Neurodegenerative Lysosomal Storage Disorders: TPC2 Comes to the Rescue!
Abstract
:1. Introduction
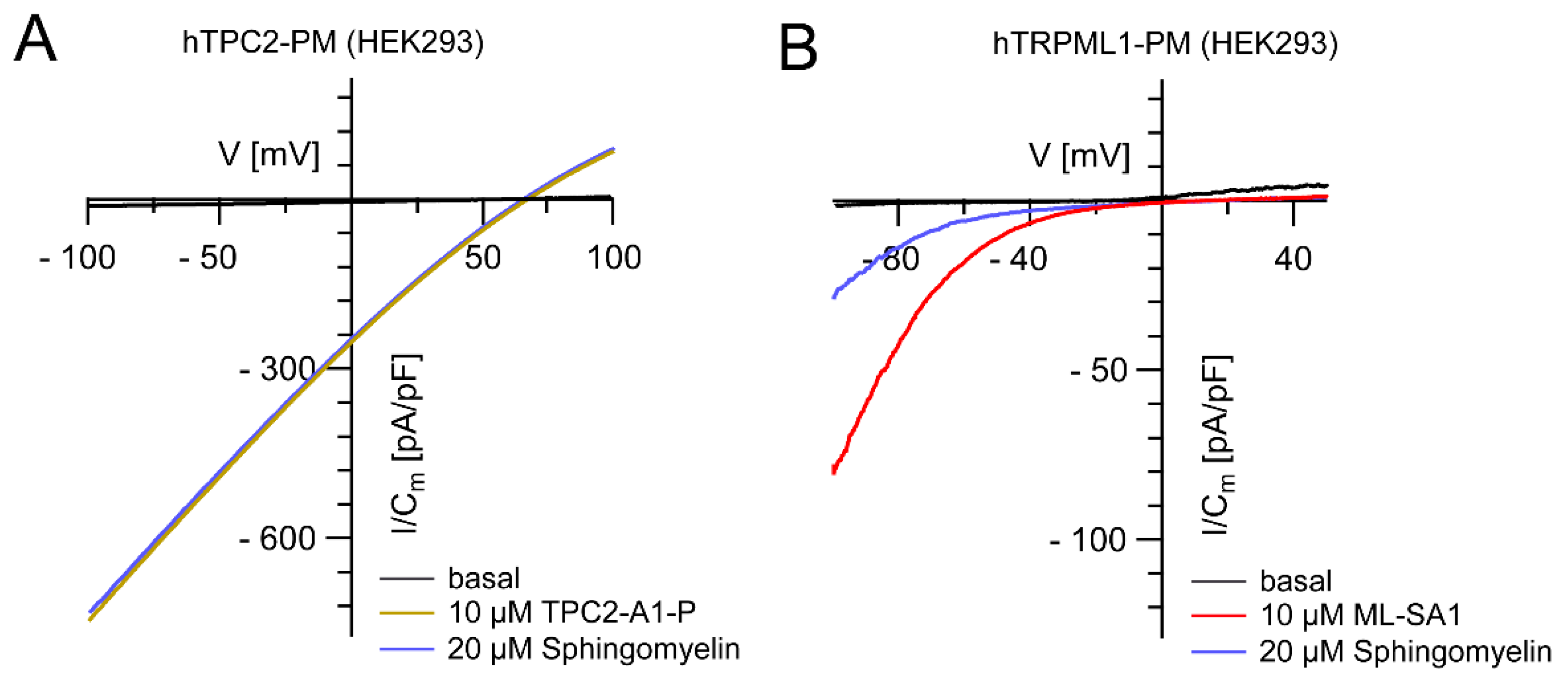
2. Candidates for LSD Therapy: TRPML1 versus TPC2
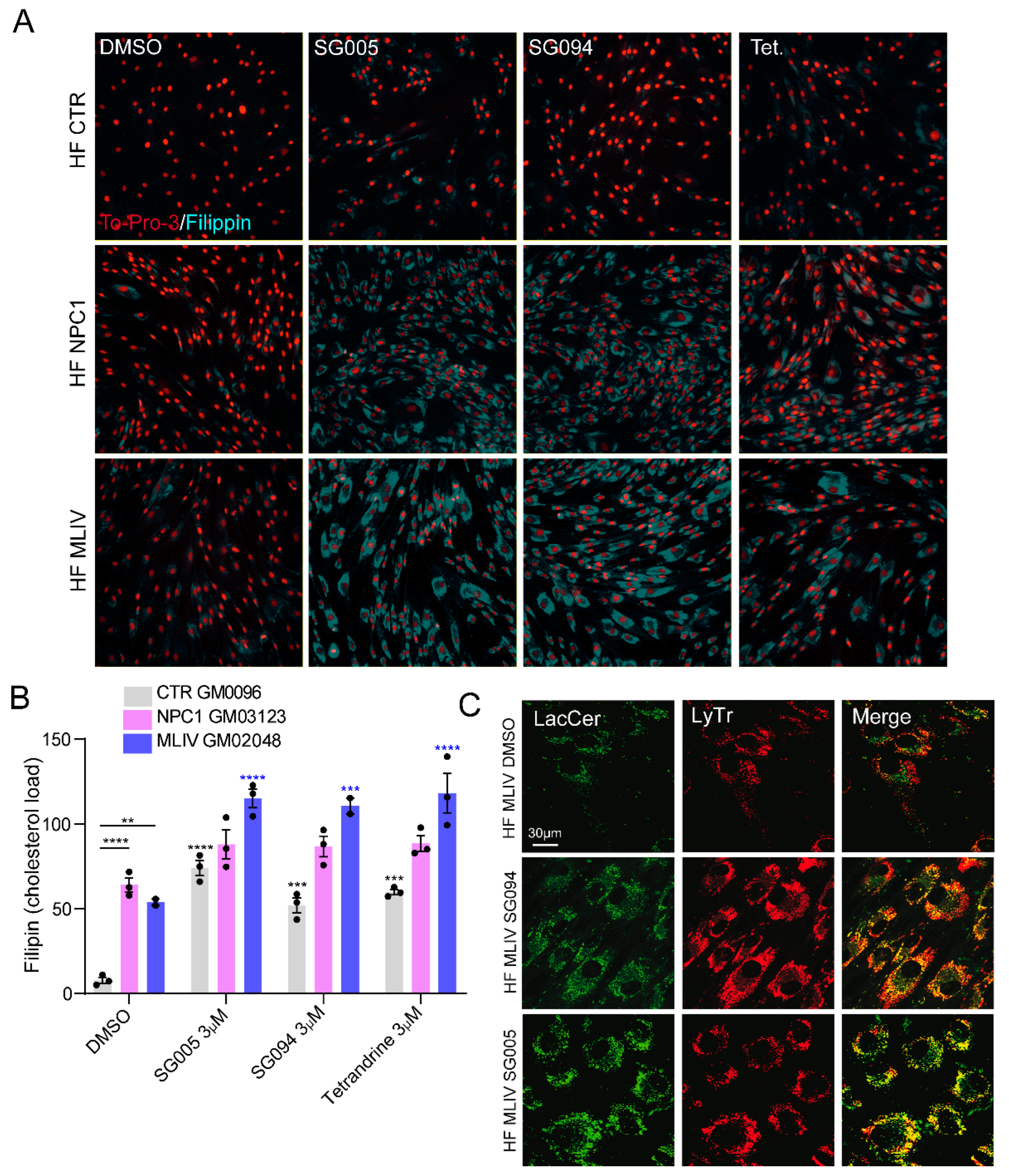
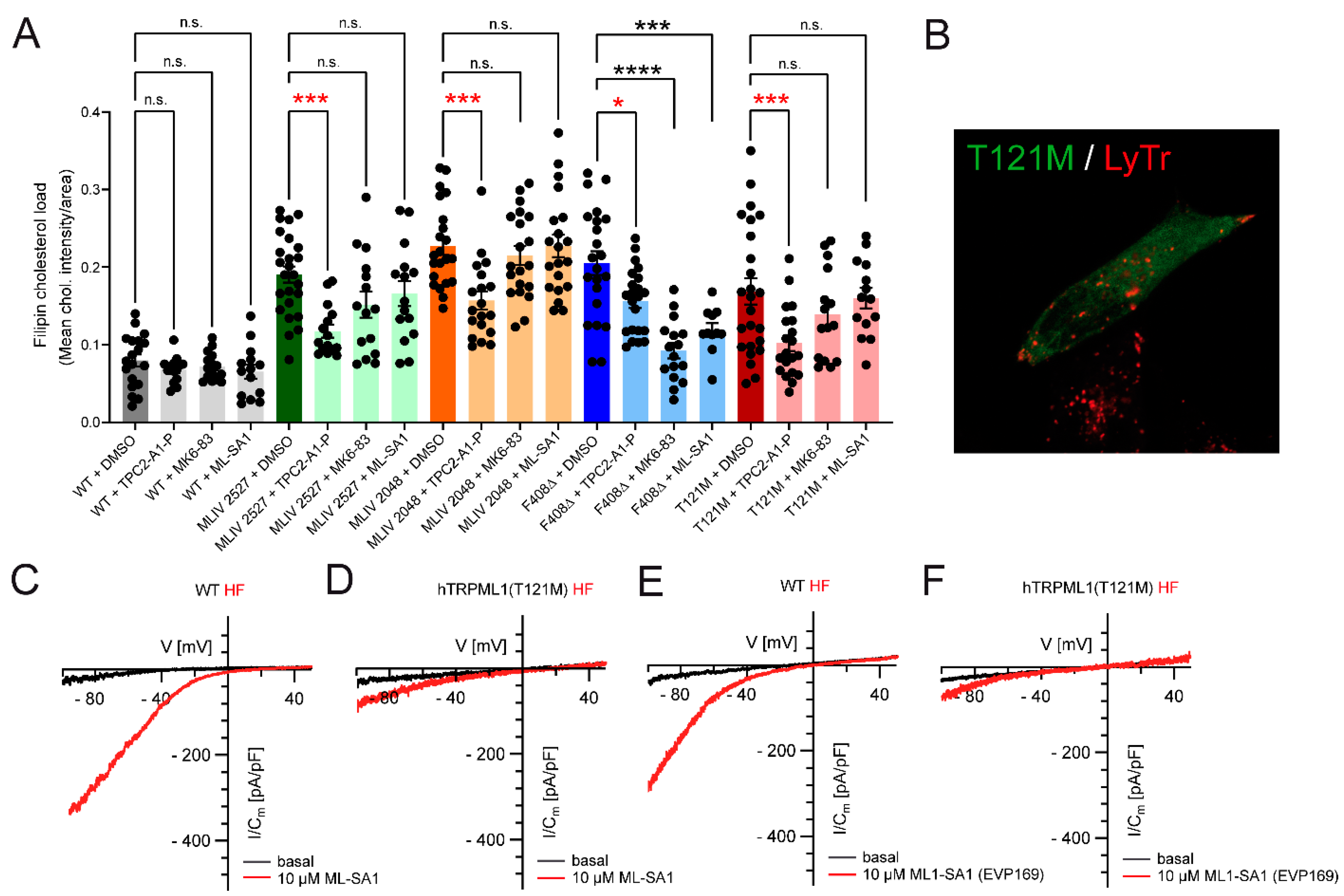
3. TPC2-A1-P Rescues Phenotypes of Several LSDs In Vitro and In Vivo
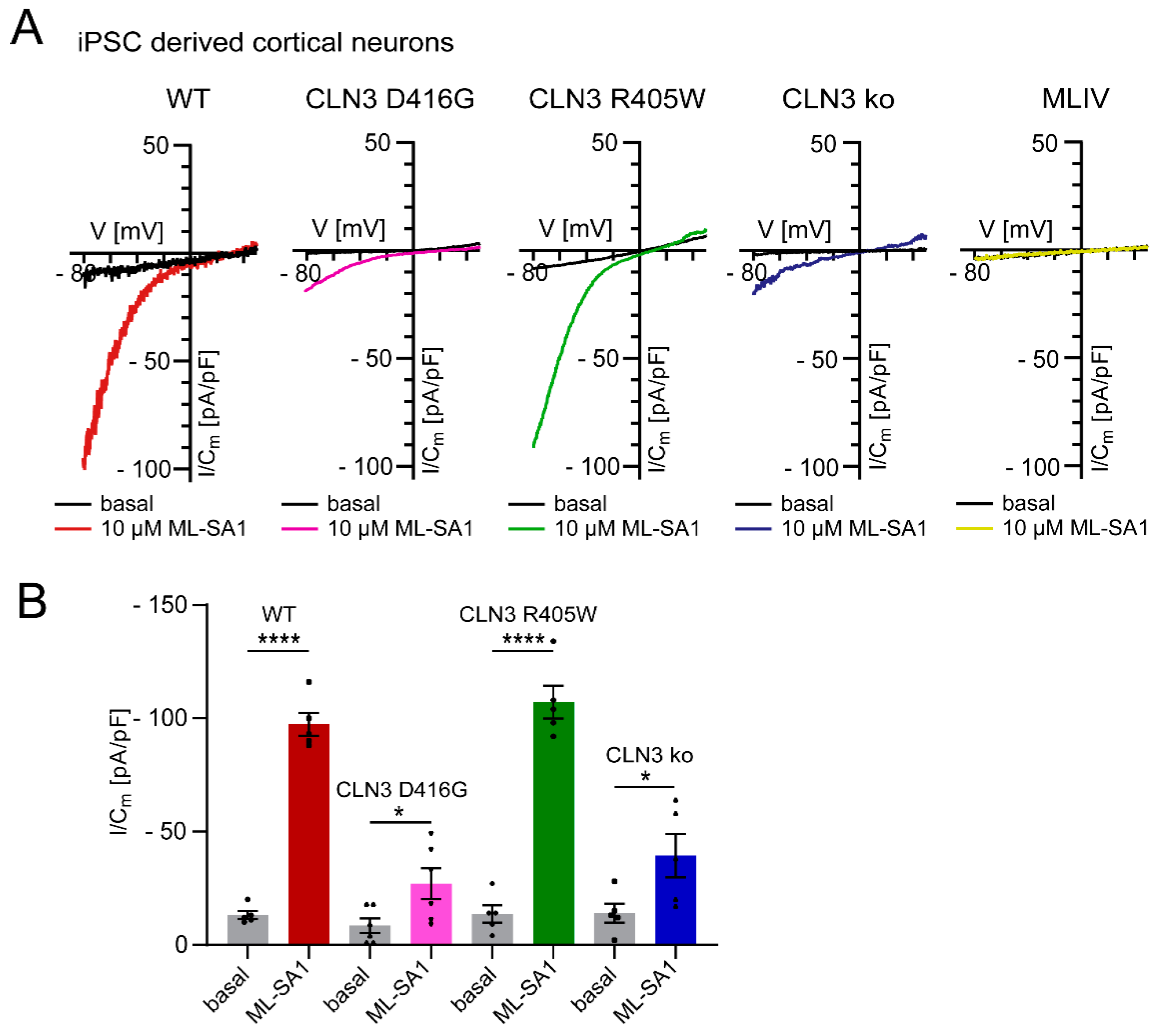
4. Is It All Clear Then?
5. Discussion and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosato, A.S.; Krogsaeter, E.K.; Jaslan, D.; Abrahamian, C.; Montefusco, S.; Soldati, C.; Spix, B.; Pizzo, M.T.; Grieco, G.; Bock, J.; et al. TPC2 Rescues Lysosomal Storage in Mucolipidosis type IV, Niemann-Pick type C1, and Batten Disease. EMBO Mol. Med. 2022, 14, e15377. [Google Scholar] [CrossRef]
- Grimm, C.; Holdt, L.M.; Chen, C.C.; Hassan, S.; Muller, C.; Jors, S.; Cuny, H.; Kissing, S.; Schroder, B.; Butz, E.; et al. High Susceptibility to Fatty Liver Disease in two-pore Channel 2-deficient Mice. Nat. Commun. 2014, 5, 4699. [Google Scholar] [CrossRef] [PubMed]
- Ruas, M.; Davis, L.C.; Chen, C.C.; Morgan, A.J.; Chuang, K.T.; Walseth, T.F.; Grimm, C.; Garnham, C.; Powell, T.; Platt, N.; et al. Expression of Ca(2)(+)-Permeable two-pore Channels Rescues NAADP Signalling in TPC-Deficient Cells. EMBO J. 2015, 34, 1743–1758. [Google Scholar] [CrossRef] [PubMed]
- Sokol, J.; Blanchette-Mackie, J.; Kruth, H.S.; Dwyer, N.K.; Amende, L.M.; Butler, J.D.; Robinson, E.; Patel, S.; Brady, R.O.; Comly, M.E.; et al. Type C Niemann-Pick disease. Lysosomal Accumulation and Defective Intracellular Mobilization of Low Density Lipoprotein Cholesterol. J. Biol. Chem. 1988, 263, 3411–3417. [Google Scholar] [CrossRef]
- Shen, D.; Wang, X.; Li, X.; Zhang, X.; Yao, Z.; Dibble, S.; Dong, X.P.; Yu, T.; Lieberman, A.P.; Showalter, H.D.; et al. Lipid Storage Disorders Block Lysosomal Trafficking by Inhibiting a TRP Channel and Lysosomal Calcium Release. Nat. Commun. 2012, 3, 731. [Google Scholar] [CrossRef] [PubMed]
- Vitner, E.B.; Platt, F.M.; Futerman, A.H. Common and Uncommon Pathogenic Cascades in Lysosomal Storage Diseases. J. Biol. Chem. 2010, 285, 20423–20427. [Google Scholar] [CrossRef]
