Vitronectin and Its Interaction with PAI-1 Suggests a Functional Link to Vascular Changes in AMD Pathobiology
Abstract
:1. Introduction
2. Material and Methods
2.1. Description of Datasets
2.2. Association Analysis
2.3. Linear Regression Analysis of Gene Expression
2.4. Cell Culture
2.5. RNA Isolation and Reverse Transcription (RT) into Complementary DNA (cDNA)
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Expression Constructs
2.8. Purification of the Recombinant Proteins PAI-1 and Vitronectin
2.9. SDS-PAGE and Western Blot Analysis
2.10. Antibodies
2.11. Affinity Chromatography of Vitronectin Isoforms to Immobilized PAI-1
2.12. PAI-1 Activity Assay
2.13. Endogenous PAI-1 Expression and Secretion by Vascular Endothelial Cells after Exposure to Recombinant Vitronectin Isoforms
2.14. Analysis of Vitronectin-Dependent Deposition of Endogenous PAI-1 in ARPE19-Derived ECM
2.15. Statistical Analysis
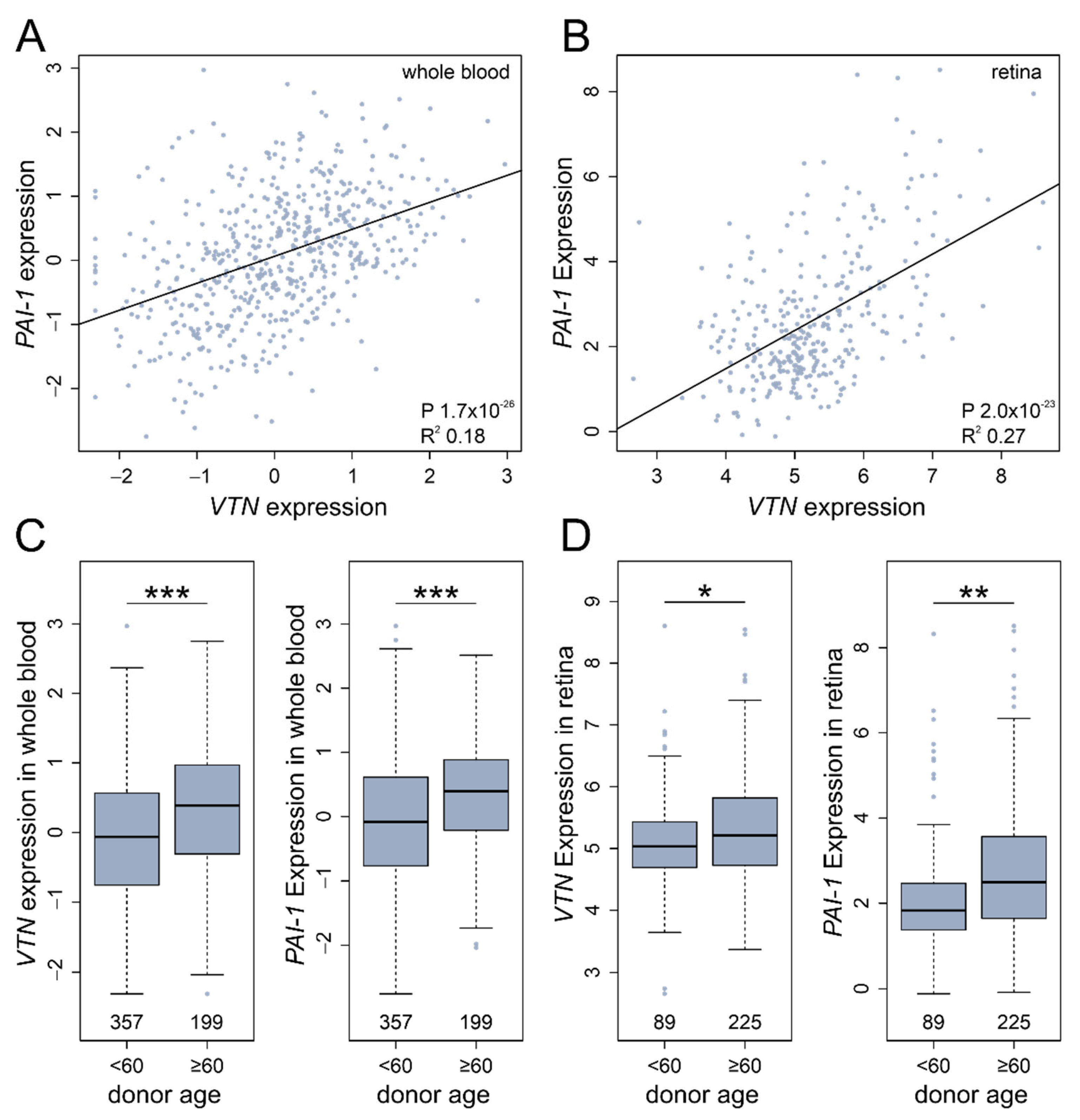
3. Results
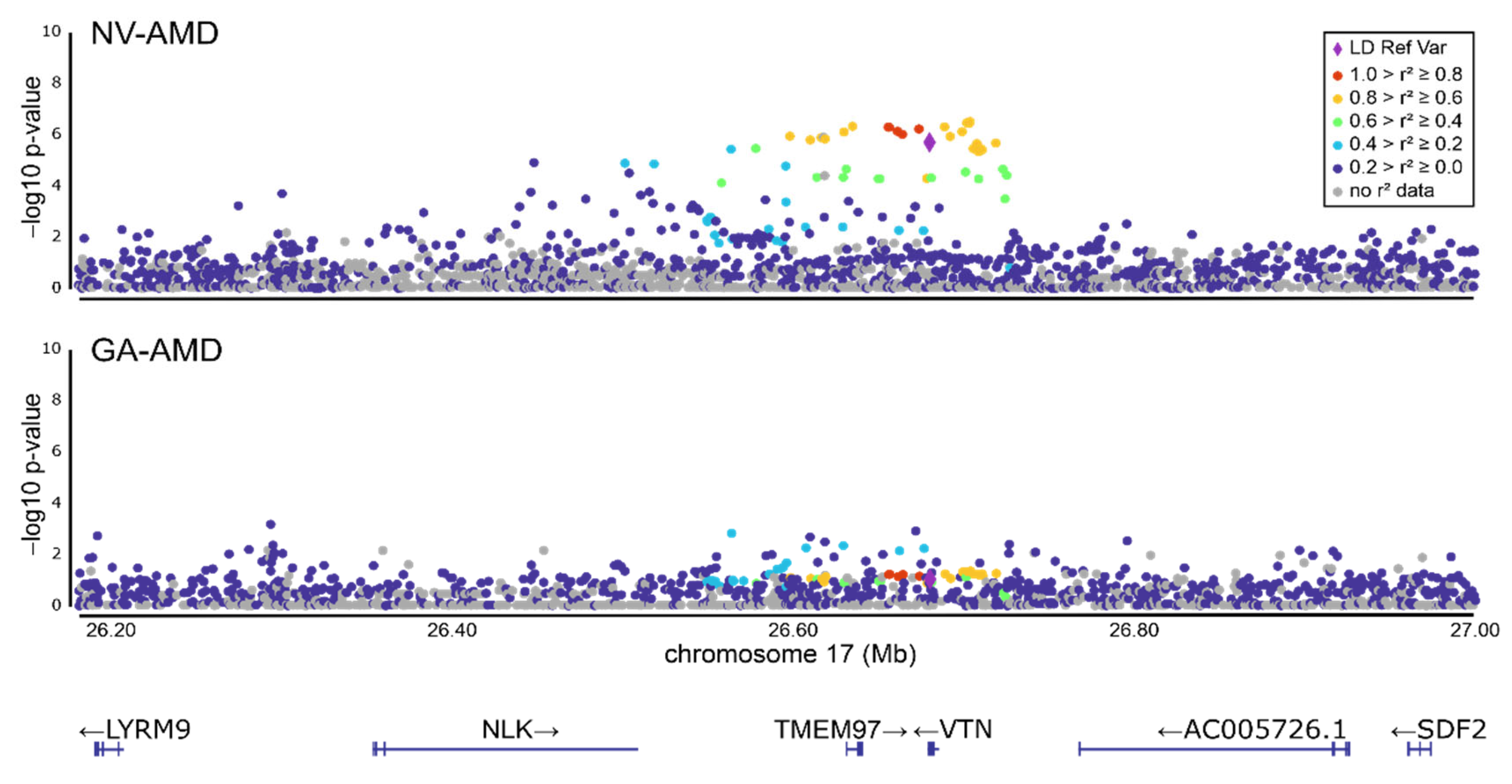
3.1. Refinement of the AMD-Associated rs704 Signal at the VTN Gene Locus by Subgroup Analysis
3.2. Functional Relationship between Vitronectin and Angiogenesis Regulator PAI-1
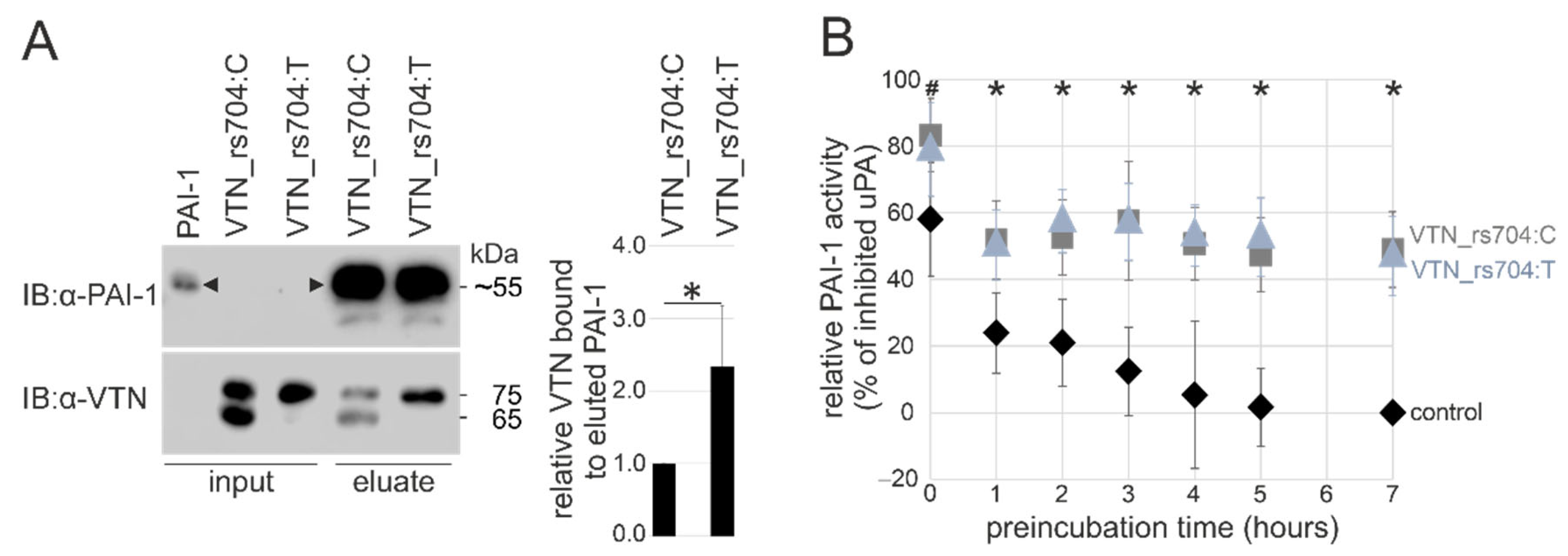
3.3. Genetic Variant rs704 and Vitronectin Binding to PAI-1
3.4. Genetic Variant rs704 and the Capacity of Vitronectin to Stabilize PAI-1 Activity
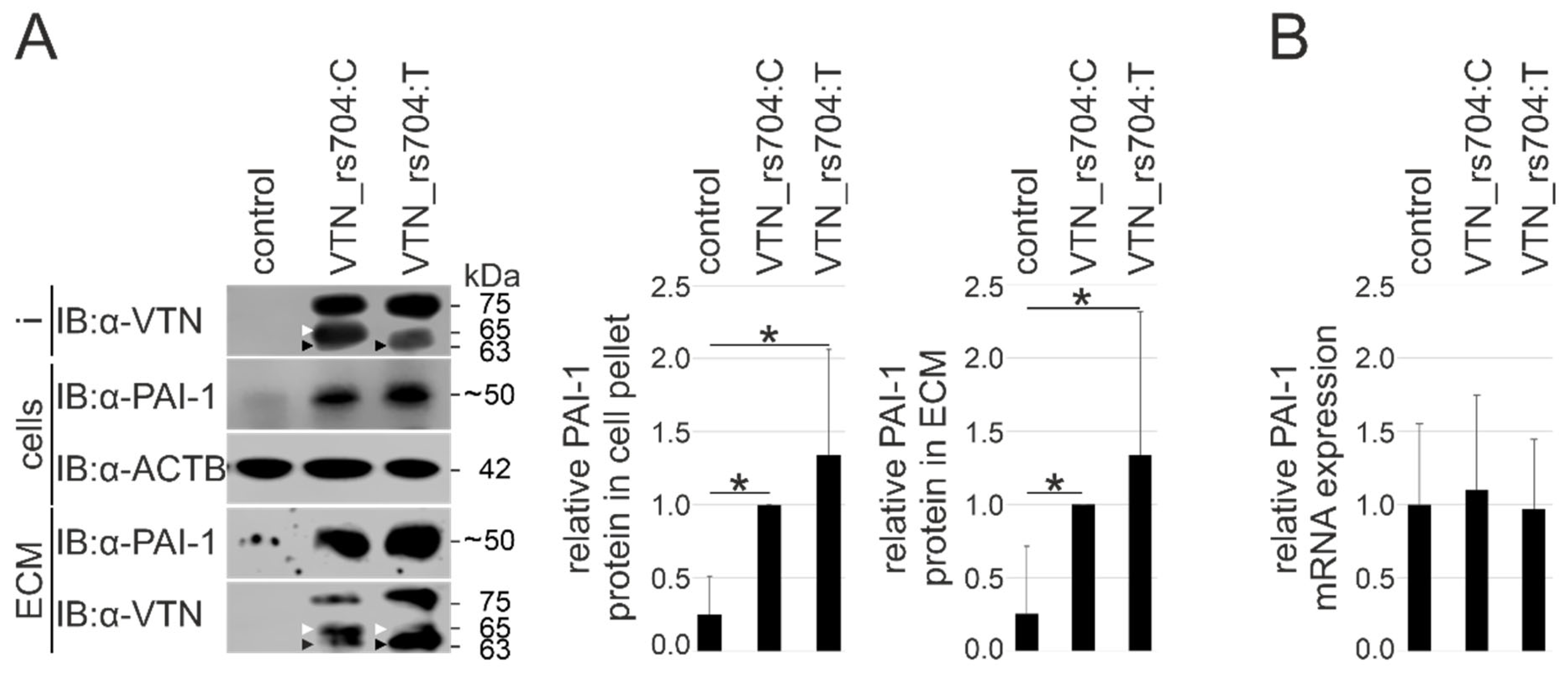
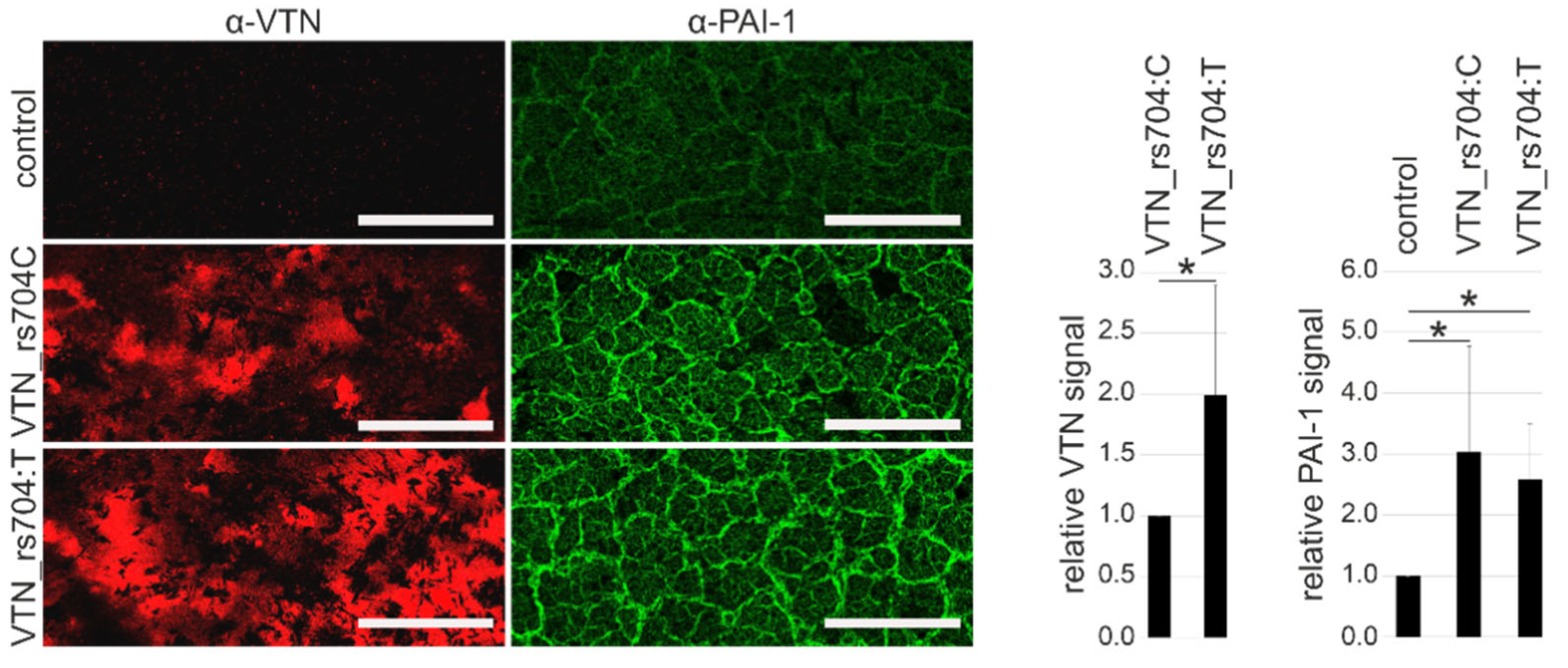
3.5. Vitronectin and Cellular PAI-1 Protein Expression and ECM Deposition
3.6. In Silico VTN and PAI-1 Expression Profiles
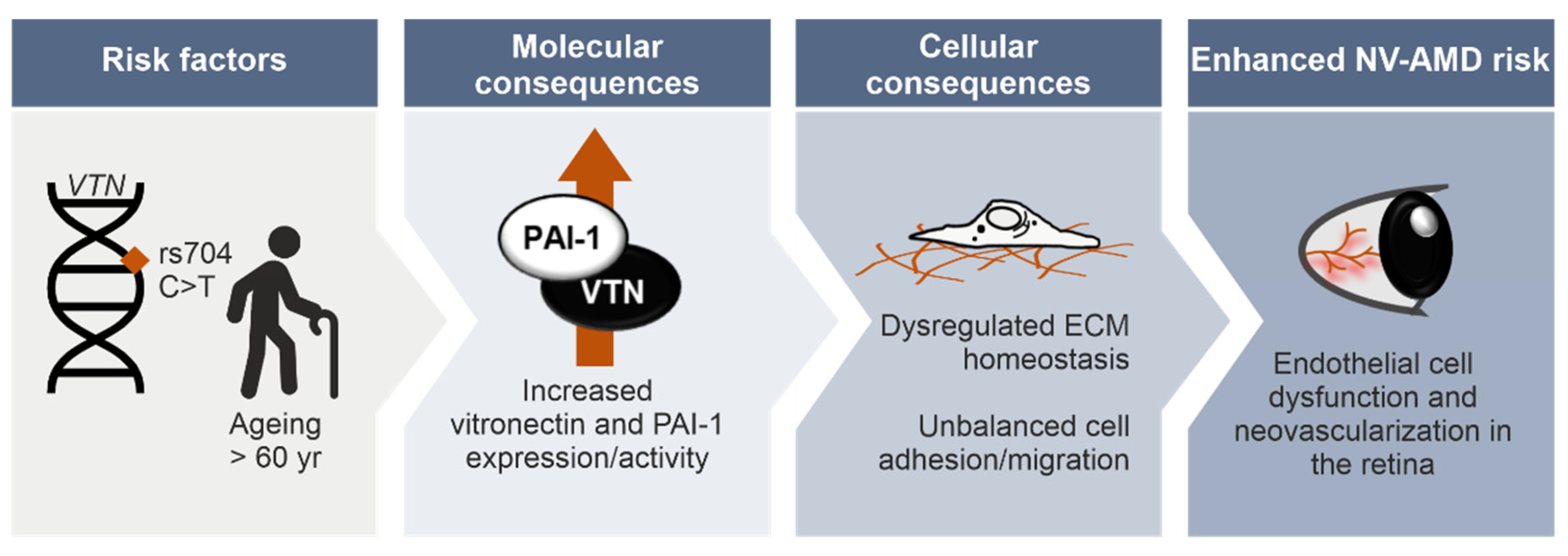
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bedell, M.; Zhang, K. Age-related macular degeneration: Genetic and environmental factors of disease. Mol. Interv. 2010, 10, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.H.; Hageman, G.S.; Mullins, R.F.; Neitz, M.; Neitz, J.; Ozaki, S.; Preissner, K.T.; Johnson, L.V. Vitronectin gene expression in the adult human retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 3305–3315. [Google Scholar]
- Dejana, E.; Conforti, G.; Zanetti, A.; Lampugnani, M.G.; Marchisio, P.C. Receptors for Extracellular Matrix Proteins in Endothelial Cells. In Vascular Endothelium; Catravas, J.D., Gillis, C.N., Ryan, U.S., Eds.; Springer: Boston, MA, USA, 1989; pp. 141–147. [Google Scholar]
- Curcio, C.A.; Johnson, M. Structure, Function, and Pathology of Bruch’s Membrane; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 1, p. 17. [Google Scholar]
- Biasella, F.; Plössl, K.; Karl, C.; Weber, B.H.F.; Friedrich, U. Altered Protein Function Caused by AMD-associated Variant rs704 Links Vitronectin to Disease Pathology. Investig. Ophthalmol. Vis. Sci. 2020, 61, 2. [Google Scholar] [CrossRef] [PubMed]
- Vial, D.; McKeown-Longo, P.J. PAI1 stimulates assembly of the fibronectin matrix in osteosarcoma cells through crosstalk between the alphavbeta5 and alpha5beta1 integrins. J. Cell Sci. 2008, 121, 1661–1670. [Google Scholar] [CrossRef] [Green Version]
- Zhou, A.; Huntington, J.A.; Pannu, N.S.; Carrell, R.W.; Read, R.J. How vitronectin binds PAI-1 to modulate fibrinolysis and cell migration. Nat. Struct. Biol. 2003, 10, 541–544. [Google Scholar] [CrossRef]
