Neurons: The Interplay between Cytoskeleton, Ion Channels/Transporters and Mitochondria
Abstract
1. Introduction
2. Neurons and Their Cytoskeleton
3. Cytoskeleton, Neuronal Excitability and Ion Channels
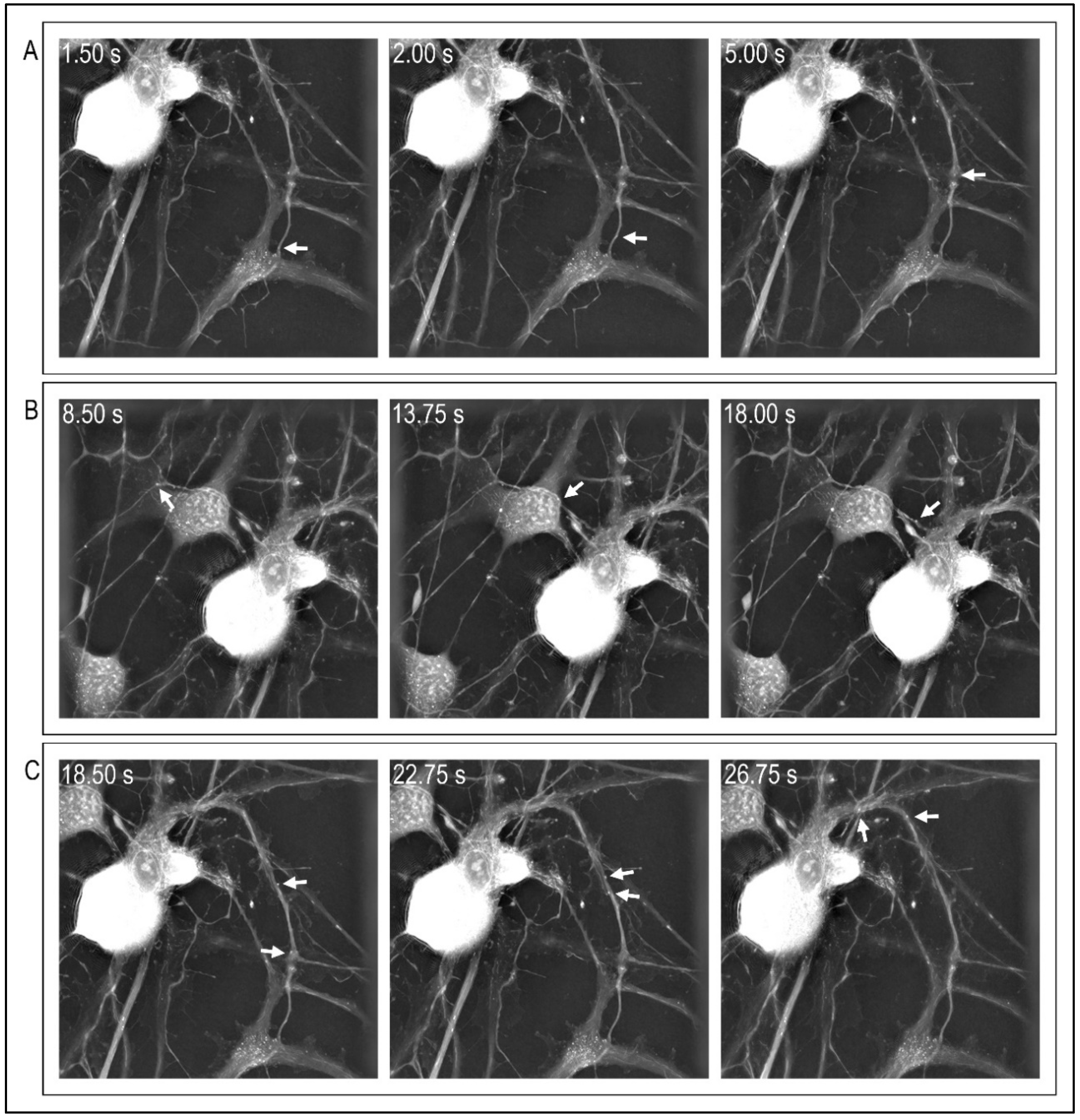
4. Microscopic Approaches to Visualize and Assess Cytoskeleton Function
5. Examples of Human Pathology Related to Cytoskeleton Dysfunction
5.1. Epilepsy
5.2. Intellectutal Disability (ID)
5.3. Peripheral Neuropathies (PN)
6. Cytoskeleton Components as a Possible Biomarker for Human Pathology? The Virtuous Example of Neurofilament Light Chain
7. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marek, K.W.; Davis, G.W. Controlling the active properties of excitable cells. Curr. Opin. Neurobiol. 2003, 13, 607–611. [Google Scholar] [CrossRef]
- López-Bendito, G.; Arlotta, P. Cell replacement therapies for nervous system regeneration. Dev. Neurobiol. 2012, 72, 145–152. [Google Scholar] [CrossRef]
- Frade, J.M.; Ovejero-Benito, M.C. Neuronal cell cycle: The neuron itself and its circumstances. Cell Cycle 2015, 14, 712–720. [Google Scholar] [CrossRef]
- Sheng, Z.H. The Interplay of Axonal Energy Homeostasis and Mitochondrial Trafficking and Anchoring. Trends Cell Biol. 2017, 27, 403–416. [Google Scholar] [CrossRef]
- Sheng, Z.H. Mitochondrial trafficking and anchoring in neurons: New insight and implications. J. Cell Biol. 2014, 204, 1087–1098. [Google Scholar] [CrossRef]
- Dogterom, M.; Koenderink, G.H. Actin-microtubule crosstalk in cell biology. Nat. Rev. Mol. Cell Biol. 2019, 20, 38–54. [Google Scholar] [CrossRef]
- Kapitein, L.C.; Hoogenraad, C.C. Building the Neuronal Microtubule Cytoskeleton. Neuron 2015, 87, 492–506. [Google Scholar] [CrossRef]
- Gomez, T.M.; Letourneau, P.C. Actin dynamics in growth cone motility and navigation. J. Neurochem. 2014, 129, 221–234. [Google Scholar] [CrossRef]
- Bennison, S.A.; Blazejewski, S.M.; Smith, T.H.; Toyo-Oka, K. Protein kinases: Master regulators of neuritogenesis and therapeutic targets for axon regeneration. Cell Mol. Life Sci. 2020, 77, 1511–1530. [Google Scholar] [CrossRef]
- Suter, D.M.; Miller, K.E. The emerging role of forces in axonal elongation. Prog. Neurobiol. 2011, 94, 91–101. [Google Scholar] [CrossRef]
- Georges, P.C.; Hadzimichalis, N.M.; Sweet, E.S.; Firestein, B.L. The yin-yang of dendrite morphology: Unity of actin and microtubules. Mol. Neurobiol. 2008, 38, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhong, G.; Zhuang, X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 2013, 339, 452–456. [Google Scholar] [CrossRef] [PubMed]
- van Bommel, B.; Konietzny, A.; Kobler, O.; Bär, J.; Mikhaylova, M. F-actin patches associated with glutamatergic synapses control positioning of dendritic lysosomes. EMBO J. 2019, 38, e101183. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, G.; Monfrini, M.; Scuteri, A. Axonal Transport Impairment in Chemotherapy-Induced Peripheral Neuropathy. Toxics 2015, 3, 322–341. [Google Scholar] [CrossRef]
- Gibbs, K.L.; Greensmith, L.; Schiavo, G. Regulation of Axonal Transport by Protein Kinases. Trends Biochem. Sci. 2015, 40, 597–610. [Google Scholar] [CrossRef]
- Chevalier-Larsen, E.; Holzbaur, E.L. Axonal transport and neurodegenerative disease. Biochim. Biophys Acta 2006, 1762, 1094–1108. [Google Scholar] [CrossRef]
- Vale, R.D. Myosin V motor proteins: Marching stepwise towards a mechanism. J. Cell Biol. 2003, 163, 445–450. [Google Scholar] [CrossRef]
- Arnold, D.B.; Gallo, G. Structure meets function: Actin filaments and myosin motors in the axon. J. Neurochem. 2014, 129, 213–220. [Google Scholar] [CrossRef]
- Kapitein, L.C.; Hoogenraad, C.C. Which way to go? Cytoskeletal organization and polarized transport in neurons. Mol. Cell NeuroSci. 2011, 46, 9–20. [Google Scholar] [CrossRef]
- Hirokawa, N.; Takemura, R. Molecular motors and mechanisms of directional transport in neurons. Nat. Rev. NeuroSci. 2005, 6, 201–214. [Google Scholar] [CrossRef]
- Maday, S.; Twelvetrees, A.E.; Moughamian, A.J.; Holzbaur, E.L. Axonal transport: Cargo-specific mechanisms of motility and regulation. Neuron 2014, 84, 292–309. [Google Scholar] [CrossRef]
- Yellen, G. Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. J. Cell Biol. 2018, 217, 2235–2246. [Google Scholar] [CrossRef]
- Coelho, P.; Fão, L.; Mota, S.; Rego, A.C. Mitochondrial function and dynamics in neural stem cells and neurogenesis: Implications for neurodegenerative diseases. Ageing Res. Rev. 2022, 80, 101667. [Google Scholar] [CrossRef]
- Quintanilla, R.A.; Tapia-Monsalves, C.; Vergara, E.H.; Pérez, M.J.; Aranguiz, A. Truncated Tau Induces Mitochondrial Transport Failure Through the Impairment of TRAK2 Protein and Bioenergetics Decline in Neuronal Cells. Front. Cell NeuroSci. 2020, 14, 175. [Google Scholar] [CrossRef]
- Cheng, X.T.; Sheng, Z.H. Developmental regulation of microtubule-based trafficking and anchoring of axonal mitochondria in health and diseases. Dev. Neurobiol. 2021, 81, 284–299. [Google Scholar] [CrossRef]
- Fenton, A.R.; Jongens, T.A.; Holzbaur, E.L.F. Mitochondrial adaptor TRAK2 activates and functionally links opposing kinesin and dynein motors. Nat. Commun. 2021, 12, 4578. [Google Scholar] [CrossRef]
