Mesenchymal Stem Cell Sheet Centrifuge-Assisted Layering Augments Pro-Regenerative Cytokine Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Umbilical Cord Mesenchymal Stem Cell (hUC-MSC) Culture
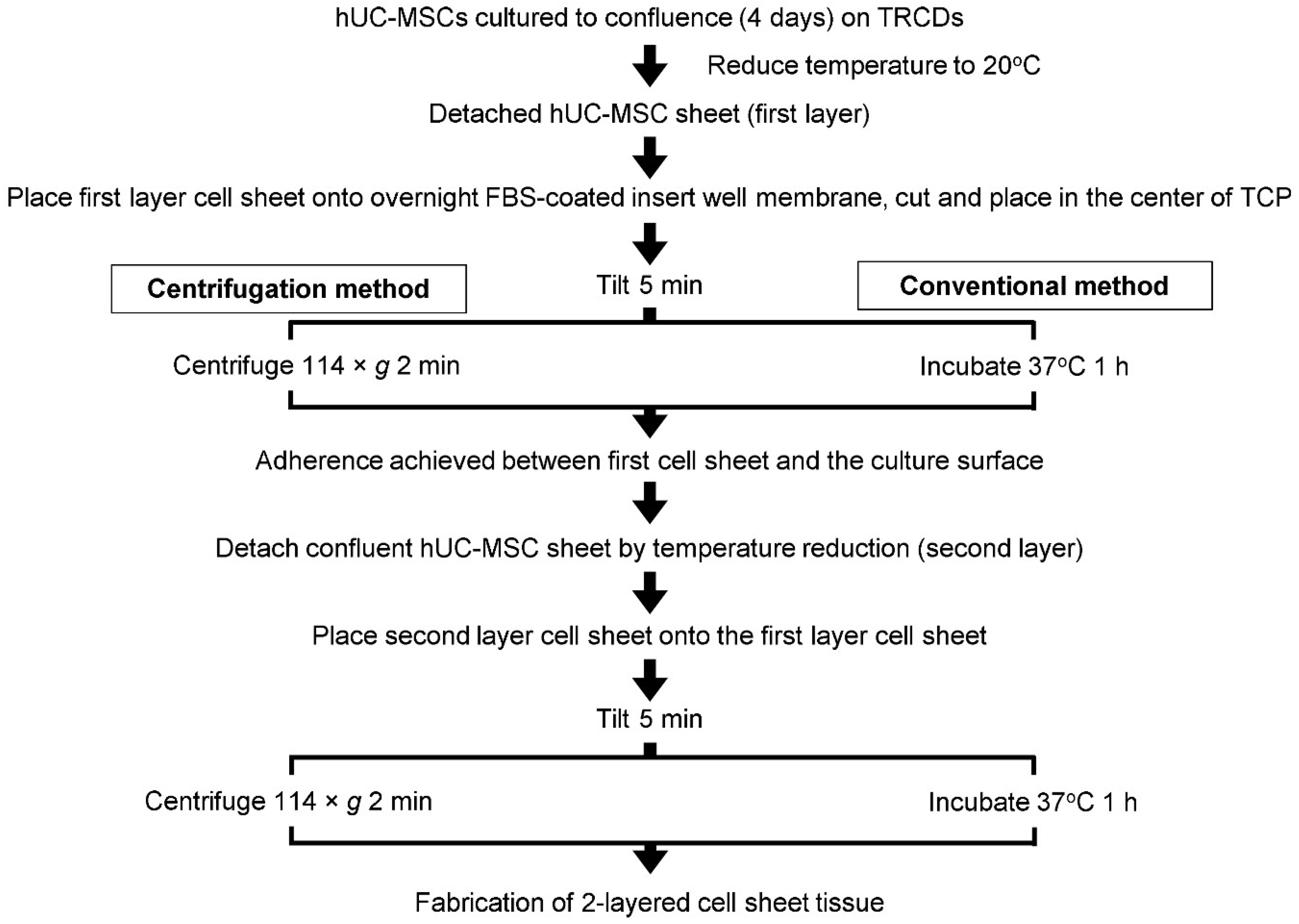
2.2. hUC-MSC Sheet Fabrication
2.3. Cell Sheet Tilting Optimization to Remove Excess Culture Media
2.4. Single Layer Cell Sheet Centrifugation
2.5. Preparation of Layered Cell Sheets by Conventional and Centrifugation Methods
2.6. Histological Analysis
2.7. Cell Proliferation Rate
2.8. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.9. Soluble Cytokine Secretion Quantification
2.10. Statistical Analysis
3. Results
3.1. Optimization of Cell Sheet-to-Surface Interactions
3.2. Centrifugation Alters Cellular Structures
3.3. Centrifugation Enhances Gene Expression Related to Cellular Interactions in One-Layer Cell Sheets
3.4. Enhanced Tissue Interactions Due to Centrifugation Increases MSC Pro-Regenerative Cytokine Production
3.5. Comparative Assessment of Two-Layer Cell Sheet Structure and Viability Fabricated by Conventional and Centrifugation Layering Methods
3.6. Centrifugation Enhances Cellular Function of Layered Cell Sheets
3.7. Cell Sheet Layering Augments MSC Cytokine Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baraniak, P.R.; McDevitt, T.C. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res. 2011, 347, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An Injury Drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.D.S.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.B.O.G.; Reis, R.L.G.; Sousa, N.J.C.; Gimble, J.M. Adipose Tissue Derived Stem Cells Secretome: Soluble Factors and Their Roles in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Iwata, T.; Morikawa, S.; Yamato, M.; Okano, T.; Uchigata, Y. Allogeneic Transplantation of an Adipose-Derived Stem Cell Sheet Combined With Artificial Skin Accelerates Wound Healing in a Rat Wound Model of Type 2 Diabetes and Obesity. Diabetes 2015, 64, 2723–2734. [Google Scholar] [CrossRef]
- Lee, D.E.; Ayoub, N.; Agrawal, D.K. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K.; et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 2006, 12, 459–465. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the Mesenchymal Stem Cell Secretome for the Treatment of Cardiovascular Disease. Cell Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef]
