Capturing Transitional Pluripotency through Proline Metabolism
Abstract
:1. Introduction
2. Proline Levels Influence Pluripotency
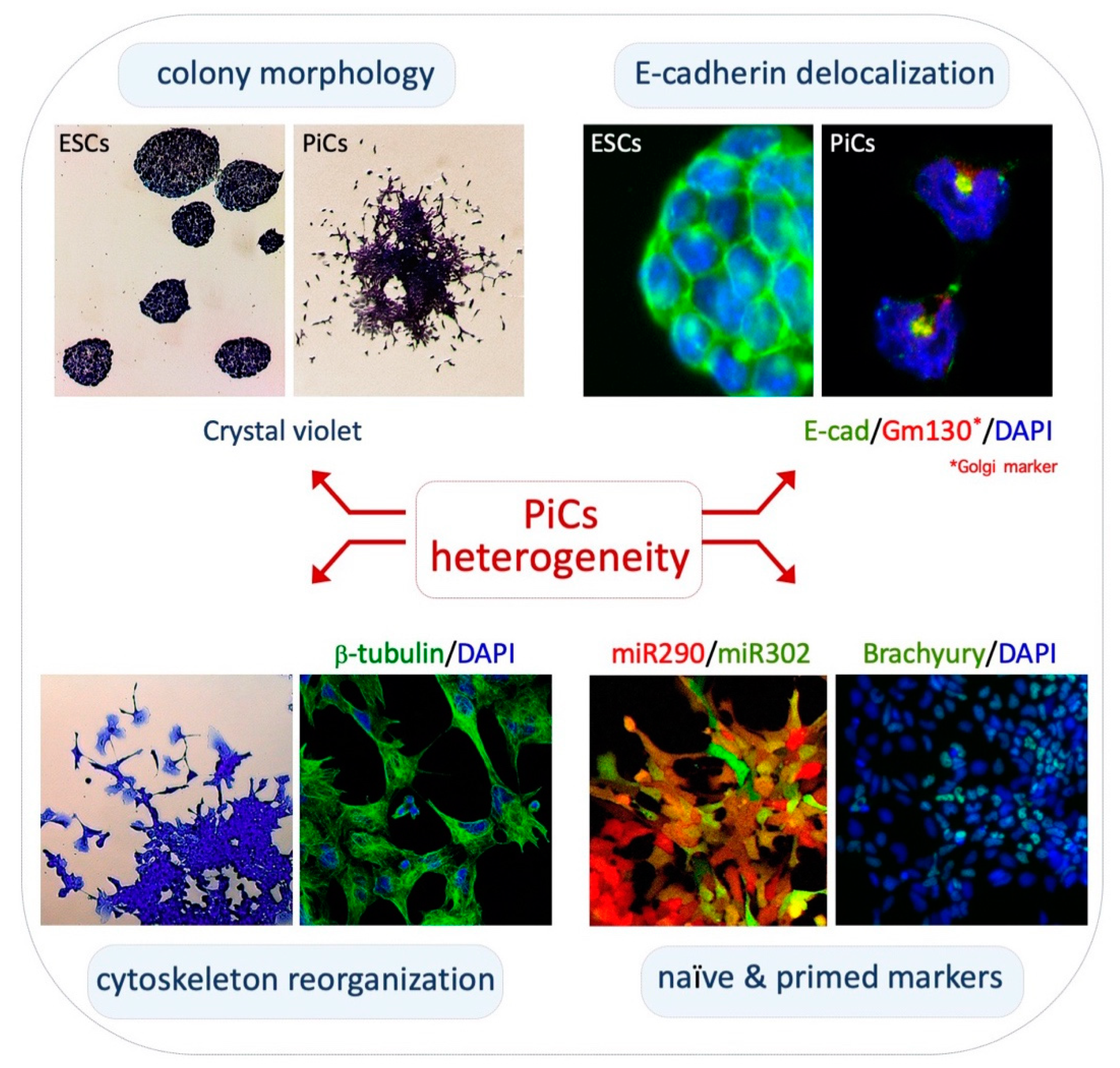
2.1. Phenotypic Heterogeneity and Metastability of PiCs
2.2. Transcriptomic and Epigenomic Landscapes of PiCs
2.3. Amino Acid Starvation Stress and Energy Metabolism in the ESC-to-PiC Transition
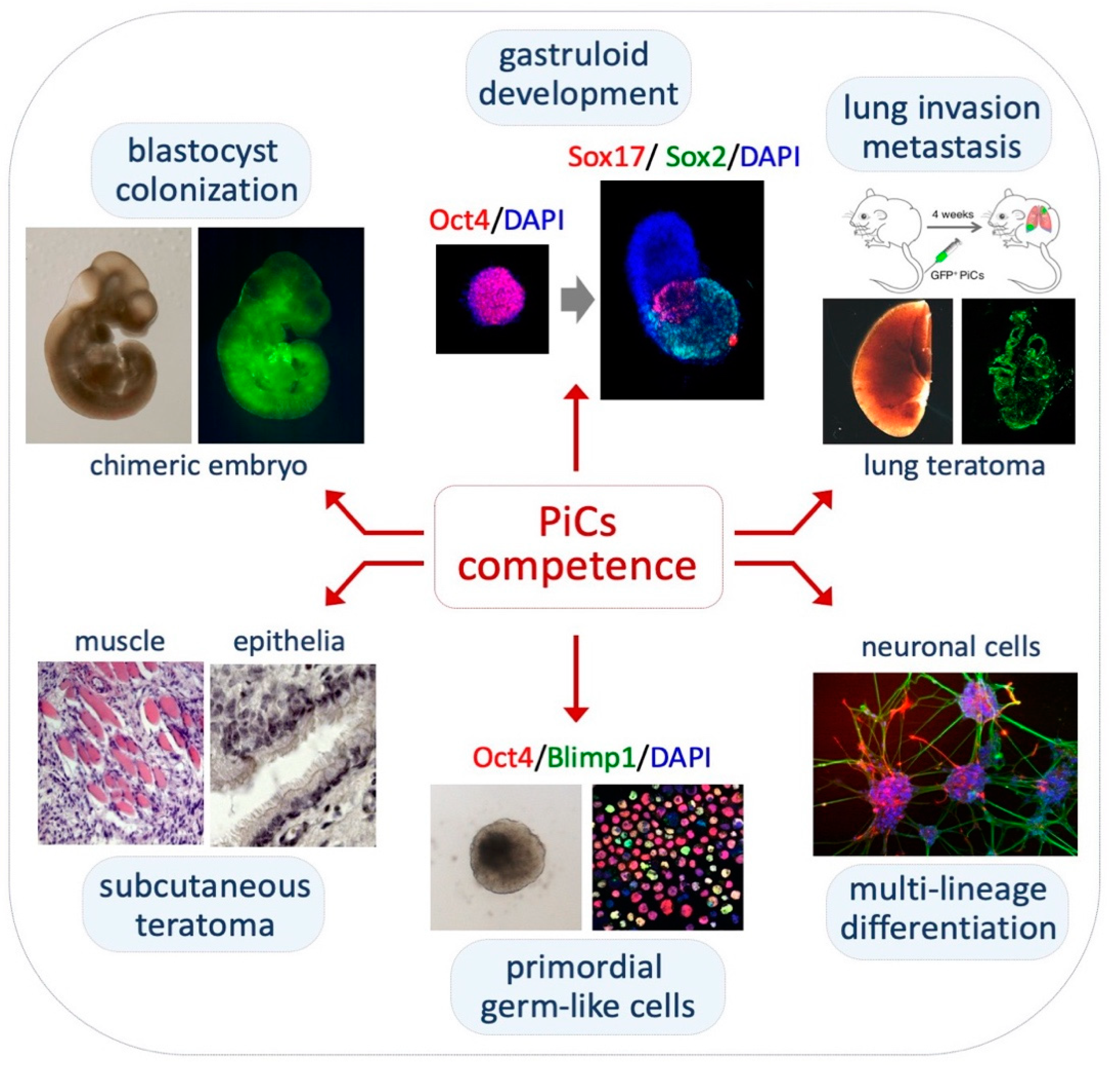
2.4. Pluripotency Features of PiCs
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAR | amino acid response |
| A–P | anterior–posterior |
| Atf4 | stress-activated transcription factor |
| BMP | bone morphogenetic protein |
| Cyr61 | cysteine-rich angiogenic inducer 61 |
| DEGs | differentially expressed genes |
| DMRs | differentially methylated regions |
| EBs | embryoid bodies |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| eGFP | enhanced green fluorescent protein |
| EpiSCs | epiblast-like cells |
| EPL | primitive ectoderm-like cells |
| ER | endoplasmic reticulum |
| ESC | embryonic stem cell |
| esMT | embryonic stem cell-to-mesenchymal-like transition |
| F/A | fibroblast growth factor plus Activin A |
| GFAP | glial fibrillary acidic protein |
| GSH | reduced glutathione |
| ICM | inner cell mass |
| Lif | leukemia inhibitory factor |
| α-MHC | myosin heavy chain |
| 5mC | 5-methylcytosine, 5hmC, 5-hydroxy-methylcytosine |
| MesT | mesenchymal-to-embryonic stem transition |
| PGCLCs | primordial germ cell-like cells |
| PRS | prolyl-tRNA synthetase |
| PSC | pluripotent stem cell |
| RISC | RNA-induced silencing complex |
| SDF-1 | stromal cell-derived factor-1 |
References
- Dundes, C.E.; Loh, K.M. Bridging naive and primed pluripotency. Nat. Cell Biol. 2020, 22, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Smith, A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neagu, A.; van Genderen, E.; Escudero, I.; Verwegen, L.; Kurek, D.; Lehmann, J.; Stel, J.; Dirks, R.A.M.; van Mierlo, G.; Maas, A.; et al. In Vitro capture and characterization of embryonic rosette-stage pluripotency between naive and primed states. Nat. Cell Biol. 2020, 22, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.L.; Wray, J.; Nichols, J.; Batlle-Morera, L.; Doble, B.; Woodgett, J.; Cohen, P.; Smith, A. The ground state of embryonic stem cell self-renewal. Nature 2008, 453, 519–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brons, I.G.; Smithers, L.E.; Trotter, M.W.; Rugg-Gunn, P.; Sun, B.; Chuva de Sousa Lopes, S.M.; Howlett, S.K.; Clarkson, A.; Ahrlund-Richter, L.; Pedersen, R.A.; et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 2007, 448, 191–195. [Google Scholar] [CrossRef]