- López-Doménech, G.; Covill-Cooke, C.; Ivankovic, D.; Halff, E.F.; Sheehan, D.F.; Norkett, R.; Birsa, N.; Kittler, J.T. Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution. EMBO J. 2018, 37, 321–336. [Google Scholar] [CrossRef]
- Celestino, R.; Henen, M.A.; Gama, J.B.; Carvalho, C.; McCabe, M.; Barbosa, D.J.; Born, A.; Nichols, P.J.; Carvalho, A.X.; Gassmann, R.; et al. A transient helix in the disordered region of dynein light intermediate chain links the motor to structurally diverse adaptors for cargo transport. PLoS Biol. 2019, 17, e3000100. [Google Scholar] [CrossRef]
- Cason, S.E.; Carman, P.J.; Van Duyne, C.; Goldsmith, J.; Dominguez, R.; Holzbaur, E.L.F. Sequential dynein effectors regulate axonal autophagosome motility in a maturation-dependent pathway. J. Cell Biol. 2021, 220, e202010179. [Google Scholar] [CrossRef]
- van Spronsen, M.; Mikhaylova, M.; Lipka, J.; Schlager, M.A.; van den Heuvel, D.J.; Kuijpers, M.; Wulf, P.S.; Keijzer, N.; Demmers, J.; Kapitein, L.C.; et al. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron 2013, 77, 485–502. [Google Scholar] [CrossRef]
- Henrichs, V.; Grycova, L.; Barinka, C.; Nahacka, Z.; Neuzil, J.; Diez, S.; Rohlena, J.; Braun, M.; Lansky, Z. Mitochondria-adaptor TRAK1 promotes kinesin-1 driven transport in crowded environments. Nat. Commun. 2020, 11, 3123. [Google Scholar] [CrossRef]
- Canty, J.T.; Tan, R.; Kusakci, E.; Fernandes, J.; Yildiz, A. Structure and Mechanics of Dynein Motors. Annu. Rev. Biophys 2021, 50, 549–574. [Google Scholar] [CrossRef]
- Armstrong, L.C.; Komiya, T.; Bergman, B.E.; Mihara, K.; Bornstein, P. Metaxin is a component of a preprotein import complex in the outer membrane of the mammalian mitochondrion. J. Biol. Chem. 1997, 272, 6510–6518. [Google Scholar] [CrossRef]
- Armstrong, L.C.; Saenz, A.J.; Bornstein, P. Metaxin 1 interacts with metaxin 2, a novel related protein associated with the mammalian mitochondrial outer membrane. J. Cell Biochem. 1999, 74, 11–22. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, E.; Wang, W.; Hsieh, C.H.; Wang, X.; Feng, W.; Shen, K. Metaxins are core components of mitochondrial transport adaptor complexes. Nat. Commun. 2021, 12, 83. [Google Scholar] [CrossRef]
- Kang, J.S.; Tian, J.H.; Pan, P.Y.; Zald, P.; Li, C.; Deng, C.; Sheng, Z.H. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell 2008, 132, 137–148. [Google Scholar] [CrossRef]
- Gutnick, A.; Banghart, M.R.; West, E.R.; Schwarz, T.L. The light-sensitive dimerizer zapalog reveals distinct modes of immobilization for axonal mitochondria. Nat. Cell Biol. 2019, 21, 768–777. [Google Scholar] [CrossRef]
- Basu, H.; Pekkurnaz, G.; Falk, J.; Wei, W.; Chin, M.; Steen, J.; Schwarz, T.L. FHL2 anchors mitochondria to actin and adapts mitochondrial dynamics to glucose supply. J. Cell Biol. 2021, 220, e201912077. [Google Scholar] [CrossRef]
- Eberhardt, E.L.; Ludlam, A.V.; Tan, Z.; Cianfrocco, M.A. Miro: A molecular switch at the center of mitochondrial regulation. Protein Sci. 2020, 29, 1269–1284. [Google Scholar] [CrossRef]
- DeSelm, C.J.; Brown, J.R.; Lu, R.; Langford, G.M. Rab-GDI inhibits myosin V-dependent vesicle transport in squid giant axon. Biol. Bull. 2003, 205, 190–191. [Google Scholar] [CrossRef]
- Brady, S.T.; Lasek, R.J.; Allen, R.D. Fast axonal transport in extruded axoplasm from squid giant axon. Science 1982, 218, 1129–1131. [Google Scholar] [CrossRef]
- Allen, R.D.; Metuzals, J.; Tasaki, I.; Brady, S.T.; Gilbert, S.P. Fast axonal transport in squid giant axon. Science 1982, 218, 1127–1129. [Google Scholar] [CrossRef]
- Reese, T.S. The molecular basis of axonal transport in the squid giant axon. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1987, 65, 89–102. [Google Scholar]
- Yi, G.; Wang, J.; Wei, X.; Che, Y. Energy Cost of Action Potential Generation and Propagation in Thalamocortical Relay Neurons During Deep Brain Stimulation. IEEE Trans. Biomed. Eng. 2019, 66, 3457–3471. [Google Scholar] [CrossRef]
- Morachevskaya, E.A.; Sudarikova, A.V. Actin dynamics as critical ion channel regulator: ENaC and Piezo in focus. Am. J. Physiol. Cell Physiol. 2021, 320, C696–C702. [Google Scholar] [CrossRef]
- Nelson, A.D.; Jenkins, P.M. Axonal Membranes and Their Domains: Assembly and Function of the Axon Initial Segment and Node of Ranvier. Front. Cell NeuroSci. 2017, 11, 136. [Google Scholar] [CrossRef]
- Sasaki, S.; Yui, N.; Noda, Y. Actin directly interacts with different membrane channel proteins and influences channel activities: AQP2 as a model. Biochim. Biophys Acta 2014, 1838, 514–520. [Google Scholar] [CrossRef]
- Keynes, R.D. The ionic channels in excitable membranes. Ciba Found. Symp 1975, 31, 191–203. [Google Scholar] [CrossRef]
- Dzhashiashvili, Y.; Zhang, Y.; Galinska, J.; Lam, I.; Grumet, M.; Salzer, J.L. Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J. Cell Biol. 2007, 177, 857–870. [Google Scholar] [CrossRef]
- Kole, M.H.; Ilschner, S.U.; Kampa, B.M.; Williams, S.R.; Ruben, P.C.; Stuart, G.J. Action potential generation requires a high sodium channel density in the axon initial segment. Nat. NeuroSci. 2008, 11, 178–186. [Google Scholar] [CrossRef]
- Yu, Y.; Shu, Y.; McCormick, D.A. Cortical action potential backpropagation explains spike threshold variability and rapid-onset kinetics. J. NeuroSci. 2008, 28, 7260–7272. [Google Scholar] [CrossRef] [PubMed]
