across Rare and Common Genetic Variants, Traits, and Psychiatric Conditions. bioRxiv 2021. [Google Scholar] [CrossRef]
- Velmeshev, D.; Schirmer, L.; Jung, D.; Haeussler, M.; Perez, Y.; Mayer, S.; Bhaduri, A.; Goyal, N.; Rowitch, D.H.; Kriegstein, A.R. Single-Cell Genomics Identifies Cell Type-Specific Molecular Changes in Autism. Science 2019, 364, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, G.; Won, H.; Gandal, M.J.; Haney, J.; Wang, J.C.; Wong, C.C.Y.; Sun, W.; Prabhakar, S.; Mill, J.; Geschwind, D.H. Integrative Genomics Identifies a Convergent Molecular Subtype That Links Epigenomic with Transcriptomic Differences in Autism. Nat. Commun. 2020, 11, 4873. [Google Scholar] [CrossRef]
- de la Torre-Ubieta, L.; Won, H.; Stein, J.L.; Geschwind, D.H. Advancing the Understanding of Autism Disease Mechanisms through Genetics. Nat. Med. 2016, 22, 345–361. [Google Scholar] [CrossRef]
- Chen, J.A.; Peñagarikano, O.; Belgard, T.G.; Swarup, V.; Geschwind, D.H. The Emerging Picture of Autism Spectrum Disorder: Genetics and Pathology. Annu. Rev. Pathol. 2015, 10, 111–144. [Google Scholar] [CrossRef]
- Ruzicka, W.B.; Mohammadi, S.; Davila-Velderrain, J.; Subburaju, S.; Tso, D.R.; Hourihan, M.; Kellis, M. Single-Cell Dissection of Schizophrenia Reveals Neurodevelopmental-Synaptic Axis and Transcriptional Resilience. bioRxiv 2020. [Google Scholar] [CrossRef]
- Bhaduri, A.; Andrews, M.G.; Kriegstein, A.R.; Nowakowski, T.J. Are Organoids Ready for Prime Time? Cell Stem Cell 2020, 27, 361–365. [Google Scholar] [CrossRef]
- Willsey, H.R.; Exner, C.R.T.; Xu, Y.; Everitt, A.; Sun, N.; Wang, B.; Dea, J.; Schmunk, G.; Zaltsman, Y.; Teerikorpi, N.; et al. Parallel in Vivo Analysis of Large-Effect Autism Genes Implicates Cortical Neurogenesis and Estrogen in Risk and Resilience. Neuron 2021, 109, 788–804.e8. [Google Scholar] [CrossRef]
- Ortiz-Virumbrales, M.; Moreno, C.L.; Kruglikov, I.; Marazuela, P.; Sproul, A.; Jacob, S.; Zimmer, M.; Paull, D.; Zhang, B.; Schadt, E.E.; et al. CRISPR/Cas9-Correctable Mutation-Related Molecular and Physiological Phenotypes in iPSC-Derived Alzheimer’s PSEN2 N141I Neurons. Acta Neuropathol. Commun. 2017, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Cooper, O.; Seo, H.; Andrabi, S.; Guardia-Laguarta, C.; Graziotto, J.; Sundberg, M.; McLean, J.R.; Carrillo-Reid, L.; Xie, Z.; Osborn, T.; et al. Pharmacological Rescue of Mitochondrial Deficits in iPSC-Derived Neural Cells from Patients with Familial Parkinson’s Disease. Sci. Transl. Med. 2012, 4, 141ra90. [Google Scholar] [CrossRef] [PubMed]