- Tesar, P.J.; Chenoweth, J.G.; Brook, F.A.; Davies, T.J.; Evans, E.P.; Mack, D.L.; Gardner, R.L.; McKay, R.D. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 2007, 448, 196–199. [Google Scholar] [CrossRef]
- Smith, A. Formative pluripotency: The executive phase in a developmental continuum. Development 2017, 144, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Morgani, S.; Nichols, J.; Hadjantonakis, A.K. The many faces of Pluripotency: In vitro adaptations of a continuum of in vivo states. BMC Dev. Biol. 2017, 17, 7. [Google Scholar] [CrossRef] [Green Version]
- Pera, M.F.; Rossant, J. The exploration of pluripotency space: Charting cell state transitions in peri-implantation development. Cell Stem Cell 2021, 28, 1896–1906. [Google Scholar] [CrossRef]
- Kinoshita, M.; Smith, A. Pluripotency Deconstructed. Dev. Growth Differ. 2018, 60, 44–52. [Google Scholar] [CrossRef]
- Lackner, A.; Sehlke, R.; Garmhausen, M.; Giuseppe Stirparo, G.; Huth, M.; Titz-Teixeira, F.; van der Lelij, P.; Ramesmayer, J.; Thomas, H.F.; Ralser, M.; et al. Cooperative genetic networks drive embryonic stem cell transition from naive to formative pluripotency. EMBO J. 2021, 40, e105776. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, T.; Olova, N.; Roode, M.; Mulas, C.; Lee, H.J.; Nett, I.; Marks, H.; Walker, R.; Stunnenberg, H.G.; Lilley, K.S.; et al. Tracking the embryonic stem cell transition from ground state pluripotency. Development 2017, 144, 1221–1234. [Google Scholar] [CrossRef] [Green Version]
- Ohinata, Y.; Ohta, H.; Shigeta, M.; Yamanaka, K.; Wakayama, T.; Saitou, M. A signaling principle for the specification of the germ cell lineage in mice. Cell 2009, 137, 571–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitou, M. Mammalian Germ Cell Development: From Mechanism to In Vitro Reconstitution. Stem Cell Rep. 2021, 16, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiang, Y.; Yu, Y.; Wang, R.; Zhang, Y.; Xu, Q.; Sun, H.; Zhao, Z.A.; Jiang, X.; Wang, X.; et al. Formative pluripotent stem cells show features of epiblast cells poised for gastrulation. Cell Res. 2021, 31, 526–541. [Google Scholar] [CrossRef]
- Kinoshita, M.; Barber, M.; Mansfield, W.; Cui, Y.; Spindlow, D.; Stirparo, G.G.; Dietmann, S.; Nichols, J.; Smith, A. Capture of Mouse and Human Stem Cells with Features of Formative Pluripotency. Cell Stem Cell 2021, 28, 453–471. [Google Scholar] [CrossRef]
