Non-Coding RNAs in the Therapeutic Landscape of Pathological Cardiac Hypertrophy
Abstract
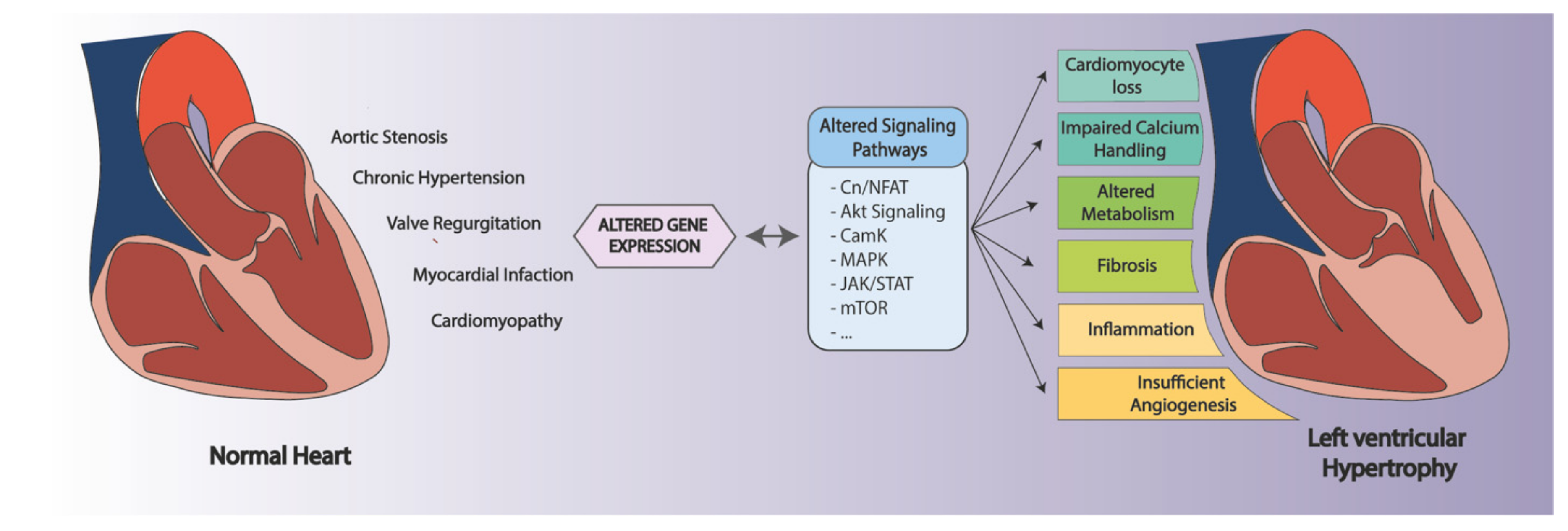
:1. Introduction
2. Non-Coding RNAs (ncRNAs)
3. ncRNAs in Pressure-Overload-Induced Cardiac Hypertrophy
3.1. ncRNAs Involved in Specific Cardiac Hypertrophy-Related Pathways
3.1.1. Calcineurin/NFAT Signaling
3.1.2. CaMKII Signaling
3.1.3. MAPK Signaling
3.1.4. Akt Signaling
3.1.5. JAK/STAT and Wnt Signaling Pathways
3.2. ncRNAs Involved in Specific Pathophysiological Processes
3.2.1. ncRNAs in Cardiomyocyte Remodeling and Loss
3.2.2. ncRNAs in Cardiac-Calcium Handling
3.2.3. ncRNAs in Cardiac Metabolic Changes
3.2.4. ncrNAs in Cardiac Fibrosis
3.2.5. ncRNAs in Cardiac Inflammation
3.2.6. ncRNAs in Cardiac Angiogenesis
4. Therapeutic Delivery of Drugs Targeting Non-Coding RNAs
4.1. Overexpression of ncRNAs
4.2. Inhibition of ncRNAs
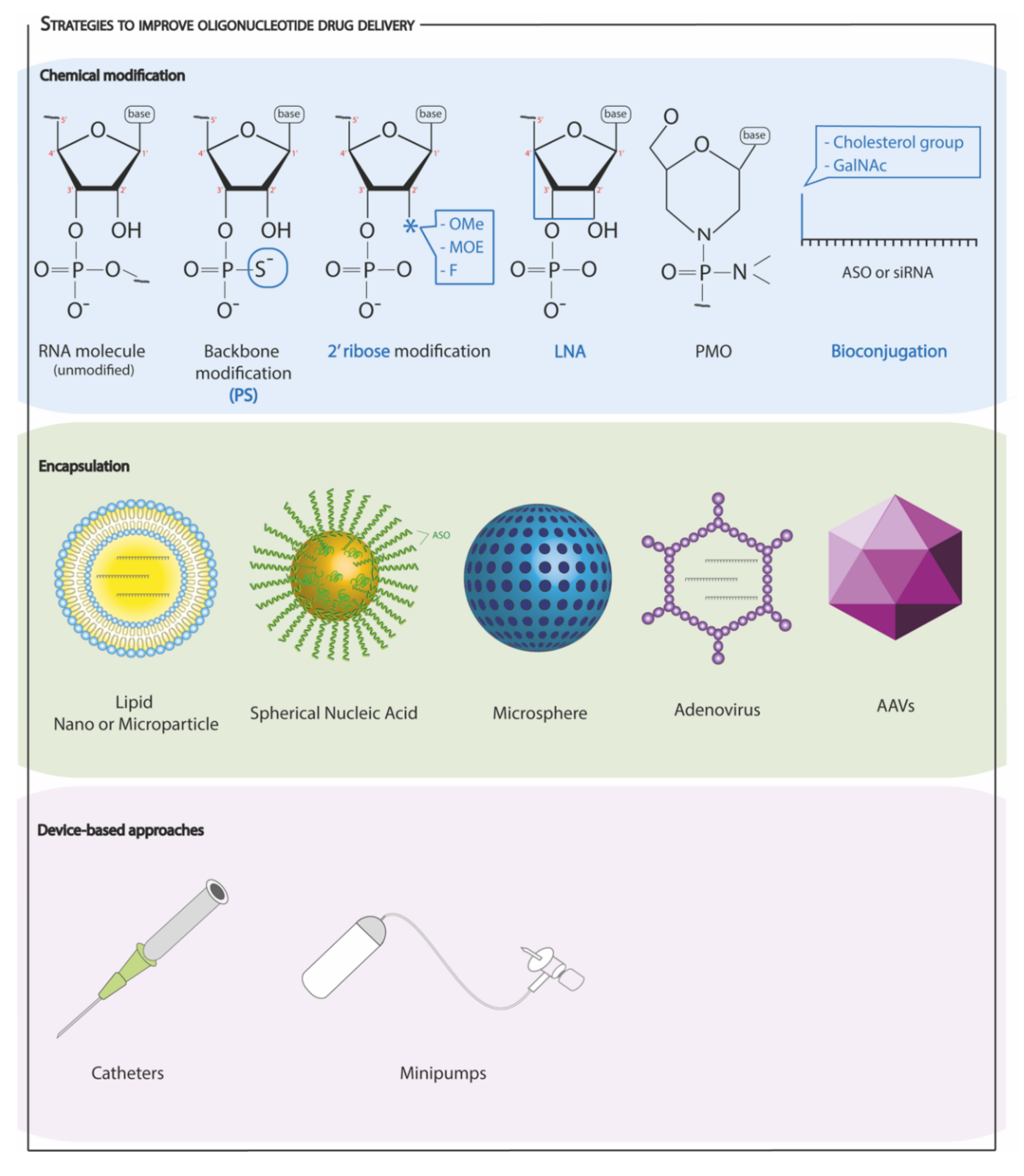
4.3. Strategies to Improve Oligonucleotide Drug Delivery
4.3.1. Chemical Modifications
4.3.2. Encapsulation and Other Approaches
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tham, Y.K.; Bernardo, B.C.; Ooi, J.Y.Y.; Weeks, K.L.; McMullen, J.R. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015, 89, 1401–1438. [Google Scholar] [CrossRef] [PubMed]
- Samak, M.; Fatullayev, J.; Sabashnikov, A.; Zeriouh, M.; Schmack, B.; Farag, M.; Popov, A.F.; Dohmen, P.M.; Choi, Y.H.; Wahlers, T.; et al. Cardiac Hypertrophy: An Introduction to Molecular and Cellular Basis. Med. Sci. Monit. Basic Res. 2016, 22, 75–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, J.F.; Pawloski-Dahm, C.; Davis, M.G.; Ball, N.; Dorn, G.W.; Walsh, R.A. The role of the cytoskeleton in left ventricular pressure overload hypertrophy and failure. J. Mol. Cell. Cardiol. 1996, 28, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Grossman, W.; Jones, D.; McLaurin, L.P. Wall Stress and Patterns of Hypertrophy in the Human Left Ventricle. J. Clin. Investig. 1975, 56, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.A.; Carillo, S.V. Cardiac hypertrophy due to pressure and volume overload: Distinctly different biological phenomena? Int. J. Cardiol. 1991, 31, 133–141. [Google Scholar] [CrossRef]
- Wong, K.; Boheler, K.R.; Petrou, M.; Yacoub, M.H. Pharmacological Modulation of Pressure-Overload Cardiac Hypertrophy. Circulation 1997, 96, 2239–2246. [Google Scholar] [CrossRef]
- Breisch, E.A.; White, F.C.; Nimmo, L.E.; Bloor, C.M. Cardiac vasculature and flow during pressure-overload hypertrophy. Am. J. Physiol.-Heart Circ. Physiol. 1986, 251, H1031–H1037. [Google Scholar] [CrossRef]
- Cohn, J.N. Structural basis for heart failure: Ventricular remodeling and its pharmacological inhibition. Circulation 1995, 91, 2504–2507. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, Z.; Xu, H.; Armah, M.A.; Teng, P.; Li, W.; Jian, D.; Ma, L.; Ni, Y. Pressure overload-induced cardiac hypertrophy varies according to different ligation needle sizes and body weights in mice. Arq. Bras. Cardiol. 2018, 110, 568–576. [Google Scholar] [CrossRef]
- Schiattarella, G.; Hill, J.A. Is inhibition of hypertrophy a good therapeutic strategy in ventricular pressure overload? Circulation 2015, 131, 1435–1447. [Google Scholar] [CrossRef] [Green Version]
- Gaasch, W.H.; Zile, M.R. Left Ventricular Structural Remodeling in Health and Disease: With Special Emphasis on Volume, Mass, and Geometry. J. Am. Coll. Cardiol. 2011, 58, 1733–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauta, J.F.; Hummel, Y.M.; Tromp, J.; Ouwerkerk, W.; van der Meer, P.; Jin, X.; Lam, C.S.P.; Bax, J.J.; Metra, M.; Samani, N.J.; et al. Concentric vs. eccentric remodelling in heart failure with reduced ejection fraction: Clinical characteristics, pathophysiology and response to treatment. Eur. J. Heart Fail. 2020, 22, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Perrino, C.; Prasad, S.V.; Mao, L.; Noma, T.; Yan, Z.; Kim, H.S.; Smithies, O.; Rockman, H.A. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J. Clin. Investig. 2006, 116, 1547–1560. [Google Scholar] [CrossRef] [Green Version]
- Devereux, R.B.; Roman, M.J.; Paranicas, M.; O’Grady, M.J.; Lee, E.T.; Welty, T.K.; Fabsitz, R.R.; Robbins, D.; Rhoades, E.R.; Howard, B.V. Impact of diabetes on cardiac structure and function: The Strong Heart Study. Circulation 2000, 101, 2271–2276. [Google Scholar] [CrossRef] [Green Version]
- Turkbey, E.B.; McClelland, R.L.; Kronmal, R.A.; Burke, G.L.; Bild, D.E.; Tracy, R.P.; Arai, A.E.; Lima, J.A.C.; Bluemke, D.A. The Impact of Obesity on the Left Ventricle. The Multi-Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc. Imaging 2010, 3, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef]
- Hombach, S.; Kretz, M. Non-coding RNAs: Classification, biology and functioning. Adv. Exp. Med. Biol. 2016, 937, 3–17. [Google Scholar] [CrossRef]
