Novel Functions of Integrins as Receptors of CD154: Their Role in Inflammation and Apoptosis
Abstract
:1. Introduction
2. CD154
3. CD40: The Classical CD154 Receptor
4. Novel Receptors of CD154
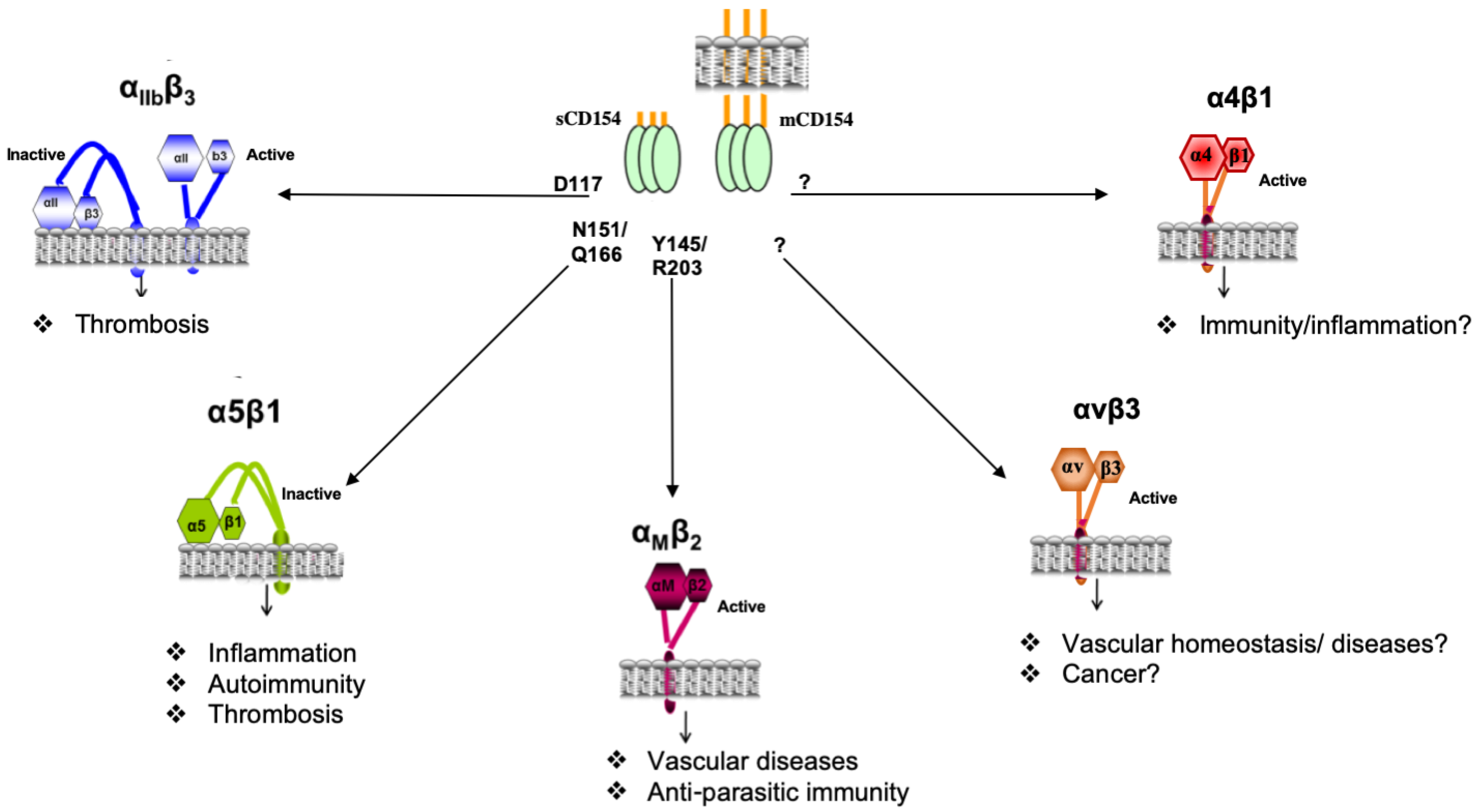
4.1. Structural Interaction of CD154 with Its Receptors
4.2. The αIIbβ3 Integrin as a Receptor of CD154
4.3. The αMβ2 Integrin as a Receptor for CD154
4.4. The α5β1 Integrin as a Receptor for CD154
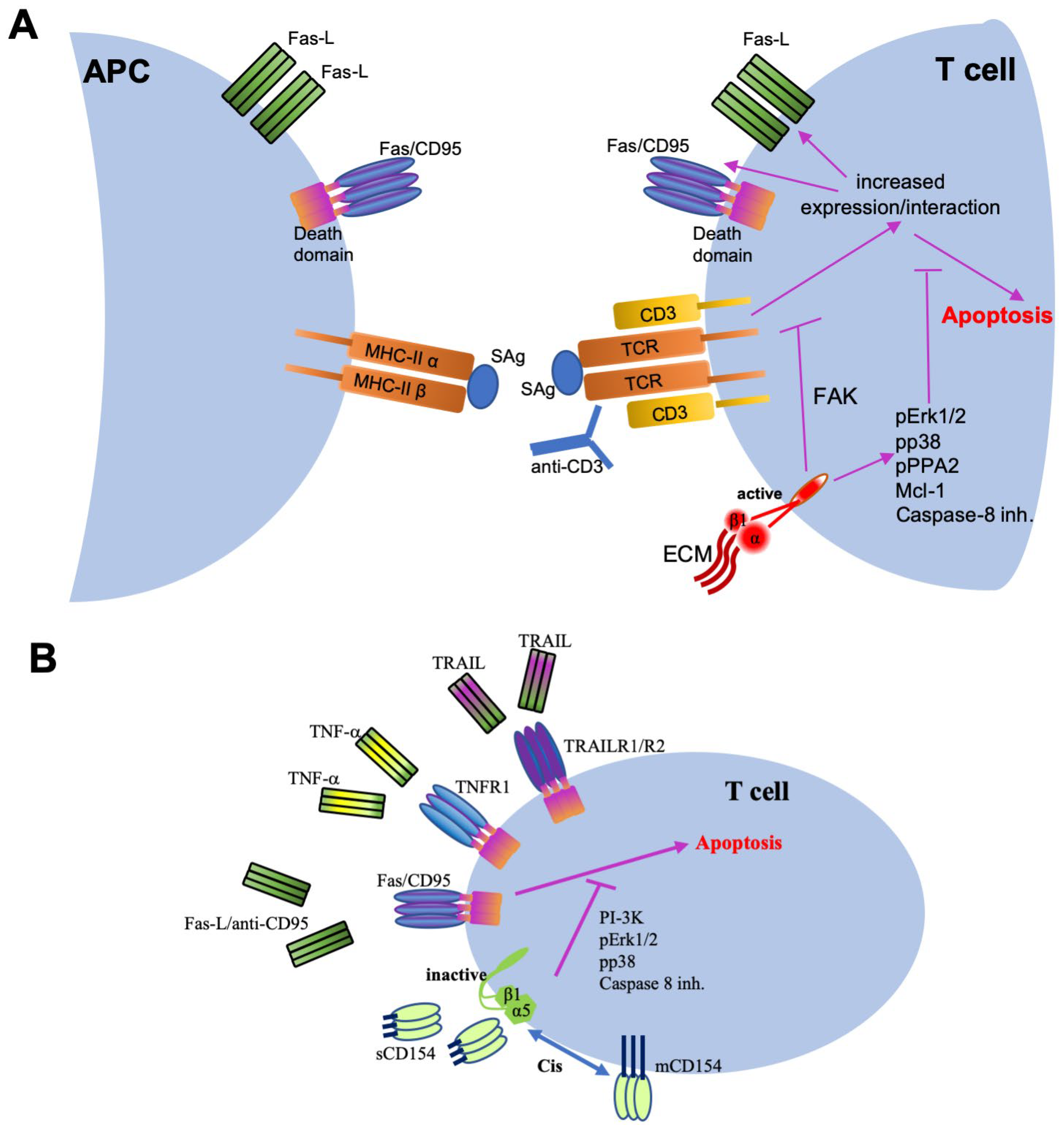
4.4.1. Inflammatory Function of the CD154–α5β1 Dyad
4.4.2. Role of the CD154–α5β1 Interaction in Cell Survival
4.5. The αVβ3 Integrin as a Receptor for CD154
4.6. The α4β1 Integrin as a Receptor for CD154
5. Conclusive Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Yamamoto, A.; Leung, P.C.; Yamamoto, F. Gene expression analysis of an integrin family of genes by systematic multiplex reverse transcription-polymerase chain reaction. Electrophoresis 2004, 25, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Cheng, K.; Krohkin, O.; Mould, A.P.; Humphries, M.J.; Ens, W.; Standing, K.; Wilkins, J.A. Evidence for the presence of a low-mass β1 integrin on the cell surface. J. Cell Sci. 2005, 118, 4009–4016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiang, Y.W.; Kitagawa, M.; Higashi, M.; Ishii, G.; Morimoto, C.; Harigaya, K. Activation of mitogen-activated protein kinase through α5/β1 integrin is required for cell cycle progression of B progenitor cell line, Reh, on human marrow stromal cells. Exp. Hematol. 2000, 28, 1147–1157. [Google Scholar] [CrossRef]
- Zeisel, M.B.; Druet, V.A.; Wachsmann, D.; Sibilia, J. MMP-3 expression and release by rheumatoid arthritis fibroblast-like synoviocytes induced with a bacterial ligand of integrin α5β1. Arthritis Res. Ther. 2005, 7, R118–R126. [Google Scholar] [CrossRef] [Green Version]
- Pulai, J.I.; Chen, H.; Im, H.J.; Kumar, S.; Hanning, C.; Hegde, P.S.; Loeser, R.F. NF-κ B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J. Immunol. 2005, 174, 5781–5788. [Google Scholar] [CrossRef] [Green Version]
- Cordes, N.; Seidler, J.; Durzok, R.; Geinitz, H.; Brakebusch, C. β1-integrin-mediated signaling essentially contributes to cell survival after radiation-induced genotoxic injury. Oncogene 2006, 25, 1378–1390. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Ray, R.M.; Johnson, L.R. Integrin β3-mediated Src activation regulates apoptosis in IEC-6 cells via Akt and STAT3. Biochem. J. 2006, 397, 437–447. [Google Scholar] [CrossRef]
- Rice, J.; Courter, D.L.; Giachelli, C.M.; Scatena, M. Molecular Mediators of αvβ3-Induced Endothelial Cell Survival. J. Vasc. Res. 2006, 43, 422–436. [Google Scholar] [CrossRef]
- Andre, P.; Prasad, K.S.; Denis, C.V.; He, M.; Papalia, J.M.; Hynes, R.O.; Phillips, D.R.; Wagner, D.D. CD40L stabilizes arterial thrombi by a β3 integrin—dependent mechanism. Nat. Med. 2002, 8, 247–252. [Google Scholar] [CrossRef]
- Zirlik, A.; Maier, C.; Gerdes, N.; MacFarlane, L.; Soosairajah, J.; Bavendiek, U.; Ahrens, I.; Ernst, S.; Bassler, N.; Missiou, A.; et al. CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation 2007, 115, 1571–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leveille, C.; Bouillon, M.; Guo, W.; Bolduc, J.; Sharif-Askari, E.; El-Fakhry, Y.; Reyes-Moreno, C.; Lapointe, R.; Merhi, Y.; Wilkins, J.A.; et al. CD40 Ligand Binds to α5β1 Integrin and Triggers Cell Signaling. J. Biol. Chem. 2007, 282, 5143–5151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takada, Y.K.; Yu, J.; Shimoda, M.; Takada, Y. Integrin Binding to the Trimeric Interface of CD40L Plays a Critical Role in CD40/CD40L Signaling. J. Immunol. 2019, 203, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.K.; Shimoda, M.; Maverakis, E.; Felding, B.H.; Cheng, R.H.; Takada, Y. Soluble CD40L activates soluble and cell-surface integrin αvβ3, α5β1, and α4β1 by binding to the allosteric ligand-binding site (site 2). J. Biol. Chem. 2021, 296, 100399. [Google Scholar] [CrossRef] [PubMed]
- Van Kooten, C.; Banchereau, J. CD40-CD40 ligand. J. Leukoc. Biol. 2000, 67, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Karnell, J.L.; Rieder, S.A.; Ettinger, R.; Kolbeck, R. Targeting the CD40-CD40L pathway in autoimmune diseases: Humoral immunity and beyond. Adv. Drug Deliv. Rev. 2019, 141, 92–103. [Google Scholar] [CrossRef]
