Metabolic Profiling of Mice with Deletion of the Orphan G Protein-Coupled Receptor, GPR37L1
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Generation and Maintenance of the Gpr37l1 Knockout Mouse Line
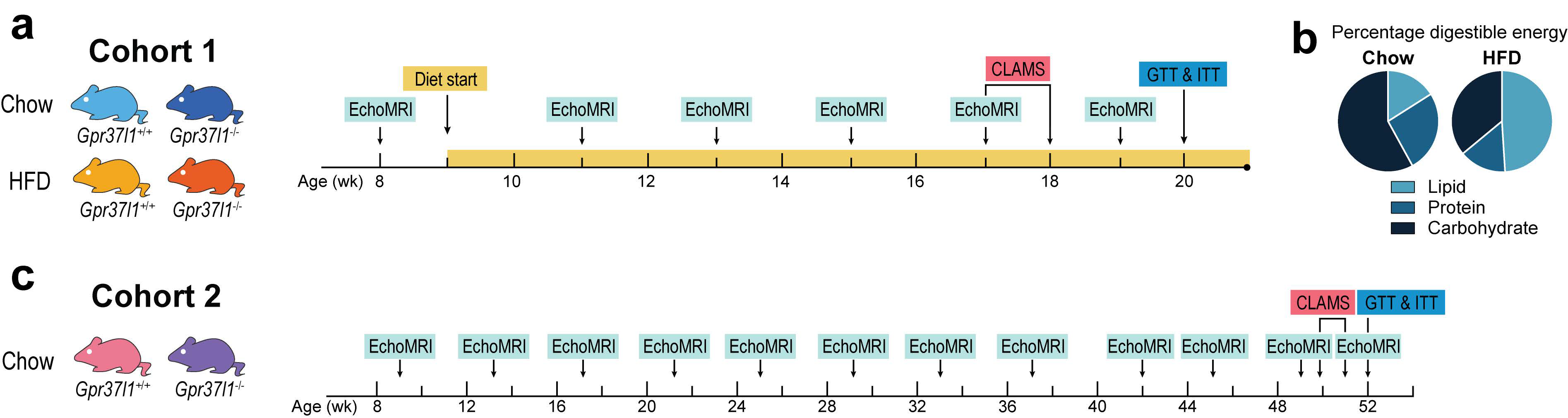
2.3. Study Design
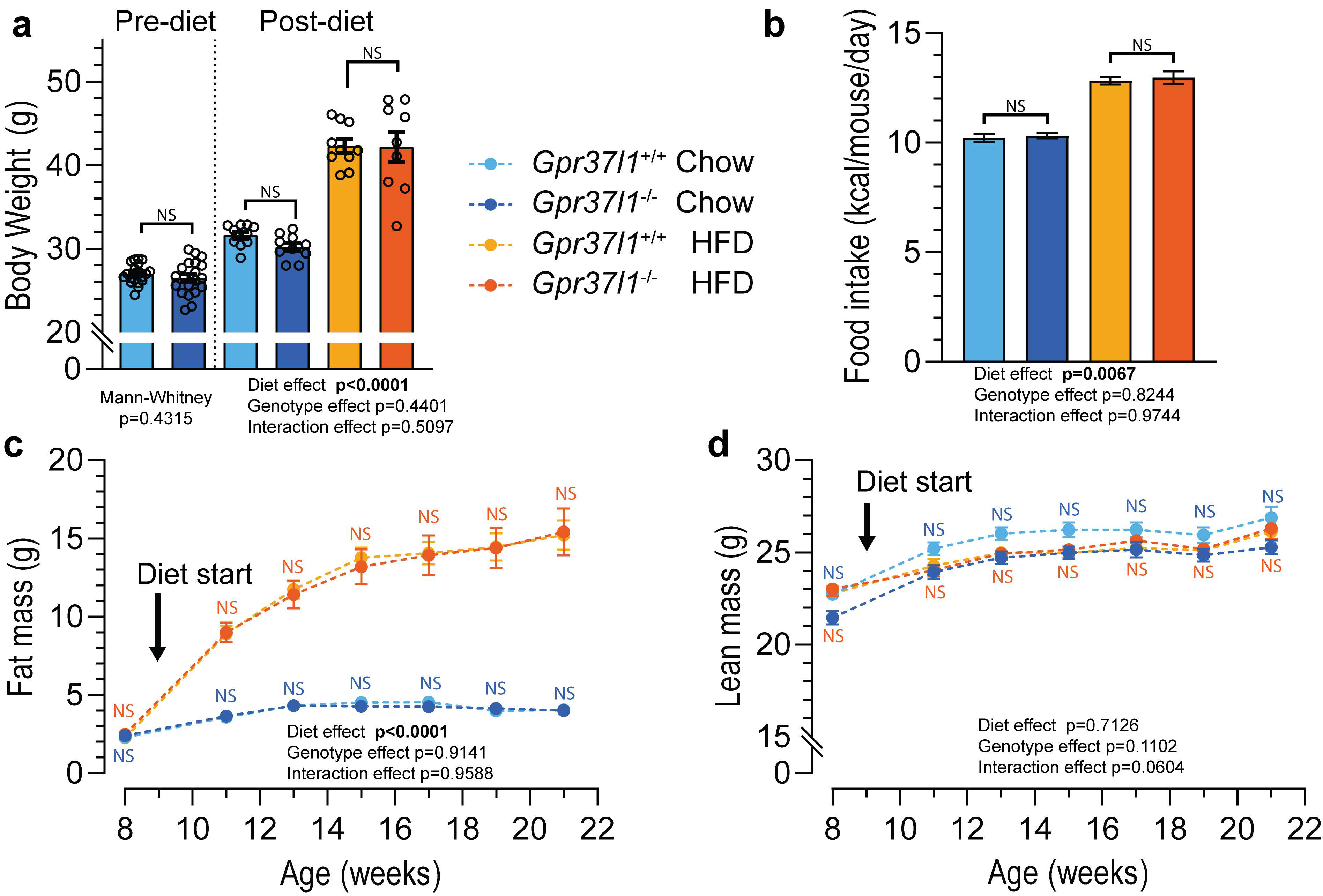
2.4. Mouse Diets and Food Intake Monitoring
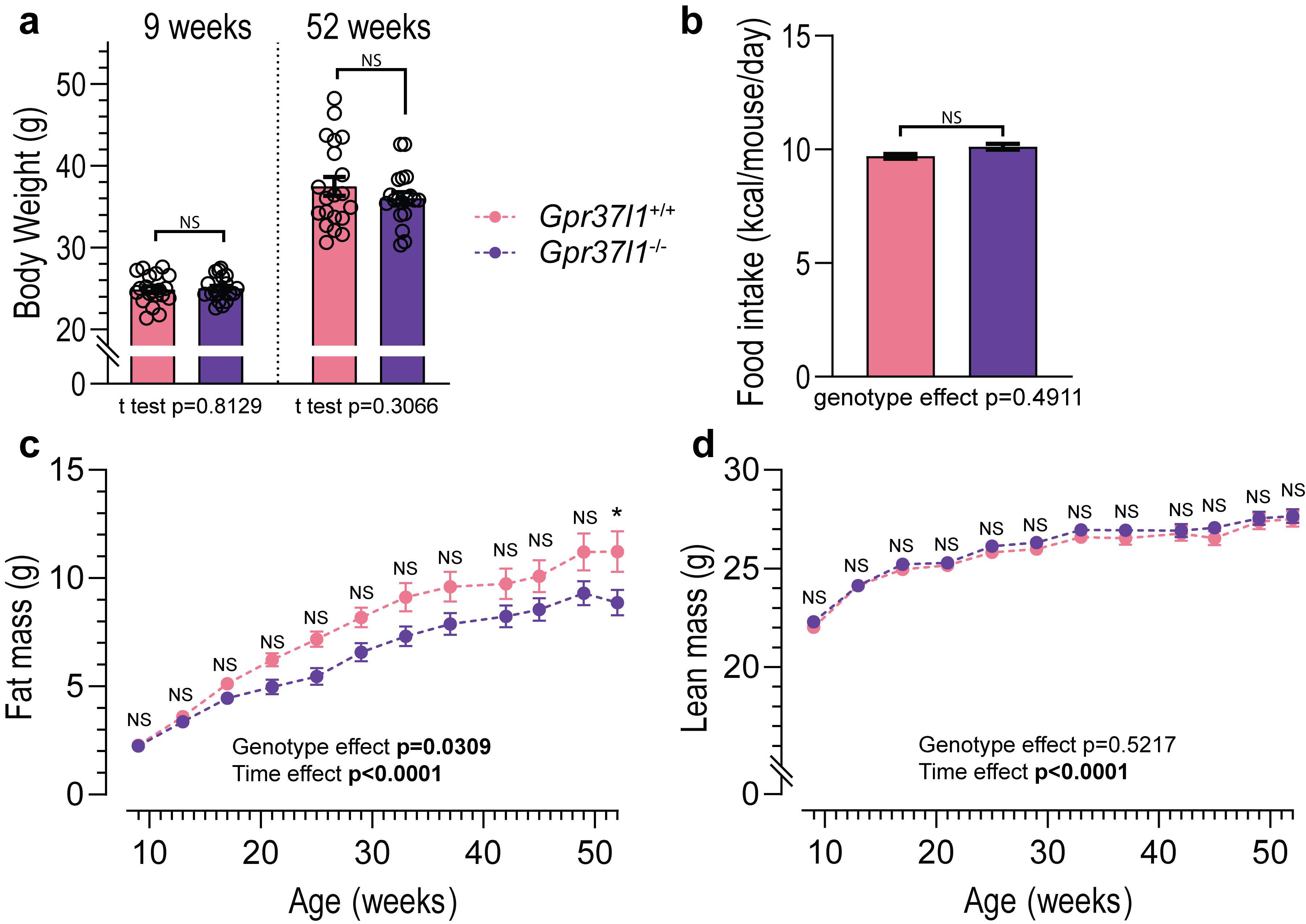
2.5. Body Composition Analysis
2.6. Glucose Tolerance Test
2.7. Insulin Tolerance Test
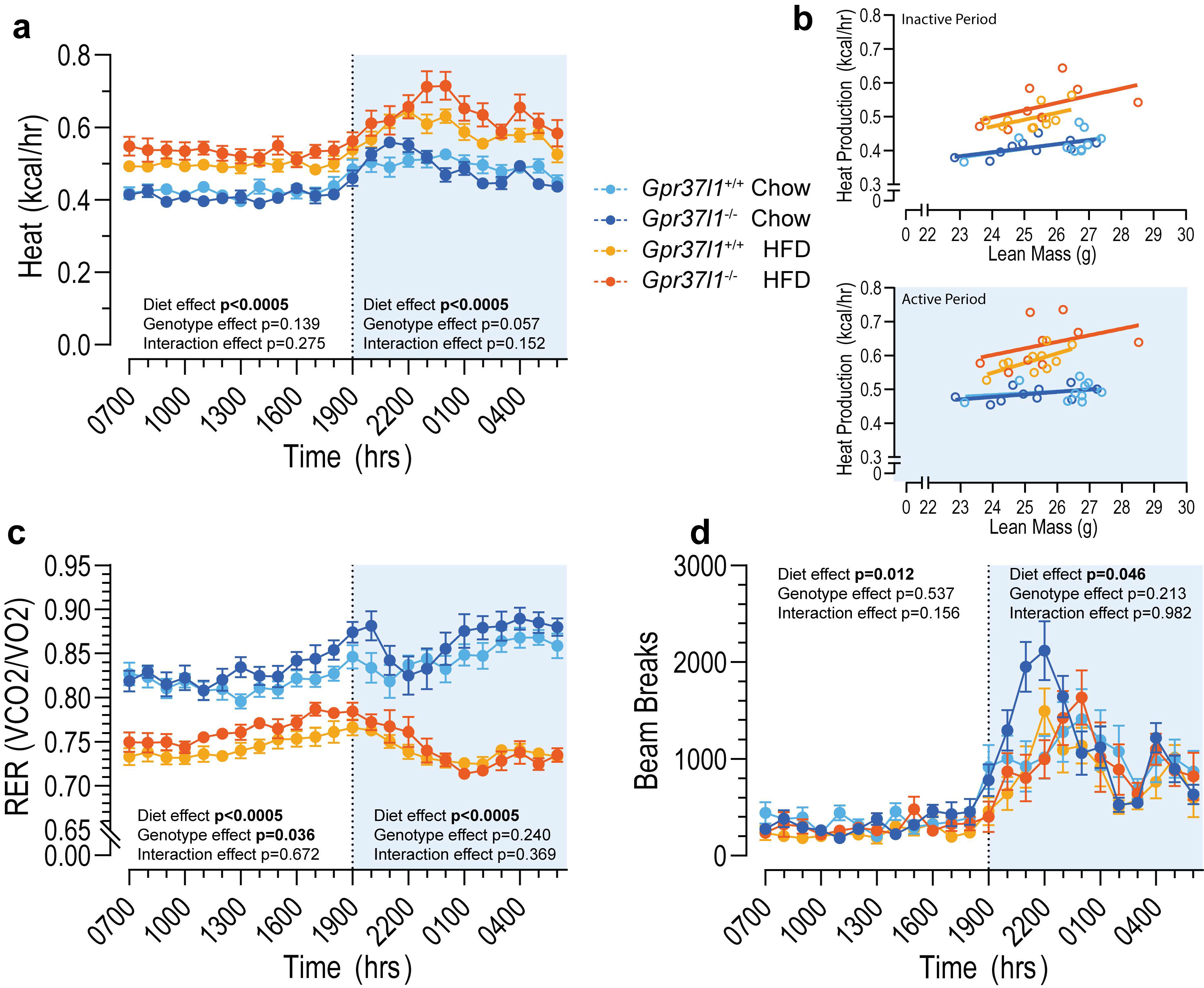
2.8. Indirect Calorimetry
2.9. Tissue Collection and Morphometry
2.10. Triglyceride and Non-Esterified Fatty Acid Assays
2.11. Statistics and Data Analysis
3. Results
3.1. High-Fat Diet Induces Obesity in Gpr37l1−/− Mice
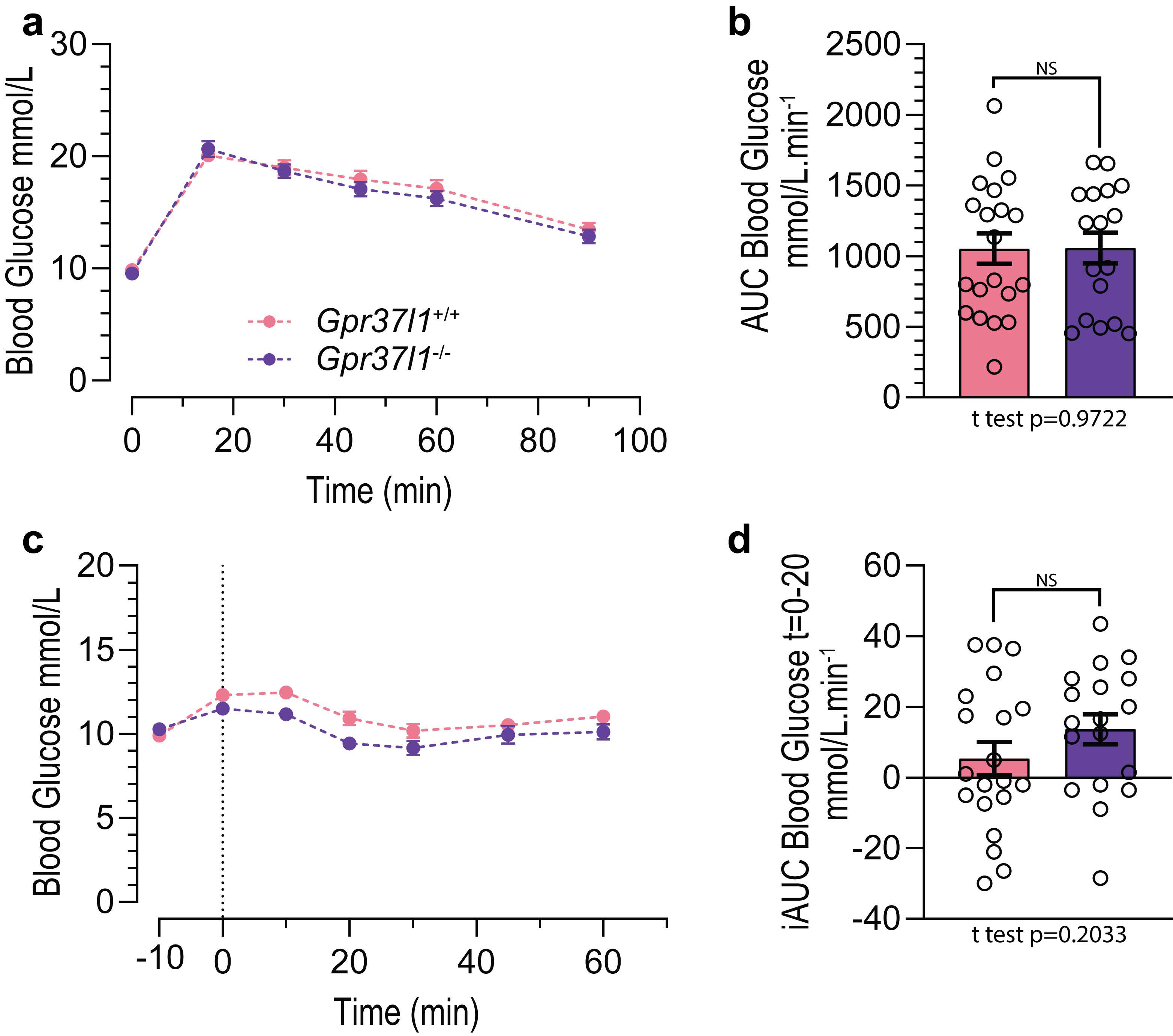
3.2. Glucose Tolerance and Insulin Sensitivity in HFD-Fed Mice Is Not Affected by Gpr37l1 Deletion
3.3. Metabolic Profile of Gpr37l1−/− Mice Is Not Different from Wildtype Controls
3.4. Effects of Ageing on Metabolic Profile of Gpr37l1−/− Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Beydoun, M.A.; Min, J.; Xue, H.; Kaminsky, L.A.; Cheskin, L.J. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int. J. Epidemiol. 2020, 49, 810–823. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Tremmel, M.; Gerdtham, U.-G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Greenway, F.L. Obesity medications in development. Expert Opin. Investig. Drugs 2020, 29, 63–71. [Google Scholar] [CrossRef]
- Williams, D.M.; Nawaz, A.; Evans, M. Drug Therapy in Obesity: A Review of Current and Emerging Treatments. Diabetes Ther. 2020, 11, 1199–1216. [Google Scholar] [CrossRef]
- Ahmad, R.; Wojciech, S.; Jockers, R. Hunting for the function of orphan GPCRs–beyond the search for the endogenous ligand. Br. J. Pharmacol. 2015, 172, 3212–3228. [Google Scholar] [CrossRef]
- Marazziti, D.; Di Pietro, C.; Golini, E.; Mandillo, S.; La Sala, G.; Matteoni, R.; Tocchini-Valentini, G.P. Precocious cerebellum development and improved motor functions in mice lacking the astrocyte cilium-, patched 1-associated Gpr37l1 receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 16486–16491. [Google Scholar] [CrossRef]
- Jolly, S.; Bazargani, N.; Quiroga, A.C.; Pringle, N.P.; Attwell, D.; Richardson, W.D.; Li, H. G protein-coupled receptor 37-like 1 modulates astrocyte glutamate transporters and neuronal NMDA receptors and is neuroprotective in ischemia. Glia 2018, 66, 47–61. [Google Scholar] [CrossRef]
