Abstract
Worldwide, the prevalence of surgery under general anesthesia has significantly increased, both because of modern anesthetic and pain-control techniques and because of better diagnosis and the increased complexity of surgical techniques. Apart from developing new concepts in the surgical field, researchers and clinicians are now working on minimizing the impact of surgical trauma and offering minimal invasive procedures due to the recent discoveries in the field of cellular and molecular mechanisms that have revealed a systemic inflammatory and pro-oxidative impact not only in the perioperative period but also in the long term, contributing to more difficult recovery, increased morbidity and mortality, and a negative financial impact. Detailed molecular and cellular analysis has shown an overproduction of inflammatory and pro-oxidative species, responsible for augmenting the systemic inflammatory status and making postoperative recovery more difficult. Moreover, there are a series of changes in certain epigenetic structures, the most important being the microRNAs. This review describes the most important molecular and cellular mechanisms that impact the surgical patient undergoing general anesthesia, and it presents a series of antioxidant therapies that can reduce systemic inflammation.
1. Introduction
Anesthesia allows the performing of surgical procedures in a rapid, safe, and pleasant manner, producing analgesia, absence of awareness, and adequate muscle relaxation when needed [1,2,3]. A critical aspect of perioperative anesthetic care is the maintenance of homeostasis, including hemodynamic stability, oxygenation, ventilation, and temperature. The World Health Organization (WHO, www.who.int/, accessed on 20 May 2022) and the World Bank (WB, www.worldbank.org, accessed on 20 May 2022) expect that by 2026, the burden of diseases requiring surgery and anesthesia will exceed that of HIV, tuberculosis, and malaria, measured in disability-adjusted life years. As anesthesia providers are an integral part of the delivery of safe and effective surgical care, it is imperative to develop the necessary tools to minimize mortality and morbidity in the perioperative period [4,5].
Surgical stress induces an immuno-inflammatory response not necessarily proportional to the degree of tissue damage. In addition to the surgical injury, other factors may be contributing to the inflammatory response in the perioperative period, including the general anesthesia itself, mechanical ventilation, the administration of blood products, and antiemetic drugs [6]. Moreover, numerous studies have highlighted a number of molecular changes induced by anesthetic substances, involving the expression of inflammation and hypermetabolism during general anesthesia. An abnormal over- or under-expressed immuno-inflammatory response has been associated with various relevant postoperative conditions, including infections, pulmonary complications, delirium and postoperative cognitive dysfunction [7], renal injury [8], and cancer recurrence [9,10,11]. Another important molecular and cellular effect is the unbalance of the expression of antioxidants in relation to the activity of reactive oxygen and nitrogen species (ROS, RNS). This phenomenon is called oxidative stress (OS) and refers to redox activity or reduction–oxidation activity [9,10].
This review article aims to integrate current knowledge regarding the effects of commonly used anesthetic agents on the inflammatory response developed in the postoperative period.
2. Redox Disturbance and Inflammation during General Anesthesia and Surgery Procedures
A degree of inflammation during surgery is unavoidable and represents the first necessary mechanism in wound healing. It involves complex pathways that alter endocrine, hemodynamic, metabolic, redox, and immune responses [12]. The main link between localized injury and a systemic inflammatory response is represented by the damage-associated molecular pattern (DAMP) molecules, also called alarmins [13]. These molecules have a physiological role inside the cell but acquire additional functions when exposed to the extracellular environment: they can activate antigen-presenting cells (APCs); have chemotactic properties; and exhibit immunoenhancing activity, stimulating both the innate and adaptive immune system [14]. Alarmins include high mobility group box 1 (HMGB1), heat shock proteins (HSPs), defensins, cathelicidin, eosinophil-derived neurotoxin (EDN), S100 proteins, purine metabolites, and DNA or RNA located outside the nucleus or mitochondria. Hence, alarmins represent a diverse and structurally different group of molecules released by damaged or dying cells [15]. Interestingly, these “danger”-sensing molecules exert their functions by interacting with specific receptors expressed on damaged or dying cells but not on apoptotic cells [16].
As the first line of defense following tissue injury, migrating macrophages and granulocytes produce a pro-inflammatory reaction at the site of injury, which stimulates the release of various pro-inflammatory cytokines [17,18,19]. This response is rapidly contained by an anti-inflammatory response that aims to fine-tune and produce a balance between the defense and healing processes [20].
From a biochemical viewpoint, oxidative stress is characterized by an imbalance between oxidant and antioxidant factors, although pro-oxidative species have a higher share. The most reactive of all these species are hydrogen peroxide, hydroxyl radicals, superoxide anions, reactive nitrogen species, reactive lipid species, and oxidative fragments resulting from protein denaturation [21,22,23]. Free radicals can be formed both intra- and extracellularly due to certain exogen factors that manage to penetrate human biological systems. The most common intracellular sources of oxidant substances are nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, mitochondria, the endoplasmic reticulum, lysosomes, cytochrome P450, and peroxisomes [24]. The body has a series of enzymatic systems, such as superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT), as well as certain antioxidant molecules, such as glutathione (GSH), vitamin E, vitamin C, melatonin, uric acid, and a series of polyphenols, with important antioxidant properties. The most well-known and widely researched oxidative reduction mechanism is that of SOD, responsible for the molecular conversion of superoxide anions to hydrogen peroxide, which leads to the formation of water through enzymatic catalyzation induced by CAT and GPX [25,26]. The reduction of oxidative species by GSH with the formation of glutathione disulfide (GSSG) is another mechanism of oxidative reduction [27]. Furthermore, reactive oxygen species help modify the cellular signaling pathways responsible for cellular proliferation, differentiation, autophagia, and apoptosis. Responsible for this is the activation of pro-inflammatory mechanisms, which leads to the formation of cytokines by activating nuclear factor κB (NF-κB), AMP-activated protein kinase, hypoxia-inducible factor, and Kelch-like ECH-associated protein 1 (KEAP1) [28]. During pro-oxidative states, at a cellular level, the mitochondria are responsible for generating oxidative factors involved in the augmentation of the redox state, which has further molecular and cellular effects. The mechanism is known as mitochondrial reactive oxidative species production (mitoROS) [29,30]. In the mitochondria, complexes I, II, and III are involved in the production of reactive oxygen species. From a molecular point of view, complex I is responsible for the entrance of electrons from the NADH complex into the respiratory chain. Therefore, the interaction of flavinmononucleotides with oxygen will result in the hydroxyl anion that will afterward be released in the mitochondrial matrix [31,32,33]. Complex II helps produce reactive species through the oxidation of succinate to fumarate, a part of the Krebs cycle. Increased amounts of ROS are produced by complex II when complexes I and III are blocked at a molecular level. Following redox activity, complex III leads to the production of high amounts of hydrogen peroxide, which is able to diffuse in the mitochondrial matrix [34,35]. Once the biological systems responsible for the adequate functioning of the mitochondria have been pro-oxidatively damaged, increased amounts of ROS will lead to mitochondrial death and the accumulation of highly oxidative species inside the cell. Physiologically, the mechanisms responsible for inhibiting the pro-oxidative destructive mechanisms inside the cell are GSH, thioredoxin (Trx), and glutaredoxin (Grx) [36]. Recent studies have shown increased oxidative reduction activity inside the mitochondria for SOD, which functions by blocking the redox activity of oxygen ions in hydrogen peroxide. According to Ribas et al., during enzymatic antioxidant activity in the mitochondria, the most important activity is that of SOD2 (manganese-dependent superoxide dismutase, MnSOD) [37], while in the intermembrane space, it is that of SOD1 (Cu, Zn-SOD). Through the activity of the SOD molecular family, reactive oxygen species are transformed into hydrogen peroxide, which has a less pronounced oxidative character. Enzymatic antioxidant species such as glutathione reductases, peroxidases, and peroxiredoxins are activated for the total inhibition of redox activity. They will dissociate hydrogen peroxide into water and oxygen. Regarding the Trx and Grx antioxidant enzymes, researchers have discovered different species, such as Trx2 and thioredoxin reductase-2 (TrxR2), responsible reducing oxidative stress by modulating NADPH mechanisms and taking control of electron migration inside the mitochondria [38,39,40,41]. The family of Grx antioxidant enzymes includes Grx2 and Grx5, responsible for modulating molecular mechanisms that produce oxygen ions in complex I by catalyzing thiol groups in GSH [42]. An increase in the redox state at the cellular organelle level will lead to a decrease in ATP production due to the migration of electrons toward the water used in the formation of hydrogen peroxide. Other important molecular sources of oxidative species inside the cell are characterized by the interaction between reactive oxygen ions with D-aspartate oxidase, xanthine oxidase, polyamine oxidase [43,44], (ACOX), L-αhydroxyacid oxidase, D-amino acid oxidase, and L-pipecolic oxidase. Due to insufficient electrons for ATP production at the mitochondrial level, the whole cell will be affected by the so-called energy failure phenomenon, which has important implications for the whole body and will have the utmost clinical impact on critically ill patients [45,46,47,48,49].
An important goal during surgery and general anesthesia is maintaining an adequate cerebral blood flow that will sustain normal cerebral metabolic activity and sufficient brain oxygenation. The literature has shown a 2–10% rate of severe cerebral ischemia cases during cardiovascular surgery and 0.05–7% during non-cardiac surgery [50,51,52]. Biochemically, important amounts of ROS and RNS are produced during ischemia–reperfusion. Moreover, an important increase in the intracellular Ca2+ levels has also been demonstrated, as well as an exacerbation of the neurotoxic properties of glutamate. Wang et al. studied the molecular effects induced by curcumin-encapsulated nanoparticles on oxidative stress in cerebral tissue that had undergone ischemia–reperfusion injury. They proved a decrease in the oxidative attachment on the endothelial tissue, as well as a decrease in the blood–brain barrier (BBB) [53,54,55].
Recent studies have discussed different theories regarding cerebral metabolism and the regional cerebral blood flow (eCBF) [56,57]. Maintaining an optimal cerebral blood flow is crucial for ensuring continuous global or regional oxygenation. Weiss et al., in an experimental study regarding cerebral oxygen consumption during ischemia and reperfusion, identified a direct correlation between O2 supply/consumption and ischemia–reperfusion syndrome. The authors used two study groups of laboratory animals, one group (n = 9) where cerebral ischemia was induced and one group (n = 9) where reperfusion was induced. The study animals received 90% isoflurane anesthesia with a mixture of oxygen and gas through an ETT tube, with the endpoint of the partial pressure of oxygen of over 100 mmHg. To achieve cerebral ischemia, the authors blocked the medial cerebral artery for 1 h in the case of group 1, while for group 2, they reperfused the same artery for 2 h. Cerebral blood flow was determined using the C14-iodoantipyrine autoradiographic technique [58].
In a study on cerebral protection bestowed by anesthetic agents, Sakai et al. reported improved neurological function after focal ischemia. During the study, the researchers induced cerebral ischemia in lab animals through the temporary occlusion of the medial cerebral artery. They proved a reduced incidence of neurological deficits in the animals that suffered from cerebral ischemia under general anesthesia with isoflurane, compared to the control group. Moreover, the authors showed that the cerebral protection induced by isoflurane persists up to eight weeks after ischemia [59].
Another important oxidative mechanism in the case of IR injury is based on the iNOS activity and on increased NO concentrations. The redox implications of NO are interesting as they have an increased reactivity toward other molecules. A specificity of this pathway is the formation of peroxynitrite free radicals following the interaction between NO and the superoxide radical [60,61]. At the cellular level, peroxynitrite attacks the mitochondrial membrane and DNA oxidation, blocking DNA repair mechanisms and leading to cell energy failure [62,63].
Neuronal OS occurring during cerebral ischemia is divided into three major groups of nitric oxide synthases: end endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS) [64,65]. Recent studies have shown an increase in the activity of these enzymes during cerebral ischemia, with a significant increase in NO production. In a study of the expression of NO in patients suffering from Moyamoya disease who had undergone bypass surgery with a temporary occlusion of the M4 branch of the middle cerebral artery, Silver et al. showed increased expression of mixed venous nitride (NO2) immediately after the occlusion of the cerebral artery. The authors concluded that NO2 expression is directly proportional to the degree of cerebral ischemia [66].
During cerebral ischemia, the released OS and the increased flow of reactive species can influence the BBB, leading to a migration of T lymphocytes, macrophages, natural killer cells, and polymorphonuclear leucocytes. Among the most widely studied reactive species involved in the BBB destruction are transforming growth factor beta (TGF-β), interleukin 6 (IL-6), interleukin 10 (IL-10), interleukin 1-beta (IL-1β), interferon beta (IFN-β), and tumor necrosis factor alpha (TNF-α) [50,67,68,69,70]. Boutin et al. proved the destructive impact of Il-1 in ischemic brain injury. Numerous mechanisms correlate increased IL-1β production with an increase in BBB permeability [71].
The most representative chemokines involved in the molecular mechanisms of IR are the monocyte chemoattractant protein-1 (MCP-1/CCL2). The main activity of MCP-1 is the central recruitment of activated lymphocytes, macrophages, and monocytes. Hartley et al. studied the implications of CCL2 in the changes that occur at the BBB level after ischemia. Blocking CCL2 with an antibody significantly reduces the permeability of the BBB [72], together with the distribution of the tight-junction proteins. A similar study carried out by Stamatovic et al. reported that MCP-1 plays an essential role in leukocyte recruitment and in the increase in BBB permeability [73].
One of the main aspects of general anesthesia is mechanical ventilation, which has significant implications for OS expression in the surgical patient. The most common trigger for OS expression at cellular and molecular levels is lung ischemia–reperfusion injury (LIRI). By reducing or even interrupting pulmonary blood flow, the reactive oxygen species production in the endothelium will be accelerated. Another site for excessive ROS production is the alveolar macrophage. The reperfusion phase is characterized by pro-inflammatory cytokine release, the activation of NOS, and neutrophile recruitment [73,74,75,76,77,78] (Figure 1).
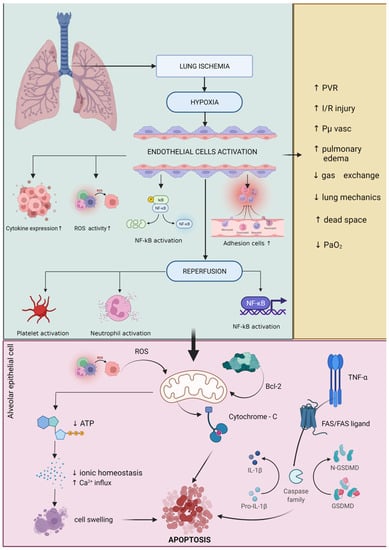
Figure 1.
Schematic representation of lung ischemia–reperfusion injury. Green border: Lung ischemia causes a high degree of hypoxia that directly affects endothelial tissue with cytokine activation, increased cell adhesion activity, NF-κB activation, and accelerated generation of reactive oxygen species (ROS). Following reperfusion, a number of other biological mechanisms responsible for increasing pro-inflammatory status (e.g., NF-κB activation), platelet activation, and neutrophil activation. Pink border: these mechanisms directly affect cellular activity by altering mitochondria-specific biochemical pathways. The direct attack of ROS on the mitochondria lowers ATP biosynthesis and affects mitochondrial electrolytic homeostasis, especially by increasing the influx of Ca2+ leading to cell swelling and apoptosis. Another biochemical pathway with a significant negative effect on mitochondrial activity is the activation of TNF-α, which activates caspase family via FAS/FAS-ligand and generates increased amounts of IL-1β by catalyzing pro-IL-1β factor. Another mechanism found in cellular apoptosis due to damage to alveolar epithelial cells is the direct action of bcl-2 on the mitochondria leading to cytochrome-C overexpression and finally cellular apoptosis. Yellow border: all these biochemical mechanisms that are involved in the process of mitochondrial and cellular denaturation induced by the phenomenon of ischemia–reperfusion of lung tissue lead to increased pulmonary vascular resistance (PVR), increased pulmonary edema, increased oncotic pressure of the vascular capacity, decreased lung mechanics, increased dead space and decreased adequate oxygenation. Increased expression of reactive oxygen species leads to changes in the cellular and molecular activities in lung tissue, mainly affecting the expression of calcium/calmodulin-dependent NO, nicotinamide adenine dinucleotide phosphate (NADPH), and nuclear factor kappa B (NF-κB). The acceleration of these processes inside the cell leads to pulmonary edema, resulting from an increase in pulmonary vascular resistance and endothelial damage. During mechanical ventilation, these secondary phenomena lead to decreased ventilation and impaired gas exchange in the alveoli. After reperfusion, pulmonary vascular resistance can increase by up to 100%, mainly due to vasoconstriction and endothelial damage in the lung microvasculature. Increased vascular resistance, worsening pulmonary edema, and increased extravascular lung water lead to impaired gas exchange and impaired lung mechanics. All these phenomena negatively impact the clinical status of the patient through a sudden decrease in arterial partial oxygen pressure and an increase in the peak airway pressure and the alveolar–arterial oxygen gradient (A-a/DO2) [78,79]. Recent studies have shown that during pulmonary reperfusion, microvascular permeability can increase by up to 10 times. It was suggested that the initial stage depends on the production of interleukin-8 (IL-8), interleukin 12 (IL-12), interleukin-18 (IL-18), and TNF-α, while the second stage is responsible for the production of activated neutrophiles and pro-oxidative factors, such as interleukin-8 (IL-8), leukocyte adhesion molecule CD18, endothelial P-selectin, and endothelial intracellular adhesion molecule-1 [74].