- Devaux, J.J.; Kleopa, K.A.; Cooper, E.C.; Scherer, S.S. KCNQ2 is a nodal K+ channel. J. NeuroSci. 2004, 24, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Kao, T.; Horvath, Z.; Lemos, J.; Sul, J.Y.; Cranstoun, S.D.; Bennett, V.; Scherer, S.S.; Cooper, E.C. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J. NeuroSci. 2006, 26, 2599–2613. [Google Scholar] [CrossRef]
- Rasmussen, H.B.; Frøkjaer-Jensen, C.; Jensen, C.S.; Jensen, H.S.; Jørgensen, N.K.; Misonou, H.; Trimmer, J.S.; Olesen, S.P.; Schmitt, N. Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment. J. Cell Sci. 2007, 120, 953–963. [Google Scholar] [CrossRef]
- Dedek, K.; Kunath, B.; Kananura, C.; Reuner, U.; Jentsch, T.J.; Steinlein, O.K. Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel. Proc. Natl. Acad. Sci. USA 2001, 98, 12272–12277. [Google Scholar] [CrossRef]
- Schwarz, J.R.; Glassmeier, G.; Cooper, E.C.; Kao, T.C.; Nodera, H.; Tabuena, D.; Kaji, R.; Bostock, H. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J. Physiol. 2006, 573, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Yaari, Y. Axo-somatic and apical dendritic Kv7/M channels differentially regulate the intrinsic excitability of adult rat CA1 pyramidal cells. J. NeuroPhysiol. 2006, 95, 3480–3495. [Google Scholar] [CrossRef]
- Chung, H.J.; Jan, Y.N.; Jan, L.Y. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc. Natl. Acad. Sci. USA 2006, 103, 8870–8875. [Google Scholar] [CrossRef]
- Ogiwara, I.; Miyamoto, H.; Morita, N.; Atapour, N.; Mazaki, E.; Inoue, I.; Takeuchi, T.; Itohara, S.; Yanagawa, Y.; Obata, K.; et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: A circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J. NeuroSci. 2007, 27, 5903–5914. [Google Scholar] [CrossRef]
- Garrido, J.J.; Giraud, P.; Carlier, E.; Fernandes, F.; Moussif, A.; Fache, M.P.; Debanne, D.; Dargent, B. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 2003, 300, 2091–2094. [Google Scholar] [CrossRef]
- Lemaillet, G.; Walker, B.; Lambert, S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J. Biol. Chem. 2003, 278, 27333–27339. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.S.; Palygin, O.; Bhasin, N.; Hund, T.J.; Boyden, P.A.; Shibata, E.; Anderson, M.E.; Mohler, P.J. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J. Cell Biol. 2008, 180, 173–186. [Google Scholar] [CrossRef]
- Eshed, Y.; Feinberg, K.; Poliak, S.; Sabanay, H.; Sarig-Nadir, O.; Spiegel, I.; Bermingham, J.R.; Peles, E. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron 2005, 47, 215–229. [Google Scholar] [CrossRef]
- Sherman, D.L.; Tait, S.; Melrose, S.; Johnson, R.; Zonta, B.; Court, F.A.; Macklin, W.B.; Meek, S.; Smith, A.J.; Cottrell, D.F.; et al. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron 2005, 48, 737–742. [Google Scholar] [CrossRef]
- Hedstrom, K.L.; Rasband, M.N. Intrinsic and extrinsic determinants of ion channel localization in neurons. J. Neurochem. 2006, 98, 1345–1352. [Google Scholar] [CrossRef]
- Zhou, D.; Lambert, S.; Malen, P.L.; Carpenter, S.; Boland, L.M.; Bennett, V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 1998, 143, 1295–1304. [Google Scholar] [CrossRef]
- Komada, M.; Soriano, P. [Beta]IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J. Cell Biol. 2002, 156, 337–348. [Google Scholar] [CrossRef]
- Shaw, J.E.; Koleske, A.J. Functional interactions of ion channels with the actin cytoskeleton: Does coupling to dynamic actin regulate NMDA receptors? J. Physiol. 2021, 599, 431–441. [Google Scholar] [CrossRef]
- de Oliveira, R.B.; Petiz, L.L.; Lim, R.; Lipski, J.; Gravina, F.S.; Brichta, A.M.; Callister, R.J.; Leão, R.N.; van Helden, D.F. Crosstalk between mitochondria, calcium channels and actin cytoskeleton modulates noradrenergic activity of locus coeruleus neurons. J. Neurochem. 2019, 149, 471–487. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, X.; Zhou, W.; Wang, Y.; Zhao, H.; Li, J.; Zhou, X.; Zhang, H.; Zhao, T.; Li, P. Protective Role of Tangshen Formula on the Progression of Renal Damage in. J. Diabetes Res. 2020, 2020, 3634974. [Google Scholar] [CrossRef]
- Guillery, R.W. Observations of synaptic structures: Origins of the neuron doctrine and its current status. Philos Trans. R Soc. Lond B Biol. Sci. 2005, 360, 1281–1307. [Google Scholar] [CrossRef]
- Leterrier, C. A Pictorial History of the Neuronal Cytoskeleton. J. NeuroSci. 2021, 41, 11–27. [Google Scholar] [CrossRef]
- Marchisio, P.C.; Osborn, M.; Weber, K. The intracellular organization of actin and tubulin in cultured C-1300 mouse neuroblastoma cells (clone NB41A3). J. Neurocytol 1978, 7, 571–582. [Google Scholar] [CrossRef]
- Cáceres, A.; Banker, G.A.; Binder, L. Immunocytochemical localization of tubulin and microtubule-associated protein 2 during the development of hippocampal neurons in culture. J. NeuroSci. 1986, 6, 714–722. [Google Scholar] [CrossRef]
- Allison, D.W.; Chervin, A.S.; Gelfand, V.I.; Craig, A.M. Postsynaptic scaffolds of excitatory and inhibitory synapses in hippocampal neurons: Maintenance of core components independent of actin filaments and microtubules. J. NeuroSci. 2000, 20, 4545–4554. [Google Scholar] [CrossRef]
