Restoration of BDNF, DARPP32, and D2R Expression Following Intravenous Infusion of Human Immature Dental Pulp Stem Cells in Huntington’s Disease 3-NP Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Cell Culture
2.3. Cell Characterization
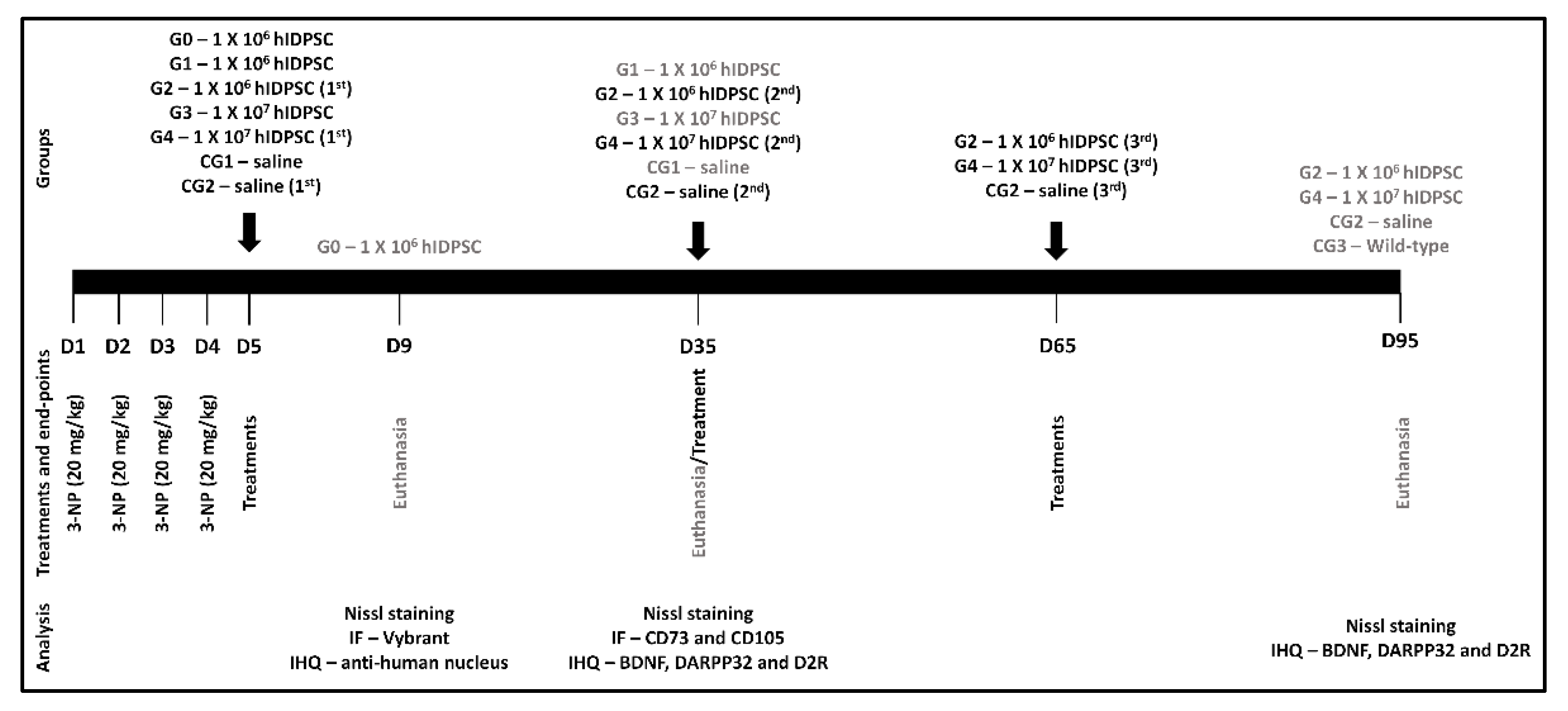
2.4. 3-NP Rat Model Obtainig and Experimental Design
2.5. Nissl Staining
2.6. Vybrant-Labeling
2.7. Immunoistochemistry (IHC)
2.8. Indirect Immunofluorescence
2.9. Quantitive Analysis Using Image J Software
2.10. Statistical Analysis
3. Results
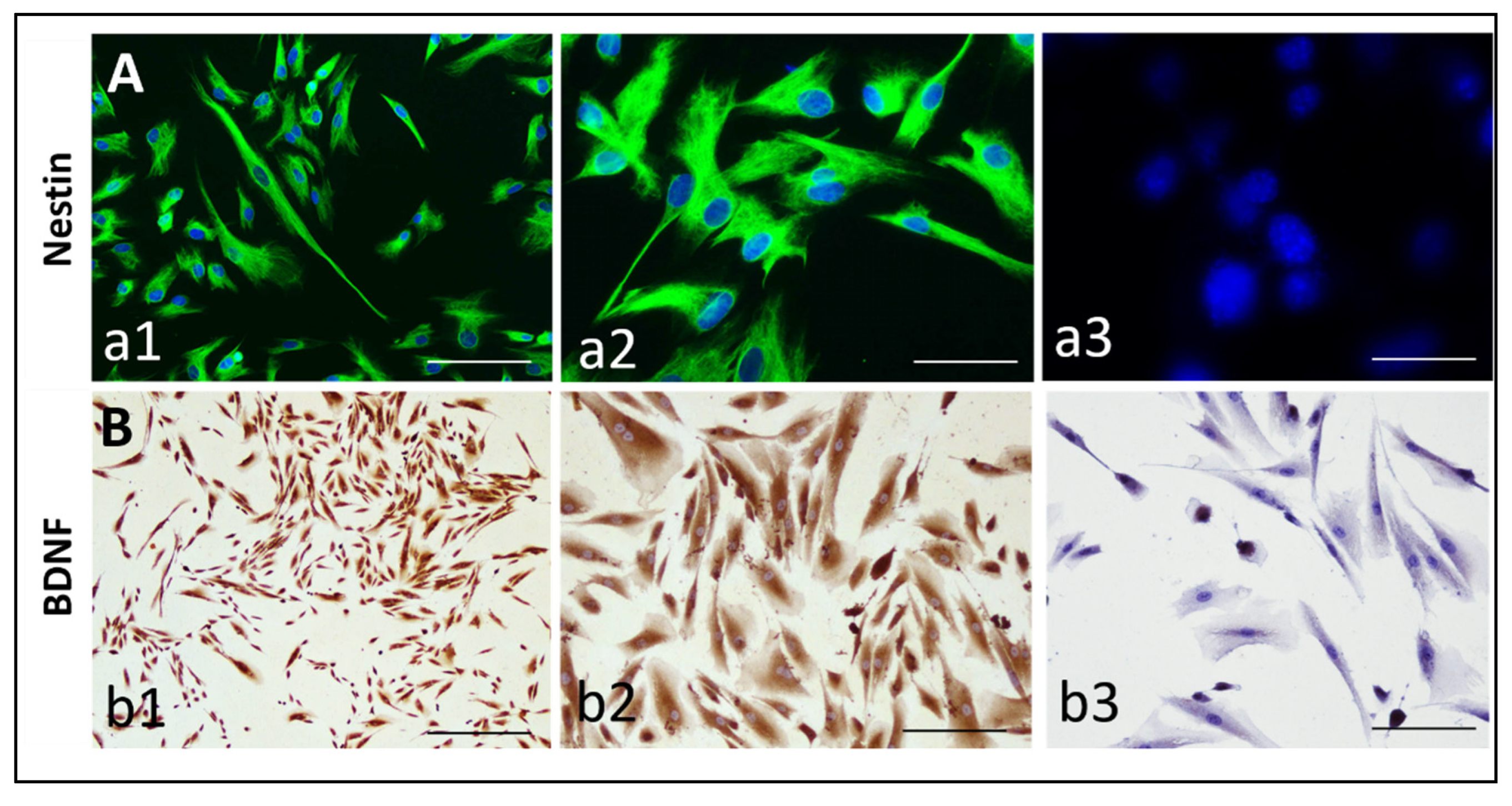
3.1. Expression of Nestin and BDNF by hIDPSCs
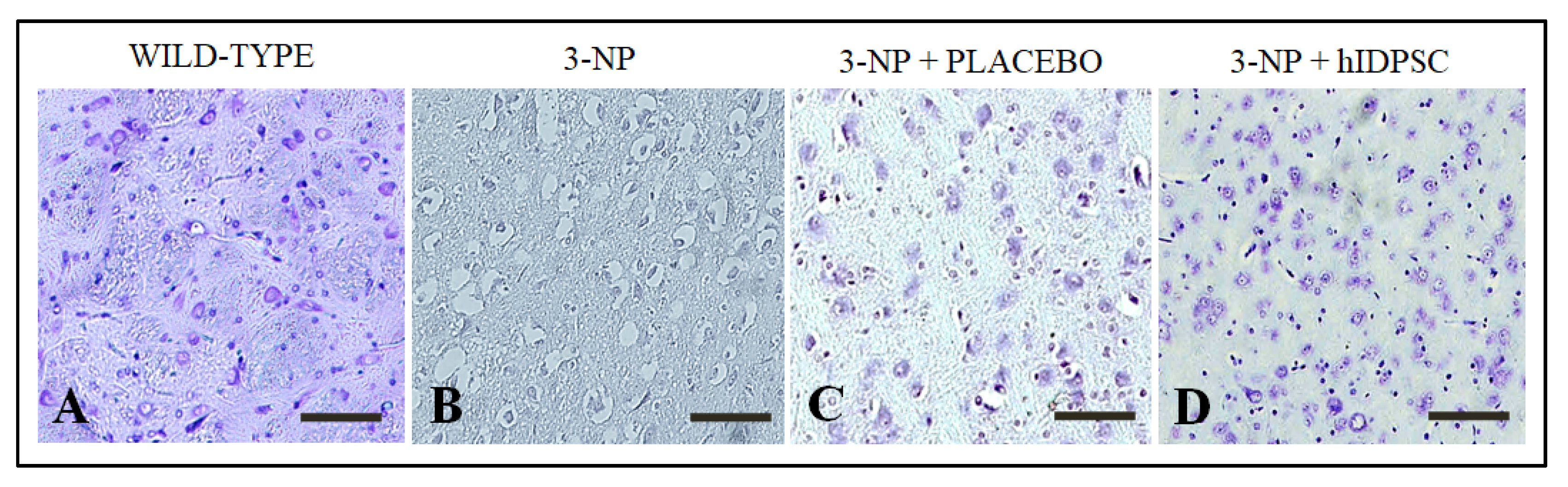
3.2. 3-NP Promoted Striatal Damages
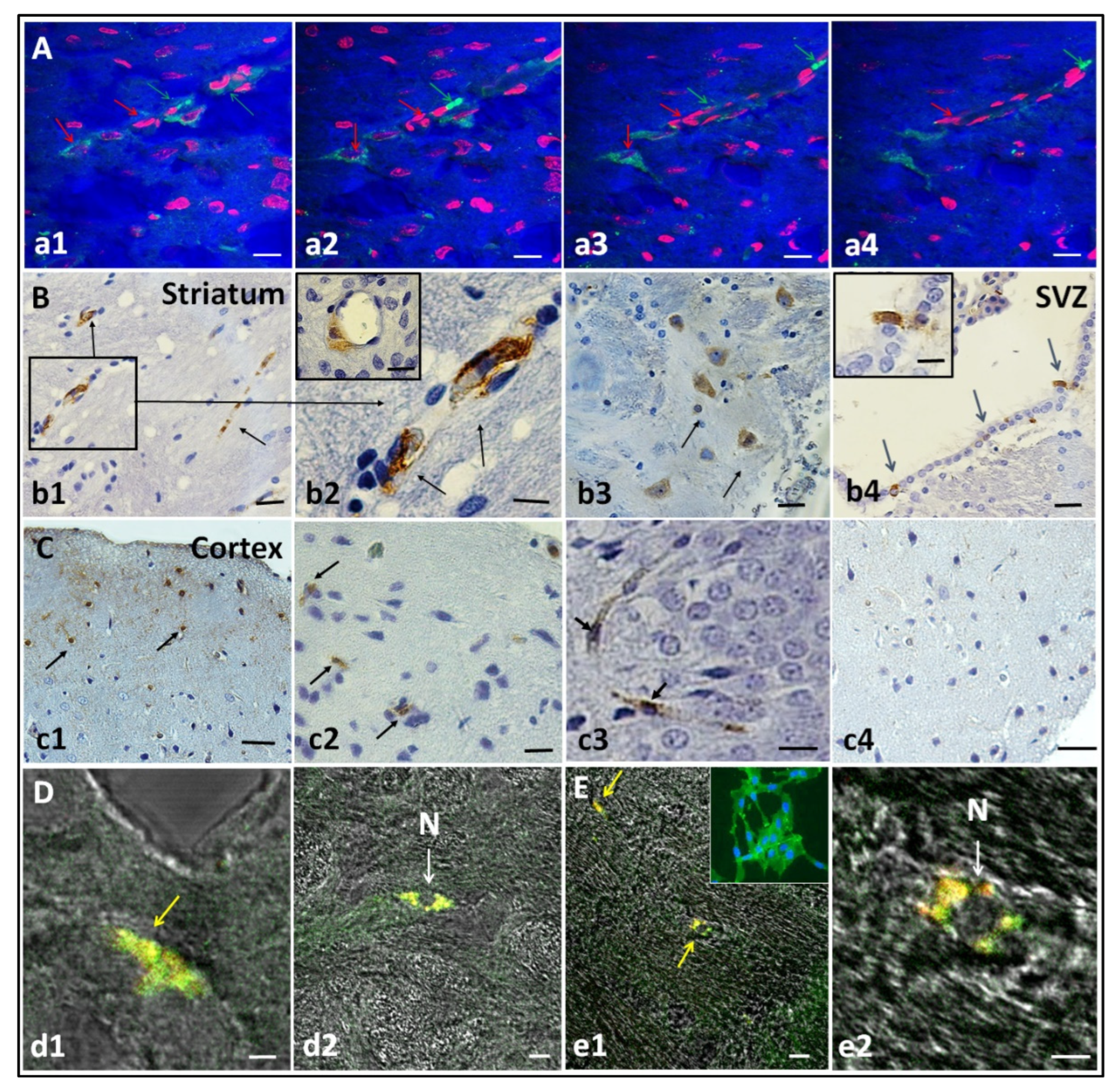
3.3. hIDPSC Homing in the HD Striatum, Cortex, and Subventricular Zone
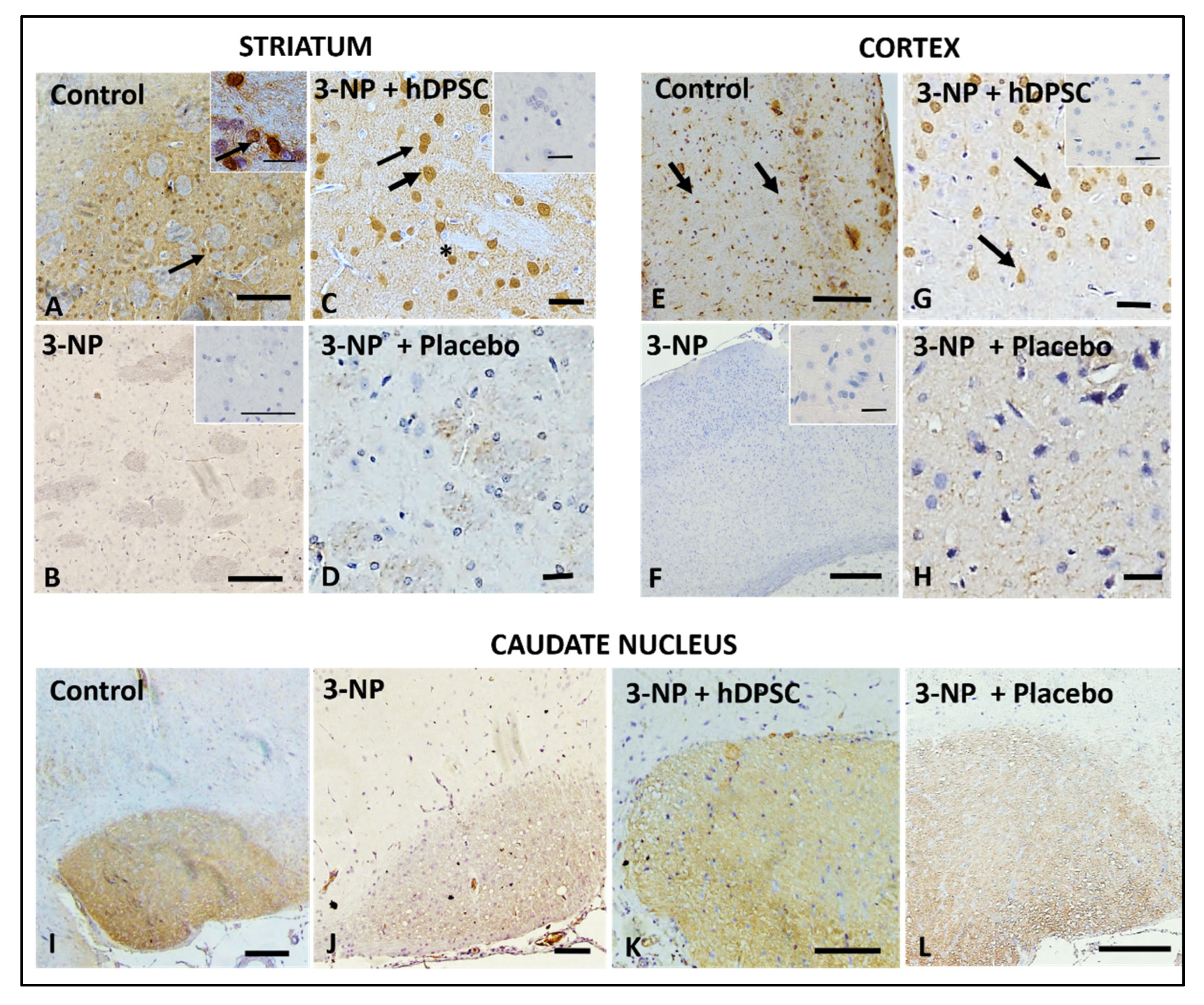
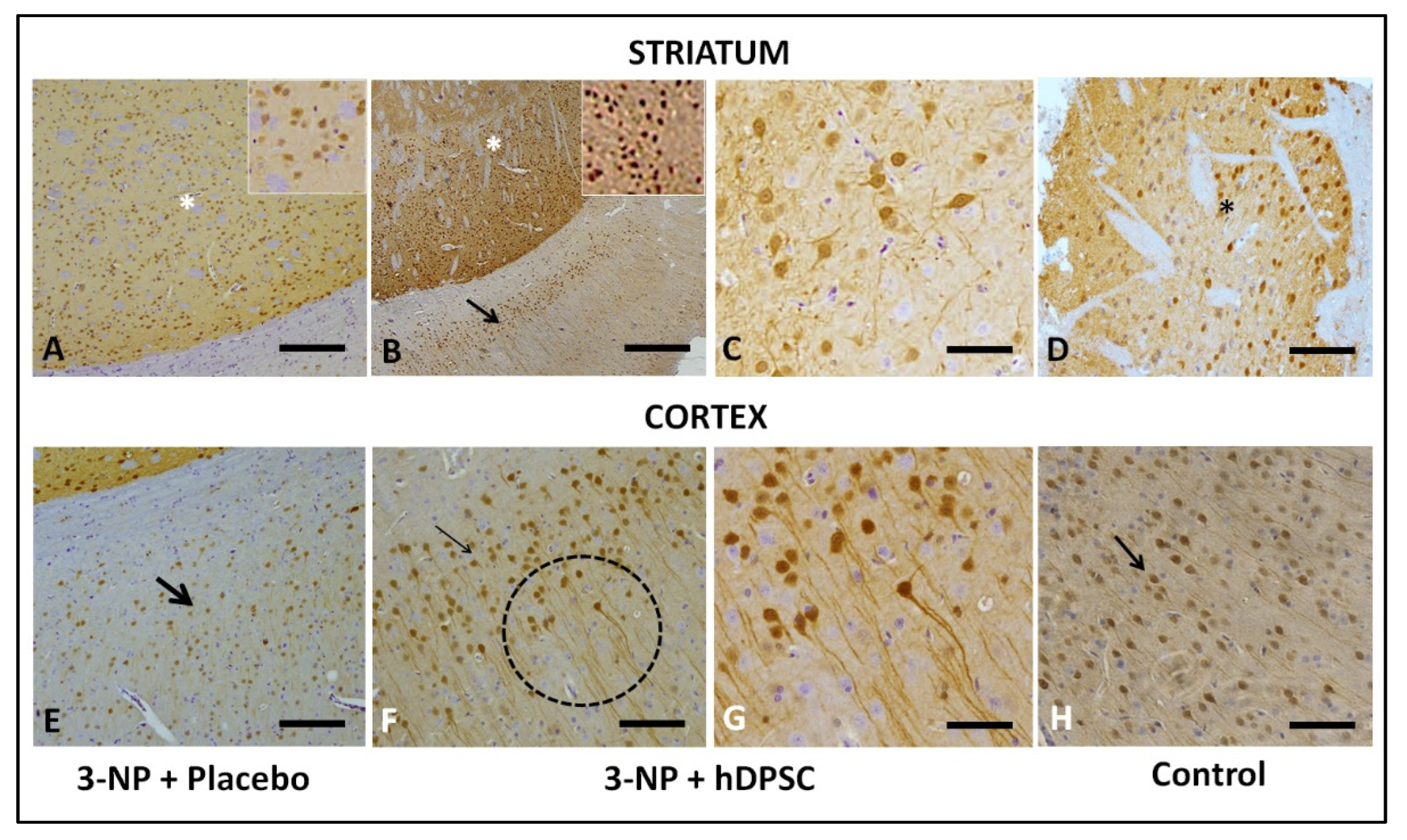
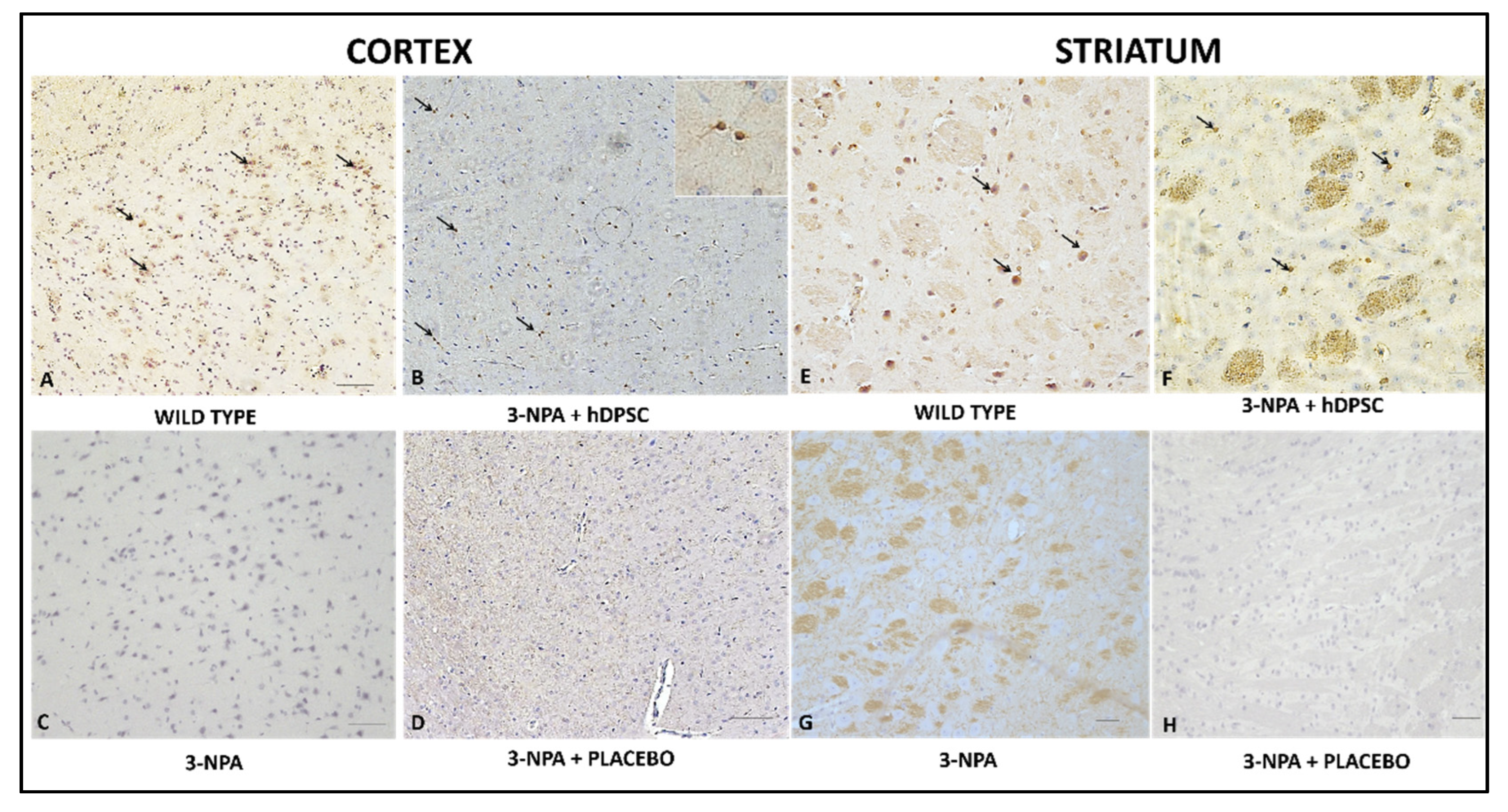
3.4. Qualitative Immunohistochemical Staining Assessment for BDNF, DARPP32 and D2R
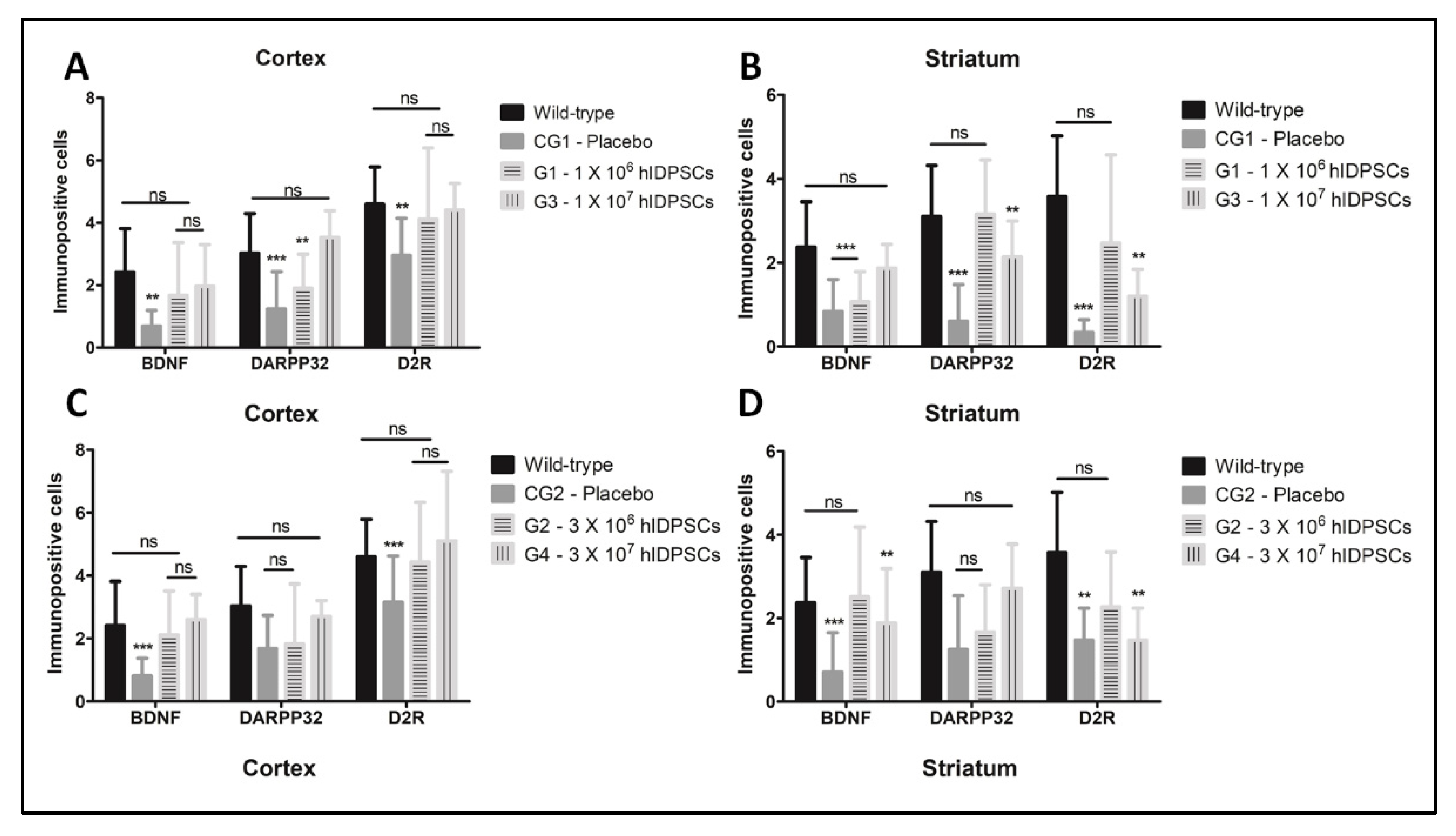
3.5. Quantitative Immunohistochemical Staining Assessment for BDNF, DARPP32 and D2R
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerkis, I.; Araldi, R.; Wenceslau, C.; Mendes, T. Advances in cellular and cell-free therapy medicinal products for Huntigton’s disease treatment. In From Physiopatology to Treatment of Huntigton’s Disease; InTech Open: London, UK, 2022; pp. 1–27. [Google Scholar]
- Lee, J.-M.; Correia, K.; Loupe, J.; Kim, K.-H.; Barker, D.; Hong, E.P.; Chao, M.J.; Long, J.D.; Lucente, D.; Vonsattel, J.P.G.; et al. CAG repeat not polyglutamine length determines timing of Huntington’s disease onset. Cell 2019, 178, 887–900.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerkis, I.; Macedo da Silva, J.; Valverde Wenceslau, C.; Caroline Mambelli-Lisboa, N.; Osorio Frare, E. Brain-derived neurotrophic factor and stem cell-based technologies in Huntington’s disease therapy. In Neurodegenerative Diseases-Molecular Mechanisms and Current Therapeutic Approaches [Working Title]; IntechOpen: London, UK, 2020. [Google Scholar]