- Yang, J.X.; Rastetter, R.H.; Wilhelm, D. Non-coding RNAs: An introduction. Adv. Exp. Med. Biol. 2016, 886, 13–32. [Google Scholar] [CrossRef]
- Ghildiyal, M.; Zamore, P.D. Small silencing RNAs: An expanding universe. Nat. Rev. Genet. 2009, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Luo, Y.; Song, J.; Tan, T.; Zhu, H. Non-coding RNAs and Pathological Cardiac Hypertrophy. Adv. Exp. Med. Biol. 2020, 1229, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, I.; Ishtiaq, A.; Ali, T.; Jan, M.I.; Murtaza, I. An Overview of Non-coding RNAs and Cardiovascular System. Adv. Exp. Med. Biol. 2020, 1229, 3–45. [Google Scholar] [CrossRef] [PubMed]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, T.; Bonauer, A.; Dimmeler, S. RNA therapeutics in cardiovascular disease. Circ. Res. 2018, 123, 205–220. [Google Scholar] [CrossRef]
- Creemers, E.E.; Tijsen, A.J.; Pinto, Y.M. Circulating MicroRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012, 110, 483–495. [Google Scholar] [CrossRef]
- Lopes, E.C.P.; Paim, L.R.; Carvalho-Romano, L.F.R.S.; Marques, E.R.; Minin, E.O.Z.; Vegian, C.F.L.; Pio-Magalhães, J.A.; Velloso, L.A.; Coelho-Filho, O.R.; Sposito, A.C.; et al. Relationship between Circulating MicroRNAs and Left Ventricular Hypertrophy in Hypertensive Patients. Front. Cardiovasc. Med. 2022, 9, 798954. [Google Scholar] [CrossRef]
- Kung, J.T.Y.; Colognori, D.; Lee, J.T. Long noncoding RNAs: Past, present, and future. Genetics 2013, 193, 651–669. [Google Scholar] [CrossRef] [Green Version]
- Bassett, A.R.; Akhtar, A.; Barlow, D.P.; Bird, A.P.; Brockdorff, N.; Duboule, D.; Ephrussi, A.; Ferguson-Smith, A.C.; Gingeras, T.R.; Haerty, W.; et al. Considerations when investigating lncRNA function in vivo. eLife 2014, 3, 1–14. [Google Scholar] [CrossRef]
- Ulitsky, I.; Shkumatava, A.; Jan, C.H.; Sive, H.; Bartel, D.P. Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell 2011, 147, 1537–1550. [Google Scholar] [CrossRef] [Green Version]
- Kay, M.; Soltani, B.M. LncRNAs in Cardiomyocyte Maturation: New Window for Cardiac Regenerative Medicine. Non-Coding RNA 2021, 7, 20. [Google Scholar] [CrossRef]
- Beermann, J.; Piccoli, M.T.; Viereck, J.; Thum, T. Non-coding rnas in development and disease: Background, mechanisms, and therapeutic approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajan, K.S.; Velmurugan, G.; Gopal, P.; Ramprasath, T.; Babu, D.D.V.; Krithika, S.; Jenifer, Y.C.; Freddy, A.; William, G.; Kalpana, K.; et al. Abundant and Altered Expression of PIWI-Interacting RNAs during Cardiac Hypertrophy. Heart Lung Circ. 2016, 25, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Xu, S.; Liu, J.; Ponnusamy, M.; Zhao, Y.; Zhang, Y.; Wang, Q.; Li, P.; Wang, K. Non-coding RNA-linked epigenetic regulation in cardiac hypertrophy. Int. J. Biol. Sci. 2018, 14, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Q.; Zhang, Y.H.; Liu, F.; Ponnusamy, M.; Zhao, X.M.; Zhou, L.Y.; Zhai, M.; Liu, C.Y.; Li, X.M.; Wang, M.; et al. The piRNA CHAPIR regulates cardiac hypertrophy by controlling METTL3-dependent N 6-methyladenosine methylation of Parp10 mRNA. Nat. Cell Biol. 2020, 22, 1319–1331. [Google Scholar] [CrossRef]
- Sobala, A.; Hutvagner, G. Small RNAs derived from the 5′ end of tRNAs can inhibit protein translation in human cells. RNA Biol. 2013, 10, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Gan, M.; Tan, Z.; Jiang, D.; Jiang, Y.; Li, M.; Wang, J.; Li, X.; Zhang, S.; Zhu, L. A novel class of tRNA-derived small non-coding RNAs respond to myocardial hypertrophy and contribute to intergenerational inheritance. Biomolecules 2018, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Marian, A.J.; Braunwald, E. Hypertrophic cardiomyopathy: Genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Luo, J.F.; Yu, X.J.; Zhu, J.N.; Huang, L.; Yang, J.; Fu, Y.H.; Li, T.; Xue, Y.M.; Feng, Y.Q.; et al. Targeting myocyte-specific enhancer factor 2D contributes to the suppression of cardiac hypertrophic growth by miR-92b-3p in mice. Oncotarget 2017, 8, 92079–92089. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Phan, D.; van Rooij, E.; Wang, D.Z.; Mcanally, J.; Qi, X.; Richardson, J.A.; Hill, J.A.; Bassel-Duby, R.; Olson, E.N. The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J. Clin. Investig. 2008, 118, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Raso, A.; Dirkx, E.; Sampaio-Pinto, V.; el Azzouzi, H.; Cubero, R.J.; Sorensen, D.W.; Ottaviani, L.; Olieslagers, S.; Huibers, M.M.; de Weger, R.; et al. A microRNA program regulates the balance between cardiomyocyte hyperplasia and hypertrophy and stimulates cardiac regeneration. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Z.; Guo, G.; Xu, C.; Song, Z.; Li, K.; Zhong, K.; Wang, D. Circ_nuclear factor I X (circNfix) attenuates pressure overload-induced cardiac hypertrophy via regulating miR-145-5p/ATF3 axis. Bioengineered 2021, 12, 5373–5385. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Zhang, C.; Li, P.; Wu, Y.; Wang, C.; Lau, W.B.; Ma, X.L.; Du, J. Cardiac Fibroblast-Specific Activating Transcription Factor 3 Protects against Heart Failure by Suppressing MAP2K3-p38 Signaling. Circulation 2017, 135, 2041. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Li, W.; Lin, C.H.; Yang, J.; Shang, C.; Nurnberg, S.T.; Jin, K.K.; Xu, W.; Lin, C.Y.; Lin, C.J.; et al. A long noncoding RNA protects the heart from pathological hypertrophy. Nature 2014, 514, 102–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zhang, X.-J.; Ji, Y.-X.; Zhang, P.; Deng, K.Q.; Gong, J.; Ren, S.; Wang, X.; Chen, I.; Wang, H.; et al. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat. Med. 2016, 22, 1131–1139. [Google Scholar] [CrossRef]
- Subrova, J.; Böhme, K.; Gillespie, A.; Orphal, M.; Plum, C.; Kreutz, R.; Eisenreich, A. MiRNA-29b and miRNA-497 Modulate the Expression of Carboxypeptidase X Member 2, a Candidate Gene Associated with Left Ventricular Hypertrophy. Int. J. Mol. Sci. 2022, 23, 2263. [Google Scholar] [CrossRef]
- Da Costa Martins, P.A.; de Windt, L.J. MicroRNAs in control of cardiac hypertrophy. Cardiovasc. Res. 2012, 93, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Ruwhof, C.; van der Laarse, A. Mechanical stress-induced cardiac hypertrophy: Mechanisms and signal transduction pathways. Cardiovasc. Res. 2000, 47, 23–37. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Wu, J.; Zhang, Q.; Ye, Y.; Wang, S.; Huang, J.; Liu, H.; Wang, X.; Zhang, W.; Bu, L.; et al. Differential cardiac hypertrophy and signaling pathways in pressure versus volume overload. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H552–H562. [Google Scholar] [CrossRef] [Green Version]
- Frey, N.; Katus, H.A.; Olson, E.N.; Hill, J.A. Hypertrophy of the Heart: A New Therapeutic Target? Circulation 2004, 109, 1580–1589. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, S.; He, A.; Kong, S.W.; Lu, J.; Bejar, R.; Bodyak, N.; Lee, K.H.; Ma, Q.; Kang, P.M.; Golub, T.R.; et al. MicroRNA-1 Negatively Regulates Expression of the Hypertrophy-Associated Calmodulin and Mef2a Genes. Mol. Cell. Biol. 2009, 29, 2193–2204. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef]
- Dong, D.L.; Chen, C.; Huo, R.; Wang, N.; Li, Z.; Tu, Y.J.; Hu, J.T.; Chu, X.; Huang, W.; Yang, B.F. Reciprocal repression between MicroRNA-133 and calcineurin regulates cardiac hypertrophy: A novel mechanism for progressive cardiac hypertrophy. Hypertension 2010, 55, 946–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, T.B.; Aliwarga, E.; Luu, T.D.A.; Li, Y.P.; Ng, S.L.; Annadoray, L.; Sian, S.; Ackers-Johnson, M.A.; Foo, R.S.Y. Targeting the highly abundant circular RNA circSlc8a1 in cardiomyocytes attenuates pressure overload induced hypertrophy. Cardiovasc. Res. 2019, 115, 1998–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, B.; Wang, P.; Zhang, D.; Wu, L. m6A modification promotes miR-133a repression during cardiac development and hypertrophy via IGF2BP2. Cell Death Discov. 2021, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- da Costa Martins, P.A.; Salic, K.; Gladka, M.M.; Armand, A.S.; Leptidis, S.; El Azzouzi, H.; Hansen, A.; Coenen-de Roo, C.J.; Bierhuizen, M.F.; Van Der Nagel, R.; et al. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat. Cell Biol. 2010, 12, 1220–1227. [Google Scholar] [CrossRef]
- Ni, Y.G.; Berenji, K.; Wang, N.; Oh, M.; Sachan, N.; Dey, A.; Cheng, J.; Lu, G.; Morris, D.J.; Castrillon, D.H.; et al. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation 2006, 114, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- Hudson, M.B.; Rahnert, J.A.; Zheng, B.; Woodworth-Hobbs, M.E.; Franch, H.A.; Russ Price, S. miR-182 attenuates atrophy-related gene expression by targeting FoxO3 in skeletal muscle. Am. J. Physiol. Physiol. 2014, 307, C314–C319. [Google Scholar] [CrossRef] [Green Version]
- Zhi, Y.; Xu, C.; Sui, D.; Du, J.; Xu, F.; Li, Y. Effective Delivery of Hypertrophic miRNA Inhibitor by Cholesterol-Containing Nanocarriers for Preventing Pressure Overload Induced Cardiac Hypertrophy. Adv. Sci. 2019, 6, 1900023. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Lin, Z.; Long, B.; Li, J.H.; Zhou, J.; Li, P.F. Cardiac hypertrophy is positively regulated by microRNA miR-23a. J. Biol. Chem. 2012, 287, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Ucar, A.; Gupta, S.K.; Fiedler, J.; Erikci, E.; Kardasinski, M.; Batkai, S.; Dangwal, S.; Kumarswamy, R.; Bang, C.; Holzmann, A.; et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat. Commun. 2012, 3, 1078. [Google Scholar] [CrossRef] [PubMed]
- Hinkel, R.; Batkai, S.; Bähr, A.; Bozoglu, T.; Straub, S.; Borchert, T.; Viereck, J.; Howe, A.; Hornaschewitz, N.; Oberberger, L.; et al. AntimiR-132 Attenuates Myocardial Hypertrophy in an Animal Model of Percutaneous Aortic Constriction. J. Am. Coll. Cardiol. 2021, 77, 2923–2935. [Google Scholar] [CrossRef] [PubMed]