Changes in the redox balance represent another frequent phenomenon in the case of LIRI, as an excessive production of reactive oxygen species (ROS) occurs right after the reperfusion. In the lung, there are a series of potential ROS generators, such as activated xanthine oxidase, neutrophiles, mitochondria, the NADPH oxidase system, and NO synthase. In a study, Chen et al. showed that mitochondrial damage plays a more important role in the ischemia process than in the reperfusion process. The oxidative phenomenon is augmented by reduced oxygen delivery in the mitochondria, which leads to a decreased oxidative phosphorylation process and ATP production [80]. Decreased ATP production will lead to a dramatic decrease in the mitochondrial membrane potential, leading to calcium transfer outside the mitochondria and the initiation of cellular apoptosis. Damage to the mitochondrial membrane and permeabilization of the membrane will lead to an increased destruction of certain proteins that are vital for the normal functioning of the mitochondrial system, such as cytochrome-C. Furthermore, the release of cytochrome-C in the cytosol will lead to the activation of caspase-9 and an increase in the apoptotic process at the cellular level [81,82]. NOS found in the lung can be subdivided into four species: endothelial NOS (eNOS), inducible NOS (iNOS), neuronal NOS (nNOS), and mtNOS. Studies have shown an increased pro-inflammatory activity for mtNOS, as well as an increased anti-inflammatory activity for iNOS and eNOS [83,84,85,86].
In their study, Fischer et al. showed that apoptosis is only present after the reperfusion of lung tissue, that the activity increases dramatically, and that the peak is reached after 2 h. Apoptosis is initiated by the activation of both intrinsic and extrinsic mechanisms, with mitochondria as the modulating factor in both pathways [87]. The intrinsic pathway is also known as the mitochondrial pathway and is initiated by the action of cytokines and free radicals. It is characterized by changes in mitochondrial membrane permeability through bcl-2 proteins. The extrinsic pathway, however, is characterized by the activation of certain signaling proteins, such as the TNF receptor, angiotensin 2, and the FAS/FAS ligand [88,89].
Ischemia in the lung tissue activates caspase-8 through the FAS/FAS-ligand system. The next phase is represented by the activation of caspase-7, caspase-6, and caspase-3, leading to DNA fragmentation through the poly-ADP ribose polymerase [88]. During ischemia–reperfusion syndrome, research has shown a rapid release of vasoconstrictor and vasodilatory mediators inside the lung, all derived from arachidonic acid: C3 and C5a complement fragments; thrombin; transcription factor; and a series of pro- and anti-inflammatory cytokines, such as IL-1β, interleukin-2 (IL-2), IL-6, IL-8, interleukin-9 (IL-9), IL-10, IL-18, interferon-γ (INF-γ), and TNF-α.
The consequences of various anesthetic drugs for immune cells have been extensively investigated in in vitro studies. Cultures of immune cells, such as neutrophils, lymphocytes, and/or NK-cells, are separated and exposed to clinical concentrations of anesthetics. Simultaneously, the cells are isolated from the whole network and interaction with other immune cells or mediators that may not reflect the clinical situation.
Several studies have shown that propofol has immuno-inhibitory activity through actions on the non-specific immune system, impairing monocyte and neutrophil functions such as chemotaxis, phagocytosis, and oxidative burst. Some studies have suggested that this activity is due to the lipid solvents found in the formula. The chemotactic and phagocytic function of neutrophils may be inhibited by a decrease in intracellular calcium. The effects of propofol seem to be dose-dependent and occur at clinically relevant concentrations [90,91]. Propofol has been found to suppress the functions of polymorphonuclears, lymphocyte proliferation, and cytokine release in response to endotoxemia only in patients already immunocompromised, but not in healthy volunteers. Hoff et al. investigated the effects of propofol and ketamine on TNF-α gene expression in peripheral blood mononuclear cells and found that lipopolysaccharide endotoxin stimulated TNF-α and that mRNA steady-state transcripts were significantly increased in the presence of propofol (+42%) and decreased in the presence of ketamine (−31%) compared to control cell groups [92].
The effects of most anesthetics are at least partially mediated through GABA-A receptors. Several studies have demonstrated that these receptors can be found on the immune cell membranes and can be influenced by anesthetic drugs. GABA can activate ion channels on macrophages and monocytes and decrease cytokine secretion and T-cell proliferation. Moreover, drugs that influence GABA concentration, such as vigabatrin and muscinol, seem to have the same effects. There is extensive crosstalk between the central nervous system and the immune system, and these findings offer a possible explanation as to why chronic propofol administration in intensive care patients increases the risk of infection. On the contrary, monocyte GABA-A receptors are not influenced by diazepam. Therefore, the use of benzodiazepines as sedative agents may be more appropriate where infection is a life-threatening problem.
The effects of inhalational anesthetics are also mainly inhibitory, for example, suppressed neutrophil function, altered lymphocyte proliferation, and decreased cytokine release by mononuclear cells. In contrast to halogenated inhalational anesthetics, which are known to suppress pro-inflammatory cytokines in murine pulmonary cells, volatile anesthetics induce increased gene expression of pro-inflammatory cytokines. Moreover, some studies have shown that the effects of inhalational anesthetics are dose- and time-dependent [93,94,95].
A 2013 study suggested that the NF-κB signaling pathway contributes to sevoflurane- and isoflurane-induced neuroinflammation by increasing the levels of IL-6 (isoflurane by 410% and sevoflurane by 290%) and the nuclear levels of NF-κB (isoflurane by 170% and sevoflurane by 320%). Other studies have confirmed that isoflurane can increase the levels of IL-6, which has been associated with learning and memory impairment in animals [96,97].
Several in vitro studies have reported changes in the immune and inflammatory expression of morphine and other opioids. Among the effects of morphine are altered cytokine release and lymphocyte proliferation, activated enzymes involved in macrophage and lymphocyte apoptosis, inhibition of cell adhesion, and increased secretion of stress hormones. Synthetic opioids, except for fentanyl, seem to have a less pronounced impact on the immune system, probably because they do not interact with the µ2 opioid receptors on immune-competent cells [98,99].
Intravenous rocuronium bromide induces pain during injection and/or withdrawal movement, the exact mechanism of which is not yet understood. One study found that rocuronium bromide induces inflammation in calf cells by inhibiting endothelial nitric oxide synthase, suppressing nitric oxide production, activating cyclo-oxygenase-2, and increasing prostaglandin E2 synthesis, which may be the cause of pain during injection [100].
Challenges in isolating various immune variables from the complex immunological network in clinical scenarios lead to far fewer in vivo than in vitro studies. Due to their volatile nature, inhalational agents are important not only to the patients but also to the personnel attending the operating theater. Chronic occupational exposure to low doses of isoflurane were correlated with fewer total T and T-helper (CD4+) lymphocytes in a dose- and time-dependent manner [101]. The in vivo effects of sevoflurane and isoflurane largely reflect the in vitro studies. Several studies have demonstrated that these two agents can reduce the levels of reactive species of oxygen, inhibit chemotaxis, and inhibit the activation of neutrophils, leading to decreased neutrophil adhesion to human endothelial cells, decreased IL-6 and TNF-α, suppressed PMN migration and function through various pathways, altered cytokine release from macrophages, and decreased NK cytotoxic activity. A study comparing two anesthetic techniques, TIVA and balanced inhalational anesthesia, found that perioperative cell-mediated immunity was less influenced by TIVA, suggesting that the stress response in patients undergoing anesthesia with a volatile agent is more pronounced [102,103,104,105,106,107].
A 2019 study on the effects of propofol on neutrophil function, lipid peroxidation, and the inflammatory response during elective coronary artery bypass grafting in patients with impaired ventricular function found that the addition of propofol contributes to a lesser increase in IL-6 levels and a greater decrease in IL-10 compared to placebo. No effects were seen on IL-8. In addition, malondialdehyde (MDA), a marker of lipid peroxidation and free-radical injury, measured in the coronary artery sinus, was lower in the propofol group at 1, 3, 5, and 60 min after reperfusion (p < 0.01), suggesting that propofol attenuates free-radical-mediated lipid peroxidation and systemic inflammation in patients with impaired myocardial function undergoing CABG [108].
Potocnik et al. studied patients undergoing thoracic surgery and one-lung ventilation where either propofol or sevoflurane was used to maintain the anesthesia. They found that IL-6, IL-8, and CRP levels were higher in the propofol group, while IL-10 was higher in the sevoflurane group. The oxygenation index 6 h after the surgery was lower in the propofol group compared to the sevoflurane group (p = 0.02), maintained 24 h after the surgery (p = 0.019). The number of postoperative adverse events was significantly higher in the propofol group (p < 0.05) and included ARDS, pneumonia, and SIRS [109]. In a similar manner, but on patients undergoing craniotomy, propofol proved to be less inflammatory than sevoflurane, with a lower IL-6/IL-10 concentration ratio during and at the end of surgery (p = 0.0001) and higher IL-10 [110].
A 2019 study compared the effects of propofol with those of desflurane on inflammation and ischemia–reperfusion syndrome during robot-assisted laparoscopic radical prostatectomy (RALRP) and found that propofol-based anesthesia significantly attenuated the increase in IL-6 levels during RALRP (4.68 ± 2.76 pg/mL vs. 8.57 ± 3.72 pg/mL; p < 0.001), but with similar effects on TNF-α, CRP, and NO levels [111].
The American Heart Association (AHA) recommends that patients at risk of myocardial ischemia during surgery who are hemodynamically stable should undergo general anesthesia with inhalational agents [112].
These recommendations and the benefits of these agents have been emphasized in numerous studies. A study conducted on 74 patients scheduled for coronary artery bypass graft surgery under cardioplegic arrest showed that 10 min of preconditioning with sevoflurane decreased the release of brain natriuretic peptide in the perioperative period. Moreover, the levels of plasma cystatin C were lower in sevoflurane-preconditioned patients, suggesting improvement in both cardiac and renal function after major heart surgery [113].
Fukazawa et al. presented extensive evidence, both in vitro and in vivo, that modern inhalational halogenated anesthetics play an important role in the pathophysiology leading to AKI by inhibiting renal tubular and endothelial cell necrosis and apoptosis, and inducing anti-inflammatory effects at this level by activating pathways responsible for anti-inflammatory and cytoprotective signaling molecules, such as releasing TGF-β1, activating CD73, inducing IL-11, and generating adenosine [114]. A systematic review concluded that patients undergoing cardiac surgery had a significantly lower increase in creatinine levels in the first two days after surgery and lower intensive care unit stay and hospitalization when anesthetized with volatile anesthetics compared to TIVA propofol. However, there was no statistically significant difference in mortality between the groups [115]. Furthermore, current anesthetics seem to offer neuroprotection, sustained for 2–4 weeks, against mild or moderate ischemic injury but have no influence on severe ischemia. These effects are mediated by antagonizing postsynaptic glutamate receptors and enhancing GABA-A-mediated hyperpolarization and by inhibiting several processes that lead to neural apoptosis (increased levels of antiapoptotic proteins, such as bcl-2 and reduced cytochrome-C release) [116,117,118].
The benefits of inhalational anesthetics extend beyond the operating theater, with extensive research suggesting that sedation with sevoflurane or isoflurane in the intensive care unit plays an important role in preventing ventilatory-associated lung injury (VILI). The release of pro-inflammatory markers, such as IL-1b and MIP-1β, and reactive oxygen species, as well as neutrophil transmigration into the alveolar compartments of the lungs are rapidly prevented in the early stages of VILI if sedation is switched from intravenous to inhalational [119]. Moreover, inhalational anesthetics seem to influence glycemic control in the perioperative period. Blood glucose levels were found to be higher with volatile agents, such as sevoflurane and isoflurane, compared to propofol-maintained anesthesia. This response was attributed to impaired glucose clearance and increased glucose production and inhibition of normal insulin production.
Kalimeris et al. studied the antioxidant effects of propofol on patients undergoing carotid endarterectomy. The study protocol included a control group, with 21 patients who were administered general anesthesia (GA) using sevoflurane, and a study group, in which GA was administered using propofol (n = 23). The biomarkers used for the quantification of oxidative stress included the expression of P-selectin and S100B protein, the lactate levels, nitrate, nitrite, and malondialdehyde. The study showed statistically significant differences regarding S100B levels that were lower in the propofol group. Moreover, it showed lower levels of malondialdehyde (MDA), nitrates, and nitrites in the same propofol group [120]. Yang et al. carried out a similar study regarding the inflammatory influence of sevoflurane vs. isoflurane in an experimental ischemia–reperfusion model. To quantify the impact on OS, they analyzed certain biomarkers, such as superoxide dismutase (SOD), MDA, nitric oxide (NO), and complement C3, C5a, and C6. The study reported lower expressions of OS in the sevoflurane group [121] (Table 1).

Table 1.
The impact of anesthetic drugs on inflammatory expression and cellular pathways activity.
3. Implications of Genetic and Epigenetic Expression on Inflammation and Oxidative Pathways
microRNA species are biosynthesized in the cell nucleus through the action of RNA polymerase II on specific genes. The initial species that result after the first interaction are called pri-microRNAs [152]. The chain of biochemical processes continues with the attack of RNA polymerase III endonuclease (Drosha) on the pri-microRNAs, leading to the formation of pre-microRNAs. This reaction is catalyzed by DiGeorge Syndrome Critical Region 8 (DGCR8) [153]. The biosynthesis process continues in the cell cytoplasm, where pre-microRNAs are transported from the nucleus to the cytoplasm with the help of Exportin-5. The final microRNA species result from a reaction between the pre-microRNAs and Enase III endonuclease (Dicer) and the RNA binding protein (TRBP). The newly formed epigenetic species are then transported inside the cell as microvesicles, exosomes, ribonucleoprotein complexes, and high-density lipoproteins [154]. Recent studies have shown that anesthetic drugs induce a series of molecular changes that are especially involved in the modulation of the inflammatory and oxidative chains. Kim et al. studied the process and identified a decrease in the expression of microRNA-27a, microRNA-194, microRNA-23b, microRNA-133a, microRNA-199a, microRNA 101, microRNA-219-5p, microRNA-30b, microRNA-92b, microRNA-127, and microRNA-204. In the same study, they reported increases in the expression of microRNA-370, microRNA-134, microRNA-302a, microRNA-107, microRNA-92a, microRNA-202, microRNA-190b, microRNA-29b, microRNA-216a, microRNA-181d, and microRNA208b [155]. A similar study was carried out by Twaroski et al. and showed essential changes in the expression of miRNA-21 in patients who had received propofol [156].
The brain is one of the most sensitive organs that must be protected during GA. Anesthetic management and perioperative protocols especially focus on maintaining adequate cerebral perfusion and oxygen delivery. The pathophysiological mechanisms involved in maintaining a constant and adequate cerebral perfusion are complex and not fully understood. It is, however, a known fact that cerebral autoregulation maintains a constant cerebral blood flow despite the changes in mean arterial pressure. Biological functions are extremely sensitive, even to the slightest change in mean arterial pressure, and therefore the cerebral autoregulation mechanisms will be activated to prevent cellular anoxia [157]. From an epigenetic point of view, scientists have identified different involvements of microRNAs in the biological pathways related to cerebral ischemia and reperfusion. In a study regarding the expression of microRNAs in cerebral ischemia in experimental animals, Gusar et al. identified statistically significant changes up to 48 h after cerebral ischemia for a series of epigenetic species, such as microRNA-30c-2-3p, microRNA-125b-5p, microRNA-300-5p, microRNA-107-3p, microRNA-497-5p, microRNA-27a-3p, microRNA-30b-3p, microRNA-29c-3p, microRNA-124-3p, microRNA-330-5p, microRNA-181b-5p, microRNA-29a-3p, let-7i-3p, microRNA-22-3p, let-7i-5p, microRNA-212-3p, microRNA-26a-5p, microRNA-99a-3p, microRNA-221-3p, microRNA-27b-3p, microRNA-24-3p, microRNA-22a-5p, microRNA-29a-5p, microRNA-186-5p, microRNA-99a-5p, microRNA-128-3p, microRNA-23a-3p, microRNA-376b-5p, microRNA-30c-5p, microRNA-9a-5p, microRNA-30a-3p, let-7f-5p, microRNA-21-5p, and microRNA-223-3p [158]. A molecular mechanism associated with cell survivor apoptosis is phosphatase and tensin homologous protein (PTEN) and its involvement in the biochemical activity of PI3K/AKT [159]. In a study on the cellular and epigenetic mechanisms involved in initiating, augmenting, and maintaining cerebral ischemic injury, Wang et al. showed that microRNA-32-5p activity is directly proportional to the expression of PTEN/P13K/AKT, which is responsible for protecting cerebral tissue. Huang et al. performed a similar study to present the role of microRNA-326-5P in reducing the signal transducer and activator signal of transcription-3 (STAT3) and the protection of cerebral tissue during ischemia–reperfusion syndrome [160].