- Yu, L.; Wei, Y.; Sun, H.X.; Mahdi, A.K.; Pinzon Arteaga, C.A.; Sakurai, M.; Schmitz, D.A.; Zheng, C.; Ballard, E.D.; Li, J.; et al. Derivation of Intermediate Pluripotent Stem Cells Amenable to Primordial Germ Cell Specification. Cell Stem Cell 2021, 28, 550–567. [Google Scholar] [CrossRef]
- Han, D.W.; Tapia, N.; Joo, J.Y.; Greber, B.; Arauzo-Bravo, M.J.; Bernemann, C.; Ko, K.; Wu, G.; Stehling, M.; Do, J.T.; et al. Epiblast stem cell subpopulations represent mouse embryos of distinct pregastrulation stages. Cell 2010, 143, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.H.; Li, M. Clonal isolation of an intermediate pluripotent stem cell state. Stem Cells 2013, 31, 918–927. [Google Scholar] [CrossRef]
- Wei, M.; Chen, Y.; Zhao, C.; Zheng, L.; Wu, B.; Chen, C.; Li, X.; Bao, S. Establishment of Mouse Primed Stem Cells by Combination of Activin and LIF Signaling. Front. Cell Dev. Biol. 2021, 9, 713503. [Google Scholar] [CrossRef]
- Tsukiyama, T.; Ohinata, Y. A modified EpiSC culture condition containing a GSK3 inhibitor can support germline-competent pluripotency in mice. PLoS ONE 2014, 9, e95329. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Tang, W.W.; Wu, B.; Kim, S.; Li, J.; Li, L.; Kobayashi, T.; Lee, C.; Chen, Y.; Wei, M.; et al. Derivation of hypermethylated pluripotent embryonic stem cells with high potency. Cell Res. 2018, 28, 22–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, P.; Pirouz, M.; Choi, J.; Huebner, A.J.; Clement, K.; Meissner, A.; Hochedlinger, K.; Gregory, R.I. An Intermediate Pluripotent State Controlled by MicroRNAs is Required for the Naive-to-Primed Stem Cell Transition. Cell Stem Cell 2018, 22, 851–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornacchia, D.; Zhang, C.; Zimmer, B.; Chung, S.Y.; Fan, Y.; Soliman, M.A.; Tchieu, J.; Chambers, S.M.; Shah, H.; Paull, D.; et al. Lipid Deprivation Induces a Stable, Naive-to-Primed Intermediate State of Pluripotency in Human PSCs. Cell Stem Cell 2019, 25, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Choi, M.; Margineantu, D.; Margaretha, L.; Hesson, J.; Cavanaugh, C.; Blau, C.A.; Horwitz, M.S.; Hockenbery, D.; Ware, C.; et al. HIF1alpha induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J. 2012, 31, 2103–2116. [Google Scholar] [CrossRef]
