Science CommuniCa2+tion Developing Scientific Literacy on Calcium: The Involvement of CRAC Currents in Human Health and Disease
Abstract
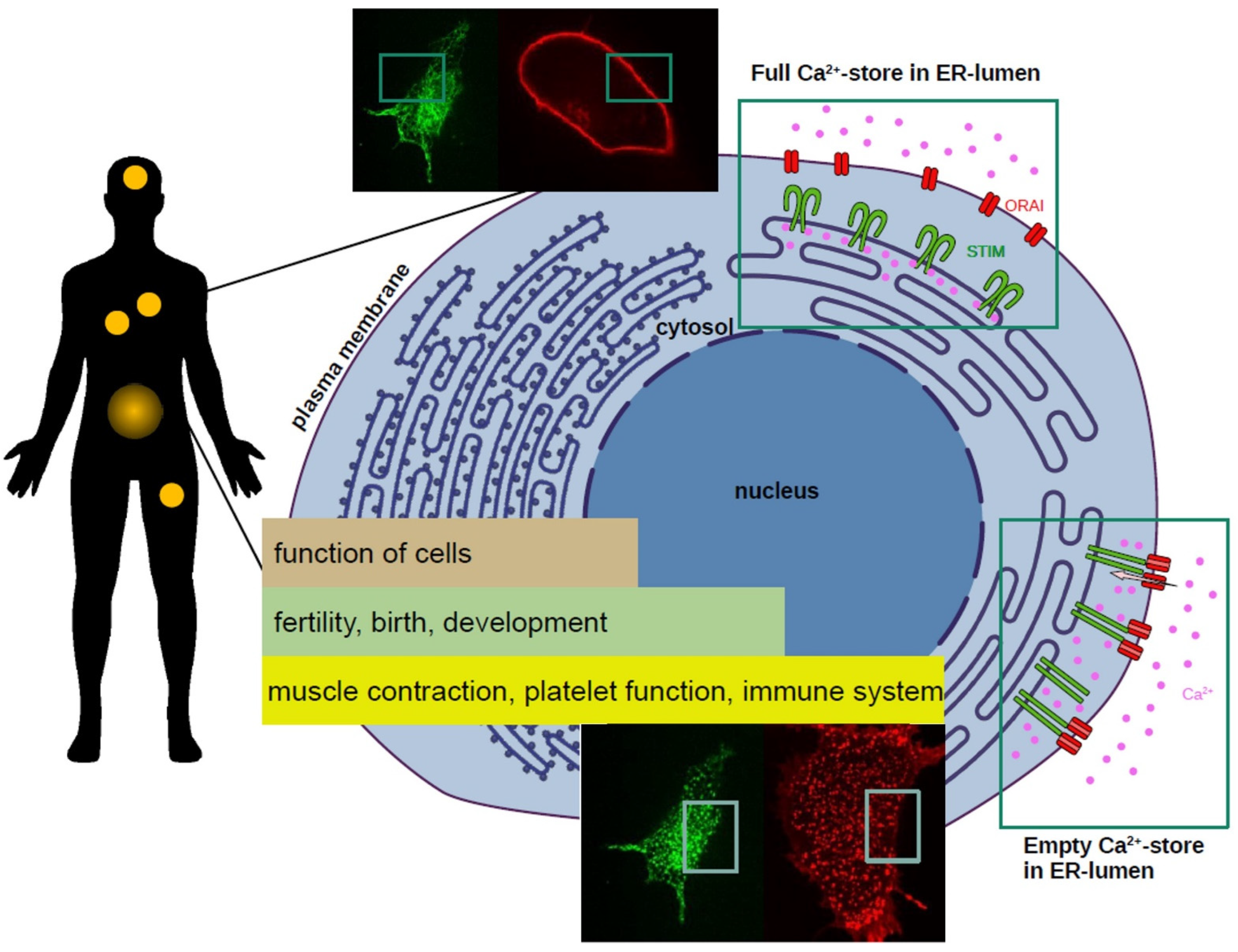
:1. Aim of This Work and the Importance of Calcium in Life
2. Calcium Uptake into the Cell—The CRAC Channel
3. Calcium and Its Role in Human Diseases
4. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santella, L.; Lim, D.; Moccia, F. Calcium and fertilization: The beginning of life. Trends Biochem. Sci. 2004, 29, 400–408. [Google Scholar] [CrossRef] [PubMed]
- United Kingdom National Health Service, Department of Health and social care UK Vitamins and minerals—Calcium.
