Myh9 Plays an Essential Role in the Survival and Maintenance of Hematopoietic Stem/Progenitor Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Flow Cytometry
2.3. Bone Marrow Transplant Experiments
2.4. Complete Blood Counts and Blood Smear
2.5. Real-Time Quantitative PCR
2.6. RNA-Sequencing and Data Analysis
2.7. Statistical Analysis
3. Results
3.1. Conditional Deletion of Myh9 Leads to Pancytopenia and Fatal Bone Marrow Failure
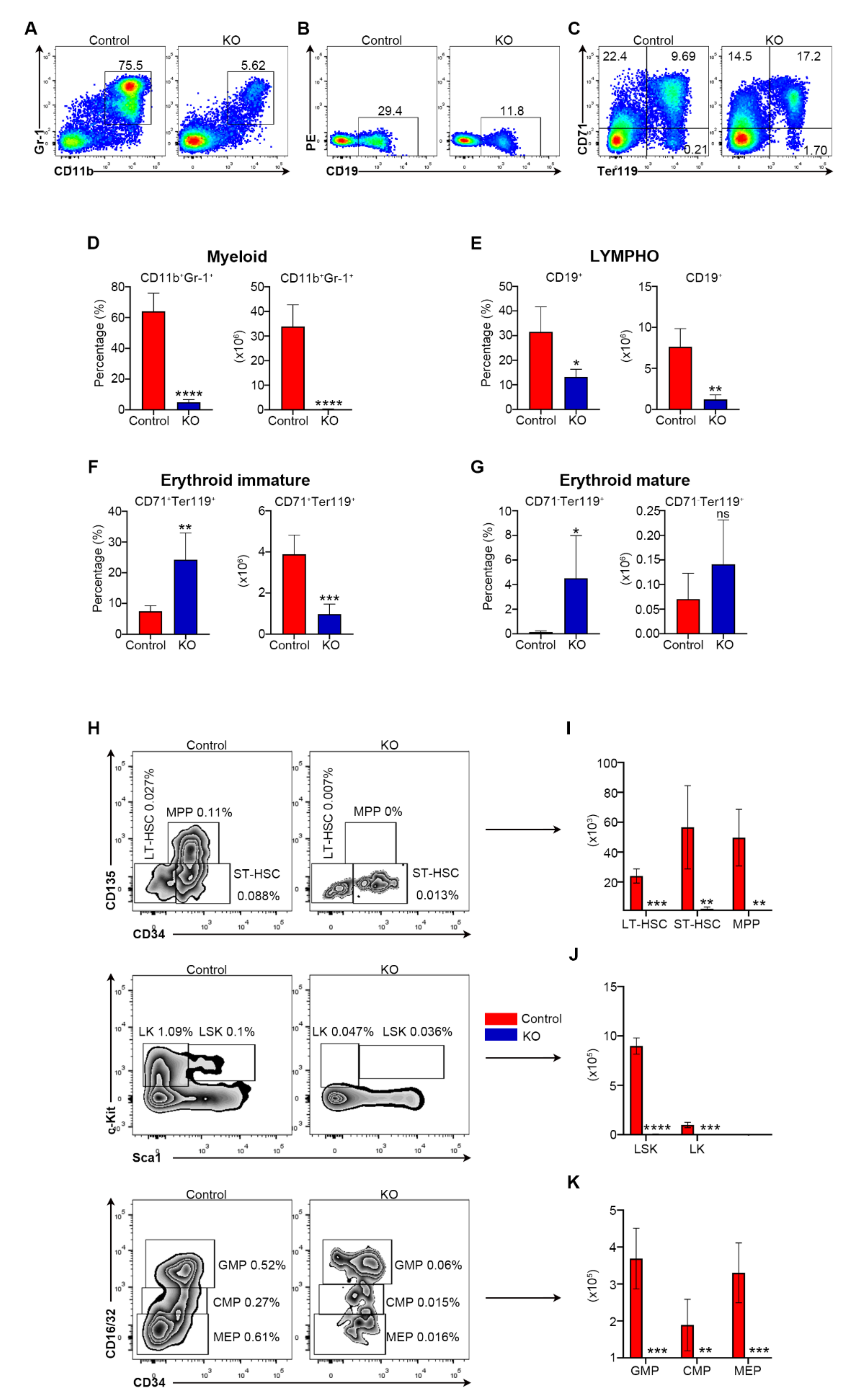
3.2. Myh9 Deficiency Causes Deletion of HSCs and Multiple Hematopoietic Lineage Cells
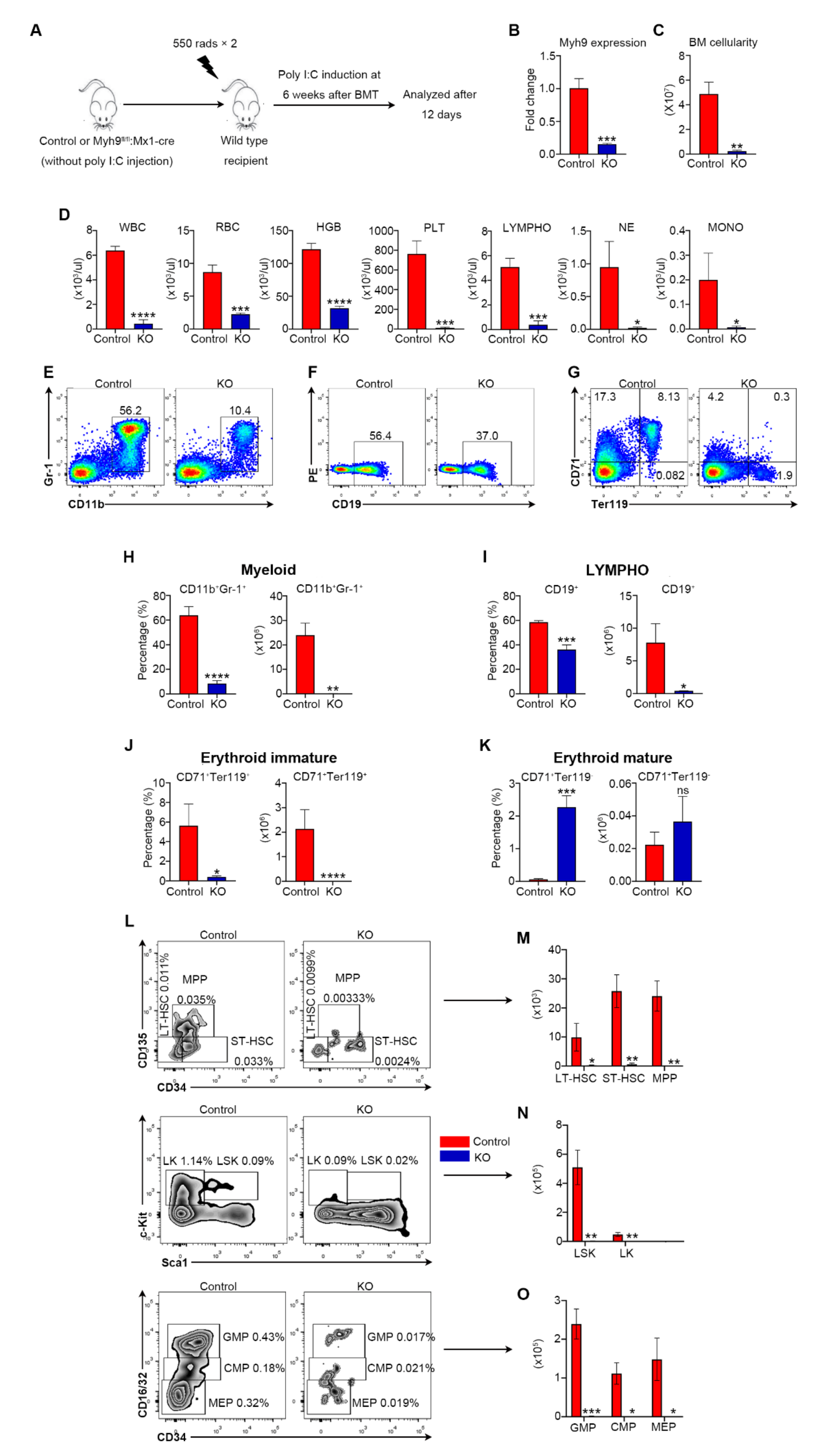
3.3. Myh9 Function in Hematopoiesis Is Hsc Intrinsic
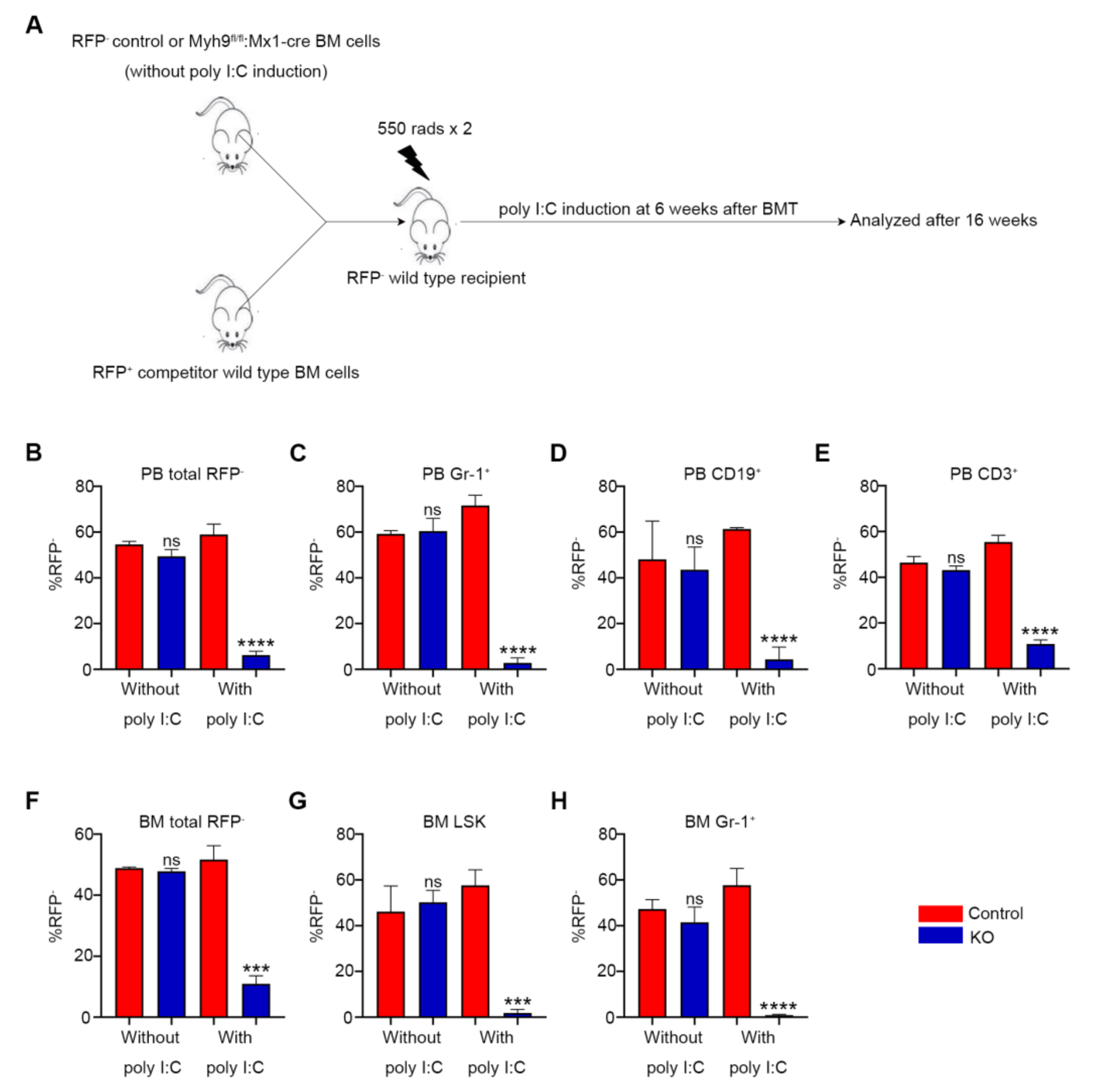
3.4. Loss of Myh9 Results in Impaired Repopulation of HSCs
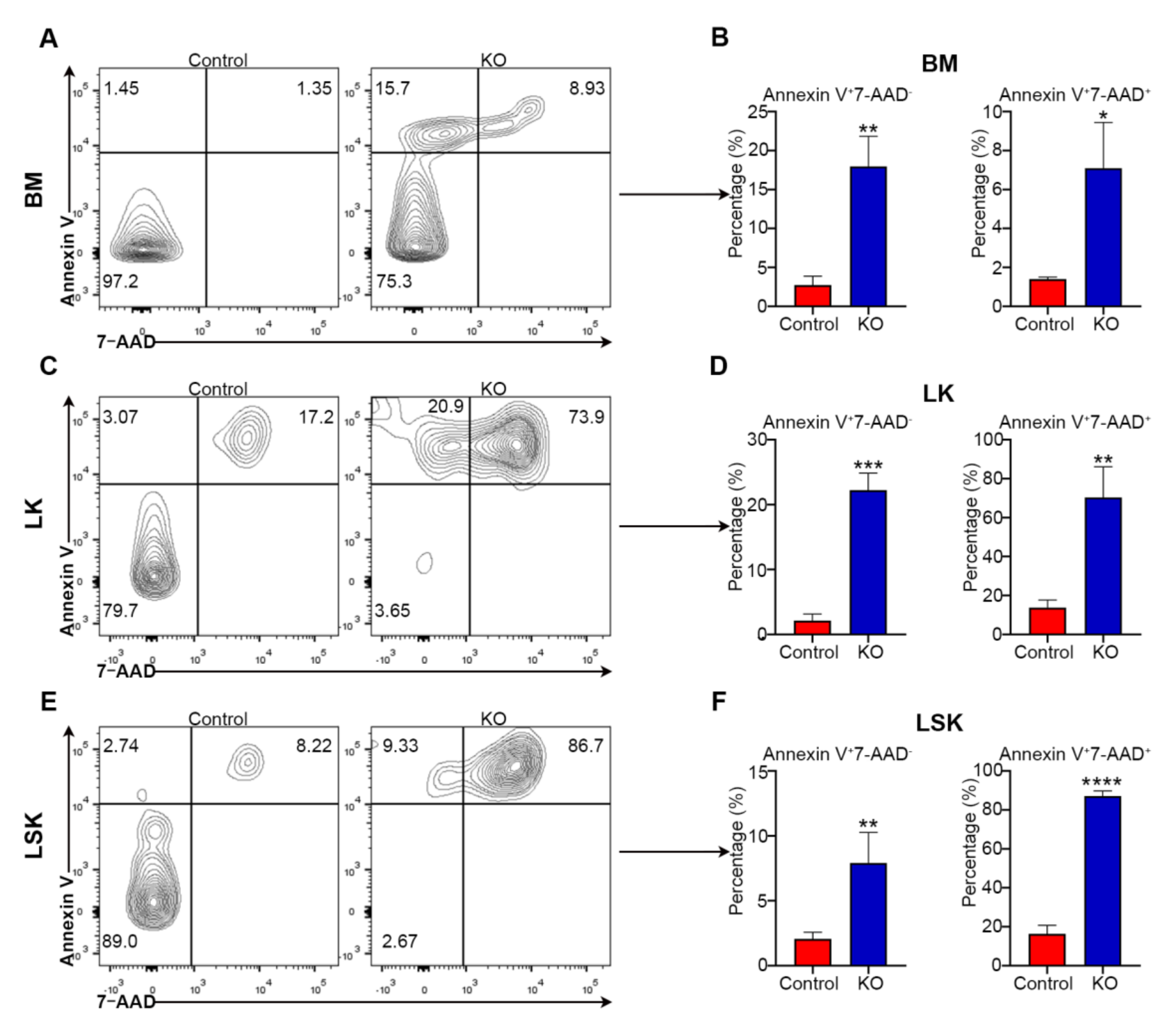
3.5. Loss of Myh9 Leads to Apoptosis of Hematopoietic Stem/Progenitor Cells
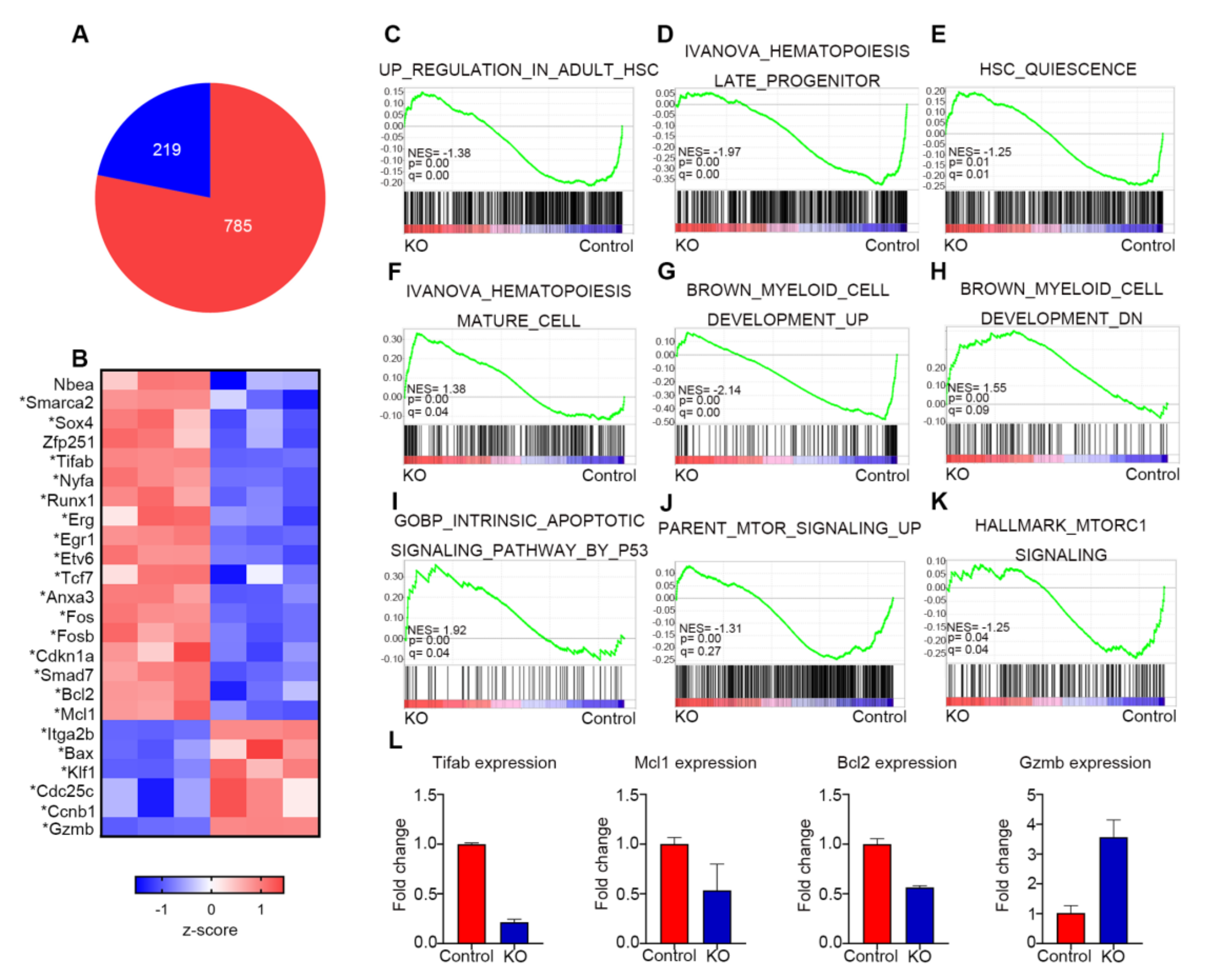
3.6. Myh9 Deletion Alters Expression of Genes Responsible for HSC Maintenance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kondo, M.; Weissman, I.L.; Akashi, K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 1997, 91, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Akashi, K.; Traver, D.; Miyamoto, T.; Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000, 404, 193–197. [Google Scholar] [CrossRef]
