Uterus: A Unique Stem Cell Reservoir Able to Support Cardiac Repair via Crosstalk among Uterus, Heart, and Bone Marrow
Abstract
1. Introduction
2. The Female Advantage in Myocardial Infarction
3. Stem Cell Crosstalk between Organs
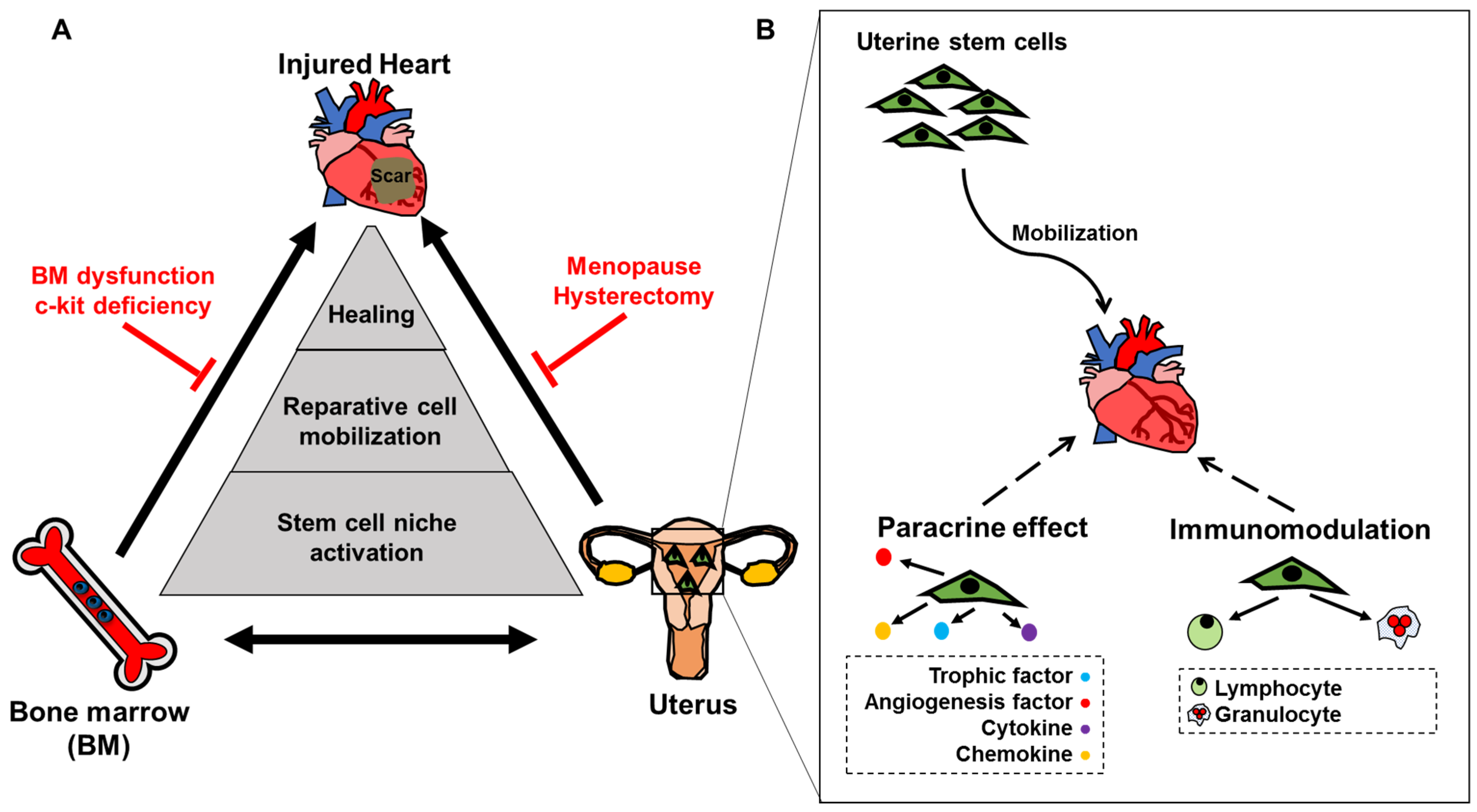
4. Utero-Cardiac Axis Involves a 3-Way Relationship between Heart, Uterus and Bone Marrow
4.1. Uterine Cells Traffic to the Injured Heart to Promote Healing: The Utero-Cardiac Axis
4.2. The BM-Cardiac Axis
4.3. Utero-BM Axis
5. The Implications of the Utero-Cardiac Axis with Respect to Preventive Hysterectomy
6. The Effects of the Utero-Cardiac Axis for Developing Uterine Cell Therapies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shlipak, M.G.; Simon, J.A.; Vittinghoff, E.; Lin, F.; Barrett-Connor, E.; Knopp, R.H.; Levy, R.I.; Hulley, S.B. Estrogen and progestin, lipoprotein(a), and the risk of recurrent coronary heart disease events after menopause. JAMA 2000, 283, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Honigberg, M.C.; Zekavat, S.M.; Aragam, K.; Finneran, P.; Klarin, D.; Bhatt, D.L.; Januzzi, J.L., Jr.; Scott, N.S.; Natarajan, P. Association of Premature Natural and Surgical Menopause with Incident Cardiovascular Disease. JAMA 2019, 322, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Hsia, J.; Johnson, K.C.; Rossouw, J.E.; Assaf, A.R.; Lasser, N.L.; Trevisan, M.; Black, H.R.; Heckbert, S.R.; Detrano, R.; et al. Estrogen plus progestin and the risk of coronary heart disease. N. Engl. J. Med. 2003, 349, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.L.; Limacher, M.; Assaf, A.R.; Bassford, T.; Beresford, S.A.; Black, H.; Bonds, D.; Brunner, R.; Brzyski, R.; Caan, B.; et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004, 291, 1701–1712. [Google Scholar] [CrossRef]
- Xaymardan, M.; Sun, Z.; Hatta, K.; Tsukashita, M.; Konecny, F.; Weisel, R.D.; Li, R.K. Uterine cells are recruited to the infarcted heart and improve cardiac outcomes in female rats. J. Mol. Cell Cardiol. 2012, 52, 1265–1273. [Google Scholar] [CrossRef]
- Prianishnikov, V.A. A functional model of the structure of the epithelium of normal, hyperplastic and malignant human endometrium: A review. Gynecol. Oncol. 1978, 6, 420–428. [Google Scholar] [CrossRef]
- De Miguel-Gomez, L.; Lopez-Martinez, S.; Frances-Herrero, E.; Rodriguez-Eguren, A.; Pellicer, A.; Cervello, I. Stem Cells and the Endometrium: From the Discovery of Adult Stem Cells to Pre-Clinical Models. Cells 2021, 10, 595. [Google Scholar] [CrossRef]
- Padykula, H.A. Regeneration in the primate uterus: The role of stem cells. Ann. N. Y. Acad. Sci. 1991, 622, 47–56. [Google Scholar] [CrossRef]
- Santamaria, X.; Mas, A.; Cervello, I.; Taylor, H.; Simon, C. Uterine stem cells: From basic research to advanced cell therapies. Hum. Reprod. Update 2018, 24, 673–693. [Google Scholar] [CrossRef]
- Cervello, I.; Martinez-Conejero, J.A.; Horcajadas, J.A.; Pellicer, A.; Simon, C. Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum. Reprod. 2007, 22, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.W.; Gargett, C.E. Identification of label-retaining cells in mouse endometrium. Stem Cells 2006, 24, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Chan, R.W.; Yeung, W.S. Label-retaining stromal cells in mouse endometrium awaken for expansion and repair after parturition. Stem Cells Dev. 2015, 24, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.W.; Kaitu’u-Lino, T.; Gargett, C.E. Role of label-retaining cells in estrogen-induced endometrial regeneration. Reprod. Sci. 2012, 19, 102–114. [Google Scholar] [CrossRef]
- Kaitu’u-Lino, T.J.; Ye, L.; Salamonsen, L.A.; Girling, J.E.; Gargett, C.E. Identification of label-retaining perivascular cells in a mouse model of endometrial decidualization, breakdown, and repair. Biol. Reprod. 2012, 86, 184. [Google Scholar] [CrossRef]
- Deane, J.A.; Ong, Y.R.; Cain, J.E.; Jayasekara, W.S.; Tiwari, A.; Carlone, D.L.; Watkins, D.N.; Breault, D.T.; Gargett, C.E. The mouse endometrium contains epithelial, endothelial and leucocyte populations expressing the stem cell marker telomerase reverse transcriptase. Mol. Hum. Reprod. 2016, 22, 272–284. [Google Scholar] [CrossRef]
- Wang, Y.; Sacchetti, A.; van Dijk, M.R.; van der Zee, M.; van der Horst, P.H.; Joosten, R.; Burger, C.W.; Grootegoed, J.A.; Blok, L.J.; Fodde, R. Identification of quiescent, stem-like cells in the distal female reproductive tract. PLoS ONE 2012, 7, e40691. [Google Scholar] [CrossRef]
