MFAP4-Mediated Effects in Elastic Fiber Homeostasis, Integrin Signaling and Cancer, and Its Role in Teleost Fish
Abstract
:1. Elastic Fiber Structure and Assembly
2. FReD Protein Superfamily
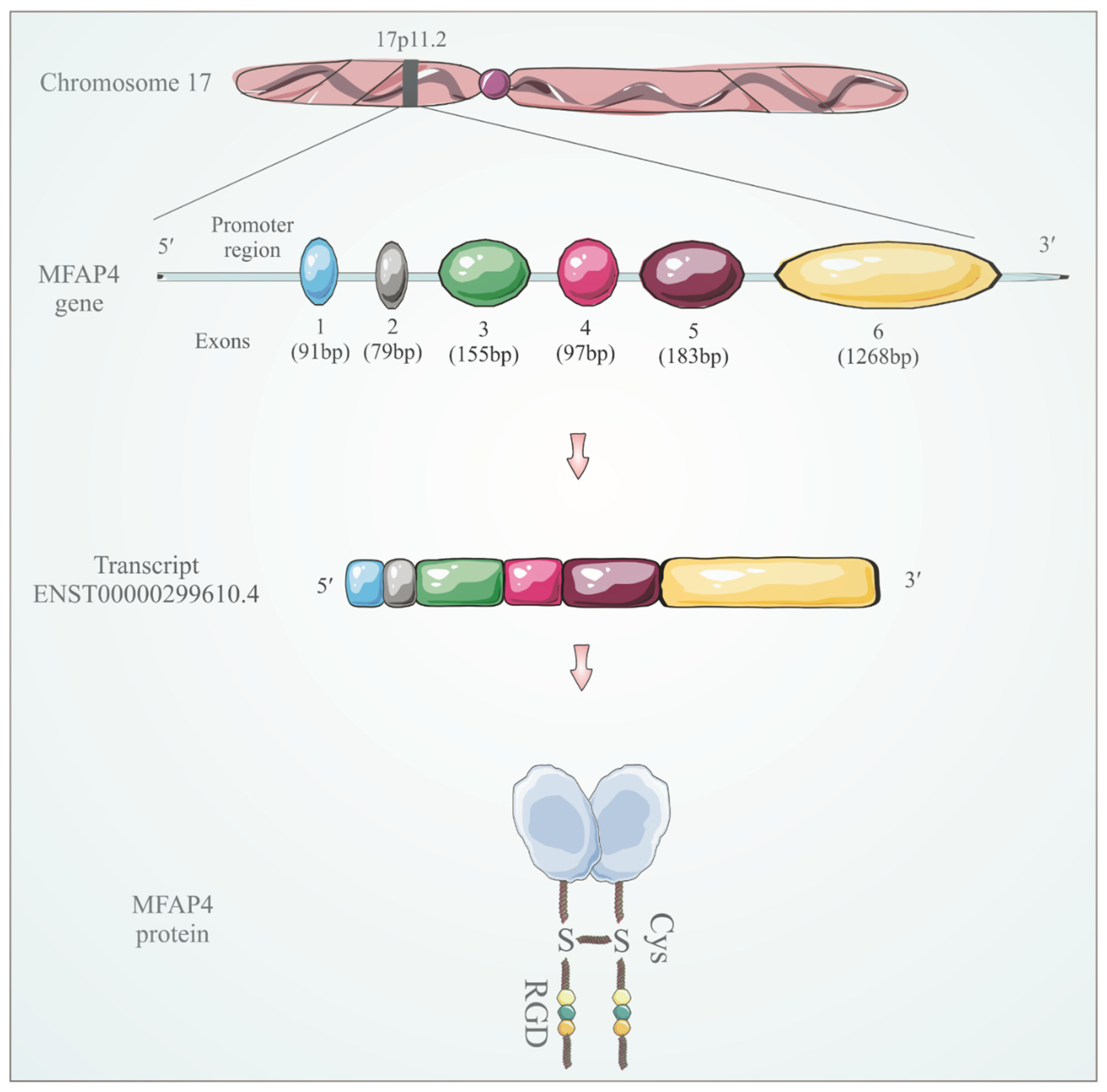
3. MFAP4 Identification and Structure
4. MFAP4 Localization and Tissue Expression
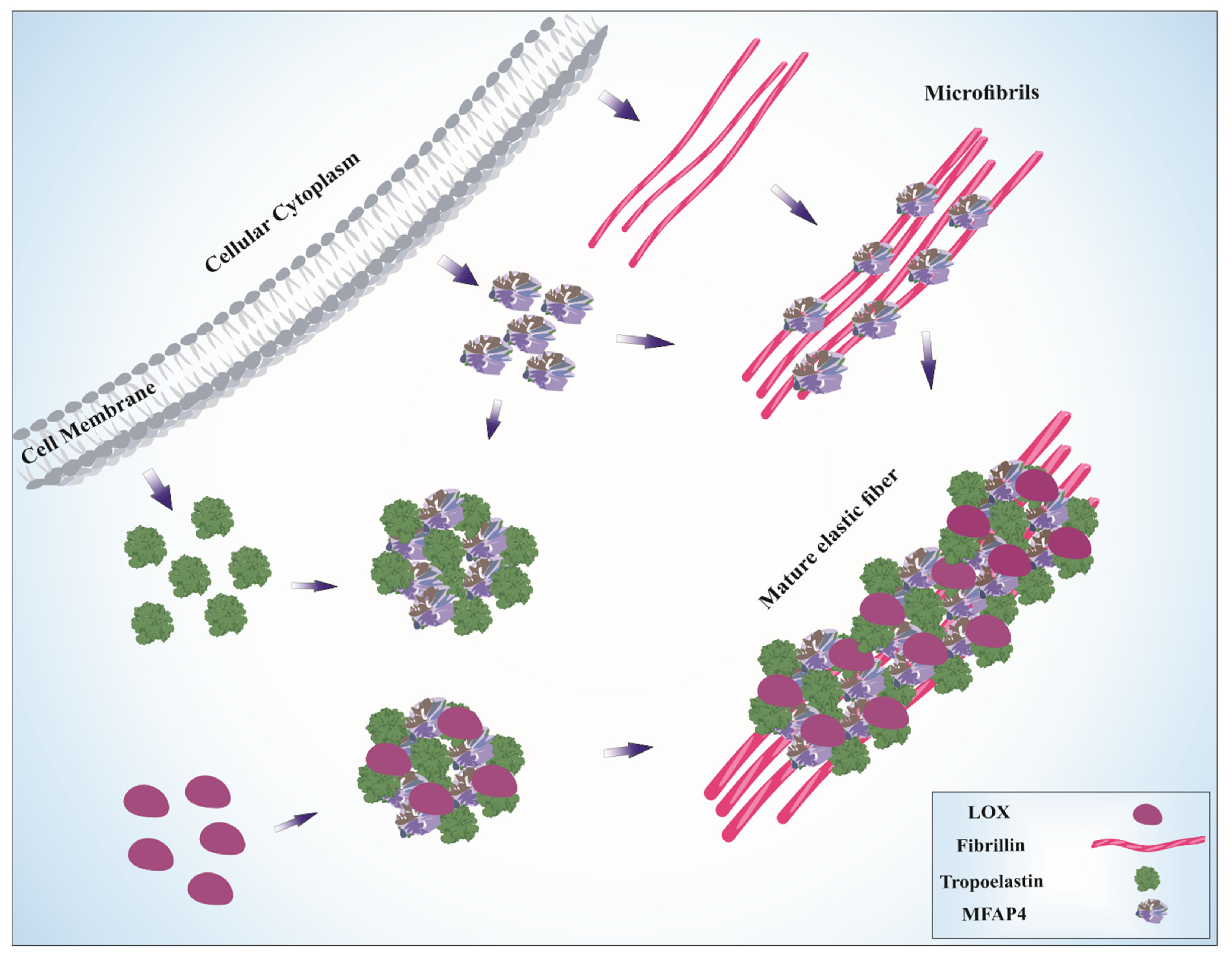
5. Role of MFAP4 in Elastic Fiber Assembly
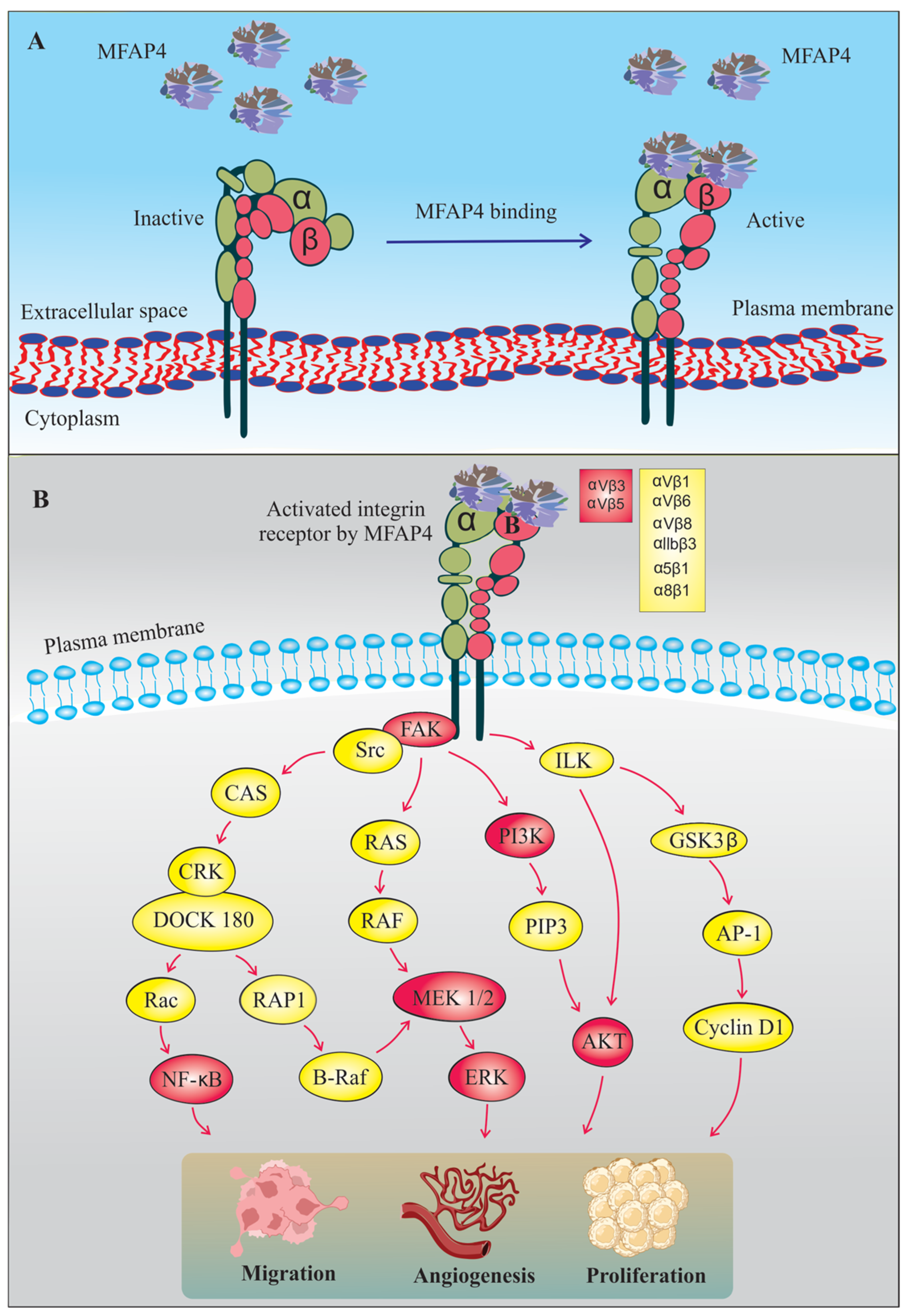
6. MFAP4-Mediated Integrin Signaling
7. The Role of MFAP4 in Tissue Fibrosis
8. MFAP4 in Cancer
| References | Sample Size | Type of Analysis | MFAP4 Expression | Sample Type | Cancer Type |
|---|---|---|---|---|---|
| [91] |
| Bioinformatic (TCGA) | Downregulation | mRNA | Breast cancer |
| [94] |
| Tissue samples | Downregulation | Protein | Breast cancer |
| [91] |
| Bioinformatic (TCGA) | Downregulation | mRNA | Bladder cancer |
| [91] |
| Bioinformatic (TCGA) | Downregulation | mRNA | Colorectal cancer |
| [91] |
| Bioinformatic (TCGA) | Downregulation | mRNA | Cervical cancer |
| [91] |
| Bioinformatic (TCGA) | Downregulation | mRNA | Head and neck cancer |
| [91] |
| Bioinformatic (TCGA) | Downregulation | mRNA | Kidney cancer |
| [91] |
| UALCAN database | Downregulation | mRNA | Lung cancer |
| [95,96] |
| Bioinformatic (TCGA) | Downregulation | mRNA | Lung adenocarcinoma |
| [91] |
| UALCAN database | Downregulation | mRNA | Liver cancer |
| [91] |
| Bioinformatic (TCGA) | Downregulation | mRNA | Ovarian cancer |
| [98] |
| Bioinformatic (GSE25099) | Downregulation | mRNA | Oral squamous cell carcinoma |
| [92] |
| Tissue Samples | Downregulation | Protein | Prostate cancer |
| [91] |
| Bioinformatic (UALCAN) | Downregulation | mRNA | Stomach cancer |
| [93] |
| Tissue Samples | Downregulation | mRNA | Urinary bladder cancer |
| [91] |
| Bioinformatic (TCGA) | Upregulation | mRNA | Brain cancer |
| [91] |
| Bioinformatic (TCGA) | Upregulation | mRNA | Esophageal cancer |
| [102] |
| Serum samples | Upregulation | Protein | Hepatocellular carcinoma |
| [91] |
| Bioinformatic (TCGA) | Upregulation | mRNA | Leukemia |
| [91] |
| Bioinformatic (TCGA) | Upregulation | mRNA | Lymphoma |
| [104] |
| Cohort study | Upregulation | mRNA | Neuroblastoma |
| [105] |
| Tissue Samples | Upregulation | Protein | Pancreatic adenocarcinoma |
| [108] |
| Tissue Samples | Upregulation | mRNA | Pleomorphic adenoma |
9. MFAP4 and Its Role in Teleost Fish
10. Conclusions
11. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muiznieks, L.D.; Weiss, A.S.; Keeley, F.W. Structural disorder and dynamics of elastin. Biochem. Cell Biol. 2010, 88, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Mecham, R.P.; Gibson, M.A. The microfibril-associated glycoproteins (MAGPs) and the microfibrillar niche. Matrix Biol. 2015, 47, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Horowitz, J.C.; Naba, A.; Ambalavanan, N.; Atabai, K.; Balestrini, J.; Bitterman, P.B.; Corley, R.A.; Ding, B.S.; Engler, A.J.; et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 2018, 73, 77–104. [Google Scholar] [CrossRef] [Green Version]
- Halper, J.; Kjaer, M. Basic components of connective tissues and extracellular matrix: Elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv. Exp. Med. Biol. 2014, 802, 31–47. [Google Scholar] [CrossRef]
- Manou, D.; Caon, I.; Bouris, P.; Triantaphyllidou, I.E.; Giaroni, C.; Passi, A.; Karamanos, N.K.; Vigetti, D.; Theocharis, A.D. The Complex Interplay Between Extracellular Matrix and Cells in Tissues. Methods Mol. Biol. 2019, 1952, 1–20. [Google Scholar] [CrossRef]
- Heinz, A. Elastic fibers during aging and disease. Ageing Res. Rev. 2021, 66, 101255. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Simpson, A.; Steer, R.; Cain, S.A.; Kielty, C.M. Elastic fibres in health and disease. Expert Rev. Mol. Med. 2013, 15, e8. [Google Scholar] [CrossRef] [Green Version]
- Vrhovski, B.; Weiss, A.S. Biochemistry of tropoelastin. Eur. J. Biochem. 1998, 258, 1–18. [Google Scholar] [CrossRef]
- Toonkool, P.; Jensen, S.A.; Maxwell, A.L.; Weiss, A.S. Hydrophobic domains of human tropoelastin interact in a context-dependent manner. J. Biol. Chem. 2001, 276, 44575–44580. [Google Scholar] [CrossRef] [Green Version]
