Retinal Proteomic Alterations and Combined Transcriptomic-Proteomic Analysis in the Early Stages of Progression of a Mouse Model of X-Linked Retinoschisis
Abstract
1. Introduction
2. Results
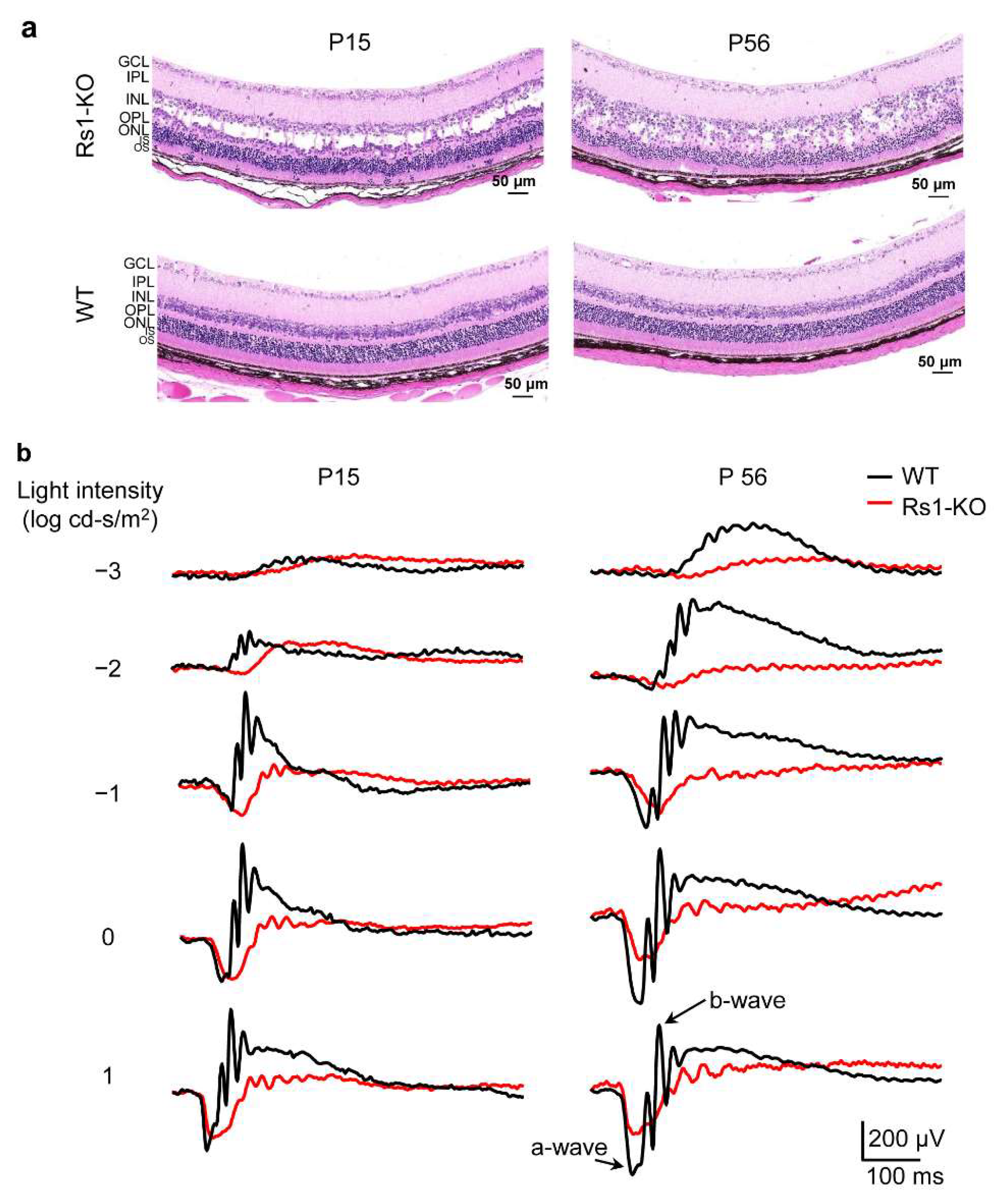
2.1. Morphological and Functional Evaluation of RS1-KO Retinas in Development
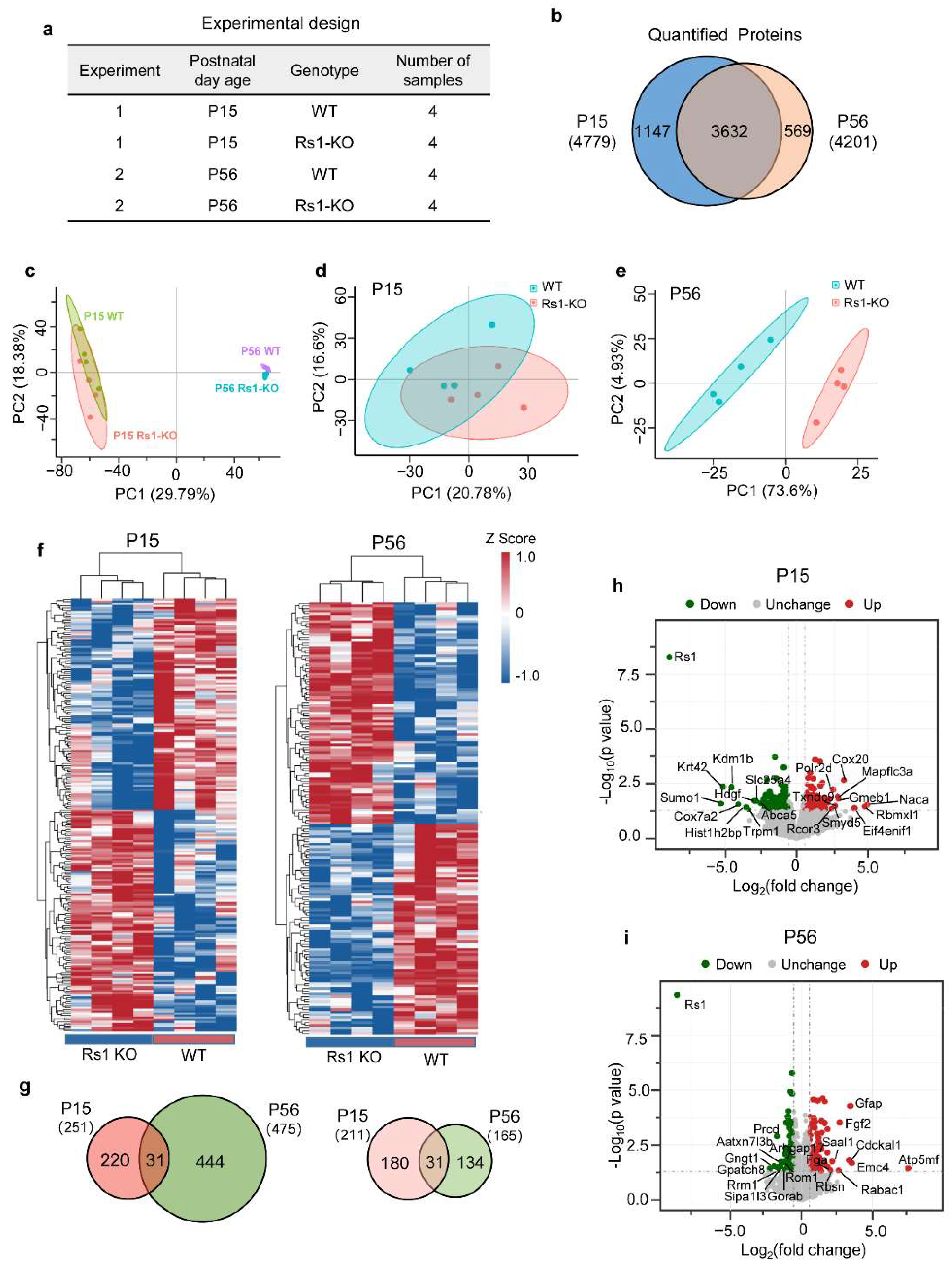
2.2. Proteomic Features of Retina in XLRS Mice
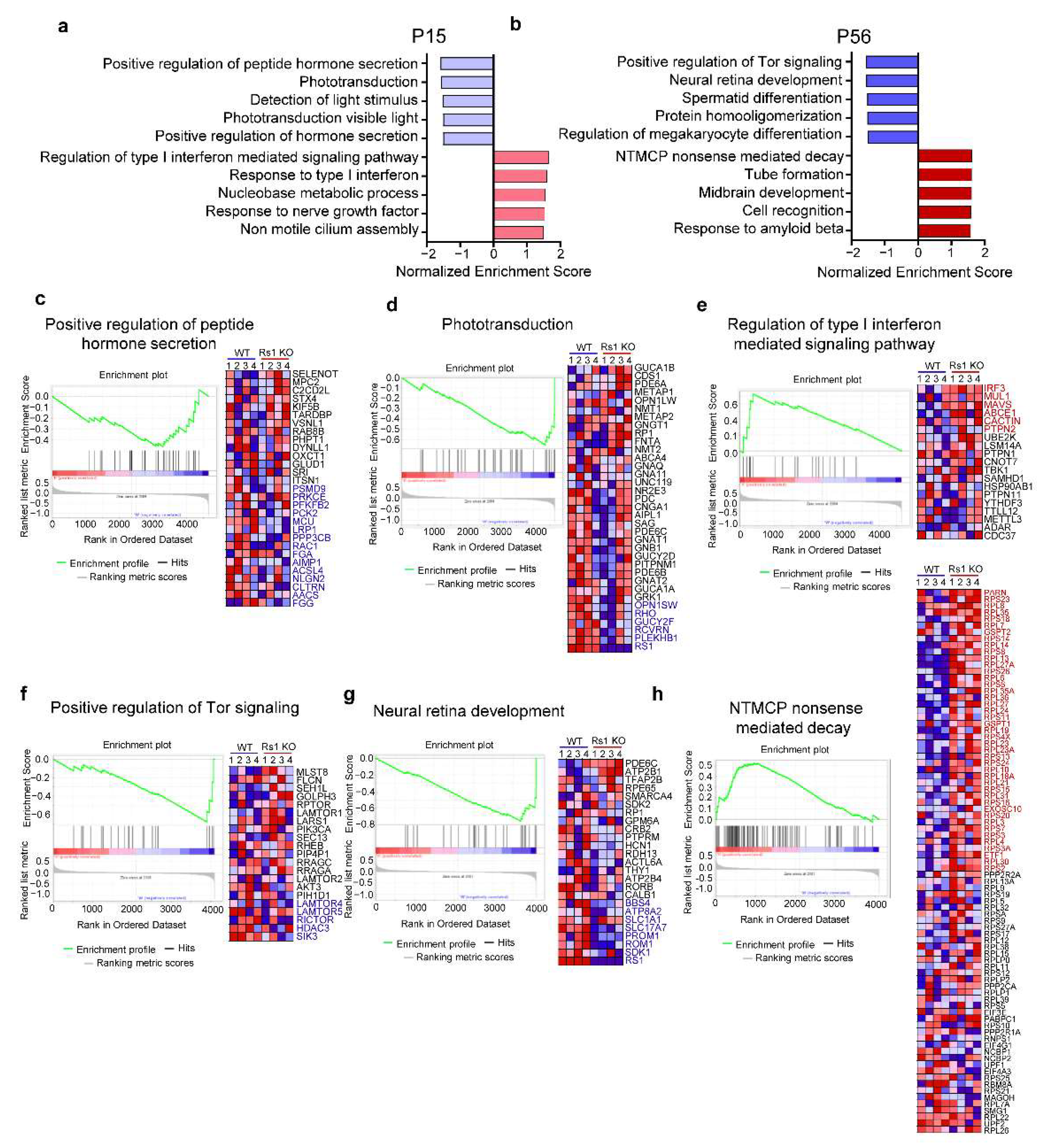
2.3. Differentially Expressed Gene Sets in XLRS Onset
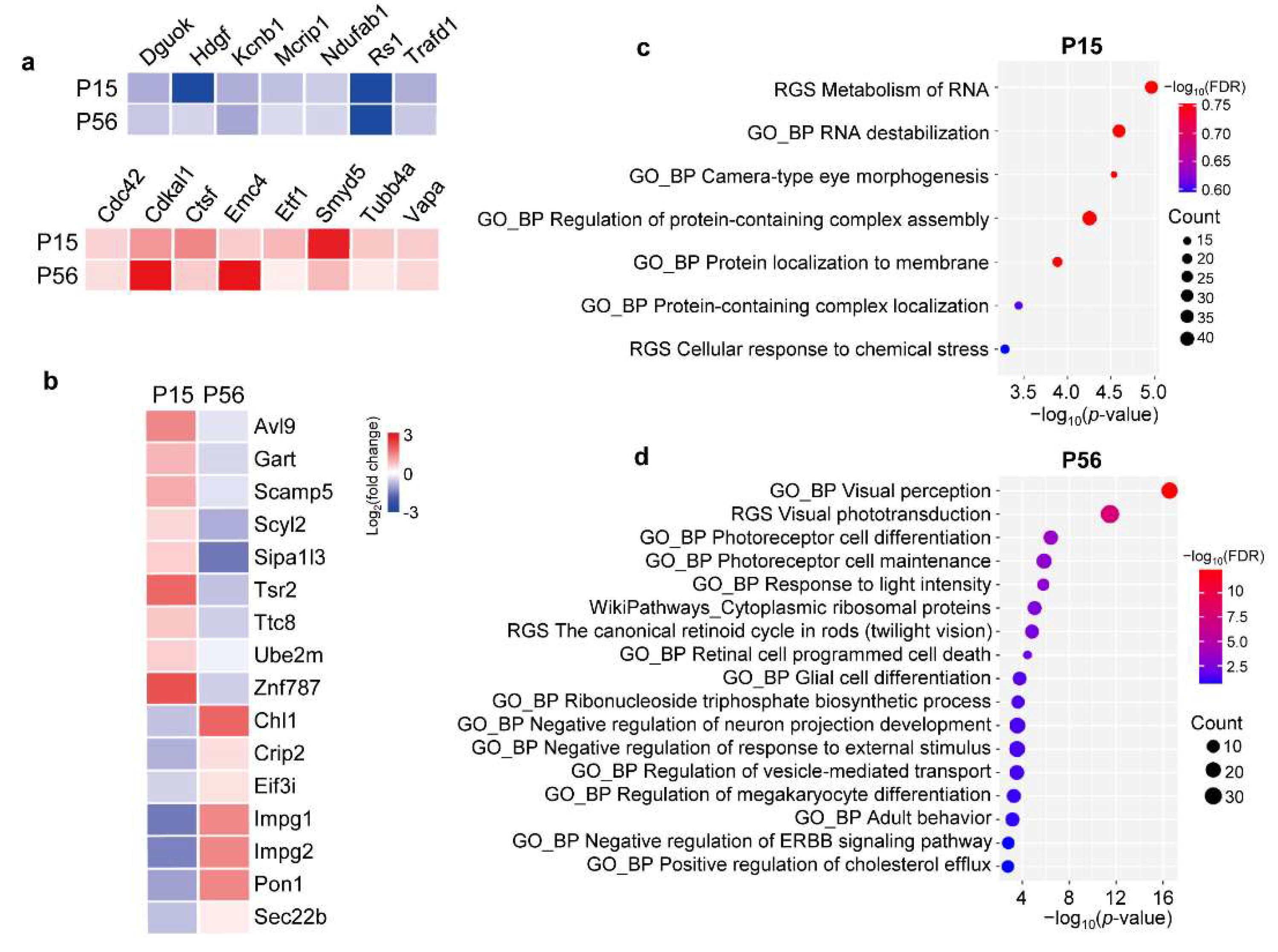
2.4. Differentially Expressed Gene Sets in XLRS Early Progression
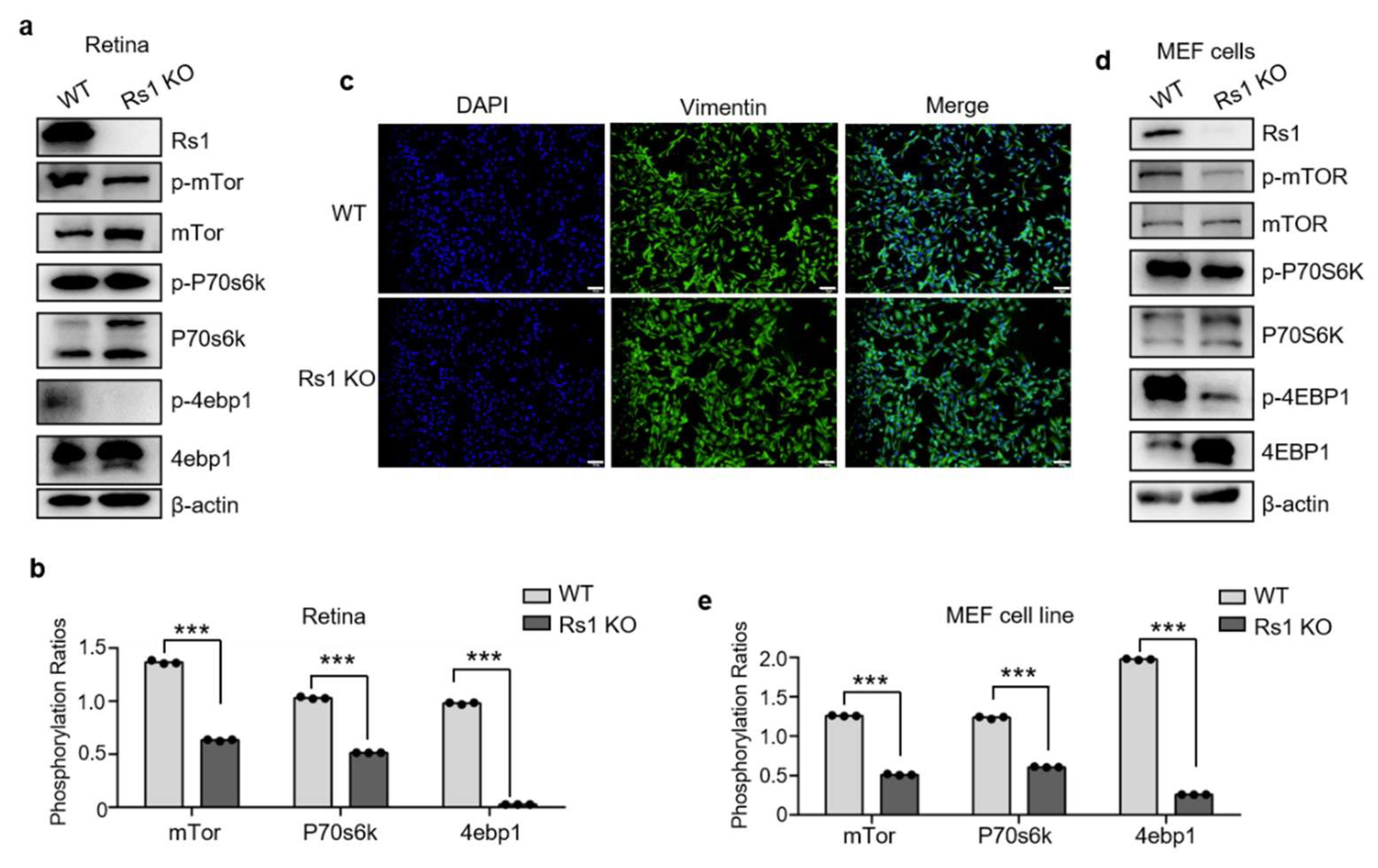
2.5. RS1 Deletion Itself Induces the Downregulation of Tor Pathway
2.6. Same and Contrasting DEPs at the Onset and Early Progression of XLRS
2.7. Functional Enrichment Analysis of the DEPs in XLRS Progression
2.8. Changes in Subcellular Location in XLRS Progression
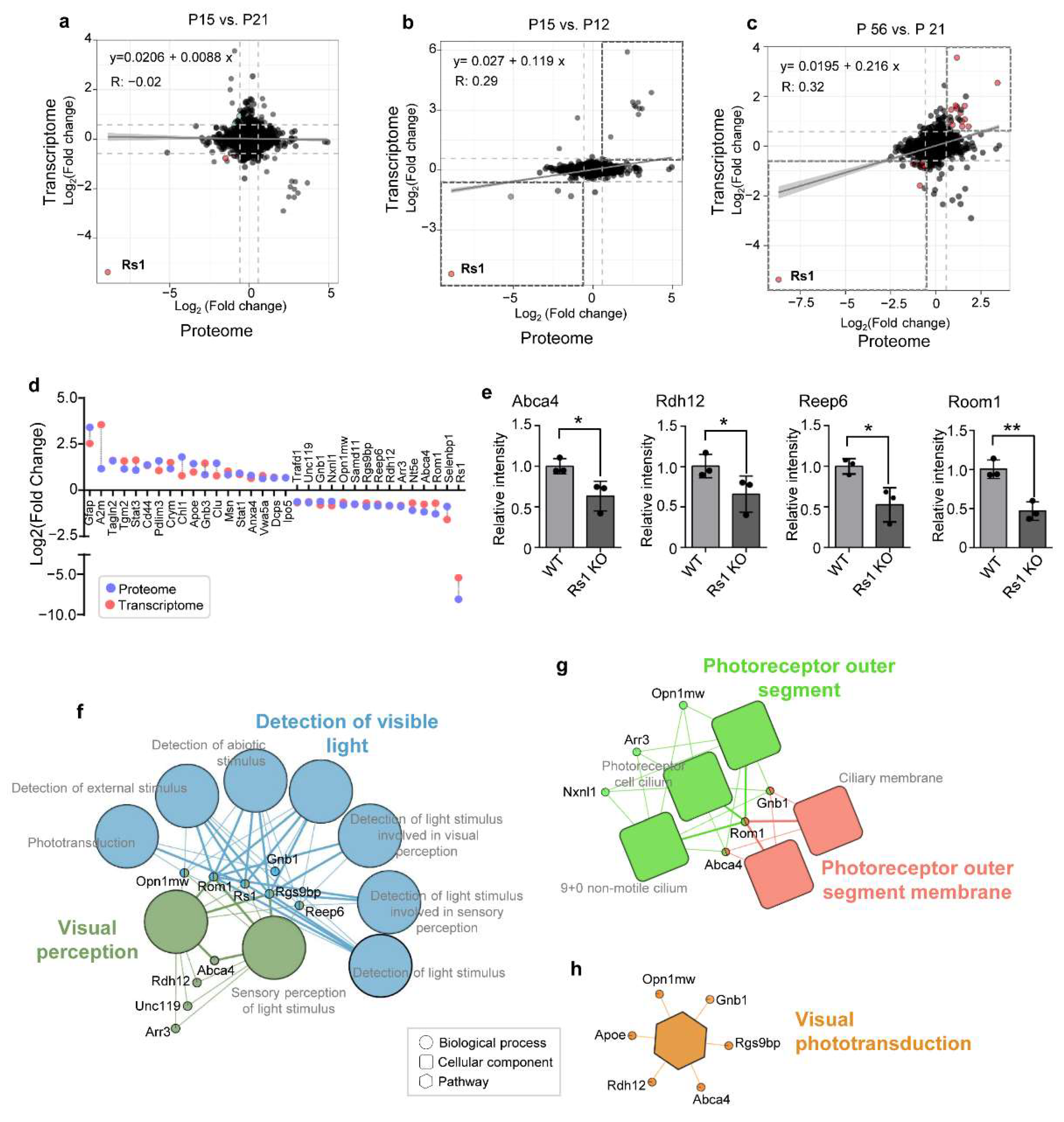
2.9. Correlation between the Proteome and Published Transcriptome Data in RS1-KO Retinas
3. Discussion
4. Materials and Methods
4.1. Experimental Mice
4.2. Histologic Analysis
4.3. ERG Assessment
4.4. Generation of MEF Cells
4.5. Real-Time PCR
4.6. Sample Preparation for Proteomic Analysis
4.7. LC–MS/MS Analysis
4.8. LC–MS/MS Data Processing
4.9. Bioinformatic and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vijayasarathy, C.; Pasha, S.P.B.S.; Sieving, P.A. Of men and mice: Human X-linked retinoschisis and fidelity in mouse modeling. Prog. Retin. Eye Res. 2021, 11, 100999. [Google Scholar] [CrossRef] [PubMed]
- Hahn, L.C.; van Schooneveld, M.J.; Wesseling, N.L.; Florijn, R.J.; Jacoline, B.; Lissenberg-Witte, B.I.; Strubbe, I.; Meester-Smoor, M.A.; Thiadens, A.A.; Diederen, R.M.; et al. X-linked retinoschisis: Novel clinical observations and genetic spectrum in 340 patients. Ophthalmology 2022, 129, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Sun, W.; Xiao, X.; Li, S.; Luo, H.; Jia, X.; Ouyang, J.; Li, X.; Wang, Y.; Jiang, Y.; et al. Clinical and genetic features of retinoschisis in 120 families with RS1 mutations. Br. J. Ophthalmol. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Kjellström, S.; Vijayasarathy, C.; Ponjavic, V.; Sieving, P.A.; Andréasson, S. Long-term 12 year follow-up of X-linked congenital retinoschisis. Ophthalmic Genet. 2010, 31, 114–125. [Google Scholar] [CrossRef]