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Carafoli, E. Calcium signaling: A tale for all seasons. Proc. Natl. Acad. Sci. USA 2002, 99, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Brini, M.; Ottolini, D.; Calì, T.; Carafoli, E. Calcium in Health and Disease. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 81–137. [Google Scholar]
- Nieto-Torres, J.L.; Verdiá-Báguena, C.; Jimenez-Guardeno, J.M.J.; Regla-Nava, J.A.; Castaño-Rodriguez, C.; Fernandez-Delgado, R.; Torres, J.; Aguilella, V.M.; Enjuanes, L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology 2015, 485, 330–339. [Google Scholar] [CrossRef] [Green Version]
- DeHaven, W.I.; Smyth, J.T.; Boyles, R.R.; Putney, J.W., Jr. Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J. Biol. Chem. 2007, 282, 17548–17556. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Ockinger, J.; Yu, J.; Byles, V.; McColl, A.; Hofer, A.M.; Horng, T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA 2012, 109, 11282–11287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, O.H.; Sutton, R. Ca2+ signalling and pancreatitis: Effects of alcohol, bile and coffee. Trends Pharmacol. Sci. 2006, 27, 113–120. [Google Scholar] [CrossRef]
- Petersen, O.H.; Tepikin, A.V. Polarized Calcium Signaling in Exocrine Gland Cells. Annu. Rev. Physiol. 2008, 70, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, R.; Vriens, J.; Voets, T.; Karashima, Y.; Owsianik, G.; Vennekens, R.; Lieben, L.; Torrekens, S.; Moermans, K.; Bosch, A.V.; et al. TRPV4-Mediated Calcium Influx Regulates Terminal Differentiation of Osteoclasts. Cell Metab. 2008, 8, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.S. The molecular choreography of a store-operated calcium channel. Nature 2007, 446, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.; Digregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Liou, J.; Kim, M.L.; Do Heo, W.; Jones, J.T.; Myers, J.W.; Ferrell, J.E., Jr.; Meyer, T. STIM Is a Ca2+ Sensor Essential for Ca2+-Store-Depletion-Triggered Ca2+ Influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.-H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef]
- Vig, M.; Peinelt, C.; Beck, A.; Koomoa, D.L.; Rabah, D.; Koblan-Huberson, M.; Kraft, S.; Turner, H.; Fleig, A.; Penner, R.; et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006, 312, 1220–1223. [Google Scholar] [CrossRef] [Green Version]
- Oritani, K.; Kincade, P.W. Identification of stromal cell products that interact with pre-B cells. J. Cell Biol. 1996, 134, 771–782. [Google Scholar] [CrossRef]
- Muik, M.; Frischauf, I.; Derler, I.; Fahrner, M.; Bergsmann, J.; Eder, P.; Schindl, R.; Hesch, C.; Polzinger, B.; Fritsch, R.; et al. Dynamic Coupling of the Putative Coiled-coil Domain of ORAI1 with STIM1 Mediates ORAI1 Channel Activation. J. Biol. Chem. 2008, 283, 8014–8022. [Google Scholar] [CrossRef] [Green Version]