- Coppen, E.M.; Jacobs, M.; van den Berg-Huysmans, A.A.; van der Grond, J.; Roos, R.A.C. Grey matter volume loss is associated with specific clinical motor signs in Huntington’s disease. Parkinsonism Relat. Disord. 2018, 46, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.J.; Shaler, T.A.; Woodman, B.; Ryu, K.-Y.; Zaitseva, T.S.; Becker, C.H.; Bates, G.P.; Schulman, H.; Kopito, R.R. Global changes to the ubiquitin system in Huntington’s disease. Nature 2007, 448, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Ha, A.D.; Fung, V.S.C. Huntington’s disease. Curr. Opin. Neurol. 2012, 25, 491–498. [Google Scholar] [CrossRef]
- Harper, P. The epidemiology of Huntington’s disease. Hum. Genet. 1992, 89. [Google Scholar] [CrossRef]
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- Soloveva, M.V.; Jamadar, S.D.; Poudel, G.; Georgiou-Karistianis, N. A critical review of brain and cognitive reserve in Huntington’s disease. Neurosci. Biobehav. Rev. 2018, 88, 155–169. [Google Scholar] [CrossRef]
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Landles, C.; Bates, G.P. Huntingtin and the molecular pathogenesis of Huntington’s disease. EMBO Rep. 2004, 5, 958–963. [Google Scholar] [CrossRef]
- Xie, Y.; Hayden, M.R.; Xu, B. BDNF overexpression in the forebrain rescues Huntington’s disease phenotypes in YAC128 mice. J. Neurosci. 2010, 30, 14708–14718. [Google Scholar] [CrossRef]
- Giampà, C.; Montagna, E.; Dato, C.; Melone, M.A.B.; Bernardi, G.; Fusco, F.R. Systemic delivery of recombinant brain derived neurotrophic factor (BDNF) in the R6/2 mouse model of Huntington’s disease. PLoS ONE 2013, 8, e64037. [Google Scholar] [CrossRef] [PubMed]