- Coleman, J.L.J.; Mouat, M.A.; Wu, J.; Jancovski, N.; Bassi, J.K.; Chan, A.Y.; Humphreys, D.T.; Mrad, N.; Yu, Z.-Y.; Ngo, T.; et al. Orphan receptor GPR37L1 contributes to the sexual dimorphism of central cardiovascular control. Biol. Sex Differ. 2018, 9, 14. [Google Scholar] [CrossRef]
- Mouat, M.A.; Jackson, K.L.; Coleman, J.L.J.; Paterson, M.R.; Graham, R.M.; Head, G.A.; Smith, N.J. Deletion of Orphan G Protein-Coupled Receptor GPR37L1 in Mice Alters Cardiovascular Homeostasis in a Sex-Specific Manner. Front. Pharmacol. 2021, 11, 2384. [Google Scholar] [CrossRef]
- Marazziti, D.; Golini, E.; Mandillo, S.; Magrelli, A.; Witke, W.; Matteoni, R.; Tocchini-Valentini, G.P. Altered dopamine signaling and MPTP resistance in mice lacking the Parkinson’s disease-associated GPR37/parkin-associated endothelin-like receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 10189. [Google Scholar] [CrossRef]
- Mouat, M.A.; Coleman, J.L.J.; Wu, J.; dos Remedios, C.G.; Feneley, M.P.; Graham, R.M.; Smith, N.J. Involvement of GPR37L1 in murine blood pressure regulation and human cardiac disease pathophysiology. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H807–H817. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Valdenaire, O.; Giller, T.; Breu, V.; Ardati, A.; Schweizer, A.; Richards, J.G. A new family of orphan G protein-coupled receptors predominantly expressed in the brain. FEBS Lett. 1998, 424, 193–196. [Google Scholar] [CrossRef]
- Korovesi, A.G.; Anagnostopoulos, A.K.; Pierros, V.; Stravopodis, D.J.; Tsangaris, G.T.J.C.G.-P. Normal Mouse Brain Proteome II: Analysis of Brain Regions by High-resolution Mass Spectrometry. Cancer Genom. Proteom. 2020, 17, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Chaboub, L.S.; Manalo, J.M.; Lee, H.K.; Glasgow, S.M.; Chen, F.; Kawasaki, Y.; Akiyama, T.; Kuo, C.T.; Creighton, C.J.; Mohila, C.A.; et al. Temporal Profiling of Astrocyte Precursors Reveals Parallel Roles for Asef during Development and after injury. J. Neurosci. 2016, 36, 11904–11917. [Google Scholar] [CrossRef] [PubMed]
- Lein, E.S.; Hawrylycz, M.J.; Ao, N.; Ayres, M.; Bensinger, A.; Bernard, A.; Boe, A.F.; Boguski, M.S.; Brockway, K.S.; Byrnes, E.J.; et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007, 445, 168–176. [Google Scholar] [CrossRef]
