Integrated Excitatory/Inhibitory Imbalance and Transcriptomic Analysis Reveals the Association between Dysregulated Synaptic Genes and Anesthetic-Induced Cognitive Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Propofol Exposure
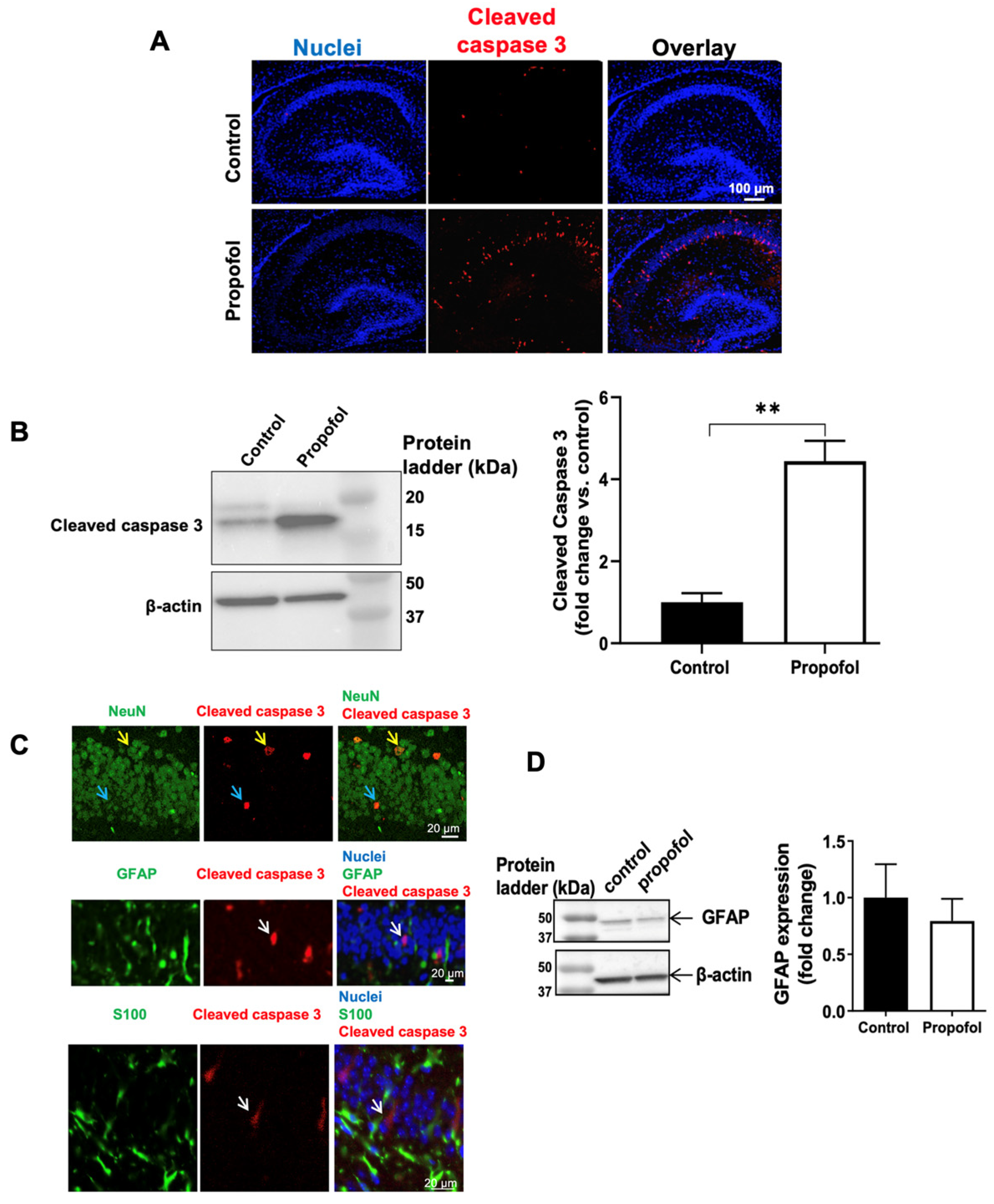
2.2. Immunofluorescence Staining
2.3. Western Blot
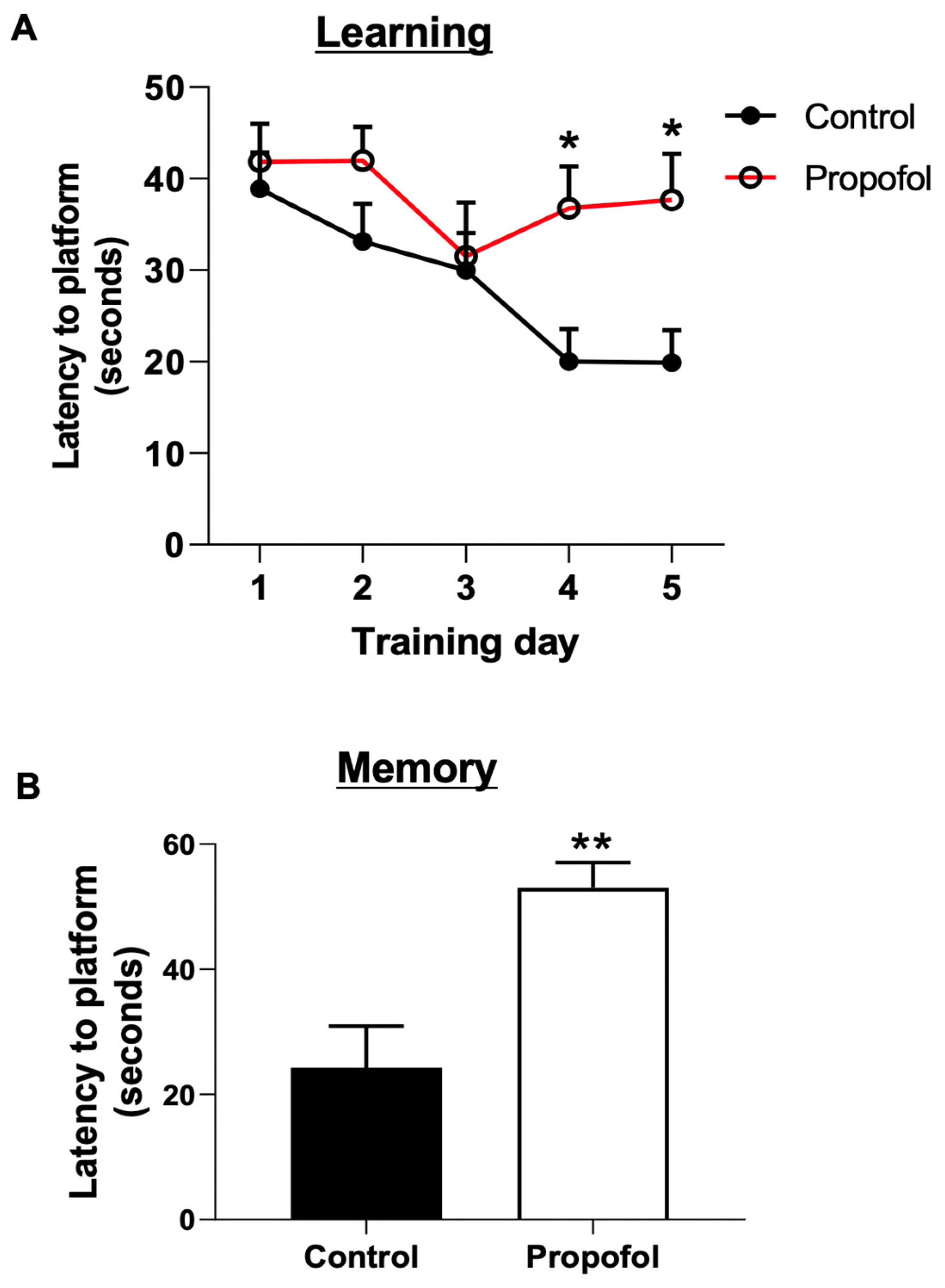
2.4. Cognitive Function Assay
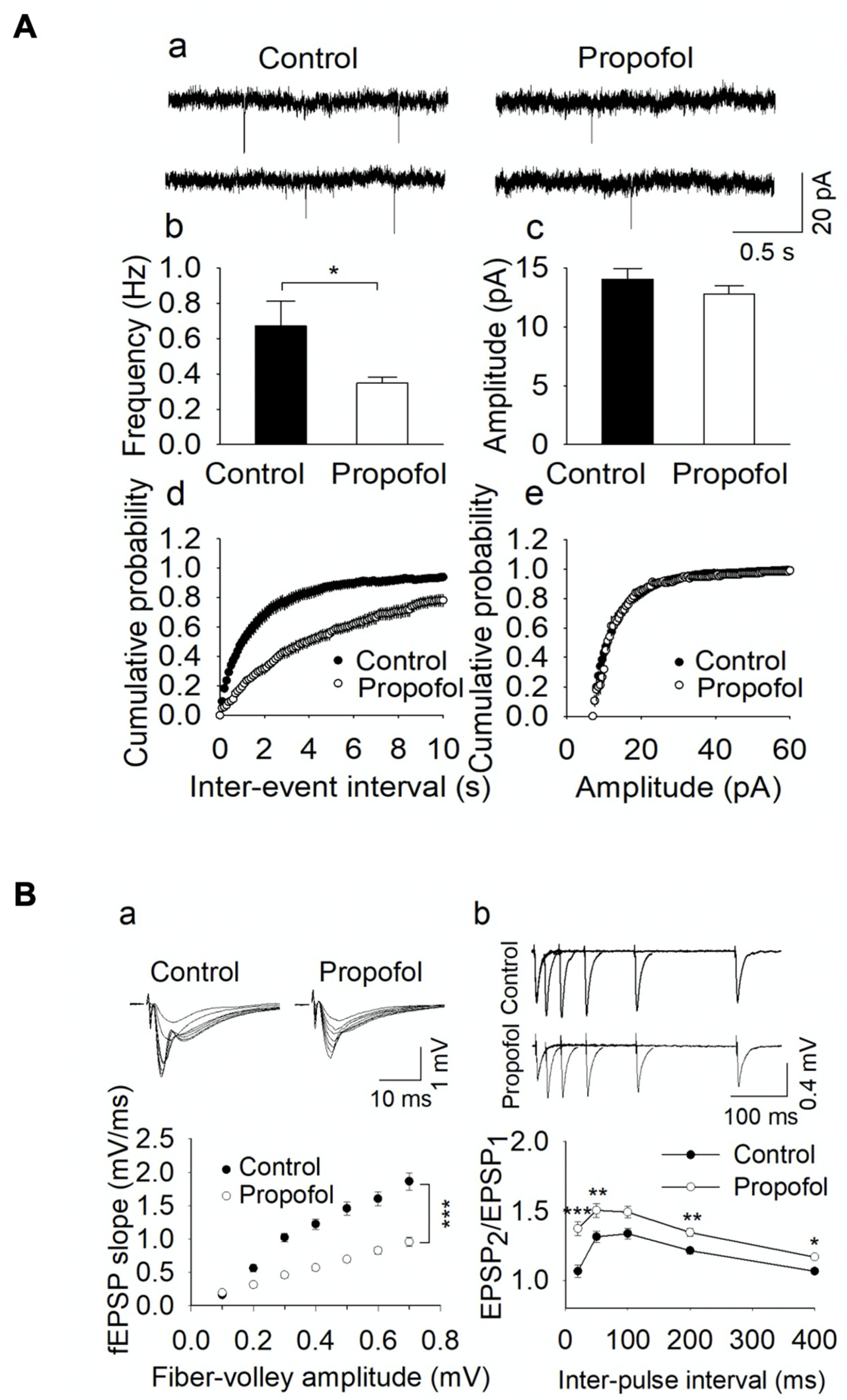
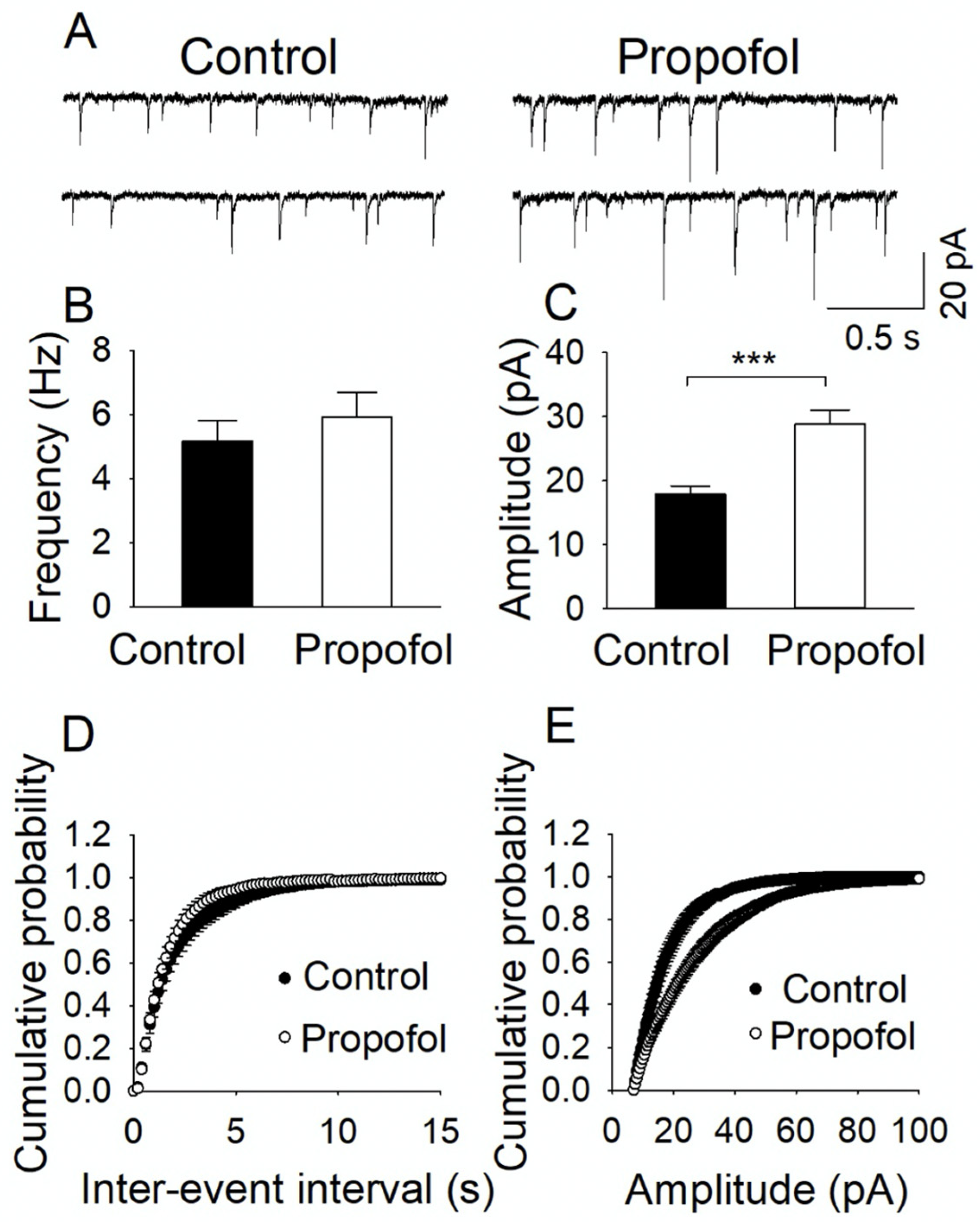
2.5. Electrophysiological Assays
2.6. Microarray Assay of Messenger RNA (mRNA) Profiling
2.7. RT-PCR
2.8. Bioinformatic Analysis of Propofol-Induced Dysregulated Synaptic Genes and the Related Pathways/Functions
2.9. Statistic Analysis
3. Results
3.1. Propofol Exposure Induces Acute Neuroapoptosis in Neonatal Mouse Hippocampi
3.2. Developmental Propofol Exposure Leads to the Impaired Learning and Memory in P60 Mice
3.3. Propofol Exposure to P7 Mice Leads to the Disruption of E/I Balance in the Hippocampal Slices from P60 Mice
3.4. P7 Propofol Exposure Induces the Alteration of mRNA Profiles in P60 Mouse Hippocampi
3.5. Propofol Induces Dysregulation of Signaling Pathways Related to Synaptic Activity and Cognitive Dysfunction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosnjak, Z.J.; Logan, S.; Liu, Y.; Bai, X. Recent Insights Into Molecular Mechanisms of Propofol-Induced Developmental Neurotoxicity: Implications for the Protective Strategies. Anesth. Analg. 2016, 123, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.J.; Ing, C. Neurotoxic Effects of Anesthetics on the Developing Brain; UptoDate: Alphen aan den Rijn, The Netherlands, 2022. [Google Scholar]
- Neudecker, V.; Perez-Zoghbi, J.F.; Brambrink, A.M. Recent advances in understanding cognitive and behavioural alterations after early-in-life anaesthesia exposure and new mitigation/alternative strategies in preclinical studies. Curr. Opin. Anaesthesiol. 2021, 34, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chu, W.; Sheng, R.; Song, S.; Yang, J.; Ji, F.; Jin, X. Maternal anesthesia with sevoflurane during the mid-gestation induces social interaction deficits in offspring C57BL/6 mice. Biochem. Biophys. Res. Commun. 2021, 553, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Joksimovic, S.M.; Faisant, A.; Jevtovic-Todorovic, V. Early exposure to general anesthesia impairs social and emotional development in rats. Mol. Neurobiol. 2020, 57, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Liu, Y.; Hu, Y.; Wang, B.; Zhu, Z.; Jiang, Y.; Suo, Y.; Hu, M.; Gao, J.; Ullah, R.; et al. Neonatal sevoflurane exposure induces impulsive behavioral deficit through disrupting excitatory neurons in the medial prefrontal cortex in mice. Transl. Psychiatry 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Wirak, G.S.; Gabel, C.V.; Connor, C.W. Isoflurane exposure in juvenile caenorhabditis elegans causes persistent changes in neuron dynamics. Anesthesiology 2020, 133, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xie, Z.; Brambrink, A.M.; Yang, G. Behavioural impairments after exposure of neonatal mice to propofol are accompanied by reductions in neuronal activity in cortical circuitry. Br. J. Anaesth. 2021, 126, 1141–1156. [Google Scholar] [CrossRef]