- Madsen, C.D.; Ferraris, G.M.; Andolfo, A.; Cunningham, O.; Sidenius, N. uPAR-induced cell adhesion and migration: Vitronectin provides the key. J. Cell Biol. 2007, 177, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Sen, A.; Ta, M. Vitronectin acts as a key regulator of adhesion and migration in umbilical cord-derived MSCs under different stress conditions. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zuchtriegel, G.; Uhl, B.; Pick, R.; Ramsauer, M.; Dominik, J.; Mittmann, L.A.; Canis, M.; Kanse, S.; Sperandio, M.; Krombach, F.; et al. Vitronectin stabilizes intravascular adhesion of neutrophils by coordinating β2 integrin clustering. Haematologica 2021, 106, 2641–2653. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Luo, M.; Ren, M.; Chen, N.; Xia, J.; Deng, X.; Zeng, M.; Yan, K.; Luo, T.; Wu, J. Vitronectin regulation of vascular endothelial growth factor-mediated angiogenesis. J. Vasc. Res. 2014, 51, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Preissner, K.T.; Reuning, U. Vitronectin in vascular context: Facets of a multitalented matricellular protein. Semin. Thromb. Hemost. 2011, 37, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Hageman, G.S.; Mullins, R.F.; Russell, S.R.; Johnson, L.V.; Anderson, D.H. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB J. 1999, 13, 477–484. [Google Scholar] [CrossRef]
- Rudolf, M.; Malek, G.; Messinger, J.D.; Clark, M.E.; Wang, L.; Curcio, C.A. Sub-retinal drusenoid deposits in human retina: Organization and composition. Exp. Eye Res. 2008, 87, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Clark, M.E.; Crossman, D.K.; Kojima, K.; Messinger, J.D.; Mobley, J.A.; Curcio, C.A. Abundant lipid and protein components of drusen. PLoS ONE 2010, 5, e10329. [Google Scholar] [CrossRef]
- Shin, K.; Kent, J.E.; Singh, C.; Fujimoto, L.M.; Yu, J.; Tian, Y.; Im, W.; Marassi, F.M. Calcium and hydroxyapatite binding site of human vitronectin provides insights to abnormal deposit formation. Proc. Natl. Acad. Sci. USA 2020, 117, 18504–18510. [Google Scholar] [CrossRef]
- Aguet, F.; Anand, S.; Ardlie, K.G.; Gabriel, S.; Getz, G.A.; Graubert, A.; Hadley, K.; Handsaker, R.E.; Huang, K.H.; Kashin, S.; et al. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- Strunz, T.; Kellner, M.; Kiel, C.; Weber, B.H.F. Assigning Co-Regulated Human Genes and Regulatory Gene Clusters. Cells 2021, 10, 2395. [Google Scholar] [CrossRef]
- Strunz, T.; Kiel, C.; Grassmann, F.; Ratnapriya, R.; Kwicklis, M.; Karlstetter, M.; Fauser, S.; Arend, N.; Swaroop, A.; Langmann, T.; et al. A mega-analysis of expression quantitative trait loci in retinal tissue. PLoS Genet. 2020, 16, e1008934. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 15 February 2022).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Pollard, K.; Dudoit, S.; Van Der Laan, M. Multiple Testing Procedures: The multtest Package and Applications to Genomics. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Statistics for Biology and Health; Gentleman, R., Carey, V.J., Huber, W., Irizarry, R.A., Dudoit, S., Eds.; Springer: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Pruim, R.J.; Welch, R.P.; Sanna, S.; Teslovich, T.M.; Chines, P.S.; Gliedt, T.P.; Boehnke, M.; Abecasis, G.R.; Willer, C.J. LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics 2010, 26, 2336–2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- My.LocusZoom.org. Available online: https://my.locuszoom.org/ (accessed on 20 October 2021).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kingston, R.E.; Chen, C.A.; Okayama, H. Calcium phosphate transfection. Curr. Protoc. Immunol. 2001, 10, 10–13. [Google Scholar] [CrossRef]