- Fahrner, M.; Derler, I.; Jardin, I.; Romanin, C. The STIM1/Orai signaling machinery. Channels 2013, 7, 330–343. [Google Scholar] [CrossRef] [Green Version]
- Frischauf, I.; Zayats, V.; Deix, M.; Hochreiter, A.; Jardin, I.; Muik, M.; Lackner, B.; Svobodová, B.; Pammer, T.; Litviňuková, M.; et al. A calcium-accumulating region, CAR, in the channel Orai1 enhances Ca2+ permeation and SOCE-induced gene transcription. Sci. Signal. 2015, 8, ra131. [Google Scholar] [CrossRef] [Green Version]
- Parekh, A.B. Calcium signalling in health and disease. Semin. Cell Dev. Biol. 2019, 94, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Feske, S. CRAC channels and disease – From human CRAC channelopathies and animal models to novel drugs. Cell Calcium 2019, 80, 112–116. [Google Scholar] [CrossRef]
- Feske, S.; Giltnane, J.; Dolmetsch, R.; Staudt, L.M.; Rao, A. Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2001, 2, 316–324. [Google Scholar] [CrossRef]
- Feske, S.; Müller, J.M.; Graf, D.; Kroczek, R.A.; Dräger, R.; Niemeyer, C.; Baeuerle, P.A.; Peter, H.-H.; Schlesier, M. Severe combined immunodeficiency due to defective binding of the nuclear factor of activated T cells in T lymphocytes of two male siblings. Eur. J. Immunol. 1996, 26, 2119–2126. [Google Scholar] [CrossRef]
- Le Deist, F.; Hivroz, C.; Partiseti, M.; Thomas, C.; Buc, H.A.; Oleastro, M.; Belohradsky, B.; Choquet, D.; Fischer, A. A pri-mary T-cell immunodeficiency associated with defective transmembrane calcium influx. Blood 1995, 85, 1053–1062. [Google Scholar] [CrossRef] [Green Version]
- Partiseti, M.; Le Deist, F.; Hivroz, C.; Fischer, A.; Korn, H.; Choquet, D. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J. Biol. Chem. 1994, 269, 32327–32335. [Google Scholar] [CrossRef]
- Zhang, S.L.; Yeromin, A.V.; Zhang, X.H.-F.; Yu, Y.; Safrina, O.; Penna, A.; Roos, J.; Stauderman, K.A.; Cahalan, M.D. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl. Acad. Sci. USA 2006, 103, 9357–9362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaeth, M.; Feske, S. Ion channelopathies of the immune system. Curr. Opin. Immunol. 2018, 52, 39–50. [Google Scholar] [CrossRef]
- Vandamme, T.F. Use of rodents as models of human diseases. J. Pharm. Bioallied Sci. 2014, 6, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, S.B.; Monteith, G.R. ORAI channels and cancer. Cell Calcium 2018, 74, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.; Trebak, M. ORAI channels in cellular remodeling of cardiorespiratory disease. Cell Calcium 2019, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mammadova-Bach, E.; Nagy, M.; Heemskerk, J.W.; Nieswandt, B.; Braun, A. Store-operated calcium entry in thrombosis and thrombo-inflammation. Cell Calcium 2018, 77, 39–48. [Google Scholar] [CrossRef]