- Young, J.T.; Vlasova, R.M.; Howell, B.R.; Knickmeyer, R.C.; Morin, E.; Kuitchoua, K.I.; Lubach, G.R.; Noel, J.; Hu, X.; Shi, Y.; et al. General anaesthesia during infancy reduces white matter micro-organisation in developing rhesus monkeys. Br. J. Anaesth. 2021, 126, 845–853. [Google Scholar] [CrossRef]
- Ing, C.; Jackson, W.M.; Zaccariello, M.J.; Goldberg, T.E.; McCann, M.E.; Grobler, A.; Davidson, A.; Sun, L.; Li, G.; Warner, D.O. Prospectively assessed neurodevelopmental outcomes in studies of anaesthetic neurotoxicity in children: A systematic review and meta-analysis. Br. J. Anaesth. 2021, 126, 433–444. [Google Scholar] [CrossRef]
- Yang, Y.L.; Wang, L.J.; Chang, J.C.; Ho, S.C.; Kuo, H.C. A national population cohort study showed that exposure to general anesthesia in early childhood is associated with an increase in the risk of developmental delay. Children 2021, 8, 840. [Google Scholar] [CrossRef]
- Warner, D.O.; Zaccariello, M.J.; Katusic, S.K.; Schroeder, D.R.; Hanson, A.C.; Schulte, P.J.; Buenvenida, S.L.; Gleich, S.J.; Wilder, R.T.; Sprung, J.; et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: The Mayo Anesthesia Safety in Kids (MASK) study. Anesthesiology 2018, 129, 89–105. [Google Scholar] [CrossRef]
- Chemaly, M.; El-Rajab, M.A.; Ziade, F.M.; Naja, Z.M. Effect of one anesthetic exposure on long-term behavioral changes in children. J. Clin. Anesth. 2014, 26, 551–556. [Google Scholar] [CrossRef]
- Sprung, J.; Flick, R.P.; Wilder, R.T.; Katusic, S.K.; Pike, T.L.; Dingli, M.; Gleich, S.J.; Schroeder, D.R.; Barbaresi, W.J.; Hanson, A.C.; et al. Anesthesia for cesarean delivery and learning disabilities in a population-based birth cohort. Anesthesiology 2009, 111, 302–310. [Google Scholar] [CrossRef]
- Zaccariello, M.J.; Frank, R.D.; Lee, M.; Kirsch, A.C.; Schroeder, D.R.; Hanson, A.C.; Schulte, P.J.; Wilder, R.T.; Sprung, J.; Katusic, S.K.; et al. Patterns of neuropsychological changes after general anaesthesia in young children: Secondary analysis of the Mayo Anesthesia Safety in Kids study. Br. J. Anaesth. 2019, 122, 671–681. [Google Scholar] [CrossRef]
- Andropoulos, D.B. Effect of Anesthesia on the Developing Brain: Infant and Fetus. Fetal Diagn. Ther. 2018, 43, 1–11. [Google Scholar] [CrossRef]
- Sun, M.; Xie, Z.; Zhang, J.; Leng, Y. Mechanistic insight into sevoflurane-associated developmental neurotoxicity. Cell Biol. Toxicol. 2021. [Google Scholar] [CrossRef]
- Sohn, H.M.; Kim, H.Y.; Park, S.; Han, S.H.; Kim, J.H. Isoflurane decreases proliferation and differentiation, but none of the effects persist in human embryonic stem cell-derived neural progenitor cells. J. Anesth. 2017, 31, 36–43. [Google Scholar] [CrossRef]
- Ju, X.; Cui, J.; Lee, Y.; Park, S.; Hong, B.; Yoo, S.; Kim, Y.H.; Ko, Y.; Lim, C.; Lee, S.Y.; et al. Increasing the interval between repeated anesthetic exposures reduces long-lasting synaptic changes in late post-natal mice. J. Neurochem. 2021, 156, 76–87. [Google Scholar] [CrossRef]
