hLMSC Secretome Affects Macrophage Activity Differentially Depending on Lung-Mimetic Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of hLMSC
2.2. Preparation of the Lung Scaffolds
2.3. Preparation of the Lung-Derived Hydrogels
2.4. Stretch Device and Functionalization
2.5. Physiomimetic Culture of hLMSC
2.6. Confocal Immunofluorescence
2.7. Multiplex Secretome Evaluation
2.8. Phagocytosis Assay
2.9. Phenotype Assessment of Macrophages Exposed to MSC Secretome
2.10. qPCR for Mechanosensory Markers
2.11. Statistical Analysis
3. Result
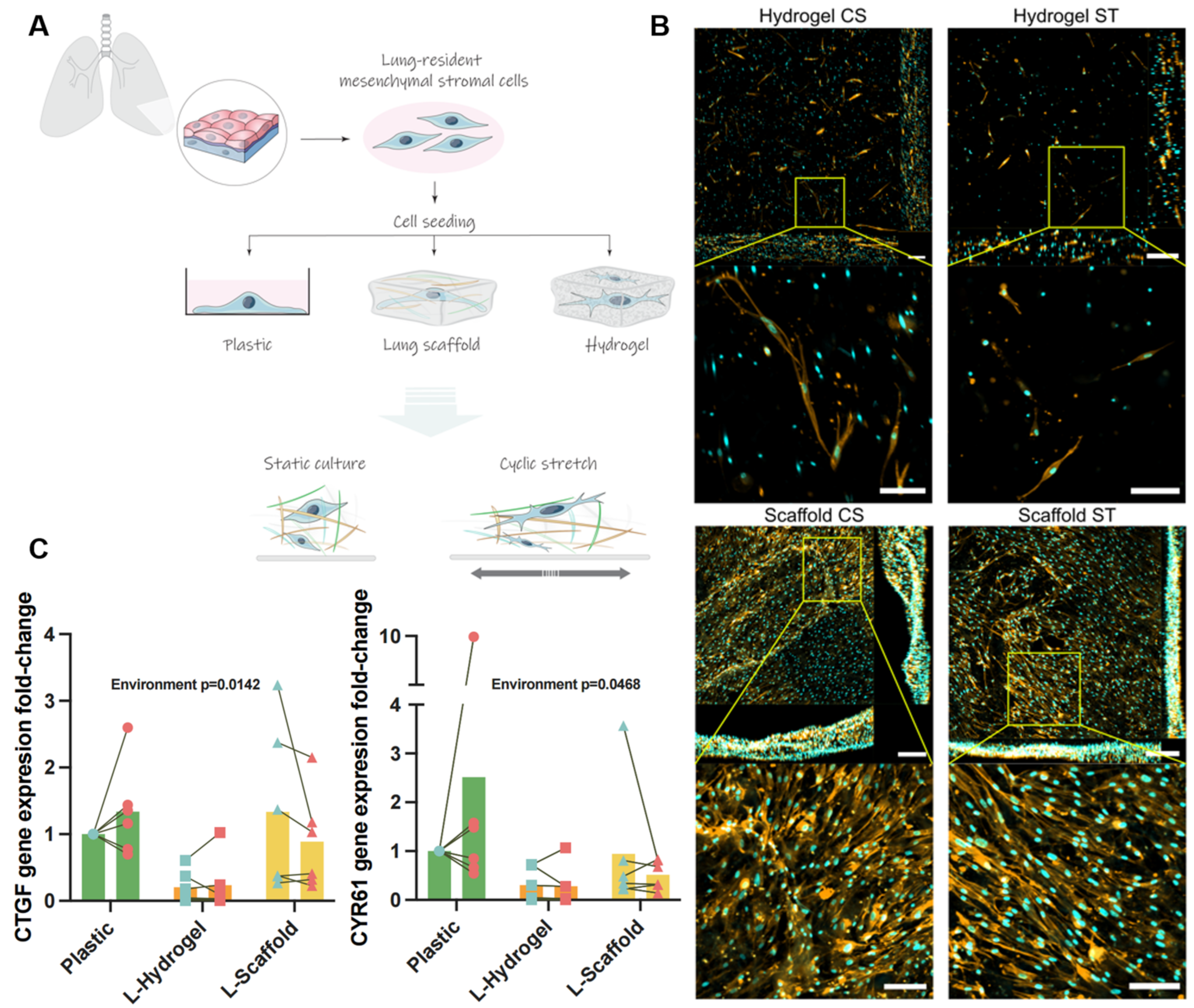
3.1. Lung-Derived Biomaterials Harness Differential Mechanical Microenvironments
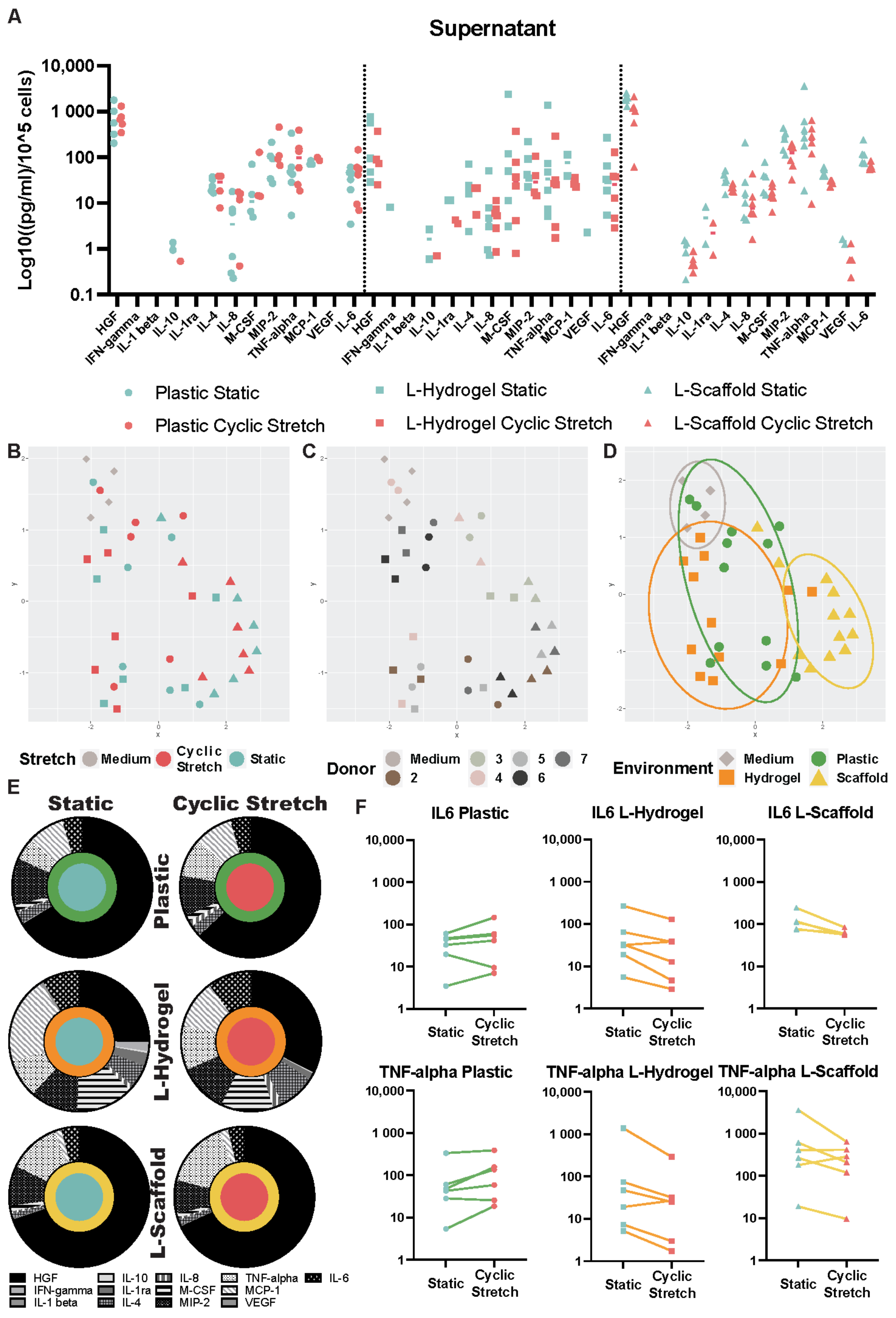
3.2. Secretome Content Is Influenced by the Microenvironment Due to the Physiomimetic Culture
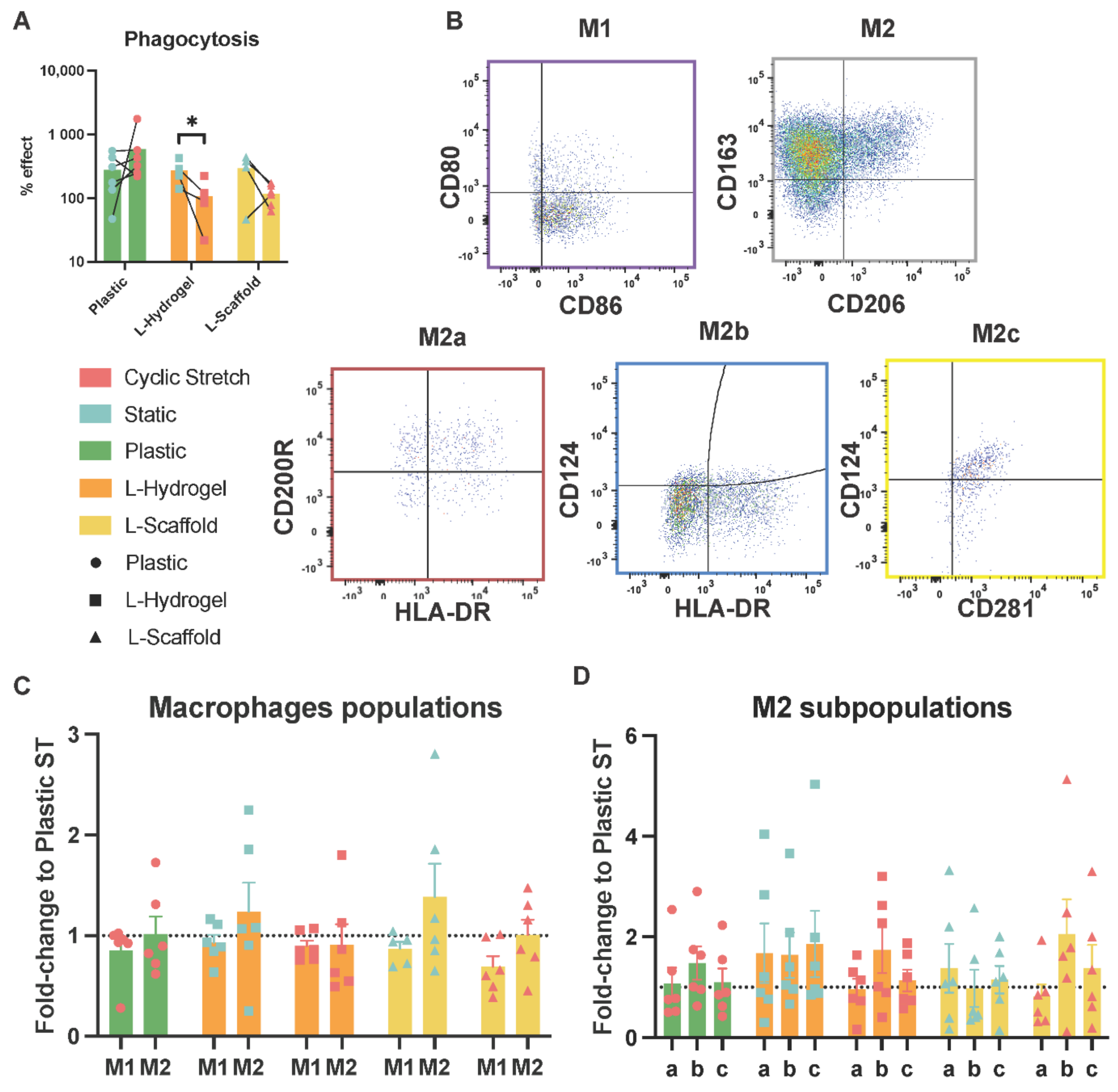
3.3. Secretome from Physiomimetic Environments Attenuates Phagocytosis and the Stretch Increases M2b Subpopulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wechsler, M.E.; Rao, V.V.; Borelli, A.N.; Anseth, K.S. Engineering the MSC Secretome: A Hydrogel Focused Approach. Adv. Healthc. Mater. 2021, 10, 2001948. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Castro, E.; Cunningham, C.J.; Miller, J.; Brown, H.; Allan, S.M.; Pinteaux, E. Changes in the secretome of tri-dimensional spheroid-cultured human mesenchymal stem cells in vitro by interleukin-1 priming. Stem Cell Res. Ther. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoury, M.; Cuenca, J.; Cruz, F.F.; Figueroa, F.E.; Rocco, P.R.M.; Weiss, D.J. Current status of cell-based therapies for respiratory virus infections: Applicability to COVID-19. Eur. Respir. J. 2020, 55, 2000858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudlik, G.; Hegyi, B.; Czibula, Á; Monostori, É; Buday, L.; Uher, F. Mesenchymal stem cells promote macrophage polarization toward M2b-like cells. Exp. Cell Res. 2016, 348, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci. Rep. 2016, 6, 38308. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Joel, M.D.M.; Yuan, J.; Wang, J.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. MSC: Immunoregulatory effects, roles on neutrophils and evolving clinical potentials. Am. J. Transl. Res. 2019, 11, 3890–3904. [Google Scholar]
- Wang, Y.; Yi, H.; Song, Y. The safety of MSC therapy over the past 15 years: A meta-analysis. Stem Cell Res. Ther. 2021, 12, 545. [Google Scholar] [CrossRef]
- A Matthay, M.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; E Levitt, J.; Rogers, A.J.; E Gotts, J.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2018, 7, 154–162. [Google Scholar] [CrossRef]
- Nonaka, P.N.; Falcones, B.; Farre, R.; Artigas, A.; Almendros, I.; Navajas, D. Biophysically Preconditioning Mesenchymal Stem Cells Improves Treatment of Ventilator-Induced Lung Injury. Arch. Bronconeumol. 2019, 56, 179–181. [Google Scholar] [CrossRef]
- Wong, S.W.; Lenzini, S.; Cooper, M.H.; Mooney, D.J.; Shin, J.-W. Soft extracellular matrix enhances inflammatory activation of mesenchymal stromal cells to induce monocyte production and trafficking. Sci. Adv. 2020, 6, eaaw0158. [Google Scholar] [CrossRef] [Green Version]
- Battiston, K.; Labow, R.; Simmons, C.; Santerre, J. Immunomodulatory polymeric scaffold enhances extracellular matrix production in cell co-cultures under dynamic mechanical stimulation. Acta Biomater. 2015, 24, 74–86. [Google Scholar] [CrossRef]
- Darnell, M.; O’Neil, A.; Mao, A.; Gu, L.; Rubin, L.L.; Mooney, D.J. Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells. Proc. Natl. Acad. Sci. USA 2018, 115, E8368–E8377. [Google Scholar] [CrossRef] [Green Version]
- Elosegui-Artola, A.; Trepat, X.; Roca-Cusachs, P. Control of Mechanotransduction by Molecular Clutch Dynamics. Trends Cell Biol. 2018, 28, 356–367. [Google Scholar] [CrossRef]