- Woodard, C.M.; Campos, B.A.; Kuo, S.-H.; Nirenberg, M.J.; Nestor, M.W.; Zimmer, M.; Mosharov, E.V.; Sulzer, D.; Zhou, H.; Paull, D.; et al. iPSC-Derived Dopamine Neurons Reveal Differences between Monozygotic Twins Discordant for Parkinson’s Disease. Cell Rep. 2014, 9, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Ochalek, A.; Mihalik, B.; Avci, H.X.; Chandrasekaran, A.; Téglási, A.; Bock, I.; Giudice, M.L.; Táncos, Z.; Molnár, K.; László, L.; et al. Neurons Derived from Sporadic Alzheimer’s Disease iPSCs Reveal Elevated TAU Hyperphosphorylation, Increased Amyloid Levels, and GSK3B Activation. Alzheimers. Res. Ther. 2017, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.H.; Kim, Y.H.; Hebisch, M.; Sliwinski, C.; Lee, S.; D’Avanzo, C.; Chen, H.; Hooli, B.; Asselin, C.; Muffat, J.; et al. A Three-Dimensional Human Neural Cell Culture Model of Alzheimer’s Disease. Nature 2014, 515, 274–278. [Google Scholar] [CrossRef]
- Penney, J.; Ralvenius, W.T.; Tsai, L.-H. Modeling Alzheimer’s Disease with iPSC-Derived Brain Cells. Mol. Psychiatry 2020, 25, 148–167. [Google Scholar] [CrossRef]
- Alić, I.; Goh, P.A.; Murray, A.; Portelius, E.; Gkanatsiou, E.; Gough, G.; Mok, K.Y.; Koschut, D.; Brunmeir, R.; Yeap, Y.J.; et al. Correction: Patient-Specific Alzheimer-like Pathology in Trisomy 21 Cerebral Organoids Reveals BACE2 as a Gene Dose-Sensitive AD Suppressor in Human Brain. Mol. Psychiatry 2021, 26, 5789. [Google Scholar] [CrossRef]
- Gonzalez, C.; Armijo, E.; Bravo-Alegria, J.; Becerra-Calixto, A.; Mays, C.E.; Soto, C. Modeling Amyloid Beta and Tau Pathology in Human Cerebral Organoids. Mol. Psychiatry 2018, 23, 2363–2374. [Google Scholar] [CrossRef]
- Meharena, H.S.; Marco, A.; Dileep, V.; Lockshin, E.R.; Akatsu, G.Y.; Mullahoo, J.; Watson, L.A.; Ko, T.; Guerin, L.N.; Abdurrob, F.; et al. Down-Syndrome-Induced Senescence Disrupts the Nuclear Architecture of Neural Progenitors. Cell Stem Cell 2022, 29, 116–130.e7. [Google Scholar] [CrossRef]
- Tian, R.; Abarientos, A.; Hong, J.; Hashemi, S.H.; Yan, R.; Dräger, N.; Leng, K.; Nalls, M.A.; Singleton, A.B.; Xu, K.; et al. Genome-Wide CRISPRi/a Screens in Human Neurons Link Lysosomal Failure to Ferroptosis. Nat. Neurosci. 2021, 24, 1020–1034. [Google Scholar] [CrossRef]
- Tian, R.; Gachechiladze, M.A.; Ludwig, C.H.; Laurie, M.T.; Hong, J.Y.; Nathaniel, D.; Prabhu, A.V.; Fernandopulle, M.S.; Patel, R.; Abshari, M.; et al. CRISPR Interference-Based Platform for Multimodal Genetic Screens in Human iPSC-Derived Neurons. Neuron 2019, 104, 239–255.e12. [Google Scholar] [CrossRef] [PubMed]
- Dräger, N.M.; Sattler, S.M.; Huang, C.T.-L.; Teter, O.M.; Leng, K.; Hashemi, S.H.; Hong, J.; Aviles, G.; Clelland, C.D.; Zhan, L.; et al. A CRISPRi/a Platform in iPSC-Derived Microglia Uncovers Regulators of Disease States. bioRxiv 2022. bioRxiv:2021.06.16.448639. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Y.; Xu, L.; Wang, J.; Hong, Y.; Wang, Y.; Zhu, Q.; Wang, D.; Zhang, X.-Y.; Liu, C.-Y.; Fang, K.-H.; et al. DSCAM/PAK1 Pathway Suppression Reverses Neurogenesis Deficits in iPSC-Derived Cerebral Organoids from Patients with Down Syndrome. J. Clin. Investig. 2021, 131, e135763. [Google Scholar] [CrossRef] [PubMed]