- Jackson, J.; Jambrina, E.; Li, J.; Marston, H.; Menzies, F.; Phillips, K.; Gilmour, G. Targeting the Synapse in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 735. [Google Scholar] [CrossRef]
- Lepeta, K.; Lourenco, M.V.; Schweitzer, B.C.; Martino Adami, P.V.; Banerjee, P.; Catuara-Solarz, S.; de La Fuente Revenga, M.; Guillem, A.M.; Haidar, M.; Ijomone, O.M.; et al. Synaptopathies: Synaptic dysfunction in neurological disorders—A review from students to students. J. Neurochem. 2016, 138, 785–805. [Google Scholar] [CrossRef]
- Megias, M.; Emri, Z.; Freund, T.F.; Gulyas, A.I. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 2001, 102, 527–540. [Google Scholar] [CrossRef]
- Eichler, S.A.; Meier, J.C. E-I balance and human diseases—From molecules to networking. Front. Mol. Neurosci. 2008, 1, 2. [Google Scholar] [CrossRef]
- Xue, M.; Atallah, B.V.; Scanziani, M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature 2014, 511, 596–600. [Google Scholar] [CrossRef]
- Taub, A.H.; Katz, Y.; Lampl, I. Cortical balance of excitation and inhibition is regulated by the rate of synaptic activity. J. Neurosci. 2013, 33, 14359–14368. [Google Scholar] [CrossRef]
- Mapelli, J.; Gandolfi, D.; Giuliani, E.; Casali, S.; Congi, L.; Barbieri, A.; D’Angelo, E.; Bigiani, A. The effects of the general anesthetic sevoflurane on neurotransmission: An experimental and computational study. Sci. Rep. 2021, 11, 4335. [Google Scholar] [CrossRef]
- Ghatak, S.; Talantova, M.; McKercher, S.R.; Lipton, S.A. Novel Therapeutic Approach for Excitatory/Inhibitory Imbalance in Neurodevelopmental and Neurodegenerative Diseases. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 701–721. [Google Scholar] [CrossRef]
- Gao, R.; Penzes, P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr. Mol. Med. 2015, 15, 146–167. [Google Scholar] [CrossRef]
- Canter, R.G.; Penney, J.; Tsai, L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef]
- Calvin, O.L.; Redish, A.D. Global disruption in excitation-inhibition balance can cause localized network dysfunction and Schizophrenia-like context-integration deficits. PLoS Comput. Biol. 2021, 17, e1008985. [Google Scholar] [CrossRef]
- Berchtold, N.C.; Coleman, P.D.; Cribbs, D.H.; Rogers, J.; Gillen, D.L.; Cotman, C.W. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1653–1661. [Google Scholar] [CrossRef]
- Williams, J.B.; Cao, Q.; Yan, Z. Transcriptomic analysis of human brains with Alzheimer’s disease reveals the altered expression of synaptic genes linked to cognitive deficits. Brain Commun. 2021, 3, fcab123. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Xie, A.; Bruzik, K.S.; Frolund, B.; Qian, H.; Pepperberg, D.R. Potentiating action of propofol at GABAA receptors of retinal bipolar cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2497–2509. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Irifune, M.; Takarada, T.; Shimizu, Y.; Endo, C.; Katayama, S.; Dohi, T.; Kawahara, M. Propofol-induced anesthesia in mice is mediated by gamma-aminobutyric acid-A and excitatory amino acid receptors. Anesth. Analg. 