- Sárközy, M.; Gáspár, R.; Zvara, Á.; Siska, A.; Kővári, B.; Szűcs, G.; Márványkövi, F.; Kovács, M.G.; Diószegi, P.; Bodai, L.; et al. Chronic kidney disease induces left ventricular overexpression of the pro-hypertrophic microRNA-212. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bao, J.; Lu, Y.; She, Q.; Dou, W.; Tang, R.; Xu, X.; Zhang, M.; Zhu, L.; Zhou, Q.; Li, H.; et al. MicroRNA-30 regulates left ventricular hypertrophy in chronic kidney disease. JCI Insight 2021, 6. [Google Scholar] [CrossRef]
- Liu, L.; An, X.; Li, Z.; Song, Y.; Li, L.; Zuo, S.; Liu, N.; Yang, G.; Wang, H.; Cheng, X.; et al. The H19 long noncoding RNA is a novel negative regulator of cardiomyocyte hypertrophy. Cardiovasc. Res. 2016, 111, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Chen, G.; Lv, F.; Liu, Y.; Tian, H.; Tao, R.; Jiang, R.; Zhang, W.; Zhuo, C. LncRNA TINCR attenuates cardiac hypertrophy by epigenetically silencing CaMKII. Oncotarget 2017, 8, 47565–47573. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Gu, H.; Chen, X.; Fu, S.; Wang, C.; Xu, H.; Feng, Q.; Ni, Y. Cardiac hypertrophy and dysfunction induced by overexpression of miR-214 in vivo. J. Surg. Res. 2014, 192, 317–325. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, H.; Gao, W.; Zhang, L.; Ye, Y.; Yuan, L.; Ding, Z.; Wu, J.; Kang, L.; Zhang, X.; et al. MicroRNA-378 suppresses myocardial fibrosis through a paracrine mechanism at the early stage of cardiac hypertrophy following mechanical stress. Theranostics 2018, 8, 2565–2582. [Google Scholar] [CrossRef]
- Ganesan, J.; Ramanujam, D.; Sassi, Y.; Ahles, A.; Jentzsch, C.; Werfel, S.; Leierseder, S.; Loyer, X.; Giacca, M.; Zentilin, L.; et al. MiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation 2013, 127, 2097–2106. [Google Scholar] [CrossRef] [Green Version]
- Matkovich, S.J.; Hu, Y.; Eschenbacher, W.H.; Dorn, L.E.; Dorn, G.W. Direct and indirect involvement of MicroRNA-499 in clinical and experimental cardiomyopathy. Circ. Res. 2012, 111, 521–531. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Jiang, Y.; Guo, X.; Zhang, B.; Wu, J.; Sun, J.; Liang, H.; Shan, H.; Zhang, Y.; Liu, J.; et al. Long non-coding RNA cardiac hypertrophy-associated regulator governs cardiac hypertrophy via regulating miR-20b and the downstream PTEN/AKT pathway. J. Cell. Mol. Med. 2019, 23, 7685–7698. [Google Scholar] [CrossRef] [Green Version]
- Kumarswamy, R.; Volkmann, I.; Jazbutyte, V.; Dangwal, S.; Park, D.H.; Thum, T. Transforming growth factor-β-induced endothelial-to-mesenchymal transition is partly mediated by MicroRNA-21. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Nie, X.; Fan, J.; Li, H.; Yin, Z.; Zhao, Y.; Dai, B.; Dong, N.; Chen, C.; Wang, D.W. miR-217 Promotes Cardiac Hypertrophy and Dysfunction by Targeting PTEN. Mol. Ther.-Nucleic Acids 2018, 12, 254–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raso, A.; Dirkx, E.; Philippen, L.E.; Fernandez-Celis, A.; De Majo, F.; Sampaio-Pinto, V.; Sansonetti, M.; Juni, R.; el Azzouzi, H.; Calore, M.; et al. Therapeutic Delivery of miR-148a Suppresses Ventricular Dilation in Heart Failure. Mol. Ther. 2019, 27, 584–599. [Google Scholar] [CrossRef] [Green Version]
- Sassi, Y.; Avramopoulos, P.; Ramanujam, D.; Grüter, L.; Werfel, S.; Giosele, S.; Brunner, A.D.; Esfandyari, D.; Papadopoulou, A.S.; De Strooper, B.; et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat. Commun. 2017, 8, 1614. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, T.; Zhao, X.; Cheng, L. MicroRNAs modulate the Wnt signaling pathway through targeting its inhibitors. Biochem. Biophys. Res. Commun. 2011, 408, 259–264. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Salido-Medina, A.B.; Gil, A.; Merino, D.; Gómez, J.; Villar, A.V.; González-Vílchez, F.; Hurlé, M.A.; Nistal, J.F. Sex-Specific Regulation of miR-29b in the Myocardium under Pressure Overload is Associated with Differential Molecular, Structural and Functional Remodeling Patterns in Mice and Patients with Aortic Stenosis. Cells 2020, 9, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Lu, Q.; Hu, Z.; Yu, Y.; Chen, Q.; Wang, Q.K. A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat. Commun. 2017, 8, 133. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Long, B.; Liu, F.; Wang, J.X.; Liu, C.Y.; Zhao, B.; Zhou, L.Y.; Sun, T.; Wang, M.; Yu, T.; et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Heart J. 2016, 37, 2602–2611. [Google Scholar] [CrossRef]
- Rothermel, B.A.; Hill, J.A. Autophagy in load-induced heart disease. Circ. Res. 2008, 103, 1363–1369. [Google Scholar] [CrossRef] [Green Version]