- Yeo, G.C.; Keeley, F.W.; Weiss, A.S. Coacervation of tropoelastin. Adv. Colloid Interface Sci. 2011, 167, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Dyksterhuis, L.B.; Weiss, A.S. Homology models for domains 21-23 of human tropoelastin shed light on lysine crosslinking. Biochem. Biophys. Res. Commun. 2010, 396, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Bedell-Hogan, D.; Trackman, P.; Abrams, W.; Rosenbloom, J.; Kagan, H. Oxidation, cross-linking, and insolubilization of recombinant tropoelastin by purified lysyl oxidase. J. Biol. Chem. 1993, 268, 10345–10350. [Google Scholar] [CrossRef]
- Trask, T.M.; Trask, B.C.; Ritty, T.M.; Abrams, W.R.; Rosenbloom, J.; Mecham, R.P. Interaction of tropoelastin with the amino-terminal domains of fibrillin-1 and fibrillin-2 suggests a role for the fibrillins in elastic fiber assembly. J. Biol. Chem. 2000, 275, 24400–24406. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, F.; Sakai, L.Y. Biogenesis and function of fibrillin assemblies. Cell Tissue Res. 2010, 339, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, M.; Braghetta, P.; Sabatelli, P.; Mura, I.; Doliana, R.; Colombatti, A.; Volpin, D.; Bonaldo, P.; Bressan, G.M. EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol. Cell Biol. 2004, 24, 638–650. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Ye, L.; Bennett, S.; Xu, H.; He, D.; Xu, J. Molecular structure and function of microfibrillar-associated proteins in skeletal and metabolic disorders and cancers. J. Cell Physiol. 2021, 236, 41–48. [Google Scholar] [CrossRef]
- Cain, S.A.; Baldwin, A.K.; Mahalingam, Y.; Raynal, B.; Jowitt, T.A.; Shuttleworth, C.A.; Couchman, J.R.; Kielty, C.M. Heparan sulfate regulates fibrillin-1 N- and C-terminal interactions. J. Biol. Chem. 2008, 283, 27017–27027. [Google Scholar] [CrossRef] [Green Version]
- Kinsey, R.; Williamson, M.R.; Chaudhry, S.; Mellody, K.T.; McGovern, A.; Takahashi, S.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J. Cell Sci. 2008, 121, 2696–2704. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, R.; McGovern, A.; Ridley, C.; Cain, S.A.; Baldwin, A.; Wang, M.C.; Guo, C.; Mironov, A., Jr.; Drymoussi, Z.; Trump, D.; et al. Differential regulation of elastic fiber formation by fibulin-4 and -5. J. Biol. Chem. 2009, 284, 24553–24567. [Google Scholar] [CrossRef] [Green Version]
- Cirulis, J.T.; Keeley, F.W. Kinetics and morphology of self-assembly of an elastin-like polypeptide based on the alternating domain arrangement of human tropoelastin. Biochemistry 2010, 49, 5726–5733. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.A.; Leavesley, D.I.; Ashman, L.K. Microfibril-associated glycoprotein-2 specifically interacts with a range of bovine and human cell types via alphaVbeta3 integrin. J. Biol. Chem. 1999, 274, 13060–13065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, M.; Polewski, R.; Fanning, J.C.; Gibson, M.A. Microfibril-associated glycoprotein-1 (MAGP-1) is specifically located on the beads of the beaded-filament structure for fibrillin-containing microfibrils as visualized by the rotary shadowing technique. J. Histochem. Cytochem. 1996, 44, 1389–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaire, R.; Bayle, J.; Mecham, R.P.; Lafyatis, R. Microfibril-associated MAGP-2 stimulates elastic fiber assembly. J. Biol. Chem. 2007, 282, 800–808. [Google Scholar] [CrossRef] [Green Version]
- Craft, C.S.; Broekelmann, T.J.; Zou, W.; Chappel, J.C.; Teitelbaum, S.L.; Mecham, R.P. Oophorectomy-induced bone loss is attenuated in MAGP1-deficient mice. J. Cell Biochem. 2012, 113, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Craft, C.S.; Zou, W.; Watkins, M.; Grimston, S.; Brodt, M.D.; Broekelmann, T.J.; Weinbaum, J.S.; Teitelbaum, S.L.; Pierce, R.A.; Civitelli, R.; et al. Microfibril-associated glycoprotein-1, an extracellular matrix regulator of bone remodeling. J. Biol. Chem. 2010, 285, 23858–23867. [Google Scholar] [CrossRef] [Green Version]
- Weinbaum, J.S.; Broekelmann, T.J.; Pierce, R.A.; Werneck, C.C.; Segade, F.; Craft, C.S.; Knutsen, R.H.; Mecham, R.P. Deficiency in microfibril-associated glycoprotein-1 leads to complex phenotypes in multiple organ systems. J. Biol. Chem. 2008, 283, 25533–25543. [Google Scholar] [CrossRef] [Green Version]