2003, 97, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Logan, S.; Yan, Y.; Inagaki, Y.; Arzua, T.; Ma, P.; Lu, S.; Bosnjak, Z.J.; Bai, X. Signaling network between the dysregulated expression of microRNAs and mRNAs in propofol-induced developmental neurotoxicity in mice. Sci. Rep. 2018, 8, 14172. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, Y.; Inagaki, Y.; Logan, S.; Bosnjak, Z.J.; Bai, X. Insufficient Astrocyte-Derived Brain-Derived Neurotrophic Factor Contributes to Propofol-Induced Neuron Death Through Akt/Glycogen Synthase Kinase 3beta/Mitochondrial Fission Pathway. Anesth. Analg. 2017, 125, 241–254. [Google Scholar] [CrossRef]
- Zuo, Y.; Chang, Y.; Thirupathi, A.; Zhou, C.; Shi, Z. Prenatal sevoflurane exposure: Effects of iron metabolic dysfunction on offspring cognition and potential mechanism. Int. J. Dev. Neurosci. 2021, 81, 1–9. [Google Scholar] [CrossRef]
- Yan, Y.; Qiao, S.; Kikuchi, C.; Zaja, I.; Logan, S.; Jiang, C.; Arzua, T.; Bai, X. Propofol Induces Apoptosis of Neurons but Not Astrocytes, Oligodendrocytes, or Neural Stem Cells in the Neonatal Mouse Hippocampus. Brain Sci. 2017, 7, 130. [Google Scholar] [CrossRef]
- Cattano, D.; Young, C.; Straiko, M.M.; Olney, J.W. Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth. Analg. 2008, 106, 1712–1714. [Google Scholar] [CrossRef]
- Logan, S.; Jiang, C.; Yan, Y.; Inagaki, Y.; Arzua, T.; Bai, X. Propofol Alters Long Non-Coding RNA Profiles in the Neonatal Mouse Hippocampus: Implication of Novel Mechanisms in Anesthetic-Induced Developmental Neurotoxicity. Cell Physiol. Biochem. 2018, 49, 2496–2510. [Google Scholar] [CrossRef]
- Arzua, T.; Yan, Y.; Jiang, C.; Logan, S.; Allison, R.L.; Wells, C.; Kumar, S.N.; Schafer, R.; Bai, X. Modeling alcohol-induced neurotoxicity using human induced pluripotent stem cell-derived three-dimensional cerebral organoids. Transl. Psychiatry 2020, 10, 347. [Google Scholar] [CrossRef]
- Twaroski, D.M.; Yan, Y.; Olson, J.M.; Bosnjak, Z.J.; Bai, X. Down-regulation of microRNA-21 is involved in the propofol-induced neurotoxicity observed in human stem cell-derived neurons. Anesthesiology 2014, 121, 786–800. [Google Scholar] [CrossRef]
- Jiang, C.; Arzua, T.; Yan, Y.; Bai, X. Expression signature of lncRNAs and mRNAs in sevoflurane-induced mouse brain injury: Implication of involvement of wide molecular networks and pathways. Int. J. Mol. Sci. 2021, 22, 1389. [Google Scholar] [CrossRef]
- Anand, K.S.; Dhikav, V. Hippocampus in health and disease: An overview. Ann. Indian Acad. Neurol. 2012, 15, 239–246. [Google Scholar] [CrossRef]
- Rubin, R.D.; Watson, P.D.; Duff, M.C.; Cohen, N.J. The role of the hippocampus in flexible cognition and social behavior. Front. Hum. Neurosci. 2014, 8, 742. [Google Scholar] [CrossRef]
- Vickstrom, C.R.; Liu, X.; Zhang, Y.; Mu, L.; Kelly, T.J.; Yan, X.; Hu, M.M.; Snarrenberg, S.T.; Liu, Q.S. T-Type Calcium Channels Contribute to Burst Firing in a Subpopulation of Medial Habenula Neurons. eNeuro 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Pan, B.; Wang, W.; Zhong, P.; Blankman, J.L.; Cravatt, B.F.; Liu, Q.S. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J. Neurosci. 2011, 31, 13420–13430. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Zhong, P.; Liu, S.J.; Long, J.Z.; Zhao, L.; Gao, H.Q.; Cravatt, B.F.; Liu, Q.S. Blockade of 2-arachidonoylglycerol hydrolysis produces antidepressant-like effects and enhances adult hippocampal neurogenesis and synaptic plasticity. Hippocampus 2015, 25, 16–26. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Zhong, P.; Wilkinson, B.; Qi, J.; Olsen, C.M.; Bayer, K.U.; Liu, Q.S. CaMKII activity in the ventral tegmental area gates cocaine-induced synaptic plasticity in the nucleus accumbens. Neuropsychopharmacology 2014, 39, 989–999. [Google Scholar] [CrossRef][Green Version]
- Tong, J.; Liu, X.; Vickstrom, C.; Li, Y.; Yu, L.; Lu, Y.; Smrcka, A.V.; Liu, Q.S. The Epac-Phospholipase Cepsilon Pathway Regulates Endocannabinoid Signaling and Cocaine-Induced Disinhibition of Ventral Tegmental Area Dopamine Neurons. J. Neurosci. 2017, 37, 3030–3044. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Tong, J.; Reynolds, A.M.; Proudfoot, S.C.; Qi, J.; Penzes, P.; Lu, Y.; Liu, Q.S. Epac Signaling Is Required for Cocaine-Induced Change in AMPA Receptor Subunit Composition in the Ventral Tegmental Area. J. Neurosci. 2016, 36, 4802–4815. [Google Scholar] [CrossRef]
- Koopmans, F.; van Nierop, P.; Andres-Alonso, M.; Byrnes, A.; Cijsouw, T.; Coba, M.P.; Cornelisse, L.N.; Farrell, R.J.; Goldschmidt, H.L.; Howrigan, D.P.; et al. SynGO: An Evidence-Based, Expert-Curated Knowledge Base for the Synapse. Neuron 2019, 103, 217–234.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Pearn, M.L.; Hu, Y.; Niesman, I.R.; Patel, H.H.; Drummond, J.C.; Roth, D.M.; Akassoglou, K.; Patel, P.M.; Head, B.P. Propofol Neurotoxicity Is Mediated by p75 Neurotrophin Receptor Activation. Anesthesiology 2012, 116, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Dholakia, U.; Clark-Price, S.C.; Keating, S.C.J.; Stern, A.W. Anesthetic effects and body weight changes associated with ketamine-xylazine-lidocaine administered to CD-1 mice. PLoS ONE 2017, 12, e0184911. [Google Scholar] [CrossRef]
- Lange, J.; Haslett, L.J.; Lloyd-Evans, E.; Pocock, J.M.; Sands, M.S.; Williams, B.P.; Cooper, J.D. Compromised astrocyte function and survival negatively impact neurons in infantile neuronal ceroid lipofuscinosis. Acta Neuropathol. Commun. 2018, 6, 74. [Google Scholar] [CrossRef]
- Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The Role of Microglia and Astrocytes in Huntington’s Disease. Front. Mol. Neurosci. 2019, 12, 258. [Google Scholar] [CrossRef]
- Zucker, R.S.; Regehr, W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef]
- Hruska, M.; Dalva, M.B. Ephrin regulation of synapse formation, function and plasticity. Mol. Cell Neurosci. 2012, 50, 35–44. [Google Scholar] [CrossRef]
- Jevtovic-Todorovic, V. Exposure of Developing Brain to General Anesthesia: What Is the Animal Evidence? Anesthesiology 2018, 128, 832–839. [Google Scholar] [CrossRef]
- Bai, X.; Yan, Y.; Canfield, S.; Muravyeva, M.Y.; Kikuchi, C.; Zaja, I.; Corbett, J.A.; Bosnjak, Z.J. Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth. Analg. 2013, 116, 869–880. [Google Scholar] [CrossRef]