- Fiorenzano, A.; Pascale, E.; D’Aniello, C.; Acampora, D.; Bassalert, C.; Russo, F.; Andolfi, G.; Biffoni, M.; Francescangeli, F.; Zeuner, A.; et al. Cripto is essential to capture mouse epiblast stem cell and human embryonic stem cell pluripotency. Nat. Commun. 2016, 7, 12589. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, C.; Cermola, F.; Patriarca, E.J.; Minchiotti, G. Metabolic-Epigenetic Axis in Pluripotent State Transitions. Epigenomes 2019, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Reid, M.A.; Dai, Z.; Locasale, J.W. The impact of cellular metabolism on chromatin dynamics and epigenetics. Nat. Cell Biol. 2017, 19, 1298–1306. [Google Scholar] [CrossRef]
- Ryall, J.G.; Cliff, T.; Dalton, S.; Sartorelli, V. Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell 2015, 17, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Alexander, P.; Wu, L.; Hammer, R.; Cleaver, O.; McKnight, S.L. Dependence of mouse embryonic stem cells on threonine catabolism. Science 2009, 325, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Shiraki, N.; Shiraki, Y.; Tsuyama, T.; Obata, F.; Miura, M.; Nagae, G.; Aburatani, H.; Kume, K.; Endo, F.; Kume, S. Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab. 2014, 19, 780–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathjen, J.; Lake, J.A.; Bettess, M.D.; Washington, J.M.; Chapman, G.; Rathjen, P.D. Formation of a primitive ectoderm like cell population, EPL cells, from ES cells in response to biologically derived factors. J. Cell Sci. 1999, 112, 601–612. [Google Scholar] [CrossRef]
- Washington, J.M.; Rathjen, J.; Felquer, F.; Lonic, A.; Bettess, M.D.; Hamra, N.; Semendric, L.; Tan, B.S.; Lake, J.A.; Keough, R.A.; et al. L-Proline induces differentiation of ES cells: A novel role for an amino acid in the regulation of pluripotent cells in culture. Am. J. Physiol. Cell Physiol. 2010, 298, C982–C992. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.S.; Lonic, A.; Morris, M.B.; Rathjen, P.D.; Rathjen, J. The amino acid transporter SNAT2 mediates L-proline-induced differentiation of ES cells. Am. J. Physiol. Cell Physiol. 2011, 300, C1270–C1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lake, J.; Rathjen, J.; Remiszewski, J.; Rathjen, P.D. Reversible programming of pluripotent cell differentiation. J. Cell Sci. 2000, 113, 555–566. [Google Scholar] [CrossRef]
- Stead, E.; White, J.; Faast, R.; Conn, S.; Goldstone, S.; Rathjen, J.; Dhingra, U.; Rathjen, P.; Walker, D.; Dalton, S. Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene 2002, 21, 8320–8333. [Google Scholar] [CrossRef] [Green Version]