- Peitsch, M.C.; Jongeneel, C.V. A 3-D model for the CD40 ligand predicts that it is a compact trimer similar to the tumor necrosis factors. Int. Immunol. 1993, 5, 233–238. [Google Scholar] [CrossRef]
- Karpusas, M.; Hsu, Y.M.; Wang, J.H.; Thompson, J.; Lederman, S.; Chess, L.; Thomas, D. 2 A crystal structure of an extracellular fragment of human CD40 ligand. Structure 1995, 3, 1426. [Google Scholar] [CrossRef] [Green Version]
- Fanslow, W.C.; Srinivasan, S.; Paxton, R.; Gibson, M.G.; Spriggs, M.K.; Armitage, R.J. Structural characteristics of CD40 ligand that determine biological function. Semin. Immunol. 1994, 6, 267–278. [Google Scholar] [CrossRef]
- Pietravalle, F.; Henchoz, S.L.; Blasey, H.; Aubry, J.P.; Elson, G.; Edgerton, M.D.; Bonnefoy, J.Y.; Gauchat, J.F. Human native soluble CD40L is a biologically active trimer, processed inside microsomes. J. Biol. Chem. 1996, 271, 5965–5967. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Nonoyama, S.; Morio, T.; Imai, K.; Ochs, H.D.; Mizutani, S. Characterization of soluble CD40 ligand released from human activated platelets. J. Med. Dent. Sci. 2001, 48, 23–27. [Google Scholar] [PubMed]
- Henn, V.; Steinbach, S.; Buchner, K.; Presek, P.; Kroczek, R.A. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood 2001, 98, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Nannizzi-Alaimo, L.; Alves, V.L.; Phillips, D.R. Inhibitory effects of glycoprotein IIb/IIIa antagonists and aspirin on the release of soluble CD40 ligand during platelet stimulation. Circulation 2003, 107, 1123–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salti, S.; Al-Zoobi, L.; Darif, Y.; Hassan, G.S.; Mourad, W. CD154 Resistant to Cleavage from Intracellular Milieu and Cell Surface Induces More Potent CD40-Mediated Responses. J. Immunol. 2021, 206, 1793–1805. [Google Scholar] [CrossRef]
- Salti, S.; Al-Zoobi, L.; Darif, Y.; Hassan, G.S.; Mourad, W. Monoclonal Antibody Targeting the CD154 Cleavage Site Inhibits CD40-Dependent and -independent Cleavage of CD154 from the Cell Surface. Immunohorizons 2021, 5, 590–601. [Google Scholar] [CrossRef]
- Vakkalanka, R.K.; Woo, C.; Kirou, K.A.; Koshy, M.; Berger, D.; Crow, M.K. Elevated levels and functional capacity of soluble CD40 ligand in systemic lupus erythematosus sera. Arthritis Rheum. 1999, 42, 871–881. [Google Scholar] [CrossRef]
- Danese, S.; Sans, M.; Fiocchi, C. The CD40/CD40L costimulatory pathway in inflammatory bowel disease. Gut 2004, 53, 1035–1043. [Google Scholar] [CrossRef] [Green Version]
- Kowal, K.; Pampuch, A.; Kowal-Bielecka, O.; Iacoviello, L.; Bodzenta-Lukaszyk, A. Soluble CD40 ligand in asthma patients during allergen challenge. J. Thromb. Haemost. 2006, 4, 2718–2720. [Google Scholar] [CrossRef]
- Elmetwali, T.; Young, L.S.; Palmer, D.H. CD40 ligand-induced carcinoma cell death: A balance between activation of TNFR-associated factor (TRAF) 3-dependent death signals and suppression of TRAF6-dependent survival signals. J. Immunol. 2010, 184, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Elmetwali, T.; Salman, A.; Palmer, D.H. NORE1A induction by membrane-bound CD40L (mCD40L) contributes to CD40L-induced cell death and G1 growth arrest in p21-mediated mechanism. Cell Death Dis. 2016, 7, e2146. [Google Scholar] [CrossRef] [Green Version]
- Elmetwali, T.; Salman, A.; Wei, W.; Hussain, S.A.; Young, L.S.; Palmer, D.H. CD40L membrane retention enhances the immunostimulatory effects of CD40 ligation. Sci. Rep. 2020, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Bachsais, M.; Salti, S.; Zaoui, K.; Hassan, G.S.; Aoudjit, F.; Mourad, W. CD154 inhibits death of T cells via a Cis interaction with the α5β1 integrin. PLoS ONE 2020, 15, e0235753. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Hohmann, J.D.; Wiedemann, A.; Bledzka, K.; Blankenbach, H.; Marchini, T.; Gutte, K.; Zeschky, K.; Bassler, N.; Hoppe, N.; et al. Binding of CD40L to Mac-1′s I-domain involves the EQLKKSKTL motif and mediates leukocyte recruitment and atherosclerosis—but does not affect immunity and thrombosis in mice. Circ. Res. 2011, 109, 1269–1279. [Google Scholar] [CrossRef] [Green Version]
- Grammer, A.C.; Lipsky, P.E. CD40-mediated regulation of immune responses by TRAF-dependent and TRAF-independent signaling mechanisms. Adv. Immunol. 2000, 76, 61–178. [Google Scholar] [PubMed]
