LSD1 Inhibition Enhances the Immunogenicity of Mesenchymal Stromal Cells by Eliciting a dsRNA Stress Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethics
2.2. Cell Lines and Reagents
2.3. Bioinformatics Analysis
2.4. Generation of Primary Bone Marrow (BM)-Derived MSCs
2.5. Identification of the TC Maximum Tolerated Dose (MTD) and Subsequent Treatments
2.6. RNA Extraction
2.7. Assessment of dsRNA via Confocal Micropscopy
2.8. Antigen Presentation Assay
2.9. Cytokine Quantification
2.10. Monitoring Peptide: MHC Complex Stability
2.11. Assessing ROS Production in Response to TC Treatment
2.12. Treatment with IFNγ and IDO-1 Quantification
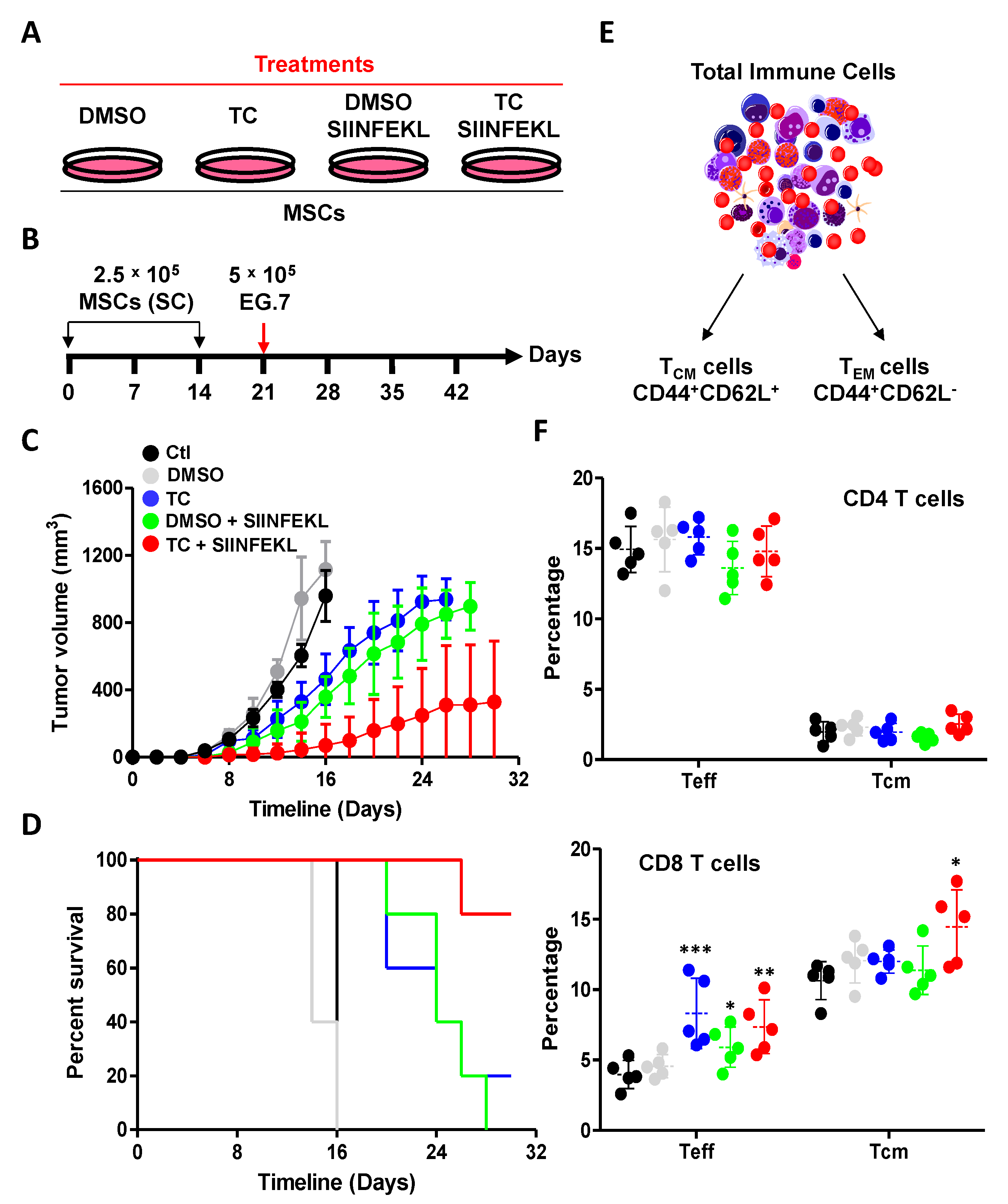
2.13. Design of the Prophylactic Vaccination Study
2.14. Statistical Analysis
3. Results
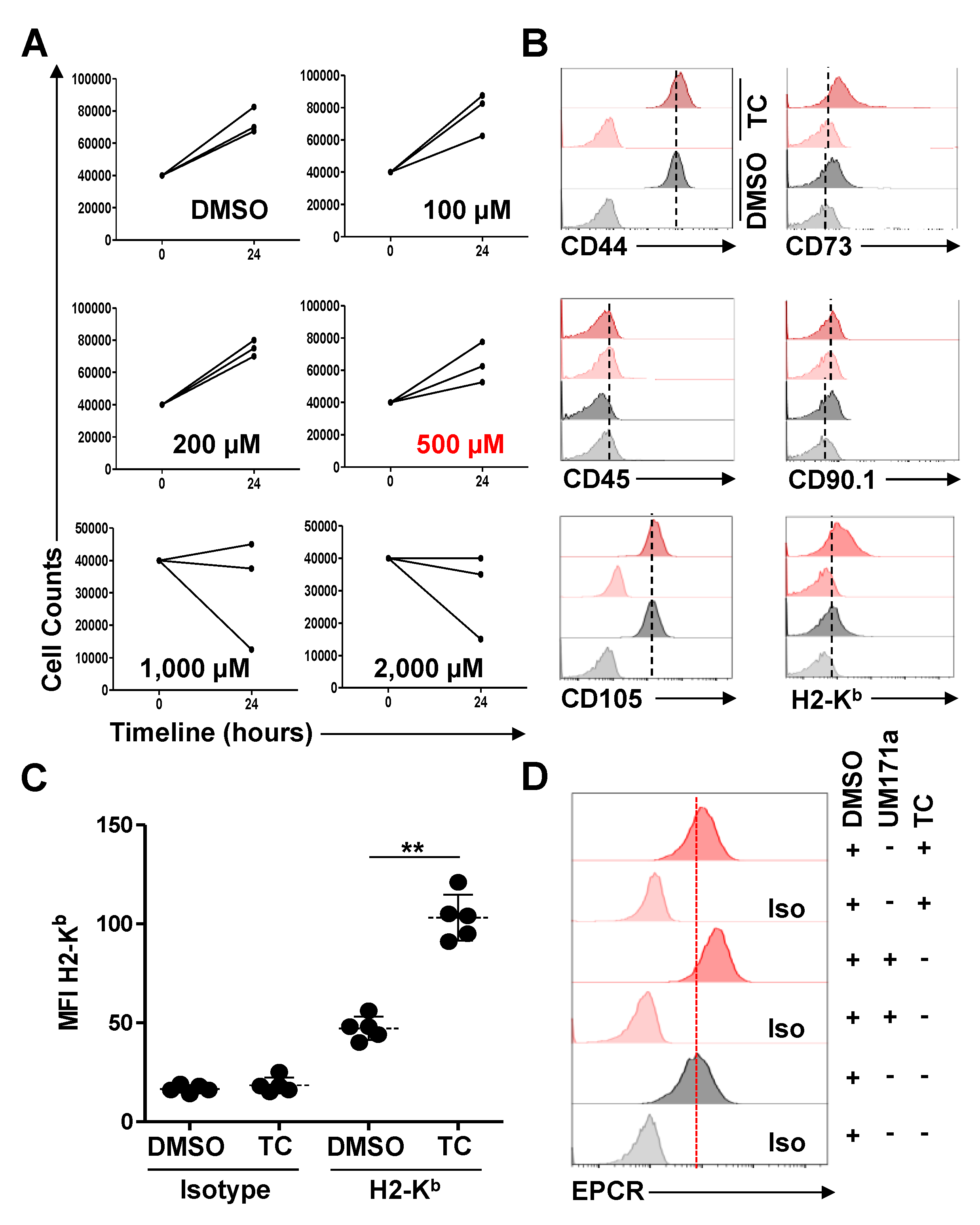
3.1. TC-Treated MSCs Exhibit a Significant Increase in H2-Kb Expression
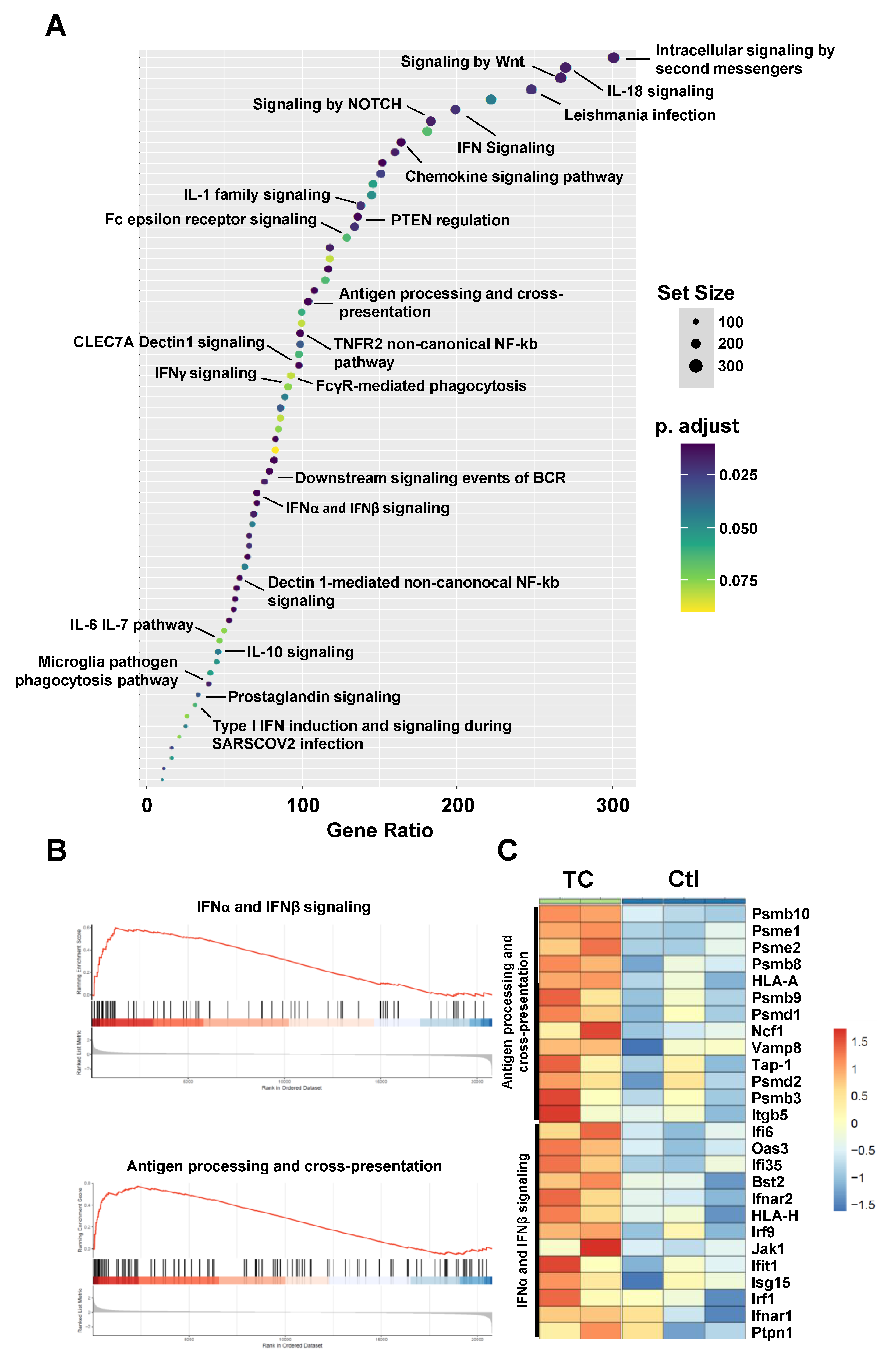
3.2. Bioinformatics Analysis Predicts Induced Type-I IFN Signaling and Antigen Presentation in Response to TC Treatment
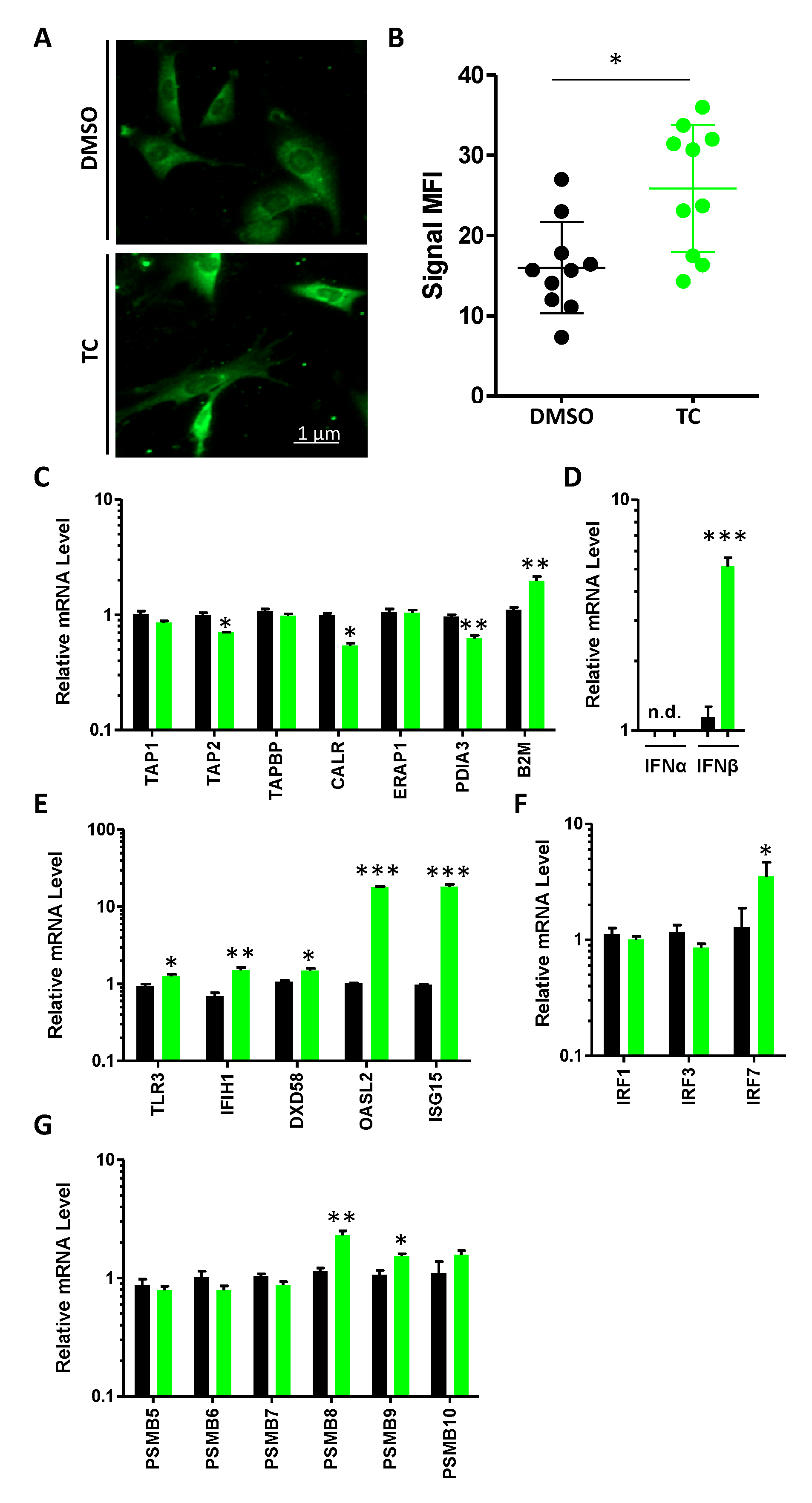
3.3. MSCs Express dsRNA and Their Cognate Sensing Elements in Response to TC Treatment
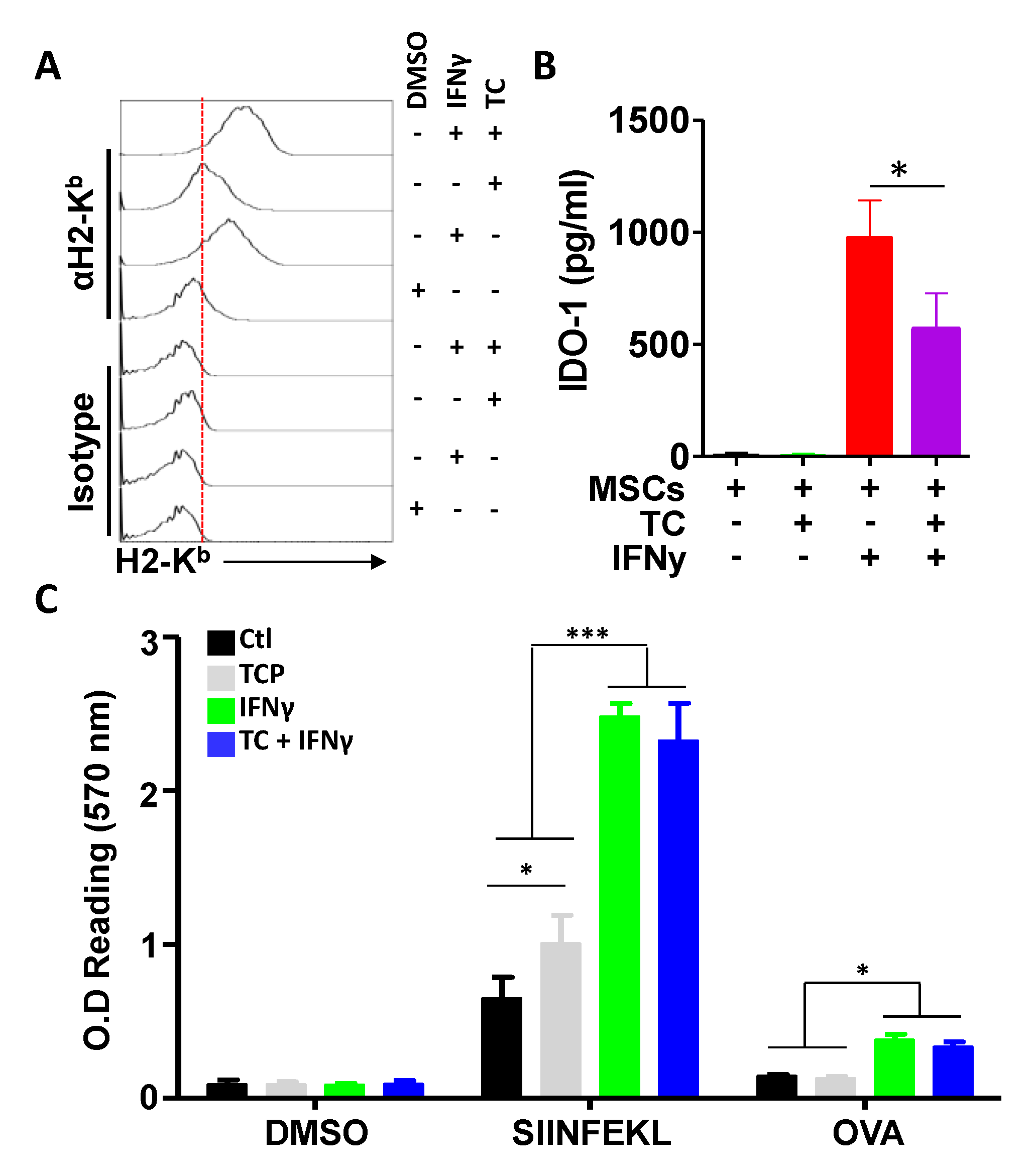
