Deconstructing Sox2 Function in Brain Development and Disease
Abstract
1. Introduction
2. SOX2 Function in Different Brain Regions
2.1. Telencephalon
2.1.1. Hippocampus
2.1.2. Medial Ganglionic Eminence (MGE)
2.2. Diencephalon
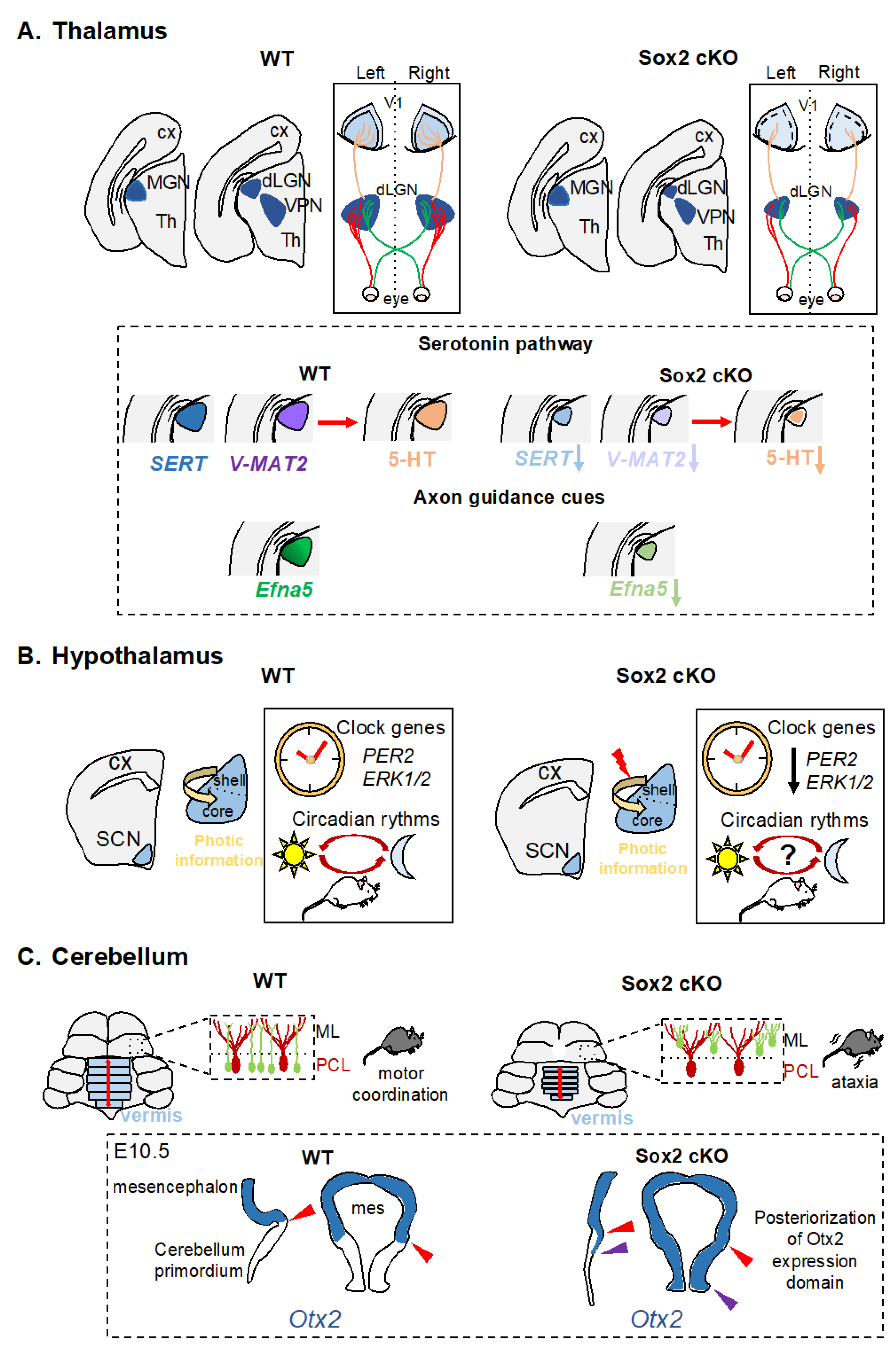
2.2.1. Thalamus
2.2.2. Hypothalamus
2.3. Cerebellum
3. Sox2 in Neural Stem Cells
4. Specific Partnerships as a Basis for SOX2 Cell-Type-Specific Functions in the CNS?
5. SOX2 Dysfunction in Human Disease: Insights from Mouse Models
6. A “Dark Side” for SOX2 Function in Neural Disease: Maintenance of Tumor-Initiating Cells in Gliomas
7. Conclusions and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wegner, M. All purpose Sox: The many roles of Sox proteins in gene expression. Int. J. Biochem. Cell Biol. 2010, 42, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Avilion, A.A.; Nicolis, S.K.; Pevny, L.H.; Perez, L.; Vivian, N.; Lovell-Badge, R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003, 17, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, S.; Serra, L.; Nicolis, S.K. More than just Stem Cells: Functional Roles of the Transcription Factor Sox2 in Differentiated Glia and Neurons. Int. J. Mol. Sci. 2019, 20, 4540. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 2016, 17, 183–193. [Google Scholar] [CrossRef]
- Elliott, K.; Pavlínková, G.; Chizhikov, V.; Yamoah, E.; Fritzsch, B. Development in the Mammalian Auditory System Depends on Transcription Factors. Int. J. Mol. Sci. 2021, 22, 4189. [Google Scholar] [CrossRef]
- Kondoh, H.; Uchikawa, M.; Kamachi, Y. Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation. Int. J. Dev. Biol. 2004, 48, 819–827. [Google Scholar] [CrossRef]
- Kondoh, H.; Lovel-Badge, R. (Eds.) Sox2: Biology and Role in Development and Disease; Elsevier, Associated Press: New York, NY, USA, 2016; ISBN 978-0-12-800352-7. [Google Scholar]
- Kelberman, D.; de Castro, S.C.P.; Huang, S.; Crolla, J.A.; Palmer, R.; Gregory, J.W.; Taylor, D.; Cavallo, L.; Faienza, M.F.; Fischetto, R.; et al. SOX2 Plays a Critical Role in the Pituitary, Forebrain, and Eye during Human Embryonic Development. J. Clin. Endocrinol. Metab. 2008, 93, 1865–1873. [Google Scholar] [CrossRef]
- Kelberman, D.; Rizzoti, K.; Avilion, A.; Bitner-Glindzicz, M.; Cianfarani, S.; Collins, J.; Chong, W.K.; Kirk, J.M.; Achermann, J.C.; Ross, R.; et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J. Clin. Investig. 2006, 116, 2442–2455. [Google Scholar] [CrossRef]
- Hébert, J.M.; McConnell, S.K. Targeting of cre to the Foxg1 (BF-1) Locus Mediates loxP Recombination in the Telencephalon and Other Developing Head Structures. Dev. Biol. 2000, 222, 296–306. [Google Scholar] [CrossRef]