- Zeheb, R.; Rafferty, U.M.; Rodriguez, M.A.; Andreasen, P.; Gelehrter, T.D. Immunoaffinity purification of HTC rat hepatoma cell plasminogen activator-inhibitor-1. Thromb. Haemost. 1987, 58, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Le Magueresse-Battistoni, B.; Pernod, G.; Sigillo, F.; Kolodié, L.; Benahmed, M. Plasminogen activator inhibitor-1 is expressed in cultured rat Sertoli cells. Biol. Reprod. 1998, 59, 591–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tollefsen, D.M.; Weigel, C.J.; Kabeer, M.H. The presence of methionine or threonine at position 381 in vitronectin is correlated with proteolytic cleavage at arginine 379. J. Biol. Chem. 1990, 265, 9778–9781. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Friedrich, U.; Myers, C.A.; Fritsche, L.G.; Milenkovich, A.; Wolf, A.; Corbo, J.C.; Weber, B.H. Risk- and non-risk-associated variants at the 10q26 AMD locus influence ARMS2 mRNA expression but exclude pathogenic effects due to protein deficiency. Hum. Mol. Genet. 2011, 20, 1387–1399. [Google Scholar] [CrossRef]
- Plössl, K.; Weber, B.H.; Friedrich, U. The X-linked juvenile retinoschisis protein retinoschisin is a novel regulator of mitogen-activated protein kinase signalling and apoptosis in the retina. J. Cell. Mol. Med. 2017, 21, 768–780. [Google Scholar] [CrossRef] [Green Version]
- Sillen, M.; Declerck, P.J. Targeting PAI-1 in Cardiovascular Disease: Structural Insights Into PAI-1 Functionality and Inhibition. Front. Cardiovasc. Med. 2020, 7, 622473. [Google Scholar] [CrossRef] [PubMed]
- McLenachan, S.; Hao, E.; Zhang, D.; Zhang, L.; Edel, M.; Chen, F. Bioengineered Bruch’s-like extracellular matrix promotes retinal pigment epithelial differentiation. Biochem. Biophys. Rep. 2017, 10, 178–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandl, C.; Zimmermann, S.J.; Milenkovic, V.M.; Rosendahl, S.M.; Grassmann, F.; Milenkovic, A.; Hehr, U.; Federlin, M.; Wetzel, C.H.; Helbig, H.; et al. In-depth characterisation of Retinal Pigment Epithelium (RPE) cells derived from human induced pluripotent stem cells (hiPSC). Neuromol. Med. 2014, 16, 551–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambati, J.; Fowler, B.J. Mechanisms of Age-Related Macular Degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Declerck, P.J.; De Mol, M.; Alessi, M.C.; Baudner, S.; Pâques, E.P.; Preissner, K.T.; Müller-Berghaus, G.; Collen, D. Purification and characterization of a plasminogen activator inhibitor 1 binding protein from human plasma. Identification as a multimeric form of S protein (vitronectin). J. Biol. Chem. 1988, 263, 15454–15461. [Google Scholar] [CrossRef]
- Sillen, M.; Declerck, P.J. A Narrative Review on Plasminogen Activator Inhibitor-1 and Its (Patho)Physiological Role: To Target or Not to Target? Int. J. Mol. Sci. 2021, 22, 2721. [Google Scholar] [CrossRef]
- Lambert, V.; Munaut, C.; Noël, A.; Frankenne, F.; Bajou, K.; Gerard, R.; Carmeliet, P.; Defresne, M.P.; Foidart, J.M.; Rakic, J.M. Influence of plasminogen activator inhibitor type 1 on choroidal neovascularization. FASEB J. 2001, 15, 1021–1027. [Google Scholar] [CrossRef] [Green Version]
- Lambert, V.; Munaut, C.; Carmeliet, P.; Gerard, R.D.; Declerck, P.J.; Gils, A.; Claes, C.; Foidart, J.M.; Noël, A.; Rakic, J.M. Dose-dependent modulation of choroidal neovascularization by plasminogen activator inhibitor type I: Implications for clinical trials. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2791–2797. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.; Risk, K. Evaluation of Homocysteine and Plasminogen Activator Inhibitor-1 Changes in Age Related Macular Degeneration. Bull. Egypt. Soc. Physiol. Sci. 2008, 28, 253–264. [Google Scholar] [CrossRef]
- Lin, Z.; Jiang, L.; Yuan, C.; Jensen, J.K.; Zhang, X.; Luo, Z.; Furie, B.C.; Furie, B.; Andreasen, P.A.; Huang, M. Structural basis for recognition of urokinase-type plasminogen activator by plasminogen activator inhibitor-1. J. Biol. Chem. 2011, 286, 7027–7032. [Google Scholar] [CrossRef] [Green Version]