- Boisvert, M.M.; Erikson, G.A.; Shokhirev, M.N.; Allen, N.J. The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Rep. 2018, 22, 269–285. [Google Scholar] [CrossRef]
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 2020, 367, eaay5947. [Google Scholar] [CrossRef]
- Ito, J.; Ito, M.; Nambu, H.; Fujikawa, T.; Tanaka, K.; Iwaasa, H.; Tokita, S. Anatomical and histological profiling of orphan G-protein-coupled receptor expression in gastrointestinal tract of C57BL/6J mice. Cell Tissue Res. 2009, 338, 257–269. [Google Scholar] [CrossRef]
- Leng, N.; Gu, G.; Simerly, R.B.; Spindel, E.R. Molecular cloning and characterization of two putative G protein-coupled receptors which are highly expressed in the central nervous system. Brain Res. Mol. Brain Res. 1999, 69, 73–83. [Google Scholar] [CrossRef]
- Ngo, T.; Ilatovskiy, A.V.; Stewart, A.G.; Coleman, J.L.J.; McRobb, F.M.; Riek, R.P.; Graham, R.M.; Abagyan, R.; Kufareva, I.; Smith, N.J. Orphan receptor ligand discovery by pickpocketing pharmacological neighbors. Nat. Chem. Biol. 2017, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.; Wilkins, B.P.; So, S.S.; Keov, P.; Chahal, K.K.; Finch, A.M.; Coleman, J.L.J.; Kufareva, I.; Smith, N.J. Orphan receptor GPR37L1 remains unliganded. Nat. Chem. Biol. 2021, 17, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Ishii, Y.; Halford, J.C.G.; Blundell, J.E. Orexins and appetite regulation. Neuropeptides 2002, 36, 303–325. [Google Scholar] [CrossRef]
- Parker, J.A.; Bloom, S.R. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 2012, 63, 18–30. [Google Scholar] [CrossRef]
- Botticelli, L.; Micioni Di Bonaventura, E.; Ubaldi, M.; Ciccocioppo, R.; Cifani, C.; Micioni Di Bonaventura, M.V. The Neural Network of Neuropeptide S (NPS): Implications in Food Intake and Gastrointestinal Functions. Pharmaceuticals 2021, 14, 293. [Google Scholar] [CrossRef]
- Skarnes, W.C.; Rosen, B.; West, A.P.; Koutsourakis, M.; Bushell, W.; Iyer, V.; Mujica, A.O.; Thomas, M.; Harrow, J.; Cox, T.; et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature 2011, 474, 337–342. [Google Scholar] [CrossRef]