- Schonbeck, U.; Libby, P. The CD40/CD154 receptor/ligand dyad. Cell. Mol. Life Sci. 2001, 58, 4–43. [Google Scholar] [PubMed]
- Noelle, R.J.; Ledbetter, J.A.; Aruffo, A. CD40 and its ligand, an essential ligand-receptor pair for thymus-dependent B-cell activation. Immunol. Today 1992, 13, 431–443. [Google Scholar] [CrossRef]
- Pullen, S.S.; Dang, T.T.; Crute, J.J.; Kehry, M.R. CD40 signaling through tumor necrosis factor receptor-associated factors (TRAFs). Binding site specificity and activation of downstream pathways by distinct TRAFs. J. Biol. Chem. 1999, 274, 14246–14254. [Google Scholar] [CrossRef] [Green Version]
- Bishop, G.A.; Hostager, B.S.; Brown, K.D. Mechanisms of TNF receptor-associated factor (TRAF) regulation in B lymphocytes. J. Leukoc. Biol. 2002, 72, 19–23. [Google Scholar]
- Fotin-Mleczek, M.; Henkler, F.; Hausser, A.; Glauner, H.; Samel, D.; Graness, A.; Scheurich, P.; Mauri, D.; Wajant, H. Tumor necrosis factor receptor-associated factor (TRAF) 1 regulates CD40-induced TRAF2-mediated NF-κB activation. J. Biol. Chem. 2004, 279, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Xie, P.; Hostager, B.S.; Munroe, M.E.; Moore, C.R.; Bishop, G.A. Cooperation between TNF receptor-associated factors 1 and 2 in CD40 signaling. J. Immunol. 2006, 176, 5388–5400. [Google Scholar] [CrossRef] [Green Version]
- Blotta, M.H.; Marshall, J.D.; DeKruyff, R.H.; Umetsu, D.T. Cross-linking of the CD40 ligand on human CD4+ T lymphocytes generates a costimulatory signal that up-regulates IL-4 synthesis. J. Immunol. 1996, 156, 3133–3140. [Google Scholar] [PubMed]
- Brenner, B.; Koppenhoefer, U.; Grassme, H.; Kun, J.; Lang, F.; Gulbins, E. Evidence for a novel function of the CD40 ligand as a signalling molecule in T-lymphocytes. FEBS Lett. 1997, 417, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Blair, P.J.; Riley, J.L.; Harlan, D.M.; Abe, R.; Tadaki, D.K.; Hoffmann, S.C.; White, L.; Francomano, T.; Perfetto, S.J.; Kirk, A.D.; et al. CD40 ligand (CD154) triggers a short-term CD4+ T cell activation response that results in secretion of immunomodulatory cytokines and apoptosis. J. Exp. Med. 2000, 191, 651–660. [Google Scholar] [CrossRef] [PubMed]
- El Fakhry, Y.; Alturaihi, H.; Diallo, D.; Merhi, Y.; Mourad, W. Critical role of lipid rafts in CD154-mediated T cell signaling. Eur. J. Immunol. 2010, 40, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Benslimane, N.; Hassan, G.S.; Yacoub, D.; Mourad, W. Requirement of transmembrane domain for CD154 association to lipid rafts and subsequent biological events. PLoS ONE 2012, 7, e43070. [Google Scholar] [CrossRef] [Green Version]
- Yacoub, D.; Benslimane, N.; Al-Zoobi, L.; Hassan, G.; Nadiri, A.; Mourad, W. CD154 is released from T-cells by a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) and ADAM17 in a CD40 protein-dependent manner. J. Biol. Chem. 2013, 288, 36083–36093. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, N.; Seki, M.; Notarangelo, L.D.; Geha, R.S. The hyper-IgM (HIM) syndrome. Springer Semin. Immunopathol. 1998, 19, 383–399. [Google Scholar] [CrossRef]
- Hassan, G.S.; Merhi, Y.; Mourad, W.M. CD154 and its receptors in inflammatory vascular pathologies. Trends Immunol. 2009, 30, 165–172. [Google Scholar] [CrossRef]
- Hassan, G.S.; Rana, M.; Léveillé, C.; Nadiri, A.; Jundi, M.; Polyak, M.; El-Fakhry, Y.; Mourad, W.M. Implication of CD154/CD40 Interaction in Healthy and Autoimmune Responses. Curr. Immunol. Rev. 2009, 5, 285–299. [Google Scholar] [CrossRef]
- Lutgens, E.; Daemen, M.J. CD40-CD40L interactions in atherosclerosis. Trends Cardiovasc. Med. 2002, 12, 27–32. [Google Scholar] [CrossRef]
- Lutgens, E.; Lievens, D.; Beckers, L.; Wijnands, E.; Soehnlein, O.; Zernecke, A.; Seijkens, T.; Engel, D.; Cleutjens, J.; Keller, A.M.; et al. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J. Exp. Med. 2010, 207, 391–404. [Google Scholar] [CrossRef]
- Yanagawa, Y.; Onoe, K. Distinct regulation of CD40-mediated interleukin-6 and interleukin-12 productions via mitogen-activated protein kinase and nuclear factor κB-inducing kinase in mature dendritic cells. Immunology 2006, 117, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Josien, R.; Wong, B.R.; Li, H.L.; Steinman, R.M.; Choi, Y. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J. Immunol. 1999, 162, 2562–2568. [Google Scholar] [PubMed]