- Chen, C.C.; Keller, M.; Hess, M.; Schiffmann, R.; Urban, N.; Wolfgardt, A.; Schaefer, M.; Bracher, F.; Biel, M.; Wahl-Schott, C.; et al. A Small Molecule Restores Function to TRPML1 Mutant Isoforms Responsible for Mucolipidosis Type IV. Nat. Commun. 2014, 5, 4681. [Google Scholar] [CrossRef]
- Kasapkara, Ç.S.; Ceylan, A.C.; Yılmaz, D.; Kıreker Köylü, O.; Yürek, B.; Civelek Ürey, B.; Gündüz, M. CLN3-Associated NCL Case with a Preliminary Diagnosis of Niemann Pick Type, C. Mol. Syndromol. 2022. [Google Scholar] [CrossRef]
- Zhong, X.Z.; Sun, X.; Cao, Q.; Dong, G.; Schiffmann, R.; Dong, X.P. BK Channel Agonist Represents a Potential Therapeutic Approach for Lysosomal Storage Diseases. Sci. Rep. 2016, 6, 33684. [Google Scholar] [CrossRef]
- Chow, C.Y.; Zhang, Y.; Dowling, J.J.; Jin, N.; Adamska, M.; Shiga, K.; Szigeti, K.; Shy, M.E.; Li, J.; Zhang, X.; et al. Mutation of FIG4 Causes Neurodegeneration in the Pale Tremor Mouse and Patients with CMT4J. Nature 2007, 448, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Hu, B.; Arpag, S.; Yan, Q.; Hamilton, A.; Zeng, Y.S.; Vanoye, C.G.; Li, J. Reactivation of Lysosomal Ca2+ Efflux Rescues Abnormal Lysosomal Storage in FIG4-Deficient Cells. J. Neurosci. 2015, 35, 6801–6812. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Cang, C.; Fenske, S.; Butz, E.; Chao, Y.K.; Biel, M.; Ren, D.; Wahl-Schott, C.; Grimm, C. Patch-clamp Technique to Characterize Ion Channels in Enlarged Individual Endolysosomes. Nat. Protoc. 2017, 12, 1639–1658. [Google Scholar] [CrossRef] [PubMed]
- Spix, B.; Butz, E.S.; Chen, C.C.; Rosato, A.S.; Tang, R.; Jeridi, A.; Kudrina, V.; Plesch, E.; Wartenberg, P.; Arlt, E.; et al. Lung Emphysema and Impaired Macrophage Elastase Clearance in Mucolipin 3 Deficient Mice. Nat. Commun. 2022, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Sillence, D.J. Glucosylceramide Modulates endolysosomal pH in Gaucher disease. Mol. Genet. Metab. 