- Mazzarini, M.; Falchi, M.; Bani, D.; Migliaccio, A.R. Evolution and new frontiers of histology in bio-medical research. Microsc. Res. Tech. 2021, 84, 217–237. [Google Scholar] [CrossRef]
- Leterrier, C. The Axon Initial Segment, 50Years Later: A Nexus for Neuronal Organization and Function. Curr. Top. Membr. 2016, 77, 185–233. [Google Scholar] [CrossRef]
- Letourneau, P.C. Differences in the organization of actin in the growth cones compared with the neurites of cultured neurons from chick embryos. J. Cell Biol. 1983, 97, 963–973. [Google Scholar] [CrossRef]
- Nagele, R.G.; Kosciuk, M.C.; Hunter, E.T.; Bush, K.T.; Lee, H. Immunoelectron microscopic localization of actin in neurites of cultured embryonic chick dorsal root ganglia: Actin is a component of granular, microtubule-associated crossbridges. Brain Res. 1988, 474, 279–286. [Google Scholar] [CrossRef]
- Alonso, G.; Gabrion, J.; Travers, E.; Assenmacher, I. Ultrastructural organization of actin filaments in neurosecretory axons of the rat. Cell Tissue Res. 1981, 214, 323–341. [Google Scholar] [CrossRef]
- DE Winter, D.A.M.; Hsieh, C.; Marko, M.; Hayles, M.F. Cryo-FIB preparation of whole cells and tissue for cryo-TEM: Use of high-pressure frozen specimens in tubes and planchets. J. Microsc. 2021, 281, 125–137. [Google Scholar] [CrossRef]
- Danev, R.; Yanagisawa, H.; Kikkawa, M. Cryo-Electron Microscopy Methodology: Current Aspects and Future Directions. Trends Biochem. Sci. 2019, 44, 837–848. [Google Scholar] [CrossRef]
- Lukinavičius, G.; Reymond, L.; D’Este, E.; Masharina, A.; Göttfert, F.; Ta, H.; Güther, A.; Fournier, M.; Rizzo, S.; Waldmann, H.; et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat. Methods 2014, 11, 731–733. [Google Scholar] [CrossRef]
- Stepanova, T.; Slemmer, J.; Hoogenraad, C.C.; Lansbergen, G.; Dortland, B.; De Zeeuw, C.I.; Grosveld, F.; van Cappellen, G.; Akhmanova, A.; Galjart, N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J. NeuroSci. 2003, 23, 2655–2664. [Google Scholar] [CrossRef]
- Ladt, K.; Ganguly, A.; Roy, S. Axonal actin in action: Imaging actin dynamics in neurons. Methods Cell Biol. 2016, 131, 91–106. [Google Scholar] [CrossRef]
- Mimori-Kiyosue, Y.; Shiina, N.; Tsukita, S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr. Biol. 2000, 10, 865–868. [Google Scholar] [CrossRef]
- Chou, V.T.; Yesilyurt, H.G.; Lai, H.; Long, J.B.; Arnes, M.; Obbad, K.; Jones, M.; Sasaki, H.; Lucas, L.A.G.; Alworth, S.; et al. 3D Particle Tracking for Noninvasive In Vivo Analysis of Synaptic Microtubule Dynamics in Dendrites and Neuromuscular Junctions of Drosophila. J. Vis. Exp. 2020, 159, e61159. [Google Scholar] [CrossRef]
- Morrison, E.E.; Moncur, P.M.; Askham, J.M. EB1 identifies sites of microtubule polymerisation during neurite development. Brain Res. Mol. Brain Res. 2002, 98, 145–152. [Google Scholar] [CrossRef]
- Leterrier, C.; Dubey, P.; Roy, S. The nano-architecture of the axonal cytoskeleton. Nat. Rev. NeuroSci. 2017, 18, 713–726. [Google Scholar] [CrossRef]
- Poole, J.J.A.; Mostaço-Guidolin, L.B. Optical Microscopy and the Extracellular Matrix Structure: A Review. Cells 2021, 10, 1760. [Google Scholar] [CrossRef]
- D’Este, E.; Kamin, D.; Göttfert, F.; El-Hady, A.; Hell, S.W. STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Rep. 2015, 10, 1246–1251. [Google Scholar] [CrossRef]
- Leduc, C.; Etienne-Manneville, S. Regulation of microtubule-associated motors drives intermediate filament network polarization. J. Cell Biol. 2017, 216, 1689–1703. [Google Scholar] [CrossRef]
- Uchida, A.; Çolakoğlu, G.; Wang, L.; Monsma, P.C.; Brown, A. Severing and end-to-end annealing of neurofilaments in neurons. Proc. Natl. Acad. Sci. USA 2013, 110, E2696–E2705. [Google Scholar] [CrossRef]
- Shelden, E.A.; Colburn, Z.T.; Jones, J.C. Focusing super resolution on the cytoskeleton. F1000Res 2016, 5, F1000. [Google Scholar] [CrossRef][Green Version]
- Maglione, M.; Sigrist, S.J. Seeing the forest tree by tree: Super-resolution light microscopy meets the neurosciences. Nat. NeuroSci. 2013, 16, 790–797. [Google Scholar] [CrossRef]
- Tam, J.; Merino, D. Stochastic optical reconstruction microscopy (STORM) in comparison with stimulated emission depletion (STED) and other imaging methods. J. Neurochem. 2015, 135, 643–658. [Google Scholar] [CrossRef]
- Almada, P.; Culley, S.; Henriques, R. PALM and STORM: Into large fields and high-throughput microscopy with sCMOS detectors. Methods 2015, 88, 109–121. [Google Scholar] [CrossRef]
- Nägerl, U.V.; Willig, K.I.; Hein, B.; Hell, S.W.; Bonhoeffer, T. Live-cell imaging of dendritic spines by STED microscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 18982–18987. [Google Scholar] [CrossRef]
- Urban, N.T.; Willig, K.I.; Hell, S.W.; Nägerl, U.V. STED nanoscopy of actin dynamics in synapses deep inside living brain slices. Biophys. J. 2011, 101, 1277–1284. [Google Scholar] [CrossRef]
- Berning, S.; Willig, K.I.; Steffens, H.; Dibaj, P.; Hell, S.W. Nanoscopy in a living mouse brain. Science 2012, 335, 551. [Google Scholar] [CrossRef]
- Willig, K.I.; Steffens, H.; Gregor, C.; Herholt, A.; Rossner, M.J.; Hell, S.W. Nanoscopy of filamentous actin in cortical dendrites of a living mouse. Biophys. J. 2014, 106, L01–L03. [Google Scholar] [CrossRef]