- Pilakka-Kanthikeel, S.; Atluri, V.S.R.; Sagar, V.; Saxena, S.K.; Nair, M. Targeted brain derived neurotropic factors (BDNF) delivery across the blood-brain barrier for neuro-protection using magnetic nano carriers: An in-vitro study. PLoS ONE 2013, 8, e62241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Eckert, M.A.; Riazifar, H.; Kang, D.-K.; Agalliu, D.; Zhao, W. From blood to the brain: Can S systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cells Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Do, P.T.; Wu, C.-C.; Chiang, Y.-H.; Hu, C.-J.; Chen, K.-Y. Mesenchymal stem/stromal cell therapy in blood–brain barrier preservation following ischemia: Molecular mechanisms and prospects. Int. J. Mol. Sci. 2021, 22, 10045. [Google Scholar] [CrossRef] [PubMed]
- Pollock, K.; Dahlenburg, H.; Nelson, H.; Fink, K.D.; Cary, W.; Hendrix, K.; Annett, G.; Torrest, A.; Deng, P.; Gutierrez, J.; et al. Human mesenchymal stem cells genetically Engineered to overexpress brain-derived neurotrophic factor improve outcomes in Huntington’s disease mouse models. Mol. Ther. 2016, 24, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Kerkis, A.; Dozortsev, D.; Stukart-Parsons, G.C.; Gomes Massironi, S.M.; Pereira, L.V.; Caplan, A.I.; Cerruti, H.F. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 2006, 184, 105–116. [Google Scholar] [CrossRef]
- Lizier, N.F.; Kerkis, A.; Gomes, C.M.; Hebling, J.; Oliveira, C.F.; Caplan, A.I.; Kerkis, I. Scaling-up of dental pulp stem cells isolated from multiple niches. PLoS ONE 2012, 7, e39885. [Google Scholar] [CrossRef] [Green Version]
- Kerkis, I.; Caplan, A.I. Stem cells in dental pulp of deciduous teeth. Tissue Eng. Part B Rev. 2012, 18, 129–138. [Google Scholar] [CrossRef]
- BORLONGAN, C. 3-Nitropropionic acid animal model and Huntington’ s disease. Neurosci. Biobehav. Rev. 1997, 21, 289–293. [Google Scholar] [CrossRef]