- De Los Angeles, A.; Ferrari, F.; Xi, R.; Fujiwara, Y.; Benvenisty, N.; Deng, H.; Hochedlinger, K.; Jaenisch, R.; Lee, S.; Leitch, H.; et al. Hallmarks of pluripotency. Nature 2015, 525, 469–478. [Google Scholar] [CrossRef]
- Spitz, F.; Furlong, E.E. Transcription factors: From enhancer binding to developmental control. Nat. Rev. Genet. 2012, 13, 613–626. [Google Scholar] [CrossRef]
- Asensio-Juárez, G.; Llorente-González, C.; Vicente-Manzanares, M. Linking the landscape of MYH9-related diseases to the molecular mechanisms that control non-muscle myosin II-A function in cells. Cells 2020, 9, 1458. [Google Scholar] [CrossRef]
- Hartman, M.A.; Spudich, J.A. The myosin superfamily at a glance. J. Cell Sci. 2012, 125, 1627–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heissler, S.M.; Sellers, J.R. Kinetic adaptations of myosins for their diverse cellular functions. Traffic 2016, 17, 839–859. [Google Scholar] [CrossRef] [Green Version]
- Masters, T.A.; Kendrick-Jones, J.; Buss, F. Myosins: Domain organisation, motor properties, physiological roles and cellular functions. Actin Cytoskelet. 2016, 235, 77–122. [Google Scholar]
- Vicente-Manzanares, M.; Ma, X.; Adelstein, R.S.; Horwitz, A.R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 778–790. [Google Scholar]