- Chaqour, B.; Goppelt-Struebe, M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J. 2006, 273, 3639–3649. [Google Scholar] [CrossRef]
- Rolandsson, S.; Sjöland, A.A.; Brune, J.C.; Li, H.; Kassem, M.; Mertens, F.; Westergren, A.; Eriksson, L.; Hansson, L.; Skog, I.; et al. Primary mesenchymal stem cells in human transplanted lungs are CD90/CD105 perivascularly located tissue-resident cells. BMJ Open Respir. Res. 2014, 1, e000027. [Google Scholar] [CrossRef]
- Rosmark, O.; Åhrman, E.; Müller, C.; Rendin, L.E.; Eriksson, L.; Malmström, A.; Hallgren, O.; Larsson-Callerfelt, A.-K.; Westergren-Thorsson, G.; Malmström, J. Quantifying extracellular matrix turnover in human lung scaffold cultures. Sci. Rep. 2018, 8, 5409. [Google Scholar] [CrossRef] [Green Version]
- Pouliot, R.A.; Link, P.; Mikhaiel, N.S.; Schneck, M.B.; Valentine, M.S.; Gninzeko, F.J.K.; Herbert, J.A.; Sakagami, M.; Heise, R.L. Development and characterization of a naturally derived lung extracellular matrix hydrogel. J. Biomed. Mater. Res. Part A 2016, 104, 1922–1935. [Google Scholar] [CrossRef]
- Campillo, N.; Jorba, I.; Schaedel, L.; Casals, B.; Gozal, D.; Farre, R.; Almendros, I.; Navajas, D. A Novel Chip for Cyclic Stretch and Intermittent Hypoxia Cell Exposures Mimicking Obstructive Sleep Apnea. Front. Physiol. 2016, 7, 319. [Google Scholar] [CrossRef] [Green Version]
- Genchi, G.G.; Ciofani, G.; Liakos, I.; Ricotti, L.; Ceseracciu, L.; Athanassiou, A.; Mazzolai, B.; Menciassi, A.; Mattoli, V. Bio/non-bio interfaces: A straightforward method for obtaining long term PDMS/muscle cell biohybrid constructs. Colloids Surfaces B Biointerfaces 2013, 105, 144–151. [Google Scholar] [CrossRef]