- Mei, Y.; Barrett, J.E.; Hu, H. Calcium release-activated calcium channels and pain. Cell Calcium 2018, 74, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Wegierski, T.; Kuznicki, J. Neuronal calcium signaling via store-operated channels in health and disease. Cell Calcium 2018, 74, 102–111. [Google Scholar] [CrossRef]
- Morin, G.; Bruechle, N.O.; Singh, A.R.; Knopp, C.; Jedraszak, G.; Elbracht, M.; Bremond-Gignac, D.; Hartmann, K.A.; Sevestre, H.; Deutz, P.; et al. Gain-of-Function Mutation in STIM1 (P.R304W) Is Associated with Stormorken Syndrome. Hum. Mutat. 2014, 35, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Böhm, J.; Laporte, J. Gain-of-function mutations in STIM1 and ORAI1 causing tubular aggregate myopathy and Stormorken syndrome. Cell Calcium 2018, 76, 1–9. [Google Scholar] [CrossRef]
- Michelucci, A.; García-Castañeda, M.; Boncompagni, S.; Dirksen, R.T. Role of STIM1/ORAI1-mediated store-operated Ca2+ entry in skeletal muscle physiology and disease. Cell Calcium 2018, 76, 101–115. [Google Scholar] [CrossRef]
- Endo, Y.; Noguchi, S.; Hara, Y.; Hayashi, Y.K.; Motomura, K.; Miyatake, S.; Murakami, N.; Tanaka, S.; Yamashita, S.; Kizu, R.; et al. Dominant mutations in ORAI1 cause tubular aggregate myopathy with hypocalcemia via constitutive activation of store-operated Ca2+ channels. Hum. Mol. Genet. 2014, 24, 637–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onouchi, Y.; Fukazawa, R.; Yamamura, K.; Suzuki, H.; Kakimoto, N.; Suenaga, T.; Takeuchi, T.; Hamada, H.; Honda, T.; Yasukawa, K.; et al. Variations in ORAI1 Gene Associated with Kawasaki Disease. PLoS ONE 2016, 11, e0145486. [Google Scholar] [CrossRef] [Green Version]
- Clemens, R.A.; Lowell, C.A. CRAC channel regulation of innate immune cells in health and disease. Cell Calcium 2019, 78, 56–65. [Google Scholar] [CrossRef]
- Feske, S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007, 7, 690–702. [Google Scholar] [CrossRef]
- Berlansky, S.; Sallinger, M.; Grabmayr, H.; Humer, C.; Bernhard, A.; Fahrner, M.; Frischauf, I. Calcium Signals during SARS-CoV-2 Infection: Assessing the Potential of Emerging Therapies. Cells 2022, 11, 253. [Google Scholar] [CrossRef]
- Zhou, Y.; Xue, S.; Yang, J.J. Calcium and Viruses. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 415–424. [Google Scholar]
- Lai, A.L.; Freed, J.H. SARS-CoV-2 Fusion Peptide has a Greater Membrane Perturbating Effect than SARS-CoV with Highly Specific Dependence on Ca2+. J. Mol. Biol. 2021, 433, 166946. [Google Scholar] [CrossRef] [PubMed]
- Nathan, L.; Lai, A.L.; Millet, J.K.; Straus, M.R.; Freed, J.H.; Whittaker, G.R.; Daniel, S. Calcium Ions Directly Interact with the Ebola Virus Fusion Peptide To Promote Structure–Function Changes That Enhance Infection. ACS Infect. Dis. 2019, 6, 250–260. [Google Scholar] [CrossRef]
- WHO, Cancer. 3 February 2022.
- World Cancer Research Fund, Global cancer statistics for the most common cancers in the world.