- Albig, A.R.; Becenti, D.J.; Roy, T.G.; Schiemann, W.P. Microfibril-associate glycoprotein-2 (MAGP-2) promotes angiogenic cell sprouting by blocking notch signaling in endothelial cells. Microvasc. Res. 2008, 76, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, A.; Lau, R.; Hein, P.W.; Shipley, J.M.; Weinmaster, G. Microfibrillar proteins MAGP-1 and MAGP-2 induce Notch1 extracellular domain dissociation and receptor activation. J. Biol. Chem. 2006, 281, 10089–10097. [Google Scholar] [CrossRef] [Green Version]
- Nehring, L.C.; Miyamoto, A.; Hein, P.W.; Weinmaster, G.; Shipley, J.M. The extracellular matrix protein MAGP-2 interacts with Jagged1 and induces its shedding from the cell surface. J. Biol. Chem. 2005, 280, 20349–20355. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Gao, X.; Luo, J.; Huang, L.; Teng, Y.; Horvitz, H.R. The Caenorhabditis elegans gene mfap-1 encodes a nuclear protein that affects alternative splicing. PLoS Genet. 2012, 8, e1002827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, D.S.; Tapon, N. Drosophila MFAP1 is required for pre-mRNA processing and G2/M progression. J. Biol. Chem. 2008, 283, 31256–31267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas-Armenteros, I.; Barroso, S.I.; Rondon, A.G.; Perez, M.; Andujar, E.; Luna, R.; Aguilera, A. Depletion of the MFAP1/SPP381 Splicing Factor Causes R-Loop-Independent Genome Instability. Cell Rep. 2019, 28, 1551–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrams, W.R.; Ma, R.I.; Kucich, U.; Bashir, M.M.; Decker, S.; Tsipouras, P.; McPherson, J.D.; Wasmuth, J.J.; Rosenbloom, J. Molecular cloning of the microfibrillar protein MFAP3 and assignment of the gene to human chromosome 5q32-q33.2. Genomics 1995, 26, 47–54. [Google Scholar] [CrossRef]
- Kang, B.; Hao, C.; Wang, H.; Zhang, J.; Xing, R.; Shao, J.; Li, W.; Xu, N.; Lu, Y.; Liu, S. Evaluation of hepatic-metastasis risk of colorectal cancer upon the protein signature of PI3K/AKT pathway. J. Proteome Res. 2008, 7, 3507–3515. [Google Scholar] [CrossRef]
- Lou, X.; Kang, B.; Zhang, J.; Hao, C.; Tian, X.; Li, W.; Xu, N.; Lu, Y.; Liu, S. MFAP3L activation promotes colorectal cancer cell invasion and metastasis. Biochim Biophys. Acta 2014, 1842, 1423–1432. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.H.; Tian, X.Y.; Hao, C.Y. The significance of a group of molecular markers and clinicopathological factors in identifying colorectal liver metastasis. Hepatogastroenterology 2011, 58, 1182–1188. [Google Scholar] [CrossRef]
- Doolittle, R.F.; McNamara, K.; Lin, K. Correlating structure and function during the evolution of fibrinogen-related domains. Protein Sci. 2012, 21, 1808–1823. [Google Scholar] [CrossRef] [Green Version]
- Doolittle, R.F. A detailed consideration of a principal domain of vertebrate fibrinogen and its relatives. Protein Sci. 1992, 1, 1563–1577. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Le, Y. Ficolins and the fibrinogen-like domain. Immunobiology 1998, 199, 190–199. [Google Scholar] [CrossRef]
- Yee, V.C.; Pratt, K.P.; Cote, H.C.; Trong, I.L.; Chung, D.W.; Davie, E.W.; Stenkamp, R.E.; Teller, D.C. Crystal structure of a 30 kDa C-terminal fragment from the gamma chain of human fibrinogen. Structure 1997, 5, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Moeller, J.B.; Leonardi, I.; Schlosser, A.; Flamar, A.L.; Bessman, N.J.; Putzel, G.G.; Thomsen, T.; Hammond, M.; Jepsen, C.S.; Skjodt, K.; et al. Modulation of the fungal mycobiome is regulated by the chitin-binding receptor FIBCD1. J. Exp. Med. 2019, 216, 2689–2700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Ali, M.A. Ficolins: Structure, function and associated diseases. Adv. Exp. Med. Biol. 2008, 632, 105–115. [Google Scholar] [PubMed]
- Jones, P.F. Not just angiogenesis--wider roles for the angiopoietins. J. Pathol. 2003, 201, 515–527. [Google Scholar] [CrossRef]
- Santulli, G. Angiopoietin-like proteins: A comprehensive look. Front. Endocrinol. 2014, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.I.; Aoki, H. The Roles of Tenascins in Cardiovascular, Inflammatory, and Heritable Connective Tissue Diseases. Front. Immunol. 2020, 11, 609752. [Google Scholar] [CrossRef]