- Kim, M.-D.; Kim, S.-S.; Cha, H.-Y.; Jang, S.-H.; Chang, D.-Y.; Kim, W.H.; Suh-Kim, H.; Lee, J.-H. Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp. Mol. Med. 2014, 46, e110. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, M.; Ma, N.; Yin, G.; Cui, C.; Zhao, S. Dynamic tracking of injected mesenchymal stem cells after myocardial in-farction in rats: A serial 7T MRI study. Stem Cells Int. 2016, 2016, 4656539. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.M.; Teo, G.S.L. Mesenchymal Stem Cell Homing: The Devil Is in the Details. Cell Stem Cell 2009, 4, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Sart, S.; Tsai, A.-C.; Li, Y.; Ma, T. Three-Dimensional Aggregates of Mesenchymal Stem Cells: Cellular Mechanisms, Biological Properties, and Applications. Tissue Eng. Part B Rev. 2014, 20, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Wahl, E.A.; Fierro, F.A.; Peavy, T.R.; Hopfner, U.; Dye, J.F.; Machens, H.-G.; Egaña, J.T.; Schenck, T.L. In Vitro Evaluation of Scaffolds for the Delivery of Mesenchymal Stem Cells to Wounds. BioMed Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Mooney, D.J.; Duda, G.N.; Geissler, S. Biomaterials that promote cell-cell interactions enhance the paracrine function of MSCs. Biomaterials 2017, 140, 103–114. [Google Scholar] [CrossRef]
- Thomas, D.; Marsico, G.; Isa, I.L.M.; Thirumaran, A.; Chen, X.; Lukasz, B.; Fontana, G.; Rodriguez, B.; Marchetti-Deschmann, M.; O’Brien, T.; et al. Temporal changes guided by mesenchymal stem cells on a 3D microgel platform enhance angiogenesis in vivo at a low-cell dose. Proc. Natl. Acad. Sci. USA 2020, 117, 19033–19044. [Google Scholar] [CrossRef]
- Thomas, D.; O’Brien, T.; Pandit, A. Toward customized extracellular niche engineering: Progress in cell-entrapment technologies. Adv. Mater. 2018, 30, 1703948. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H.; Sakurai, Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 1995, 16, 297–303. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef]
- Bou-Ghannam, S.; Kim, K.; Grainger, D.W.; Okano, T. 3D cell sheet structure augments mesenchymal stem cell cytokine production. Sci. Rep. 2021, 11, 1–11. [Google Scholar]
- Thorp, H.; Kim, K.; Kondo, M.; Grainger, D.W.; Okano, T. Fabrication of hyaline-like cartilage constructs using mesenchymal stem cell sheets. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Yamato, M.; Tsuchioka, H.; Takagi, R.; Mukobata, S.; Washio, K.; Okano, T.; Ishikawa, I. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials 2009, 30, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef]
- Ohki, T.; Yamato, M.; Ota, M.; Takagi, R.; Murakami, D.; Kondo, M.; Sasaki, R.; Namiki, H.; Okano, T.; Yamamoto, M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 2012, 143, 582–588.e2. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Isomoto, H.; Kobayashi, S.; Kanai, N.; Kanetaka, K.; Sakai, Y.; Kasai, Y.; Takagi, R.; Ohki, T.; Fukuda, H.; et al. Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yamato, M.; Morino, T.; Sugiyama, H.; Takagi, R.; Yaguchi, Y.; Okano, T.; Kojima, H. Middle ear mucosal regeneration by tissue-engineered cell sheet transplantation. NPJ Regen. Med. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Kim, K.; Bou-Ghannam, S.; Okano, T. Cell sheet tissue engineering for scaffold-free three-dimensional (3D) tissue reconstruction. Methods Cell Biol. 2020, 157, 143–167. [Google Scholar]