- Wood, E.H.; Lertjirachai, I.; Ghiam, B.K.; Koulisis, N.; Moysidis, S.N.; Dirani, A.; Drenser, K.A.; Capone, A.; Trese, M.T. The natural history of congenital X-linked retinoschisis and conversion between phenotypes over time. Ophthalmol. Retin. 2019, 3, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Vijayasarathy, C.; Zeng, Y.; Brooks, M.J.; Fariss, R.N.; Sieving, P.A. Genetic rescue of X-linked retinoschisis mouse (Rs1(-/y)) retina induces quiescence of the retinal microglial inflammatory state following AAV8-RS1 gene transfer and identifies gene networks underlying retinal recovery. Hum. Gene Ther. 2021, 32, 667–681. [Google Scholar] [CrossRef]
- Cukras, C.; Wiley, H.E.; Jeffrey, B.G.; Sen, H.N.; Turriff, A.; Zeng, Y.; Vijayasarathy, C.; Marangoni, D.; Ziccardi, L.; Kjellstrom, S.; et al. Retinal AAV8-RS1 gene therapy for X-linked retinoschisis: Initial findings from a phase I/IIa trial by intravitreal delivery. Mol.Ther. J. Am. Soc. Gene Ther. 2018, 26, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Bush, R.A.; Zeng, Y.; Colosi, P.; Kjellstrom, S.; Hiriyanna, S.; Vijayasarathy, C.; Santos, M.; Li, J.; Wu, Z.; Sieving, P.A. Preclinical dose-escalation study of intravitreal aav-rs1 gene therapy in a mouse model of x-linked retinoschisis: Dose-dependent expression and improved retinal structure and function. Hum. Gene Ther. 2016, 27, 376–389. [Google Scholar] [CrossRef]
- Zeng, Y.; Qian, H.; Campos, M.M.; Li, Y.; Vijayasarathy, C.; Sieving, P.A. Rs1h(-/y) exon 3-del rat model of X-linked retinoschisis with early onset and rapid phenotype is rescued by RS1 supplementation. Gene Ther. 2021, 2021. [Google Scholar] [CrossRef]
- Sauer, C.G.; Gehrig, A.; Warneke-Wittstock, R.; Marquardt, A.; Ewing, C.C.; Gibson, A.; Lorenz, B.; Jurklies, B.; Weber, B.H. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat. Genet. 1997, 17, 164–170. [Google Scholar] [CrossRef]