3.4. TC-Mediated LSD1 Inhibition Improves MSC Antigen Presentation through Cell Surface MHC:Peptide Complex Stabilization
3.5. IFNγ Treatment Does Not Synergise with TC at Enhancing Antigen Presentation
3.6. TC-Mediated LSD1 Inhibition Enhances the Anti-Tumoral Property of MSCs
3.7. TC Treatment of Human-Derived UC-MSCs Triggers Similar Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017, 9, eaam7828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.; Shojaee, M.; Crow, J.M.; Khanabdali, R. From Mesenchymal Stromal Cells to Engineered Extracellular Vesicles: A New Therapeutic Paradigm. Front. Cell Dev. Biol. 2021, 9, 705676. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.H.M.; D’Rozario, J.; Mendonca, S.; Bhuvan, T.; Payne, N.L.; Zheng, D.; Hisana, A.; Wallis, G.; Barugahare, A.; Powell, D.; et al. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nat. Commun. 2021, 12, 6495. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salame, N.; Bikorimana, J.P.; El-Hachem, N.; Saad, W.; Kurdi, M.; Zhao, J.; Eliopoulos, N.; Shammaa, R.; Rafei, M. UM171A-induced ROS promote antigen cross-presentation of immunogenic peptides by bone marrow-derived mesenchymal stromal cells. Stem Cell Res. Ther. 2022, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Stagg, J.; Pommey, S.; Eliopoulos, N.; Galipeau, J. Interferon-gamma-stimulated