- Patterson, A.L.; Pru, J.K. Long-term label retaining cells localize to distinct regions within the female reproductive epithelium. Cell Cycle 2013, 12, 2888–2898. [Google Scholar] [CrossRef]
- Huang, C.C.; Orvis, G.D.; Wang, Y.; Behringer, R.R. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS ONE 2012, 7, e44285. [Google Scholar] [CrossRef]
- Hu, F.F.; Jing, X.; Cui, Y.G.; Qian, X.Q.; Mao, Y.D.; Liao, L.M.; Liu, J.Y. Isolation and characterization of side population cells in the postpartum murine endometrium. Reprod. Sci. 2010, 17, 629–642. [Google Scholar] [CrossRef]
- Chan, R.W.; Schwab, K.E.; Gargett, C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod. 2004, 70, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Betsholtz, C.; Lindblom, P.; Gerhardt, H. Role of pericytes in vascular morphogenesis. In EXS; Springer: Berlin/Heidelberg, Germany, 2005; pp. 115–125. [Google Scholar] [CrossRef]
- Spitzer, T.L.; Rojas, A.; Zelenko, Z.; Aghajanova, L.; Erikson, D.W.; Barragan, F.; Meyer, M.; Tamaresis, J.S.; Hamilton, A.E.; Irwin, J.C.; et al. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol. Reprod. 2012, 86, 58. [Google Scholar] [CrossRef]
- Li, S.; Ding, L. Endometrial Perivascular Progenitor Cells and Uterus Regeneration. J. Pers. Med. 2021, 11, 477. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, F.; Yan, G.; Hu, Y.; Sun, H.; Ding, L. Human endometrial perivascular stem cells exhibit a limited potential to regenerate endometrium after xenotransplantation. Hum. Reprod. 2021, 36, 145–159. [Google Scholar] [CrossRef]
- Masuda, H.; Anwar, S.S.; Buhring, H.J.; Rao, J.R.; Gargett, C.E. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant. 2012, 21, 2201–2214. [Google Scholar] [CrossRef]
- Cervello, I.; Gil-Sanchis, C.; Mas, A.; Delgado-Rosas, F.; Martinez-Conejero, J.A.; Galan, A.; Martinez-Romero, A.; Martinez, S.; Navarro, I.; Ferro, J.; et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS ONE 2010, 5, e10964. [Google Scholar] [CrossRef]
- Cervello, I.; Mas, A.; Gil-Sanchis, C.; Peris, L.; Faus, A.; Saunders, P.T.; Critchley, H.O.; Simon, C. Reconstruction of endometrium from human endometrial side population cell lines. PLoS ONE 2011, 6, e21221. [Google Scholar] [CrossRef]
- Valentijn, A.J.; Palial, K.; Al-Lamee, H.; Tempest, N.; Drury, J.; Von Zglinicki, T.; Saretzki, G.; Murray, P.; Gargett, C.E.; Hapangama, D.K. SSEA-1 isolates human endometrial basal glandular epithelial cells: Phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum. Reprod. 2013, 28, 2695–2708. [Google Scholar] [CrossRef]
- Nguyen, H.P.T.; Xiao, L.; Deane, J.A.; Tan, K.S.; Cousins, F.L.; Masuda, H.; Sprung, C.N.; Rosamilia, A.; Gargett, C.E. N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Hum. Reprod. 2017, 32, 2254–2268. [Google Scholar] [CrossRef] [PubMed]
- Tempest, N.; Jansen, M.; Baker, A.M.; Hill, C.J.; Hale, M.; Magee, D.; Treanor, D.; Wright, N.A.; Hapangama, D.K. Histological 3D reconstruction and in vivo lineage tracing of the human endometrium. J. Pathol. 2020, 251, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.M.; Kumar, M.; Ghosh, A.; Tomasetig, F.; Ali, A.; Whan, R.M.; Alterman, D.; Tanwar, P.S. Endometrial Axin2(+) Cells Drive Epithelial Homeostasis, Regeneration, and Cancer following Oncogenic Transformation. Cell Stem Cell 2020, 26, 64–80.e13. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Kong, Y.; Shao, Y.; Ren, C.; Yang, G. Endometrial stem/progenitor cells and their roles in immunity, clinical application, and endometriosis. Stem Cell Res. Ther. 2021, 12, 474. [Google Scholar] [CrossRef]
- Masuda, H.; Matsuzaki, Y.; Hiratsu, E.; Ono, M.; Nagashima, T.; Kajitani, T.; Arase, T.; Oda, H.; Uchida, H.; Asada, H.; et al. Stem cell-like properties of the endometrial side population: Implication in endometrial regeneration. PLoS ONE 2010, 5, e10387. [Google Scholar] [CrossRef]
- Tsuji, S.; Yoshimoto, M.; Takahashi, K.; Noda, Y.; Nakahata, T.; Heike, T. Side population cells contribute to the genesis of human endometrium. Fertil. Steril. 2008, 90, 1528–1537. [Google Scholar] [CrossRef]
- Miyazaki, K.; Maruyama, T.; Masuda, H.; Yamasaki, A.; Uchida, S.; Oda, H.; Uchida, H.; Yoshimura, Y. Stem cell-like differentiation potentials of endometrial side population cells as revealed by a newly developed in vivo endometrial stem cell assay. PLoS ONE 2012, 7, e50749. [Google Scholar] [CrossRef]
- Lopez-Perez, N.; Gil-Sanchis, C.; Ferrero, H.; Faus, A.; Diaz, A.; Pellicer, A.; Cervello, I.; Simon, C. Human Endometrial Reconstitution from Somatic Stem Cells: The Importance of Niche-Like Cells. Reprod. Sci. 2019, 26, 77–87. [Google Scholar] [CrossRef]