- Bowles, K.R.; Silva, M.C.; Whitney, K.; Bertucci, T.; Berlind, J.E.; Lai, J.D.; Garza, J.C.; Boles, N.C.; Mahali, S.; Strang, K.H.; et al. ELAVL4, Splicing, and Glutamatergic Dysfunction Precede Neuron Loss in MAPT Mutation Cerebral Organoids. Cell 2021, 184, 4547–4563.e17. [Google Scholar] [CrossRef]
- Staats, K.A.; Seah, C.; Sahimi, A.; Wang, Y.; Koutsodendris, N.; Lin, S.; Kim, D.; Chang, W.-H.; Gray, K.A.; Shi, Y.; et al. Small Molecule Inhibition of PIKFYVE Kinase Rescues Gain- and Loss-of-Function C9ORF72 ALS/FTD Disease Processes in Vivo. bioRxiv 2019. bioRxiv:685800. [Google Scholar]
- Elston, G.N. Cortex, Cognition and the Cell: New Insights into the Pyramidal Neuron and Prefrontal Function. Cereb. Cortex 2003, 13, 1124–1138. [Google Scholar] [CrossRef]
- Kriegstein, A.; Noctor, S.; Martínez-Cerdeño, V. Patterns of Neural Stem and Progenitor Cell Division May Underlie Evolutionary Cortical Expansion. Nat. Rev. Neurosci. 2006, 7, 883–890. [Google Scholar] [CrossRef]
- Borrell, V.; Reillo, I. Emerging Roles of Neural Stem Cells in Cerebral Cortex Development and Evolution. Dev. Neurobiol. 2012, 72, 955–971. [Google Scholar] [CrossRef]
- Borrell, V.; Calegari, F. Mechanisms of Brain Evolution: Regulation of Neural Progenitor Cell Diversity and Cell Cycle Length. Neurosci. Res. 2014, 86, 14–24. [Google Scholar] [CrossRef]
- Molnár, Z.; Métin, C.; Stoykova, A.; Tarabykin, V.; Price, D.J.; Francis, F.; Meyer, G.; Dehay, C.; Kennedy, H. Comparative Aspects of Cerebral Cortical Development. Eur. J. Neurosci. 2006, 23, 921–934. [Google Scholar] [CrossRef]
- Rakic, P. Evolution of the Neocortex: A Perspective from Developmental Biology. Nat. Rev. Neurosci. 2009, 10, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P. Developmental and Evolutionary Adaptations of Cortical Radial Glia. Cereb. Cortex 2003, 13, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Monuki, E.S.; Walsh, C.A. Mechanisms of Cerebral Cortical Patterning in Mice and Humans. Nat. Neurosci. 2001, 4, 1199–1206. [Google Scholar] [CrossRef]
- Chenn, A.; Walsh, C.A. Regulation of Cerebral Cortical Size by Control of Cell Cycle Exit in Neural Precursors. Science 2002, 297, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Kuida, K.; Haydar, T.F.; Kuan, C.Y.; Gu, Y.; Taya, C.; Karasuyama, H.; Su, M.S.; Rakic, P.; Flavell, R.A. Reduced Apoptosis and Cytochrome c-Mediated Caspase Activation in Mice Lacking Caspase 9. Cell 1998, 94, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Lui, J.H.; Hansen, D.V.; Kriegstein, A.R. Development and Evolution of the Human Neocortex. Cell 2011, 146, 18–36. [Google Scholar] [CrossRef]