- Kobayashi, R.; Tashima, Y.; Masuda, H.; Shozawa, T.; Numata, Y.; Miyauchi, K.; Hayakawa, T. Isolation and characterization of a new 36-kDa microfibril-associated glycoprotein from porcine aorta. J. Biol. Chem. 1989, 264, 17437–17444. [Google Scholar] [CrossRef]
- Zhao, Z.; Lee, C.C.; Jiralerspong, S.; Juyal, R.C.; Lu, F.; Baldini, A.; Greenberg, F.; Caskey, C.T.; Patel, P.I. The gene for a human microfibril-associated glycoprotein is commonly deleted in Smith-Magenis syndrome patients. Hum. Mol. Genet. 1995, 4, 589–597. [Google Scholar] [CrossRef]
- Elsea, S.H.; Girirajan, S. Smith-Magenis syndrome. Eur. J. Hum. Genet. 2008, 16, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.J.; Chen, A.N.; Yin, X.J.; Li, C.; Lin, C.C. Human Microfibrillar-Associated Protein 4 (MFAP4) Gene Promoter: A TATA-Less Promoter That Is Regulated by Retinol and Coenzyme Q10 in Human Fibroblast Cells. Int. J. Mol. Sci. 2020, 21, 8392. [Google Scholar] [CrossRef]
- Schlosser, A.; Thomsen, T.; Shipley, J.M.; Hein, P.W.; Brasch, F.; Tornoe, I.; Nielsen, O.; Skjodt, K.; Palaniyar, N.; Steinhilber, W.; et al. Microfibril-associated protein 4 binds to surfactant protein A (SP-A) and colocalizes with SP-A in the extracellular matrix of the lung. Scand. J. Immunol. 2006, 64, 104–116. [Google Scholar] [CrossRef]
- Toyoshima, T.; Ishida, T.; Nishi, N.; Kobayashi, R.; Nakamura, T.; Itano, T. Differential gene expression of 36-kDa microfibril-associated glycoprotein (MAGP-36/MFAP4) in rat organs. Cell Tissue Res. 2008, 332, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Wulf-Johansson, H.; Lock Johansson, S.; Schlosser, A.; Trommelholt Holm, A.; Rasmussen, L.M.; Mickley, H.; Diederichsen, A.C.; Munkholm, H.; Poulsen, T.S.; Tornoe, I.; et al. Localization of microfibrillar-associated protein 4 (MFAP4) in human tissues: Clinical evaluation of serum MFAP4 and its association with various cardiovascular conditions. PLoS ONE 2013, 8, e82243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoshima, T.; Yamashita, K.; Furuichi, H.; Shishibori, T.; Itano, T.; Kobayashi, R. Ultrastructural distribution of 36-kD microfibril-associated glycoprotein (MAGP-36) in human and bovine tissues. J. Histochem. Cytochem. 1999, 47, 1049–1056. [Google Scholar] [CrossRef] [Green Version]
- Toyoshima, T.; Nishi, N.; Kusama, H.; Kobayashi, R.; Itano, T. 36-kDa microfibril-associated glycoprotein (MAGP-36) is an elastin-binding protein increased in chick aortae during development and growth. Exp. Cell Res. 2005, 307, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Hirano, E.; Fujimoto, N.; Tajima, S.; Akiyama, M.; Ishibashi, A.; Kobayashi, R.; Okamoto, K. Expression of 36-kDa microfibril-associated glycoprotein (MAGP-36) in human keratinocytes and its localization in skin. J. Dermatol. Sci. 2002, 28, 60–67. [Google Scholar] [CrossRef]
- Kasamatsu, S.; Hachiya, A.; Fujimura, T.; Sriwiriyanont, P.; Haketa, K.; Visscher, M.O.; Kitzmiller, W.J.; Bello, A.; Kitahara, T.; Kobinger, G.P.; et al. Essential role of microfibrillar-associated protein 4 in human cutaneous homeostasis and in its photoprotection. Sci. Rep. 2011, 1, 164. [Google Scholar] [CrossRef] [Green Version]
- Lausen, M.; Lynch, N.; Schlosser, A.; Tornoe, I.; Saekmose, S.G.; Teisner, B.; Willis, A.C.; Crouch, E.; Schwaeble, W.; Holmskov, U. Microfibril-associated protein 4 is present in lung washings and binds to the collagen region of lung surfactant protein D. J. Biol. Chem. 1999, 274, 32234–32240. [Google Scholar] [CrossRef] [Green Version]
- Dorn, L.E.; Lawrence, W.; Petrosino, J.M.; Xu, X.; Hund, T.J.; Whitson, B.A.; Stratton, M.S.; Janssen, P.M.L.; Mohler, P.J.; Schlosser, A.; et al. Microfibrillar-Associated Protein 4 Regulates Stress-Induced Cardiac Remodeling. Circ. Res. 2021, 128, 723–737. [Google Scholar] [CrossRef]
- Wang, L.; Yu, P.; Zhou, B.; Song, J.; Li, Z.; Zhang, M.; Guo, G.; Wang, Y.; Chen, X.; Han, L.; et al. Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat. Cell Biol. 2020, 22, 108–119. [Google Scholar] [CrossRef]