- Taylor, H.S. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 2004, 292, 81–85. [Google Scholar] [CrossRef]
- Cervello, I.; Gil-Sanchis, C.; Mas, A.; Faus, A.; Sanz, J.; Moscardo, F.; Higueras, G.; Sanz, M.A.; Pellicer, A.; Simon, C. Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS ONE 2012, 7, e30260. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Shaikh, S.; Pallavi, P.; Tal, A.; Lopez-Giraldez, F.; Lyu, F.; Fang, Y.Y.; Chinchanikar, S.; Liu, Y.; Kliman, H.J.; et al. Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy. PLoS Biol. 2019, 17, e3000421. [Google Scholar] [CrossRef]
- Ong, Y.R.; Cousins, F.L.; Yang, X.; Mushafi, A.; Breault, D.T.; Gargett, C.E.; Deane, J.A. Bone Marrow Stem Cells Do Not Contribute to Endometrial Cell Lineages in Chimeric Mouse Models. Stem Cells 2018, 36, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Kannel, W.B.; Hjortland, M.C.; McNamara, P.M. Menopause and coronary heart disease. The Framingham Study. Ann. Intern. Med. 1978, 89, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Kalin, M.F.; Zumoff, B. Sex hormones and coronary disease: A review of the clinical studies. Steroids 1990, 55, 330–352. [Google Scholar] [CrossRef]
- Kannel, W.B.; Hjortland, M.C.; McNamara, P.M.; Gordon, T. Menopause and risk of cardiovascular disease: The Framingham study. Ann. Intern. Med. 1976, 85, 447–452. [Google Scholar] [CrossRef]
- Palmer, J.R.; Rosenberg, L.; Shapiro, S. Reproductive factors and risk of myocardial infarction. Am. J. Epidemiol. 1992, 136, 408–416. [Google Scholar] [CrossRef]
- Katz, L.N.; Pick, R.; Stamler, J. Experiences in assessing estrogen anti-atherogenesis in the chick, the rabbit, and man. Ann. N. Y. Acad. Sci. 1956, 64, 596–619. [Google Scholar]
- Stamler, J.; Pick, R.; Katz, L.N.; Pick, A.; Kaplan, B.M.; Berkson, D.M.; Century, D. Effectiveness of estrogens for therapy of myocardial infarction in middle-age men. JAMA 1963, 183, 632–638. [Google Scholar] [CrossRef]
- The Coronary Drug Project. Findings leading to discontinuation of the 2.5-mg day estrogen group. The coronary Drug Project Research Group. JAMA 1973, 226, 652–657. [Google Scholar] [CrossRef]
- Meriggiola, M.C.; Berra, M. Long-term cross-sex hormone treatment is safe in transsexual subjects. Asian J. Androl. 2012, 14, 813–814. [Google Scholar] [CrossRef] [PubMed]
- Bush, T.L.; Barrett-Connor, E.; Cowan, L.D.; Criqui, M.H.; Wallace, R.B.; Suchindran, C.M.; Tyroler, H.A.; Rifkind, B.M. Cardiovascular mortality and noncontraceptive use of estrogen in women: Results from the Lipid Research Clinics Program Follow-up Study. Circulation 1987, 75, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Willett, W.C.; Stampfer, M.J.; Rosner, B.; Speizer, F.E.; Hennekens, C.H. Menopause and the risk of coronary heart disease in women. N. Engl. J. Med. 1987, 316, 1105–1110. [Google Scholar] [CrossRef]
- Falkeborn, M.; Persson, I.; Adami, H.O.; Bergstrom, R.; Eaker, E.; Lithell, H.; Mohsen, R.; Naessen, T. The risk of acute myocardial infarction after oestrogen and oestrogen-progestogen replacement. Br. J. Obstet. Gynaecol. 1992, 99, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, E.; Lip, G.Y. Cardiovascular risk in women: The cardiologist’s perspective. QJM 2000, 93, 135–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hulley, S.; Grady, D.; Bush, T.; Furberg, C.; Herrington, D.; Riggs, B.; Vittinghoff, E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998, 280, 605–613. [Google Scholar] [CrossRef]
- Wilson, P.W.; Garrison, R.J.; Castelli, W.P. Postmenopausal estrogen use, cigarette smoking, and cardiovascular morbidity in women over 50. The Framingham Study. N. Engl. J. Med. 1985, 313, 1038–1043. [Google Scholar] [CrossRef]
- Siddle, N.; Sarrel, P.; Whitehead, M. The effect of hysterectomy on the age at ovarian failure: Identification of a subgroup of women with premature loss of ovarian function and literature review. Fertil. Steril. 1987, 47, 94–100. [Google Scholar] [CrossRef]
- Khalil, R.A. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem. Pharmacol. 2013, 86, 1627–1642. [Google Scholar] [CrossRef]
- Centerwall, B.S. Premenopausal hysterectomy and cardiovascular disease. Am. J. Obstet. Gynecol. 1981, 139, 58–61. [Google Scholar] [CrossRef]
- Falkeborn, M.; Schairer, C.; Naessen, T.; Persson, I. Risk of myocardial infarction after oophorectomy and hysterectomy. J. Clin. Epidemiol. 2000, 53, 832–837. [Google Scholar] [CrossRef]