- van Mourik, J.A.; Lawrence, D.A.; Loskutoff, D.J. Purification of an inhibitor of plasminogen activator (antiactivator) synthesized by endothelial cells. J. Biol. Chem. 1984, 259, 14914–14921. [Google Scholar] [CrossRef]
- Andreasen, P.A.; Nielsen, L.S.; Kristensen, P.; Grøndahl-Hansen, J.; Skriver, L.; Danø, K. Plasminogen activator inhibitor from human fibrosarcoma cells binds urokinase-type plasminogen activator, but not its proenzyme. J. Biol. Chem. 1986, 261, 7644–7651. [Google Scholar] [CrossRef]
- Philips, M.; Juul, A.G.; Thorsen, S. Human endothelial cells produce a plasminogen activator inhibitor and a tissue-type plasminogen activator-inhibitor complex. Biochim. Biophys. Acta 1984, 802, 99–110. [Google Scholar] [CrossRef]
- Simpson, A.J.; Booth, N.A.; Moore, N.R.; Bennett, B. Distribution of plasminogen activator inhibitor (PAI-1) in tissues. J. Clin. Pathol. 1991, 44, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chain, D.; Kreizman, T.; Shapira, H.; Shaltiel, S. Plasmin cleavage of vitronectin. Identification of the site and consequent attenuation in binding plasminogen activator inhibitor-1. FEBS Lett. 1991, 285, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996, 62, 155–169. [Google Scholar] [CrossRef]
- Kiel, C.; Strunz, T.; International Amd Genomics Consortium Project Manager Susan Blanton; Grassmann, F.; Weber, B.H.F. Pleiotropic Locus 15q24.1 Reveals a Gender-Specific Association with Neovascular but Not Atrophic Age-Related Macular Degeneration (AMD). Cells 2020, 9, 2257. [Google Scholar] [CrossRef]
- Huang, J.S.; Lin, C.M.; Cheng, Y.C.; Hung, K.L.; Chien, C.C.; Chen, S.K.; Chang, C.J.; Chen, C.W.; Huang, C.J. A vitronectin M381T polymorphism increases risk of hemangioblastoma in patients with VHL gene defect. J. Mol. Med. 2009, 87, 613–622. [Google Scholar] [CrossRef]
- Hammes, H.P.; Brownlee, M.; Jonczyk, A.; Sutter, A.; Preissner, K.T. Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat. Med. 1996, 2, 529–533. [Google Scholar] [CrossRef]
- Mahabeleshwar, G.H.; Feng, W.; Reddy, K.; Plow, E.F.; Byzova, T.V. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ. Res. 2007, 101, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Bafetti, L.M.; Young, T.N.; Itoh, Y.; Stack, M.S. Intact vitronectin induces matrix metalloproteinase-2 and tissue inhibitor of metalloproteinases-2 expression and enhanced cellular invasion by melanoma cells. J. Biol. Chem. 1998, 273, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devy, L.; Blacher, S.; Grignet-Debrus, C.; Bajou, K.; Masson, V.; Gerard, R.D.; Gils, A.; Carmeliet, G.; Carmeliet, P.; Declerck, P.J.; et al. The pro- or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. FASEB J. 2002, 16, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, A.; Menicucci, G.; Maestas, J.; Das, A.; McGuire, P. Plasminogen activator inhibitor-1 (PAI-1) facilitates retinal angiogenesis in a model of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4974–4981. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.B.; Spoerri, P.E.; Player, D.W.; Bush, D.M.; Ellis, E.A.; Caballero, S.; Robison, W.G. Plasminogen activator inhibitor (PAI)-1 overexpression in retinal microvessels of PAI-1 transgenic mice. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2296–2302. [Google Scholar]
- Stefánsson, E.; Geirsdóttir, A.; Sigurdsson, H. Metabolic physiology in age related macular degeneration. Prog. Retin. Eye Res. 2011, 30, 72–80. [Google Scholar] [CrossRef]