- Coleman, J.L.; Brennan, K.; Ngo, T.; Balaji, P.; Graham, R.M.; Smith, N.J. Rapid Knockout and Reporter Mouse Line Generation and Breeding Colony Establishment Using EUCOMM Conditional-Ready Embryonic Stem Cells: A Case Study. Front. Endocrinol. 2015, 6, 105. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Osborne, B.; Brandon, A.E.; O’Reilly, L.; Fiveash, C.E.; Brown, S.H.J.; Wilkins, B.P.; Samsudeen, A.; Yu, J.; Devanapalli, B.; et al. Regulation of mitochondrial metabolism in murine skeletal muscle by the medium-chain fatty acid receptor Gpr84. FASEB J. 2019, 33, 12264–12276. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Davenport, A.P.; Alexander, S.P.; Sharman, J.L.; Pawson, A.J.; Benson, H.E.; Monaghan, A.E.; Liew, W.C.; Mpamhanga, C.P.; Bonner, T.I.; Neubig, R.R.; et al. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: Recommendations for new pairings with cognate ligands. Pharmacol. Rev. 2013, 65, 967–986. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D. The Concise Guide to Pharmacology 2021/22: G protein-coupled receptors. Br. J. Pharmacol. 2021, 178, S27–S156. [Google Scholar] [CrossRef] [PubMed]
- Giddens, M.M.; Wong, J.C.; Schroeder, J.P.; Farrow, E.G.; Smith, B.M.; Owino, S.; Soden, S.E.; Meyer, R.C.; Saunders, C.; LePichon, J.B.; et al. GPR37L1 modulates seizure susceptibility: Evidence from mouse studies and analyses of a human GPR37L1 variant. Neurobiol. Dis. 2017, 106, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, C.; La Sala, G.; Matteoni, R.; Marazziti, D.; Tocchini-Valentini, G.P. Genetic ablation of Gpr37l1 delays tumor occurrence in Ptch1+/− mouse models of medulloblastoma. Exp. Neurol. 2019, 312, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.; Wu, S.; Ling, L.; Danao, J.; Li, Y.; Yeh, W.-C.; Tian, H.; Baribault, H. G-protein-coupled receptor GPR21 knockout mice display improved glucose tolerance and increased insulin response. Biochem. Biophys. Res. Commun. 2012, 418, 1–5. [Google Scholar] [CrossRef]
- Osborn, O.; Oh, D.Y.; McNelis, J.; Sanchez-Alavez, M.; Talukdar, S.; Lu, M.; Li, P.; Thiede, L.; Morinaga, H.; Kim, J.J.; et al. G protein–coupled receptor 21 deletion improves insulin sensitivity in diet-induced obese mice. J. Clin. Investig. 2012, 122, 2444–2453. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Bechtold, D.A.; Dupré, S.M.; Brennand, J.; Barrett, P.; Luckman, S.M.; Loudon, A.S.I. Altered metabolism in the melatonin-related receptor (GPR50) knockout mouse. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E176–E182. [Google Scholar] [CrossRef]
- Engel, K.M.Y.; Schröck, K.; Teupser, D.; Holdt, L.M.; Tönjes, A.; Kern, M.; Dietrich, K.; Kovacs, P.; Krügel, U.; Scheidt, H.A. Reduced food intake and body weight in mice deficient for the G protein-coupled receptor GPR82. PLoS ONE 2011, 6, e29400. [Google Scholar] [CrossRef][Green Version]