- Yi, C.X.; Tschop, M.H. Brain-gut-adipose-tissue communication pathways at a glance. Dis. Model. Mech. 2012, 5, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.J. Mechanisms of soluble cytokine receptor generation. J. Immunol. 2004, 173, 5343–5348. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Ratajczak, M.Z. A novel view of the adult bone marrow stem cell hierarchy and stem cell trafficking. Leukemia 2015, 29, 776–782. [Google Scholar] [CrossRef]
- Prianishnikov, V.A. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception 1978, 18, 213–223. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Y.; Brunt, K.R.; Wu, J.; Li, S.H.; Fazel, S.; Weisel, R.D.; Keating, A.; Li, R.K. An adult uterine hemangioblast: Evidence for extramedullary self-renewal and clonal bilineage potential. Blood 2010, 116, 2932–2941. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, J.; Li, S.H.; Zhang, Y.; Xaymardan, M.; Wen, X.Y.; Weisel, R.D.; Keating, A.; Li, R.K. Uterine-derived stem cells reconstitute the bone marrow of irradiated mice. Stem Cells Dev. 2015, 24, 938–947. [Google Scholar] [CrossRef]
- Fazel, S.; Cimini, M.; Chen, L.; Li, S.; Angoulvant, D.; Fedak, P.; Verma, S.; Weisel, R.D.; Keating, A.; Li, R.K. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J. Clin. Investig. 2006, 116, 1865–1877. [Google Scholar] [CrossRef]
- Cimini, M.; Fazel, S.; Zhuo, S.; Xaymardan, M.; Fujii, H.; Weisel, R.D.; Li, R.K. c-kit dysfunction impairs myocardial healing after infarction. Circulation 2007, 116, I-77–I-82. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Sun, Z.; Brunt, K.R.; Shi, X.; Chen, M.S.; Weisel, R.D.; Li, R.K. Reconstitution of aged bone marrow with young cells repopulates cardiac-resident bone marrow-derived progenitor cells and prevents cardiac dysfunction after a myocardial infarction. Eur. Heart J. 2013, 34, 1157–1167. [Google Scholar] [CrossRef][Green Version]
- Li, S.H.; Sun, L.; Yang, L.; Li, J.; Shao, Z.; Du, G.Q.; Wu, J.; Weisel, R.D.; Li, R.K. Young Bone-Marrow Sca-1(+) Stem Cells Rejuvenate the Aged Heart and Improve Function after Injury through PDGFRbeta-Akt pathway. Sci. Rep. 2017, 7, 41756. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, S.H.; Dong, J.; Alibhai, F.J.; Zhang, C.; Shao, Z.B.; Song, H.F.; He, S.; Yin, W.J.; Wu, J.; et al. Long-term repopulation of aged bone marrow stem cells using young Sca-1 cells promotes aged heart rejuvenation. Aging Cell 2019, 18, e13026. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Taylor, H.S. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 2007, 25, 2082–2086. [Google Scholar] [CrossRef]
- Ayach, B.B.; Yoshimitsu, M.; Dawood, F.; Sun, M.; Arab, S.; Chen, M.; Higuchi, K.; Siatskas, C.; Lee, P.; Lim, H.; et al. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc. Natl. Acad. Sci. USA 2006, 103, 2304–2309. [Google Scholar] [CrossRef]
- Hu, X.; Wei, L.; Taylor, T.M.; Wei, J.; Zhou, X.; Wang, J.A.; Yu, S.P. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. Am. J. Physiol. Cell Physiol. 2011, 301, C362–C372. [Google Scholar] [CrossRef]
- Simoes, F.C.; Riley, P.R. Immune cells in cardiac repair and regeneration. Development 2022, 149. [Google Scholar] [CrossRef]
- Cheng, M.; Huang, K.; Zhou, J.; Yan, D.; Tang, Y.L.; Zhao, T.C.; Miller, R.J.; Kishore, R.; Losordo, D.W.; Qin, G. A critical role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow progenitor cell recruitment to the ischemic heart. J. Mol. Cell Cardiol. 2015, 81, 49–53. [Google Scholar] [CrossRef]
- Finan, A.; Sopko, N.; Dong, F.; Turturice, B.; Kiedrowski, M.; Penn, M.S. Bone marrow SSEA1+ cells support the myocardium in cardiac pressure overload. PLoS ONE 2013, 8, e68528. [Google Scholar] [CrossRef]
- Ludke, A.; Wu, J.; Nazari, M.; Hatta, K.; Shao, Z.; Li, S.H.; Song, H.; Ni, N.C.; Weisel, R.D.; Li, R.K. Uterine-derived progenitor cells are immunoprivileged and effectively improve cardiac regeneration when used for cell therapy. J. Mol. Cell Cardiol. 2015, 84, 116–128. [Google Scholar] [CrossRef]
- Hatta, K.; Zhang, Y.; Wu, J.; Sun, Z.; Weisel, R.D.; Li, R.K. Uterine-Derived CD11b Cells Significantly Increase Vasculogenesis and Promote Myocardial Healing in Ischemic Cardiomyopathy. Cell Transplant. 2016, 25, 1665–1674. [Google Scholar] [CrossRef]
- Fan, X.; He, S.; Song, H.; Yin, W.; Zhang, J.; Peng, Z.; Yang, K.; Zhai, X.; Zhao, L.; Gong, H.; et al. Human endometrium-derived stem cell improves cardiac function after myocardial ischemic injury by enhancing angiogenesis and myocardial metabolism. Stem. Cell Res. Ther. 2021, 12, 344. [Google Scholar] [CrossRef]