- Takahashi, H.; Itoga, K.; Shimizu, T.; Yamato, M.; Okano, T. Human Neural Tissue Construct Fabrication Based on Scaffold-Free Tissue Engineering. Adv. Health Mater. 2016, 5, 1931–1938. [Google Scholar] [CrossRef]
- Ebihara, G.; Sato, M.; Yamato, M.; Mitani, G.; Kutsuna, T.; Nagai, T.; Ito, S.; Ukai, T.; Kobayashi, M.; Kokubo, M.; et al. Cartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig model. Biomaterials 2012, 33, 3846–3851. [Google Scholar] [CrossRef]
- Sekine, H.; Shimizu, T.; Dobashi, I.; Matsuura, K.; Hagiwara, N.; Takahashi, M.; Kobayashi, E.; Yamato, M.; Okano, T. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng. Part A 2011, 17, 2973–2980. [Google Scholar] [CrossRef]
- Nakao, M.; Kim, K.; Nagase, K.; Grainger, D.W.; Kanazawa, H.; Okano, T. Phenotypic traits of mesenchymal stem cell sheets fabricated by temperature-responsive cell culture plate: Structural characteristics of MSC sheets. Stem Cell Res. Ther. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Thorp, H.; Kim, K.; Bou-Ghannam, S.; Kondo, M.; Maak, T.; Grainger, D.W.; Okano, T. Enhancing chondrogenic potential via mesenchymal stem cell sheet multilayering. Regen. Ther. 2021, 18, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Motlekar, N.A.; Stein, A.; Diamond, S.L.; Shore, E.M.; Mauck, R.L. High-Throughput Screening for Modulators of Mesenchymal Stem Cell Chondrogenesis. Ann. Biomed. Eng. 2008, 36, 1909–1921. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Kouroupis, D.; Correa, D. Increased Mesenchymal Stem Cell Functionalization in Three-Dimensional Manufacturing Settings for Enhanced Therapeutic Applications. Front. Bioeng. Biotechnol. 2021, 9, 621748. [Google Scholar] [CrossRef]
- Markway, B.; Tan, G.-K.; Brooke, G.; Hudson, J.E.; Cooper-White, J.J.; Doran, M.R. Enhanced Chondrogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells in Low Oxygen Environment Micropellet Cultures. Cell Transpl. 2010, 19, 29–42. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Hasegawa, A.; Matsuura, K.; Kobayashi, M.; Iwana, S.-I.; Kabetani, Y.; Shimizu, T. Three-Dimensional Human Cardiac Tissue Engineered by Centrifugation of Stacked Cell Sheets and Cross-Sectional Observation of Its Synchronous Beatings by Optical Coherence Tomography. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Kagawa, Y.; Hasegawa, A.; Kubo, H.; Shimizu, T. Rapid fabrication of detachable three-dimensional tissues by layering of cell sheets with heating centrifuge. Biotechnol. Prog. 2018, 34, 692–701. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Matsuura, K.; Kagawa, Y.; Hasegawa, A.; Kubo, H.; Shimizu, T. Rapid creation system of morphologically and functionally communicative three-dimensional cell-dense tissue by centrifugation. Biotechnol. Prog. 2018, 34, 1447–1453. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Shimizu, T.; Sasagawa, T.; Sekine, H.; Sakaguchi, K.; Kikuchi, T.; Sekine, W.; Sekiya, S.; Yamato, M.; Umezu, M.; et al. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat. Protoc. 2012, 7, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Haraguchi, Y.; Shimizu, T.; Okano, T. Rapid fabrication system for three-dimensional tissues using cell sheet engineering and centrifugation. J. Biomed. Mater. Res. Part A 2015, 103, 3825–3833. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, Y.; Kagawa, Y.; Kubo, H.; Shimizu, T. Analysis of force vector field during centrifugation for optimizing cell sheet adhesion. Biotechnol. Prog. 2019, 35, e2857. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Kameishi, S.; Kim, K.; Metzler, N.F.; Maak, T.G.; Hutchinson, D.T.; Wang, A.A.; Maehara, M.; Sato, M.; Grainger, D.W.; et al. Safety and efficacy of human juvenile chondrocyte-derived cell sheets for osteochondral defect treatment. NPJ Regen. Med. 2021, 6, 1–11. [Google Scholar] [CrossRef]
- Baczynska, D.; Bombik, I.; Malicka-Błaszkiewicz, M. β-Catenin expression regulates cell migration of human colonic adenocarcinoma cells through gelsolin. Anticancer Res. 2016, 36, 5249–5256. [Google Scholar] [CrossRef][Green Version]
- Cai, S.X.; Liu, A.R.; Chen, S.; He, H.L.; Chen, Q.H.; Xu, J.Y.; Pan, C.; Yang, Y.; Guo, F.; Huang, Y.; et al. Activation of Wnt/β-catenin signalling promotes mesenchymal stem cells to repair injured alveolar epithelium induced by lipopolysaccharide in mice. Stem Cell Res. Ther. 2015, 6, 1–11. [Google Scholar]
- Mills, W.R.; Mal, N.; Kiedrowski, M.J.; Unger, R.; Forudi, F.; Popovic, Z.B.; Penn, M.S.; Laurita, K.R. Stem cell therapy enhances electrical viability in myocardial infarction. J. Mol. Cell. Cardiol. 2007, 42, 304–314. [Google Scholar] [CrossRef]
- Galbraith, C.G.; Davidson, M.W.; Galbraith, J.A. Coupling integrin dynamics to cellular adhesion behaviors. Biol. Open 2018, 7, bio036806. [Google Scholar] [CrossRef]
- Docheva, D.; Popov, C.; Mutschler, W.; Schieker, M. Human mesenchymal stem cells in contact with their environment: Surface characteristics and the integrin system. J. Cell. Mol. Med. 2007, 11, 21–38. [Google Scholar] [CrossRef]
- Kim, J.H.; Jekarl, D.W.; Kim, M.; Oh, E.J.; Kim, Y.; Park, I.Y.; Shin, J.C. Effects of ECM protein mimetics on adhesion and proliferation of chorion derived mesenchymal stem cells. Int. J. Med. Sci. 2014, 11, 298. [Google Scholar] [CrossRef]