- Casalino, L.; Comes, S.; Lambazzi, G.; De Stefano, B.; Filosa, S.; De Falco, S.; De Cesare, D.; Minchiotti, G.; Patriarca, E.J. Control of embryonic stem cell metastability by L-proline catabolism. J. Mol. Cell Biol. 2011, 3, 108–122. [Google Scholar] [CrossRef] [Green Version]
- Glover, H.J.; Shparberg, R.A.; Morris, M.B. L-Proline Supplementation Drives Self-Renewing Mouse Embryonic Stem Cells to a Partially Primed Pluripotent State: The Early Primitive Ectoderm-Like Cell. Methods Mol. Biol. 2022, 2490, 11–24. [Google Scholar]
- Comes, S.; Gagliardi, M.; Laprano, N.; Fico, A.; Cimmino, A.; Palamidessi, A.; De Cesare, D.; De Falco, S.; Angelini, C.; Scita, G.; et al. L-Proline induces a mesenchymal-like invasive program in embryonic stem cells by remodeling H3K9 and H3K36 methylation. Stem Cell Rep. 2013, 1, 307–321. [Google Scholar] [CrossRef] [Green Version]
- Cermola, F.; D’Aniello, C.; Tate, R.; De Cesare, D.; Martinez-Arias, A.; Minchiotti, G.; Patriarca, E.J. Gastruloid Development Competence Discriminates Different States of Pluripotency. Stem Cell Rep. 2021, 16, 354–369. [Google Scholar] [CrossRef]
- D’Aniello, C.; Habibi, E.; Cermola, F.; Paris, D.; Russo, F.; Fiorenzano, A.; Di Napoli, G.; Melck, D.J.; Cobellis, G.; Angelini, C.; et al. Vitamin C and l-Proline Antagonistic Effects Capture Alternative States in the Pluripotency Continuum. Stem Cell Rep. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Aniello, C.; Fico, A.; Casalino, L.; Guardiola, O.; Di Napoli, G.; Cermola, F.; De Cesare, D.; Tate, R.; Cobellis, G.; Patriarca, E.J.; et al. A novel autoregulatory loop between the Gcn2-Atf4 pathway and (L)-Proline [corrected] metabolism controls stem cell identity. Cell Death Differ. 2015, 22, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Fico, A.; Fiorenzano, A.; Pascale, E.; Patriarca, E.J.; Minchiotti, G. Long non-coding RNA in stem cell pluripotency and lineage commitment: Functions and evolutionary conservation. Cell Mol. Life Sci. 2019, 76, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Fiorenzano, A.; Pascale, E.; Patriarca, E.J.; Minchiotti, G.; Fico, A. LncRNAs and PRC2: Coupled Partners in Embryonic Stem Cells. Epigenomes 2019, 3, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorenzano, A.; Pascale, E.; Gagliardi, M.; Terreri, S.; Papa, M.; Andolfi, G.; Galasso, M.; Tagliazucchi, G.M.; Taccioli, C.; Patriarca, E.J.; et