Other mechanisms involved in cerebral injury following ischemia and reperfusion are the excessive production of free radicals, mitochondrial disfunction, lipid degeneration, protein oxidation, DNA structure modification, and finally cerebral tissue death. In an experimental study, Guo et al. reported a series of neuronal protection mechanisms through the activity of microRNA-25 [161]. Huang et al. described that ischemia and reperfusion lead to augmented tissue damage and accelerated cell death [162]. In a complex study on the actions of microRNA-25 in neuronal protection during cerebral ischemia, Zang et al. reported that the increased expression of microRNA-25 inhibits Fas/FasL activity and reduces apoptosis induced by ischemic injury [163]. Mao et al. reported protective effects at the cerebral level when p38 mitogen-activated protein kinase (MAPK) activity is inhibited through microRNA-128-3p. Moreover, they showed that higher levels of microRNA-128-3p are able to block the post-transcription activity of p38α and can contribute to the survival of neurons during cerebral ischemia [164].
An experimental study carried out on laboratory animals by Zhang et al., investigating the expression of certain biological markers, such as microRNAs-144, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), and glycogen synthase kinase-3β (GSK3β) in LIRI, showed that the overexpression of genes was directly proportional to the intensity of the ischemic injury. Regarding the epigenetic expression of microRNA-144, the authors reported a decrease in its concentration [165]. Li et al. studied the expression of microRNA-146a in LIRI and showed a decrease in the epigenetic expression in pulmonary ischemic injury. The group identified that microRNA-146a activity is directly proportional to the overproduction of IL-1β, IFN-γ, TGF-β1, and TNF-α [166]. Zhou et al. reported an increase in the expression of microRNA-155 during LIRI, accompanied by an overexpression of IL-6, IL-1β, TNF-α, 8-isoprostaglandin F2α (8-iso PGD2α), and 8-hydroxy-2-deoxyguanosine (8-OHdG) [167]. Overexpressed in LIRI, microRNA-223 is involved in the hypoxia-inducible factor-2α (HIF2α) pathway, being responsible for worsening the pro-inflammatory status in the lung [168]. Cai et al. carried out a study on the expression of microRNAs in pulmonary ischemia in laboratory animals that had undergone pulmonary transplantation, discovering increased microRNA-206 levels [169]. Another experimental study reported decreased levels of microRNA-18a-5p in ischemic pulmonary tissue [170]. Qin et al. studied the expression of microRNAs in multiorgan ischemia caused by hemorrhagic shock and identified an increased expression of microRNA-24a in the animal group in which hemorrhagic shock had been induced (p < 0.05). Qin et al. also identified a statistically positive correlation between increased microRNA-34a levels and serum malondialdehyde (MDA) [171]. Li et al. showed that the expression of microRNA-21-5p and IL-1β, IL-6, IL-8, IL-17, and TNF-α bioproduction by macrophages are statistically dependent in the case of laboratory animals suffering from pulmonary ischemia [172].
During general anesthesia, one of the main pathophysiological factors the anesthetic team needs to consider is the maintenance of blood-pressure homeostasis in order to ensure the adequate oxygenation of vital organs. An important marker for the adequate perfusion of tissues is provided by kidney function, assessed through the excretion capacity. In many clinical scenarios, the kidneys suffer both due to tissue hypoperfusion and due to other factors, such as inflammation, rhabdomyolysis, and hemorrhagic shock. One of the main causes of acute kidney injury is ischemia–reperfusion syndrome. From a pathophysiological standpoint, kidney ischemia–reperfusion syndrome is complex and multifactorial, with not all causes being fully elucidated at this point. Among the basic causes of kidney IR injury is the destruction of renal tubular cells, leading to the destruction of renal tubules. Clinically, once these pathophysiological mechanisms have exerted their effects, the glomerular filtration rate (GFR) has already been affected. Dixon et al. identified a specific mechanism for the destruction of renal cells, characterized by lipid peroxidation, condensation of mitochondrial membranes, and iron accumulation. The process, named ferroptosis, has been recently identified and explained in other studies as being involved in different pathological states, including myocardial infarction [173,174,175]. Inside the cell, there are a series of enzymes and proteins responsible for the inhibition of ferroptosis, such as glutathione peroxidase 4 (GPX4), responsible for diminishing the effects of lipid peroxidation, a fact proven by Yang et al. in a study regarding ferroptosis mechanisms in cancer cells [176]. Fang et al. studied another mechanism possibly responsible for ferroptosis inhibition, the reduced activity of lipid peroxidation through the transport of cystine in the cytosol and through the stimulation of GSH production through Solute carrier family 7 member 11 (SLC7A11). Another molecular and epigenetic mechanism involved in increasing renal disfunction is represented by microRNAs [177]. According to Drobna et al., it is possible to augment molecular mechanisms through the action of genes and microRNA-20b-5p and microRNA 363-3p [178]. In a similar study, Ding et al. proved the direct involvement of microRNA-182-5p and microRNA-378a-3p in intensifying the ferroptosis process and renal function deterioration due to ischemia and reperfusion [179].
In an experimental study on the role of microRNAs in kidney ischemia, Chiba et al. indicated that the subjects presented high levels of microRNA-17, microRNA-18a, microRNA-19a, microRNA-20a, microRNA19b-1, and microRNA-92a-1 [180]. MicroRNA-24 was identified as a promoter of ischemic kidney injury, acting by stimulating the biological mechanisms of H2A-histone family and oxygenase 1 [181]. Wang et al. also discovered increased expressions of microRNA-466 and microRNA-709 in kidney disfunction induced by ischemia [182]. Furthermore, this group of authors reported the important involvement of genes Mcm5, Oip5, E2f8, Myc, E2f1, and Mdm2 in increasing the severity of ischemic injury. A similar study was carried out by Cao et al., and it identified significant implications of microRNA-125b-5p in ischemic kidney injury [183]. MicroRNA-489 was found to be a protective epigenetic species against kidney disfunction induced by ischemia through hypoxia-inducible factor-1 [184]. Other studies have found a series of microRNA species whose expression was significantly modified by acute renal injury, such as microRNA-4640, microRNA-4270, microRNA-4321, microRNA-10a-5p, microRNA-26b-5p, microRNA-16, microRNA-27a-3p, microRNA-93-3p, microRNA-29a-3p, microRNA-126-3p, microRNA-127-3p, microRNA-200c, microRNA-210-3p, microRNA-210, microRNA-423, microRNA-21, microRNA-30e, and microRNA-30a [181,185,186,187,188,189,190,191]. In a study on microRNA expression in patients presenting with septic shock and acute kidney injury (AKI), Zang et al. identified decreased plasma and urine levels for microRNA-22-3p in patients who did not survive. The statistical analysis based on the Kaplan–Maier survival curves and Cox regression analysis has identified a statistically significant correlation between a decrease in this microRNA species and 28-day mortality for patients presenting with septic shock and AKI [192]. In the case of critically ill patients, one of the most widely studied mechanisms of kidney injury is the formation of oxalic acid and calcium oxalate (CaC2O4), which produce tubular obstruction and crystallization in epithelial cells. Shihana et al. identified increased expressions of microRNA-20a, microRNA-93, microRNA-92a, microRNA-195, and microRNA-451 in acute kidney injury caused by oxalate [193]. Another mechanism responsible for worsening kidney tissue injury is the activation of mitogen-activated protein kinase ½ (MEK1/2) through phosphorylation. Recent studies have reported the implications of MEK1/2 in differentiation, migration, proliferation, metabolism, and cellular death. Collier et al. studied all these complex mechanisms and reported the active involvement of microRNA-34a in decreasing MEK1/2 activity and initiating ischemic renal injury [194]. Another microRNA species involved in pathogenetic mechanisms is microRNA-21 [195]. Zhang et al. showed that microRNA-192 expression increases in patients with AKI. Moreover, there is an increase in the concentration at 3 h after the ischemic event in the kidney, with a peak value at 12 h, and a decrease in its levels during the next 24 h (AUC-ROC 0.673, 95% CI 0.540–0.806, and p = 0.014) [196]. The Wnt/β-catenin pathway is another mechanism involved in kidney ischemia and is responsible for fibrosis in the kidney [197,198]. Apart from the aforementioned microRNAs, there is a molecular complex responsible for modulating the activity of Wnt/β-catenin, which includes Dickkopf (DKK) 1, 2, 3, and 4 (DKK1, DKK2, DKK3, and DKK4) [199]. Yi et al. showed that microRNA-214 is directly involved in inhibiting the activity of Wnt/β by directly blocking β-catenin [200]. Zu et al. identified the direct molecular processes of microRNA-214 in kidney injury through the modulation of Wnt/β-catenin activity and the expression of DKK3 [201].
4. Perioperative Antioxidant Therapy
Numerous studies in the preceding decades have focused on reducing the pro-oxidative profile in surgical patients [202]. Different antioxidant therapies have been proposed, targeting either the inhibition of biological processes responsible for the production of certain molecules or the neutralization of the redox activity of free radicals by complexing them in molecular formulas with no chemical activity [203,204,205]. One such widely studied molecule is hydrogen sulfide (H2S), which due to its structural properties can easily diffuse through cell membranes and through the membranes of organelles. From a biochemical standpoint, the antioxidant activity of H2S is represented by the inhibition of reactive oxygen species through nuclear factor erythroid 2-related factor 2 (Nrf2) activation and Kelch-like ECH-associated protein-1 (Keap1) inactivation and by an increase in GSH and Trx expression. The main sources of H2S biosynthesis in the body are cystathione β-synthase (CBS), predominantly found in the central nervous system (CNS), and cystathionine γ-lyase (CSE), found in the cardiovascular system (CVS). The biosynthesis mechanism is based on the desulfydration of cysteine through catalyst biological mechanisms given by the pyridoxal-5′-phosphate-dependent enzymes. Tripatara et al. carried out an experimental study on 74 lab animals (Wistar rats) on the effect of endogen and exogen H2S on the ischemia–reperfusion syndrome in the kidney tissue. Exogen H2S sources were represented by the administered sodium hydrosulfide, which reduced the incidence and severity of tubular and glomerular dysfunction in the experimental model that had undergone 45 min of ischemia and 6 h of reperfusion. A histochemical analysis showed a significant reduction in the histological score for acute tubular necrosis and, importantly, a decrease in the expression of redox mechanisms [206]. Bos et al., in a similar study, reported a protective effect of H2S for renal function, apoptosis, and kidney tissue inflammation under experimental conditions of ischemia–reperfusion injury. They highlighted that by inducing a hypermetabolic status through H2S, the kidney can be protected from hypoxia [207]. The most recent studies have focused on blocking biochemical mechanisms of free radical production inside the mitochondria. In this regard, one of the most widely studied pharmacological formulas is represented by SS-31, also known as Bendavia, Elamipretide, or MTP-131. Structurally, it is a synthetic tetrapeptide with the formula D-Arg-2′6′dimethylTyr-Lys-Phe-NH2 [208]. SS-31 can selectively attack cardiolipin from inside the mitochondrial membrane and is able to block the transient mitochondrial permeability mechanism that is in action during ischemia–reperfusion, leading to increased ATP biosynthesis and reduced ischemic injury [209]. Szeto et al. identified low levels of IL-18, IL-1β, and TGF-β in ischemic tissue. They reported a decrease in macrophage infiltration and TNF-α activity, with the restoration of glomerular capillaries and the podocyte structure [210]. Birk et al. carried out a similar study and reported the recovery of the ATP production mechanism in the mitochondria during kidney ischemia–reperfusion syndrome [209]. In addition, 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl, also known as Tempol, was investigated for reducing cellular redox activity, due to its capacity to block reactive oxygen species by reducing intracellular Fe2+ concentrations [211]. Chatterjee et al., in an experimental study on Wistar rats on the effects of Tempor on redox activity in the proximal renal tubules, showed that there was a significant decrease in renal disfunction and kidney injury due to ischemia–reperfusion syndrome. The mechanisms of action that were identified in this case are characterized by a reduction in hydrogen peroxide biosynthesis in the respiratory chain and an increase in lactate dehydrogenase (LDH) [212].
Patients under surgical stress undergo a series of time-related changes at a metabolic level. More et al., in their book published in 1959, described the stages of surgical stress as follows: Phase 1—stress period that can continue for 2–4 days; Phase II—turning point, which will be followed by the anabolic phase; Phase III—regeneration of proteins and muscle, which happens a few weeks after surgery; and Phase IV—accumulation of fat [213]. During these phases, muscular proteins are broken down to activate gluconeogenesis, energy production, and tissue repair, some of the reasons for which the nutrition of the surgical patients is of the utmost importance before and after surgery [214,215]. Miyachi et al., in a study on the impact of cysteine administration (700 mg) and theanine (280 mg) for four days before surgery and five days after surgery, in oncological patients undergoing distal gastrectomy, showed reduced postoperative inflammation and faster recovery [216]. A similar experimental study carried out by Shibakusa et al. reported a decrease in IL-6 expression in laboratory animals that had received cystine/theanine five days before surgery [217]. Tanaka et al. came to similar conclusions, stating that cystine administration leads to a decrease in the concentration of IL-6 and IL-10 in lab animals with induced sepsis [218].
The most widely studied molecule with antioxidant properties is vitamin C. From a chemical perspective, ascorbic acid (AscH2) is a water-soluble ketolactone that expresses two ionizable hydroxyl groups (Sven-Olaf Kuhn). At physiological pH, the predominant chemical type is the AscH− anion. The redox capacity of ascorbate is given by its reduction in two steps [219,220,221,222]: (i) the donation of an electron and (ii) the formation of the ascorbate radical (Asc−) and of dehydroascorbic acid (DHA) [223]. Physiologically, the concentration of ascorbate in plasma in healthy individuals is between 40 and 80 microM, a level capable of ensuring endogenous antioxidant activity. The main reactive species to which ascorbate donates an electron leading to a blockage in redox activity are the alkoxyl radical (RO), thiol radical (GS), hydroxyl radical (HO), peroxyl radical (LOO), and tocopheroxyl radical (TO) [224,225,226]. Recent studies have demonstrated that ascorbate can inhibit lipid oxidation processes in endothelial cells and macrophages [227]. Du et al. showed in their experimental study the ability of vitamin C to inhibit inflammatory and pro-oxidative processes mediated by NF-κB [228]. Brown et al. performed a similar study regarding the impact that vitamin E and vitamin C antioxidant therapy has on pancreatic transplantation and showed a significant decrease in the inflammatory and oxidative expressions [229]. Moon et al. carried out a prospective, randomized study that included 132 patients scheduled for gynecological surgery. For statistical analysis and for underlining the antioxidant effects of vitamin C, the authors divided the patients into four groups as follows: Group 1 (n = 33), which received magnesium sulfate 40 mg/kg; Group 2 (n = 33), which received vitamin C 50 mg/kg; Group 3, which received magnesium sulfate 40 mg/kg and vitamin C 50 mg/kg; and Group 4, which received isotonic saline solution 40 mL. The monitored clinical effects were the postoperative pain score, postoperative nausea and vomiting (PONV), and total fentanyl requirements. The study showed significantly lower fentanyl requirements in the groups that received magnesium sulfate and vitamin C (Group 1, Group 2, and Group 3). The incidence of PONV was lower in the group that received both magnesium sulfate and vitamin C [230]. Mohamed et al. studied the impact of perioperative intravenous administration of vitamin C and N-acetylcysteine on the production of malondialdehyde, IL-6, and IL-8 as markers for oxidative stress and systemic inflammation in patients needing a tourniquet during surgery; the study included 60 patients who had undergone surgical intervention of the upper limb and received 1 g of vitamin C and 10 mg/kg N-acetyl cysteine. Following statistical analysis, there was an increased expression of IL-6, IL-8, and malondialdehyde in patients who had not received antioxidant substances [231]. Rumelin et al. studied the plasmatic concentration of vitamin C in the pre- and postoperative period following the administration of 1 g of single-dose vitamin C. For the study, the authors divided the patients into two groups: one group that received 1000 mg vitamin C preoperatively and one group that received a placebo. The vitamin C levels in plasma were determined in both groups on the first postoperative day. The study concluded that the administration of 1 g of vitamin C in the preoperative period does not ensure a constant increased plasma concentration [232]. Jeon et al. carried out a similar study with 100 patients undergoing laparoscopic colectomy who received 50 mg/kg intravenous vitamin C immediately after the induction of anesthesia. The authors wanted to monitor the postoperative morphine requirements, as well as the pain scores, at 2, 4, and 24 h after surgery. They identified a decrease in morphine requirements 2 h after surgery in patients who had received antioxidant therapy, as well as a lower pain score at 24 h [233].