- Schlosser, A.; Pilecki, B.; Hemstra, L.E.; Kejling, K.; Kristmannsdottir, G.B.; Wulf-Johansson, H.; Moeller, J.B.; Fuchtbauer, E.M.; Nielsen, O.; Kirketerp-Moller, K.; et al. MFAP4 Promotes Vascular Smooth Muscle Migration, Proliferation and Accelerates Neointima Formation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 122–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molleken, C.; Sitek, B.; Henkel, C.; Poschmann, G.; Sipos, B.; Wiese, S.; Warscheid, B.; Broelsch, C.; Reiser, M.; Friedman, S.L.; et al. Detection of novel biomarkers of liver cirrhosis by proteomic analysis. Hepatology 2009, 49, 1257–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terkelsen, M.K.; Bendixen, S.M.; Hansen, D.; Scott, E.A.H.; Moeller, A.F.; Nielsen, R.; Mandrup, S.; Schlosser, A.; Andersen, T.L.; Sorensen, G.L.; et al. Transcriptional Dynamics of Hepatic Sinusoid-Associated Cells After Liver Injury. Hepatology 2020, 72, 2119–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, M.; Zhang, C.; Mear, L.; Zhong, W.; Digre, A.; Katona, B.; Sjostedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef]
- Ponten, F.; Jirstrom, K.; Uhlen, M. The Human Protein Atlas—A tool for pathology. J. Pathol. 2008, 216, 387–393. [Google Scholar] [CrossRef]
- Pilecki, B.; Holm, A.T.; Schlosser, A.; Moeller, J.B.; Wohl, A.P.; Zuk, A.V.; Heumuller, S.E.; Wallis, R.; Moestrup, S.K.; Sengle, G.; et al. Characterization of Microfibrillar-associated Protein 4 (MFAP4) as a Tropoelastin- and Fibrillin-binding Protein Involved in Elastic Fiber Formation. J. Biol. Chem. 2016, 291, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.R.; Sordillo, J.E.; Kraft, P.; Asgari, M.M. Sex-Stratified Polygenic Risk Score Identifies Individuals at Increased Risk of Basal Cell Carcinoma. J. Investig. Dermatol. 2020, 140, 971–975. [Google Scholar] [CrossRef]
- Holm, A.T.; Wulf-Johansson, H.; Hvidsten, S.; Jorgensen, P.T.; Schlosser, A.; Pilecki, B.; Ormhoj, M.; Moeller, J.B.; Johannsen, C.; Baun, C.; et al. Characterization of spontaneous air space enlargement in mice lacking microfibrillar-associated protein 4. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L1114–L1124. [Google Scholar] [CrossRef]
- Brandsma, C.A.; van den Berge, M.; Postma, D.S.; Jonker, M.R.; Brouwer, S.; Pare, P.D.; Sin, D.D.; Bosse, Y.; Laviolette, M.; Karjalainen, J.; et al. A large lung gene expression study identifying fibulin-5 as a novel player in tissue repair in COPD. Thorax 2015, 70, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Wanga, S.; Fellows, A.L.; Barallobre-Barreiro, J.; Lu, R.; Davaapil, H.; Franken, R.; Fava, M.; Baig, F.; Skroblin, P.; et al. Glycoproteomic Analysis of the Aortic Extracellular Matrix in Marfan Patients. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1859–1873. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.D.; Cheresh, D.A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulos, S.N.; Blaikie, P.; Yoshioka, T.; Guo, W.; Puri, C.; Tacchetti, C.; Giancotti, F.G. Targeted deletion of the integrin beta4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-kappaB, causing defects in epidermal growth and migration. Mol. Cell Biol. 2005, 25, 6090–6102. [Google Scholar] [CrossRef] [Green Version]
- Shishido, S.; Bonig, H.; Kim, Y.M. Role of integrin alpha4 in drug resistance of leukemia. Front. Oncol. 2014, 4, 99. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H. Pull and push: Talin activation for integrin signaling. Cell Res. 2012, 22, 1512–1514. [Google Scholar] [CrossRef] [Green Version]
- Razia, S.; Park, H.; Shin, E.; Shim, K.S.; Cho, E.; Kang, M.C.; Kim, S.Y. Synergistic effect of Aloe vera flower and Aloe gel on cutaneous wound healing targeting MFAP4 and its associated signaling pathway: In-vitro study. J. Ethnopharmacol. 2022, 290, 115096. [Google Scholar] [CrossRef]
- Pilecki, B.; de Carvalho, P.; Kirketerp-Moller, K.L.; Schlosser, A.; Kejling, K.; Dubik, M.; Madsen, N.P.; Stubbe, J.; Hansen, P.B.L.; Andersen, T.L.; et al. MFAP4 Deficiency Attenuates Angiotensin II-Induced Abdominal Aortic Aneurysm Formation Through Regulation of Macrophage Infiltration and Activity. Front. Cardiovasc. Med. 2021, 8, 764337. [Google Scholar] [CrossRef]
- Wang, H.B.; Yang, J.; Shuai, W.; Yang, J.; Liu, L.B.; Xu, M.; Tang, Q.Z. Deletion of Microfibrillar-Associated Protein 4 Attenuates Left Ventricular Remodeling and Dysfunction in Heart Failure. J. Am. Heart Assoc. 2020, 9, e015307. [Google Scholar] [CrossRef]