- Bhuiyan, S.; Pattison, J.S.; Osinska, H.; James, J.; Gulick, J.; McLendon, P.M.; Hill, J.A.; Sadoshima, J.; Robbins, J. Enhanced autophagy ameliorates cardiac proteinopathy. J. Clin. Investig. 2013, 123, 5284–5297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viereck, J.; Kumarswamy, R.; Foinquinos, A.; Xiao, K.; Avramopoulos, P.; Kunz, M.; Dittrich, M.; Maetzig, T.; Zimmer, K.; Remke, J.; et al. Long noncoding RNA Chast promotes cardiac remodeling. Sci. Transl. Med. 2016, 8, 326ra22. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y.; Chen, Y.; Isobe, M.; Gustafsson, Å.B.; Kitsis, R.N.; Sadoshima, J.; Åb, G. Recent progress in research on molecular mechanisms of autophagy in the heart. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Qi, H.; Liu, Y.; Chen, Y.; Shi, P.; Zhang, S.; Ren, J.; Wang, L.; Cao, Y.; Sun, H. Inhibition of lncRNA Gm15834 Attenuates Autophagy-Mediated Myocardial Hypertrophy via the miR-30b-3p/ULK1 Axis in Mice. Mol. Ther. 2021, 29, 1120–1137. [Google Scholar] [CrossRef]
- Hasenfuss, G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc. Res. 1998, 37, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Kawase, Y.; Ly, H.Q.; Prunier, F.; Lebeche, D.; Shi, Y.; Jin, H.; Hadri, L.; Yoneyama, R.; Hoshino, K.; Takewa, Y.; et al. Reversal of Cardiac Dysfunction after Long-Term Expression of SERCA2a by Gene Transfer in a Pre-Clinical Model of Heart Failure. J. Am. Coll. Cardiol. 2008, 51, 1112–1119. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.G.; Watanabe, S.; Lee, A.; Gorski, P.A.; Lee, P.; Jeong, D.; Liang, L.; Liang, Y.; Baccarini, A.; Sahoo, S.; et al. MiR-146a Suppresses SUMO1 Expression and Induces Cardiac Dysfunction in Maladaptive Hypertrophy. Circ. Res. 2018, 123, 673–685. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Gao, X.; Zhang, R.; Zhang, Y.; Liang, H.; Xu, C.; Du, W.; Zhang, Y.; Liu, X.; et al. MicroRNA-328 as a regulator of cardiac hypertrophy. Int. J. Cardiol. 2014, 173, 268–276. [Google Scholar] [CrossRef]
- Wahlquist, C.; Jeong, D.; Rojas-Muñoz, A.; Kho, C.; Lee, A.; Mitsuyama, S.; Van Mil, A.; Jin, P.W.; Sluijter, J.P.G.; Doevendans, P.A.F.; et al. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature 2014, 508, 531–535. [Google Scholar] [CrossRef]
- Yang, L.; Deng, J.; Ma, W.; Qiao, A.; Xu, S.; Yu, Y.; Boriboun, C.; Kang, X.; Han, D.; Ernst, P.; et al. Ablation of lncRNA Miat attenuates pathological hypertrophy and heart failure. Theranostics 2021, 11, 7995. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Wang, X. Silencing of circHIPK3 Inhibits Pressure Overload-Induced Cardiac Hypertrophy and Dysfunction by Sponging miR-185-3p. Drug Des. Dev. Ther. 2020, 14, 5699. [Google Scholar] [CrossRef] [PubMed]
- Taegtmeyer, H. Switching metabolic genes to build a better heart. Circulation 2002, 106, 2043–2045. [Google Scholar] [CrossRef] [Green Version]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, S.; Horn, M.; Cramer, M.; Harre, K.; Newell, J.B.; Pabst, T.; Ertl, G.; Hahn, D.; Ingwall, J.S.; Kochsiek, K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997, 96, 2190–2196. [Google Scholar] [CrossRef]
- Neubauer, S. The Failing Heart—An Engine Out of Fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Shao, Y.; Guo, H.C.; Zhi, Y.; Qiao, B.; Ma, K.; Du, J.; Lai, Y.Q.; Li, Y. MicroRNA-27b-3p down-regulates FGF1 and aggravates pathological cardiac remodelling. Cardiovasc. Res. 2021, cvab248. [Google Scholar] [CrossRef]
- Ding, Y.Q.; Zhang, Y.H.; Lu, J.; Li, B.; Yu, W.J.; Yue, Z.B.; Hu, Y.H.; Wang, P.X.; Li, J.Y.; Cai, S.D.; et al. MicroRNA-214 contributes to Ang II-induced cardiac hypertrophy by targeting SIRT3 to provoke mitochondrial malfunction. Acta Pharmacol. Sin. 2020, 42, 1422–1436. [Google Scholar] [CrossRef]
- Hall, C.; Gehmlich, K.; Denning, C.; Pavlovic, D. Complex Relationship between Cardiac Fibroblasts and Cardiomyocytes in Health and Disease. J. Am. Heart Assoc. 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Khan, R.; Sheppard, R. Fibrosis in heart disease: Understanding the role of transforming growth factor-β1 in cardiomyopathy, valvular disease and arrhythmia. Immunology 2006, 118, 10–24. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Bang, C.; Batkai, S.; Dangwal, S.; Gupta, S.K.; Foinquinos, A.; Holzmann, A.; Just, A.; Remke, J.; Zimmer, K.; Zeug, A.; et al. Cardiac fibroblast–derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J. Clin. Investig. 2014, 124, 2136. [Google Scholar] [CrossRef] [PubMed]
- Hinkel, R.; Ramanujam, D.; Kaczmarek, V.; Howe, A.; Klett, K.; Beck, C.; Dueck, A.; Thum, T.; Laugwitz, K.L.; Maegdefessel, L.; et al. AntimiR-21 Prevents Myocardial Dysfunction in a Pig Model of Ischemia/Reperfusion Injury. J. Am. Coll. Cardiol. 2020, 75, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Karakikes, I.; Chaanine, A.H.; Kang, S.; Mukete, B.N.; Jeong, D.; Zhang, S.; Hajjar, R.J.; Lebeche, D. Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J. Am. Heart Assoc. 2013, 2, e000078. [Google Scholar] [CrossRef] [Green Version]