- Krendel, M.; Mooseker, M.S. Myosins: Tails (and heads) of functional diversity. Physiology 2005, 20, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Loubery, S.; Coudrier, E. Myosins in the secretory pathway: Tethers or transporters? Cell. Mol. Life Sci. CMLS 2008, 65, 2790–2800. [Google Scholar] [PubMed]
- Breshears, L.M.; Wessels, D.; Soll, D.R.; Titus, M.A. An unconventional myosin required for cell polarization and chemotaxis. Proc. Natl. Acad. Sci. USA 2010, 107, 6918–6923. [Google Scholar]
- Woolner, S.; Bement, W.M. Unconventional myosins acting unconventionally. Trends Cell Biol. 2009, 19, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Althaus, K.; Greinacher, A. MYH9 related platelet disorders: Strategies for management and diagnosis. Transfus. Med. Hemother. 2010, 37, 260–267. [Google Scholar]
- Pecci, A.; Ma, X.; Savoia, A.; Adelstein, R.S. MYH9: Structure, functions and role of non-muscle myosin ⅡA in human disease. Gene 2018, 664, 152–167. [Google Scholar] [PubMed]
- Mhatre, A.N.; Li, Y.; Bhatia, N.; Wang, K.H.; Atkin, G.; Lalwani, A.K. Generation and characterization of mice with Myh9 deficiency. Neuromol. Med. 2007, 9, 205–215. [Google Scholar] [CrossRef]
- Moura, P.L.; Hawley, B.R.; Mankelow, T.J.; Griffiths, R.E.; Dobbe, J.G.G.; Streekstra, G.J.; Anstee, D.J.; Satchwell, T.J.; Toye, A.M. Non-muscle myosin II drives vesicle loss during human reticulocyte maturation. Haematologica 2018, 103, 1997. [Google Scholar] [CrossRef] [Green Version]