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Tajada, S.; Villalobos, C. Calcium Permeable Channels in Cancer Hallmarks. Front. Pharmacol. 2020, 11, 968. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Ouadid-Ahidouch, H.; Skryma, R.; Shuba, Y. Remodelling of Ca2+ transport in cancer: How it contributes to cancer hallmarks? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130097. [Google Scholar] [CrossRef] [Green Version]
- Monteith, G.R.; Prevarskaya, N.; Roberts-Thomson, S.J. The calcium–cancer signalling nexus. Nat. Rev. Cancer 2017, 17, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef] [Green Version]
- So, C.L.; Saunus, J.M.; Roberts-Thomson, S.J.; Monteith, G.R. Calcium signalling and breast cancer. Semin. Cell Dev. Biol. 2018, 94, 74–83. [Google Scholar] [CrossRef]
- Shuba, Y. Ca2+ channel-forming ORAI proteins: Cancer foes or cancer allies? Exp. Oncol. 2019, 41, 200–206. [Google Scholar] [CrossRef]
- Batra, S.; Alenfall, J. Effect of diverse categories of drugs on human colon tumour cell proliferation. Anticancer Res. 1991, 11, 1221–1224. [Google Scholar] [PubMed]
- Taylor, J.M.; Simpson, R.U. Inhibition of cancer cell growth by calcium channel antagonists in the athymic mouse. Cancer Res. 1992, 52, 2413–2418. [Google Scholar]
- Lee, S.C.; Deutsch, C.; Beck, W.T. Comparison of ion channels in multidrug-resistant and -sensitive human leukemic cells. Proc. Natl. Acad. Sci. USA 1988, 85, 2019–2023. [Google Scholar] [CrossRef] [Green Version]
- Dolmetsch, R.E.; Xu, K.; Lewis, R.S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 1998, 392, 933–936. [Google Scholar] [CrossRef]
- Li, W.-H.; Llopis, J.; A Whitney, M.; Zlokarnik, G.; Tsien, R.Y. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 1998, 392, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Kupzig, S.; Bouyoucef, D.; Davies, L.C.; Tsuboi, T.; Bivona, T.G.; Cozier, G.; Lockyer, P.J.; Buckler, A.; Rutter, G.; et al. Identification of a Ras GTPase-activating protein regulated by receptor-mediated Ca2+ oscillations. EMBO J. 2004, 23, 1749–1760. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Maller, J.L. Calcium Elevation at Fertilization Coordinates Phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to Release Metaphase Arrest by Cytostatic Factor. Curr. Biol. 2005, 15, 1458–1468. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Deng, Y.; Ye, J.; Luo, Y.; Weng, J.; He, Q.; Liu, F.; Li, M.; Liang, R.; Lin, Y.; et al. Store-operated Ca2+ entry as a key oncogenic Ca2+ signaling driving tumor invasion-metastasis cascade and its translational potential. Cancer Lett. 2021, 516, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.I.E.; James, A.D. Targeting the Calcium Signalling Machinery in Cancer. Cancers 2020, 12, 2351. [Google Scholar] [CrossRef]
- Motiani, R.K.; Hyzinski-García, M.C.; Zhang, X.; Henkel, M.M.; Abdullaev, I.F.; Kuo, Y.-H.; Matrougui, K.; Mongin, A.A.; Trebak, M. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflügers Archiv-Eur. J. Physiol. 2013, 465, 1249–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAndrew, D.; Grice, D.M.; Peters, A.A.; Davis, F.M.; Stewart, T.; Rice, M.; Smart, C.E.; Brown, M.A.; Kenny, P.A.; Roberts-Thomson, S.J.; et al. ORAI1-Mediated Calcium Influx in Lactation and in Breast Cancer. Mol. Cancer Ther. 2011, 10, 448–460. [Google Scholar] [CrossRef] [Green Version]