- Verjans, R.; Peters, T.; Beaumont, F.J.; Van Leeuwen, R.; Van Herwaarden, T.; Verhesen, W.; Munts, C.; Bijnen, M.; Henkens, M.; Diez, J.; et al. MicroRNA-221/222 family counteracts myocardial fibrosis in pressure overload-induced heart failure. Hypertension 2018, 71, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ. Res. 2002, 91, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. The emerging role of innate immunity in the heart and vascular system: For whom the cell tolls. Circ. Res. 2011, 108, 1133–1145. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 1. [Google Scholar] [CrossRef]
- Ehara, N.; Ono, K.; Morimoto, T.; Kawamura, T.; Abe, M.; Hasegawa, K. The possible role of peroxisome proliferator-activated receptor gamma in heart failure. Exp. Clin. Cardiol. 2004, 9, 169–173. [Google Scholar]
- Wang, J.; Song, Y.; Zhang, Y.; Xiao, H.; Sun, Q.; Hou, N.; Guo, S.; Wang, Y.; Fan, K.; Zhan, D.; et al. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell Res. 2012, 22, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Liu, F.; Zhou, L.Y.; Long, B.; Yuan, S.M.; Wang, Y.; Liu, C.Y.; Sun, T.; Zhang, X.J.; Li, P.F. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ. Res. 2014, 114, 1377–1388. [Google Scholar] [CrossRef] [Green Version]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, Hypertension, and Cardiac Dysfunction: Novel Roles of Immunometabolism in Macrophage Activation and Inflammation. Circ. Res. 2020, 126, 789. [Google Scholar] [CrossRef] [PubMed]
- Heymans, S.; Corsten, M.F.; Verhesen, W.; Carai, P.; Van Leeuwen, R.E.W.; Custers, K.; Peters, T.; Hazebroek, M.; Stöger, L.; Wijnands, E.; et al. Macrophage MicroRNA-155 promotes cardiac hypertrophy and failure. Circulation 2013, 128, 1420–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiojima, I.; Sato, K.; Izumiya, Y.; Schiekofer, S.; Ito, M.; Liao, R.; Colucci, W.S.; Walsh, K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Investig. 2005, 115, 2108–2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banquet, S.; Gomez, E.; Nicol, L.; Edwards-Lévy, F.; Henry, J.P.; Cao, R.; Schapman, D.; Dautreaux, B.; Lallemand, F.; Bauer, F.; et al. Arteriogenic therapy by intramyocardial sustained delivery of a novel growth factor combination prevents chronic heart failure. Circulation 2011, 124, 1059–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huusko, J.; Lottonen, L.; Merentie, M.; Gurzeler, E.; Anisimov, A.; Miyanohara, A.; Alitalo, K.; Tavi, P.; Ylä-Herttuala, S. AAV9-mediated VEGF-B gene transfer improves systolic function in progressive left ventricular hypertrophy. Mol. Ther. 2012, 20, 2212–2221. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wu, J.; You, J.; Shi, H.; Xue, X.; Huang, J.; Xu, L.; Jiang, G.; Yuan, L.; Gong, X.; et al. HSF1 deficiency accelerates the transition from pressure overload-induced cardiac hypertrophy to heart failure through endothelial miR-195a-3p-mediated impairment of cardiac angiogenesis. J. Mol. Cell. Cardiol. 2018, 118, 193–207. [Google Scholar] [CrossRef]
- Peacock, H.; Fucini, R.V.; Jayalath, P.; Ibarra-Soza, J.M.; Haringsma, H.J.; Flanagan, W.M.; Willingham, A.; Beal, P.A. Nucleobase and ribose modifications control immunostimulation by a MicroRNA-122-mimetic RNA. J. Am. Chem. Soc. 2011, 133, 9200–9203. [Google Scholar] [CrossRef] [Green Version]
- Lucas, T.; Dimmeler, S. RNA Therapeutics for Treatment of Cardiovascular Diseases: Promises and Challenges. Circ. Res. 2016, 119, 794–797. [Google Scholar] [CrossRef] [Green Version]
- Geisler, A.; Fechner, H. MicroRNA-regulated viral vectors for gene therapy. World J. Exp. Med. 2016, 6, 37. [Google Scholar] [CrossRef]
- Gabisonia, K.; Prosdocimo, G.; Aquaro, G.D.; Carlucci, L.; Zentilin, L.; Secco, I.; Ali, H.; Braga, L.; Gorgodze, N.; Bernini, F.; et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardialinfarction in pigs. Nature 2019, 569, 418. [Google Scholar] [CrossRef]
- Greenberg, B.; Butler, J.; Felker, G.M.; Ponikowski, P.; Voors, A.A.; Desai, A.S.; Barnard, D.; Bouchard, A.; Jaski, B.; Lyon, A.R.; et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): A randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 2016, 387, 1178–1186. [Google Scholar] [CrossRef]
- Rao, D.D.; Vorhies, J.S.; Senzer, N.; Nemunaitis, J. siRNA vs. shRNA: Similarities and differences. Adv. Drug Deliv. Rev. 2009, 61, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Second RNAi drug approved. Nat. Biotechnol. 2020, 38, 385. [CrossRef] [PubMed] [Green Version]
- Hoy, S.M. Patisiran: First Global Approval. Drugs 2018, 78, 1625–1631. [Google Scholar] [CrossRef]
- FDA Approves First Drug to Treat Rare Metabolic Disorder|FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treat-rare-metabolic-disorder (accessed on 26 October 2021).