- Mercurio, S.; Alberti, C.; Serra, L.; Meneghini, S.; Berico, P.; Bertolini, J.; Becchetti, A.; Nicolis, S.K. An early Sox2-dependent gene expression programme required for hippocampal dentate gyrus development. Open Biol. 2021, 11, 200339. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.A.; Talley, T.; Qiu, M.; Puelles, L.; Rubenstein, J.L.R.; Jones, K. Cortical Excitatory Neurons and Glia, But Not GABAergic Neurons, Are Produced in the Emx1-Expressing Lineage. J. Neurosci. 2002, 22, 6309–6314. [Google Scholar] [CrossRef] [PubMed]
- Dubois, N.C.; Hofmann, D.; Kaloulis, K.; Bishop, J.; Trumpp, A. Nestin-Cre transgenic mouse line Nes-Cre1 mediates highly efficient Cre/loxP mediated recombination in the nervous system, kidney, and somite-derived tissues. Genesis 2006, 44, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Favaro, R.; Valotta, M.; Ferri, A.L.; Latorre, E.; Mariani, J.; Giachino, C.; Lancini, C.; Tosetti, V.; Ottolenghi, S.; Taylor, V.; et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat. Neurosci. 2009, 12, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, V.; Mercurio, S.; Leto, K.; Fucà, E.; Hoxha, E.; Bottes, S.; Pagin, M.; Milanese, M.; Ngan, C.-Y.; Concina, G.; et al. Sox2 conditional mutation in mouse causes ataxic symptoms, cerebellar vermis hypoplasia, and postnatal defects of Bergmann glia. Glia 2018, 66, 1929–1946. [Google Scholar] [CrossRef]
- Danielian, P.; Muccino, D.; Rowitch, D.; Michael, S.K.; McMahon, A.P. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 1998, 8, 1323–1326. [Google Scholar] [CrossRef]
- Chou, S.-J.; Babot, Z.; Leingärtner, A.; Studer, M.; Nakagawa, Y.; O’Leary, D.D.M. Geniculocortical Input Drives Genetic Distinctions between Primary and Higher-Order Visual Areas. Science 2013, 340, 1239–1242. [Google Scholar] [CrossRef]
- Mercurio, S.; Serra, L.; Motta, A.; Gesuita, L.; Sanchez-Arrones, L.; Inverardi, F.; Foglio, B.; Barone, C.; Kaimakis, P.; Martynoga, B.; et al. Sox2 Acts in Thalamic Neurons to Control the Development of Retina-Thalamus-Cortex Connectivity. iScience 2019, 15, 257–273. [Google Scholar] [CrossRef]
- Li, G.; Pleasure, S.J. Morphogenesis of the Dentate Gyrus: What We Are Learning from Mouse Mutants. Dev. Neurosci. 2005, 27, 93–99. [Google Scholar] [CrossRef]
- Hatami, M.; Conrad, S.; Naghsh, P.; Alvarez-Bolado, G.; Skutella, T. Cell-Biological Requirements for the Generation of Dentate Gyrus Granule Neurons. Front. Cell. Neurosci. 2018, 12, 402. [Google Scholar] [CrossRef]
- Subramanian, L.; Remedios, R.; Shetty, A.; Tole, S. Signals from the edges: The cortical hem and antihem in telencephalic development. Semin. Cell Dev. Biol. 2009, 20, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, L.; Tole, S. Mechanisms Underlying the Specification, Positional Regulation, and Function of the Cortical Hem. Cereb. Cortex 2009, 19, i90–i95. [Google Scholar] [CrossRef] [PubMed]
- Amelio, I.; Panatta, E.; Niklison-Chirou, M.V.; Steinert, J.R.; Agostini, M.; Morone, N.; Knight, R.A.; Melino, G. The C terminus of p73 is essential for hippocampal development. Proc. Natl. Acad. Sci. USA 2020, 117, 15694–15701. [Google Scholar] [CrossRef] [PubMed]