- Gao, Y.; Chu, S.; Li, J.; Zhang, Z.; Yan, J.; Wen, Z.; Xia, C.; Mou, Z.; Wang, Z.; He, W.; et al. Protopanaxtriol protects against 3-nitropropionic acid-induced oxidative stress in a rat model of Huntington’s disease. Acta Pharmacol. Sin. 2015, 36, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Araldi, R.P.; Prezoto, B.C.; Gonzaga, V.; Policiquio, B.; Mendes, T.B.; D’Amélio, F.; Vigerelli, H.; Viana, M.; Valverde, C.W.; Pagani, E.; et al. Advanced cell therapy with low tissue factor loaded product NestaCell® does not confer thrombogenic risk for critically ill COVID-19 heparin-treated patients. Biomed. Pharmacother. 2022, 112920. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Krampera, M.; Barrett, J.; Dazzi, F.; Deans, R.J.; DeBruijn, J.; Dominici, M.; Fibbe, W.E.; Gee, A.P.; Gimble, J.M.; et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy 2016, 18, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida, F.M.; Marques, S.A.; Ramalho, B.D.S.; Rodrigues, R.F.; Cadilhe, D.V.; Furtado, D.; Kerkis, I.; Pereira, L.V.; Rehen, S.K.; Martinez, A.M.B. Human dental pulp cells: A new source of cell therapy in a mouse model of compressive spinal cord injury. J. Neurotrauma 2011, 28, 1939–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef]
- Gaspar, P.; Bloch, B.; Moine, C. D1 and D2 receptor gene expression in the rat frontal cortex: Cellular localization in different classes of efferent neurons. Eur. J. Neurosci. 1995, 7, 1050–1063. [Google Scholar] [CrossRef]
- Colle, D.; Santos, D.B.; Moreira, E.L.G.; Hartwig, J.M.; dos Santos, A.A.; Zimmermann, L.T.; Hort, M.A.; Farina, M. Probucol increases striatal glutathione peroxidase activity and protects against 3-nitropropionic acid-induced pro-oxidative damage in rats. PLoS ONE 2013, 8, e67658. [Google Scholar] [CrossRef] [Green Version]
- Achilleos, A.; Trainor, P.A. Neural crest stem cells: Discovery, properties and potential for therapy. Cell Res. 2012, 22, 288–304. [Google Scholar] [CrossRef] [Green Version]