- Meng, X.; Ichim, T.E.; Zhong, J.; Rogers, A.; Yin, Z.; Jackson, J.; Wang, H.; Ge, W.; Bogin, V.; Chan, K.W.; et al. Endometrial regenerative cells: A novel stem cell population. J. Transl. Med. 2007, 5, 57. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, R.; Li, W.; Lin, J.; Stamm, C.; Steinhoff, G.; Ma, N. Therapeutic potential of menstrual blood-derived endometrial stem cells in cardiac diseases. Cell Mol. Life Sci. 2019, 76, 1681–1695. [Google Scholar] [CrossRef]
- Huang, M.L.; Tian, H.; Wu, J.; Matsubayashi, K.; Weisel, R.D.; Li, R.K. Myometrial cells induce angiogenesis and salvage damaged myocardium. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2057–H2066. [Google Scholar] [CrossRef][Green Version]
- Bockeria, L.; Bogin, V.; Bockeria, O.; Le, T.; Alekyan, B.; Woods, E.J.; Brown, A.A.; Ichim, T.E.; Patel, A.N. Endometrial regenerative cells for treatment of heart failure: A new stem cell enters the clinic. J. Transl. Med. 2013, 11, 56. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.A.; Xu, Y.; Jiang, Z.; Wu, R.; Wang, L.; Chen, P.; Hu, X.; Yu, H. Menstrual blood derived mesenchymal cells ameliorate cardiac fibrosis via inhibition of endothelial to mesenchymal transition in myocardial infarction. Int. J. Cardiol. 2013, 168, 1711–1714. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, Z.; Webster, K.A.; Chen, J.; Hu, H.; Zhou, Y.; Zhao, J.; Wang, L.; Wang, Y.; Zhong, Z.; et al. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem Cells Transl. Med. 2017, 6, 209–222. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Gu, X.; Zhang, B.; Tian, W.; Han, H.; Sun, P.; Du, C.; Wang, H. Prolongation of Cardiac Allograft Survival by Endometrial Regenerative Cells: Focusing on B-Cell Responses. Stem Cells Transl. Med. 2017, 6, 778–787. [Google Scholar] [CrossRef]
- Lan, X.; Wang, G.; Xu, X.; Lu, S.; Li, X.; Zhang, B.; Shi, G.; Zhao, Y.; Du, C.; Wang, H. Stromal Cell-Derived Factor-1 Mediates Cardiac Allograft Tolerance Induced by Human Endometrial Regenerative Cell-Based Therapy. Stem Cells Transl. Med. 2017, 6, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, R.B.; Shivakumar, S.B.; Choe, Y.H.; Son, Y.B.; Lee, H.J.; Ullah, I.; Jang, S.J.; Ock, S.A.; Lee, S.L.; Rho, G.J. CD105(+) Porcine Endometrial Stromal Mesenchymal Stem Cells Possess Differentiation Potential Toward Cardiomyocyte-Like Cells and Insulin-Producing beta Cell-Like Cells In Vitro. Reprod. Sci. 2019, 26, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Sun, L.; Chen, W.; Liu, K.Y.; Zhang, C.F.; Wang, F.; Zhang, G.H.; Huang, Y.; Li, J.X.; Gao, Y.; et al. Evidence for the existence of CD34(+) angiogenic stem cells in human first-trimester decidua and their therapeutic for ischaemic heart disease. J. Cell Mol. Med. 2020, 24, 11837–11848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Yu, D.; Hu, Y.; Jin, W.; Qin, Y.; Kong, D.; Wang, H.; Li, G.; Alessandrini, A.; et al. Galectin-9 is required for endometrial regenerative cells to induce long-term cardiac allograft survival in mice. Stem Cell Res. Ther. 2020, 11, 471. [Google Scholar] [CrossRef]
- Hu, Y.; Kong, D.; Qin, Y.; Yu, D.; Jin, W.; Li, X.; Zhao, Y.; Wang, H.; Li, G.; Hao, J.; et al. CD73 expression is critical to therapeutic effects of human endometrial regenerative cells in inhibition of cardiac allograft rejection in mice. Stem Cells Transl. Med. 2021, 10, 465–478. [Google Scholar] [CrossRef]
- Wojakowski, W.; Landmesser, U.; Bachowski, R.; Jadczyk, T.; Tendera, M. Mobilization of stem and progenitor cells in cardiovascular diseases. Leukemia 2012, 26, 23–33. [Google Scholar] [CrossRef]
- Forcillo, J.; Stevens, L.M.; Mansour, S.; Prieto, I.; Salem, R.; Baron, C.; Roy, D.C.; Larose, E.; Masckauchan, D.; Noiseux, N. Implantation of CD133+ stem cells in patients undergoing coronary bypass surgery: IMPACT-CABG pilot trial. Can. J. Cardiol. 2013, 29, 441–447. [Google Scholar] [CrossRef]
- Gargett, C.E. Uterine stem cells: What is the evidence? Hum. Reprod. Update 2007, 13, 87–101. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Lyu, F.; Abuwala, N.; Tal, A.; Chen, A.Y.; Taylor, H.S.; Tal, R. Chemokine C-X-C receptor 4 (CXCR4) mediates recruitment of bone marrow-derived nonhematopoietic and immune cells to the pregnant uterus. Biol. Reprod. 2022, 106, 1083–1097. [Google Scholar] [CrossRef]