- Hodge, R.D.; Garcia, A.J.; Elsen, G.E.; Nelson, B.R.; Mussar, K.E.; Reiner, S.L.; Ramirez, J.-M.; Hevner, R.F. Tbr2 Expression in Cajal-Retzius Cells and Intermediate Neuronal Progenitors Is Required for Morphogenesis of the Dentate Gyrus. J. Neurosci. 2013, 33, 4165–4180. [Google Scholar] [CrossRef]
- Ferri, A.L.; Cavallaro, M.; Braida, D.; Di Cristofano, A.; Canta, A.; Vezzani, A.; Ottolenghi, S.; Pandolfi, P.P.; Sala, M.; DeBiasi, S.; et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 2004, 131, 3805–3819. [Google Scholar] [CrossRef]
- Hodge, R.D.; Hevner, R.F. Expression and actions of transcription factors in adult hippocampal neurogenesis. Dev. Neurobiol. 2011, 71, 680–689. [Google Scholar] [CrossRef]
- Medina, D.L.; Sciarretta, C.; Calella, A.M.; Halbach, O.V.B.U.; Unsicker, K.; Minichiello, L. TrkB regulates neocortex formation through the Shc/PLCγ-mediated control of neuronal migration. EMBO J. 2004, 23, 3803–3814. [Google Scholar] [CrossRef]
- Feng, R.; Zhou, S.; Liu, Y.; Song, D.; Luan, Z.; Dai, X.; Li, Y.; Tang, N.; Wen, J.; Li, L. Sox2 protects neural stem cells from apoptosis via up-regulating survivin expression. Biochem. J. 2013, 450, 459–468. [Google Scholar] [CrossRef]
- Bertolini, J.A.; Favaro, R.; Zhu, Y.; Pagin, M.; Ngan, C.Y.; Wong, C.H.; Tjong, H.; Vermunt, M.W.; Martynoga, B.; Barone, C.; et al. Mapping the Global Chromatin Connectivity Network for Sox2 Function in Neural Stem Cell Maintenance. Cell Stem Cell 2019, 24, 462–476.e6. [Google Scholar] [CrossRef]
- Kormish, J.D.; Sinner, D.; Zorn, A.M. Interactions between SOX factors and Wnt/β-catenin signaling in development and disease. Dev. Dyn. 2009, 239, 56–68. [Google Scholar] [CrossRef]
- Hu, J.S.; Vogt, D.; Sandberg, M.; Rubenstein, J.L. Cortical interneuron development: A tale of time and space. Development 2017, 144, 3867–3878. [Google Scholar] [CrossRef] [PubMed]
- Marín, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, G.; Fishell, G. GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb. Cortex 2011, 21, 845–852. [Google Scholar] [CrossRef]
- Cavallaro, M.; Mariani, J.; Lancini, C.; Latorre, E.; Caccia, R.; Gullo, F.; Valotta, M.; DeBiasi, S.; Spinardi, L.; Ronchi, A.; et al. Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development 2008, 135, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Ferri, A.; Favaro, R.; Beccari, L.; Bertolini, J.A.; Mercurio, S.; Nieto-Lopez, F.; Verzeroli, C.; La Regina, F.; Tonelli, D.D.P.; Ottolenghi, S.; et al. Sox2 is required for embryonic development of the ventral telencephalon through the activation of the ventral determinants Nkx2.1 and Shh. Development 2013, 140, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; El-Jaick, K.; Roessler, E.; Muenke, M.; Epstein, D.J. A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers. Development 2006, 133, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Sussel, L.; Marin, O.; Kimura, S.; Rubenstein, J. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: Evidence for a transformation of the pallidum into the striatum. Development 1999, 126, 3359–3370. [Google Scholar] [CrossRef]