- Chirco, K.R.; Sohn, E.H.; Stone, E.M.; Tucker, B.A.; Mullins, R.F. Structural and molecular changes in the aging choroid: Implications for age-related macular degeneration. Eye 2017, 31, 10–25. [Google Scholar] [CrossRef]
- Yeo, N.J.Y.; Chan, E.J.J.; Cheung, C. Choroidal Neovascularization: Mechanisms of Endothelial Dysfunction. Front. Pharmacol. 2019, 10, 1363. [Google Scholar] [CrossRef] [Green Version]
- Isogai, C.; Laug, W.E.; Shimada, H.; Declerck, P.J.; Stins, M.F.; Durden, D.L.; Erdreich-Epstein, A.; DeClerck, Y.A. Plasminogen activator inhibitor-1 promotes angiogenesis by stimulating endothelial cell migration toward fibronectin. Cancer Res. 2001, 61, 5587–5594. [Google Scholar]
- Wu, J.; Strawn, T.L.; Luo, M.; Wang, L.; Li, R.; Ren, M.; Xia, J.; Zhang, Z.; Ma, W.; Luo, T.; et al. Plasminogen activator inhibitor-1 inhibits angiogenic signaling by uncoupling vascular endothelial growth factor receptor-2-αVβ3 integrin cross talk. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Czekay, R.P.; Loskutoff, D.J. Plasminogen activator inhibitors regulate cell adhesion through a uPAR-dependent mechanism. J. Cell. Physiol. 2009, 220, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Gibson, A.D.; Peterson, C.B. Full-length and truncated forms of vitronectin provide insight into effects of proteolytic processing on function. Biochim. Biophys. Acta 2001, 1545, 289–304. [Google Scholar] [CrossRef]
- Barnes, D.W.; Silnutzer, J. Isolation of human serum spreading factor. J. Biol. Chem. 1983, 258, 12548–12552. [Google Scholar] [CrossRef]
- Hayman, E.G.; Pierschbacher, M.D.; Ohgren, Y.; Ruoslahti, E. Serum spreading factor (vitronectin) is present at the cell surface and in tissues. Proc. Natl. Acad. Sci. USA 1983, 80, 4003–4007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chain, D.; Korc-Grodzicki, B.; Kreizman, T.; Shaltiel, S. The phosphorylation of the two-chain form of vitronectin by protein kinase A is heparin dependent. FEBS Lett. 1990, 269, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Seiffert, D.; Loskutoff, D.J. Type 1 plasminogen activator inhibitor induces multimerization of plasma vitronectin. A suggested mechanism for the generation of the tissue form of vitronectin in vivo. J. Biol. Chem. 1996, 271, 29644–29651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minor, K.H.; Peterson, C.B. Plasminogen activator inhibitor type 1 promotes the self-association of vitronectin into complexes exhibiting altered incorporation into the extracellular matrix. J. Biol. Chem. 2002, 277, 10337–10345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickey, S.W.; Baker, R.P.; Cho, S.; Urban, S. Proteolysis inside the membrane is a rate-governed reaction not driven by substrate affinity. Cell 2013, 155, 1270–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grulich-Henn, J.; Mullerberghaus, G.; Preissner, K.T. The influence of growth substratum and cell activation on the deposition of plasminogen activator inhibitor-1 in the extracellular matrix of human endothelial cells. Fibrinolysis 1992, 6, 131–137. [Google Scholar]
- Underwood, P.A.; Bean, P.A. The Effect of Vitronectin and Other Extracellular Matrix Molecules on Endothelial Expansion and Plasminogen Activation. Cells Mater. 1996, 6, 20. [Google Scholar]
- Vogel, C.; Abreu Rde, S.; Ko, D.; Le, S.Y.; Shapiro, B.A.; Burns, S.C.; Sandhu, D.; Boutz, D.R.; Marcotte, E.M.; Penalva, L.O. Sequence signatures and mRNA concentration can explain two-thirds of protein abundance variation in a human cell line. Mol. Syst. Biol. 2010, 6, 400. [Google Scholar] [CrossRef]