- Chamlali, M.; Rodat-Despoix, L.; Ouadid-Ahidouch, H. Store-Independent Calcium Entry and Related Signaling Pathways in Breast Cancer. Genes 2021, 12, 994. [Google Scholar] [CrossRef]
- Zhou, W.; Pan, H.; Xia, T.; Xue, J.; Cheng, L.; Fan, P.; Zhang, Y.; Zhu, W.; Xue, Y.; Liu, X.; et al. Up-regulation of S100A16 expression promotes epithelial-mesenchymal transition via Notch1 pathway in breast cancer. J. Biomed. Sci. 2014, 21, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umemura, M.; Baljinnyam, E.; Feske, S.; De Lorenzo, M.S.; Xie, L.-H.; Feng, X.; Oda, K.; Makino, A.; Fujita, T.; Yokoyama, U.; et al. Store-Operated Ca2+ Entry (SOCE) Regulates Melanoma Proliferation and Cell Migration. PLoS ONE 2014, 9, e89292. [Google Scholar] [CrossRef]
- Stanisz, H.; Stark, A.; Kilch, T.; Schwarz, E.C.; Müller, C.S.; Peinelt, C.; Hoth, M.; Niemeyer, B.A.; Vogt, T.; Bogeski, I. ORAI1 Ca2+ Channels Control Endothelin-1-Induced Mitogenesis and Melanogenesis in Primary Human Melanocytes. J. Investig. Dermatol. 2012, 132, 1443–1451. [Google Scholar] [CrossRef] [Green Version]
- Gueguinou, M.; Crottès, D.; Chantôme, A.; Rapetti-Mauss, R.; Potier-Cartereau, M.; Clarysse, L.; Girault, A.; Fourbon, Y.; Jézéquel, P.; Guérin-Charbonnel, C.; et al. The SigmaR1 chaperone drives breast and colorectal cancer cell migration by tuning SK3-dependent Ca2+ homeostasis. Oncogene 2017, 36, 3640–3647. [Google Scholar] [CrossRef]
- Leverrier-Penna, S.; Destaing, O.; Penna, A. Insights and perspectives on calcium channel functions in the cockpit of cancerous space invaders. Cell Calcium 2020, 90, 102251. [Google Scholar] [CrossRef] [PubMed]
- Skryma, R.; Mariot, P.; Bourhis, X.; Coppenolle, F.; Shuba, Y.; Abeele, F.V.; Legrand, G.; Humez, S.; Boilly, B.; Prevarskaya, N. Store depletion and store-operated Ca2+ current in human prostate cancer LNCaP cells: Involvement in apoptosis. J. Physiol. 2000, 527, 71–83. [Google Scholar] [CrossRef]
- Perrouin-Verbe, M.A.; Schoentgen, N.; Talagas, M.; Garlantezec, R.; Uguen, A.; Doucet, L.; Rosec, S.; Marcorelles, P.; Potier-Cartereau, M.; Vandier, C.; et al. Overexpression of certain transient receptor potential and Orai channels in prostate cancer is associated with decreased risk of systemic recurrence after radical prostatectomy. Prostate 2019, 79, 1793–1804. [Google Scholar] [CrossRef]
- Motta, F.; Valera, E.; Lucio-Eterovic, A.; Queiroz, R.; Neder, L.; Scrideli, C.; Machado, H.; Carlotti-Junior, C.; Marie, S.; Tone, L. Differential expression of E-cadherin gene in human neuroepithelial tumors. Genet. Mol. Res. 2008, 7, 295–304. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Y.; Gong, C.; Yu, F.; Su, S.; Chen, J.; Liu, B.; Deng, H.; Wang, F.; Lin, L.; et al. CCL18 from Tumor-Associated Macrophages Promotes Breast Cancer Metastasis via PITPNM3. Cancer Cell 2011, 19, 541–555. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Koh, W.-P.; Jin, A.-Z.; Yuan, J.-M.; Yu, M.C.; Butler, L.M. Calcium intake is not related to breast cancer risk among Singapore Chinese women. Int. J. Cancer 2013, 133, 680–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Cao, L.; Liu, B.; McCaig, C.D.; Pu, J. The Transition from Proliferation to Differentiation in Colorectal Cancer Is Regulated by the Calcium Activated Chloride Channel A1. PLoS ONE 2013, 8, e60861. [Google Scholar] [CrossRef] [Green Version]