- Sawa, A.; Wiegand, G.W.; Cooper, J.; Margolis, R.L.; Sharp, A.H.; Lawler, J.F.; Greenamyre, J.T.; Snyder, S.H.; Ross, C.A. Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nat. Med. 1999, 5, 1194–1198. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Workman, J.; Hart, P.E.; Mangiarini, L.; Mahal, A.; Bates, G.; Cooper, J.M.; Schapira, A.H.V. Mitochondrial dysfunction and free radical damage in the Huntington R6/2 transgenic mouse. Ann. Neurol. 2000, 47, 80–86. [Google Scholar] [CrossRef]
- Eylert, G.; Dolp, R.; Parousis, A.; Cheng, R.; Auger, C.; Holter, M.; Lang-Olip, I.; Reiner, V.; Kamolz, L.-P.; Jeschke, M.G. Skin regeneration is accelerated by a lower dose of multipotent mesenchymal stromal/stem cells—a paradigm change. Stem Cell Res. Ther. 2021, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, W.; Li, J.J.; Chai, S.; Xing, D.; Yu, H.; Zhang, Y.; Yan, W.; Xu, Z.; Zhao, B.; et al. A low dose cell therapy system for treating osteoarthritis: In vivo study and in vitro mechanistic investigations. Bioact. Mater. 2022, 7, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.-I.; Xu, H.; Igarashi, S.; Fujimuro, M.; Agrawal, N.; Taya, Y.; Hayward, S.D.; Moran, T.H.; Montell, C.; Ross, C.A.; et al. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease. Neuron 2005, 47, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdanov, Y.; Balaban, P.; Poteryaev, D.; Zakharov, I.; Belyavsky, A. Putative neuropeptides and an EF-hand motif region are encoded by a novel gene expressed in the four giant interneurons of the terrestrial snail. Neuroscience 1998, 85, 637–647. [Google Scholar] [CrossRef]
- Panov, A.V.; Gutekunst, C.-A.; Leavitt, B.R.; Hayden, M.R.; Burke, J.R.; Strittmatter, W.J.; Greenamyre, J.T. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat. Neurosci. 2002, 5, 731–736. [Google Scholar] [CrossRef]
- Hart, Y.; Reich-Zeliger, S.; Antebi, Y.E.; Zaretsky, I.; Mayo, A.E.; Alon, U.; Friedman, N. Paradoxical signaling by a secreted molecule leads to homeostasis of cell levels. Cell 2014, 158, 1022–1032. [Google Scholar] [CrossRef] [Green Version]