al. An Ultraconserved Element Containing lncRNA Preserves Transcriptional Dynamics and Maintains ESC Self-Renewal. Stem Cell Rep. 2018, 10, 1102–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascale, E.; Beclin, C.; Fiorenzano, A.; Andolfi, G.; Erni, A.; De Falco, S.; Minchiotti, G.; Cremer, H.; Fico, A. Long Non-coding RNA T-UCstem1 Controls Progenitor Proliferation and Neurogenesis in the Postnatal Mouse Olfactory Bulb through Interaction with miR-9. Stem Cell Rep. 2020, 15, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Parchem, R.J.; Ye, J.; Judson, R.L.; LaRussa, M.F.; Krishnakumar, R.; Blelloch, A.; Oldham, M.C.; Blelloch, R. Two miRNA clusters reveal alternative paths in late-stage reprogramming. Cell Stem Cell 2014, 14, 617–631. [Google Scholar] [CrossRef] [Green Version]
- Habibi, E.; Stunnenberg, H.G. Transcriptional and epigenetic control in mouse pluripotency: Lessons from in vivo and in vitro studies. Curr. Opin. Genet. Dev. 2017, 46, 114–122. [Google Scholar] [CrossRef]
- Lu, F.; Liu, Y.; Jiang, L.; Yamaguchi, S.; Zhang, Y. Role of Tet proteins in enhancer activity and telomere elongation. Genes Dev. 2014, 28, 2103–2119. [Google Scholar] [CrossRef] [Green Version]
- Pandolfini, L.; Luzi, E.; Bressan, D.; Ucciferri, N.; Bertacchi, M.; Brandi, R.; Rocchiccioli, S.; D’Onofrio, M.; Cremisi, F. RISC-mediated control of selected chromatin regulators stabilizes ground state pluripotency of mouse embryonic stem cells. Genome Biol. 2016, 17, 94. [Google Scholar] [CrossRef] [Green Version]
- Patriarca, E.J.; Cermola, F.; D’Aniello, C.; Fico, A.; Guardiola, O.; De Cesare, D.; Minchiotti, G. The Multifaceted Roles of Proline in Cell Behavior. Front. Cell Dev. Biol. 2021, 9, 2236. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, C.; Patriarca, E.J.; Phang, J.M.; Minchiotti, G. Proline Metabolism in Tumor Growth and Metastatic Progression. Front. Oncol. 2020, 10, 776. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.G.K.; Biasibetti-Brendler, H.; Sidegum, D.S.V.; Loureiro, S.O.; Figueiro, F.; Wyse, A.T.S. Effect of Proline on Cell Death, Cell Cycle, and Oxidative Stress in C6 Glioma Cell Line. Neurotox. Res. 2021, 39, 327–334. [Google Scholar] [CrossRef]