- Heymann, J.B.; Vijayasarathy, C.; Huang, R.K.; Dearborn, A.D.; Sieving, P.A.; Steven, A.C. Cryo-EM of retinoschisin branched networks suggests an intercellular adhesive scaffold in the retina. J. Cell Biol. 2019, 218, 1027–1038. [Google Scholar] [CrossRef]
- Ramsay, E.P.; Collins, R.F.; Owens, T.W.; Siebert, C.A.; Jones, R.P.; Wang, T.; Roseman, A.M.; Baldock, C. Structural analysis of X-linked retinoschisis mutations reveals distinct classes which differentially effect retinoschisin function. Hum. Mol. Genet. 2016, 25, 5311–5320. [Google Scholar] [CrossRef][Green Version]
- Molday, L.L.; Hicks, D.; Sauer, C.G.; Weber, B.H.; Molday, R.S. Expression of X-linked retinoschisis protein RS1 in photoreceptor and bipolar cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 816–825. [Google Scholar]
- Reid, S.N.; Yamashita, C.; Farber, D.B. Retinoschisin, a photoreceptor-secreted protein, and its interaction with bipolar and muller cells. J. Neurosci. 2003, 23, 6030–6040. [Google Scholar] [CrossRef]
- De Silva, S.R.; Arno, G.; Robson, A.G.; Fakin, A.; Pontikos, N.; Mohamed, M.D.; Bird, A.C.; Moore, A.T.; Michaelides, M.; Webster, A.R.; et al. The X-linked retinopathies: Physiological insights, pathogenic mechanisms, phenotypic features and novel therapies. Prog. Retin. Eye Res. 2021, 82, 100898. [Google Scholar] [CrossRef]
- Tolun, G.; Vijayasarathy, C.; Huang, R.; Zeng, Y.; Li, Y.; Steven, A.C.; Sieving, P.A.; Heymann, J.B. Paired octamer rings of retinoschisin suggest a junctional model for cell-cell adhesion in the retina. Proc. Natl. Acad. Sci. USA 2016, 113, 5287–5292. [Google Scholar] [CrossRef]
- Liu, Y.; Kinoshita, J.; Ivanova, E.; Sun, D.; Li, H.; Liao, T.; Cao, J.; Bell, B.A.; Wang, J.M.; Tang, Y.; et al. Mouse models of X-linked juvenile retinoschisis have an early onset phenotype, the severity of which varies with genotype. Hum. Mol. Genet. 2019, 28, 3072–3090. [Google Scholar] [CrossRef]
- Chen, D.; Xu, T.; Tu, M.; Xu, J.; Zhou, C.; Cheng, L.; Yang, R.; Yang, T.; Zheng, W.; He, X.; et al. Recapitulating X-linked juvenile retinoschisis in mouse model by knock-in patient-specific novel mutation. Front. Mol. Neurosci. 2017, 10, 453. [Google Scholar] [CrossRef]