- Pilecki, B.; Schlosser, A.; Wulf-Johansson, H.; Trian, T.; Moeller, J.B.; Marcussen, N.; Aguilar-Pimentel, J.A.; de Angelis, M.H.; Vestbo, J.; Berger, P.; et al. Microfibrillar-associated protein 4 modulates airway smooth muscle cell phenotype in experimental asthma. Thorax 2015, 70, 862–872. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann-Petersen, B.; Suffolk, R.; Petersen, J.J.H.; Petersen, T.H.; Arendt, K.; Host, A.; Halken, S.; Sorensen, G.L.; Agertoft, L. Microfibrillar-associated protein 4 in serum is associated with asthma in Danish adolescents and young adults. Immun. Inflamm. Dis. 2019, 7, 150–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemstra, L.E.; Schlosser, A.; Lindholt, J.S.; Sorensen, G.L. Microfibrillar-associated protein 4 variation in symptomatic peripheral artery disease. J. Transl. Med. 2018, 16, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, S.L.; Roberts, N.B.; Schlosser, A.; Andersen, C.B.; Carlsen, J.; Wulf-Johansson, H.; Saekmose, S.G.; Titlestad, I.L.; Tornoe, I.; Miller, B.; et al. Microfibrillar-associated protein 4: A potential biomarker of chronic obstructive pulmonary disease. Respir. Med. 2014, 108, 1336–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracht, T.; Molleken, C.; Ahrens, M.; Poschmann, G.; Schlosser, A.; Eisenacher, M.; Stuhler, K.; Meyer, H.E.; Schmiegel, W.H.; Holmskov, U.; et al. Evaluation of the biomarker candidate MFAP4 for non-invasive assessment of hepatic fibrosis in hepatitis C patients. J. Transl. Med. 2016, 14, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, B.S.; Thiele, M.; Detlefsen, S.; Sorensen, M.D.; Kjaergaard, M.; Moller, L.S.; Rasmussen, D.N.; Schlosser, A.; Holmskov, U.; Trebicka, J.; et al. Prediction of liver fibrosis severity in alcoholic liver disease by human microfibrillar-associated protein 4. Liver Int. 2020, 40, 1701–1712. [Google Scholar] [CrossRef]
- Saekmose, S.G.; Mossner, B.; Christensen, P.B.; Lindvig, K.; Schlosser, A.; Holst, R.; Barington, T.; Holmskov, U.; Sorensen, G.L. Microfibrillar-Associated Protein 4: A Potential Biomarker for Screening for Liver Fibrosis in a Mixed Patient Cohort. PLoS ONE 2015, 10, e0140418. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M.; Wang, X.; Shuai, W.; Fu, H. MFAP4 deletion attenuates the progression of angiotensin II-induced atrial fibrosis and atrial fibrillation. Europace 2022, 24, 340–347. [Google Scholar] [CrossRef]
- Decaris, M.L.; Gatmaitan, M.; FlorCruz, S.; Luo, F.; Li, K.; Holmes, W.E.; Hellerstein, M.K.; Turner, S.M.; Emson, C.L. Proteomic analysis of altered extracellular matrix turnover in bleomycin-induced pulmonary fibrosis. Mol. Cell Proteom. 2014, 13, 1741–1752. [Google Scholar] [CrossRef] [Green Version]
- Molleken, C.; Poschmann, G.; Bonella, F.; Costabel, U.; Sitek, B.; Stuhler, K.; Meyer, H.E.; Schmiegel, W.H.; Marcussen, N.; Helmer, M.; et al. MFAP4: A candidate biomarker for hepatic and pulmonary fibrosis? Sarcoidosis Vasc. Diffus. Lung Dis. 2016, 33, 41–50. [Google Scholar]
- Pan, Z.; Yang, K.; Wang, H.; Xiao, Y.; Zhang, M.; Yu, X.; Xu, T.; Bai, T.; Zhu, H. MFAP4 deficiency alleviates renal fibrosis through inhibition of NF-kappaB and TGF-beta/Smad signaling pathways. FASEB J. 2020, 34, 14250–14263. [Google Scholar] [CrossRef]
- Yang, J.; Song, H.; Chen, L.; Cao, K.; Zhang, Y.; Li, Y.; Hao, X. Integrated analysis of microfibrillar-associated proteins reveals MFAP4 as a novel biomarker in human cancers. Epigenomics 2019, 11, 1635–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davalieva, K.; Kostovska, I.M.; Kiprijanovska, S.; Markoska, K.; Kubelka-Sabit, K.; Filipovski, V.; Stavridis, S.; Stankov, O.; Komina, S.; Petrusevska, G.; et al. Proteomics analysis of malignant and benign prostate tissue by 2D DIGE/MS reveals new insights into proteins involved in prostate cancer. Prostate 2015, 75, 1586–1600. [Google Scholar] [CrossRef] [PubMed]