Recently, the antioxidant vitamin D has been intensely studied, especially due to the inflammatory mechanisms that are induced upon infection by the new SARS-CoV-2 coronavirus [234,235]. Recent studies have shown that decreased plasma concentrations for vitamin D are associated with an increase in pro-inflammatory and pro-oxidative factors, such as TNF-α, IL-6, fibrinogen, and C reactive protein [236]. It was shown that the vascular and microvascular systems contain a number of important vitamin D receptors (vitamin D), explaining its ability to modulate pro-inflammatory mechanisms at the vascular level. Nieuwland et al., in an experimental study regarding the impact of vitamin D on vascular inflammation, identified a decrease in CD4+ T-helper cell count expression. A reduction in the biosynthesis of IL-2, IL-4, and IL-10 associated with T-cell activity was also proven [237]. Martinez-Moreno et al., in a similar study, identified a decrease in pro-inflammatory cytokines, such as IL-6, IL-8, IL-1β, and TNF-α, when human aortic smooth muscle cells are exposed to increased concentrations of vitamin D [238]. The importance of vitamin D was also noticed in the case of orthopedic surgery. Bogunovic et al. carried out a retrospective study that included 723 patients who had undergone orthopedic surgery. In these patients, the levels of 25-hydroxyvitamin D were determined in the preoperative period. The prevalence for vitamin D plasma concentrations was expressed as follows: normal (≥32 ng/mL), insufficient (<32 ng/mL), and deficit (<20 ng/mL). It was shown that 40% of the patients had vitamin D levels <32 ng/mL, and out of these patients, half had levels <20 ng/mL. The authors found a statistically significant correlation between vitamin D levels in plasma and bone fractures or degeneration, underlining the importance of this vitamin in maintaining normal muscle and skeletal functions [239]. Maniar et al. studied the impact vitamin D has on recovery after total knee arthroplasty. The study included 120 patients, out of which 64 had vitamin D levels <30 ng/mL preoperatively. In the postoperative period, all patients received vitamin D antioxidant therapy. Patients with a vitamin D deficit before surgery presented slower recovery in comparison to the others. Analysis performed three months after surgery showed that the functional scores were similar in the two groups [240]. Mouli et al. carried out a similar study, including 174 patients with vitamin D deficit (<30 ng/mL) who received vitamin D antioxidant therapy with the following protocol: (1) daily vitamin D supplementation with an increasing dose between 1000 and 6000 IU and (2) one weekly dose of 50,000 IU, administered for four weeks, followed by the maintenance of a daily dose of 2000 IU. In the surgical patients included in the study, the authors recommended a vitamin D antioxidant therapy with 50,000 IU of vitamin D weekly for four weeks, followed by a maintenance dose, as a better solution than the administration of lower, daily doses [241]. Hajimohammadebrahim-Ketabforoush et al. studied the impact of vitamin D administration in deficient patients that had undergone craniectomy for brain tumor resection. The study included two patient groups that had lower-than-normal vitamin D levels (<20 ng/mL). One of the groups received intramuscular vitamin D 300.000 IU in the preoperative period. In the study group, the vitamin D concentration increased significantly at five days after surgery (p < 0.01). The ICU admission time and the duration of hospital stay for patients who received vitamin D supplementation in the preoperative period were also significantly shorter. The authors of the study concluded that antioxidant therapy with vitamin D in the perioperative period is beneficial in patients with brain tumors [242] (Table 2).

Table 2.
Impact of perioperative antioxidant therapy on clinical outcomes in surgery.
5. Conclusions
The pro-inflammatory and pro-oxidative profiles of surgical patients undergoing general anesthesia are similar to those of patients presenting with sepsis or septic shock. One reason is that the pathophysiological mechanisms are similar and mainly represented by an increase in the inflammatory profile, as well as microvascular injury due to ischemia and reperfusion. The epigenetic analysis of the microRNA expression shows a series of molecular and cellular mechanisms responsible for either the initiation or the augmentation of this systemic inflammatory profile. Once identified, these pathways can be blocked and/or certain mechanisms can be minimized in order to reduce the systemic impact on the surgical patient. Moreover, the administration of antioxidant therapies indicates a beneficial clinical impact, the most important benefit being reduced postoperative adverse effects. However, further studies are needed regarding the administration of modern antioxidant therapies targeted at the cellular and molecular levels.
Author Contributions
Conceptualization, S.A.R. and A.F.R.; methodology, D.C.; software, S.E.P. and R.V.; validation, D.S., A.P., M.P. and D.C.; formal analysis, D.T., O.H.B. and R.I.I.; investigation, S.A.R. and R.I.I.; resources, E.M.P.; data curation, E.M.P.; writing—original draft preparation, A.F.R. and L.M.B.; writing—review and editing, A.F.R. and D.N.G.; visualization, D.N.G.; supervision, A.P. and D.S.; project administration, M.P. and O.H.B.; funding acquisition, A.F.R. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kirov, K.; Motamed, C.; Ndoko, S.K.; Dhonneur, G. TOF Count at Corrugator Supercilii Reflects Abdominal Muscles Relaxation Better than at Adductor Pollicis. Br. J. Anaesth. 2007, 98, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Honing, M.; Martini, C.; van Velzen, M.; Niesters, M.; Dahan, A.; Boon, M. Cholinergic Chemotransmission and Anesthetic Drug Effects at the Carotid Bodies. Molecules 2020, 25, 5974. [Google Scholar] [CrossRef] [PubMed]
- Duţu, M.; Ivaşcu, R.; Tudorache, O.; Morlova, D.; Stanca, A.; Negoiţă, S.; Corneci, D. Neuromuscular monitoring: An update. Rom. J. Anaesth. Intensive Care 2018, 25, 55–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Funcke, S.; Saugel, B.; Koch, C.; Schulte, D.; Zajonz, T.; Sander, M.; Gratarola, A.; Ball, L.; Pelosi, P.; Spadaro, S.; et al. Individualized, Perioperative, Hemodynamic Goal-Directed Therapy in Major Abdominal Surgery (IPEGASUS Trial): Study Protocol for a Randomized Controlled Trial. Trials 2018, 19, 273. [Google Scholar] [CrossRef] [PubMed]
- Bedreag, O.H.; Rogobete, A.F.; Sarandan, M.; Cradigati, A.C.; Papurica, M.; Dumbuleu, M.C.; Chira, A.M.; Rosu, O.M.; Sandesc, D. Oxidative Stress in Severe Pulmonary Trauma in Critical Ill Patients. Antioxidant Therapy in Patients with Multiple Trauma—A Review. Anestezjol. Intensywna Ter. 2015, 47, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Hsing, C.H.; Wang, J.J. Clinical implication of perioperative inflammatory cytokine alteration. Acta Anaesthesiol. Taiwan 2015, 53, 23–28. [Google Scholar] [CrossRef]
- Boehm, O.; Baumgarten, G.; Hoeft, A. Epidemiology of the High-Risk Population: Perioperative Risk and Mortality after Surgery. Curr. Opin. Crit. Care 2015, 21, 322–327. [Google Scholar] [CrossRef]
- Riedel, B.; Browne, K.; Silbert, B. Cerebral Protection: Inflammation, Endothelial Dysfunction, and Postoperative Cognitive Dysfunction. Curr. Opin. Anaesthesiol. 2014, 27, 89–97. [Google Scholar] [CrossRef]
- Romagnoli, S.; Ricci, Z. Postoperative Acute Kidney Injury. Minerva Anestesiol. 2014, 81, 684–696. [Google Scholar]
- Snyder, G.L.; Greenberg, S. Effect of Anaesthetic Technique and Other Perioperative Factors on Cancer Recurrence. Br. J. Anaesth. 2010, 105, 106–115. [Google Scholar] [CrossRef]
- Busti, A.J.; Hooper, J.S.; Amaya, C.J.; Kazi, S. Effects of Perioperative Antiinflammatory and Immunomodulating Therapy on Surgical Wound Healing. Pharmacotherapy 2005, 25, 1566–1591. [Google Scholar] [CrossRef] [PubMed]
- Papurica, M.; Rogobete, A.F.; Sandesc, D.; Dumache, R.; Nartita, R.; Sarandan, M.; Cradigati, A.C.; Luca, L.; Vernic, C.; Bedreag, O.H. Redox Changes Induced by General Anesthesia in Critically Ill Patients with Multiple Traumas. Mol. Biol. Int. 2015, 2015, 238586. [Google Scholar] [CrossRef] [PubMed]
- Denk, S.; Perl, M.; Huber-Lang, M. Damage- and Pathogen-Associated Molecular Patterns and Alarmins: Keys to Sepsis? Eur. Surg. Res. 2012, 48, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, J.J.; Yang, D. Alarmins: Chemotactic Activators of Immune Responses. Curr. Opin. Immunol. 2005, 17, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Roth, J.; Oppenheim, J.J.; Tracey, K.J.; Vogl, T.; Feldmann, M.; Horwood, N.; Nanchahal, J. Alarmins: Awaiting a Clinical Response. J. Clin. Invest. 2012, 122, 2711–2719. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of Chromatin Protein HMGB1 by Necrotic Cells Triggers Inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef]
- Tbahriti, H.F.; Meknassi, D.; Moussaoui, R.; Messaoudi, A.; Zemour, L.; Kaddous, A.; Bouchenak, M.; Mekki, K. Inflammatory Status in Chronic Renal Failure: The Role of Homocysteinemia and Pro-Inflammatory Cytokines. World J. Nephrol. 2013, 2, 31–37. [Google Scholar] [CrossRef]
- Little, J.P.; Simtchouk, S.; Schindler, S.M.; Villanueva, E.B.; Gill, N.E.; Walker, D.G.; Wolthers, K.R.; Klegeris, A. Mitochondrial Transcription Factor A (Tfam) Is a pro-Inflammatory Extracellular Signaling Molecule Recognized by Brain Microglia. Mol. Cell. Neurosci. 2014, 60, 88–96. [Google Scholar] [CrossRef]
- Kirchhoff, C.; Biberthaler, P.; Mutschler, W.E.; Faist, E.; Jochum, M.; Zedler, S. Early Down-Regulation of the pro-Inflammatory Potential of Monocytes Is Correlated to Organ Dysfunction in Patients after Severe Multiple Injury: A Cohort Study. Crit. Care 2009, 13, R88. [Google Scholar] [CrossRef]
- Phillipson, M.; Kubes, P. The Neutrophil in Vascular Inflammation. Nat. Med. 2011, 17, 1381–1390. [Google Scholar] [CrossRef]
- Reis, G.S.; Augusto, V.S.; Silveira, A.P.C.; Jordão, A.A.; Baddini-Martinez, J.; Poli Neto, O.; Rodrigues, A.J.; Evora, P.R.B. Oxidative-Stress Biomarkers in Patients with Pulmonary Hypertension. Pulmonary 2013, 3, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Hafner, S.; Radermacher, P.; Frick, M.; Dietl, P.; Calzia, E. Hyperglycemia, Oxidative Stress, and the Diaphragm: A Link between Chronic Co-Morbidity and Acute Stress? Crit. Care 2014, 18, 149. [Google Scholar] [CrossRef] [PubMed]
- Cellular, O.S.; Overload, C. Sevoflurane Protects Ventricular Myocytes against Oxidative Stress-Induced Cellular Ca2+ Overload and Hypercontracture. Anesthesiology 2013, 119, 606–620. [Google Scholar]
- Breitenbach, M.; Rinnerthaler, M.; Weber, M.; Breitenbach-Koller, H.; Karl, T.; Cullen, P.; Basu, S.; Haskova, D.; Hasek, J. The Defense and Signaling Role of NADPH Oxidases in Eukaryotic Cells: Review. Wien. Med. Wochenschr. 2018, 168, 286–299. [Google Scholar] [CrossRef]
- Ren, X.; Wang, M.; Wang, Y.; Huang, A. Superoxide anion generation response to wound in Arabidopsis hypocotyl cutting. Plant Signal Behav. 2021, 16, 1848086. [Google Scholar] [CrossRef]
- Constantino, L.; Gonçalves, R.C.; Giombelli, V.R.; Tomasi, C.D.; Vuolo, F.; Kist, L.W.; Medeiros, G.; de Oliveira, T.; Augusto, M.; Pasquali, D.B.; et al. Regulation of Lung Oxidative Damage by Endogenous Superoxide Dismutase in Sepsis. Intensive Care Med. Exp. 2014, 2, 17. [Google Scholar] [CrossRef]
- Milkovic, L.; Gasparovic, A.C.; Cindric, M.; Mouthuy, P.A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef]
- Lv, P.; Xue, P.; Dong, J.; Peng, H.; Clewell, R.; Wang, A.; Wang, Y.; Peng, S.; Qu, W.; Zhang, Q.; et al. Keap1 Silencing Boosts Lipopolysaccharide-Induced Transcription of Interleukin 6 via Activation of Nuclear Factor ΚB in Macrophages. Toxicol. Appl. Pharmacol. 2013, 272, 697–702. [Google Scholar] [CrossRef]
- Pagano, G.; Talamanca, A.A.; Castello, G.; Cordero, M.D.; Ischia, M.; Gadaleta, M.N.; Pallardó, F.V.; Petrović, S.; Tiano, L.; Zatterale, A. Oxidative Stress and Mitochondrial Dysfunction across Broad-Ranging Pathologies: Toward Mitochondria-Targeted Clinical Strategies. Oxidative Med. Cell. Longev. 2014, 2014, 541230. [Google Scholar] [CrossRef]
- Waldbaum, S.; Patel, M. Mitochondria, Oxidative Stress, and Temporal Lobe Epilepsy. Epilepsy Res. 2010, 88, 23–45. [Google Scholar] [CrossRef]
- Zang, Q.S.; Martinez, B.; Yao, X.; Maass, D.L.; Ma, L.; Wolf, S.E.; Minei, J.P. Sepsis-Induced Cardiac Mitochondrial Dysfunction Involves Altered Mitochondrial-Localization of Tyrosine Kinase Src and Tyrosine Phosphatase SHP2. PLoS ONE 2012, 7, e43424. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.M.; Williams, J.A.; Ding, W.X. Mitochondrial Dynamics and Mitochondrial Quality Control. Redox Biol. 2015, 4, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Bahrami, S.; Calzia, E.; Dungel, P.; Gille, L.; Kuznetsov, A.V.; Troppmair, J. Mitochondrial Dysfunction and Biogenesis: Do ICU Patients Die from Mitochondrial Failure? Ann. Intensive Care 2011, 1, 41. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Bao, Y.; Korthuis, R.J. Mitochondrial Reactive Oxygen Species: A Double Edged Sword in Ischemia/Reperfusion vs Preconditioning. Redox Biol. 2014, 2, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hu, F.; Wu, J.; Zhang, S. Cannabidiol Attenuates OGD/R-Induced Damage by Enhancing Mitochondrial Bioenergetics and Modulating Glucose Metabolism via Pentose-Phosphate Pathway in Hippocampal Neurons. Redox Biol. 2017, 11, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Gorelenkova Miller, O.; Behring, J.B.; Siedlak, S.L.; Jiang, S.; Matsui, R.; Bachschmid, M.M.; Zhu, X.; Mieyal, J.J. Upregulation of Glutaredoxin-1 Activates Microglia and Promotes Neurodegeneration: Implications for Parkinson’s Disease. Antioxid. Redox Signal. 2016, 25, 967–982. [Google Scholar] [CrossRef]