- Lee, M.J.; Yaffe, M.B. Protein Regulation in Signal Transduction. Cold Spring Harb. Perspect. Biol. 2016, 8, a005918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, D.I.; Xu, H.; Vaughan, D.E. Cathepsin D-like aspartyl protease activity mediates the degradation of tissue-type plasminogen activator/plasminogen activator inhibitor-1 complexes in human monocytes. Biochim. Biophys. Acta 1995, 1268, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Seiffert, D.; Mimuro, J.; Schleef, R.R.; Loskutoff, D.J. Interactions between type 1 plasminogen activator inhibitor, extracellular matrix and vitronectin. Cell Differ. Dev. 1990, 32, 287–292. [Google Scholar] [CrossRef]
- Olson, D.; Pöllänen, J.; Høyer-Hansen, G.; Rønne, E.; Sakaguchi, K.; Wun, T.C.; Appella, E.; Danø, K.; Blasi, F. Internalization of the urokinase-plasminogen activator inhibitor type-1 complex is mediated by the urokinase receptor. J. Biol. Chem. 1992, 267, 9129–9133. [Google Scholar] [CrossRef]
- Gettins, P.G.; Dolmer, K. The High Affinity Binding Site on Plasminogen Activator Inhibitor-1 (PAI-1) for the Low Density Lipoprotein Receptor-related Protein (LRP1) Is Composed of Four Basic Residues. J. Biol. Chem. 2016, 291, 800–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.J.; Ito, S.; Kai, H.; Nagamine, K.; Nagai, N.; Nishizawa, M.; Abe, T.; Kaji, H. Microfluidic co-cultures of retinal pigment epithelial cells and vascular endothelial cells to investigate choroidal angiogenesis. Sci. Rep. 2017, 7, 3538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Chen, C.; Guo, W.; Liu, K.; Zhao, Q.; Lu, P.; Yu, F.; Xu, X. EFEMP1 Overexpression Contributes to Neovascularization in Age-Related Macular Degeneration. Front. Pharmacol. 2020, 11, 547436. [Google Scholar] [CrossRef]
- Latifi-Navid, H.; Soheili, Z.S.; Samiei, S.; Sadeghi, M.; Taghizadeh, S.; Pirmardan, E.R.; Ahmadieh, H. Network analysis and the impact of Aflibercept on specific mediators of angiogenesis in HUVEC cells. J. Cell. Mol. Med. 2021, 25, 8285–8299. [Google Scholar] [CrossRef]
- Lou, D.A.; Hu, F.N. Specific antigen and organelle expression of a long-term rhesus endothelial cell line. Vitr. Cell. Dev. Biol. J. Tissue Cult. Assoc. 1987, 23, 75–85. [Google Scholar] [CrossRef]
- Canfield, A.E.; Schor, A.M.; Loskutoff, D.J.; Schor, S.L.; Grant, M.E. Plasminogen activator inhibitor-type I is a major biosynthetic product of retinal microvascular endothelial cells and pericytes in culture. Biochem. J. 1989, 259, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.R.; David, L.L.; Appukuttan, B.; Wilmarth, P.A. Angiogenic and Immunologic Proteins Identified by Deep Proteomic Profiling of Human Retinal and Choroidal Vascular Endothelial Cells: Potential Targets for New Biologic Drugs. Am. J. Ophthalmol. 2018, 193, 197–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brazionis, L.; Yau, J.; Rowley, K.; Itsiopoulos, C.; O’Dea, K.; Wong, T.Y.; Jenkins, A. Plasminogen activator inhibitor-1 (PAI-1) activity and retinal vascular calibre in type 2 diabetes. Diabetes Res. Clin. Pract. 2010, 87, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Bergen, A.A.; Arya, S.; Koster, C.; Pilgrim, M.G.; Wiatrek-Moumoulidis, D.; van der Spek, P.J.; Hauck, S.M.; Boon, C.J.F.; Emri, E.; Stewart, A.J.; et al. On the origin of proteins in human drusen: The meet, greet and stick hypothesis. Prog. Retin. Eye Res. 2019, 70, 55–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, A.C. Pathogenetic Mechanisms in Age-Related Macular Degeneration. In Retina, 5th ed.; Wilkinson, C.P., Hinton, D.R., Sadda, S.R., Wiedemann, P., Ryan, S.J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 1145–1149. [Google Scholar] [CrossRef]