- Wegner, W.; Ilgen, P.; Gregor, C.; van Dort, J.; Mott, A.C.; Steffens, H.; Willig, K.I. In vivo mouse and live cell STED microscopy of neuronal actin plasticity using far-red emitting fluorescent proteins. Sci. Rep. 2017, 7, 11781. [Google Scholar] [CrossRef]
- Ter Veer, M.J.T.; Pfeiffer, T.; Nägerl, U.V. Two-Photon STED Microscopy for Nanoscale Imaging of Neural Morphology In Vivo. Methods Mol. Biol. 2017, 1663, 45–64. [Google Scholar] [CrossRef]
- Masch, J.M.; Steffens, H.; Fischer, J.; Engelhardt, J.; Hubrich, J.; Keller-Findeisen, J.; D’Este, E.; Urban, N.T.; Grant, S.G.N.; Sahl, S.J.; et al. Robust nanoscopy of a synaptic protein in living mice by organic-fluorophore labeling. Proc. Natl. Acad. Sci. USA 2018, 115, E8047–E8056. [Google Scholar] [CrossRef]
- Pero, M.E.; Meregalli, C.; Qu, X.; Shin, G.J.; Kumar, A.; Shorey, M.; Rolls, M.M.; Tanji, K.; Brannagan, T.H.; Alberti, P.; et al. Pathogenic role of delta 2 tubulin in bortezomib-induced peripheral neuropathy. Proc. Natl. Acad. Sci. USA 2021, 118, e2012685118. [Google Scholar] [CrossRef]
- Mou, Y.; Mukte, S.; Chai, E.; Dein, J.; Li, X.J. Analyzing Mitochondrial Transport and Morphology in Human Induced Pluripotent Stem Cell-Derived Neurons in Hereditary Spastic Paraplegia. J. Vis. Exp. 2020, 9, 156. [Google Scholar] [CrossRef]
- Gavini, C.K.; White, C.R.; Mansuy-Aubert, V.; Aubert, G. Loss of C2 Domain Protein (C2CD5) Alters Hypothalamic Mitochondrial Trafficking, Structure, and Function. Neuroendocrinology 2021, 112, 324–337. [Google Scholar] [CrossRef]
- Ligon, L.A.; Steward, O. Movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J. Comp. Neurol 2000, 427, 340–350. [Google Scholar] [CrossRef]
- Wang, X.; Schwarz, T.L. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell 2009, 136, 163–174. [Google Scholar] [CrossRef]
- Jin, D.; Zhou, R.; Yaqoob, Z.; So, P.T.C. Tomographic phase microscopy: Principles and applications in bioimaging [Invited]. J. Opt. Soc. Am. B 2017, 34, B64–B77. [Google Scholar] [CrossRef]
- Lee, K.; Kim, K.; Jung, J.; Heo, J.; Cho, S.; Lee, S.; Chang, G.; Jo, Y.; Park, H.; Park, Y. Quantitative phase imaging techniques for the study of cell pathophysiology: From principles to applications. Sensors 2013, 13, 4170–4191. [Google Scholar] [CrossRef]
- Debailleul, M.; Georges, V.; Simon, B.; Morin, R.; Haeberlé, O. High-resolution three-dimensional tomographic diffractive microscopy of transparent inorganic and biological samples. Opt. Lett. 2009, 34, 79–81. [Google Scholar] [CrossRef]
- Park, S.; Ahn, J.W.; Jo, Y.; Kang, H.Y.; Kim, H.J.; Cheon, Y.; Kim, J.W.; Park, Y.; Lee, S.; Park, K. Label-Free Tomographic Imaging of Lipid Droplets in Foam Cells for Machine-Learning-Assisted Therapeutic Evaluation of Targeted Nanodrugs. ACS Nano 2020, 14, 1856–1865. [Google Scholar] [CrossRef]
- Popescu, G.; Park, Y.; Lue, N.; Best-Popescu, C.; Deflores, L.; Dasari, R.R.; Feld, M.S.; Badizadegan, K. Optical imaging of cell mass and growth dynamics. Am. J. Physiol. Cell Physiol. 2008, 295, C538–C544. [Google Scholar] [CrossRef]
- Bromfield, E.B.; Cavazos, J.E.; Sirven, J.I. An Introduction to Epilepsy; West Hartford (CT); American Epilepsy Society: Chicago, IL, USA, 2006. [Google Scholar]
- Ngugi, A.K.; Bottomley, C.; Kleinschmidt, I.; Sander, J.W.; Newton, C.R. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach. Epilepsia 2010, 51, 883–890. [Google Scholar] [CrossRef]
- Glauser, T.; Ben-Menachem, E.; Bourgeois, B.; Cnaan, A.; Guerreiro, C.; Kälviäinen, R.; Mattson, R.; French, J.A.; Perucca, E.; Tomson, T.; et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2013, 54, 551–563. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Berry, D.J.; Bourgeois, B.F.; Cloyd, J.C.; Glauser, T.A.; Johannessen, S.I.; Leppik, I.E.; Tomson, T.; Perucca, E. Antiepileptic drugs--best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008, 49, 1239–1276. [Google Scholar] [CrossRef]
- Craddock, T.J.; Tuszynski, J.A.; Priel, A.; Freedman, H. Microtubule ionic conduction and its implications for higher cognitive functions. J. Integr NeuroSci. 2010, 9, 103–122. [Google Scholar] [CrossRef]
- Whatley, V.J.; Harris, R.A. The cytoskeleton and neurotransmitter receptors. Int. Rev. Neurobiol. 1996, 39, 113–143. [Google Scholar] [CrossRef]
- Gardiner, J.; Marc, J. Disruption of normal cytoskeletal dynamics may play a key role in the pathogenesis of epilepsy. Neuroscientist 2010, 16, 28–39. [Google Scholar] [CrossRef]
- Xu, X.; Hu, Y.; Xiong, Y.; Li, Z.; Wang, W.; Du, C.; Yang, Y.; Zhang, Y.; Xiao, F.; Wang, X. Association of Microtubule Dynamics with Chronic Epilepsy. Mol. Neurobiol. 2016, 53, 5013–5024. [Google Scholar] [CrossRef] [PubMed]
- Compagnucci, C.; Piermarini, E.; Sferra, A.; Borghi, R.; Niceforo, A.; Petrini, S.; Piemonte, F.; Bertini, E. Cytoskeletal dynamics during in vitro neurogenesis of induced pluripotent stem cells (iPSCs). Mol. Cell NeuroSci. 2016, 77, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Cushion, T.D.; Paciorkowski, A.R.; Pilz, D.T.; Mullins, J.G.; Seltzer, L.E.; Marion, R.W.; Tuttle, E.; Ghoneim, D.; Christian, S.L.; Chung, S.K.; et al. De novo mutations in the beta-tubulin gene TUBB2A cause simplified gyral patterning and infantile-onset epilepsy. Am. J. Hum. Genet. 2014, 94, 634–641. [Google Scholar] [CrossRef]