- Porfire, A.S.; Leucuţa, S.E.; Kiss, B.; Loghin, F.; Pârvu, A.E. Investigation into the Role of Cu/Zn-SOD Delivery System on Its Antioxidant and Antiinflammatory Activity in Rat Model of Peritonitis. Pharmacol. Rep. 2014, 66, 670–676. [Google Scholar] [CrossRef]
- Edem, V.F.; Kosoko, A.; Akinyoola, S.B.; Owoeye, O.; Rahamon, S.K.; Arinola, O.G. Plasma Antioxidant Enzymes, Lipid Peroxidation and Hydrogen Peroxide in Wistar Rats Exposed to Dichlorvos Insecticide. Sch. Res. Libr. Arch. Appl. Sci. Res. 2012, 4, 1778–1781. [Google Scholar]
- Risnes, S.F.; Hartwig, A. Impact of Cadmium on Antioxidant Enzymes in HCT116 Cells and Protective Interaction by Selenium. Perspect. Sci. 2015, 3, 55. [Google Scholar] [CrossRef]
- Samarghandian, S.; Afshari, R.; Farkhondeh, T. Effect of Long-Term Treatment of Morphine on Enzymes, Oxidative Stress Indices and Antioxidant Status in Male Rat Liver. Int. J. Clin. Exp. Med. 2014, 7, 1449–1453. [Google Scholar]
- Lee, B.-J.; Lin, J.-S.; Lin, Y.-C.; Lin, P.-T. Effects of L-Carnitine Supplementation on Oxidative Stress and Antioxidant Enzymes Activities in Patients with Coronary Artery Disease: A Randomized, Placebo-Controlled Trial. Nutr. J. 2014, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Redox Biology Effects of N-Acetylcysteine, Oral Glutathione (GSH) and a Novel Sublingual Form of GSH on Oxidative Stress Markers: A Comparative Crossover Study. Redox Biol. 2015, 6, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Babu, D.; Leclercq, G.; Goossens, V.; Vanden, T.; van Hamme, E.; Vandenabeele, P.; Lefebvre, R.A. Mitochondria and NADPH Oxidases Are the Major Sources of TNF-α/Cycloheximide-Induced Oxidative Stress in Murine Intestinal Epithelial MODE-K Cells. Cell. Signal. 2015, 27, 1141–1158. [Google Scholar] [CrossRef]
- Jaganjac, M.; Milkovic, L.; Zarkovic, N.; Zarkovic, K. Oxidative Stress and Regeneration. Free Radic. Biol. Med. 2022, 181, 154–165. [Google Scholar] [CrossRef]
- Li, P.; Stetler, R.A.; Leak, R.K.; Shi, Y.; Li, Y.; Yu, W.; Bennett, M.V.L.; Chen, J. Oxidative Stress and DNA Damage after Cerebral Ischemia: Potential Therapeutic Targets to Repair the Genome and Improve Stroke Recovery. Neuropharmacology 2018, 134, 208–217. [Google Scholar] [CrossRef]
- Carcy, R.; Cougnon, M.; Poet, M.; Durandy, M.; Sicard, A.; Counillon, L.; Blondeau, N.; Hauet, T.; Tauc, M.; Pisani, D.F. Targeting Oxidative Stress, a Crucial Challenge in Renal Transplantation Outcome. Free Radic. Biol. Med. 2021, 169, 258–270. [Google Scholar] [CrossRef]
- Yuan, Q.; Yuan, Y.; Zheng, Y.; Sheng, R.; Liu, L.; Xie, F.; Tan, J. Anti-Cerebral Ischemia Reperfusion Injury of Polysaccharides: A Review of the Mechanisms. Biomed. Pharmacother. 2021, 137, 111303. [Google Scholar] [CrossRef]
- Padmavathi, G.; Ramkumar, K.M. MicroRNA Mediated Regulation of the Major Redox Homeostasis Switch, Nrf2, and Its Impact on Oxidative Stress-Induced Ischemic/Reperfusion Injury. Arch. Biochem. Biophys. 2021, 698, 108725. [Google Scholar] [CrossRef]
- Hu, Y.; Deng, H.; Xu, S.; Zhang, J. MicroRNAs Regulate Mitochondrial Function in Cerebral Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2015, 16, 24895–24917. [Google Scholar] [CrossRef]
- Chi, O.Z.; Barsoum, S.; Rah, K.H.; Liu, X.; Weiss, H.R. Local O2 Balance in Cerebral Ischemia-Reperfusion Improved during Pentobarbital Compared with Isoflurane Anesthesia. J. Stroke Cerebrovasc. Dis. 2015, 24, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.; Zhang, X.; Guan, Y.; Yang, X.; Li, Y.; Song, G.; Guan, L. Expression Profiles of MicroRNAs after Focal Cerebral Ischemia/Reperfusion Injury in Rats. Neural Regen. Res. 2012, 7, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.R.; Keller, J.N. Oxidative Stress and Cerebral Endothelial Cells: Regulation of the Blood-Brain-Barrier and Antioxidant Based Interventions. Biochim. Biophys. Acta-Mol. Basis Dis. 2012, 1822, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, J.; Shi, H. Neurobiology of Disease Role of Hypoxia Inducible Factor 1 in Hyperglycemia-Exacerbated Blood-Brain Barrier Disruption in Ischemic Stroke. Neurobiol. Dis. 2016, 95, 82–92. [Google Scholar] [CrossRef]
- Xia, H.; Cheng, Z.; Cheng, Y.; Xu, Y. Investigating the Passage of Tetramethylpyrazine-Loaded Liposomes across Blood-Brain Barrier Models in Vitro and Ex Vivo. Mater. Sci. Eng. C 2016, 69, 1010–1017. [Google Scholar] [CrossRef]
- Robertson, C.S.; Narayan, R.K.; Gokaslan, Z.L.; Pahwa, R.; Grossman, R.G.; Caram, P.J.; Allen, E. Cerebral Arteriovenous Oxygen Difference as an Estimate of Cerebral Blood Flow in Comatose Patients. J. Neurosurg. 1989, 70, 222–230. [Google Scholar] [CrossRef]
- Bouzat, P.; Sala, N.; Payen, J.-F.; Oddo, M. Beyond Intracranial Pressure: Optimization of Cerebral Blood Flow, Oxygen, and Substrate Delivery after Traumatic Brain Injury. Ann. Intensive Care 2013, 3, 23. [Google Scholar] [CrossRef]
- Weiss, H.R.; Grayson, J.; Liu, X.; Barsoum, S.; Shah, H.; Chi, O.Z. Cerebral Ischemia and Reperfusion Increases the Heterogeneity of Local Oxygen Supply/Consumption Balance. Stroke 2013, 44, 2553–2558. [Google Scholar] [CrossRef]
- Sakai, H.; Sheng, H.; Yates, R.B.; Ishida, K.; Pearlstein, R.D.; Warner, D.S. Isoflurane Provides Long-Term Protection against Focal Cerebral Ischemia in the Rat. Anesthesiology 2007, 106, 92–99. [Google Scholar] [CrossRef]
- Gambim, M.; de Oliveira do Carmo, A.; Marti, L.; Veríssimo-Filho, S.; Lopes, L.; Janiszewski, M. Platelet-Derived Exosomes Induce Endothelial Cell Apoptosis through Peroxynitrite Generation: Experimental Evidence for a Novel Mechanism of Septic Vascular Dysfunction. Crit. Care 2007, 11, R107. [Google Scholar] [CrossRef]
- Carnes, C.A.; Chung, M.K.; Nakayama, T.; Nakayama, H.; Baliga, R.S.; Piao, S.; Kanderian, A.; Pavia, S.; Hamlin, R.L.; McCarthy, P.M.; et al. Ascorbate Attenuates Atrial Pacing-Induced Peroxynitrite Formation and Electrical Remodeling and Decreases the Incidence of Postoperative Atrial Fibrillation. Circ. Res. 2001, 89, e32–e38. [Google Scholar] [CrossRef] [PubMed]
- Erecinska, M.; Thoresen, M.; Silver, I.A. Effects of Hypothermia on Energy Metabolism in Mammalian Central Nervous System. J. Cereb. Blood Flow Metab. 2003, 23, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Scheufler, K.M.; Lehnert, A.; Rohrborn, H.J.; Nadstawek, J.; Thees, C. Individual Value of Brain Tissue Oxygen Pressure, Microvascular Oxygen Saturation, Cytochrome Redox Level, and Energy Metabolites in Detecting Critically Reduced Cerebral Energy State during Acute Changes in Global Cerebral Perfusion. J. Neurosurg. Anesthesiol. 2004, 16, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Tousoulis, D.; Vasiliadou, C.; Pitsavos, C.; Toutouza, M.; Tentolouris, C.; Marinou, K.; Stefanadis, C. Genetic Polymorphisms G894T on the ENOS Gene Is Associated with Endothelial Function and VWF Levels in Premature Myocardial Infarction Survivors. Int. J. Cardiol. 2006, 107, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Abd-elbaset, M.; Arafa, E.A. Quercetin Modulates INOS, ENOS and NOSTRIN Expressions and Attenuates Oxidative Stress in Warm Hepatic Ischemia-Reperfusion Injury in Rats. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 246–255. [Google Scholar] [CrossRef]
- Silver, J.H.; Jaffe, R.A.; López, J.R. Plasma Nitrite as an Indicator of Cerebral Ischemia during Extracranial/Intracranial Bypass Surgery in Moyamoya Patients. J. Stroke Cerebrovasc. Dis. 2020, 29, 104830. [Google Scholar] [CrossRef]
- Suzuki, H.; Kanamaru, H.; Kawakita, F.; Asada, R.; Fujimoto, M.; Shiba, M. Cerebrovascular pathophysiology of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Histol. Histopathol. 2021, 36, 143–158. [Google Scholar] [CrossRef]
- Washington, C.W.; Zipfel, G.J. Detection and Monitoring of Vasospasm and Delayed Cerebral Ischemia: A Review and Assessment of the Literature. Neurocrit. Care 2011, 15, 312–317. [Google Scholar] [CrossRef]
- Liu, T.-J.; Zhang, J.-C.; Gao, X.-Z.; Tan, Z.-B.; Wang, J.-J.; Zhang, P.-P.; Cheng, A.-B.; Zhang, S.-B. Effect of Sevoflurane on the ATPase Activity of Hippocampal Neurons in a Rat Model of Cerebral Ischemia-Reperfusion Injury via the CAMP-PKA Signaling Pathway. Kaohsiung J. Med. Sci. 2018, 34, 22–33. [Google Scholar] [CrossRef]
- Coles, J.P.; Fryer, T.D.; Smielewski, P.; Chatfield, D.A.; Steiner, L.A.; Johnston, A.J.; Downey, S.P.; Williams, G.B.; Aigbirhio, F.; Hutchinson, P.J.; et al. Incidence and Mechanisms of Cerebral Ischemia in Early Clinical Head Injury. J. Cereb. Blood Flow Metab. 2004, 24, 202–211. [Google Scholar] [CrossRef]
- Drake, C.; Boutin, H.; Jones, M.S.; Denes, A.; Mccoll, B.W.; Selvarajah, J.R.; Hulme, S.; Georgiou, R.F.; Hinz, R.; Gerhard, A.; et al. Brain, Behavior, and Immunity Brain Inflammation Is Induced by Co-Morbidities and Risk Factors for Stroke. Brain Behav. Immun. 2011, 25, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Hartley, O.; Offord, R.E. Engineering Chemokines to Develop Optimized HIV Inhibitors. Curr. Protein Pept. Sci. 2005, 6, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Phillips, C.M.; Martinez-Revollar, G.; Keep, R.F.; Andjelkovic, A.V. Involvement of Epigenetic Mechanisms and Non-Coding RNAs in Blood-Brain Barrier and Neurovascular Unit Injury and Recovery after Stroke. Front. Neurosci. 2019, 13, 864. [Google Scholar] [CrossRef] [PubMed]
- De Perrot, M.; Liu, M.; Waddell, T.K.; Keshavjee, S. Ischemia-Reperfusion-Induced Lung Injury. Am. J. Respir. Crit. Care Med. 2003, 167, 490–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, Z.; Du, L.; Huang, Y.; Ge, J.; Deng, Y.; Mei, Z. The Potential Role of MicroRNA-124 in Cerebral Ischemia Injury. Int. J. Mol. Sci. 2020, 21, 120. [Google Scholar] [CrossRef]
- Stoica, L.; Dobrescu, A.; Isaic, A.; Verdeæ, G.; Taråa, C.; Lazãr, F. Metabolic and Hormonal Changes after Sleeve Gastrectomy and Mini Gastric Bypass in a Rat Model of Induced Type 2 Diabetes Mellitus and Obesity. Chirurgia 2019, 114, 732–738. [Google Scholar] [CrossRef]
- Rogobete, A.F.; Grintescu, I.M.; Bratu, T.; Bedreag, O.H.; Papurica, M.; Crainiceanu, Z.P.; Popovici, S.E.; Sandesc, D. Assessment of Metabolic and Nutritional Imbalance in Mechanically Ventilated Multiple Trauma Patients: From Molecular to Clinical Outcomes. Diagnostics 2019, 9, 171. [Google Scholar] [CrossRef]
- Eppinger, M.J.; Deeb, G.M.; Bolling, S.F.; Ward, P.A. Mediators of ischemia-reperfusion injury of rat lung. Am. J. Pathol. 1997, 150, 1773–1784. [Google Scholar]
- Qayumi, A.K.; Jamieson, W.R.E.; Godin, D.V.; Lam, S.; Ko, K.M.; Germann, E.; van den Broek, J. Response to Allopurinol Pretreatment in a Swine Model of Heart-Lung Transplantation. J. Investig. Surg. 1990, 3, 331–340. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, J.; Li, L.; Li, H.; Mao, S.; Zhang, F.; Zen, K.; Zhang, C.; Zhang, Q. MicroRNA-23a/b and MicroRNA-27a/b Suppress Apaf-1 Protein and Alleviate Hypoxia-Induced Neuronal Apoptosis. Cell Death Dis. 2014, 5, e1132. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell Death: Critical Control Points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Borutaite, V.; Jekabsone, A.; Morkuniene, R.; Brown, G.C. Inhibition of Mitochondrial Permeability Transition Prevents Mitochondrial Dysfunction, Cytochrome c Release and Apoptosis Induced by Heart Ischemia. J. Mol. Cell. Cardiol. 2003, 35, 357–366. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial Formation of Reactive Oxygen Species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Cobelens, P.M.; van Putte, B.P.; Kavelaars, A.; Heijnen, C.J.; Kesecioglu, J. Inflammatory Consequences of Lung Ischemia-Reperfusion Injury and Low-Pressure Ventilation. J. Surg. Res. 2008, 153, 295–301. [Google Scholar] [CrossRef]
- Hickey, M.J.; Sharkey, K.A.; Sihota, E.G.; Reinhardt, P.H.; Macmicking, J.D.; Nathan, C.; Kubes, P. Inducible Nitric Oxide Synthase-Deficient Mice Have Enhanced Leukocyte-Endothelium Interactions in Endotoxemia. FASEB J. 1997, 11, 955–964. [Google Scholar] [CrossRef]
- Ovechkin, A.V.; Lominadze, D.; Sedoris, K.C.; Robinson, T.W.; Tyagi, S.C.; Roberts, A.M. Lung Ischemia-Reperfusion Injury: Implications of Oxidative Stress and Platelet-Arteriolar Wall Interactions. Arch. Physiol. Biochem. 2007, 113, 1–12. [Google Scholar] [CrossRef]
- Saelens, X.; Festjens, N.; van de Walle, L.; van Gurp, M.; van Loo, G.; Vandenabeele, P. Toxic Proteins Released from Mitochondria in Cell Death. Oncogene 2004, 23, 2861–2874. [Google Scholar] [CrossRef]
- Scarabelli, T.M.; Stephanou, A.; Pasini, E.; Comini, L.; Raddino, R.; Knight, R.A.; Latchman, D.S. Different Signaling Pathways Induce Apoptosis in Endothelial Cells and Cardiac Myocytes during Ischemia/Reperfusion Injury. Circ. Res. 2002, 90, 745–748. [Google Scholar] [CrossRef]
- Zhang, X.; Lemasters, J.J. Free Radical Biology and Medicine Translocation of Iron from Lysosomes to Mitochondria during Ischemia Predisposes to Injury after Reperfusion in Rat Hepatocytes. Free Radic. Biol. Med. 2013, 63, 243–253. [Google Scholar] [CrossRef]
- Mikawa, K.; Akamatsu, H.; Nishina, K.; Shiga, M.; Maekawa, N.; Obara, H.; Niwa, Y. Propofol Inhibits Human Neutrophil Functions. Anesth. Analg. 1998, 87, 695–700. [Google Scholar] [CrossRef]