- Twaroski, D.M.; Yan, Y.; Zaja, I.; Clark, E.; Bosnjak, Z.J.; Bai, X. Altered Mitochondrial Dynamics Contributes to Propofol-induced Cell Death in Human Stem Cell-derived Neurons. Anesthesiology 2015, 123, 1067–1083. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Useinovic, N.; Maksimovic, S.; Near, M.; Quillinan, N.; Jevtovic-Todorovic, V. Do We Have Viable Protective Strategies against Anesthesia-Induced Developmental Neurotoxicity? Int. J. Mol. Sci. 2022, 23, 1128. [Google Scholar] [CrossRef]
- Sun, W.; Wang, J.; Cai, D.; Pei, L. Neuroprotection of the Developing Brain by Dexmedetomidine Is Mediated by Attenuating Single Propofol-induced Hippocampal Apoptosis and Synaptic Plasticity Deficits. Exp. Neurobiol. 2020, 29, 356–375. [Google Scholar] [CrossRef]
- Sanchez, V.; Feinstein, S.D.; Lunardi, N.; Joksovic, P.M.; Boscolo, A.; Todorovic, S.M.; Jevtovic-Todorovic, V. General Anesthesia Causes Long-term Impairment of Mitochondrial Morphogenesis and Synaptic Transmission in Developing Rat Brain. Anesthesiology 2011, 115, 992–1002. [Google Scholar] [CrossRef]
- Wan, J.; Shen, C.M.; Wang, Y.; Wu, Q.Z.; Wang, Y.L.; Liu, Q.; Sun, Y.M.; Cao, J.P.; Wu, Y.Q. Repeated exposure to propofol in the neonatal period impairs hippocampal synaptic plasticity and the recognition function of rats in adulthood. Brain Res. Bull. 2021, 169, 63–72. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, B.; Chen, Y.; Zhang, J. Learning, memory and synaptic plasticity in hippocampus in rats exposed to sevoflurane. Int. J. Dev. Neurosci. 2016, 48, 38–49. [Google Scholar] [CrossRef]
- Vico Varela, E.; Etter, G.; Williams, S. Excitatory-inhibitory imbalance in Alzheimer’s disease and therapeutic significance. Neurobiol. Dis. 2019, 127, 605–615. [Google Scholar] [CrossRef]
- Mu, L.; Liu, X.; Yu, H.; Hu, M.; Friedman, V.; Kelly, T.J.; Zhao, L.; Liu, Q.S. Ibudilast attenuates cocaine self-administration and prime- and cue-induced reinstatement of cocaine seeking in rats. Neuropharmacology 2021, 201, 108830. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, P.; Vickstrom, C.; Li, Y.; Liu, Q.S. PDE4 Inhibition Restores the Balance Between Excitation and Inhibition in VTA Dopamine Neurons Disrupted by Repeated In Vivo Cocaine Exposure. Neuropsychopharmacology 2017, 42, 1991–1999. [Google Scholar] [CrossRef]
- Gatto, C.L.; Broadie, K. Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models. Front. Synaptic Neurosci. 2010, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Rothberg, B.S.; Brenner, R. Mechanism of increased BK channel activation from a channel mutation that causes epilepsy. J. Gen. Physiol. 2009, 133, 283–294. [Google Scholar] [CrossRef]
- Hussain, N.K.; Hsin, H.; Huganir, R.L.; Sheng, M. MINK and TNIK differentially act on Rap2-mediated signal transduction to regulate neuronal structure and AMPA receptor function. J. Neurosci. 2010, 30, 14786–14794. [Google Scholar] [CrossRef]
- Coba, M.P.; Komiyama, N.H.; Nithianantharajah, J.; Kopanitsa, M.V.; Indersmitten, T.; Skene, N.G.; Tuck, E.J.; Fricker, D.G.; Elsegood, K.A.; Stanford, L.E.; et al. TNiK is required for postsynaptic and nuclear signaling pathways and cognitive function. J. Neurosci. 2012, 32, 13987–13999. [Google Scholar] [CrossRef]