- Li, W.; Germain, R.N.; Gerner, M.Y. High-dimensional cell-level analysis of tissues with Ce3D multiplex volume imaging. Nat. Protoc. 2019, 14, 1708–1733. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Trepat, X.; Wasserman, M.R.; Angelini, T.E.; Millet, E.; Weitz, D.A.; Butler, J.P.; Fredberg, J.J. Physical forces during collective cell migration. Nat. Phys. 2009, 5, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Rendin, L.E.; Löfdahl, A.; Åhrman, E.; Müller, C.; Notermans, T.; Michaliková, B.; Rosmark, O.; Zhou, X.-H.; Dellgren, G.; Silverborn, M.; et al. Matrisome Properties of Scaffolds Direct Fibroblasts in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2019, 20, 4013. [Google Scholar] [CrossRef] [Green Version]
- Falcones, B.; Sanz-Fraile, H.; Marhuenda, E.; Mendizábal, I.; Cabrera-Aguilera, I.; Malandain, N.; Uriarte, J.; Almendros, I.; Navajas, D.; Weiss, D.; et al. Bioprintable Lung Extracellular Matrix Hydrogel Scaffolds for 3D Culture of Mesenchymal Stromal Cells. Polymers 2021, 13, 2350. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, L.; Ling, L.; Meng, X.; Chu, F.; Zhang, S.; Zhou, F. The Crosstalk Between Hippo-YAP Pathway and Innate Immunity. Front. Immunol. 2020, 11, 323. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-H.K. Aging of mesenchymal stem cells: Implication in regenerative medicine. Regen. Ther. 2018, 9, 120–122. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Wang, Z.; Gao, W.; Chen, W.; Zhang, H.; Jing, B.; Zhu, X.; Chen, L.; Zheng, C.; et al. Eradication of specific donor-dependent variations of mesenchymal stem cells in immunomodulation to enhance therapeutic values. Cell Death Dis. 2021, 12, 357. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017, 26, 617–631. [Google Scholar] [CrossRef]