- Baydyuk, M.; Xu, B. BDNF signaling and survival of striatal neurons. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.-H.J. Transcriptional signatures in Huntington’s disease. Prog. Neurobiol. 2007, 83, 228–248. [Google Scholar] [CrossRef] [Green Version]
- Ivkovic, S.; Ehrlich, M.E. Expression of the striatal DARPP-32/ARPP-21 phenotype in GABAergic neurons requires neurotrophins in vivo and in vitro. J. Neurosci. 1999, 19, 5409–5419. [Google Scholar] [CrossRef] [Green Version]
- Runne, H.; Regulier, E.; Kuhn, A.; Zala, D.; Gokce, O.; Perrin, V.; Sick, B.; Aebischer, P.; Deglon, N.; Luthi-Carter, R. Dysregulation of gene expression in primary neuron models of Huntington’s disease shows that polyglutamine-related effects on the striatal transcriptome may not be dependent on brain circuitry. J. Neurosci. 2008, 28, 9723–9731. [Google Scholar] [CrossRef]
- Svenningsson, P.; Nishi, A.; Fisone, G.; Girault, J.-A.; Nairn, A.C.; Greengard, P. DARPP-32: An integrator of neurotransmission. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-W.; Cao, R.; Rao, Z.-R.; Chen, L.-W. Differential expression of NMDA and AMPA receptor subunits in DARPP-32-containing neurons of the cerebral cortex, hippocampus and neostriatum of rats. Brain Res. 2004, 998, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Young, D.; Mayer, F.; Vidotto, N.; Schweizer, T.; Berth, R.; Abramowski, D.; Shimshek, D.R.; van der Putten, P.H.; Schmid, P. Mutant Huntingtin gene-dose impacts on aggregate deposition, DARPP32 expression and neuroinflammation in HdhQ150 mice. PLoS ONE 2013, 8, e75108. [Google Scholar] [CrossRef]
- Ehrlich, M.E. Huntington’s disease and the striatal medium spiny neuron: Cell-autonomous and non-cell-autonomous mechanisms of disease. Neurotherapeutics 2012, 9, 270–284. [Google Scholar] [CrossRef] [Green Version]
- Metzler, M.; Gan, L.; Mazarei, G.; Graham, R.K.; Liu, L.; Bissada, N.; Lu, G.; Leavitt, B.R.; Hayden, M.R. Phosphorylation of Huntingtin at Ser421 in YAC128 neurons is associated with protection of YAC128 neurons from NMDA-mediated excitotoxicity and Is modulated by PP1 and PP2A. J. Neurosci. 2010, 30, 14318–14329. [Google Scholar] [CrossRef] [Green Version]