- Dubins, J.S.; Sanchez-Alavez, M.; Zhukov, V.; Sanchez-Gonzalez, A.; Moroncini, G.; Carvajal-Gonzalez, S.; Hadcock, J.R.; Bartfai, T.; Conti, B. Downregulation of GPR83 in the hypothalamic preoptic area reduces core body temperature and elevates circulating levels of adiponectin. Metabolism 2012, 61, 1486–1493. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Yang, H.; Gao, C.; Du, Q.; Zhang, C.; Zhu, L.; Li, Q. MMP9, CXCR1, TLR6, and MPO participant in the progression of coronary artery disease. J. Cell. Physiol. 2020, 235, 8283–8292. [Google Scholar] [CrossRef]
- Bang, S.; Xie, Y.-K.; Zhang, Z.-J.; Wang, Z.; Xu, Z.-Z.; Ji, R.-R. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J. Clin. Investig. 2018, 128, 3568–3582. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Donnelly, C.R.; Luo, X.; Toro-Moreno, M.; Tao, X.; Wang, Z.; Chandra, S.; Bortsov, A.V.; Derbyshire, E.R.; Ji, R.-R. Activation of GPR37 in macrophages confers protection against infection-induced sepsis and pain-like behaviour in mice. Nat. Commun. 2021, 12, 1704. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Kitade, H.; Ni, Y.; Ota, T. Roles of Chemokines and Chemokine Receptors in Obesity-Associated Insulin Resistance and Nonalcoholic Fatty Liver Disease. Biomolecules 2015, 5, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, K.; Jokkala, J.; Ahonen, I.; Auriola, S.; Kolehmainen, M.; Hanhineva, K.; Tiihonen, K. High-Fat Diet, Betaine, and Polydextrose Induce Changes in Adipose Tissue Inflammation and Metabolism in C57BL/6J Mice. Mol. Nutr. Food Res. 2018, 62, 1800455. [Google Scholar] [CrossRef] [PubMed]
- Siersbæk, M.S.; Ditzel, N.; Hejbøl, E.K.; Præstholm, S.M.; Markussen, L.K.; Avolio, F.; Li, L.; Lehtonen, L.; Hansen, A.K.; Schrøder, H.D.; et al. C57BL/6J substrain differences in response to high-fat diet intervention. Sci. Rep. 2020, 10, 14052. [Google Scholar] [CrossRef]
- Yang, Y.; Smith, D.L., Jr.; Keating, K.D.; Allison, D.B.; Nagy, T.R. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity 2014, 22, 2147–2155. [Google Scholar] [CrossRef]
- Lin, S.; Thomas, T.C.; Storlien, L.H.; Huang, X.F. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int. J. Obes. 2000, 24, 639–646. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Hallahan, N.L.; Brown, S.H.; Liu, M.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 2013, 56, 1129–1139. [Google Scholar] [CrossRef]