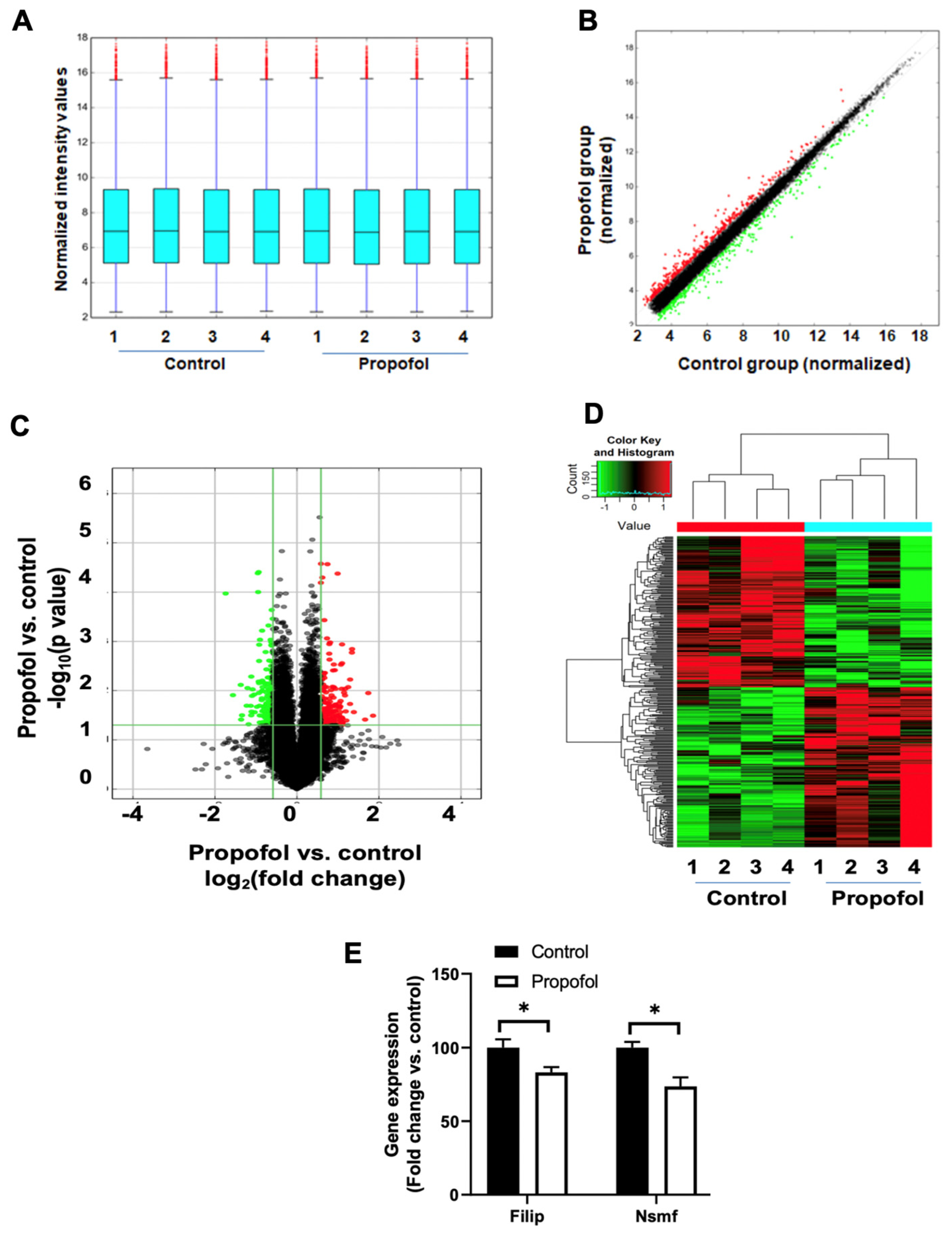
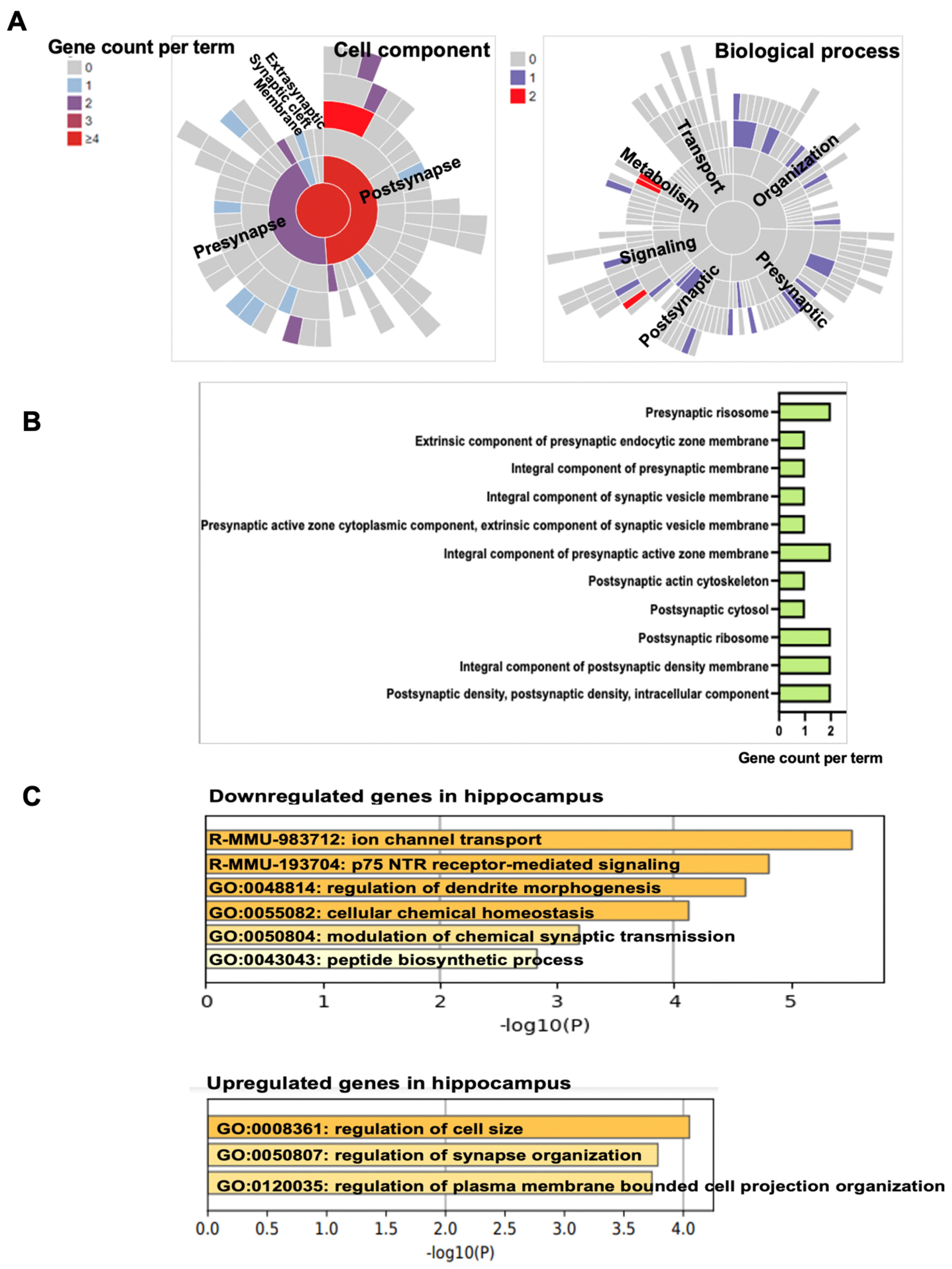
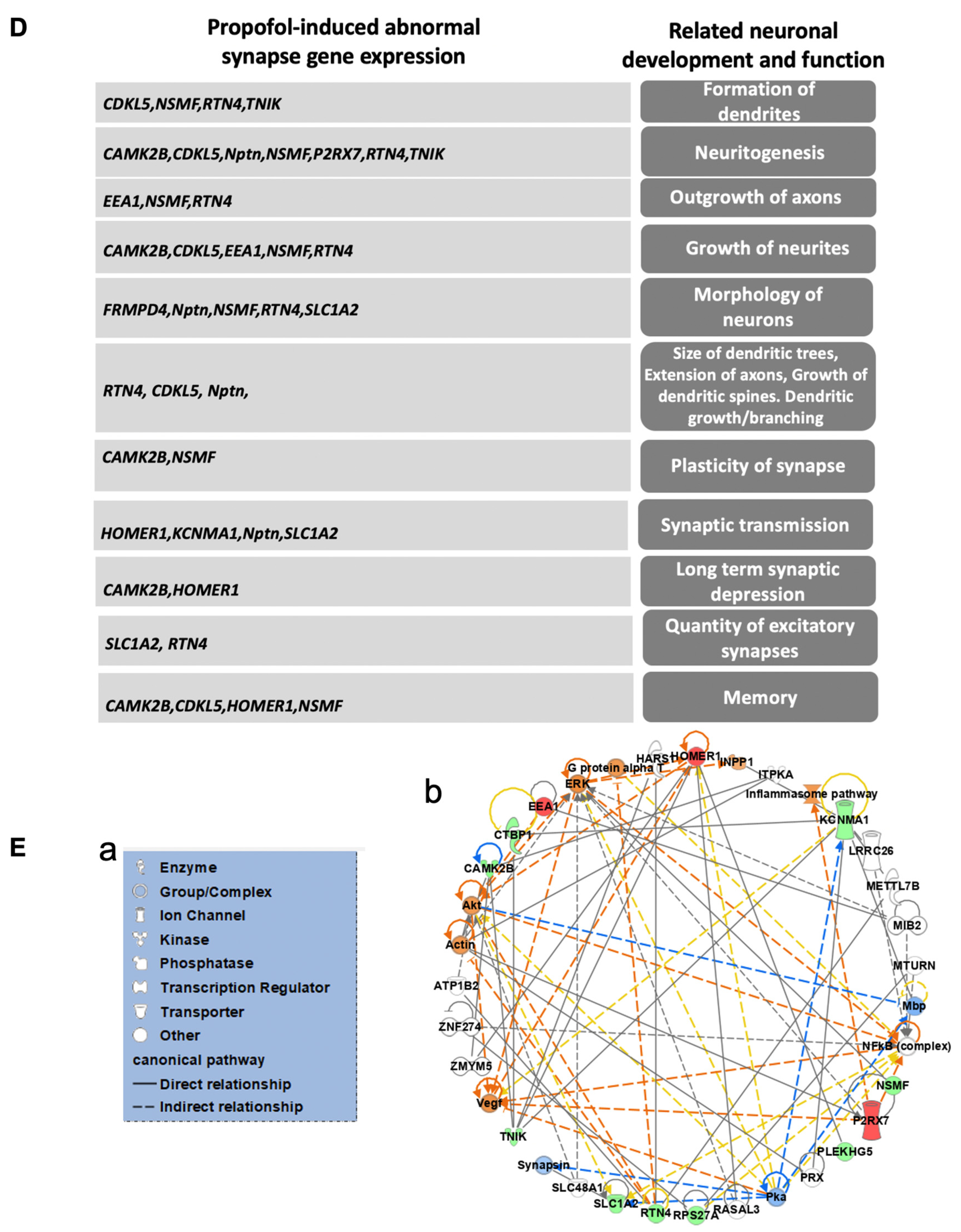
| Gene Symbol | Gene Name | Location of Cellular Component | Related Biological Process of Synapse | Expression Change (Propofol vs. Control) |
|---|---|---|---|---|
| ADRBK1 | G protein-coupled receptor kinase 2 | postsynaptic density, presynapse | down | |
| ATP6V0C | ATPase H+ transporting V0 subunit c | integral component of synaptic vesicle membrane | down | |
| ATP6V1G1 | ATPase H+ transporting V1 subunit G1 | extrinsic component of synaptic vesicle membrane | synaptic vesicle proton loading | down |
| CAMK2B | calcium/calmodulin dependent protein kinase II beta | postsynaptic density | regulation of synapse maturation, structural constituent of postsynaptic actin cytoskeleton | down |
| CTBP1 | C-terminal binding protein 1 | presynaptic active zone cytoplasmic component, extrinsic component of presynaptic endocytic zone membrane | synaptic vesicle endocytosis, synaptic vesicle clustering, synaptic vesicle clustering, presynapse to nucleus signaling pathway | down |
| FILIP1 | filamin A interacting protein 1 | postsynapse, postsynaptic actin cytoskeleton | modification of postsynaptic structure | down |
| KCNMA1 | potassium calcium-activated channel subfamily M alpha 1 | integral component of presynaptic active zone membrane | ligand-gated ion channel activity involved in regulation of presynaptic membrane potential | down |
| NSMF | NMDA receptor synaptonuclear signaling and neuronal migration factor | postsynapse | postsynapse to nucleus signaling pathway | down |
| Plekhg5 | pleckstrin homology domain containing, family G (with RhoGef domain) member 5 | pleckstrin homology and RhoGEF domain containing G5 | down | |
| RPL38 | ribosomal protein L38 | postsynaptic density, synapse, postsynaptic ribosome, presynaptic ribosome | translation at presynapse | down |
| RPS27A | ribosomal protein S27a | synapse, postsynaptic ribosome | translation at presynapse, translation at postsynapse | down |
| RTN4 | reticulon 4 | postsynapse, integral component of postsynaptic density membrane | modulation of chemical synaptic transmission | down |
| SLC1A2 | solute carrier family 1 member 2 | integral component of presynaptic membrane | neurotransmitter reuptake | down |
| TNIK | TRAF2 and NCK interacting kinase | presynapse, postsynaptic density, intracellular component | regulation of neurotransmitter receptor localization to postsynaptic specialization membrane | down |
| ANO6 | anoctamin 6 | integral component of synaptic membrane | regulation of postsynaptic membrane potential | up |
| CDKL5 | cyclin dependent kinase like 5 | postsynaptic density, intracellular component | modulation of chemical synaptic transmission, regulation of postsynapse organization | up |
| EEA1 | early endosome antigen 1 | postsynapse | postsynaptic process involved in chemical synaptic transmission | up |
| ELAVL2 | ELAV like RNA binding protein 2 | synapse | regulation of synapse assembly | up |
| FRMPD4 | FERM and PDZ domain containing 4 | postsynaptic density | postsynaptic actin cytoskeleton organization | up |