2013, 109, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Bourdenx, M.; Daniel, J.; Genin, E.; Soria, F.N.; Blanchard-Desce, M.; Bezard, E.; Dehay, B. Nanoparticles Restore Lysosomal Acidification Defects: Implications for Parkinson and Other Lysosomal-related Diseases. Autophagy 2016, 12, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, J.; Gegg, M.E.; Migdalska-Richards, A.; Doherty, M.K.; Whitfield, P.D.; Schapira, A.H. Autophagic Lysosome Reformation Dysfunction in Glucocerebrosidase Deficient Cells: Relevance to Parkinson Disease. Hum. Mol. Genet. 2016, 25, 3432–3445. [Google Scholar] [CrossRef]
- Chakraborty, K.; Leung, K.; Krishnan, Y. High Lumenal Chloride in the Lysosome is critical for lysosome function. Elife 2017, 6, e28862. [Google Scholar] [CrossRef]
- de la Mata, M.; Cotan, D.; Oropesa-Avila, M.; Villanueva-Paz, M.; de Lavera, I.; Alvarez-Cordoba, M.; Luzon-Hidalgo, R.; Suarez-Rivero, J.M.; Tiscornia, G.; Sanchez-Alcazar, J.A. Coenzyme Q10 partially restores pathological alterations in a macrophage model of Gaucher disease. Orphanet J. Rare Dis. 2017, 12, 23. [Google Scholar] [CrossRef]
- Tharkeshwar, A.K.; Trekker, J.; Vermeire, W.; Pauwels, J.; Sannerud, R.; Priestman, D.A.; Te Vruchte, D.; Vints, K.; Baatsen, P.; Decuypere, J.P.; et al. A novel approach to analyze lysosomal dysfunctions through subcellular proteomics and lipidomics: The case of NPC1 deficiency. Sci. Rep. 2017, 7, 41408. [Google Scholar] [CrossRef]
- Wheeler, S.; Haberkant, P.; Bhardwaj, M.; Tongue, P.; Ferraz, M.J.; Halter, D.; Sprong, H.; Schmid, R.; Aerts, J.; Sullo, N.; et al. Cytosolic glucosylceramide regulates endolysosomal function in Niemann-Pick type C disease. Neurobiol. Dis. 2019, 127, 242–252. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Dong, X.P.; Samie, M.; Li, X.; Cheng, X.; Goschka, A.; Shen, D.; Zhou, Y.; Harlow, J.; et al. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell 2012, 151, 372–383. [Google Scholar] [CrossRef]