marrow stromal cells: A new type of nonhematopoietic antigen-presenting cell. Blood 2006, 107, 2570–2577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikorimana, J.P.; Abusarah, J.; Salame, N.; El-Hachem, N.; Shammaa, R.; Rafei, M. Humoral Immunity to Allogeneic Immunoproteasome-Expressing Mesenchymal Stromal Cells Requires Efferocytosis by Endogenous Phagocytes. Cells 2022, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Abusarah, J.; Khodayarian, F.; El-Hachem, N.; Salame, N.; Olivier, M.; Balood, M.; Roversi, K.; Talbot, S.; Bikorimana, J.P.; Chen, J.; et al. Engineering immunoproteasome-expressing mesenchymal stromal cells: A potent cellular vaccine for lymphoma and melanoma in mice. Cell Rep. Med. 2021, 2, 100455. [Google Scholar] [CrossRef]
- Wen, R.; Dong, C.; Xu, C.; Zhao, L.; Yang, Y.; Zhang, Z.; Chen, Y.; Duan, L.; Chen, H.; Yang, Z.; et al. UM171 promotes expansion of autologous peripheral blood hematopoietic stem cells from poorly mobilizing lymphoma patients. Int. Immunopharmacol. 2020, 81, 106266. [Google Scholar] [CrossRef]
- Subramaniam, A.; Žemaitis, K.; Talkhoncheh, M.S.; Yudovich, D.; Bäckström, A.; Debnath, S.; Chen, J.; Jain, M.V.; Galeev, R.; Gaetani, M.; et al. Lysine-specific demethylase 1A restricts ex vivo propagation of human HSCs and is a target of UM171. Blood 2020, 136, 2151–2161. [Google Scholar] [CrossRef]