- Phang, J.M.; Donald, S.P.; Pandhare, J.; Liu, Y. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids 2008, 35, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, T.L.; Zocco, D.; Sundrud, M.S.; Hendrick, M.; Edenius, M.; Yum, J.; Kim, Y.J.; Lee, H.K.; Cortese, J.F.; Wirth, D.F.; et al. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat. Chem. Biol. 2012, 8, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Aniello, C.; Cermola, F.; Palamidessi, A.; Wanderlingh, L.G.; Gagliardi, M.; Migliaccio, A.; Varrone, F.; Casalino, L.; Matarazzo, M.R.; De Cesare, D.; et al. Collagen Prolyl Hydroxylation-Dependent Metabolic Perturbation Governs Epigenetic Remodeling and Mesenchymal Transition in Pluripotent and Cancer Cells. Cancer Res. 2019, 79, 3235–3250. [Google Scholar] [CrossRef] [Green Version]
- Fico, A.; Manganelli, G.; Simeone, M.; Guido, S.; Minchiotti, G.; Filosa, S. High-throughput screening-compatible single-step protocol to differentiate embryonic stem cells in neurons. Stem Cells Dev. 2008, 17, 573–584. [Google Scholar] [CrossRef]
- Casalino, L.; Magnani, D.; De Falco, S.; Filosa, S.; Minchiotti, G.; Patriarca, E.J.; De Cesare, D. An automated high throughput screening-compatible assay to identify regulators of stem cell neural differentiation. Mol. Biotechnol. 2012, 50, 171–180. [Google Scholar] [CrossRef]
- Arias, A.M.; Marikawa, Y.; Moris, N. Gastruloids: Pluripotent stem cell models of mammalian gastrulation and embryo engineering. Dev. Biol. 2022, 488, 35–46. [Google Scholar] [CrossRef]
- Hayashi, K.; Saitou, M. Generation of eggs from mouse embryonic stem cells and induced pluripotent stem cells. Nat. Protoc. 2013, 8, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, C.; Yamanaka, Y.; Pawson, T.; Rossant, J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev. Cell 2006, 10, 615–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagida, A.; Corujo-Simon, E.; Revell, C.K.; Sahu, P.; Stirparo, G.G.; Aspalter, I.M.; Winkel, A.K.; Peters, R.; De Belly, H.; Cassani, D.A.D.; et al. Cell surface fluctuations regulate early embryonic lineage sorting. Cell 2022, 185, 777–793. [Google Scholar] [CrossRef]
- Allegre, N.; Chauveau, S.; Dennis, C.; Renaud, Y.; Meistermann, D.; Estrella, L.V.; Pouchin, P.; Cohen-Tannoudji, M.; David, L.; Chazaud, C. NANOG initiates epiblast fate through the coordination of pluripotency genes expression. Nat. Commun. 2022, 13, 3550. [Google Scholar] [CrossRef]