- Cheng, A.H.; Bouchard-Cannon, P.; Hegazi, S.; Lowden, C.; Fung, S.W.; Chiang, C.-K.; Ness, R.W.; Cheng, H.-Y.M. SOX2-Dependent Transcription in Clock Neurons Promotes the Robustness of the Central Circadian Pacemaker. Cell Rep. 2019, 26, 3191–3202.e8. [Google Scholar] [CrossRef]
- Hoefflin, S.; Carter, D.A. Neuronal expression of SOX2 is enriched in specific hypothalamic cell groups. J. Chem. Neuroanat. 2014, 61–62, 153–160. [Google Scholar] [CrossRef][Green Version]
- Sugiura, A.; Shimizu, T.; Kameyama, T.; Maruo, T.; Kedashiro, S.; Miyata, M.; Mizutani, K.; Takai, Y. Identification of Sox2 and NeuN Double-Positive Cells in the Mouse Hypothalamic Arcuate Nucleus and Their Reduction in Number with Aging. Front. Aging Neurosci. 2021, 12, 515. [Google Scholar] [CrossRef]
- Taranova, O.V.; Magness, S.T.; Fagan, B.M.; Wu, Y.; Surzenko, N.; Hutton, S.R.; Pevny, L.H. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006, 20, 1187–1202. [Google Scholar] [CrossRef] [PubMed]
- Kamachi, Y.; Uchikawa, M.; Collignon, J.; Lovell-Badge, R.; Kondoh, H. Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development 1998, 125, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Le, R.D.; Rayner, K.; Rex, M.; Wigmore, P.M.; Scotting, P.J. The transcription factor cSox2 and Neuropeptide Y define a novel subgroup of amacrine cells in the retina. J. Anat. 2002, 200, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Kamaid, A.; Alsina, B.; Giraldez, F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. J. Comp. Neurol. 2007, 503, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.; Giangreco, A.; Jensen, K.; Mulder, K.W.; Watt, F.M. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development 2009, 136, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffenberger, C.; Yamada, J.; Feldheim, D.A. Ephrin-As and Patterned Retinal Activity Act Together in the Development of Topographic Maps in the Primary Visual System. J. Neurosci. 2006, 26, 12873–12884. [Google Scholar] [CrossRef] [PubMed]
- Huberman, A.D.; Murray, K.D.; Warland, D.K.; Feldheim, D.A.; Chapman, B. Ephrin-As mediate targeting of eye-specific projections to the lateral geniculate nucleus. Nat. Neurosci. 2005, 8, 1013–1021. [Google Scholar] [CrossRef]
- Gaspar, P.; Cases, O.; Maroteaux, L. The developmental role of serotonin: News from mouse molecular genetics. Nat. Rev. Neurosci. 2003, 4, 1002–1012. [Google Scholar] [CrossRef]