- Esser, P.; Bresgen, M.; Weller, M.; Heimann, K.; Wiedemann, P. The significance of vitronectin in proliferative diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 1994, 232, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Bartha, K.; Declerck, P.J.; Moreau, H.; Nelles, L.; Collen, D. Synthesis and secretion of plasminogen activator inhibitor 1 by human endothelial cells in vitro. Effect of active site mutagenized tissue-type plasminogen activator. J. Biol. Chem. 1991, 266, 792–797. [Google Scholar] [CrossRef]
- Daniel, A.E.; Timmerman, I.; Kovacevic, I.; Hordijk, P.L.; Adriaanse, L.; Paatero, I.; Belting, H.G.; van Buul, J.D. Plasminogen Activator Inhibitor-1 Controls Vascular Integrity by Regulating VE-Cadherin Trafficking. PLoS ONE 2015, 10, e0145684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Fang, W.; Liang, J.; Lin, C.; Wu, S.; Yan, S.; Hu, C.; Ke, X. Apelin/APJ axis improves angiotensin II-induced endothelial cell senescence through AMPK/SIRT1 signaling pathway. Arch. Med. Sci. 2018, 14, 725–734. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Godino, R.; Bujakowska, K.M.; Pierce, E.A. Changes in extracellular matrix cause RPE cells to make basal deposits and activate the alternative complement pathway. Hum. Mol. Genet. 2018, 27, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Cai, H.; Noggle, S.; Paull, D.; Rizzolo, L.J.; Del Priore, L.V.; Fields, M.A. Stem cell-derived retinal pigment epithelium from patients with age-related macular degeneration exhibit reduced metabolism and matrix interactions. Stem Cells Transl. Med. 2020, 9, 364–376. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhuo, Y.; Fukuhara, M.; Rizzolo, L.J. Effects of culture conditions on heterogeneity and the apical junctional complex of the ARPE-19 cell line. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3644–3655. [Google Scholar] [CrossRef] [Green Version]
- Ablonczy, Z.; Dahrouj, M.; Tang, P.H.; Liu, Y.; Sambamurti, K.; Marmorstein, A.D.; Crosson, C.E. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8614–8620. [Google Scholar] [CrossRef] [PubMed]
- Constantine, N.A. Regression analysis and causal inference: Cause for concern? Perspect. Sex. Reprod. Health 2012, 44, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.N.; Brunet, A. The Aging Epigenome. Mol. Cell 2016, 62, 728–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stegeman, R.; Weake, V.M. Transcriptional Signatures of Aging. J. Mol. Biol. 2017, 429, 2427–2437. [Google Scholar] [CrossRef]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Tomasini-Johansson, B.R.; Milbrink, J.; Pejler, G. Vitronectin expression in rheumatoid arthritic synovia--inhibition of plasmin generation by vitronectin produced in vitro. Br. J. Rheumatol. 1998, 37, 620–629. [Google Scholar] [CrossRef]
- Jang, Y.C.; Tsou, R.; Gibran, N.S.; Isik, F.F. Vitronectin deficiency is associated with increased wound fibrinolysis and decreased microvascular angiogenesis in mice. Surgery 2000, 127, 696–704. [Google Scholar] [CrossRef]
- Dufourcq, P.; Couffinhal, T.; Alzieu, P.; Daret, D.; Moreau, C.; Duplàa, C.; Bonnet, J. Vitronectin is up-regulated after vascular injury and vitronectin blockade prevents neointima formation. Cardiovasc. Res. 2002, 53, 952–962. [Google Scholar] [CrossRef] [Green Version]
- Morrow, G.B.; Whyte, C.S.; Mutch, N.J. A Serpin With a Finger in Many PAIs: PAI-1’s Central Function in Thromboinflammation and Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 653655. [Google Scholar] [CrossRef]