- Ohmizo, H.; Iwama, H.; Sugita, T. Complement Activation by Propofol and Its Effect during Propofol Anaesthesia. Anaesth. Intensive Care 1999, 27, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Hoff, G.; Bauer, I.; Larsen, B.; Bauer, M. Modulation of Endotoxin-Stimulated TNF-Alpha Gene Expression by Ketamine and Propofol in Cultured Human Whole Blood. Anaesthesist 2001, 50, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhata, H.; Shimizu, R.; Yokoyama, M.M. Suppressive Effects of Volatile Anesthetics on Cytokine Release in Human Peripheral Blood Mononuclear Cells. Int. J. Immunopharmacol. 1995, 17, 529–534. [Google Scholar] [CrossRef]
- Giraud, O.; Molliex, S.; Rolland, C.; Leçon-Malas, V.; Desmonts, J.-M.; Aubier, M.; Dehoux, M. Halogenated Anesthetics Reduce Interleukin-1beta-Induced Cytokine Secretion by Rat Alveolar Type II Cells in Primary Culture. Anesthesiology 2003, 98, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Kotani, N.; Takahashi, S.; Sessler, D.I.; Hashiba, E.; Kubota, T.; Hashimoto, H.; Matsuki, A. Volatile Anesthetics Augment Expression of Proinflammatory Cytokines in Rat Alveolar Macrophages during Mechanical Ventilation. Anesthesiology 1999, 91, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Yang, L.; Dong, Y.; Zhang, Y.; Xie, Z. Isoflurane and Sevoflurane Increase Interleukin-6 Levels through the Nuclear Factor-Kappa B Pathway in Neuroglioma Cells. Br. J. Anaesth. 2013, 110 (Suppl. S1), i82–i91. [Google Scholar] [CrossRef]
- Hudetz, J.A.; Gandhi, S.D.; Iqbal, Z.; Patterson, K.M.; Pagel, P.S. Elevated Postoperative Inflammatory Biomarkers Are Associated with Short- and Medium-Term Cognitive Dysfunction after Coronary Artery Surgery. J. Anesth. 2011, 25, 1–9. [Google Scholar] [CrossRef]
- Manfreda, S.E.; Dunzendorfer, S.; Schratzberger, P.; Buratti, T.; Reinisch, N.; Kähler, C.M.; List, W.F.; Wiedermann, C.J. The Chemotaxis of Human Peripheral Blood B Lymphocytes by Beta-Endorphin Is Reversible by Naloxone. Anesth. Analg. 1998, 86, 670–672. [Google Scholar] [CrossRef]
- Yeager, M.P.; Yu, C.T.; Campbell, A.S.; Moschella, M.; Guyre, P.M. Effect of Morphine and Beta-Endorphin on Human Fc Receptor-Dependent and Natural Killer Cell Functions. Clin. Immunol. Immunopathol. 1992, 62, 336–343. [Google Scholar] [CrossRef]
- Baek, S.B.; Shin, M.S.; Han, J.H.; Moon, S.W.; Chang, B.; Jeon, J.W.; Yi, J.W.; Chung, J.Y. Rocuronium Bromide Inhibits Inflammation and Pain by Suppressing Nitric Oxide Production and Enhancing Prostaglandin E(2) Synthesis in Endothelial Cells. Int. Neurourol. J. 2016, 20, 296–303. [Google Scholar] [CrossRef]
- Bargellini, A.; Rovesti, S.; Barbieri, A.; Vivoli, R.; Roncaglia, R.; Righi, E.; Borella, P. Effects of Chronic Exposure to Anaesthetic Gases on Some Immune Parameters. Sci. Total Environ. 2001, 270, 149–156. [Google Scholar] [CrossRef]
- Yamamoto, S.; Niida, S.; Azuma, E.; Yanagibashi, T.; Muramatsu, M.; Huang, T.T.; Sagara, H.; Higaki, S.; Ikutani, M.; Nagai, Y.; et al. Inflammation-Induced Endothelial Cell-Derived Extracellular Vesicles Modulate the Cellular Status of Pericytes. Sci. Rep. 2015, 5, 8505. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-ΚB, Inflammation, and Metabolic Disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, B.; Chen, S.; Jiang, F.; Meng, W. Association Study of Two Inflammation-Related Polymorphisms with Susceptibility to Hepatocellular Carcinoma: A Meta-Analysis. BMC Med. Genet. 2014, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Papurica, M.; Sandesc, D.; Rogobete, A.F.; Nartita, R.; Vernic, C.; Popovici, S.E.; Bedreag, O.H. Cardioprotective Effects Induced by Preconditioning with Halogenated Anesthetics. J. Interdiscip. Med. 2016, 1, 23–31. [Google Scholar] [CrossRef]
- Tretter, V.; Hochreiter, B.; Zach, M.L.; Krenn, K.; Klein, K.U. Understanding Cellular Redox Homeostasis: A Challenge for Precision Medicine. Int. J. Mol. Sci. 2022, 23, 106. [Google Scholar] [CrossRef]
- Bedreag, O.H.; Rogobete, A.F.; Cradigati, C.A.; Sarandan, M.; Nartita, R.; Horhat, F.G.; Popovici, S.E.; Sandesc, D.; Papurica, M. A Novel Evaluation of Microvascular Damage in Critically Ill Polytrauma Patients by Using Circulating MicroRNAs. Rev. Română Med. Lab. 2016, 24, 21–30. [Google Scholar] [CrossRef]
- Corcoran, T.B.; Engel, A.; Sakamoto, H.; O’Shea, A.; O’Callaghan-Enright, S.; Shorten, G.D. The Effects of Propofol on Neutrophil Function, Lipid Peroxidation and Inflammatory Response during Elective Coronary Artery Bypass Grafting in Patients with Impaired Ventricular Function. Br. J. Anaesth. 2006, 97, 825–831. [Google Scholar] [CrossRef]
- Potočnik, I.; Janković, V.N.; Šostarič, M.; Jerin, A.; Štupnik, T.; Skitek, M.; Markovič-Božič, J.; Klokočovnik, T. Antiinflammatory Effect of Sevoflurane in Open Lung Surgery with One-Lung Ventilation. Croat. Med. J. 2014, 55, 628–637. [Google Scholar] [CrossRef]
- Markovic-Bozic, J.; Karpe, B.; Potocnik, I.; Jerin, A.; Vranic, A.; Novak-Jankovic, V. Effect of Propofol and Sevoflurane on the Inflammatory Response of Patients Undergoing Craniotomy. BMC Anesthesiol. 2016, 16, 18. [Google Scholar] [CrossRef]
- Roh, G.U.; Song, Y.; Park, J.; Ki, Y.M.; Han, D.W. Effects of Propofol on the Inflammatory Response during Robot-Assisted Laparoscopic Radical Prostatectomy: A Prospective Randomized Controlled Study. Sci. Rep. 2019, 9, 5242. [Google Scholar] [CrossRef] [PubMed]
- Wijeysundera, D.N.; Committee, E.R.; Duncan, D.; Nkonde-price, C.; Virani, S.S.; Washam, J.B.; Fleischmann, K.E.; Vice, P.G.; Fleisher, L.A.; Guideline, P.; et al. Perioperative Beta Blockade in Noncardiac Surgery: A Systematic Review for the 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2014, 64, 2246–2264. [Google Scholar] [CrossRef] [PubMed]
- Julier, K.; da Silva, R.; Garcia, C.; Bestmann, L.; Frascarolo, P.; Zollinger, A.; Chassot, P.-G.; Schmid, E.R.; Turina, M.I.; von Segesser, L.K.; et al. Preconditioning by Sevoflurane Decreases Biochemical Markers for Myocardial and Renal Dysfunction in Coronary Artery Bypass Graft Surgery: A Double-Blinded, Placebo-Controlled, Multicenter Study. Anesthesiology 2003, 98, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, K.; Lee, H.T. Volatile Anesthetics and AKI: Risks, Mechanisms, and a Potential Therapeutic Window. J. Am. Soc. Nephrol. 2014, 25, 884–892. [Google Scholar] [CrossRef]
- Cai, J.; Xu, R.; Yu, X.; Fang, Y.; Ding, X. Volatile Anesthetics in Preventing Acute Kidney Injury after Cardiac Surgery: A Systematic Review and Meta-Analysis. J. Thorac. Cardiovasc. Surg. 2014, 148, 3127–3136. [Google Scholar] [CrossRef][Green Version]
- Kempson, S.A.; Zhou, Y.; Danbolt, N.C. The Betaine/GABA Transporter and Betaine: Roles in Brain, Kidney, and Liver. Front. Physiol. 2014, 5, 159. [Google Scholar] [CrossRef]
- Bagley, E.E. Opioid and GABAB Receptors Differentially Couple to an Adenylyl Cyclase/Protein Kinase a Downstream Effector after Chronic Morphine Treatment. Front. Pharmacol. 2014, 5, 148. [Google Scholar] [CrossRef]
- Adermark, L.; Söderpalm, B.; Burkhardt, J.M. Brain Region Specific Modulation of Ethanol-Induced Depression of GABAergic Neurons in the Brain Reward System by the Nicotine Receptor Antagonist Mecamylamine. Alcohol 2014, 48, 455–461. [Google Scholar] [CrossRef][Green Version]
- Wagner, J.; Strosing, K.M.; Spassov, S.G.; Lin, Z.; Engelstaedter, H.; Tacke, S.; Hoetzel, A.; Faller, S. Sevoflurane Posttreatment Prevents Oxidative and Inflammatory Injury in Ventilator-Induced Lung Injury. PLoS ONE 2018, 13, e0192896. [Google Scholar] [CrossRef]
- Kalimeris, K.; Kouni, S.; Kostopanagiotou, G.; Nomikos, T.; Fragopoulou, E.; Kakisis, J.; Vasdekis, S.; Matsota, P.; Pandazi, A. Cognitive Function and Oxidative Stress after Carotid Endarterectomy: Comparison of Propofol to Sevoflurane Anesthesia. J. Cardiothorac. Vasc. Anesth. 2013, 27, 1246–1252. [Google Scholar] [CrossRef]
- Yang, P.; Du, Y.; Zeng, H.; Xing, H.; Tian, C.; Zou, X. Comparison of Inflammatory Markers between the Sevoflurane and Isoflurane Anesthesia in a Rat Model of Liver Ischemia/Reperfusion Injury. Transplant. Proc. 2019, 51, 2071–2075. [Google Scholar] [CrossRef] [PubMed]
- Gyires, K.; Budavñri, I.; Fürst, S.; Molnñr, I. Morphine Inhibits the Carrageenan-Induced Oedema and the Chemoluminescence of Leucocytes Stimulated by Zymosan. J. Pharm. Pharmacol. 1985, 37, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Bianchi, M.; Panerai, A.E. Involvement of β-Endorphin in the Modulation of Paw Inflammatory Edema in the Rat. Regul. Pept. 1996, 63, 79–83. [Google Scholar] [CrossRef]
- Planas, E.; Sánchez, S.; Rodriguez, L.; Pol, O.; Puig, M.M. Antinociceptive/Anti-Edema Effects of Liposomal Morphine during Acute Inflammation of the Rat Paw. Pharmacology 2000, 60, 121–127. [Google Scholar] [CrossRef]
- Joris, J.; Costello, A.; Dubner, R.; Hargreaves, K.M. Opiates Suppress Carrageenan-Induced Edema and Hyperthermia at Doses That Inhibit Hyperalgesia. Pain 1990, 43, 95–103. [Google Scholar] [CrossRef]
- Honoré, P.; Buritova, J.; Besson, J.M. Intraplantar Morphine Depresses Spinal C-Fos Expression Induced by Carrageenin Inflammation but Not by Noxious Heat. Br. J. Pharmacol. 1996, 118, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-X.; Lei, L.-G.; Wang, Y.; Da, D.-F.; Zhao, Z.-Q. Endomorphin-1 Reduces Carrageenan-Induced Fos Expression in the Rat Spinal Dorsal Horn. Neuropeptides 1999, 33, 281–284. [Google Scholar] [CrossRef]
- Fletcher, D.; Gentili, M.; Mazoit, J.X.; Samii, K. Additivity of Bupivacaine and Morphine for Peripheral Analgesia in Rats. Fundam. Clin. Pharmacol. 2000, 14, 327–334. [Google Scholar] [CrossRef]
- Walker, J.S. Anti-Inflammatory Effects of Opioids. Adv. Exp. Med. Biol. 2003, 521, 148–160. [Google Scholar]
- Hu, Q.; Wang, Q.; Han, C.; Yang, Y. Sufentanil Attenuates Inflammation and Oxidative Stress in Sepsisinduced Acute Lung Injury by Downregulating KNG1 Expression. Mol. Med. Rep. 2020, 22, 4298–4306. [Google Scholar] [CrossRef]
- Rahimi, S.; Dadfar, B.; Tavakolian, G.; Asadi Rad, A.; Rashid Shabkahi, A.; Siahposht-Khachaki, A. Morphine Attenuates Neuroinflammation and Blood-Brain Barrier Disruption Following Traumatic Brain Injury through the Opioidergic System. Brain Res. Bull. 2021, 176, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, X.; Shu, S.; Wang, S.; Guo, F.; Yin, Y.; Zhou, W.; Han, H.; Chai, X. Sufentanil Protects the Liver from Ischemia/Reperfusion-Induced Inflammation and Apoptosis by Inhibiting ATF4-Induced TP53BP2 Expression. Inflammation 2021, 44, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, R.; Frass, M.; Gmeiner, B.; Sandor, N.; Schumann, R.; Wagner, O.; Kaye, A.D. Effects of Remifentanil on Neutrophil Adhesion, Transmigration, and Intercellular Adhesion Molecule Expression. Acta Anaesthesiol. Scand. 2000, 44, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zhang, Y.; Su, W.; Zhou, L.; Xu, J.; Xia, Z. Remifentanil Attenuates Lipopolysaccharide-Induced Oxidative Injury by Downregulating PKCβ2 Activation and Inhibiting Autophagy in H9C2 Cardiomyocytes. Life Sci. 2018, 213, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Shen, X.; Nan, H.; Yan, L.; Zhao, H.; Yu, J.; Lv, Y. Remifentanil Protects Liver against Ischemia/Reperfusion Injury through Activation of Anti-Apoptotic Pathways. J. Surg. Res. 2013, 183, 827–834. [Google Scholar] [CrossRef]
- Hyejin, J.; Mei, L.; Seongheon, L.; Cheolwon, J.; Seokjai, K.; Hongbeom, B.; Minsun, K.; Sungsu, C.; Sanghyun, K. Remifentanil Attenuates Human Neutrophils Activation Induced by Lipopolysaccharide. Immunopharmacol. Immunotoxicol. 2013, 35, 264–271. [Google Scholar] [CrossRef]
- Maeda, S.; Andoh, T.; Onishi, R.; Tomoyasu, Y.; Higuchi, H.; Miyawaki, T. Remifentanil Suppresses Increase in Interleukin-6 MRNA in the Brain by Inhibiting Cyclic AMP Synthesis. J. Anesth. 2018, 32, 731–739. [Google Scholar] [CrossRef]
- Hasegawa, A.; Iwasaka, H.; Hagiwara, S.; Hasegawa, R.; Kudo, K.; Kusaka, J.; Asai, N.; Noguchi, T. Remifentanil and Glucose Suppress Inflammation in a Rat Model of Surgical Stress. Surg. Today 2011, 41, 1617. [Google Scholar] [CrossRef]
- Lu, Y.; Ding, X.; Wu, X.; Huang, S. Ketamine Inhibits LPS-Mediated BV2 Microglial Inflammation via NMDA Receptor Blockage. Fundam. Clin. Pharmacol. 2020, 34, 229–237. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, Y.; Chen, X.; Zhu, M.; Miao, C. Propofol Attenuates BV2 Microglia Inflammation via NMDA Receptor Inhibition. Can. J. Physiol. Pharmacol. 2017, 96, 241–248. [Google Scholar] [CrossRef]
- Inada, T.; Kamibayashi, T. Protective Effect of the Intravenous Anesthetic Propofol against a Local Inflammation in the Mouse Carrageenan-Induced Air Pouch Model. Immunopharmacol. Immunotoxicol. 2021, 43, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jin, Y.-W.; Wang, D.-Y.; Wang, Z.-G. Effect of propofol upon brain after whole-body hyperthermia in rats. Zhonghua Yi Xue Za Zhi 2009, 89, 2356–2359. [Google Scholar] [PubMed]
- Plachinta, R.V.; Hayes, J.K.; Cerilli, L.A.; Rich, G.F. Isoflurane Pretreatment Inhibits Lipopolysaccharide-Induced Inflammation in Rats. Anesthesiology 2003, 98, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, L.; Wang, Y.; Song, S. Isoflurane Upregulates MicroRNA-9-3p to Protect Rats from Hepatic Ischemia-Reperfusion Injury through Inhibiting Fibronectin Type III Domain Containing 3B. Cell Cycle 2021, 20, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, X.; Feng, Z.; Chen, Y.; Wen, D.; Liu, Z. The Protective Effect of Isoflurane Pretreatment on Liver IRI by Suppressing Noncanonical Pyroptosis of Liver Macrophages. Int. Immunopharmacol. 2021, 99, 107977. [Google Scholar] [CrossRef]
- Blondonnet, R.; Simand, L.-A.; Vidal, P.; Borao, L.; Bourguignon, N.; Morand, D.; Bernard, L.; Roszyk, L.; Audard, J.; Godet, T.; et al. Design and Rationale of the Sevoflurane for Sedation in Acute Respiratory Distress Syndrome (SESAR) Randomized Controlled Trial. J. Clin. Med. 2022, 11, 2796. [Google Scholar] [CrossRef]