- Zehrer, A.; Pick, R.; Salvermoser, M.; Boda, A.; Miller, M.; Stark, K.; Weckbach, L.T.; Walzog, B.; Begandt, D. A fundamental role of Myh9 for neutrophil migration in innate immunity. J. Immunol. 2018, 201, 1748–1764. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wei, G.; Cao, Y.; Deng, Q.; Han, X.; Huang, X.; Huo, Y.; He, Y.; Chen, L.; Luo, J. Myosin IIa is critical for cAMP-mediated endothelial secretion of von Willebrand factor. Blood J. Am. Soc. Hematol. 2018, 131, 686–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Dong, Y.; Lu, X.; Li, W.; Zhang, Y.; Mao, B.; Pan, X.; Li, X.; Zhou, Y.; An, Q.; et al. Inhibition of aryl hydrocarbon receptor signaling promotes the terminal differentiation of human erythrocytes. J. Mol. Cell Biol. 2022, 14, mjac001. [Google Scholar] [CrossRef]
- Dong, Y.; Bai, J.; Zhang, Y.; Zhou, Y.; Pan, X.; Li, X.; Zhou, Q.; Chen, Y.; Lai, M.; Mao, B.; et al. Alpha lipoic acid promotes development of hematopoietic progenitors derived from human embryonic stem cells by antagonizing ROS signals. J. Leukoc. Biol. 2020, 108, 1711–1725. [Google Scholar] [CrossRef]
- Dutta, A.; Yang, Y.; Le, B.T.; Zhang, Y.; Abdel-Wahab, O.; Zang, C.; Mohi, G. U2af1 is required for survival and function of hematopoietic stem/progenitor cells. Leukemia 2021, 35, 2382–2398. [Google Scholar] [CrossRef] [PubMed]