- Vaughan, D.E.; Rai, R.; Khan, S.S.; Eren, M.; Ghosh, A.K. Plasminogen Activator Inhibitor-1 Is a Marker and a Mediator of Senescence. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1446–1452. [Google Scholar] [CrossRef] [Green Version]
- Chakravarthy, U.; Evans, J.; Rosenfeld, P.J. Age related macular degeneration. BMJ 2010, 340, c981. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.B.; Maranville, J.C.; Peters, J.E.; Stacey, D.; Staley, J.R.; Blackshaw, J.; Burgess, S.; Jiang, T.; Paige, E.; Surendran, P.; et al. Genomic atlas of the human plasma proteome. Nature 2018, 558, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Wiman, B.; Almquist, A.; Sigurdardottir, O.; Lindahl, T. Plasminogen activator inhibitor 1 (PAI) is bound to vitronectin in plasma. FEBS Lett. 1988, 242, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Völker, W.; Hess, S.; Vischer, P.; Preissner, K.T. Binding and processing of multimeric vitronectin by vascular endothelial cells. J. Histochem. Cytochem. 1993, 41, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, M.; Franzone, F.; Lambiase, A.; Taurone, S.; Artico, M.; Gharbiya, M.; Greco, A.; Polimeni, A. Complement Mediators in Development to Treat Age-Related Macular Degeneration. Drugs Aging 2022, 39, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hartnett, M.E. Regulation of signaling events involved in the pathophysiology of neovascular AMD. Mol. Vis. 2016, 22, 189–202. [Google Scholar] [PubMed]
- GTEx Portal Datasets. Available online: https://www.gtexportal.org/home/datasets (accessed on 7 July 2021).
| Cases (n) | Controls (n) | OR [95% CI] | p-Value | Q-Value | |
|---|---|---|---|---|---|
| Early stage AMD | 6.657 | 17.832 | 0.978 [0.939–1.018] | 0.286 | 0.286 |
| Late-stage AMD | 16.144 | 17.832 | 1.078 [1.044–1.113] | 3.97 × 10−6 | 1.79 × 10−5 |
| Geographic atrophy (GA) | 3.235 | 17.832 | 1.048 [0.992–1.106] | 0.092 | 0.118 |
| Neovascularization (NV) | 10.749 | 17.832 | 1.09 [1.052–1.13] | 2.02 × 10−6 | 1.79 × 10−5 |
| Combined, GA & NV | 2.160 | 17.832 | 1.053 [0.987–1.124] | 0.120 | 0.135 |
| Male | 6.532 | 7.820 | 1.084 [1.034–1.139] | 1.03 × 10−3 | 2.17 × 10−3 |
| Female | 9.612 | 10.012 | 1.073 [1.027–1.119] | 1.21 × 10−3 | 2.17 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biasella, F.; Strunz, T.; Kiel, C.; on behalf of the International AMD Genomics Consortium (IAMDGC); Weber, B.H.F.; Friedrich, U. Vitronectin and Its Interaction with PAI-1 Suggests a Functional Link to Vascular Changes in AMD Pathobiology. Cells 2022, 11, 1766. https://doi.org/10.3390/cells11111766
Biasella F, Strunz T, Kiel C, on behalf of the International AMD Genomics Consortium (IAMDGC), Weber BHF, Friedrich U. Vitronectin and Its Interaction with PAI-1 Suggests a Functional Link to Vascular Changes in AMD Pathobiology. Cells. 2022; 11(11):1766. https://doi.org/10.3390/cells11111766
Chicago/Turabian StyleBiasella, Fabiola, Tobias Strunz, Christina Kiel, on behalf of the International AMD Genomics Consortium (IAMDGC), Bernhard H. F. Weber, and Ulrike Friedrich. 2022. "Vitronectin and Its Interaction with PAI-1 Suggests a Functional Link to Vascular Changes in AMD Pathobiology" Cells 11, no. 11: 1766. https://doi.org/10.3390/cells11111766
APA StyleBiasella, F., Strunz, T., Kiel, C., on behalf of the International AMD Genomics Consortium (IAMDGC), Weber, B. H. F., & Friedrich, U. (2022). Vitronectin and Its Interaction with PAI-1 Suggests a Functional Link to Vascular Changes in AMD Pathobiology. Cells, 11(11), 1766. https://doi.org/10.3390/cells11111766