- Inclisiran Approved in Europe for Lowering LDL Cholesterol|tctmd.com. Available online: https://www.tctmd.com/news/inclisiran-approved-europe-lowering-ldl-cholesterol (accessed on 21 March 2021).
- FDA Passes on Inclisiran, Citing Manufacturing Site Problems|tctmd.com. Available online: https://www.tctmd.com/news/fda-passes-inclisiran-citing-manufacturing-site-problems (accessed on 21 March 2021).
- Ohrt, T.; Merkle, D.; Birkenfeld, K.; Echeverri, C.J.; Schwille, P. In situ fluorescence analysis demonstrates active siRNA exclusion from the nucleus by Exportin 5. Nucleic Acids Res. 2006, 34, 1369–1380. [Google Scholar] [CrossRef]
- Sliva, K.; Schnierle, B.S. Selective gene silencing by viral delivery of short hairpin RNA. Virol. J. 2010, 7, 248. [Google Scholar] [CrossRef] [Green Version]
- Mathew, V.; Wang, A.K. Inotersen: New promise for the treatment of hereditary transthyretin amyloidosis. Drug Des. Dev. Ther. 2019, 13, 1515–1525. [Google Scholar] [CrossRef] [Green Version]
- Täubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: Results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 2021, 42, 178–188. [Google Scholar] [CrossRef]
- Duygu, B.; Juni, R.; Ottaviani, L.; Bitsch, N.; Wit, J.B.M.; de Windt, L.J.; da Costa Martins, P.A. Comparison of different chemically modified inhibitors of miR-199b in vivo. Biochem. Pharmacol. 2019, 159, 106–115. [Google Scholar] [CrossRef]
- Henry, S.P.; Johnson, M.; Zanardi, T.A.; Fey, R.; Auyeung, D.; Lappin, P.B.; Levin, A.A. Renal uptake and tolerability of a 2’-O-methoxyethyl modified antisense oligonucleotide (ISIS 113715) in monkey. Toxicology 2012, 301, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Philippen, L.E.; Dirkx, E.; Wit, J.B.M.; Burggraaf, K.; de Windt, L.J.; da Costa Martins, P.A. Antisense MicroRNA Therapeutics in Cardiovascular Disease: Quo Vadis? Mol. Ther. 2015, 23, 1810–1818. [Google Scholar] [CrossRef] [PubMed]
- Judge, A.D.; Sood, V.; Shaw, J.R.; Fang, D.; McClintock, K.; MacLachlan, I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005, 23, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Behlke, M.A. Chemical modification of siRNAs for in vivo use. Oligonucleotides 2008, 18, 305–319. [Google Scholar] [CrossRef] [Green Version]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in vivo with “antagomirs”. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.; Propp, S.; Freier, S.M.; Jones, L.E.; Serra, M.J.; Kinberger, G.; Bhat, B.; Swayze, E.E.; Bennett, C.F.; Esau, C. Potent inhibition of microRNA in vivo without degradation. Nucleic Acids Res. 2009, 37, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Swayze, E.E.; Siwkowski, A.M.; Wancewicz, E.V.; Migawa, M.T.; Wyrzykiewicz, T.K.; Hung, G.; Monia, B.P.; Bennett, C.F. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007, 35, 687–700. [Google Scholar] [CrossRef]
- Hinkel, R.; Penzkofer, D.; Zühlke, S.; Fischer, A.; Husada, W.; Xu, Q.F.; Baloch, E.; Van Rooij, E.; Zeiher, A.M.; Kupatt, C.; et al. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation 2013, 128, 1066–1075. [Google Scholar] [CrossRef] [Green Version]
- Juliano, R.L.; Akhtar, S. Liposomes as a Drug Delivery System for Antisense Oligonucleotides. Antisense Res. Dev. 1992, 2, 165–176. [Google Scholar] [CrossRef]
- Wisse, E.; Jacobs, F.; Topal, B.; Frederik, P.; De Geest, B. The size of endothelial fenestrae in human liver sinusoids: Implications for hepatocyte-directed gene transfer. Gene Ther. 2008, 15, 1193–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamerkar, S.; Lebleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, C.H.; Melamed, J.R.; Day, E.S. Spherical Nucleic Acid Nanoparticles: Therapeutic Potential. BioDrugs 2018, 32, 297–309. [Google Scholar] [CrossRef]
- Bellera, N.; Barba, I.; Rodriguez-Sinovas, A.; Ferret, E.; Asín, M.A.; Gonzalez-Alujas, M.T.; Pérez-Rodon, J.; Esteves, M.; Fonseca, C.; Toran, N.; et al. Single intracoronary injection of encapsulated antagomir-92a promotes angiogenesis and prevents adverse infarct remodeling. J. Am. Heart Assoc. 2014, 3, e000946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef]
- Morgan, E.S.; Tami, Y.; Hu, K.; Brambatti, M.; Mullick, A.E.; Geary, R.S.; Bakris, G.L.; Tsimikas, S. Antisense Inhibition of Angiotensinogen With IONIS-AGT-LRx: Results of Phase 1 and Phase 2 Studies. JACC Basic Transl Sci. 2021, 6, 485–496. [Google Scholar] [CrossRef]
- Hirt, M.N.; Hansen, A.; Eschenhagen, T. Cardiac tissue engineering: State of the art. Circ. Res. 2014, 114, 354–367. [Google Scholar] [CrossRef] [Green Version]
- Perbellini, F.; Thum, T. Living myocardial slices: A novel multicellular model for cardiac translational research. Eur. Heart J. 2020, 41, 2405–2408. [Google Scholar] [CrossRef]
- Huang, C.K.; Kafert-Kasting, S.; Thum, T. Preclinical and Clinical Development of Noncoding RNA Therapeutics for Cardiovascular Disease. Circ. Res. 2020, 126, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ruderisch, H.; Queirós, A.M.; Fliegner, D.; Eschen, C.; Kararigas, G.; Regitz-Zagrosek, V. Sex-specific regulation of cardiac microRNAs targeting mitochondrial proteins in pressure overload. Biol. Sex. Differ. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| ncRNA | Mechanism of Action | Modulation Strategy (In Vivo) | Ref |