- Holmfeldt, P.; Ganuza, M.; Marathe, H.; He, B.; Hall, T.; Kang, G.; Moen, J.; Pardieck, J.; Saulsberry, A.C.; Cico, A.; et al. Functional screen identifies regulators of murine hematopoietic stem cell repopulation. J. Exp. Med. 2016, 213, 433–449. [Google Scholar] [CrossRef] [Green Version]
- Blank, U.; Karlsson, G.; Moody, J.L.; Utsugisawa, T.; Magnusson, M.; Singbrant, S.; Larsson, J.; Karlsson, S. Smad7 promotes self-renewal of hematopoietic stem cells. Blood 2006, 108, 4246–4254. [Google Scholar] [CrossRef] [Green Version]
- Aranda-Orgilles, B.; Saldaña-Meyer, R.; Wang, E.; Trompouki, E.; Fassl, A.; Lau, S.; Mullenders, J.; Rocha, P.P.; Raviram, R.; Guillamot, M.; et al. MED12 regulates HSC-specific enhancers independently of mediator kinase activity to control hematopoiesis. Cell Stem Cells 2016, 19, 784–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, T.H.; Rando, T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef]
- Forsberg, E.C.; Passegué, E.; Prohaska, S.S.; Wagers, A.J.; Koeva, M.; Stuart, J.M.; Weissman, I.L. Molecular signatures of quiescent, mobilized and leukemia-initiating hematopoietic stem cells. PLoS ONE 2010, 5, e8785. [Google Scholar] [CrossRef]
- Ivanova, N.B.; Dimos, J.T.; Schaniel, C.; Hackney, J.A.; Moore, K.A.; Lemischka, I.R. A stem cell molecular signature. Science 2002, 298, 601–604. [Google Scholar] [CrossRef]