- Surwit, R.S.; Kuhn, C.M.; Cochrane, C.; McCubbin, J.A.; Feinglos, M.N. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 1988, 37, 1163–1167. [Google Scholar] [CrossRef]
- Arch, J.R.S.; Hislop, D.; Wang, S.J.Y.; Speakman, J.R. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int. J. Obes. 2006, 30, 1322–1331. [Google Scholar] [CrossRef]
- Seidell, J.C.; Muller, D.C.; Sorkin, J.D.; Andres, R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: The Baltimore Longitudinal Study on Aging. Int. J. Obes. Relat. Metab. Disord. 1992, 16, 667–674. [Google Scholar] [PubMed]
- Marra, M.; Scalfi, L.; Contaldo, F.; Pasanisi, F. Fasting respiratory quotient as a predictor of long-term weight changes in non-obese women. Ann. Nutr. Metab. 2004, 48, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Argmann, C.; Houten, S.M.; Cantó, C.; Jeninga, E.H.; Andreux, P.A.; Thomas, C.; Doenlen, R.; Schoonjans, K.; Auwerx, J. The metabolic footprint of aging in mice. Sci. Rep. 2011, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Inglis, A.; Shibin, S.; Ubungen, R.; Farooq, S.; Mata, P.; Thiam, J.; Al-Mohanna, F.A.; Collison, K.S. Strain and sex-based glucocentric & behavioral differences between KK/HlJ and C57BL/6J mice. Physiol. Behav. 2019, 210, 112646. [Google Scholar] [CrossRef]
- Ingvorsen, C.; Karp, N.A.; Lelliott, C.J. The role of sex and body weight on the metabolic effects of high-fat diet in C57BL/6N mice. Nutr. Diabetes 2017, 7, e261. [Google Scholar] [CrossRef]
- Hwang, L.L.; Wang, C.H.; Li, T.L.; Chang, S.D.; Lin, L.C.; Chen, C.P.; Chen, C.T.; Liang, K.C.; Ho, I.K.; Yang, W.S. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity 2010, 18, 463–469. [Google Scholar] [CrossRef]
- Sweeney, P.; Bedenbaugh, M.N.; Maldonado, J.; Pan, P.; Fowler, K.; Williams, S.Y.; Gimenez, L.E.; Ghamari-Langroudi, M.; Downing, G.; Gui, Y. The melanocortin-3 receptor is a pharmacological target for the regulation of anorexia. Sci. Transl. Med. 2021, 13, eabd6434. [Google Scholar] [CrossRef]
- Vassileva, G.; Hu, W.; Hoos, L.; Tetzloff, G.; Yang, S.; Liu, L.; Kang, L.; Davis, H.R.; Hedrick, J.A.; Lan, H. Gender-dependent effect of Gpbar1 genetic deletion on the metabolic profiles of diet-induced obese mice. J. Endocrinol. 2010, 205, 225. [Google Scholar] [CrossRef]