- Feng, X.; Xiong, J.; Cai, W.; Tian, J.-B.; Zhu, M.X. The Three Two-Pore Channel Subtypes from Rabbit Exhibit Distinct Sensitivity to Phosphoinositides, Voltage, and Extracytosolic pH. Cells 2022, 11, 2006. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ren, D. Lysosomal Physiology. Annu. Rev. Physiol. 2015, 77, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, S.; Medina, D.L. TRPML1-/TFEB-Dependent Regulation of Lysosomal Exocytosis. Methods Mol. Biol. 2019, 1925, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xu, M.; Wu, Y.; Rizvi Syeda, A.K.; Dong, X.P. Multiple facets of TRPML1 in Autophagy. Cell Calcium 2020, 88, 102196. [Google Scholar] [CrossRef] [PubMed]
- Krogsaeter, E.; Rosato, A.S.; Grimm, C. TRPMLs and TPCs: Targets for Lysosomal Storage and Neurodegenerative Disease Therapy? Cell Calcium 2022, 103, 102553. [Google Scholar] [CrossRef]
- Pryor, P.R.; Reimann, F.; Gribble, F.M.; Luzio, J.P. Mucolipin-1 is a Lysosomal Membrane Protein Required for Intracellular Lactosylceramide Traffic. Traffic 2006, 7, 1388–1398. [Google Scholar] [CrossRef]
- Puri, V.; Watanabe, R.; Dominguez, M.; Sun, X.; Wheatley, C.L.; Marks, D.L.; Pagano, R.E. Cholesterol Modulates Membrane Traffic along the Endocytic Pathway in Sphingolipid-storage Diseases. Nat. Cell Biol. 1999, 1, 386–388. [Google Scholar] [CrossRef]
- Mole, S.E.; Anderson, G.; Band, H.A.; Berkovic, S.F.; Cooper, J.D.; Kleine Holthaus, S.M.; McKay, T.R.; Medina, D.L.; Rahim, A.A.; Schulz, A.; et al. Clinical Challenges and Future Therapeutic Approaches for Neuronal Ceroid Lipofuscinosis. Lancet Neurol. 2019, 18, 107–116. [Google Scholar] [CrossRef]
- Soldati, C.; Lopez-Fabuel, I.; Wanderlingh, L.G.; Garcia-Macia, M.; Monfregola, J.; Esposito, A.; Napolitano, G.; Guevara-Ferrer, M.; Scotto Rosato, A.; Krogsaeter, E.K.; et al. Repurposing of tamoxifen ameliorates CLN3 and CLN7 disease phenotype. EMBO Mol. Med. 2021, 13, e13742. [Google Scholar] [CrossRef]
- Paquet, D.; Kwart, D.; Chen, A.; Sproul, A.; Jacob, S.; Teo, S.; Olsen, K.M.; Gregg, A.; Noggle, S.; Tessier-Lavigne, M. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature 2016, 533, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Kwart, D.; Paquet, D.; Teo, S.; Tessier-Lavigne, M. Precise and efficient scarless genome editing in stem cells using CORRECT. Nat. Protoc. 2017, 12, 329–354. [Google Scholar] [CrossRef]