- Upton, A.L.; Salichon, N.; Lebrand, C.; Ravary, A.; Blakely, R.; Seif, I.; Gaspar, P. Excess of Serotonin (5-HT) Alters the Segregation of Ispilateral and Contralateral Retinal Projections in Monoamine Oxidase A Knock-Out Mice: Possible Role of 5-HT Uptake in Retinal Ganglion Cells during Development. J. Neurosci. 1999, 19, 7007–7024. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Cai, D. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat. Cell Biol. 2012, 14, 999–1012. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, M.S.; Jia, B.; Yan, J.; Hertz, J.P.Z.; Han, C.; Cai, D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 2017, 548, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Molecular Analysis of Mammalian Circadian Rhythms. Annu. Rev. Physiol. 2001, 63, 647–676. [Google Scholar] [CrossRef] [PubMed]
- Basson, M.A.; Wingate, R.J. Congenital hypoplasia of the cerebellum: Developmental causes and behavioral consequences. Front. Neuroanat. 2013, 7, 29. [Google Scholar] [CrossRef]
- Leto, K.; Arancillo, M.; Becker, E.B.E.; Buffo, A.; Chiang, C.; Ding, B.; Dobyns, W.; Dusart, I.; Haldipur, P.; Hatten, M.E.; et al. Consensus Paper: Cerebellar Development. Cerebellum 2015, 15, 789–828. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.H.; Joyner, A.L. Otx2 and Gbx2 are required for refinement and not induction of mid-hindbrain gene expression. Development 2001, 128, 4979–4991. [Google Scholar] [CrossRef] [PubMed]
- Broccoli, V.; Boncinelli, E.; Wurst, W. The caudal limit of Otx2 expression positions the isthmic organizer. Nature 1999, 401, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Buffo, A.; Rossi, F. Origin, lineage and function of cerebellar glia. Prog. Neurobiol. 2013, 109, 42–63. [Google Scholar] [CrossRef]
- Custer, S.K.; Garden, G.; Gill, N.; Rueb, U.; Libby, R.T.; Schultz, C.; Guyenet, S.J.; Deller, T.; Westrum, L.E.; Sopher, B.L.; et al. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat. Neurosci. 2006, 9, 1302–1311. [Google Scholar] [CrossRef]
- Miyazaki, T.; Yamasaki, M.; Hashimoto, K.; Kohda, K.; Yuzaki, M.; Shimamoto, K.; Tanaka, K.; Kano, M.; Watanabe, M. Glutamate transporter GLAST controls synaptic wrapping by Bergmann glia and ensures proper wiring of Purkinje cells. Proc. Natl. Acad. Sci. USA 2017, 114, 7438–7443. [Google Scholar] [CrossRef]
- Alcock, J.; Lowe, J.; England, T.; Bath, P.; Sottile, V. Expression of Sox1, Sox2 and Sox9 is maintained in adult human cerebellar cortex. Neurosci. Lett. 2009, 450, 114–116. [Google Scholar] [CrossRef]
- Pevny, L.H.; Nicolis, S.K. Sox2 roles in neural stem cells. Int. J. Biochem. Cell Biol. 2010, 42, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Agullo, C.; Maurin, T.; Thompson, C.B.; Lai, E.C. Ars2 maintains neural stem-cell identity through direct transcriptional activation of Sox2. Nature 2011, 481, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.-P.; Zhang, Y.; Wang, C.; Yuan, F.; Li, H.; Yao, Y.; Chen, Y.; Li, C.; Wei, W.; Liu, C.H.; et al. Dynamic ubiquitylation of Sox2 regulates proteostasis and governs neural progenitor cell differentiation. Nat. Commun. 2018, 9, 4648. [Google Scholar] [CrossRef] [PubMed]