- Takada, Y.; Vijayasarathy, C.; Zeng, Y.; Kjellstrom, S.; Bush, R.A.; Sieving, P.A. Synaptic pathology in retinoschisis knockout (Rs1-/y) mouse retina and modification by rAAV-Rs1 gene delivery. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3677–3686. [Google Scholar] [CrossRef]
- Zeng, Y.; Takada, Y.; Kjellstrom, S.; Hiriyanna, K.; Tanikawa, A.; Wawrousek, E.; Smaoui, N.; Caruso, R.; Bush, R.A.; Sieving, P.A. RS-1 gene delivery to an adult Rs1h knockout mouse model restores ERG b-wave with reversal of the electronegative waveform of X-linked retinoschisis. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3279–3285. [Google Scholar] [CrossRef]
- Takada, Y.; Fariss, R.; Tanikawa, A.; Zeng, Y.; Carper, D.; Bush, R.; Sieving, P.A. A retinal neuronal developmental wave of retinoschisin expression begins in ganglion cells during layer formation. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3302–3312. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, M.M.; Dalke, C.; Wang, X.; Lu, L.; Manly, K.F.; Pretsch, W.; Favor, J.; Pardue, M.T.; Rinchik, E.M.; Williams, R.W.; et al. An ENU-induced mutation in Rs1h causes disruption of retinal structure and function. Mol. Vis. 2005, 11, 569–581. [Google Scholar] [PubMed]
- Johnson, B.A.; Ikeda, S.; Pinto, L.H.; Ikeda, A. Reduced synaptic vesicle density and aberrant synaptic localization caused by a splice site mutation in the Rs1h gene. Vis. Neurosci. 2006, 23, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, Y.; Li, J.; You, Y.; Liu, C.; Chen, K.; Li, S.; Lei, B. Phenotype heterogeneity and the association between visual acuity and outer retinal structure in a cohort of Chinese X-linked juvenile retinoschisis patients. Front. Genet. 2022, 322, 832814. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Wang, W.; Liu, G.; Jin, X.; Lei, B. Longitudinal photoreceptor phenotype observation and therapeutic evaluation of a carbonic anhydrase inhibitor in a mouse model of X-linked retinoschisis. Front. Med. 2022, 9, 886947. [Google Scholar] [CrossRef]