- Li, X.; Xia, C.; Wang, T.; Liu, L.; Zhao, Q.; Yang, D.; Hu, F.; Zhang, M.; Huang, K.; Geng, Y.; et al. Pyrimidoindole derivative UM171 enhances derivation of hematopoietic progenitor cells from human pluripotent stem cells. Stem Cell Res. 2017, 21, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Fares, I.; Chagraoui, J.; Lehnertz, B.; MacRae, T.; Mayotte, N.; Tomellini, E.; Aubert, L.; Roux, P.P.; Sauvageau, G. EPCR expression marks UM171-expanded CD34(+) cord blood stem cells. Blood 2017, 129, 3344–3351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fares, I.; Chagraoui, J.; Gareau, Y.; Gingras, S.; Ruel, R.; Mayotte, N.; Csaszar, E.; Knapp, D.J.; Miller, P.; Ngom, M.; et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science 2014, 345, 1509–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chagraoui, J.; Lehnertz, B.; Girard, S.; Spinella, J.F.; Fares, I.; Tomellini, E.; Mayotte, N.; Corneau, S.; MacRae, T.; Simon, L.; et al. UM171 induces a homeostatic inflammatory-detoxification response supporting human HSC self-renewal. PLoS ONE 2019, 14, e0224900. [Google Scholar] [CrossRef] [Green Version]