- Julian, L.M.; Vandenbosch, R.; Pakenham, C.A.; Andrusiak, M.G.; Nguyen, A.P.; McClellan, K.A.; Svoboda, D.S.; Lagace, D.C.; Park, D.; Leone, G.; et al. Opposing Regulation of Sox2 by Cell-Cycle Effectors E2f3a and E2f3b in Neural Stem Cells. Cell Stem Cell 2013, 12, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, M.; Sandhu, J.K.; Deb-Rinker, P.; Jezierski, A.; LeBlanc, J.; Charlebois, C.; Ribecco-Lutkiewicz, M.; Bani-Yaghoub, M.; Walker, P.R. Epigenetic modifications of SOX2 enhancers, SRR1 and SRR2, correlate with in vitro neural differentiation. J. Neurosci. Res. 2008, 86, 1680–1693. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 in cell proliferation and survival. Oncogene 2001, 20, 2390–2400. [Google Scholar] [CrossRef]
- Pagin, M.; Pernebrink, M.; Pitasi, M.; Malighetti, F.; Ngan, C.-Y.; Ottolenghi, S.; Pavesi, G.; Cantù, C.; Nicolis, S. FOS Rescues Neuronal Differentiation of Sox2-Deleted Neural Stem Cells by Genome-Wide Regulation of Common SOX2 and AP1(FOS-JUN) Target Genes. Cells 2021, 10, 1757. [Google Scholar] [CrossRef]
- Pagin, M.; Pernebrink, M.; Giubbolini, S.; Barone, C.; Sambruni, G.; Zhu, Y.; Chiara, M.; Ottolenghi, S.; Pavesi, G.; Wei, C.-L.; et al. Sox2 Controls Neural Stem Cell Self-Renewal Through a Fos-Centered Gene Regulatory Network. Stem Cells 2021, 39, 1107–1119. [Google Scholar] [CrossRef]
- Wei, C.-L.; Nicolis, S.K.; Zhu, Y.; Pagin, M. Sox2-Dependent 3D Chromatin Interactomes in Transcription, Neural Stem Cell Proliferation and Neurodevelopmental Diseases. J. Exp. Neurosci. 2019, 13, 1179069519868224. [Google Scholar] [CrossRef]
- Zhang, Y.; Wong, C.-H.; Birnbaum, R.Y.; Li, G.; Favaro, R.; Ngan, C.Y.; Lim, J.; Tai, E.; Poh, H.M.; Wong, E.; et al. Chromatin connectivity maps reveal dynamic promoter–enhancer long-range associations. Nature 2013, 504, 306–310. [Google Scholar] [CrossRef]
- Kamachi, Y.; Uchikawa, M.; Tanouchi, A.; Sekido, R.; Kondoh, H. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 2001, 15, 1272–1286. [Google Scholar] [CrossRef] [PubMed]
- Kamachi, Y.; Uchikawa, M.; Kondoh, H. Pairing SOX off: With partners in the regulation of embryonic development. Trends Genet. 2000, 16, 182–187. [Google Scholar] [CrossRef]
- Gagliardi, A.; Mullin, N.P.; Tan, Z.Y.; Colby, D.; Kousa, A.I.; Halbritter, F.; Weiss, J.T.; Felker, A.; Bezstarosti, K.; Favaro, R.; et al. A direct physical interaction between Nanog and Sox2 regulates embryonic stem cell self-renewal. EMBO J. 2013, 32, 2231–2247. [Google Scholar] [CrossRef] [PubMed]
- Lodato, M.A.; Ng, C.W.; Wamstad, J.A.; Cheng, A.W.; Thai, K.K.; Fraenkel, E.; Jaenisch, R.; Boyer, L.A. SOX2 Co-Occupies Distal Enhancer Elements with Distinct POU Factors in ESCs and NPCs to Specify Cell State. PLoS Genet. 2013, 9, e1003288. [Google Scholar] [CrossRef]