- Colletti, G.A.; Miedel, M.T.; Quinn, J.; Andharia, N.; Weisz, O.A.; Kiselyov, K. Loss of lysosomal ion channel transient receptor potential channel mucolipin-1 (TRPML1) leads to cathepsin B-dependent apoptosis. J. Biol. Chem. 2012, 287, 8082–8091. [Google Scholar] [CrossRef]
- Grishchuk, Y.; Sri, S.; Rudinskiy, N.; Ma, W.; Stember, K.G.; Cottle, M.W.; Sapp, E.; Difiglia, M.; Muzikansky, A.; Betensky, R.A.; et al. Behavioral deficits, early gliosis, dysmyelination and synaptic dysfunction in a mouse model of mucolipidosis IV. Acta Neuropathol. Commun. 2014, 2, 133. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, S.; Salani, M.; Smith, S.; Sangster, M.; Miller-Browne, V.; Wassmer, S.; Xiao, R.; Vandenberghe, L.; Slaugenhaupt, S.; Misko, A.; et al. MCOLN1 gene therapy corrects neurologic dysfunction in the mouse model of mucolipidosis IV. Hum. Mol. Genet. 2021, 30, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Micsenyi, M.C.; Dobrenis, K.; Stephney, G.; Pickel, J.; Vanier, M.T.; Slaugenhaupt, S.A.; Walkley, S.U. Neuropathology of the Mcoln1(-/-) knockout mouse model of mucolipidosis type IV. J. Neuropathol. Exp. Neurol. 2009, 68, 125–135. [Google Scholar] [CrossRef]
- Walker, M.T.; Montell, C. Suppression of the motor deficit in a mucolipidosis type IV mouse model by bone marrow transplantation. Hum. Mol. Genet. 2016, 25, 2752–2761. [Google Scholar] [CrossRef]
- Medina, D.L.; Fraldi, A.; Bouche, V.; Annunziata, F.; Mansueto, G.; Spampanato, C.; Puri, C.; Pignata, A.; Martina, J.A.; Sardiello, M.; et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell 2011, 21, 421–430. [Google Scholar] [CrossRef]
- Bae, M.; Patel, N.; Xu, H.; Lee, M.; Tominaga-Yamanaka, K.; Nath, A.; Geiger, J.; Gorospe, M.; Mattson, M.P.; Haughey, N.J. Activation of TRPML1 clears intraneuronal Abeta in preclinical models of HIV infection. J. Neurosci. 2014, 34, 11485–11503. [Google Scholar] [CrossRef]