|---|---|---|---|
| miR-92b-3p | ↓ MEF2D | agomiR-92b-3p (↑) | [38] |
| miR-106~25 cluster | ↓ MEF2D + Hand2 | AAV9-miR-106b~25 (↑) AntagomiR- (-106b, -93, -25) (↓) | [40] |
| miR-145-5p | ↓ ATF3 (↑ pro-hyp genes) | ad-circNfix (↑) | [41] |
| circNfix | Sponge for miR-145-5p (↑ ATF3; ↓ pro-hyp genes) | ||
| lncRNA Mhrt | ↓ Chromatin Remodeling; (↓ pro-hyp genes) | Mhrt779 transgenic mice (↑) | [43] |
| lncRNA Chaer | ↓ EZH2; ↓ H3K27me (↑ pro-hyp genes) | siChaer (↓) Chaer KO mice (↓) | [44] |
| ncRNA | Mechanism of Action | In Vivo Delivery or Gain/Loss of Function Strategy | Ref |
|---|---|---|---|
| Calcineurin/NFAT Signaling | |||
| miR-1 | ↓ Cn/NFAT SP 1 | Ad-miR-1 (↑) | [50] |
| miR-133 | antagomiR-133 (↓) Ad133 (↑) | [52] | |
| circSLC8A1-1 | Sponge for miR-133 (↑ Cn/NFAT SP) | AAV9-circSlc8a1 (↑) AAV9-sh-circSlc8a1 (↓) | [54] |
| miR-182 | ↑ Cn/NFAT SP | miR-182 agomir (↑) CHO-PGEA/miR-182 (↑) CHO-PGEA/miR-182-in (↓) | [58] [59] |
| miR-23a | antagomiR-23a (↓) | [60] | |
| miR-212/132 | antagomiR-132 (↓) | [61] | |
| miR-132 | antimiR-132 (↓) | [62] | |
| miR-199b | antagomiR-199b (↓) | [56] | |
| piRNA CHAPIR | ↓ m6A methylation of Parp10 (↑ Cn/NFAT SP) | CHAPIR mimic (↑) CHAPIR antagomiR (↓) | [34] |
| CaMKII Signaling | |||
| miR-675 | ↓ CamKII | antagomiR-675 (↓) | [65] |
| lncRNA H19 | Sponge for miR-675 (↑ CamKII) | ||
| lncRNA TINCR | ↑ EZH2; ↑ H3K27me3 (↓ CamKII) | Lentivirus pcDNA-TINCR (↑) | [66] |
| miR-214 | ↓ EZH2 | antagomiR-214 (↓) | [67] |
| MAPK Signaling | |||
| miR-378 | ↓ MAPK SP | AgomiR-378 (↑) AntagomiR (↓) AAV9–miR-378 (↑) | [68,69] |
| miR-499 | ↑ MAPK SP | miR-499 transgenic mice (↑) | [70] |
| Akt Signaling | |||
| lncRNA CHAR | Sponge for miR-20b | Lenti-CHAR (↑) Lenti-sh-CHAR (↓) | [71] |
| miR-20b | ↓ PTEN; ↑ Akt SP | ||
| miR-21 | antagomiR-21 (↓) | [72] | |
| miR-217 | rAAV9-miR-217 (↑) rAAV9-miR-217-TUD (↓) | [73] | |
| JAK/STAT Signaling | |||
| miR-148a | ↓JAK/STAT SP | AAV9-148a (↑) AntagomiR-148a-3p (↓) | [74] |
| Wnt Signaling | |||
| miR-29 | ↑ Wnt SP | AAV9-miR-29a (↑) antimiR-29 (↓) | [75,77] |
| ncRNA | Mechanism of Action | In Vivo Delivery or Gain/Loss of Function Strategy | Ref |
|---|---|---|---|
| Cardiomyocyte remodeling and loss | |||
| mir-183-5p | ↓ Apoptosis | miR-183 mimics (↑) ASO inhibitor (↓) | [78] |
| miR-223 | ↑ Apoptosis | Ad-HRCR (↑) | [79] |
| HRCR | Sponge for miR-223; ↓ Apoptosis | ||
| lncRNA Chast | Regulation of the expression of adjacent protein (Plekhm1) (↓ Autophagy) | AAV9-Chast (↑) LNA GapmeR-Chast (↓) | [82] |
| miR-30b-3p | ↓ Autophagy | AAV9-sh-Gm15834 (↓) | [84] |
| Gm15834 | Sponge for miR-30b-3p (↑ Autophagy) | ||
| Calcium Handling | |||
| miR-146a | ↓ SERCA2a SUMOylation; (↓ calcium handling) | rAAV9_premir-146a (↑) rAAV9_decoy-146a (↓) | [87] |
| miR-328 | ↓ SERCA2a (↓ calcium handling) | LNA-antimiR-328 (↓) | [88] |
| miR-25 | AAV9-miR-25 (↑) Anti-miR-25 (↓) | [89] | |
| lncRNA MIAT | MIAT KO mice | [90] | |
| miR-185-3p | ↓ CaSR; (↑ calcium handling) | ad-si-circHIPK3 (↓) | [91] |
| circHIPK3 | Sponge for miR-185-3p; ↑ CaSR (↓ calcium handling) | ||
| Metabolism | |||
| miR-27b-3p | ↓ FGF1; (↓ mitochondrial metabolism) | miR-27b-3p KO mice (↓) | [96] |
| miR-214 | ↓ SIRT3; (↓ mitochondrial metabolism) | agomiR-214 (↑) antagomiR-214 (↓) | [97] |
| Fibrosis | |||
| miR-21 | ↑ Akt SP (↑ fibrosis) | antagomiR-21 (↓) | [72] |
| ↑ MAPK SP (↑ fibrosis) | [100] | ||
| ↓ SORBS2 ↓ PDLIM5 | [101] | ||
| miR-1 | ↓ fibrosis | AAV9-miR-1 (↑) | [103] |
| miR-221/222 | ↓ fibrosis | AntagomiRs (↓) | [104] |
| Inflammation | |||
| miR-27b | ↑ TNF-α (↑ Inflammation) | antagomiR-27b (↓) | [109] |
| miR-489 | ↓ Myd88 (↓ Inflammation) | miR-489-3p mimic (↑) antagomiR-489 (↓) | [110] |
| lncRNA CHRF | Sponge for miR-489 (↑ Inflammation) | Adenoviral CHRF (↑) Adenoviral CHRF siRNA (↓) | |
| miR-155 | ↓ SOCS1 (↑ Inflammation) | miR-155 KO mice (↓) | [112] |
| Angiogenesis | |||
| miR-195-3p | ↓ angiogenesis | miR-195a-3p mimic (↑) miR-195a-3p antagomiR (↓) | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.; da Costa Martins, P.A. Non-Coding RNAs in the Therapeutic Landscape of Pathological Cardiac Hypertrophy. Cells 2022, 11, 1805. https://doi.org/10.3390/cells11111805
Silva J, da Costa Martins PA. Non-Coding RNAs in the Therapeutic Landscape of Pathological Cardiac Hypertrophy. Cells. 2022; 11(11):1805. https://doi.org/10.3390/cells11111805
Chicago/Turabian StyleSilva, Joana, and Paula A. da Costa Martins. 2022. "Non-Coding RNAs in the Therapeutic Landscape of Pathological Cardiac Hypertrophy" Cells 11, no. 11: 1805. https://doi.org/10.3390/cells11111805
APA StyleSilva, J., & da Costa Martins, P. A. (2022). Non-Coding RNAs in the Therapeutic Landscape of Pathological Cardiac Hypertrophy. Cells, 11(11), 1805. https://doi.org/10.3390/cells11111805