- Dehay, C.; Giroud, P.; Berland, M.; Killackey, H.P.; Kennedy, H. Phenotypic Characterisation of Respecified Visual Cortex Subsequent to Prenatal Enucleation in the Monkey: Development of Acetylcholinesterase and Cytochrome Oxidase Patterns. J. Comp. Neurol. 1996, 376, 386–402. [Google Scholar] [CrossRef]
- Sulovari, A.; Li, R.; Audano, P.A.; Porubsky, D.; Vollger, M.R.; Logsdon, G.A.; Human Genome Structural Variation Consortium; Warren, W.C.; Pollen, A.A.; Chaisson, M.J.P.; et al. Human-Specific Tandem Repeat Expansion and Differential Gene Expression during Primate Evolution. Proc. Natl. Acad. Sci. USA 2019, 116, 23243–23253. [Google Scholar] [CrossRef]
- Dougherty, M.L.; Underwood, J.G.; Nelson, B.J.; Tseng, E.; Munson, K.M.; Penn, O.; Nowakowski, T.J.; Pollen, A.A.; Eichler, E.E. Transcriptional Fates of Human-Specific Segmental Duplications in Brain. Genome Res. 2018, 28, 1566–1576. [Google Scholar] [CrossRef]
- Kronenberg, Z.N.; Fiddes, I.T.; Gordon, D.; Murali, S.; Cantsilieris, S.; Meyerson, O.S.; Underwood, J.G.; Nelson, B.J.; Chaisson, M.J.P.; Dougherty, M.L.; et al. High-Resolution Comparative Analysis of Great Ape Genomes. Science 2018, 360, eaar6343. [Google Scholar] [CrossRef]
- Pollard, K.S.; Salama, S.R.; Lambert, N.; Lambot, M.-A.; Coppens, S.; Pedersen, J.S.; Katzman, S.; King, B.; Onodera, C.; Siepel, A.; et al. An RNA Gene Expressed during Cortical Development Evolved Rapidly in Humans. Nature 2006, 443, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Fiddes, I.T.; Lodewijk, G.A.; Mooring, M.; Bosworth, C.M.; Ewing, A.D.; Mantalas, G.L.; Novak, A.M.; van den Bout, A.; Bishara, A.; Rosenkrantz, J.L.; et al. Human-Specific NOTCH2NL Genes Affect Notch Signaling and Cortical Neurogenesis. Cell 2018, 173, 1356–1369.e22. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I.K.; Gacquer, D.; Van Heurck, R.; Kumar, D.; Wojno, M.; Bilheu, A.; Herpoel, A.; Lambert, N.; Cheron, J.; Polleux, F.; et al. Human-Specific NOTCH2NL Genes Expand Cortical Neurogenesis through Delta/Notch Regulation. Cell 2018, 173, 1370–1384.e16. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, C.A.; Rice, E.S.; Schaefer, N.K.; Chaim, I.A.; Wheeler, E.C.; Madrigal, A.A.; Buchanan, J.; Preissl, S.; Wang, A.; Negraes, P.D.; et al. Reintroduction of the Archaic Variant of NOVA1 in Cortical Organoids Alters Neurodevelopment. Science 2021, 371, eaax2537. [Google Scholar] [CrossRef]
- Gallego Romero, I.; Pavlovic, B.J.; Hernando-Herraez, I.; Zhou, X.; Ward, M.C.; Banovich, N.E.; Kagan, C.L.; Burnett, J.E.; Huang, C.H.; Mitrano, A.; et al. A Panel of Induced Pluripotent Stem Cells from Chimpanzees: A Resource for Comparative Functional Genomics. Elife 2015, 4, e07103. [Google Scholar] [CrossRef]
- Otani, T.; Marchetto, M.C.; Gage, F.H.; Simons, B.D.; Livesey, F.J. 2D and 3D Stem Cell Models of Primate Cortical Development Identify Species-Specific Differences in Progenitor Behavior Contributing to Brain Size. Cell Stem Cell 2016, 18, 467–480. [Google Scholar] [CrossRef]