- Song, P.; Han, T.; Xiang, X.; Wang, Y.; Fang, H.; Niu, Y.; Shen, C. The role of hepatocyte growth factor in mesenchymal stem cell-induced recovery in spinal cord injured rats. Stem Cell Res. Ther. 2020, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, H.; Mahon, B.; English, K. Human mesenchymal stromal cells exert HGF dependent cytoprotective effects in a human relevant pre-clinical model of COPD. Sci. Rep. 2016, 6, 38207. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, H.; Takahashi, M. Role of Monocyte Chemoattractant Protein-1 in Myocardial Infarction. Int. J. Biomed. Sci. IJBS 2007, 3, 159–167. [Google Scholar] [PubMed]
- Asakura, E.; Hanamura, T.; Umemura, A.; Yada, K.; Yamauchi, T.; Tanabe, T. Effects of Macrophage Colony-Stimulating Factor (M-CSF) on Lipopolysaccharide (LPS)-induced Mediator Production from Monocytes in vitro. Immunobiology 1996, 195, 300–313. [Google Scholar] [CrossRef]
- Saldaña, L.; Bensiamar, F.; Vallés, G.; Mancebo, F.J.; García-Rey, E.; Vilaboa, N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res. Ther. 2019, 10, 58. [Google Scholar] [CrossRef]
- Jackson, M.V.; Morrison, T.J.; Doherty, D.F.; McAuley, D.F.; Matthay, M.A.; Kissenpfennig, A.; OߣKane, C.M.; Krasnodembskaya, A.D. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells 2016, 34, 2210–2223. [Google Scholar] [CrossRef] [Green Version]
- Adutler-Lieber, S.; Ben-Mordechai, T.; Naftali-Shani, N.; Asher, E.; Loberman, D.; Raanani, E.; Leor, J. Human Macrophage Regulation Via Interaction With Cardiac Adipose Tissue-Derived Mesenchymal Stromal Cells. J. Cardiovasc. Pharmacol. Ther. 2012, 18, 78–86. [Google Scholar] [CrossRef]
- Chu, S.-Y.; Chou, C.-H.; Huang, H.-D.; Yen, M.-H.; Hong, H.-C.; Chao, P.-H.; Wang, Y.-H.; Chen, P.-Y.; Nian, S.-X.; Chen, Y.-R.; et al. Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Liu, X.; Guo, P.; Li, X.; He, Z.; Li, Z.; Stoddart, M.J.; Grad, S.; Tian, W.; Chen, D.; et al. Effect of cyclic mechanical loading on immunoinflammatory microenvironment in biofabricating hydroxyapatite scaffold for bone regeneration. Bioact. Mater. 2021, 6, 3097–3108. [Google Scholar] [CrossRef]