- Guallar, D.; Wang, J. Taking the RISC of exiting naive pluripotency. Genome Biol. 2016, 17, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mali, P.; Chou, B.K.; Yen, J.; Ye, Z.; Zou, J.; Dowey, S.; Brodsky, R.A.; Ohm, J.E.; Yu, W.; Baylin, S.B.; et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells 2010, 28, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Shyh-Chang, N.; Locasale, J.W.; Lyssiotis, C.A.; Zheng, Y.; Teo, R.Y.; Ratanasirintrawoot, S.; Zhang, J.; Onder, T.; Unternaehrer, J.J.; Zhu, H.; et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 2013, 339, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Esteban, M.A.; Wang, T.; Qin, B.; Yang, J.; Qin, D.; Cai, J.; Li, W.; Weng, Z.; Chen, J.; Ni, S.; et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 2010, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Loh, Y.H.; Zhang, W.; Chen, X.; George, J.; Ng, H.H. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 2007, 21, 2545–2557. [Google Scholar] [CrossRef] [Green Version]
- Blaschke, K.; Ebata, K.T.; Karimi, M.M.; Zepeda-Martinez, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Hirst, M.; Rao, A.; et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013, 500, 222–226. [Google Scholar] [CrossRef]
- Phang, J.M. Perspectives, past, present and future: The proline cycle/proline-collagen regulatory axis. Amino Acids 2021, 53, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Cermola, F.; Patriarca, E.J.; Minchiotti, G. Generation of Gastruloids from Epiblast-Like Cells. Methods Mol. Biol. 2022, 2490, 197–204. [Google Scholar] [PubMed]
- Romero, J.J.; De Rossi, M.C.; Oses, C.; Echegaray, C.V.; Verneri, P.; Francia, M.; Guberman, A.; Levi, V. Nucleus-cytoskeleton communication impacts on OCT4-chromatin interactions in embryonic stem cells. BMC Biol. 2022, 20, 6. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minchiotti, G.; D’Aniello, C.; Fico, A.; De Cesare, D.; Patriarca, E.J. Capturing Transitional Pluripotency through Proline Metabolism. Cells 2022, 11, 2125. https://doi.org/10.3390/cells11142125
Minchiotti G, D’Aniello C, Fico A, De Cesare D, Patriarca EJ. Capturing Transitional Pluripotency through Proline Metabolism. Cells. 2022; 11(14):2125. https://doi.org/10.3390/cells11142125
Chicago/Turabian StyleMinchiotti, Gabriella, Cristina D’Aniello, Annalisa Fico, Dario De Cesare, and Eduardo Jorge Patriarca. 2022. "Capturing Transitional Pluripotency through Proline Metabolism" Cells 11, no. 14: 2125. https://doi.org/10.3390/cells11142125
APA StyleMinchiotti, G., D’Aniello, C., Fico, A., De Cesare, D., & Patriarca, E. J. (2022). Capturing Transitional Pluripotency through Proline Metabolism. Cells, 11(14), 2125. https://doi.org/10.3390/cells11142125