- Marchetto, M.C.; Hrvoj-Mihic, B.; Kerman, B.E.; Yu, D.X.; Vadodaria, K.C.; Linker, S.B.; Narvaiza, I.; Santos, R.; Denli, A.M.; Mendes, A.P.; et al. Species-Specific Maturation Profiles of Human, Chimpanzee and Bonobo Neural Cells. Elife 2019, 8, e37527. [Google Scholar] [CrossRef]
- Field, A.R.; Jacobs, F.M.J.; Fiddes, I.T.; Phillips, A.P.R.; Reyes-Ortiz, A.M.; LaMontagne, E.; Whitehead, L.; Meng, V.; Rosenkrantz, J.L.; Olsen, M.; et al. Structurally Conserved Primate LncRNAs Are Transiently Expressed during Human Cortical Differentiation and Influence Cell-Type-Specific Genes. Stem Cell Reports 2019, 12, 245–257. [Google Scholar] [CrossRef]
- Mora-Bermúdez, F.; Badsha, F.; Kanton, S.; Camp, J.G.; Vernot, B.; Köhler, K.; Voigt, B.; Okita, K.; Maricic, T.; He, Z.; et al. Differences and Similarities between Human and Chimpanzee Neural Progenitors during Cerebral Cortex Development. Elife 2016, 5, e18683. [Google Scholar] [CrossRef]
- Mora-Bermúdez, F.; Kanis, P.; Macak, D.; Peters, J.; Naumann, R.; Xing, L.; Sarov, M.; Winkler, S.; Oegema, C.E.; Haffner, C.; et al. Longer Metaphase and Fewer Chromosome Segregation Errors in Modern Human than Neanderthal Brain Development. Sci. Adv. 2022, 8, eabn7702. [Google Scholar] [CrossRef]
- Benito-Kwiecinski, S.; Giandomenico, S.L.; Sutcliffe, M.; Riis, E.S.; Freire-Pritchett, P.; Kelava, I.; Wunderlich, S.; Martin, U.; Wray, G.A.; McDole, K.; et al. An Early Cell Shape Transition Drives Evolutionary Expansion of the Human Forebrain. Cell 2021, 184, 2084–2102.e19. [Google Scholar] [CrossRef] [PubMed]
- Kanton, S.; Boyle, M.J.; He, Z.; Santel, M.; Weigert, A.; Sanchís-Calleja, F.; Guijarro, P.; Sidow, L.; Fleck, J.S.; Han, D.; et al. Organoid Single-Cell Genomic Atlas Uncovers Human-Specific Features of Brain Development. Nature 2019, 574, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Agoglia, R.M.; Sun, D.; Birey, F.; Yoon, S.-J.; Miura, Y.; Sabatini, K.; Pașca, S.P.; Fraser, H.B. Primate Cell Fusion Disentangles Gene Regulatory Divergence in Neurodevelopment. Nature 2021, 592, 421–427. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowakowski, T.J.; Salama, S.R. Cerebral Organoids as an Experimental Platform for Human Neurogenomics. Cells 2022, 11, 2803. https://doi.org/10.3390/cells11182803
Nowakowski TJ, Salama SR. Cerebral Organoids as an Experimental Platform for Human Neurogenomics. Cells. 2022; 11(18):2803. https://doi.org/10.3390/cells11182803
Chicago/Turabian StyleNowakowski, Tomasz J., and Sofie R. Salama. 2022. "Cerebral Organoids as an Experimental Platform for Human Neurogenomics" Cells 11, no. 18: 2803. https://doi.org/10.3390/cells11182803
APA StyleNowakowski, T. J., & Salama, S. R. (2022). Cerebral Organoids as an Experimental Platform for Human Neurogenomics. Cells, 11(18), 2803. https://doi.org/10.3390/cells11182803