- Lv, G.; Li, C.; Wang, W.; Li, N.; Wang, K. Silencing SP1 Alleviated Sevoflurane-Induced POCD Development via Cholinergic Anti-Inflammatory Pathway. Neurochem. Res. 2020, 45, 2082–2090. [Google Scholar] [CrossRef]
- Ngamsri, K.-C.; Fabian, F.; Fuhr, A.; Gamper-Tsigaras, J.; Straub, A.; Fecher, D.; Steinke, M.; Walles, H.; Reutershan, J.; Konrad, F.M. Sevoflurane Exerts Protective Effects in Murine Peritonitis-Induced Sepsis via Hypoxia-Inducible Factor 1α/Adenosine A2B Receptor Signaling. Anesthesiology 2021, 135, 136–150. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Shao, J.; Ma, J. Sevoflurane Induces Inflammation of Microglia in Hippocampus of Neonatal Rats by Inhibiting Wnt/β-Catenin/CaMKIV Pathway. J. Pharmacol. Sci. 2021, 146, 105–115. [Google Scholar] [CrossRef]
- Shen, Q.-Y.; Wu, L.; Wei, C.-S.; Zhou, Y.-N.; Wu, H.-M. Sevoflurane Prevents Airway Remodeling via Downregulation of VEGF and TGF-Β1 in Mice with OVA-Induced Chronic Airway Inflammation. Inflammation 2019, 42, 1015–1022. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Huang, Q.; Wu, G.; Weng, X.; Lai, Z.; Lin, P. Monitoring the End-Tidal Concentration of Sevoflurane for Preventing Awareness during Anesthesia (MEETS-PANDA): A Prospective Clinical Trial. Int. J. Surg. 2017, 41, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Papurica, M.; Rogobete, A.F.; Sandesc, D.; Cradigati, C.A.; Sarandan, M.; Crisan, D.C.; Horhat, F.G.; Boruga, O.; Dumache, R.; Nilima, K.R.; et al. The Expression of Nuclear Transcription Factor Kappa B (NF-KappaB) in the Case of Critically Ill Polytrauma Patients with Sepsis and Its Interactions with MicroRNAs. Biochem. Genet. 2016, 54, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Di Raimondo, D.; Pirera, E.; Rizzo, G.; Simonetta, I.; Musiari, G.; Tuttolomondo, A. Non-Coding RNA Networks as Potential Novel Biomarker and Therapeutic Target for Sepsis and Sepsis-Related Multi-Organ Failure. Diagnostics 2022, 12, 1355. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Chen, K.-Y.; Shih, H.-J.; Chiang, M.; Huang, I.-T.; Huang, Y.-H.; Huang, C.-J. Let-7i-5p Mediates the Therapeutic Effects of Exosomes from Human Placenta Choriodecidual Membrane-Derived Mesenchymal Stem Cells on Mitigating Endotoxin-Induced Mortality and Liver Injury in High-Fat Diet-Induced Obese Mice. Pharmaceuticals 2022, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, M.; Yun, S.; Doh, J.; Greenberg, P.D.; Kim, T.-D.; Choi, I. MicroRNA-150 Regulates the Cytotoxicity of Natural Killers by Targeting Perforin-1. J. Allergy Clin. Immunol. 2014, 134, 195–203. [Google Scholar] [CrossRef]
- Acta, A.; Twaroski, D.; Zj, B.; Bai, X. Pharmaceutica MicroRNAs: New Players in Anesthetic-Induced Developmental Neurotoxicity. Pharm. Anal. Acta 2015, 6, 357. [Google Scholar] [CrossRef]
- Manquat, E.; Ravaux, H.; Kindermans, M.; Joachim, J.; Serrano, J.; Touchard, C.; Mateo, J.; Mebazaa, A.; Gayat, E.; Vallée, F.; et al. Impact of Impaired Cerebral Blood Flow Autoregulation on Electroencephalogram Signals in Adults Undergoing Propofol Anaesthesia: A Pilot Study. BJA Open 2022, 1, 100004. [Google Scholar] [CrossRef]
- Gusar, V.A.; Timofeeva, A.V.; Zhanin, I.S.; Shram, S.I.; Pinelis, V.G. Estimation of Time-Dependent MicroRNA Expression Patterns in Brain Tissue, Leukocytes, and Blood Plasma of Rats under Photochemically Induced Focal Cerebral Ischemia. Mol. Biol. 2017, 51, 602–613. [Google Scholar] [CrossRef]
- Zheng, T.; Shi, Y.; Zhang, J.; Peng, J.; Zhang, X.; Chen, K.; Chen, Y.; Liu, L. MiR-130a Exerts Neuroprotective Effects against Ischemic Stroke through PTEN/PI3K/AKT Pathway. Biomed. Pharmacother. 2019, 117, 109117. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Duan, Z.; Liang, J.; Xu, Y.; Zhang, S.; Tang, T. Restored MicroRNA-326-5p Inhibits Neuronal Apoptosis and Attenuates Mitochondrial Damage via Suppressing STAT3 in Cerebral Ischemia/Reperfusion Injury. Nanoscale Res. Lett. 2021, 16, 63. [Google Scholar] [CrossRef]
- Guo, F.; Han, X.; Zhang, J.; Zhao, X.; Lou, J.; Chen, H.; Huang, X. Repetitive Transcranial Magnetic Stimulation Promotes Neural Stem Cell Proliferation via the Regulation of MiR-25 in a Rat Model of Focal Cerebral Ischemia. PLoS ONE 2014, 9, e109267. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, X.; Cao, J.; Meng, F.; Li, M.; Chen, B.; Zhang, J. MiR-134 Regulates Ischemia/Reperfusion Injury-Induced Neuronal Cell Death by Regulating CREB Signaling. J. Mol. Neurosci. 2014, 55, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-F.; Shi, L.-L.; Zhang, L.; Zhao, Z.-H.; Liang, F.; Xu, X.; Zhao, L.-Y.; Yang, P.-B.; Zhang, J.-S.; Tian, Y.-f. MicroRNA-25 Negatively Regulates Cerebral Ischemia/Reperfusion Injury-Induced Cell Apoptosis Through Fas/FasL Pathway. J. Mol. Neurosci. 2016, 58, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Ren, P.; Wang, G.; Yan, F.; Zhang, Y. MicroRNA-128-3p Protects Mouse Against Cerebral Ischemia Through Reducing P38α Mitogen-Activated Protein Kinase Activity. J. Mol. Neurosci. 2017, 61, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-P.; Zhang, W.-J.; Yang, M.; Fang, H. Propofol Attenuates Lung Ischemia/Reperfusion Injury Though the Involvement of the MALAT1/MicroRNA-144/GSK3β Axis. Mol. Med. 2021, 27, 77. [Google Scholar] [CrossRef]
- Li, G.; Xu, M.; Wang, H.; Qi, X.; Wang, X.; Li, Y.; Sun, J.; Li, Y. MicroRNA-146a Overexpression Alleviates Intestinal Ischemia/Reperfusion-Induced Acute Lung Injury in Mice. Exp. Ther. Med. 2021, 22, 937. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, H.; Zhong, Y.; Huang, J.; Lv, J.; Li, J. The Cold-Inducible RNA-Binding Protein (CIRP) Level in Peripheral Blood Predicts Sepsis Outcome. PLoS ONE 2015, 674, e0137721. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Qi, W.; Dai, S.; Zou, G.; Liu, W.; Yu, B.; Tang, J. MicroRNA-223 Promotes Autophagy to Aggravate Lung Ischemia-Reperfusion Injury by Inhibiting the Expression of Transcription Factor HIF2α. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L1–L10. [Google Scholar] [CrossRef]
- Cai, J.; Gehrau, R.; Tu, Z.; Leroy, V.; Su, G.; Shang, J.; Mas, V.R.; Emtiazjoo, A.; Pelaez, A.; Atkinson, C.; et al. MicroRNA-206 AntagomiR—Enriched Extracellular Vesicles Attenuate Lung Ischemia—Reperfusion Injury through CXCL1 Regulation in Alveolar Epithelial Cells. J. Heart Lung Transpl. 2020, 39, 1476–1490. [Google Scholar] [CrossRef]
- Xiao, K.; Song, L.; Hu, Y.; He, W.; Hou, F.; Yan, P.; Xu, J.; Wang, K.; Tao, Y.; Li, D.; et al. Novel Role of MiR-18a-5p and Galanin in Rat Lung Ischemia Reperfusion-Mediated Response. Oxid. Med. Cell Longev. 2021, 2021, 6621921. [Google Scholar] [CrossRef]
- Qin, L.-B.; Li, Z.-Y.; Li, H.; Fan, X.-Q.; Liu, H.-G.; Dong, X.-M.; Jia, W.-Y. Inhibitive Effects of MicroRNA-34a on Protecting against Ischemia-Reperfusion Injury of Vital Organs in Hemorrhagic Shock Pregnant Mice. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1812–1818. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Wei, L.; Han, Z.; Chen, Z. Mesenchymal Stromal Cells-Derived Exosomes Alleviate Ischemia/Reperfusion Injury in Mouse Lung by Transporting Anti-Apoptotic MiR-21-5p. Eur. J. Pharmacol. 2019, 852, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Masaldan, S.; Bush, A.I.; Devos, D.; Rolland, A.S.; Moreau, C. Striking While the Iron Is Hot: Iron Metabolism and Ferroptosis in Neurodegeneration. Free Radic. Biol. Med. 2018, 133, 221–233. [Google Scholar] [CrossRef]
- Tuo, Q.-Z.; Lei, P.; Jackman, K.A.; Li, X.-L.; Xiong, H.; Li, X.-L.; Liuyang, Z.-Y.; Roisman, L.; Zhang, S.-T.; Ayton, S.; et al. Tau-Mediated Iron Export Prevents Ferroptotic Damage after Ischemic Stroke. Mol. Psychiatry 2017, 22, 1520–1530. [Google Scholar] [CrossRef]
- Dzikiewicz-Krawczyk, A.; Kok, K.; Slezak-Prochazka, I.; Robertus, J.-L.; Bruining, J.; Tayari, M.M.; Rutgers, B.; de Jong, D.; Koerts, J.; Seitz, A.; et al. ZDHHC11 and ZDHHC11B Are Critical Novel Components of the Oncogenic MYC-MiR-150-MYB Network in Burkitt Lymphoma. Leukemia 2017, 31, 1470–1473. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Cai, Z.; Wang, H.; Han, D.; Cheng, Q.; Zhang, P.; Gao, F.; Yu, Y.; Song, Z.; Wu, Q.; et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ. Res. 2020, 127, 486–501. [Google Scholar] [CrossRef]
- Drobna, M.; Szarzyńska, B.; Jaksik, R.; Sędek, Ł.; Kuchmiy, A.; Taghon, T.; van Vlierberghe, P.; Szczepański, T.; Witt, M.; Dawidowska, M. Hsa-MiR-20b-5p and Hsa-MiR-363-3p Affect Expression of PTEN and BIM Tumor Suppressor Genes and Modulate Survival of T-ALL Cells In Vitro. Cells 2020, 9, 1137. [Google Scholar] [CrossRef]
- Ding, C.; Ding, X.; Zheng, J.; Wang, B.; Li, Y.; Xiang, H.; Dou, M.; Qiao, Y.; Tian, P.; Xue, W. MiR-182-5p and MiR-378a-3p Regulate Ferroptosis in I/R-Induced Renal Injury. Cell Death Dis. 2020, 11, 929. [Google Scholar] [CrossRef]
- Chiba, T.; Cerqueira, D.M.; Li, Y.; Bodnar, A.J.; Mukherjee, E.; Pfister, K.; Phua, Y.L.; Shaikh, K.; Sanders, B.T.; Hemker, S.L.; et al. Endothelial-Derived MiR-17∽92 Promotes Angiogenesis to Protect against Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2021, 32, 553–562. [Google Scholar] [CrossRef]
- Lorenzen, J.M.; Kielstein, J.T.; Hafer, C.; Gupta, S.K.; Ku, P. Circulating MiR-210 Predicts Survival in Critically Ill Patients with Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2011, 6, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Gao, J.; Song, Y.-H.; Cai, G.-Y.; Chen, X.-M. Identification of Potential Gene and MicroRNA Biomarkers of Acute Kidney Injury. Biomed. Res. Int. 2021, 2021, 8834578. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-Y.; Wang, B.; Tang, T.-T.; Wen, Y.; Li, Z.-L.; Feng, S.-T.; Wu, M.; Liu, D.; Yin, D.; Ma, K.-L.; et al. Exosomal MiR-125b-5p Deriving from Mesenchymal Stem Cells Promotes Tubular Repair by Suppression of P53 in Ischemic Acute Kidney Injury. Theranostics 2021, 11, 5248–5266. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, Y.; Liu, P.; Hao, J.; Liang, M.; Mi, Q.-S.; Chen, J.-K.; Dong, Z. MicroRNA-489 Induction by Hypoxia-Inducible Factor-1 Protects against Ischemic Kidney Injury. J. Am. Soc. Nephrol. 2016, 27, 2784–2796. [Google Scholar] [CrossRef]
- Aguado-fraile, E.; Ramos, E.; Conde, E.; Rodríguez, M.; Martín-, L. A Pilot Study Identifying a Set of MicroRNAs as Precise Diagnostic Biomarkers of Acute Kidney Injury. PLoS ONE 2015, 10, e0127175. [Google Scholar] [CrossRef]
- Bruno, N.; ter Maaten, J.M.; Ovchinnikova, E.S.; Vegter, E.L.; Valente, M.A.E.; van der Meer, P.; de Boer, R.A.; van der Harst, P.; Schmitter, D.; Metra, M.; et al. MicroRNAs Relate to Early Worsening of Renal Function in Patients with Acute Heart Failure. Int. J. Cardiol. 2015, 203, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, K.; Saikumar, J.; Bijol, V.; Koyner, J.L.; Qian, J.; Betensky, R.A.; Waikar, S.S.; Vaidya, V.S. Human MiRNome Profiling Identifies MicroRNAs Differentially Present in the Urine after Kidney Injury. Clin. Chem. 2013, 59, 1742–1752. [Google Scholar] [CrossRef]
- Chen, H.; Lan, Y.; Li, H.; Cheng, C.; Lai, P. Urinary MiR-16 Transactivated by C / EBP β Reduces Kidney Function after Ischemia/Reperfusion—Induced Injury. Nat. Publ. Group 2016, 6, 27945. [Google Scholar] [CrossRef]
- Ge, Q.-M.; Huang, C.-M.; Zhu, X.-Y.; Bian, F.; Pan, S.-M. Differentially Expressed MiRNAs in Sepsis-Induced Acute Kidney Injury Target Oxidative Stress and Mitochondrial Dysfunction Pathways. PLoS ONE 2017, 12, e0173292. [Google Scholar] [CrossRef]
- Pavkovic, M.; Riefke, B.; Ellinger-Ziegelbauer, H. Urinary MicroRNA Profiling for Identification of Biomarkers after Cisplatin-Induced Kidney Injury. Toxicology 2014, 324, 147–157. [Google Scholar] [CrossRef]
- Gutiérrez-Escolano, A.; Santacruz-Vázquez, E.; Gómez-Pérez, F. Dysregulated MicroRNAs Involved in Contrast-Induced Acute Kidney Injury in Rat and Human. Ren. Fail. 2015, 37, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Che, L.; Wang, Y.; Zhou, H.; Gong, H.; Man, X.; Zhao, Q. Deregulated MicroRNA-22-3p in Patients with Sepsis-Induced Acute Kidney Injury Serves as a New Biomarker to Predict Disease Occurrence and 28-Day Survival Outcomes. Int. Urol. Nephrol. 2021, 53, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- Shihana, F.; Joglekar, M.V.; Raubenheimer, J.; Hardikar, A.A.; Buckley, N.A.; Seth, D. Circulating Human MicroRNA Biomarkers of Oxalic Acid-Induced Acute Kidney Injury. Arch. Toxicol. 2020, 94, 1725–1737. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.B.; Schnellmann, R.G. Extracellular Signal-Regulated Kinase 1/2 Regulates NAD Metabolism during Acute Kidney Injury through MicroRNA-34a-Mediated NAMPT Expression. Cell. Mol. Life Sci. 2020, 77, 3643–3655. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jing, Y.; Hao, J.; Frankfort, N.C.; Zhou, X.; Shen, B. MicroRNA-21 in the Pathogenesis of Acute Kidney Injury. Protein Cell 2013, 4, 813–819. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Xue, S.; Wang, X.; Dai, H.; Qian, J. Implications of Dynamic Changes in MiR-192 Expression in Ischemic Acute Kidney Injury. Int. Urol. Nephrol. 2017, 49, 541–550. [Google Scholar] [CrossRef]
- Uhlenhaut, N.H.; Treier, M. Transcriptional Regulators in Kidney Disease: Gatekeepers of Renal Homeostasis. Trends Genet. 2008, 24, 361–371. [Google Scholar] [CrossRef]
- Tan, R.J.; Zhou, D.; Zhou, L.; Liu, Y. Wnt/β-Catenin Signaling and Kidney Fibrosis. Kidney Int. Suppl. 2014, 4, 84–90. [Google Scholar] [CrossRef]
- Niehrs, C. Function and Biological Roles of the Dickkopf Family of Wnt Modulators. Oncogene 2006, 25, 7469–7481. [Google Scholar] [CrossRef]
- Yi, S.-J.; Li, L.-L.; Tu, W.-B. MiR-214 Negatively Regulates Proliferation and WNT/β-Catenin Signaling in Breast Cancer. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 5148–5154. [Google Scholar]