- Sawyer, A.A.; Hennessy, K.M.; Bellis, S.L. The effect of adsorbed serum proteins, RGD and proteoglycan-binding peptides on the adhesion of mesenchymal stem cells to hydroxyapatite. Biomaterials 2007, 28, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Shimizu, T.; Wada, M.; Yamato, M.; Okano, T. Automatic fabrication of 3-dimensional tissues using cell sheet manipulator technique. Biomaterials 2014, 35, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Li, F.; Fang, J.; Xu, L.; Sun, C.; Han, J.; Hua, T.; Zhang, Z.; Feng, Z.; Jiang, X. Hypoxia inducible factor 1α promotes survival of mesenchymal stem cells under hypoxia. Am. J. Transl. Res. 2017, 9, 1521–1529. [Google Scholar] [PubMed]
- Mathieu, P.S.; Loboa, E.G. Cytoskeletal and Focal Adhesion Influences on Mesenchymal Stem Cell Shape, Mechanical Properties, and Differentiation Down Osteogenic, Adipogenic, and Chondrogenic Pathways. Tissue Eng. Part B Rev. 2012, 18, 436–444. [Google Scholar] [CrossRef]
- Wang, D.-G.; Zhang, F.-X.; Chen, M.-L.; Zhu, H.-J.; Yang, B.; Cao, K.-J. Cx43 in mesenchymal stem cells promotes angiogenesis of the infarcted heart independent of gap junctions. Mol. Med. Rep. 2014, 9, 1095–1102. [Google Scholar] [CrossRef]
- Miranda, J.P.; Camões, S.P.; Gaspar, M.M.; Rodrigues, J.; Carvalheiro, M.; Bárcia, R.N.; da Cruz, P.E.; Cruz, H.; Simões, S.; Santos, J.M. The Secretome Derived From 3D-Cultured Umbilical Cord Tissue MSCs Counteracts Manifestations Typifying Rheumatoid Arthritis. Front. Immunol. 2019, 10, 18. [Google Scholar] [CrossRef]
- Ball, S.G.; Shuttleworth, C.A.; Kielty, C.M. Mesenchymal stem cells and neovascularization: Role of platelet-derived growth factor receptors. J. Cell. Mol. Med. 2007, 11, 1012–1030. [Google Scholar] [CrossRef]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef]
- Kim, K.; Bou-Ghannam, S.; Thorp, H.; Grainger, D.W.; Okano, T. Human mesenchymal stem cell sheets in xeno-free media for possible allogenic applications. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Grayson, W.L.; Zhao, F.; Izadpanah, R.; Bunnell, B.; Ma, T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J. Cell. Physiol. 2005, 207, 331–339. [Google Scholar] [CrossRef]
- Jin, Y.; Kato, T.; Furu, M.; Nasu, A.; Kajita, Y.; Mitsui, H.; Ueda, M.; Aoyama, T.; Nakayama, T.; Nakamura, T.; et al. Mesenchymal stem cells cultured under hypoxia escape from senescence via down-regulation of p16 and extracellular signal regulated kinase. Biochem. Biophys. Res. Commun. 2010, 391, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Sekine, W.; Haraguchi, Y.; Shimizu, T.; Umezawa, A.; Okano, T. Thickness limitation and cell viability of multi-layered cell sheets and overcoming the diffusion limit by a porous-membrane culture insert. J. Biochip Tissue Chip. S. 2011, 1, 2153-0777. [Google Scholar] [CrossRef]