- Sutton, G.M.; Trevaskis, J.L.; Hulver, M.W.; McMillan, R.P.; Markward, N.J.; Babin, M.J.; Meyer, E.A.; Butler, A.A. Diet-Genotype Interactions in the Development of the Obese, Insulin-Resistant Phenotype of C57BL/6J Mice Lacking Melanocortin-3 or -4 Receptors. Endocrinology 2006, 147, 2183–2196. [Google Scholar] [CrossRef]
- Reilly, A.M.; Zhou, S.; Panigrahi, S.K.; Yan, S.; Conley, J.M.; Sheets, P.L.; Wardlaw, S.L.; Ren, H. Gpr17 deficiency in POMC neurons ameliorates the metabolic derangements caused by long-term high-fat diet feeding. Nutr. Diabetes 2019, 9, 29. [Google Scholar] [CrossRef]
- Collins, S.; Martin, T.L.; Surwit, R.S.; Robidoux, J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: Physiological and molecular characteristics. Physiol. Behav. 2004, 81, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Champy, M.-F.; Selloum, M.; Zeitler, V.; Caradec, C.; Jung, B.; Rousseau, S.; Pouilly, L.; Sorg, T.; Auwerx, J. Genetic background determines metabolic phenotypes in the mouse. Mamm. Genome 2008, 19, 318–331. [Google Scholar] [CrossRef] [PubMed]


| Chow | HFD | Statistical Analysis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gpr37l1+/+ | Gpr37l1−/− | Gpr37l1+/+ | Gpr37l1−/− | Genotype | Diet | Interaction | |||||||||
| Tissue Weights (mg) | |||||||||||||||
| Heart | 141.39 | ± | 3.44 | 137.91 | ± | 4.91 | 136.67 | ± | 2.20 | 144.16 | ± | 4.93 | 0.6189 | 0.8495 | 0.1781 |
| Liver | 1448.63 | ± | 60.78 | 1305.17 | ± | 56.69 | 1595.43 | ± | 60.18 | 1549.70 | ± | 123.93 | 0.2313 | 0.0165 | 0.5332 |
| Inguinal WAT (1) | 161.93 | ± | 9.08 | 165.81 | ± | 7.43 | 769.42 | ± | 60.18 | 764.73 | ± | 82.54 | 0.9936 | <0.0001 | 0.9313 |
| Epididymal WAT (1) | 264.01 | ± | 19.78 | 261.89 | ± | 17.13 | 1021.78 | ± | 50.57 | 1056.57 | ± | 95.62 | 0.7589 | <0.0001 | 0.7289 |
| BAT (2) | 87.46 | ± | 3.77 | 87.71 | ± | 4.37 | 205.34 | ± | 16.83 | 201.47 | ± | 14.89 | 0.8749 | <0.0001 | 0.8578 |
| Quadriceps (1) | 223.32 | ± | 4.69 | 223.91 | ± | 4.58 | 211.10 | ± | 4.57 | 202.82 | ± | 6.22 | 0.4472 | 0.0021 | 0.3818 |
| Tibialis anterior (1) | 55.29 | ± | 0.91 | 54.76 | ± | 1.21 | 50.65 | ± | 1.30 | 49.48 | ± | 0.95 | 0.4508 | <0.0001 | 0.7753 |
| Triglyceride content (nmol/mg) | |||||||||||||||
| Liver | 3.95 | ± | 0.74 | 4.30 | ± | 0.77 | 12.90 | ± | 1.99 | 12.68 | ± | 2.59 | 0.9716 | <0.0001 | 0.8685 |
| Quadriceps | 1.98 | ± | 0.33 | 1.78 | ± | 0.19 | 3.24 | ± | 0.33 | 4.76 | ± | 0.74 | 0.1477 | <0.0001 | 0.0615 |