- Zhu, X.; Li, W.; Li, H. MiR-214 Ameliorates Acute Kidney Injury via Targeting DKK3 and Activating of Wnt/β-Catenin Signaling Pathway. Biol. Res. 2018, 51, 31. [Google Scholar] [CrossRef] [PubMed]
- Marcil, V.; Lavoie, J.C.; Emonnot, L.; Seidman, E.; Levy, E. Analysis of the Effects of Iron and Vitamin C Co-Supplementation on Oxidative Damage, Antioxidant Response and Inflammation in THP-1 Macrophages. Clin. Biochem. 2011, 44, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Reyes-García, J.; Carbajal-García, A.; di Mise, A.; Zheng, Y.M.; Wang, X.; Wang, Y.X. Important Functions and Molecular Mechanisms of Mitochondrial Redox Signaling in Pulmonary Hypertension. Antioxidants 2022, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Baev, A.Y.; Vinokurov, A.Y.; Novikova, I.N.; Dremin, V.V.; Potapova, E.V.; Abramov, A.Y. Interaction of Mitochondrial Calcium and ROS in Neurodegeneration. Cells 2022, 11, 706. [Google Scholar] [CrossRef]
- Tripatara, P.; Patel, N.S.A.; Collino, M.; Gallicchio, M.; Kieswich, J.; Castiglia, S.; Benetti, E.; Stewart, K.N.; Brown, P.A.; Yaqoob, M.M.; et al. Generation of Endogenous Hydrogen Sulfide by Cystathionine γ-Lyase Limits Renal Ischemia/Reperfusion Injury and Dysfunction. Lab. Investig. 2008, 88, 1038–1048. [Google Scholar] [CrossRef]
- Bos, E.M.; Leuvenink, H.G.D.; Snijder, P.M.; Kloosterhuis, N.J.; Hillebrands, J.-L.; Leemans, J.C.; Florquin, S.; van Goor, H. Hydrogen Sulfide-Induced Hypometabolism Prevents Renal Ischemia/Reperfusion Injury. J. Am. Soc. Nephrol. 2009, 20, 1901–1905. [Google Scholar] [CrossRef]
- Granata, S.; Votrico, V.; Spadaccino, F.; Catalano, V.; Netti, G.S.; Ranieri, E.; Stallone, G.; Zaza, G. Oxidative Stress and Ischemia/Reperfusion Injury in Kidney Transplantation: Focus on Ferroptosis, Mitophagy and New Antioxidants. Antioxidants 2022, 11, 769. [Google Scholar] [CrossRef]
- Birk, A.V.; Liu, S.; Soong, Y.; Mills, W.; Singh, P.; Warren, J.D.; Seshan, S.V.; Pardee, J.D.; Szeto, H.H. The Mitochondrial-Targeted Compound SS-31 Re-Energizes Ischemic Mitochondria by Interacting with Cardiolipin. J. Am. Soc. Nephrol. 2013, 24, 1250–1261. [Google Scholar] [CrossRef]
- Szeto, H.H.; Liu, S.; Soong, Y.; Seshan, S.V.; Cohen-Gould, L.; Manichev, V.; Feldman, L.C.; Gustafsson, T. Mitochondria Protection after Acute Ischemia Prevents Prolonged Upregulation of IL-1β and IL-18 and Arrests CKD. J. Am. Soc. Nephrol. 2016, 28, 1437–1449. [Google Scholar] [CrossRef]
- Laight, D.W.; Andrews, T.J.; Haj-Yehia, A.I.; Carrier, M.J.; Änggård, E.E. Microassay of Superoxide Anion Scavenging Activity in Vitro. Environ. Toxicol. Pharmacol. 1997, 3, 65–68. [Google Scholar] [CrossRef]
- Chatterjee, P.; Bhattacharyya, M.; Bandyopadhyay, S.; Roy, D. Studying the System-Level Involvement of MicroRNAs in Parkinson’s Disease. PLoS ONE 2014, 9, e93751. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Kurihara, S. Cystine and Theanine as Stress-Reducing Amino Acids—Perioperative Use for Early Recovery after Surgical Stress. Nutrients 2022, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Bissolati, M.; Rocchetti, S.; Beneduce, A.; Pecorelli, N.; di Carlo, V. Oral Preoperative Antioxidants in Pancreatic Surgery: A Double-Blind, Randomized, Clinical Trial. Nutrition 2012, 28, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.-Y.; van der Merwe, J.; Pepe, S.; Bailey, M.; Perkins, A.; Lymbury, R.; Esmore, D.; Marasco, S.; Rosenfeldt, F. Perioperative Metabolic Therapy Improves Redox Status and Outcomes in Cardiac Surgery Patients: A Randomised Trial. Heart Lung Circ. 2010, 19, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, T.; Tsuchiya, T.; Oyama, A.; Tsuchiya, T.; Abe, N.; Sato, A.; Chiba, Y.; Kurihara, S.; Shibakusa, T.; Mikami, T. Perioperative Oral Administration of Cystine and Theanine Enhances Recovery after Distal Gastrectomy: A Prospective Randomized Trial. J. Parenter. Enteral. Nutr. 2012, 37, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Shibakusa, T.; Mikami, T.; Kurihara, S.; Chiba, Y.; Tsuchiya, T.; Miyachi, T.; Oyama, A.; Tanaka, K.A.K.; Koyama, N. Enhancement of Postoperative Recovery by Preoperative Oral Co-Administration of the Amino Acids, Cystine and Theanine, in a Mouse Surgical Model. Clin. Nutr. 2012, 31, 555–561. [Google Scholar] [CrossRef]
- Tanaka, K.A.; Mazzeffi, M.; Durila, M. Role of Prothrombin Complex Concentrate in Perioperative Coagulation Therapy. J. Intensive Care 2014, 2, 60. [Google Scholar] [CrossRef][Green Version]
- Dingchao, H.; Zhiduan, Q.; Liye, H.; Xiaodong, F. The Protective Effects of High-Dose Ascorbic Acid on Myocardium against Reperfusion Injury during and after Cardiopulmonary Bypass. Thorac. Cardiovasc. Surg. 1994, 42, 276–278. [Google Scholar] [CrossRef]
- Nathens, A.B.; Neff, M.J.; Jurkovich, G.J.; Klotz, P.; Farver, K.; Ruzinski, J.T.; Radella, F.; Garcia, I.; Maier, R.V. Randomized, Prospective Trial of Antioxidant Supplementation in Critically Ill Surgical Patients. Ann. Surg. 2002, 236, 814–822. [Google Scholar] [CrossRef]
- Tanaka, H.; Matsuda, T.; Miyagantani, Y.; Yukioka, T.; Matsuda, H.; Shimazaki, S. Reduction of Resuscitation Fluid Volumes in Severely Burned Patients Using Ascorbic Acid Administration: A Randomized, Prospective Study. Arch. Surg. 2000, 135, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.A.; Beers, R.J.; Lentz, C.W. Resuscitation after Severe Burn Injury Using High-Dose Ascorbic Acid: A Retrospective Review. J. Burn Care Res. 2011, 32, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Shaw, G.M.; Fowler, A.A.; Natarajan, R. Ascorbate-Dependent Vasopressor Synthesis: A Rationale for Vitamin C Administration in Severe Sepsis and Septic Shock? Crit. Care 2015, 19, 418. [Google Scholar] [CrossRef] [PubMed]
- Oakes, B.; Bolia, I.K.; Weber, A.E.; Petrigliano, F.A. Vitamin C in Orthopedic Practices: Current Concepts, Novel Ideas, and Future Perspectives. J. Orthop. Res. 2021, 39, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Suter, M. Positive End-Expiratory Pressure During Induction of General Anesthesia Increases Duration of Nonhypoxic Apnea in Morbidly Obese Patients. Anesth. Analg. 2005, 100, 580–584. [Google Scholar] [CrossRef]
- Chen, S.; Roffey, D.M.; Dion, C.-A.; Arab, A.; Wai, E.K. Effect of Perioperative Vitamin C Supplementation on Postoperative Pain and the Incidence of Chronic Regional Pain Syndrome: A Systematic Review and Meta-Analysis. Clin. J. Pain 2016, 32, 179–185. [Google Scholar] [CrossRef]
- Ngo, B.; van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting Cancer Vulnerabilities with High-Dose Vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef]
- Du, Y.T.; Long, Y.; Tang, W.; Liu, X.F.; Dai, F.; Zhou, B. Prooxidative Inhibition against NF-ΚB-Mediated Inflammation by Pharmacological Vitamin C. Free Radic. Biol. Med. 2022, 180, 85–94. [Google Scholar] [CrossRef]
- Brown, M.L.; Braun, M.; Cicalese, L.; Rastellini, C. Effect of Perioperative Antioxidant Therapy on Suboptimal Islet Transplantation in Rats. Transplant. Proc. 2005, 37, 217–219. [Google Scholar] [CrossRef]
- Moon, S.; Lim, S.H.; Cho, K.; Kim, M.H.; Lee, W.; Cho, Y.H. The Efficacy of Vitamin C on Postlaparoscopic Shoulder Pain: A Double-Blind Randomized Controlled Trial. Anesth. Pain Med. 2019, 14, 202–207. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Hamawy, T.Y. Comparative Evaluation between Ascorbic Acid and N-Acetyl Cysteine for Preventing Tourniquet Induced Ischaemic Reperfusion Injury during Lower Limb Surgery, a Randomized Controlled Trial. Egypt. J. Anaesth. 2016, 32, 103–109. [Google Scholar] [CrossRef][Green Version]
- Rümelin, A.; Dörr, S.; Fauth, U. Single Preoperative Oral Application of Ascorbic Acid Does Not Affect Postoperative Plasma Levels of Ascorbic Acid. Ann. Nutr. Metab. 2002, 46, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Park, J.S.; Moon, S.; Yeo, J. Effect of Intravenous High Dose Vitamin C on Postoperative Pain and Morphine Use after Laparoscopic Colectomy: A Randomized Controlled Trial. Pain Res. Manag. 2016, 2016, 9147279. [Google Scholar] [CrossRef] [PubMed]
- Holford, P.; Carr, A.C.; Jovic, T.H.; Ali, S.R.; Whitaker, I.S.; Marik, P.E.; Smith, A.D. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients 2020, 12, 3760. [Google Scholar] [CrossRef] [PubMed]
- Martín Giménez, V.M.; Chuffa, L.G.A.; Simão, V.A.; Reiter, R.J.; Manucha, W. Protective Actions of Vitamin D, Anandamide and Melatonin during Vascular Inflammation: Epigenetic Mechanisms Involved. Life Sci. 2022, 288, 120191. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Nieuwland, A.J.; Kokje, V.B.C.; Koning, O.H.; Hamming, J.F.; Szuhai, K.; Claas, F.H.J.; Lindeman, J.H.N. Activation of the Vitamin D Receptor Selectively Interferes with Calcineurin-Mediated Inflammation: A Clinical Evaluation in the Abdominal Aortic Aneurysm. Lab. Investig. 2016, 96, 784–790. [Google Scholar] [CrossRef]
- Martínez-Moreno, J.M.; Herencia, C.; de Oca, A.M.; Díaz-Tocados, J.M.; Vergara, N.; Gómez-Luna, M.J.; López-Argüello, S.D.; Camargo, A.; Peralbo-Santaella, E.; Rodríguez-Ortiz, M.E.; et al. High Phosphate Induces a Pro-Inflammatory Response by Vascular Smooth Muscle Cells and Modulation by Vitamin D Derivatives. Clin. Sci. 2017, 131, 1449–1463. [Google Scholar] [CrossRef]
- Bogunovic, L.; Kim, A.D.; Beamer, B.S.; Nguyen, J.; Lane, J.M. Hypovitaminosis D in Patients Scheduled to Undergo Orthopaedic Surgery: A Single-Center Analysis. J. Bone Joint Surg. Am. 2010, 92, 2300–2304. [Google Scholar] [CrossRef]
- Maniar, R.N.; Patil, A.M.; Maniar, A.R.; Gangaraju, B.; Singh, J. Effect of Preoperative Vitamin D Levels on Functional Performance after Total Knee Arthroplasty. Clin. Orthop. Surg. 2016, 8, 153–156. [Google Scholar] [CrossRef]
- Mouli, V.H.; Schudrowitz, N.; Carrera, C.X.; Uzosike, A.C.; Fitz, W.; Rajaee, S.S. High-Dose Vitamin D Supplementation Can Correct Hypovitaminosis D Prior to Total Knee Arthroplasty. J. Arthroplast. 2022, 37, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Hajimohammadebrahim-Ketabforoush, M.; Shahmohammadi, M.; Keikhaee, M.; Eslamian, G.; Vahdat Shariatpanahi, Z. Single High-Dose Vitamin D3 Injection and Clinical Outcomes in Brain Tumor Resection: A Randomized, Controlled Clinical Trial. Clin. Nutr. ESPEN 2021, 41, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Alshafey, M.K.; Elrakhawy, H.M.; Rezk, M.E.; Moustafa, H.M. Role of Ascorbic Acid in Reduction of the Incidence of the Atrial Fibrillation in Patients under B-Blocker and Undergoing Coronary Artery Bypass Graft Operation in Early Post-Operative Period. J. Egypt. Soc. Cardio-Thorac. Surg. 2017, 25, 198–203. [Google Scholar] [CrossRef]
- Gunes, T.; Bozok, S.; Kestelli, M.; Yurekli, I.; Ilhan, G.; Ozpak, B.; Bademci, M.; Ozcem, B.; Sahin, A. α-Tocopherol and Ascorbic Acid in Early Postoperative Period of Cardiopulmonary Bypass. J. Cardiovasc. Med. 2012, 13, 691–699. [Google Scholar] [CrossRef]
- Castillo, R.L.; Loza, R.C. Pathophysiological Approaches of Acute Respiratory Distress Syndrome: Novel Bases for Study of Lung Injury. Open Respir. Med. J. 2015, 9, 83–91. [Google Scholar] [CrossRef]
- Angdin, M.; Settergren, G.; Starkopf, J.; Zilmer, M.; Zilmer, K.; Vaage, J. Protective Effect of Antioxidants on Pulmonary Endothelial Function after Cardiopulmonary Bypass. J. Cardiothorac. Vasc. Anesth. 2003, 17, 314–320. [Google Scholar] [CrossRef]
- Antonic, M. Effect of Ascorbic Acid on Postoperative Acute Kidney Injury in Coronary Artery Bypass Graft Patients: A Pilot Study. Heart Surg. Forum 2017, 20, E214–E218. [Google Scholar] [CrossRef]
- Das, D.; Sen, C.; Goswami, A. Effect of Vitamin C on Adrenal Suppression by Etomidate Induction in Patients Undergoing Cardiac Surgery: A Randomized Controlled Trial. Ann. Card. Anaesth. 2016, 19, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Sadeghpour, A.; Alizadehasl, A.; Kyavar, M.; Sadeghi, T.; Moludi, J. Impact of Vitamin C Supplementation on Post-Cardiac Surgery ICU and Hospital Length of Stay. Anesthesiol. Pain Med. 2015, 5, e25337. [Google Scholar] [CrossRef] [PubMed]
- Samadikhah, J.; Golzari, S.E.J.; Sabermarouf, B.; Karimzadeh, I.; Tizro, P.; Khanli, H.M.; Ghabili, K. Efficacy of Combination Therapy of Statin and Vitamin C in Comparison with Statin in the Prevention of Post-CABG Atrial Fibrillation. Adv. Pharm. Bull. 2014, 4, 97–100. [Google Scholar] [CrossRef]
- Dehghani, M.R.; Majidi, N.; Rahmani, A.; Asgari, B.; Rezaei, Y. Effect of Oral Vitamin C on Atrial Fibrillation Development after Isolated Coronary Artery Bypass Grafting Surgery: A Prospective Randomized Clinical Trial. Cardiol. J. 2013, 21, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Jouybar, R.; Kabgani, H.; Kamalipour, H.; Shahbazi, S.; Allahyary, E.; Rasouli, M.; Hedayatallah Akhlagh, S.; Shafa, M.; Ghazinoor, M.; Taghi Moeinvaziri, M.; et al. The Perioperative Effect of Ascorbic Acid on Inflammatory Response in Coronary Artery Bypass Graft Surgery; A Randomized Controlled Trial Coronary Artery Bypass Graft Surgery. Int. Cardiovasc. Res. J. 2012, 6, e12835. [Google Scholar]
- Papoulidis, P.; Ananiadou, O.; Chalvatzoulis, E.; Ampatzidou, F.; Koutsogiannidis, C.; Karaiskos, T.; Madesis, A.; Drossos, G. The Role of Ascorbic Acid in the Prevention of Atrial Fibrillation after Elective On-Pump Myocardial Revascularization Surgery: A Single-Center Experience—A Pilot Study. Interact. CardioVascular Thorac. Surg. 2011, 12, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Collier, B.R.; Giladi, A.; Dossett, L.A.; Dyer, L.; Fleming, S.B.; Cotton, B.A. Impact of High-Dose Antioxidants on Outcomes in Acutely Injured Patients. J. Parenter. Enter. Nutr. 2008, 32, 384–388. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).