- Lutgens, E.; Lievens, D.; Beckers, L.; Donners, M.; Daemen, M. CD40 and its ligand in atherosclerosis. Trends Cardiovasc. Med. 2007, 17, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Pluvinet, R.; Olivar, R.; Krupinski, J.; Herrero-Fresneda, I.; Luque, A.; Torras, J.; Cruzado, J.M.; Grinyo, J.M.; Sumoy, L.; Aran, J.M. CD40: An upstream master switch for endothelial cell activation uncovered by RNAi-coupled transcriptional profiling. Blood 2008, 112, 3624–3637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmann, K.; Hughes, C.C.; Schechner, J.; Fanslow, W.C.; Pober, J.S. CD40 on human endothelial cells: Inducibility by cytokines and functional regulation of adhesion molecule expression. Proc. Natl. Acad. Sci. USA 1995, 92, 4342–4346. [Google Scholar] [CrossRef] [Green Version]
- Kotowicz, K.; Dixon, G.L.; Klein, N.J.; Peters, M.J.; Callard, R.E. Biological function of CD40 on human endothelial cells: Costimulation with CD40 ligand and interleukin-4 selectively induces expression of vascular cell adhesion molecule-1 and P-selectin resulting in preferential adhesion of lymphocytes. Immunology 2000, 100, 441–448. [Google Scholar] [CrossRef]
- Mukundan, L.; Milhorn, D.M.; Matta, B.; Suttles, J. CD40-mediated activation of vascular smooth muscle cell chemokine production through a Src-initiated, MAPK-dependent pathway. Cell. Signal. 2004, 16, 375–384. [Google Scholar] [CrossRef]
- Xu, F.; Ji, J.; Li, L.; Chen, R.; Hu, W. Activation of adventitial fibroblasts contributes to the early development of atherosclerosis: A novel hypothesis that complements the “Response-to-Injury Hypothesis” and the “Inflammation Hypothesis”. Med. Hypotheses 2007, 69, 908–912. [Google Scholar] [CrossRef]
- Quezada, S.A.; Jarvinen, L.Z.; Lind, E.F.; Noelle, R.J. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 2004, 22, 307–328. [Google Scholar] [CrossRef]
- Gerritse, K.; Laman, J.; Noelle, R.; Aruffo, A.; Ledbetter, J.; Boersma, W.; Claassen, E. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. USA 1996, 93, 2499–2504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faure, G.; Bensoussan-Lejzerowicz, D.; Bene, M.; Aubert, V.; Leclere, J. Coexpression of CD40 and class II antigen HLA-DR in Graves’ disease thyroid epithelial cells. Clin. Immunol. Immunopathol. 1997, 84, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, T.I.; Wang, J.; Karnell, J.L.; Wang, Q.; Wang, S.; Naiman, B.; Gross, P.; Brohawn, P.Z.; Morehouse, C.; Aoyama, J.; et al. Autoimmune manifestations in aged mice arise from early-life immune dysregulation. Sci. Transl. Med. 2016, 8, 361ra137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanji, S.A.; Hancock, W.W.; Luo, B.; Schur, C.D.; Pawlick, R.L.; Zhu, L.F.; Anderson, C.C.; Shapiro, A.M. Costimulation blockade of both inducible costimulator and CD40 ligand induces dominant tolerance to islet allografts and prevents spontaneous autoimmune diabetes in the NOD mouse. Diabetes 2006, 55, 27–33. [Google Scholar] [CrossRef]
- Baker, R.L.; Wagner, D.H., Jr.; Haskins, K. CD40 on NOD CD4 T cells contributes to their activation and pathogenicity. J. Autoimmun. 2008, 31, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kawaguchi, Y.; Harigai, M.; Takagi, K.; Ohta, S.; Fukasawa, C.; Hara, M.; Kamatani, N. Increased CD40 expression on muscle cells of polymyositis and dermatomyositis: Role of CD40-CD40 ligand interaction in IL-6, IL-8, IL-15, and monocyte chemoattractant protein-1 production. J. Immunol. 2000, 164, 6593–6600. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, P.S.; Bleharski, J.R.; Uyemura, K.; Kim, J.; Sieling, P.A.; Miller, A.; Brightbill, H.; Schlienger, K.; Rea, T.H.; Modlin, R.L. A role for CD40-CD40 ligand interactions in the generation of type 1 cytokine responses in human leprosy. J. Immunol. 2000, 165, 1506–1512. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Colpaert, S.; D’Haens, G.R.; Kasran, A.; de Boer, M.; Rutgeerts, P.; Geboes, K.; Ceuppens, J.L. Hyperexpression of CD40 ligand (CD154) in inflammatory bowel disease and its contribution to pathogenic cytokine production. J. Immunol. 1999, 163, 4049–4057. [Google Scholar]