- Bozzi, Y.; Borrelli, E. Dopamine in neurotoxicity and neuroprotection: What do D2 receptors have to do with it? Trends Neurosci. 2006, 29, 167–174. [Google Scholar] [CrossRef]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of brain-derived neurotrophic factor and glucocorticoid stress in neurogenesis. Int. J. Mol. Sci. 2017, 18, 2312. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 2018, 361. [Google Scholar] [CrossRef] [Green Version]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C.-Y. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013, 19, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Zuccato, C.; Ciammola, A.; Rigamonti, D.; Leavitt, B.R.; Goffredo, D.; Conti, L.; MacDonald, M.E.; Friedlander, R.M.; Silani, V.; Hayden, M.R.; et al. Loss of Huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science 2001, 293, 493–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciammola, A.; Sassone, J.; Cannella, M.; Calza, S.; Poletti, B.; Frati, L.; Squitieri, F.; Silani, V. Low brain-derived neurotrophic factor (BDNF) levels in serum of Huntington’s disease patients. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2007, 144B, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Marullo, M.; Vitali, B.; Tarditi, A.; Mariotti, C.; Valenza, M.; Lahiri, N.; Wild, E.J.; Sassone, J.; Ciammola, A.; et al. Brain-derived neurotrophic factor in patients with Huntington’s disease. PLoS ONE 2011, 6, e22966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo, J.; Pagani, E.; Wenceslau, C.; Ferrara, L.; Kerkis, I. A pahse I clinical trial on intravenous administration of immature human dental pulp stem cells (NestaCell) to Huntington’s disease patients. Cytotherapy 2021, 23, 1. [Google Scholar] [CrossRef]
| Number of Rats Used in Each Assay | |||||
|---|---|---|---|---|---|
| hIDPSC Homing | Immunodetection of | ||||