- Sun, M.; Sun, L.; Huang, C.; Chen, B.-C.; Zhou, Z. Induction of Macrophage M2b/c Polarization by Adipose Tissue-Derived Mesenchymal Stem Cells. J. Immunol. Res. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Philipp, D.; Suhr, L.; Wahlers, T.; Choi, Y.H.; Paunel-Görgülü, A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization 11 Medical and Health Sciences 1107 Immunology. Stem Cell Res. Ther. 2018, 9, 286. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcones, B.; Söderlund, Z.; Ibáñez-Fonseca, A.; Almendros, I.; Otero, J.; Farré, R.; Rolandsson Enes, S.; Elowsson Rendin, L.; Westergren-Thorsson, G. hLMSC Secretome Affects Macrophage Activity Differentially Depending on Lung-Mimetic Environments. Cells 2022, 11, 1866. https://doi.org/10.3390/cells11121866
Falcones B, Söderlund Z, Ibáñez-Fonseca A, Almendros I, Otero J, Farré R, Rolandsson Enes S, Elowsson Rendin L, Westergren-Thorsson G. hLMSC Secretome Affects Macrophage Activity Differentially Depending on Lung-Mimetic Environments. Cells. 2022; 11(12):1866. https://doi.org/10.3390/cells11121866
Chicago/Turabian StyleFalcones, Bryan, Zackarias Söderlund, Arturo Ibáñez-Fonseca, Isaac Almendros, Jordi Otero, Ramon Farré, Sara Rolandsson Enes, Linda Elowsson Rendin, and Gunilla Westergren-Thorsson. 2022. "hLMSC Secretome Affects Macrophage Activity Differentially Depending on Lung-Mimetic Environments" Cells 11, no. 12: 1866. https://doi.org/10.3390/cells11121866
APA StyleFalcones, B., Söderlund, Z., Ibáñez-Fonseca, A., Almendros, I., Otero, J., Farré, R., Rolandsson Enes, S., Elowsson Rendin, L., & Westergren-Thorsson, G. (2022). hLMSC Secretome Affects Macrophage Activity Differentially Depending on Lung-Mimetic Environments. Cells, 11(12), 1866. https://doi.org/10.3390/cells11121866