- Bosmans, L.A.; Bosch, L.; Kusters, P.J.H.; Lutgens, E.; Seijkens, T.T.P. The CD40-CD40L Dyad as Immunotherapeutic Target in Cardiovascular Disease. J. Cardiovasc. Transl. Res. 2021, 14, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Hassan, G.S.; Stagg, J.; Mourad, W. Role of CD154 in cancer pathogenesis and immunotherapy. Cancer Treat. Rev. 2015, 41, 431–440. [Google Scholar] [CrossRef]
- Mackey, M.; Gunn, J.; Ting, P.; Kikutani, H.; Dranoff, G.; Noelle, R.; Barth, R.J., Jr. Protective immunity induced by tumor vaccines requires interaction between CD40 and its ligand, CD154. Cancer Res. 1997, 57, 2569–2574. [Google Scholar] [PubMed]
- Ridge, J.P.; Di Rosa, F.; Matzinger, P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 1998, 393, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Jundi, M.; Nadiri, A.; Al-Zoobi, L.; Hassan, G.S.; Mourad, W. CD40-mediated cell death requires TRAF6 recruitment. Immunobiology 2011, 217, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Bugajska, U.; Georgopoulos, N.T.; Southgate, J.; Johnson, P.W.; Graber, P.; Gordon, J.; Selby, P.J.; Trejdosiewicz, L.K. The effects of malignant transformation on susceptibility of human urothelial cells to CD40-mediated apoptosis. J. Natl. Cancer Inst. 2002, 94, 1381–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funakoshi, S.; Longo, D.L.; Beckwith, M.; Conley, D.K.; Tsarfaty, G.; Tsarfaty, I.; Armitage, R.J.; Fanslow, W.C.; Spriggs, M.K.; Murphy, W.J. Inhibition of human B-cell lymphoma growth by CD40 stimulation. Blood 1994, 83, 2787–2794. [Google Scholar] [CrossRef] [Green Version]
- Georgopoulos, N.T.; Steele, L.P.; Thomson, M.J.; Selby, P.J.; Southgate, J.; Trejdosiewicz, L.K. A novel mechanism of CD40-induced apoptosis of carcinoma cells involving TRAF3 and JNK/AP-1 activation. Cell Death Differ. 2006, 13, 1789–1801. [Google Scholar] [CrossRef]
- Eliopoulos, A.G.; Davies, C.; Knox, P.G.; Gallagher, N.J.; Afford, S.C.; Adams, D.H.; Young, L.S. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily. Mol. Cell. Biol. 2000, 20, 5503–5515. [Google Scholar] [CrossRef] [Green Version]
- Baxendale, A.J.; Dawson, C.W.; Stewart, S.E.; Mudaliar, V.; Reynolds, G.; Gordon, J.; Murray, P.G.; Young, L.S.; Eliopoulos, A.G. Constitutive activation of the CD40 pathway promotes cell transformation and neoplastic growth. Oncogene 2005, 24, 7913–7923. [Google Scholar] [CrossRef] [Green Version]
- Pham, L.V.; Tamayo, A.T.; Yoshimura, L.C.; Lo, P.; Terry, N.; Reid, P.S.; Ford, R.J. A CD40 Signalosome anchored in lipid rafts leads to constitutive activation of NF-κB and autonomous cell growth in B cell lymphomas. Immunity 2002, 16, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Challa, A.; Eliopoulos, A.G.; Holder, M.J.; Burguete, A.S.; Pound, J.D.; Chamba, A.; Grafton, G.; Armitage, R.J.; Gregory, C.D.; Martinez-Valdez, H.; et al. Population depletion activates autonomous CD154-dependent survival in biopsylike Burkitt lymphoma cells. Blood 2002, 99, 3411–3418. [Google Scholar] [CrossRef] [Green Version]
- Bussolati, B.; Russo, S.; Deambrosis, I.; Cantaluppi, V.; Volpe, A.; Ferrando, U.; Camussi, G. Expression of CD154 on renal cell carcinomas and effect on cell proliferation, motility and platelet-activating factor synthesis. Int. J. Cancer 2002, 100, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Bajorath, J. Detailed comparison of two molecular models of the human CD40 ligand with an X-ray structure and critical assessment of model-based mutagenesis and residue mapping studies. J. Biol. Chem. 1998, 273, 24603–24609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, H.J.; Kim, Y.J.; Song, D.H.; Park, B.S.; Kim, H.M.; Lee, J.D.; Paik, S.G.; Lee, J.O.; Lee, H. Crystallographic and mutational analysis of the CD40-CD154 complex and its implications for receptor activation. J. Biol. Chem. 2011, 286, 11226–11235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, P.; Nannizzi-Alaimo, L.; Prasad, S.K.; Phillips, D.R. Platelet-derived CD40L: The switch-hitting player of cardiovascular disease. Circulation 2002, 106, 896–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etongue-Mayer, P.; Langlois, M.A.; Ouellette, M.; Li, H.; Younes, S.; Al-Daccak, R.; Mourad, W. Involvement of zinc in the binding of Mycoplasma arthritidis-derived mitogen to the proximity of the HLA-DR binding groove regardless of histidine 81 of the β chain. Eur. J. Immunol. 2002, 32, 50–58. [Google Scholar] [CrossRef]