- Zaravinos, A.; Lambrou, G.I.; Boulalas, I.; Delakas, D.; Spandidos, D.A. Identification of common differentially expressed genes in urinary bladder cancer. PLoS ONE 2011, 6, e18135. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, S.; Kume, H.; Watanabe, S.; Adachi, J.; Kuwano, M.; Sato, M.; Kawasaki, N.; Kodera, Y.; Ishitobi, M.; Inaji, H.; et al. Strategy for SRM-based verification of biomarker candidates discovered by iTRAQ method in limited breast cancer tissue samples. J. Proteome Res. 2012, 11, 4201–4210. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Y.; Liu, C.H.; Xue, Y.; Chen, Y.Y.; Wang, Y.L.; Wu, X.Z. MicroRNA-147b promotes lung adenocarcinoma cell aggressiveness through negatively regulating microfibril-associated glycoprotein 4 (MFAP4) and affects prognosis of lung adenocarcinoma patients. Gene 2020, 730, 144316. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Yang, H.; Li, X.; Wu, J.; Wang, P.; Liu, D.; Xiao, G.; Sun, X.; Ren, H. Identification of a Novel Cancer Stemness-Associated ceRNA Axis in Lung Adenocarcinoma via Stemness Indices Analysis. Oncol. Res. 2021, 28, 715–729. [Google Scholar] [CrossRef]
- Lv, R.; Wang, Y.; Lin, B.; Peng, X.; Liu, J.; Lu, W.D.; Tian, J. Targeted Luminescent Probes for Precise Upconversion/NIR II Luminescence Diagnosis of Lung Adenocarcinoma. Anal. Chem. 2021, 93, 4984–4992. [Google Scholar] [CrossRef]
- Han, Y.; Xia, K.; Su, T. Exploration of the Important Role of Microfibril-Associated Protein 4 Gene in Oral Squamous Cell Carcinoma. Med. Sci. Monit. 2021, 27, e931238. [Google Scholar] [CrossRef]
- Cao, Z.; Ao, Y.; Guo, Y.; Zhou, S. Comprehensive Analysis of mRNA Expression Profiles in Head and Neck Cancer by Using Robust Rank Aggregation and Weighted Gene Coexpression Network Analysis. BioMed Res. Int. 2020, 2020, 4908427. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, P.; Yuan, M.; Yang, X.; Chong, T. Bioinformatic screening and identification of downregulated hub genes in adrenocortical carcinoma. Exp. Ther. Med. 2020, 20, 2730–2742. [Google Scholar] [CrossRef]
- Hoshida, Y.; Fuchs, B.C.; Bardeesy, N.; Baumert, T.F.; Chung, R.T. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J. Hepatol. 2014, 61, S79–S90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salama, M.M.; Nomir, M.M.; Fahmi, M.W.; El-Gayar, A.M.; El-Shishtawy, M.M. Potential Role of Microfibrillar-Associated Protein 4, Fibrotic Indices and Oxidative Stress in Hepatocellular Carcinoma. Sci. Pharm. 2018, 86, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Sun, Q.; Li, L.; Zhou, J.; Zhang, C.; Hu, T.; Zhou, X.; Zhang, L.; Wang, B.; Li, B.; et al. High Expression Levels of AGGF1 and MFAP4 Predict Primary Platinum-Based Chemoresistance and are Associated with Adverse Prognosis in Patients with Serous Ovarian Cancer. J. Cancer 2019, 10, 397–407. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Sung, D.; Li, M.; Kosti, A.; Lin, G.; Chen, Y.; Pertsemlidis, A.; Hsiao, T.H.; Du, L. microRNA-449a functions as a tumor suppressor in neuroblastoma through inducing cell differentiation and cell cycle arrest. RNA Biol. 2015, 12, 538–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, P.E.; Duran, A.; Ortiz, M.R.; Castro, E.; Garcia-Velasco, A.; Llop, E.; Peracaula, R. Microfibril associated protein 4 (MFAP4) is a carrier of the tumor associated carbohydrate sialyl-Lewis x (sLe(x)) in pancreatic adenocarcinoma. J. Proteom. 2021, 231, 104004. [Google Scholar] [CrossRef]
- Chang, P.Y.; Liao, Y.P.; Wang, H.C.; Chen, Y.C.; Huang, R.L.; Wang, Y.C.; Yuan, C.C.; Lai, H.C. An epigenetic signature of adhesion molecules predicts poor prognosis of ovarian cancer patients. Oncotarget 2017, 8, 53432–53449. [Google Scholar] [CrossRef] [Green Version]
- Meza-Zepeda, L.A.; Kresse, S.H.; Barragan-Polania, A.H.; Bjerkehagen, B.; Ohnstad, H.O.; Namlos, H.M.; Wang, J.; Kristiansen, B.E.; Myklebost, O. Array comparative genomic hybridization reveals distinct DNA copy number differences between gastrointestinal stromal tumors and leiomyosarcomas. Cancer Res. 2006, 66, 8984–8993. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Cho, B.H.; Kim, H.J.; Kim, Y.M.; Jang, J.H. Identification of new genes of pleomorphic adenoma. Medicine 2019, 98, e18468. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef] [Green Version]