- Engelen, E.; Akinci, U.; Bryne, J.C.; Hou, J.; Gontan, C.; Moen, M.; Szumska, D.; Kockx, C.; van Ijcken, W.; Dekkers, D.H.W.; et al. Sox2 cooperates with Chd7 to regulate genes that are mutated in human syndromes. Nat. Genet. 2011, 43, 607–611. [Google Scholar] [CrossRef]
- Hagey, D.; Klum, S.; Kurtsdotter, I.; Zaouter, C.; Topcic, D.; Andersson, O.; Bergsland, M.; Muhr, J. SOX2 regulates common and specific stem cell features in the CNS and endoderm derived organs. PLoS Genet. 2018, 14, e1007224. [Google Scholar] [CrossRef]
- Malik, V.; Glaser, L.V.; Zimmer, D.; Velychko, S.; Weng, M.; Holzner, M.; Arend, M.; Chen, Y.; Srivastava, Y.; Veerapadian, V.; et al. Pluripotency reprogramming by competent and incompetent POU factors uncovers temporal dependency for Oct4 and Sox2. Nat. Commun. 2019, 10, 3477. [Google Scholar] [CrossRef]
- Fantes, J.; Ragge, N.K.; Lynch, S.-A.; McGill, N.I.; Collin, J.R.O.; Howard-Peebles, P.N.; Hayward, C.; Vivian, A.J.; Williamson, K.; van Heyningen, V.; et al. Mutations in SOX2 cause anophthalmia. Nat. Genet. 2003, 33, 462–463. [Google Scholar] [CrossRef]
- Ragge, N.K.; Lorenz, B.; Schneider, A.; Bushby, K.; de Sanctis, L.; de Sanctis, U.; Salt, A.; Collin, J.R.O.; Vivian, A.J.; Free, S.L.; et al. SOX2 anophthalmia syndrome. Am. J. Med Genet. Part A 2005, 135A, 1–7. [Google Scholar] [CrossRef]
- Sisodiya, S.M.; Ragge, N.K.; Cavalleri, G.; Hever, A.; Lorenz, B.; Schneider, A.; Williamson, K.A.; Stevens, J.M.; Free, S.L.; Thompson, P.J.; et al. Role of SOX2 Mutations in Human Hippocampal Malformations and Epilepsy. Epilepsia 2006, 47, 534–542. [Google Scholar] [CrossRef]
- Foglio, B.; Rossini, L.; Garbelli, R.; Regondi, M.C.; Mercurio, S.; Bertacchi, M.; Avagliano, L.; Bulfamante, G.; Coras, R.; Maiorana, A.; et al. Dynamic expression of NR2F1 and SOX2 in developing and adult human cortex: Comparison with cortical malformations. Anat. Embryol. 2021, 226, 1303–1322. [Google Scholar] [CrossRef] [PubMed]
- Abrous, D.N.; Wojtowicz, J.M. Interaction between Neurogenesis and Hippocampal Memory System: New Vistas. Cold Spring Harb. Perspect. Biol. 2015, 7, a018952. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Kitamura, T.; Saitoh, Y.; Ohkawa, N.; Kondo, T.; Inokuchi, K. Adult Neurogenesis Conserves Hippocampal Memory Capacity. J. Neurosci. 2018, 38, 6854–6863. [Google Scholar] [CrossRef] [PubMed]
- Dooves, S.; Bugiani, M.; Wisse, L.E.; Abbink, T.E.M.; Van Der Knaap, M.S.; Heine, V.M. Bergmann glia translocation: A new disease marker for vanishing white matter identifies therapeutic effects of Guanabenz treatment. Neuropathol. Appl. Neurobiol. 2017, 44, 391–403. [Google Scholar] [CrossRef]
- Parisi, M.A. The molecular genetics of Joubert syndrome and related ciliopathies: The challenges of genetic and phenotypic heterogeneity. Transl. Sci. Rare Dis. 2019, 4, 25–49. [Google Scholar] [CrossRef]
- Barone, C.; Pagin, M.; Serra, L.; Rigoldi, L.; Giubbolini, S.; Badiola-Sanga, A.; Mercurio, S.; Nicolis, S.K. Sox2 Functions in Neural Cancer Stem Cells: The Importance of the Context. Insights Neuro Oncol. 2018, 2, 18–26. [Google Scholar] [CrossRef]