| Group | Dose | Total of Cells | Vybrant | Anti-hNu | DARPP32, BDNF and D2R |
| G0 | Single | 1 × 106 | 3 | 3 | N/A |
| G1 | Single | 1 × 106 | N/A | 3 | 6 |
| G2 | Three | 3 × 106 | 3 | 3 | 6 |
| G3 | Single | 1 × 107 | N/A | 3 | 6 |
| G4 | Three | 3 × 107 | N/A | 3 | 6 |
| CG1 | N/A | N/A | N/A | N/A | 6 |
| CG2 | N/A | N/A | N/A | N/A | 6 |
| CG3 | N/A | N/A | N/A | 3 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenceslau, C.V.; de Souza, D.M.; Mambelli-Lisboa, N.C.; Ynoue, L.H.; Araldi, R.P.; da Silva, J.M.; Pagani, E.; Haddad, M.S.; Kerkis, I. Restoration of BDNF, DARPP32, and D2R Expression Following Intravenous Infusion of Human Immature Dental Pulp Stem Cells in Huntington’s Disease 3-NP Rat Model. Cells 2022, 11, 1664. https://doi.org/10.3390/cells11101664
Wenceslau CV, de Souza DM, Mambelli-Lisboa NC, Ynoue LH, Araldi RP, da Silva JM, Pagani E, Haddad MS, Kerkis I. Restoration of BDNF, DARPP32, and D2R Expression Following Intravenous Infusion of Human Immature Dental Pulp Stem Cells in Huntington’s Disease 3-NP Rat Model. Cells. 2022; 11(10):1664. https://doi.org/10.3390/cells11101664
Chicago/Turabian StyleWenceslau, Cristiane Valverde, Dener Madeiro de Souza, Nicole Caroline Mambelli-Lisboa, Leandro Hideki Ynoue, Rodrigo Pinheiro Araldi, Joyce Macedo da Silva, Eduardo Pagani, Monica Santoro Haddad, and Irina Kerkis. 2022. "Restoration of BDNF, DARPP32, and D2R Expression Following Intravenous Infusion of Human Immature Dental Pulp Stem Cells in Huntington’s Disease 3-NP Rat Model" Cells 11, no. 10: 1664. https://doi.org/10.3390/cells11101664
APA StyleWenceslau, C. V., de Souza, D. M., Mambelli-Lisboa, N. C., Ynoue, L. H., Araldi, R. P., da Silva, J. M., Pagani, E., Haddad, M. S., & Kerkis, I. (2022). Restoration of BDNF, DARPP32, and D2R Expression Following Intravenous Infusion of Human Immature Dental Pulp Stem Cells in Huntington’s Disease 3-NP Rat Model. Cells, 11(10), 1664. https://doi.org/10.3390/cells11101664