- Medina, D.L.; Di Paola, S.; Peluso, I.; Armani, A.; De Stefani, D.; Venditti, R.; Montefusco, S.; Scotto-Rosato, A.; Prezioso, C.; Forrester, A.; et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 2015, 17, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Rua, V.; Feijoo-Bandin, S.; Rodriguez-Penas, D.; Mosquera-Leal, A.; Abu-Assi, E.; Beiras, A.; Maria Seoane, L.; Lear, P.; Parrington, J.; Portoles, M.; et al. Endolysosomal two-pore channels regulate autophagy in cardiomyocytes. J. Physiol. 2016, 594, 3061–3077. [Google Scholar] [CrossRef]
- Grimm, C.; Butz, E.; Chen, C.C.; Wahl-Schott, C.; Biel, M. From mucolipidosis type IV to Ebola: TRPML and two-pore channels at the crossroads of endo-lysosomal trafficking and disease. Cell Calcium 2017, 67, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Wang, F.; Muller, S. Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 2019, 18, 923–948. [Google Scholar] [CrossRef]
- Tsunemi, T.; Perez-Rosello, T.; Ishiguro, Y.; Yoroisaka, A.; Jeon, S.; Hamada, K.; Rammonhan, M.; Wong, Y.C.; Xie, Z.; Akamatsu, W.; et al. Increased Lysosomal Exocytosis Induced by Lysosomal Ca(2+) Channel Agonists Protects Human Dopaminergic Neurons from alpha-Synuclein Toxicity. J. Neurosci. 2019, 39, 5760–5772. [Google Scholar] [CrossRef] [PubMed]
- Gerndt, S.; Chen, C.C.; Chao, Y.K.; Yuan, Y.; Burgstaller, S.; Scotto Rosato, A.; Krogsaeter, E.; Urban, N.; Jacob, K.; Nguyen, O.N.P.; et al. Agonist-mediated switching of ion selectivity in TPC2 differentially promotes lysosomal function. Elife 2020, 9, e54712. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.C.; Wu, A.J.; Huang, A.S.; Dong, R.; Malampati, S.; Iyaswamy, A.; Krishnamoorthi, S.; Sreenivasmurthy, S.G.; Zhu, Z.; Su, C.; et al. Lysosomal TPCN (two pore segment channel) inhibition ameliorates beta-amyloid pathology and mitigates memory impairment in Alzheimer disease. Autophagy 2022, 18, 624–642. [Google Scholar] [CrossRef]
- Ambrosio, A.L.; Boyle, J.A.; Aradi, A.E.; Christian, K.A.; Di Pietro, S.M. TPC2 controls pigmentation by regulating melanosome pH and size. Proc. Natl. Acad. Sci. USA 2016, 113, 5622–5627. [Google Scholar] [CrossRef]
- Morgan, A.J.; Galione, A. NAADP induces pH changes in the lumen of acidic Ca2+ stores. Biochem. J. 2007, 402, 301–310. [Google Scholar] [CrossRef]