- Mamillapalli, R.; Mutlu, L.; Taylor, H.S. Characterization of Bone Marrow Progenitor Cell Uterine Engraftment and Transdifferentiation. Reprod. Sci. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Lee, Y.J.; Yi, K.W. Bone marrow-derived stem cells contribute to regeneration of the endometrium. Clin. Exp. Reprod. Med. 2018, 45, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Kisa, J.; Abuwala, N.; Kliman, H.J.; Shaikh, S.; Chen, A.Y.; Lyu, F.; Taylor, H.S. Bone marrow-derived progenitor cells contribute to remodeling of the postpartum uterus. Stem Cells 2021, 39, 1489–1505. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ren, C.; Jiang, J. Effects of bone marrow mesenchymal stem cells on repair and receptivity of damaged endometrium in rats. J. Obstet. Gynaecol. Res. 2021, 47, 3223–3231. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, A.I.; Einstein, E.; Morelli, S.S. Bone Marrow-Derived Cells in Endometrial Cancer Pathogenesis: Insights from Breast Cancer. Cells 2022, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Borzekowski, D.L.; Guan, Y.; Smith, K.C.; Erby, L.H.; Roter, D.L. The Angelina effect: Immediate reach, grasp, and impact of going public. Genet. Med. 2014, 16, 516–521. [Google Scholar] [CrossRef]
- Boyle, A.J.; Schulman, S.P.; Hare, J.M.; Oettgen, P. Is stem cell therapy ready for patients? Stem Cell Therapy for Cardiac Repair. Ready for the Next Step. Circulation 2006, 114, 339–352. [Google Scholar] [CrossRef]
- Sanganalmath, S.K.; Bolli, R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res. 2013, 113, 810–834. [Google Scholar] [CrossRef]
- Fazel, S.S.; Chen, L.; Angoulvant, D.; Li, S.H.; Weisel, R.D.; Keating, A.; Li, R.K. Activation of c-kit is necessary for mobilization of reparative bone marrow progenitor cells in response to cardiac injury. FASEB J. 2008, 22, 930–940. [Google Scholar] [CrossRef]
- Ulrich, D.; Muralitharan, R.; Gargett, C.E. Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin. Biol. Ther. 2013, 13, 1387–1400. [Google Scholar] [CrossRef]
- Hida, N.; Nishiyama, N.; Miyoshi, S.; Kira, S.; Segawa, K.; Uyama, T.; Mori, T.; Miyado, K.; Ikegami, Y.; Cui, C.; et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells 2008, 26, 1695–1704. [Google Scholar] [CrossRef]
- Khatun, M.; Sorjamaa, A.; Kangasniemi, M.; Sutinen, M.; Salo, T.; Liakka, A.; Lehenkari, P.; Tapanainen, J.S.; Vuolteenaho, O.; Chen, J.C.; et al. Niche matters: The comparison between bone marrow stem cells and endometrial stem cells and stromal fibroblasts reveal distinct migration and cytokine profiles in response to inflammatory stimulus. PLoS ONE 2017, 12, e0175986. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.; Karp, J.M. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol. Med. 2010, 16, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006, 20, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Wolff, E.F.; Gao, X.B.; Yao, K.V.; Andrews, Z.B.; Du, H.; Elsworth, J.D.; Taylor, H.S. Endometrial stem cell transplantation restores dopamine production in a Parkinson’s disease model. J. Cell Mol. Med. 2011, 15, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, X.; Massasa, E.E.; Feng, Y.; Wolff, E.; Taylor, H.S. Derivation of insulin producing cells from human endometrial stromal stem cells and use in the treatment of murine diabetes. Mol. Ther. 2011, 19, 2065–2071. [Google Scholar] [CrossRef]
- Murphy, M.P.; Wang, H.; Patel, A.N.; Kambhampati, S.; Angle, N.; Chan, K.; Marleau, A.M.; Pyszniak, A.; Carrier, E.; Ichim, T.E.; et al. Allogeneic endometrial regenerative cells: An “Off the shelf solution” for critical limb ischemia? J. Transl. Med. 2008, 6, 45. [Google Scholar] [CrossRef]
- Huang, S.S.; Ling, T.Y.; Tseng, W.F.; Huang, Y.H.; Tang, F.M.; Leal, S.M.; Huang, J.S. Cellular growth inhibition by IGFBP-3 and TGF-beta1 requires LRP-1. FASEB J. 2003, 17, 2068–2081. [Google Scholar] [CrossRef]
- Vassilieva, I.; Kosheverova, V.; Vitte, M.; Kamentseva, R.; Shatrova, A.; Tsupkina, N.; Skvortsova, E.; Borodkina, A.; Tolkunova, E.; Nikolsky, N.; et al. Paracrine senescence of human endometrial mesenchymal stem cells: A role for the insulin-like growth factor binding protein 3. Aging 2020, 12, 1987–2004. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, G.; Xu, Y.; Zhao, D.; Zheng, L. Stem Cell-Based Therapy for Asherman Syndrome: Promises and Challenges. Cell Transplant. 2021, 30, 9636897211020734. [Google Scholar] [CrossRef]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef]