- Li, N.; Yang, Y.; Liang, C.; Qiu, Q.; Pan, C.; Li, M.; Yang, S.; Chen, L.; Zhu, X.; Hu, Y. Tmem30a plays critical roles in ensuring the survival of hematopoietic cells and leukemia cells in mice. Am. J. Pathol. 2018, 188, 1457–1468. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Zhang, S.; Grogg, M.; Cancelas, J.A.; Varney, M.E.; Starczynowski, D.T.; Du, W.; Yang, J.; Liu, W.; Thomas, G.; et al. Mouse gene targeting reveals an essential role of mTOR in hematopoietic stem cell engraftment and hematopoiesis. Haematologica 2013, 98, 1353. [Google Scholar] [CrossRef]
- Dzierzak, E.; Bigas, A. Blood development: Hematopoietic stem cell dependence and independence. Cell Stem Cells 2018, 22, 639–651. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Conti, M.A.; Malide, D.; Dong, F.; Wang, A.; Shmist, Y.A.; Liu, C.; Zerfas, P.; Daniels, M.P.; Chan, C.C.; et al. Mouse models of MYH9-related disease: Mutations in nonmuscle myosin II-A. Blood 2012, 119, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Kunishima, S.; Ikejiri, M.; Maruyma, S.; Sone, M.; Takagi, A.; Ikawa, M.; Okabe, M.; Kojima, T.; Saito, H.; et al. Establishment of mouse model of MYH9 disorders: Heterozygous R702C mutation provokes macrothrombocytopenia with leukocyte inclusion bodies, renal glomerulosclerosis and hearing disability. PLoS ONE 2013, 8, e71187. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Onai, N.; Yoshihara, H.; Arai, F.; Suda, T.; Ohteki, T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cell from type I interferon-dependent exhaustion. Nat. Med. 2009, 15, 696–700. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Imai, M.; Kamimura, H.; Takamura, M.; Yamagiwa, S.; Sugiyama, T.; Nomoto, M.; Heike, T.; Nagasawa, T.; Nakahata, T.; et al. Increased susceptibility to severe chronic liver damage in CXCR4 conditional knock-out mice. Dig. Dis. Sci. 2012, 57, 2892–2900. [Google Scholar] [CrossRef]
- Varney, M.E.; Niederkorn, M.; Konno, H.; Matsumura, T.; Gohda, J.; Yoshida, N.; Akiyama, T.; Christie, S.; Fang, J.; Miller, D.; et al. Loss of Tifab, a del (5q) MDS gene, alters hematopoiesis through derepression of Toll-like receptor–TRAF6 signaling. J. Exp. Med. 2015, 212, 1967–1985. [Google Scholar] [CrossRef] [PubMed]
- Carnevalli, L.S.; Scognamiglio, R.; Cabezas-Wallscheid, N.; Rahmig, S.; Laurenti, E.; Masuda, K.; Jockel, L.; Kuck, A.; Sujer, S.; Polykratis, A.; et al. Improved HSC reconstitution and protection from inflammatory stress and chemotherapy in mice lacking granzyme B. J. Exp. Med. 2014, 211, 769–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, H.; Moura, J.; Carvalho, E. mTOR signaling as a regulator of hematopoietic stem cell fate. Stem Cell Rev. Rep. 2021, 17, 1312–1322. [Google Scholar] [CrossRef]