- Alturaihi, H.; Hassan, G.S.; Al-Zoobi, L.; Salti, S.; Darif, Y.; Yacoub, D.; Akoum, S.E.; Oudghiri, M.; Merhi, Y.; Mourad, W. Interaction of CD154 with different receptors and its role in bidirectional signals. Eur. J. Immunol. 2015, 45, 592–602. [Google Scholar] [CrossRef]
- El Fakhry, Y.; Alturaihi, H.; Yacoub, D.; Liu, L.; Guo, W.; Leveille, C.; Jung, D.; Khzam, L.B.; Merhi, Y.; Wilkins, J.A.; et al. Functional Interaction of CD154 Protein with α5β1 Integrin is Totally Independent from Its Binding to αIIbβ3 Integrin and CD40 Molecules. J. Biol. Chem. 2012, 287, 18055–18066. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.S.; Andre, P.; He, M.; Bao, M.; Manganello, J.; Phillips, D.R. Soluble CD40 ligand induces β3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 12367–12371. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, S.; Varghese, S.; Vitseva, O.; Tanriverdi, K.; Freedman, J.E. CD40 ligand influences platelet release of reactive oxygen intermediates. Arter. Thromb. Vasc. Biol. 2005, 25, 2428–2434. [Google Scholar] [CrossRef] [Green Version]
- Kuijpers, M.J.; Mattheij, N.J.; Cipolla, L.; van Geffen, J.P.; Lawrence, T.; Donners, M.M.; Boon, L.; Lievens, D.; Torti, M.; Noels, H.; et al. Platelet CD40L Modulates Thrombus Growth Via Phosphatidylinositol 3-Kinase β, and Not Via CD40 and IκB Kinase α. Arter. Thromb. Vasc. Biol. 2015, 35, 1374–1381. [Google Scholar] [CrossRef] [Green Version]
- May, A.E.; Kalsch, T.; Massberg, S.; Herouy, Y.; Schmidt, R.; Gawaz, M. Engagement of glycoprotein IIb/IIIa (αIIbβ3) on platelets upregulates CD40L and triggers CD40L-dependent matrix degradation by endothelial cells. Circulation 2002, 106, 2111–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margadant, C.; Monsuur, H.N.; Norman, J.C.; Sonnenberg, A. Mechanisms of integrin activation and trafficking. Curr. Opin. Cell Biol. 2011, 23, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 1994, 76, 301–314. [Google Scholar] [CrossRef]
- Ross, G.D.; Lambris, J.D. Identification of a C3bi-specific membrane complement receptor that is expressed on lymphocytes, monocytes, neutrophils, and erythrocytes. J. Exp. Med. 1982, 155, 96–110. [Google Scholar] [CrossRef] [Green Version]
- Diamond, M.S.; Staunton, D.E.; Marlin, S.D.; Springer, T.A. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell 1991, 65, 961–971. [Google Scholar] [CrossRef]
- Altieri, D.C.; Agbanyo, F.R.; Plescia, J.; Ginsberg, M.H.; Edgington, T.S.; Plow, E.F. A unique recognition site mediates the interaction of fibrinogen with the leukocyte integrin Mac-1 (CD11b/CD18). J. Biol. Chem. 1990, 265, 12119–12122. [Google Scholar] [CrossRef]
- Kanse, S.M.; Matz, R.L.; Preissner, K.T.; Peter, K. Promotion of leukocyte adhesion by a novel interaction between vitronectin and the β2 integrin Mac-1 (αMβ2, CD11b/CD18). Arter. Thromb. Vasc. Biol. 2004, 24, 2251–2256. [Google Scholar] [CrossRef] [Green Version]
- Diamond, M.S.; Alon, R.; Parkos, C.A.; Quinn, M.T.; Springer, T.A. Heparin is an adhesive ligand for the leukocyte integrin Mac-1 (CD11b/CD1). J. Cell Biol. 1995, 130, 1473–1482. [Google Scholar] [CrossRef] [Green Version]
- Lundgren-Akerlund, E.; Olofsson, A.M.; Berger, E.; Arfors, K.E. CD11b/CD18-dependent polymorphonuclear leucocyte interaction with matrix proteins in adhesion and migration. Scand. J. Immunol. 1993, 37, 569–574. [Google Scholar] [CrossRef]
- Dunne, J.L.; Ballantyne, C.M.; Beaudet, A.L.; Ley, K. Control of leukocyte rolling velocity in TNF-α-induced inflammation by LFA-1 and Mac-1. Blood 2002, 99, 336–341. [Google Scholar] [CrossRef]