- Alkass, K.; Panula, J.; Westman, M.; Wu, T.D.; Guerquin-Kern, J.L.; Bergmann, O. No Evidence for Cardiomyocyte Number Expansion in Preadolescent Mice. Cell 2015, 163, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Maillet, M.; van Berlo, J.H.; Molkentin, J.D. Molecular basis of physiological heart growth: Fundamental concepts and new players. Nat. Rev. Mol. Cell Biol. 2013, 14, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Meckert, P.C.; Rivello, H.G.; Vigliano, C.; Gonzalez, P.; Favaloro, R.; Laguens, R. Endomitosis and polyploidization of myocardial cells in the periphery of human acute myocardial infarction. Cardiovasc. Res. 2005, 67, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Derks, W.; Bergmann, O. Polyploidy in Cardiomyocytes: Roadblock to Heart Regeneration? Circ. Res. 2020, 126, 552–565. [Google Scholar] [CrossRef]
- Das, S.K. Cell cycle regulatory control for uterine stromal cell decidualization in implantation. Reproduction 2009, 137, 889–899. [Google Scholar] [CrossRef]
- Rahman, M.A.; Li, M.; Li, P.; Wang, H.; Dey, S.K.; Das, S.K. Hoxa-10 deficiency alters region-specific gene expression and perturbs differentiation of natural killer cells during decidualization. Dev. Biol. 2006, 290, 105–117. [Google Scholar] [CrossRef][Green Version]
- Tan, J.; Raja, S.; Davis, M.K.; Tawfik, O.; Dey, S.K.; Das, S.K. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech. Dev. 2002, 111, 99–113. [Google Scholar] [CrossRef]
- Paria, B.C.; Zhao, X.; Das, S.K.; Dey, S.K.; Yoshinaga, K. Zonula occludens-1 and E-cadherin are coordinately expressed in the mouse uterus with the initiation of implantation and decidualization. Dev. Biol. 1999, 208, 488–501. [Google Scholar] [CrossRef]
- Sroga, J.M.; Ma, X.; Das, S.K. Developmental regulation of decidual cell polyploidy at the site of implantation. Front. Biosci. 2012, 4, 1475–1486. [Google Scholar] [CrossRef]
- Marinaro, F.; Gomez-Serrano, M.; Jorge, I.; Silla-Castro, J.C.; Vazquez, J.; Sanchez-Margallo, F.M.; Blazquez, R.; Lopez, E.; Alvarez, V.; Casado, J.G. Unraveling the Molecular Signature of Extracellular Vesicles From Endometrial-Derived Mesenchymal Stem Cells: Potential Modulatory Effects and Therapeutic Applications. Front. Bioeng. Biotechnol. 2019, 7, 431. [Google Scholar] [CrossRef]
- Zhang, S.; Chang, Q.; Li, P.; Tong, X.; Feng, Y.; Hao, X.; Zhang, X.; Yuan, Z.; Tan, J. Concentrated small extracellular vesicles from menstrual blood-derived stromal cells improve intrauterine adhesion, a pre-clinical study in a rat model. Nanoscale 2021, 13, 7334–7347. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhu, M.; Feng, W.; Lin, Y.; Yin, J.; Jin, J.; Wang, Y. Exosomal miRNA Let-7 from Menstrual Blood-Derived Endometrial Stem Cells Alleviates Pulmonary Fibrosis through Regulating Mitochondrial DNA Damage. Oxid. Med. Cell Longev. 2019, 2019, 4506303. [Google Scholar] [CrossRef] [PubMed]
| Method | Result | Reference | |
|---|---|---|---|
| Rats who undergone hysterectomy, followed by heterotropic GFP+ uterine transplant and MI | GFP+ uteruses were removed from GFP rats and heterotropically-transplanted into non-GFP recipients who have undergone hysterectomy; MI was then induced | Heterotropic-transplanted uterine GFP+ cells were found in recipient hearts 7 days after MI and persisted for 6 months, in which they were localized around blood vessels, and improved cardiac functioning | [6] |
| Commercially-obtained menstrual blood-derived mesenchymal stem cells and a rat MI model | 2 × 106 menstrual blood-derived mesenchymal stem cells were intramyocardially-injected into a Sprague-Dawley rat MI model | Menstrual blood-derived mesenchymal stem cells improved cardiac functioning through inhibition of the TGF-β/Smad-induced endothelial to mesenchymal transition, in turn reducing cardiac fibrosis | [89] |
| Murine uterine MHC I− and MHC I+ cells, along with a murine MI model | Murine uterine MHC I− and MHC I+ cells were isolated from C57BL/6N mice, characterized in vitro for their immuno-modulatory properties, followed by allogenic injection of 0.5 × 106 cells into a FVB mouse MI model | MHC I− cells were immuno-privileged, with lower cell death and leukocyte proliferation, as well as yielding comparable benefits to syngeneic bone marrow cell transplantation after intra-myocardial injection, with engraftment in cardiac tissue and limited recruitment of CD4 and CD8 cells | [82] |
| Rat uterine-derived CD11b cells and a rat ischemia/reperfusion model | 9 × 105 CD11b+ cells were intramyocardially injected into ischemic/re-perfused rat hearts 5 days post-injury | CD11b cells increased vasculogenesis, leading to reduced infarct size, as well as restoring myocardial functioning and perfusion | [83] |