- Cosker, F.; Cheviron, N.; Yamasaki, M.; Menteyne, A.; Lund, F.E.; Moutin, M.J.; Galione, A.; Cancela, J.M. The ecto-enzyme CD38 is a nicotinic acid adenine dinucleotide phosphate (NAADP) synthase that couples receptor activation to Ca2+ mobilization from lysosomes in pancreatic acinar cells. J. Biol. Chem. 2010, 285, 38251–38259. [Google Scholar] [CrossRef]
- Hockey, L.N.; Kilpatrick, B.S.; Eden, E.R.; Lin-Moshier, Y.; Brailoiu, G.C.; Brailoiu, E.; Futter, C.E.; Schapira, A.H.; Marchant, J.S.; Patel, S. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J. Cell Sci. 2015, 128, 232–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, Y.; Kolokoltsov, A.A.; Chen, C.C.; Tidwell, M.W.; Bauta, W.E.; Klugbauer, N.; Grimm, C.; Wahl-Schott, C.; Biel, M.; Davey, R.A. Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 2015, 347, 995–998. [Google Scholar] [CrossRef]
- Lee, J.H.; Yu, W.H.; Kumar, A.; Lee, S.; Mohan, P.S.; Peterhoff, C.M.; Wolfe, D.M.; Martinez-Vicente, M.; Massey, A.C.; Sovak, G.; et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 2010, 141, 1146–1158. [Google Scholar] [CrossRef]
- Lie, P.P.Y.; Yoo, L.; Goulbourne, C.N.; Berg, M.J.; Stavrides, P.; Huo, C.; Lee, J.H.; Nixon, R.A. Axonal transport of late endosomes and amphisomes is selectively modulated by local Ca(2+) efflux and disrupted by PSEN1 loss of function. Sci. Adv. 2022, 8, eabj5716. [Google Scholar] [CrossRef]
- Spix, B.; Chao, Y.K.; Abrahamian, C.; Chen, C.C.; Grimm, C. TRPML Cation Channels in Inflammation and Immunity. Front. Immunol. 2020, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.P.; Cheng, X.; Mills, E.; Delling, M.; Wang, F.; Kurz, T.; Xu, H. The type IV Mucolipidosis-associated Protein TRPML1 is an endolysosomal iron release channel. Nature 2008, 455, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Butz, E.S.; Chao, Y.K.; Grishchuk, Y.; Becker, L.; Heller, S.; Slaugenhaupt, S.A.; Biel, M.; Wahl-Schott, C.; Grimm, C. Small Molecules for Early Endosome-Specific Patch Clamping. Cell Chem. Biol. 2017, 24, 907–916.e4. [Google Scholar] [CrossRef] [PubMed]