- Chavakis, T.; Bierhaus, A.; Al-Fakhri, N.; Schneider, D.; Witte, S.; Linn, T.; Nagashima, M.; Morser, J.; Arnold, B.; Preissner, K.T.; et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: A novel pathway for inflammatory cell recruitment. J. Exp. Med. 2003, 198, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.; Busch, H.J.; Bourgeois, N.; Schwarz, M.; Wolf, D.; Zirlik, A.; Peter, K.; Bode, C.; von Zur Muhlen, C. Mac-1 directly binds to the endothelial protein C-receptor: A link between the protein C anticoagulant pathway and inflammation? PLoS ONE 2013, 8, e53103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, C.; Edelman, E.R.; Simon, D.I. A mAb to the β2-leukocyte integrin Mac-1 (CD11b/CD18) reduces intimal thickening after angioplasty or stent implantation in rabbits. Proc. Natl. Acad. Sci. USA 1998, 95, 10134–10139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, D.I.; Dhen, Z.; Seifert, P.; Edelman, E.R.; Ballantyne, C.M.; Rogers, C. Decreased neointimal formation in Mac-1-/- mice reveals a role for inflammation in vascular repair after angioplasty. J. Clin. Investig. 2000, 105, 293–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schober, J.M.; Chen, N.; Grzeszkiewicz, T.M.; Jovanovic, I.; Emeson, E.E.; Ugarova, T.P.; Ye, R.D.; Lau, L.F.; Lam, S.C. Identification of integrin αMβ2 as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): Immediate-early gene products expressed in atherosclerotic lesions. Blood 2002, 99, 4457–4465. [Google Scholar] [CrossRef] [Green Version]
- Wolf, D.; Anto-Michel, N.; Blankenbach, H.; Wiedemann, A.; Buscher, K.; Hohmann, J.D.; Lim, B.; Bauml, M.; Marki, A.; Mauler, M.; et al. A ligand-specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense. Nat. Commun. 2018, 9, 525. [Google Scholar] [CrossRef]
- Liu, D.; Ford, M.L. CD11b is a novel alternate receptor for CD154 during alloimmunity. Am. J. Transplant. 2020, 20, 2216–2225. [Google Scholar] [CrossRef]
- Lefort, C.T.; Rossaint, J.; Moser, M.; Petrich, B.G.; Zarbock, A.; Monkley, S.J.; Critchley, D.R.; Ginsberg, M.H.; Fassler, R.; Ley, K. Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation. Blood 2012, 119, 4275–4282. [Google Scholar] [CrossRef]
- Bouti, P.; Webbers, S.D.S.; Fagerholm, S.C.; Alon, R.; Moser, M.; Matlung, H.L.; Kuijpers, T.W. β2 Integrin Signaling Cascade in Neutrophils: More Than a Single Function. Front. Immunol. 2020, 11, 619925. [Google Scholar] [CrossRef]
- Hirahashi, J.; Mekala, D.; Van Ziffle, J.; Xiao, L.; Saffaripour, S.; Wagner, D.D.; Shapiro, S.D.; Lowell, C.; Mayadas, T.N. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity 2006, 25, 271–283. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.; Yu, S.; Song, Z.; Zhu, X.; Wang, C.; Yan, J.; Wu, F.; Nanda, A.; Granger, D.N.; Li, G. Soluble CD40 ligand stimulates CD40-dependent activation of the β2 integrin Mac-1 and protein kinase C zeda (PKCzeta) in neutrophils: Implications for neutrophil-platelet interactions and neutrophil oxidative burst. PLoS ONE 2013, 8, e64631. [Google Scholar]
- Li, G.; Sanders, J.M.; Bevard, M.H.; Sun, Z.; Chumley, J.W.; Galkina, E.V.; Ley, K.; Sarembock, I.J. CD40 Ligand Promotes Mac-1 Expression, Leukocyte Recruitment, and Neointima Formation after Vascular Injury. Am. J. Pathol. 2008, 172, 1141–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okwor, I.; Jia, P.; Uzonna, J.E. Interaction of Macrophage Antigen 1 and CD40 Ligand Leads to IL-12 Production and Resistance in CD40-Deficient Mice Infected with Leishmania major. J. Immunol. 2015, 195, 3218–3226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffner, F.; Ray, A.M.; Dontenwill, M. Integrin α5β1, the Fibronectin Receptor, as a Pertinent Therapeutic Target in Solid Tumors. Cancers 2013, 5, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Hocking, D.C.; Sottile, J.; McKeown-Longo, P.J. Activation of distinct α5β1-mediated signaling pathways by fibronectin’s cell adhesion and matrix assembly domains. J. Cell Biol. 1998, 141, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Mehlhop, P.D.; van de Rijn, M.; Brewer, J.P.; Kisselgof, A.B.; Geha, R.S.; Oettgen, H.C.; Martin, T.R. CD40L, but not CD40, is required for allergen-induced bronchial hyperresponsiveness in mice. Am. J. Respir. Cell. Mol. Biol. 2000, 23, 646–651. [Google Scholar] [CrossRef]
- Lindmark, E.; Tenno, T.; Siegbahn, A. Role of platelet P-selectin and CD40 ligand in the induction of monocytic tissue factor expression. Arter. Thromb. Vasc. Biol. 2000, 20, 2322–2328. [Google Scholar] [CrossRef] [Green Version]
- Ni, H.; Li, A.; Simonsen, N.; Wilkins, J.A. Integrin activation by dithiothreitol or Mn2+ induces a ligand-occupied conformation and exposure of a novel NH2-terminal regulatory site on the β1 integrin chain. J. Biol. Chem. 1998, 273, 7981–7987. [Google Scholar] [CrossRef] [Green Version]