- Chagraoui, J.; Girard, S.; Spinella, J.F.; Simon, L.; Bonneil, E.; Mayotte, N.; MacRae, T.; Coulombe-Huntington, J.; Bertomeu, T.; Moison, C.; et al. UM171 Preserves Epigenetic Marks that Are Reduced in Ex Vivo Culture of Human HSCs via Potentiation of the CLR3-KBTBD4 Complex. Cell Stem Cell 2021, 28, 48–62.e6. [Google Scholar] [CrossRef]
- Sheng, W.; LaFleur, M.W.; Nguyen, T.H.; Chen, S.; Chakravarthy, A.; Conway, J.R.; Li, Y.; Chen, H.; Yang, H.; Hsu, P.H.; et al. LSD1 Ablation Stimulates Anti-tumor Immunity and Enables Checkpoint Blockade. Cell 2018, 174, 549–563.e19. [Google Scholar] [CrossRef] [Green Version]
- Barth, J.; Abou-El-Ardat, K.; Dalic, D.; Kurrle, N.; Maier, A.M.; Mohr, S.; Schütte, J.; Vassen, L.; Greve, G.; Schulz-Fincke, J.; et al. LSD1 inhibition by tranylcypromine derivatives interferes with GFI1-mediated repression of PU.1 target genes and induces differentiation in AML. Leukemia 2019, 33, 1411–1426. [Google Scholar] [CrossRef]
- Schenk, T.; Chen, W.C.; Göllner, S.; Howell, L.; Jin, L.; Hebestreit, K.; Klein, H.U.; Popescu, A.C.; Burnett, A.; Mills, K.; et al. Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nat. Med. 2012, 18, 605–611. [Google Scholar] [CrossRef]