- Coen, K.; Flannagan, R.S.; Baron, S.; Carraro-Lacroix, L.R.; Wang, D.; Vermeire, W.; Michiels, C.; Munck, S.; Baert, V.; Sugita, S.; et al. Lysosomal Calcium Homeostasis Defects, not Proton Pump Defects, Cause Endo-lysosomal Dysfunction in PSEN-deficient cells. J. Cell Biol. 2012, 198, 23–35. [Google Scholar] [CrossRef]
- Narayanaswamy, N.; Chakraborty, K.; Saminathan, A.; Zeichner, E.; Leung, K.; Devany, J.; Krishnan, Y. A pH-correctable, DNA-based Fluorescent Reporter for Organellar Calcium. Nat. Methods 2019, 16, 95–102. [Google Scholar] [CrossRef]
- Burgstaller, S.; Bischof, H.; Gensch, T.; Stryeck, S.; Gottschalk, B.; Ramadani-Muja, J.; Eroglu, E.; Rost, R.; Balfanz, S.; Baumann, A.; et al. pH-Lemon, a Fluorescent Protein-Based pH Reporter for Acidic Compartments. ACS Sens. 2019, 4, 883–891. [Google Scholar] [CrossRef]
- Zhang, X.; Garbett, K.; Veeraraghavalu, K.; Wilburn, B.; Gilmore, R.; Mirnics, K.; Sisodia, S.S. A Role for Presenilins in Autophagy Revisited: Normal Cidification of Lysosomes in Cells Lacking PSEN1 and PSEN2. J. Neurosci. 2012, 32, 8633–8648. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prat Castro, S.; Kudrina, V.; Jaślan, D.; Böck, J.; Scotto Rosato, A.; Grimm, C. Neurodegenerative Lysosomal Storage Disorders: TPC2 Comes to the Rescue! Cells 2022, 11, 2807. https://doi.org/10.3390/cells11182807
Prat Castro S, Kudrina V, Jaślan D, Böck J, Scotto Rosato A, Grimm C. Neurodegenerative Lysosomal Storage Disorders: TPC2 Comes to the Rescue! Cells. 2022; 11(18):2807. https://doi.org/10.3390/cells11182807
Chicago/Turabian StylePrat Castro, Sandra, Veronika Kudrina, Dawid Jaślan, Julia Böck, Anna Scotto Rosato, and Christian Grimm. 2022. "Neurodegenerative Lysosomal Storage Disorders: TPC2 Comes to the Rescue!" Cells 11, no. 18: 2807. https://doi.org/10.3390/cells11182807
APA StylePrat Castro, S., Kudrina, V., Jaślan, D., Böck, J., Scotto Rosato, A., & Grimm, C. (2022). Neurodegenerative Lysosomal Storage Disorders: TPC2 Comes to the Rescue! Cells, 11(18), 2807. https://doi.org/10.3390/cells11182807