| Serum Analysis (mM) | |||||||||||||||
| Triglycerides | 1.40 | ± | 0.11 | 1.17 | ± | 0.15 | 1.17 | ± | 0.09 | 1.24 | ± | 0.17 | 0.5229 | 0.5625 | 0.2633 |
| NEFA | 0.54 | ± | 0.04 | 0.53 | ± | 0.04 | 0.61 | ± | 0.04 | 0.67 | ± | 0.07 | 0.5807 | 0.0367 | 0.5256 |
| 1yo Gpr37l1+/+ | 1yo Gpr37l1−/− | WT:KO | |||||
|---|---|---|---|---|---|---|---|
| Tissue Weights (mg) | |||||||
| Heart | 158.88 | ± | 3.14 | 153.66 | ± | 2.74 | 0.2233 |
| Liver | 1616.55 | ± | 62.81 | 1570.95 | ± | 44.99 | 0.9226 † |
| Inguinal WAT (1) | 470.24 | ± | 55.59 | 332.61 | ± | 31.49 | 0.1067 † |
| Epididymal WAT (1) | 663.33 | ± | 59.10 | 580.68 | ± | 44.87 | 0.2762 |
| BAT (2) | 136.74 | ± | 8.97 | 136.32 | ± | 9.66 | 0.8119 † |
| Quadriceps (1) | 210.75 | ± | 2.88 | 214.08 | ± | 3.76 | 0.4838 |
| Tibialis anterior (1) | 53.97 | ± | 0.68 | 52.51 | ± | 1.40 | 0.8621 † |
| Triglyceride content (nmol/mg) | |||||||
| Liver | 12.72 | ± | 2.02 | 14.67 | ± | 2.34 | 0.4440 † |
| Quadriceps | 7.20 | ± | 1.09 | 6.86 | ± | 0.79 | 0.9880 † |
| Serum Analysis | |||||||
| Triglycerides (mM) | 1.30 | ± | 0.09 | 1.42 | ± | 0.07 | 0.2216 † |
| NEFA (mM) | 0.89 | ± | 0.05 | 0.88 | ± | 0.05 | 0.8721 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouat, M.A.; Wilkins, B.P.; Ding, E.; Govindaraju, H.; Coleman, J.L.J.; Graham, R.M.; Turner, N.; Smith, N.J. Metabolic Profiling of Mice with Deletion of the Orphan G Protein-Coupled Receptor, GPR37L1. Cells 2022, 11, 1814. https://doi.org/10.3390/cells11111814
Mouat MA, Wilkins BP, Ding E, Govindaraju H, Coleman JLJ, Graham RM, Turner N, Smith NJ. Metabolic Profiling of Mice with Deletion of the Orphan G Protein-Coupled Receptor, GPR37L1. Cells. 2022; 11(11):1814. https://doi.org/10.3390/cells11111814
Chicago/Turabian StyleMouat, Margaret A., Brendan P. Wilkins, Eileen Ding, Hemna Govindaraju, James L. J. Coleman, Robert M. Graham, Nigel Turner, and Nicola J. Smith. 2022. "Metabolic Profiling of Mice with Deletion of the Orphan G Protein-Coupled Receptor, GPR37L1" Cells 11, no. 11: 1814. https://doi.org/10.3390/cells11111814
APA StyleMouat, M. A., Wilkins, B. P., Ding, E., Govindaraju, H., Coleman, J. L. J., Graham, R. M., Turner, N., & Smith, N. J. (2022). Metabolic Profiling of Mice with Deletion of the Orphan G Protein-Coupled Receptor, GPR37L1. Cells, 11(11), 1814. https://doi.org/10.3390/cells11111814