- Harimoto, M.; Yamato, M.; Hirose, M.; Takahashi, C.; Isoi, Y.; Kikuchi, A.; Okano, T. Novel approach for achieving double-layered cell sheets co-culture: Overlaying endothelial cell sheets onto monolayer hepatocytes utilizing temperature-responsive culture dishes. J. Biomed. Mater. Res. 2002, 62, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Utoh, R.; Ohashi, K.; Kikuchi, T.; Okano, T. Fabrication of functional 3D hepatic tissues with polarized hepatocytes by stacking endothelial cell sheets in vitro. J. Tissue Eng. Regen. Med. 2017, 11, 2071–2080. [Google Scholar] [CrossRef]
- Muraoka, M.; Shimizu, T.; Itoga, K.; Takahashi, H.; Okano, T. Control of the formation of vascular networks in 3D tissue engineered constructs. Biomaterials 2013, 34, 696–703. [Google Scholar] [CrossRef]






| Time | 5 s | 15 s | 30 s | 60 s | 120 s | |
|---|---|---|---|---|---|---|
| ×g | ||||||
| 29 | 0% (0/3 trials) | 0% (0/3 trials) | 0% (0/3 trials) | 66% (2/3 trials) | 100% (3/3 trials) | |
| 114 | 0% (0/3 trials) | 0% (0/3 trials) | 33% (1/3 trials) | 66% (2/3 trials) | 100% (3/3 trials) | |
| 458 | 33% (1/3 trials) | 66% (2/3 trials) | 100% (3/3 trials) | 100% (3/3 trials) | -- | |
| 1030 | 100% (3/3 trials) | 100% (3/3 trials) | 100% (3/3 trials) | -- | -- | |
| 1832 | Sheet deformation | -- | -- | -- | -- | |
| 1 Day | 4 Days | |
|---|---|---|
| 1-layer conventional cell sheet (1 × 104 cells) | 75 ± 9.5 | 77 ± 8.5 |
| 1-layer centrifuged cell sheet (1 × 104 cells) | 61 ± 6.6 | 72 ± 11 |
| 2-layer conventional cell sheet (1 × 104 cells) | 126 ± 7.0 | Not measurable |
| 2-layer centrifuged cell sheet (1 × 104 cells) | 123 ± 5.1 | 128 ± 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bou-Ghannam, S.; Kim, K.; Kondo, M.; Grainger, D.W.; Okano, T. Mesenchymal Stem Cell Sheet Centrifuge-Assisted Layering Augments Pro-Regenerative Cytokine Production. Cells 2022, 11, 2840. https://doi.org/10.3390/cells11182840
Bou-Ghannam S, Kim K, Kondo M, Grainger DW, Okano T. Mesenchymal Stem Cell Sheet Centrifuge-Assisted Layering Augments Pro-Regenerative Cytokine Production. Cells. 2022; 11(18):2840. https://doi.org/10.3390/cells11182840
Chicago/Turabian StyleBou-Ghannam, Sophia, Kyungsook Kim, Makoto Kondo, David W. Grainger, and Teruo Okano. 2022. "Mesenchymal Stem Cell Sheet Centrifuge-Assisted Layering Augments Pro-Regenerative Cytokine Production" Cells 11, no. 18: 2840. https://doi.org/10.3390/cells11182840
APA StyleBou-Ghannam, S., Kim, K., Kondo, M., Grainger, D. W., & Okano, T. (2022). Mesenchymal Stem Cell Sheet Centrifuge-Assisted Layering Augments Pro-Regenerative Cytokine Production. Cells, 11(18), 2840. https://doi.org/10.3390/cells11182840