- Guan, L.; Song, Y.; Gao, J.; Gao, J.; Wang, K. Inhibition of calcium-activated chloride channel ANO1 suppresses proliferation and induces apoptosis of epithelium originated cancer cells. Oncotarget 2016, 7, 78619–78630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Chen, M.; Huang, J.; Zhang, F.; Lv, Z.; Jia, Y.; Cui, Y.-Z.; Sun, L.-Z.; Wang, Y.; Tang, Y.; et al. ORAI2 Promotes Gastric Cancer Tumorigenicity and Metastasis through PI3K/Akt Signaling and MAPK-Dependent Focal Adhesion Disassembly. Cancer Res. 2020, 81, 986–1000. [Google Scholar] [CrossRef]
- Motiani, R.K.; Zhang, X.; Harmon, K.E.; Keller, R.S.; Matrougui, K.; Bennett, J.A.; Trebak, M. Orai3 is an estrogen receptor α-regulated Ca2+ channel that promotes tumorigenesis. FASEB J. 2012, 27, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Collado, J.; Lopez, J.J.; Cantonero, C.; Jardin, I.; Regodón, S.; Redondo, P.C.; Gordillo, J.; Smani, T.; Salido, G.M.; Rosado, J.A. Orai2 Modulates Store-Operated Ca2+ Entry and Cell Cycle Progression in Breast Cancer Cells. Cancers 2021, 14, 114. [Google Scholar] [CrossRef]
- Fleur-Lominy, S.S.; Maus, M.; Vaeth, M.; Lange, I.; Zee, I.; Suh, D.; Liu, C.; Wu, X.; Tikhonova, A.; Aifantis, I.; et al. STIM1 and STIM2 Mediate Cancer-Induced Inflammation in T Cell Acute Lymphoblastic Leukemia. Cell Rep. 2018, 24, 3045–3060. [Google Scholar] [CrossRef] [Green Version]
- Chamlali, M.; Kouba, S.; Rodat-Despoix, L.; Todesca, L.M.; Pethö, Z.; Schwab, A.; Ouadid-Ahidouch, H. Orai3 Calcium Channel Regulates Breast Cancer Cell Migration through Calcium-Dependent and -Independent Mechanisms. Cells 2021, 10, 3487. [Google Scholar] [CrossRef]
- Dubois, C.; Kondratska, K.; Kondratskyi, A.; Morabito, A.; Mesilmany, L.; Farfariello, V.; Toillon, R.-A.; Gelus, N.Z.; Laurenge, E.; Abeele, F.V.; et al. ORAI3 silencing alters cell proliferation and promotes mitotic catastrophe and apoptosis in pancreatic adenocarcinoma. Biochim. Biophys. Acta 2021, 1868, 119023. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Roy, S.; Pan, Z. Store-Operated Calcium Channels as Drug Target in Gastroesophageal Cancers. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humer, C.; Berlansky, S.; Grabmayr, H.; Sallinger, M.; Bernhard, A.; Fahrner, M.; Frischauf, I. Science CommuniCa2+tion Developing Scientific Literacy on Calcium: The Involvement of CRAC Currents in Human Health and Disease. Cells 2022, 11, 1849. https://doi.org/10.3390/cells11111849
Humer C, Berlansky S, Grabmayr H, Sallinger M, Bernhard A, Fahrner M, Frischauf I. Science CommuniCa2+tion Developing Scientific Literacy on Calcium: The Involvement of CRAC Currents in Human Health and Disease. Cells. 2022; 11(11):1849. https://doi.org/10.3390/cells11111849
Chicago/Turabian StyleHumer, Christina, Sascha Berlansky, Herwig Grabmayr, Matthias Sallinger, Andreas Bernhard, Marc Fahrner, and Irene Frischauf. 2022. "Science CommuniCa2+tion Developing Scientific Literacy on Calcium: The Involvement of CRAC Currents in Human Health and Disease" Cells 11, no. 11: 1849. https://doi.org/10.3390/cells11111849
APA StyleHumer, C., Berlansky, S., Grabmayr, H., Sallinger, M., Bernhard, A., Fahrner, M., & Frischauf, I. (2022). Science CommuniCa2+tion Developing Scientific Literacy on Calcium: The Involvement of CRAC Currents in Human Health and Disease. Cells, 11(11), 1849. https://doi.org/10.3390/cells11111849