| HOMER1 | homer scaffold protein 1 | postsynaptic density, postsynaptic cytosol | regulation of postsynaptic neurotransmitter receptor activity, structural constituent of postsynapse | up |
| NPTN | neuroplastin | synaptic membrane, integral component of presynaptic active zone membrane, integral component of postsynaptic density membrane | trans-synaptic signaling by trans-synaptic complex, modulating synaptic transmission, trans-synaptic signaling by trans-synaptic complex, modulating synaptic transmission, postsynapse | up |
| P2rx7 | purinergic receptor P2X, ligand-gated ion channel, 7 | purinergic receptor P2X 7 | up | |
| TDRD6 | tudor domain containing 6 | synapse | up | |
| Molecules | Diseases or Functions Annotation |
|---|---|
| CAMK2B,CDKL5,HOMER1,KCNMA1,NSMF | Conditioning |
| CAMK2B,CDKL5,NSMF | Contextual conditioning |
| CDKL5,RTN4 | Social withdrawal |
| CDKL5,HOMER1 | Working memory |
| CAMK2B,CDKL5 | Nest building behavior |
| CAMK2B,CDKL5,HOMER1,NSMF,RTN4 | Learning |
| CAMK2B,CDKL5,HOMER1,NSMF | Memory |
| CAMK2B,NSMF | Object recognition memory |
| P2RX7 | Locomotion of vesicles |
| HOMER1 | Chaining behavior |
| HOMER1,KCNMA1,P2RX7,RTN4 | Locomotion |
| HOMER1 | Lever press response |
| HOMER1 | Navigation |
| RTN4 | Perseverance behavior |
| CAMK2B,CDKL5,HOMER1 | Anxiety |
| HOMER1 | Tone fear conditioning |
| HOMER1 | Cocaine seeking behavior |
| KCNMA1 | Blinking |
| CAMK2B | Hippocampal learning |
| KCNMA1 | Eyeblink conditioning |
| P2RX7 | Coping response |
| HOMER1 | Habituation |
| HOMER1 | Despair behavior |
| KCNMA1 | Swimming behavior |
| KCNMA1 | Circling behavior |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Logan, S.; Liu, X.; Chen, B.; Jiang, C.; Arzua, T.; Ramchandran, R.; Liu, Q.-s.; Bai, X. Integrated Excitatory/Inhibitory Imbalance and Transcriptomic Analysis Reveals the Association between Dysregulated Synaptic Genes and Anesthetic-Induced Cognitive Dysfunction. Cells 2022, 11, 2497. https://doi.org/10.3390/cells11162497
Yan Y, Logan S, Liu X, Chen B, Jiang C, Arzua T, Ramchandran R, Liu Q-s, Bai X. Integrated Excitatory/Inhibitory Imbalance and Transcriptomic Analysis Reveals the Association between Dysregulated Synaptic Genes and Anesthetic-Induced Cognitive Dysfunction. Cells. 2022; 11(16):2497. https://doi.org/10.3390/cells11162497
Chicago/Turabian StyleYan, Yasheng, Sarah Logan, Xiaojie Liu, Bixuan Chen, Congshan Jiang, Thiago Arzua, Ramani Ramchandran, Qing-song Liu, and Xiaowen Bai. 2022. "Integrated Excitatory/Inhibitory Imbalance and Transcriptomic Analysis Reveals the Association between Dysregulated Synaptic Genes and Anesthetic-Induced Cognitive Dysfunction" Cells 11, no. 16: 2497. https://doi.org/10.3390/cells11162497
APA StyleYan, Y., Logan, S., Liu, X., Chen, B., Jiang, C., Arzua, T., Ramchandran, R., Liu, Q.-s., & Bai, X. (2022). Integrated Excitatory/Inhibitory Imbalance and Transcriptomic Analysis Reveals the Association between Dysregulated Synaptic Genes and Anesthetic-Induced Cognitive Dysfunction. Cells, 11(16), 2497. https://doi.org/10.3390/cells11162497