| Human endometrium-derived, bone marrow, and adipose-derived mesenchymal stem cells in a rat MI model | Human endometrium-derived, bone marrow, and adipose-derived mesenchymal stem cells were injected intra-myocardially to compare their cardio-protective capabilities | Endometrium-derived mesenchymal stem cells had greater cardioprotective capabilities and increased angiogenesis via secreting miR-21 in exosomes, which in turn activates the PTEN/Akt pathway | [90] |
| Murine heart transplant model and human menstrual blood-derived ERC | Heterotropic cardiac transplantation was conducted from C57BL/6 to BALB/c mice, followed by intravenous injection of 1 × 106 human ERCs | ERC treatment prolonged cardiac allograft survival in mice by reducing CD19+ B cell numbers and activity | [91] |
| Murine heart transplant model and human ERCs | Heterotropic cardiac transplantation was conducted from BALB/c to C57BL/6 mice, followed by intravenous injection of 1 × 106 human ERCs | Inhibition of ERC-produced SDF-1by the antagonist AMD3100, resulted in cardiac allograft rejection in recipient mice, as it was associated with increased antibodies and infiltrating immune cells | [92] |
| Porcine adipose-derived and endometrial stromal mesenchymal stem cells | Endometrial stromal and adipose-derived mesenchymal stem cells were obtained from pigs, and their markers, growth, and differentiation potential were compared to each other in vitro. | Endometrial stromal mesenchymal stem cells had higher growth rates compared to adipose-derived mesenchymal stem cells, as well as being able to differentiate into cardiomyocyte-like like cells | [93] |
| Human decidual stem cells from the first trimester of pregnancy and bone marrow stem cells in a rat MI model | Human CD34+ decidual stem cells were obtained from women who terminated during the first trimester, and compared to bone marrow stem cells obtained from cardiac surgery patients | Human CD34+ decidual stem cells had greater angiogenic capabilities, compared to bone marrow stem cells, as well as being able to increase cardiomyocyte survival and increase neo-vasculature post-MI | [94] |
| Murine heart transplant model and human ERCs | Heterotropic cardiac transplantation was conducted from BALB/c to C57BL/6 mice, followed by intravenous injection of 5 × 106 human ERCs | Human ERCs expressed Galectin-9, which suppressed immune responses, in the form of lowered Th1, Th17, CD8+ T, and B cell activity, decreased donor-specific antibody levels, and enhanced Treg, all of which contributed to prolonged cardiac allograft survival | [95] |
| Murine heart transplant model and human ERCs | Cardiac allograft transplantation was conducted from BALB/c donors to C57BL/6 mice, followed by implantation of human endometrial stem cells, either untreated or pre-treated with CD73 monoclonal antibodies | CD73 on ERCs led to decreased pro-inflammatory cytokines IFN-γ and TNF-α, increased anti-inflammatory cytokine IL-10, as well as increasing expression of protective cardiac allograft receptor A2B. By contrast, blocking CD73 led to reduced Tol-DC, M2, and Treg activity | [96] |
| Human endometrial and bone marrow stem cells in a rat MI model | Human endometrial stem cells were isolated from 22 premenopausal women, and compared to human bone marrow mesenchymal stem cells derived from 25 age-matched patients | Human endometrial stem cells had greater proliferative, migratory, and pro-angiogenic capabilities, as well as being able to preserve viable cardiomyocytes and improve cardiac functioning post-ischemic injury, compared to bone marrow stem cells | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ludke, A.; Hatta, K.; Yao, A.; Li, R.-K. Uterus: A Unique Stem Cell Reservoir Able to Support Cardiac Repair via Crosstalk among Uterus, Heart, and Bone Marrow. Cells 2022, 11, 2182. https://doi.org/10.3390/cells11142182
Ludke A, Hatta K, Yao A, Li R-K. Uterus: A Unique Stem Cell Reservoir Able to Support Cardiac Repair via Crosstalk among Uterus, Heart, and Bone Marrow. Cells. 2022; 11(14):2182. https://doi.org/10.3390/cells11142182
Chicago/Turabian StyleLudke, Ana, Kota Hatta, Alina Yao, and Ren-Ke Li. 2022. "Uterus: A Unique Stem Cell Reservoir Able to Support Cardiac Repair via Crosstalk among Uterus, Heart, and Bone Marrow" Cells 11, no. 14: 2182. https://doi.org/10.3390/cells11142182
APA StyleLudke, A., Hatta, K., Yao, A., & Li, R.-K. (2022). Uterus: A Unique Stem Cell Reservoir Able to Support Cardiac Repair via Crosstalk among Uterus, Heart, and Bone Marrow. Cells, 11(14), 2182. https://doi.org/10.3390/cells11142182