- de Nijs, L.; Lakaye, B.; Coumans, B.; Léon, C.; Ikeda, T.; Delgado-Escueta, A.V.; Grisar, T.; Chanas, G. EFHC1, a protein mutated in juvenile myoclonic epilepsy, associates with the mitotic spindle through its N-terminus. Exp. Cell Res. 2006, 312, 2872–2879. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.H.; Roberts, E.A.; Her, L.S.; Liu, X.; Williams, D.S.; Cleveland, D.W.; Goldstein, L.S. Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J. Cell Biol. 2003, 161, 55–66. [Google Scholar] [CrossRef]
- Nakajima, K.; Yin, X.; Takei, Y.; Seog, D.H.; Homma, N.; Hirokawa, N. Molecular motor KIF5A is essential for GABA(A) receptor transport, and KIF5A deletion causes epilepsy. Neuron 2012, 76, 945–961. [Google Scholar] [CrossRef]
- Lu, B.; Pang, P.T.; Woo, N.H. The yin and yang of neurotrophin action. Nat. Rev. NeuroSci. 2005, 6, 603–614. [Google Scholar] [CrossRef]
- Rex, C.S.; Lin, C.Y.; Kramár, E.A.; Chen, L.Y.; Gall, C.M.; Lynch, G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J. NeuroSci. 2007, 27, 3017–3029. [Google Scholar] [CrossRef]
- Yu, X.; Guan, Q.; Wang, Y.; Shen, H.; Zhai, L.; Lu, X.; Jin, Y. Anticonvulsant and anti-apoptosis effects of salvianolic acid B on pentylenetetrazole-kindled rats via AKT/CREB/BDNF signaling. Epilepsy Res. 2019, 154, 90–96. [Google Scholar] [CrossRef]
- Aneja, R.; Asress, S.; Dhiman, N.; Awasthi, A.; Rida, P.C.; Arora, S.K.; Zhou, J.; Glass, J.D.; Joshi, H.C. Non-toxic melanoma therapy by a novel tubulin-binding agent. Int. J. Cancer 2010, 126, 256–265. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Y.; Huang, Z.; Cai, M.; Shu, Y.; Zeng, C.; Feng, L.; Xiao, B.; Zhan, Q. The study of microtubule dynamics and stability at the postsynaptic density in a rat pilocarpine model of temporal lobe epilepsy. Ann. Transl. Med. 2020, 8, 863. [Google Scholar] [CrossRef] [PubMed]
- Liaci, C.; Camera, M.; Caslini, G.; Rando, S.; Contino, S.; Romano, V.; Merlo, G.R. Neuronal Cytoskeleton in Intellectual Disability: From Systems Biology and Modeling to Therapeutic Opportunities. Int. J. Mol. Sci. 2021, 22, 6167. [Google Scholar] [CrossRef] [PubMed]
- Sriroopreddy, R.; Sajeed, R.; Raguraman, P.; Sudandiradoss, C. Differentially expressed gene (DEG) based protein-protein interaction (PPI) network identifies a spectrum of gene interactome, transcriptome and correlated miRNA in nondisjunction Down syndrome. Int. J. Biol. Macromol. 2019, 122, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Bhambhvani, H.P.; Mueller, T.M.; Simmons, M.S.; Meador-Woodruff, J.H. Actin polymerization is reduced in the anterior cingulate cortex of elderly patients with schizophrenia. Transl. Psychiatry 2017, 7, 1278. [Google Scholar] [CrossRef] [PubMed]
- Dowjat, K.; Adayev, T.; Kaczmarski, W.; Wegiel, J.; Hwang, Y.W. Gene dosage-dependent association of DYRK1A with the cytoskeleton in the brain and lymphocytes of down syndrome patients. J. Neuropathol. Exp. Neurol. 2012, 71, 1100–1112. [Google Scholar] [CrossRef]
- Michaelsen-Preusse, K.; Feuge, J.; Korte, M. Imbalance of synaptic actin dynamics as a key to fragile X syndrome? J. Physiol. 2018, 596, 2773–2782. [Google Scholar] [CrossRef]
- Bhattacherjee, A.; Mu, Y.; Winter, M.K.; Knapp, J.R.; Eggimann, L.S.; Gunewardena, S.S.; Kobayashi, K.; Kato, S.; Krizsan-Agbas, D.; Smith, P.G. Neuronal cytoskeletal gene dysregulation and mechanical hypersensitivity in a rat model of Rett syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, E6952–E6961. [Google Scholar] [CrossRef]
- Guo, D.; Yang, X.; Shi, L. Rho GTPase Regulators and Effectors in Autism Spectrum Disorders: Animal Models and Insights for Therapeutics. Cells 2020, 9, 835. [Google Scholar] [CrossRef]
- Griesi-Oliveira, K.; Suzuki, A.M.; Alves, A.Y.; Mafra, A.C.C.N.; Yamamoto, G.L.; Ezquina, S.; Magalhães, Y.T.; Forti, F.L.; Sertie, A.L.; Zachi, E.C.; et al. Actin cytoskeleton dynamics in stem cells from autistic individuals. Sci. Rep. 2018, 8, 11138. [Google Scholar] [CrossRef]
- Higuchi, Y.; Takashima, H. Clinical genetics of Charcot-Marie-Tooth disease. J. Hum. Genet. 2022. on-line ahed of print. [Google Scholar] [CrossRef]
- Hong, Y.B.; Lee, J.H.; Park, H.J.; Choi, Y.R.; Hyun, Y.S.; Park, J.H.; Koo, H.; Chung, K.W.; Choi, B.O. A family with axonal sensorimotor polyneuropathy with TUBB3 mutation. Mol. Med. Rep. 2015, 11, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Courtemanche, N. Mechanisms of formin-mediated actin assembly and dynamics. Biophys Rev. 2018, 10, 1553–1569. [Google Scholar] [CrossRef]
- Boyer, O.; Nevo, F.; Plaisier, E.; Funalot, B.; Gribouval, O.; Benoit, G.; Huynh Cong, E.; Arrondel, C.; Tête, M.J.; Montjean, R.; et al. INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N. Engl. J. Med. 2011, 365, 2377–2388. [Google Scholar] [CrossRef]
- Rebelo, A.P.; Abrams, A.J.; Cottenie, E.; Horga, A.; Gonzalez, M.; Bis, D.M.; Sanchez-Mejias, A.; Pinto, M.; Buglo, E.; Markel, K.; et al. Cryptic Amyloidogenic Elements in the 3’ UTRs of Neurofilament Genes Trigger Axonal Neuropathy. Am. J. Hum. Genet. 2016, 98, 597–614. [Google Scholar] [CrossRef]
- Sainio, M.T.; Ylikallio, E.; Mäenpää, L.; Lahtela, J.; Mattila, P.; Auranen, M.; Palmio, J.; Tyynismaa, H. Absence of NEFL in patient-specific neurons in early-onset Charcot-Marie-Tooth neuropathy. Neurol Genet. 2018, 4, e244. [Google Scholar] [CrossRef] [PubMed]