- Chen, M.; Sun, L.; Yu, L.; Liu, J.; Sun, L.; Yang, Z.; Shu, X.; Ran, Y. MYH9 is crucial for stem cell-like properties in non-small cell lung cancer by activating mTOR signaling. Cell Death Discov. 2021, 7, 282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, Z.; Li, C.; Zhang, Y.; Li, Z.; Sun, S. NMⅡA promotes tumorigenesis and prevents chemosensitivity in colorectal cancer by activating AMPK/mTOR pathway. Exp. Cell Res. 2021, 398, 112387. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Q.; Dong, Y.; Cao, Y.; Pan, X.; Xue, Y.; Zhou, Y.; Zhang, Y.; Ma, F. Myh9 Plays an Essential Role in the Survival and Maintenance of Hematopoietic Stem/Progenitor Cells. Cells 2022, 11, 1865. https://doi.org/10.3390/cells11121865
An Q, Dong Y, Cao Y, Pan X, Xue Y, Zhou Y, Zhang Y, Ma F. Myh9 Plays an Essential Role in the Survival and Maintenance of Hematopoietic Stem/Progenitor Cells. Cells. 2022; 11(12):1865. https://doi.org/10.3390/cells11121865
Chicago/Turabian StyleAn, Quanming, Yong Dong, Yang Cao, Xu Pan, Yuan Xue, Ya Zhou, Yonggang Zhang, and Feng Ma. 2022. "Myh9 Plays an Essential Role in the Survival and Maintenance of Hematopoietic Stem/Progenitor Cells" Cells 11, no. 12: 1865. https://doi.org/10.3390/cells11121865
APA StyleAn, Q., Dong, Y., Cao, Y., Pan, X., Xue, Y., Zhou, Y., Zhang, Y., & Ma, F. (2022). Myh9 Plays an Essential Role in the Survival and Maintenance of Hematopoietic Stem/Progenitor Cells. Cells, 11(12), 1865. https://doi.org/10.3390/cells11121865