- Schmid, V.; Plossl, K.; Schmid, C.; Bernklau, S.; Weber, B.H.F.; Friedrich, U. Retinoschisin and cardiac glycoside crosstalk at the retinal Na/K-ATPase. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef]
- Plössl, K.; Royer, M.; Bernklau, S.; Tavraz, N.N.; Friedrich, T.; Wild, J.; Weber, B.H.; Friedrich, U. Retinoschisin is linked to retinal Na/K-ATPase signaling and localization. Mol. Biol. Cell 2017, 28, 2178–2189. [Google Scholar] [CrossRef]
- Gehrig, A.; Langmann, T.; Horling, F.; Janssen, A.; Bonin, M.; Walter, M.; Poths, S.; Weber, B.H.F. Genome-wide expression profiling of the retinoschisin-deficient retina in early postnatal mouse development. Investig. Ophthalmol. Vis. Sci. 2007, 48, 891–900. [Google Scholar] [CrossRef]
- Robson, J.G.; Frishman, L.J. The rod-driven a-wave of the dark-adapted mammalian electroretinogram. Prog. Retin. Eye Res. 2014, 39, 1–22. [Google Scholar] [CrossRef]
- Thomas, M.M.; Lamb, T.D. Light adaptation and dark adaptation of human rod photoreceptors measured from the a-wave of the electroretinogram. J. Physiol. 1999, 518, 479–496. [Google Scholar] [CrossRef]
- Stockton, R.A.; Slaughter, M.M. B-wave of the electroretinogram. A reflection of ON bipolar cell activity. J. Gen. Physiol. 1989, 93, 101–122. [Google Scholar] [CrossRef]
- Gurevich, L.; Slaughter, M.M. Comparison of the waveforms of the ON bipolar neuron and the b-wave of the electroretinogram. Vis. Res. 1993, 33, 2431–2435. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Subramanian, A.; Kuehn, H.; Gould, J.; Tamayo, P.; Mesirov, J.P. GSEA-P: A desktop application for Gene Set Enrichment Analysis. Bioinformatics 2007, 23, 3251–3253. [Google Scholar] [CrossRef]
- Štrbák, V. Cell volume and peptide hormone secretion. Mech. Significance Cell Vol. Regul. 2006, 152, 210–220. [Google Scholar]
- Bortner, C.D.; Cidlowski, J.A. Apoptotic volume decrease and the incredible shrinking cell. Cell Death Differ. 2002, 9, 1307–1310. [Google Scholar] [CrossRef]
- Lang, F.; Ritter, M.; Völkl, H.; Häussinger, D. The biological significance of cell volume. Ren. Physiol. Biochem. 1993, 16, 48–65. [Google Scholar] [CrossRef]