- Savina, A.; Peres, A.; Cebrian, I.; Carmo, N.; Moita, C.; Hacohen, N.; Moita, L.F.; Amigorena, S. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity 2009, 30, 544–555. [Google Scholar] [CrossRef] [Green Version]
- Savina, A.; Jancic, C.; Hugues, S.; Guermonprez, P.; Vargas, P.; Moura, I.C.; Lennon-Duménil, A.M.; Seabra, M.C.; Raposo, G.; Amigorena, S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 2006, 126, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Oberkampf, M.; Guillerey, C.; Mouriès, J.; Rosenbaum, P.; Fayolle, C.; Bobard, A.; Savina, A.; Ogier-Denis, E.; Enninga, J.; Amigorena, S.; et al. Mitochondrial reactive oxygen species regulate the induction of CD8(+) T cells by plasmacytoid dendritic cells. Nat. Commun. 2018, 9, 2241. [Google Scholar] [CrossRef] [Green Version]
- Dingjan, I.; Verboogen, D.R.; Paardekooper, L.M.; Revelo, N.H.; Sittig, S.P.; Visser, L.J.; Mollard, G.F.; Henriet, S.S.; Figdor, C.G.; Ter Beest, M.; et al. Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci. Rep. 2016, 6, 22064. [Google Scholar] [CrossRef] [Green Version]
- Shou, P.; Chen, Q.; Jiang, J.; Xu, C.; Zhang, J.; Zheng, C.; Jiang, M.; Velletri, T.; Cao, W.; Huang, Y.; et al. Type I interferons exert anti-tumor effect via reversing immunosuppression mediated by mesenchymal stromal cells. Oncogene 2016, 35, 5953–5962. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardani, F.; Saad, W.; El-Hachem, N.; Bikorimana, J.-P.; Kurdi, M.; Shammaa, R.; Talbot, S.; Rafei, M. LSD1 Inhibition Enhances the Immunogenicity of Mesenchymal Stromal Cells by Eliciting a dsRNA Stress Response. Cells 2022, 11, 1816. https://doi.org/10.3390/cells11111816
Mardani F, Saad W, El-Hachem N, Bikorimana J-P, Kurdi M, Shammaa R, Talbot S, Rafei M. LSD1 Inhibition Enhances the Immunogenicity of Mesenchymal Stromal Cells by Eliciting a dsRNA Stress Response. Cells. 2022; 11(11):1816. https://doi.org/10.3390/cells11111816
Chicago/Turabian StyleMardani, Fatemeh, Wael Saad, Nehme El-Hachem, Jean-Pierre Bikorimana, Mazen Kurdi, Riam Shammaa, Sebastien Talbot, and Moutih Rafei. 2022. "LSD1 Inhibition Enhances the Immunogenicity of Mesenchymal Stromal Cells by Eliciting a dsRNA Stress Response" Cells 11, no. 11: 1816. https://doi.org/10.3390/cells11111816
APA StyleMardani, F., Saad, W., El-Hachem, N., Bikorimana, J.-P., Kurdi, M., Shammaa, R., Talbot, S., & Rafei, M. (2022). LSD1 Inhibition Enhances the Immunogenicity of Mesenchymal Stromal Cells by Eliciting a dsRNA Stress Response. Cells, 11(11), 1816. https://doi.org/10.3390/cells11111816