- Jordanova, A.; De Jonghe, P.; Boerkoel, C.F.; Takashima, H.; De Vriendt, E.; Ceuterick, C.; Martin, J.J.; Butler, I.J.; Mancias, P.; Papasozomenos, S.C.h.; et al. Mutations in the neurofilament light chain gene (NEFL) cause early onset severe Charcot-Marie-Tooth disease. Brain 2003, 126, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Bouhy, D.; Juneja, M.; Katona, I.; Holmgren, A.; Asselbergh, B.; De Winter, V.; Hochepied, T.; Goossens, S.; Haigh, J.J.; Libert, C.; et al. A knock-in/knock-out mouse model of HSPB8-associated distal hereditary motor neuropathy and myopathy reveals toxic gain-of-function of mutant Hspb8. Acta Neuropathol. 2018, 135, 131–148. [Google Scholar] [CrossRef]
- Irobi, J.; Holmgren, A.; De Winter, V.; Asselbergh, B.; Gettemans, J.; Adriaensen, D.; Ceuterick-de Groote, C.; Van Coster, R.; De Jonghe, P.; Timmerman, V. Mutant HSPB8 causes protein aggregates and a reduced mitochondrial membrane potential in dermal fibroblasts from distal hereditary motor neuropathy patients. Neuromuscul. Disord. 2012, 22, 699–711. [Google Scholar] [CrossRef]
- d’Ydewalle, C.; Krishnan, J.; Chiheb, D.M.; Van Damme, P.; Irobi, J.; Kozikowski, A.P.; Vanden Berghe, P.; Timmerman, V.; Robberecht, W.; Van Den Bosch, L. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat. Med. 2011, 17, 968–974. [Google Scholar] [CrossRef]
- Alberti, P. Chemotherapy-induced peripheral neurotoxicity—Outcome measures: The issue. Expert Opin. Drug Metab. Toxicol. 2016, 13, 241–243. [Google Scholar] [CrossRef][Green Version]
- Velasco, R.; Alberti, P.; Bruna, J.; Psimaras, D.; Argyriou, A.A. Bortezomib and other proteosome inhibitors-induced peripheral neurotoxicity: From pathogenesis to treatment. J. Peripher. Nerv. Syst. 2019, 24 (Suppl. S2), S52–S62. [Google Scholar] [CrossRef]
- Islam, B.; Lustberg, M.; Staff, N.P.; Kolb, N.; Alberti, P.; Argyriou, A.A. Vinca alkaloids, thalidomide and eribulin-induced peripheral neurotoxicity: From pathogenesis to treatment. J. Peripher. Nerv. Syst. 2019, 24 (Suppl. S2), S63–S73. [Google Scholar] [CrossRef]
- Tamburin, S.; Park, S.B.; Alberti, P.; Demichelis, C.; Schenone, A.; Argyriou, A.A. Taxane and epothilone-induced peripheral neurotoxicity: From pathogenesis to treatment. J. Peripher. Nerv. Syst. 2019, 24 (Suppl. S2), S40–S51. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Bruna, J.; Marmiroli, P.; Cavaletti, G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit Rev. Oncol. Hematol. 2012, 82, 51–77. [Google Scholar] [CrossRef]
- Herrmann, H.; Aebi, U. Intermediate filaments: Molecular structure, assembly mechanism, and integration into functionally distinct intracellular Scaffolds. Annu. Rev. Biochem. 2004, 73, 749–789. [Google Scholar] [CrossRef]
- Herrmann, H.; Aebi, U. Intermediate Filaments: Structure and Assembly. Cold Spring Harb. Perspect. Biol. 2016, 8, a018242. [Google Scholar] [CrossRef]
- Burton, P.R.; Wentz, M.A. Neurofilaments are prominent in bullfrog olfactory axons but are rarely seen in those of the tiger salamander, Ambystoma tigrinum. J. Comp. Neurol. 1992, 317, 396–406. [Google Scholar] [CrossRef]
- Yuan, A.; Rao, M.V.; Nixon, R.A. Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb. Perspect Biol. 2017, 9, a018309. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Rohrer, J.D.; Woollacott, I.O.; Dick, K.M.; Brotherhood, E.; Gordon, E.; Fellows, A.; Toombs, J.; Druyeh, R.; Cardoso, M.J.; Ourselin, S.; et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 2016, 87, 1329–1336. [Google Scholar] [CrossRef]
- Sandelius, Å.; Zetterberg, H.; Blennow, K.; Adiutori, R.; Malaspina, A.; Laura, M.; Reilly, M.M.; Rossor, A.M. Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology 2018, 90, e518–e524. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, S.; Farinazzo, A.; Magliozzi, R.; Alberti, D.; Monaco, S.; Ferrari, S. Serum and cerebrospinal neurofilament light chain levels in patients with acquired peripheral neuropathies. J. Peripher. Nerv. Syst. 2018, 23, 174–177. [Google Scholar] [CrossRef]
- Meregalli, C.; Fumagalli, G.; Alberti, P.; Canta, A.; Carozzi, V.A.; Chiorazzi, A.; Monza, L.; Pozzi, E.; Sandelius, Å.; Blennow, K.; et al. Neurofilament light chain as disease biomarker in a rodent model of chemotherapy induced peripheral neuropathy. Exp. Neurol. 2018, 307, 129–132. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, M.K.; Park, N.Y.; Hyun, J.W.; Lee, M.Y.; Kim, H.J.; Jung, S.K.; Cha, Y. Serum neurofilament light chain levels as a biomarker of neuroaxonal injury and severity of oxaliplatin-induced peripheral neuropathy. Sci. Rep. 2020, 10, 7995. [Google Scholar] [CrossRef]
- Meregalli, C.; Fumagalli, G.; Alberti, P.; Canta, A.; Chiorazzi, A.; Monza, L.; Pozzi, E.; Carozzi, V.A.; Blennow, K.; Zetterberg, H.; et al. Neurofilament light chain: A specific serum biomarker of axonal damage severity in rat models of Chemotherapy-Induced Peripheral Neurotoxicity. Arch. Toxicol 2020, 94, 2517–2522. [Google Scholar] [CrossRef]
- Bendstrup, N.; Hejl, A.M.; Salvesen, L. Neurofilament Light Chain Levels in Frontotemporal Dementia and Progressive Supranuclear Palsy: A Systematic Review. J. Alzheimers Dis. 2022, 87, 131–140. [Google Scholar] [CrossRef]