- Stefan, K.L.; Kim, M.V.; Iwasaki, A.; Kasper, D.L. Commensal microbiota modulation of natural resistance to virus infection. Cell 2020, 183, 1312–1324. [Google Scholar] [CrossRef]
- Snell, L.M.; McGaha, T.L.; Brooks, D.G. Type I interferon in chronic virus infection and cancer. Trends Immunol. 2017, 38, 542–557. [Google Scholar] [CrossRef]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef]
- Hung, C.M.; Garcia-Haro, L.; Sparks, C.A.; Guertin, D.A. mTOR-dependent cell survival mechanisms. Cold Spring Harb. Perspect. Biol. 2012, 4, 008771. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Popp, M.W.; Maquat, L.E. Quality and quantity control of gene expression by nonsense-mediated mRNA decay. Nat. Rev. Mol. Cell Biol. 2019, 20, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Lykke-Andersen, S.; Jensen, T.H. Nonsense-mediated mRNA decay: An intricate machinery that shapes transcriptomes. Nat. Rev. Mol. Cell Biol. 2015, 16, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Maquat, L.E. Nonsense-mediated mRNA decay in humans at a glance. J. Cell Sci. 2016, 129, 461–467. [Google Scholar] [CrossRef] [PubMed]
- El Masri, R.; Delon, J. RHO GTPases: From new partners to complex immune syndromes. Nat. Rev. Immunol. 2021, 21, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Chai, R.; Zhong, G.; Zhang, C.; Cao, W.; Yan, L.; Zhang, X.; Xu, Z. Hypoxia-regulated lncRNA CRPAT4 promotes cell migration via regulating AVL9 in clear cell renal cell carcinomas. OncoTargets Ther. 2018, 11, 4537–4545. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, J.; Zhou, Z. AVL9 promotes colorectal carcinoma cell migration via regulating EGFR expression. Biol. Proced. Online 2022, 24, 1. [Google Scholar] [CrossRef]
- Leshchyns’ka, I.; Sytnyk, V.; Richter, M.; Andreyeva, A.; Puchkov, D.; Schachner, M. The adhesion molecule CHL1 regulates uncoating of clathrin-coated synaptic vesicles. Neuron 2006, 52, 1011–1025. [Google Scholar] [CrossRef]
- Felemban, M.; Dorgau, B.; Hunt, N.C.; Hallam, D.; Zerti, D.; Bauer, R.; Ding, Y.; Collin, J.; Steel, D.; Krasnogor, N.; et al. Extracellular matrix component expression in human pluripotent stem cell-derived retinal organoids recapitulates retinogenesis in vivo and reveals an important role for IMPG1 and CD44 in the development of photoreceptors and interphotoreceptor matrix. Acta Biomater. 2018, 74, 207–221. [Google Scholar] [CrossRef]