- Mansouri, S.; Nejad, R.; Karabork, M.; Ekinci, C.; Solaroglu, I.; Aldape, K.D.; Zadeh, G. Sox2: Regulation of expression and contribution to brain tumors. CNS Oncol. 2016, 5, 159–173. [Google Scholar] [CrossRef]
- Stevanovic, M.; Kovacevic-Grujicic, N.; Mojsin, M.; Milivojevic, M.; Drakulic, D. SOX transcription factors and glioma stem cells: Choosing between stemness and differentiation. World J. Stem Cells 2021, 13, 1417–1445. [Google Scholar] [CrossRef]
- Wuebben, E.L.; Rizzino, A. The dark side of SOX2: Cancer—A comprehensive overview. Oncotarget 2017, 8, 44917–44943. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, X.; Sun, Y. Functional characterization of SOX2 as an anticancer target. Signal Transduct. Target. Ther. 2020, 5, 135. [Google Scholar] [CrossRef]
- Garros-Regulez, L.; Garcia, I.; Carrasco-Garcia, E.; Lantero, A.; Aldaz, P.; Moreno-Cugnon, L.; Arrizabalaga, O.; Undabeitia, J.; Torres-Bayona, S.; Villanua, J.; et al. Targeting SOX2 as a Therapeutic Strategy in Glioblastoma. Front. Oncol. 2016, 6, 222. [Google Scholar] [CrossRef] [PubMed]
- Parmigiani, E.; Taylor, V.; Giachino, C. Oncogenic and Tumor-Suppressive Functions of NOTCH Signaling in Glioma. Cells 2020, 9, 2304. [Google Scholar] [CrossRef] [PubMed]
- Jagga, B.; Edwards, M.; Pagin, M.; Wagstaff, K.M.; Aragão, D.; Roman, N.; Nanson, J.D.; Raidal, S.R.; Dominado, N.; Stewart, M.; et al. Structural basis for nuclear import selectivity of pioneer transcription factor SOX2. Nat. Commun. 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]


| Sox2 cKO | Cre Expression Domain in CNS | Timing Sox2 Deletion | Reference |
|---|---|---|---|
| Sox2-FoxG1-Cre cKO | Telencephalon | complete by E9.5 | [11,12] |
| Sox2-Emx1-Cre cKO | Dorsal Telencephalon | complete by E10.5 | [12,13] |
| Sox2-Nestin-Cre cKO | NSC | complete by E14.5 | [12,14,15] |
| Sox2-Wnt1-Cre cKO | Midbrain and Hindbrain | complete by E9.5 | [16,17] |
| Sox2-Rora-Cre cKO | Thalamus (dLGN, VP, MG) | complete by E15.5 | [18,19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercurio, S.; Serra, L.; Pagin, M.; Nicolis, S.K. Deconstructing Sox2 Function in Brain Development and Disease. Cells 2022, 11, 1604. https://doi.org/10.3390/cells11101604
Mercurio S, Serra L, Pagin M, Nicolis SK. Deconstructing Sox2 Function in Brain Development and Disease. Cells. 2022; 11(10):1604. https://doi.org/10.3390/cells11101604
Chicago/Turabian StyleMercurio, Sara, Linda Serra, Miriam Pagin, and Silvia K. Nicolis. 2022. "Deconstructing Sox2 Function in Brain Development and Disease" Cells 11, no. 10: 1604. https://doi.org/10.3390/cells11101604
APA StyleMercurio, S., Serra, L., Pagin, M., & Nicolis, S. K. (2022). Deconstructing Sox2 Function in Brain Development and Disease. Cells, 11(10), 1604. https://doi.org/10.3390/cells11101604