- Schartl, M. Beyond the zebrafish: Diverse fish species for modeling human disease. Dis. Models Mech. 2014, 7, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Hason, M.; Bartunek, P. Zebrafish Models of Cancer-New Insights on Modeling Human Cancer in a Non-Mammalian Vertebrate. Genes 2019, 10, 935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doolittle, R.F. Structural and functional diversity of Fibrinogen-Related Domains. In The Evolution of the Immune System; Elsevier: Amsterdam, The Netherlands, 2016; pp. 275–294. [Google Scholar]
- Niu, D.; Peatman, E.; Liu, H.; Lu, J.; Kucuktas, H.; Liu, S.; Sun, F.; Zhang, H.; Feng, T.; Zhou, Z.; et al. Microfibrillar-associated protein 4 (MFAP4) genes in catfish play a novel role in innate immune responses. Dev. Comp. Immunol. 2011, 35, 568–579. [Google Scholar] [CrossRef]
- Zakrzewska, A.; Cui, C.; Stockhammer, O.W.; Benard, E.L.; Spaink, H.P.; Meijer, A.H. Macrophage-specific gene functions in Spi1-directed innate immunity. Blood 2010, 116, e1–e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begon-Pescia, C.; Boireau, S.; Boyer-Clavel, M.; Lutfalla, G.; Nguyen-Chi, M. Preparing sequencing grade RNAs from a small number of FACS-sorted larvae macrophages isolated from enzyme free dissociated zebrafish larvae. MethodsX 2022, 9, 101651. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, K.; Dong, X.; Liang, D.; Zhao, Q. Ncor1 and Ncor2 play essential but distinct roles in zebrafish primitive myelopoiesis. Dev. Dyn. 2014, 243, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Walton, E.M.; Cronan, M.R.; Beerman, R.W.; Tobin, D.M. The Macrophage-Specific Promoter mfap4 Allows Live, Long-Term Analysis of Macrophage Behavior during Mycobacterial Infection in Zebrafish. PLoS ONE 2015, 10, e0138949. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.L.M.; de Vos, I.; Meroshini, M.; Poobalan, Y.; Dunn, N.R. Microfibril-associated glycoprotein 4 (Mfap4) regulates haematopoiesis in zebrafish. Sci. Rep. 2020, 10, 11801. [Google Scholar] [CrossRef]
- Peatman, E.; Baoprasertkul, P.; Terhune, J.; Xu, P.; Nandi, S.; Kucuktas, H.; Li, P.; Wang, S.; Somridhivej, B.; Dunham, R.; et al. Expression analysis of the acute phase response in channel catfish (Ictalurus punctatus) after infection with a Gram-negative bacterium. Dev. Comp. Immunol. 2007, 31, 1183–1196. [Google Scholar] [CrossRef]
- Wu, H.; Mu, L.; Yin, X.; Han, K.; Yan, F.; Zhou, E.; Han, B.; Guo, Z.; Ye, J. A microfibril-associated glycoprotein 4 (MFAP4) from Nile tilapia (Oreochromis niloticus) possesses agglutination and opsonization ability to bacterial pathogens. Fish. Shellfish Immunol. 2020, 104, 182–191. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Q.; Chen, Y.; Liu, Q.; Wang, W.; Song, J.; Zheng, Y.; Liu, W. Integrated Analysis of mRNA- and miRNA-Seq in the Ovary of Rare Minnow Gobiocypris rarus in Response to 17alpha-Methyltestosterone. Front. Genet. 2021, 12, 695699. [Google Scholar] [CrossRef]
- Zhou, L.; Liang, H.; Zhou, X.; Jia, J.; Ye, C.; Hu, Q.; Xu, S.; Yu, Y.; Zou, G.; Hu, G. Genetic Characteristic and RNA-Seq Analysis in Transparent Mutant of Carp-Goldfish Nucleocytoplasmic Hybrid. Genes 2019, 10, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilecki, B.; Moeller, J.B. Fungal recognition by mammalian fibrinogen-related proteins. Scand. J. Immunol. 2020, 92, e12925. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, A.; Sorensen, G.L.; Pilecki, B. MFAP4-Mediated Effects in Elastic Fiber Homeostasis, Integrin Signaling and Cancer, and Its Role in Teleost Fish. Cells 2022, 11, 2115. https://doi.org/10.3390/cells11132115
Mohammadi A, Sorensen GL, Pilecki B. MFAP4-Mediated Effects in Elastic Fiber Homeostasis, Integrin Signaling and Cancer, and Its Role in Teleost Fish. Cells. 2022; 11(13):2115. https://doi.org/10.3390/cells11132115
Chicago/Turabian StyleMohammadi, Ali, Grith L. Sorensen, and Bartosz Pilecki. 2022. "MFAP4-Mediated Effects in Elastic Fiber Homeostasis, Integrin Signaling and Cancer, and Its Role in Teleost Fish" Cells 11, no. 13: 2115. https://doi.org/10.3390/cells11132115
APA StyleMohammadi, A., Sorensen, G. L., & Pilecki, B. (2022). MFAP4-Mediated Effects in Elastic Fiber Homeostasis, Integrin Signaling and Cancer, and Its Role in Teleost Fish. Cells, 11(13), 2115. https://doi.org/10.3390/cells11132115