- Xu, H.; Qu, C.; Gan, L.; Sun, K.; Tan, J.; Liu, X.; Jiang, Z.; Tian, W.; Liu, W.; Zhang, S.; et al. Deletion of the Impg2 gene causes the degeneration of rod and cone cells in mice. Hum. Mol. Genet. 2020, 29, 1624–1634. [Google Scholar] [CrossRef]
- Bandah-Rozenfeld, D.; Collin, R.W.; Banin, E.; Born, L.I.V.D.; Coene, K.; Siemiatkowska, A.M.; Zelinger, L.; Khan, M.I.; Lefeber, D.J.; Erdinest, I.; et al. Mutations in IMPG2, encoding interphotoreceptor matrix proteoglycan 2, cause autosomal-recessive retinitis pigmentosa. Am. J. Hum. Genet. 2010, 87, 199–208. [Google Scholar] [CrossRef]
- Ou, J.; Vijayasarathy, C.; Ziccardi, L.; Chen, S.; Zeng, Y.; Marangoni, D.; Pope, J.G.; Bush, R.A.; Wu, Z.; Li, W.; et al. Synaptic pathology and therapeutic repair in adult retinoschisis mouse by AAV-RS1 transfer. J. Clin. Investig. 2015, 125, 2891–2903. [Google Scholar] [CrossRef]
- Molday, R.S.; Kellner, U.; Weber, B.H. X-linked juvenile retinoschisis: Clinical diagnosis, genetic analysis, and molecular mechanisms. Prog. Retin. Eye Res. 2012, 31, 195–212. [Google Scholar] [CrossRef]
- Yeo, W.; Gautier, J. Early neural cell death: Dying to become neurons. Dev. Biol. 2004, 274, 233–244. [Google Scholar] [CrossRef]
- Jin, X.; Liu, J.; Wang, W.; Li, J.; Liu, G.; Qiu, R.; Yang, M.; Liu, M.; Yang, L.; Du, X.; et al. Identification of age-associated proteins and functional alterations in human retinal pigment epithelium. Genom. Proteom. Bioinform. 2022, 35752290. [Google Scholar] [CrossRef] [PubMed]
- Kole, A.J.; Annis, R.P.; Deshmukh, M. Mature neurons: Equipped for survival. Cell Death Dis. 2013, 4, 689. [Google Scholar] [CrossRef]
- Sun, Z.; Wei, W.; Liu, H.; Ma, J.; Hu, M.; Huang, H. Acute response of neurons: An early event of neuronal cell death after facial nerve injury. World Neurosurg. 2018, 109, 252–257. [Google Scholar] [CrossRef]
- Nguyen-Ba-Charvet, K.T.; Chédotal, A. Development of retinal layers. Comptes Rendus Biol. 2014, 337, 153–159. [Google Scholar] [CrossRef]