- Wendel, E.M.; Bertolini, A.; Kousoulos, L.; Rauchenzauner, M.; Schanda, K.; Wegener-Panzer, A.; Baumann, M.; Reindl, M.; Otto, M.; Rostásy, K. Serum neurofilament light-chain levels in children with monophasic myelin oligodendrocyte glycoprotein-associated disease, multiple sclerosis, and other acquired demyelinating syndrome. Mult. Scler. 2022, 28, 1553–1561. [Google Scholar] [CrossRef]
- van den Bosch, A.; Fransen, N.; Mason, M.; Rozemuller, A.J.; Teunissen, C.; Smolders, J.; Huitinga, I. Neurofilament Light Chain Levels in Multiple Sclerosis Correlate With Lesions Containing Foamy Macrophages and With Acute Axonal Damage. Neurol. Neuroimmunol Neuroinflamm 2022, 9, e1154. [Google Scholar] [CrossRef]
- Alberti, P. Platinum-drugs induced peripheral neurotoxicity: Clinical course and preclinical evidence. Expert Opin. Drug Metab Toxicol 2019, 15, 487–497. [Google Scholar] [CrossRef]
- Karteri, S.; Bruna, J.; Argyriou, A.A.; Mariotto, S.; Velasco, R.; Alemany, M.; Kalofonou, F.; Alberti, P.; Dinoto, A.; Velissaris, D.; et al. Prospectively Assessing Serum Neurofilament Light Chain Levels As A Biomarker Of Paclitaxel-Induced Peripheral Neurotoxicity In Breast Cancer Patients. J. Peripher Nerv. Syst. 2022, 27, 166–174. [Google Scholar] [CrossRef]
- Huehnchen, P.; Schinke, C.; Bangemann, N.; Dordevic, A.D.; Kern, J.; Maierhof, S.K.; Hew, L.; Nolte, L.; Körtvelyessy, P.; Göpfert, J.C.; et al. Neurofilament proteins as a potential biomarker in chemotherapy-induced polyneuropathy. JCI Insight 2022, 7, e154395. [Google Scholar] [CrossRef] [PubMed]
| Class of Transport Protein | Transport | Substrates |
|---|---|---|
| Myosin | They move specifically along actin filaments. They are involved in contractile forces and short-range transport [17]. | Myosin Va is involved in localization of proteins to the somatodendritic compartment and in regulating transport of mRNAs, dense core vesicles, mitochondria and neurofilaments. It is involved in the localization of axonal proteins and modulates mitochondrial movements. More study is needed to fully elucidate these roles and determine their functional significance [18]. |
| Dyneins | They move along the microtubule cytoskeleton to facilitate long-range transport [17]. Retrograde transport [19]. | It requires the dynein activator, dynactin, that binds directly to dynein and also binds directly to microtubules via Cytoskeletal Associated Protein-Glycine-rich domain [20,21]. |
| Kinesin | They move along the microtubule cytoskeleton to facilitate long-range transport [17]. Anterograde transport [19]. | Kinesin-1 family drive the transport of a wide range of cargos, including vesicles, organelles, proteins, and RNA particles. Kinesin-2 family drive fodrin-positive plasma membrane precursors, N-cadherin and β-catenin, choline acetyltransferase, and Rab7-positive late endosome-lysosome compartments. Kinesin-3 family drive the motility of synaptic vesicle precursors and dense core vesicles [20,21]. |
| Super Resolution Techniques | Resolution | Further Insights into | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| SIM 1 | 100–250 nm | Dynamic changes in cytoskeleton architecture. | Conventional fluorophores Multiple fluorophores | Reduced resolution. Longer processing time. | [90,94,95] |
| STED 2 | 50–80 nm | Cell matrix interactions. Periodic ring structure of actin in neurons. Actin dynamics in dendritic spine in living neurons. Intermediate filaments network. Microtubule in primary cilia. | Generic dye Speed of acquisition No need for complex analysis | Photobleaching. High-energy laser, often not compatible with living cells. | [90,94,96] |
| STORM 3 | 20–50 nm | Actin organization in cytoskeleton. Cell junctions (integrin keratin, plectin). Microtubule organization. | Very high resolutionSingle fluorophore, specific | Low temporal resolution. Low acquisition speed. Phototoxicity. Complex post-acquisition. Need of special fluorophores. | [90,94,96,97] |
| PALM 4 | 20–50 nm | Mitochondrial proteins. Microtubule organization and role in organelle movement in living cells. Interaction with kinesin motor proteins in neurons. | Very high resolutionSingle fluorophore, specific | Low temporal resolution. Low acquisition speed. Phototoxicity. Complex post-acquisition. Need of special fluorophores. | [90,94,97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberti, P.; Semperboni, S.; Cavaletti, G.; Scuteri, A. Neurons: The Interplay between Cytoskeleton, Ion Channels/Transporters and Mitochondria. Cells 2022, 11, 2499. https://doi.org/10.3390/cells11162499
Alberti P, Semperboni S, Cavaletti G, Scuteri A. Neurons: The Interplay between Cytoskeleton, Ion Channels/Transporters and Mitochondria. Cells. 2022; 11(16):2499. https://doi.org/10.3390/cells11162499
Chicago/Turabian StyleAlberti, Paola, Sara Semperboni, Guido Cavaletti, and Arianna Scuteri. 2022. "Neurons: The Interplay between Cytoskeleton, Ion Channels/Transporters and Mitochondria" Cells 11, no. 16: 2499. https://doi.org/10.3390/cells11162499
APA StyleAlberti, P., Semperboni, S., Cavaletti, G., & Scuteri, A. (2022). Neurons: The Interplay between Cytoskeleton, Ion Channels/Transporters and Mitochondria. Cells, 11(16), 2499. https://doi.org/10.3390/cells11162499