- Care, R.A.; Anastassov, I.A.; Kastner, D.B.; Kuo, Y.M.; Della Santina, L.; Dunn, F.A. Mature retina compensates functionally for partial loss of rod photoreceptors. Cell Rep. 2020, 31, 107730. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, H.; Chen, Z.; Zheng, C.; Lei, C.; Lei, B. Activation of liver X receptor protects inner retinal damage induced by N-methyl-D-aspartate. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1168–1180. [Google Scholar] [CrossRef]
- Yang, M.; Qiu, R.; Wang, W.; Liu, J.; Jin, X.; Li, Y.; Li, L.; Lei, B. P2X7 receptor antagonist attenuates retinal inflammation and neovascularization induced by oxidized low-density lipoprotein. Oxidative Med. Cell. Longev. 2021, 2021, 5520644. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Zhang, X.; Liu, J.; Wang, W.; Liu, M.; Yang, L.; Liu, G.; Qiu, R.; Yang, M.; Yao, S.; et al. Retinal Proteomic Alterations and Combined Transcriptomic-Proteomic Analysis in the Early Stages of Progression of a Mouse Model of X-Linked Retinoschisis. Cells 2022, 11, 2150. https://doi.org/10.3390/cells11142150
Jin X, Zhang X, Liu J, Wang W, Liu M, Yang L, Liu G, Qiu R, Yang M, Yao S, et al. Retinal Proteomic Alterations and Combined Transcriptomic-Proteomic Analysis in the Early Stages of Progression of a Mouse Model of X-Linked Retinoschisis. Cells. 2022; 11(14):2150. https://doi.org/10.3390/cells11142150
Chicago/Turabian StyleJin, Xiuxiu, Xiaoli Zhang, Jingyang Liu, Weiping Wang, Meng Liu, Lin Yang, Guangming Liu, Ruiqi Qiu, Mingzhu Yang, Shun Yao, and et al. 2022. "Retinal Proteomic Alterations and Combined Transcriptomic-Proteomic Analysis in the Early Stages of Progression of a Mouse Model of X-Linked Retinoschisis" Cells 11, no. 14: 2150. https://doi.org/10.3390/cells11142150
APA StyleJin, X., Zhang, X., Liu, J., Wang, W., Liu, M., Yang, L., Liu, G., Qiu, R., Yang, M., Yao, S., & Lei, B. (2022). Retinal Proteomic Alterations and Combined Transcriptomic-Proteomic Analysis in the Early Stages of Progression of a Mouse Model of X-Linked Retinoschisis. Cells, 11(14), 2150. https://doi.org/10.3390/cells11142150