- Loubaki, L.; Semlali, A.; Boisvert, M.; Jacques, E.; Plante, S.; Aoudjit, F.; Mourad, W.; Chakir, J. Crosstalk between T cells and bronchial fibroblasts obtained from asthmatic subjects involves CD40L/α5β1 interaction. Mol. Immunol. 2010, 47, 2112–2118. [Google Scholar] [CrossRef]
- Gros, A.; Ollivier, V.; Ho-Tin-Noe, B. Platelets in inflammation: Regulation of leukocyte activities and vascular repair. Front. Immunol. 2014, 5, 678. [Google Scholar] [CrossRef]
- Thomas, M.R.; Storey, R.F. The role of platelets in inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [PubMed]
- Bennett, J.S.; Berger, B.W.; Billings, P.C. The structure and function of platelet integrins. J. Thromb. Haemost. 2009, 7 (Suppl. S1), 200–205. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.S.; Merhi, Y.; Mourad, W. CD40 Ligand: A neo-inflammatory molecule in vascular diseases. Immunobiology 2011, 217, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Inwald, D.P.; McDowall, A.; Peters, M.J.; Callard, R.E.; Klein, N.J. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ. Res. 2003, 92, 1041–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simic, D.; Bogdan, N.; Teng, F.; Otieno, M. Blocking α5β1 Integrin Attenuates sCD40L-Mediated Platelet Activation. Clin. Appl. Thromb. Hemost. 2017, 23, 607–614. [Google Scholar] [CrossRef]
- Lin, Y.P.; Su, C.C.; Huang, J.Y.; Lin, H.C.; Cheng, Y.J.; Liu, M.F.; Yang, B.C. Aberrant integrin activation induces p38 MAPK phosphorylation resulting in suppressed Fas-mediated apoptosis in T cells: Implications for rheumatoid arthritis. Mol. Immunol. 2009, 46, 3328–3335. [Google Scholar] [CrossRef]
- Walle, T.K.; Helve, T.; Virtanen, I.; Kurki, P. Increased expression of VLA-5 adhesion molecules on synovial fluid T lymphocytes in chronic polyarthritis: A consequence of T-cell activation. Scand. J. Immunol. 1994, 39, 189–194. [Google Scholar] [CrossRef]
- Pribila, J.T.; Quale, A.C.; Mueller, K.L.; Shimizu, Y. Integrins and T cell-mediated immunity. Annu. Rev. Immunol. 2004, 22, 157–180. [Google Scholar] [CrossRef]
- Nakayamada, S.; Saito, K.; Nakano, K.; Tanaka, Y. Activation signal transduction by β1 integrin in T cells from patients with systemic lupus erythematosus. Arthritis Rheum. 2007, 56, 1559–1568. [Google Scholar] [CrossRef]
- White, D.E.; Kurpios, N.A.; Zuo, D.; Hassell, J.A.; Blaess, S.; Mueller, U.; Muller, W.J. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 2004, 6, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Radisky, D.C.; Wang, F.; Bissell, M.J. Polarity and proliferation are controlled by distinct signaling pathways downstream of PI3-kinase in breast epithelial tumor cells. J. Cell Biol. 2004, 164, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Park, C.C.; Zhang, H.; Pallavicini, M.; Gray, J.W.; Baehner, F.; Park, C.J.; Bissell, M.J. β1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006, 66, 1526–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkelstein, L.D.; Reynolds, P.J.; Hunt, S.W., 3rd; Shimizu, Y. Structural requirements for β1 integrin-mediated tyrosine phosphorylation in human T cells. J. Immunol. 1997, 159, 5355–5363. [Google Scholar] [PubMed]
- Frisch, S.M.; Ruoslahti, E. Integrins and anoikis. Curr. Opin. Cell Biol. 1997, 9, 701–706. [Google Scholar] [CrossRef]
- Wong, B.; Choi, Y. Pathways leading to cell death in T cells. Curr. Opin. Immunol. 1997, 9, 358–364. [Google Scholar] [CrossRef]
- Dhein, J.; Walczak, H.; Baumler, C.; Debatin, K.M.; Krammer, P.H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 1995, 373, 438–441. [Google Scholar] [CrossRef]
- Brunner, T.; Mogil, R.J.; LaFace, D.; Yoo, N.J.; Mahboubi, A.; Echeverri, F.; Martin, S.J.; Force, W.R.; Lynch, D.H.; Ware, C.F.; et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 1995, 373, 441–444. [Google Scholar] [CrossRef]
- Aoudjit, F.; Vuori, K. Engagement of the α2β1 integrin inhibits Fas ligand expression and activation-induced cell death in T cells in a focal adhesion kinase-dependent manner. Blood 2000, 95, 2044–2051. [Google Scholar] [CrossRef]
- Rich, S.; Van Nood, N.; Lee, H.M. Role of α5β1 integrin in TGF-β 1-costimulated CD8+ T cell growth and apoptosis. J. Immunol. 1996, 157, 2916–2923. [Google Scholar]
- Naci, D.; Aoudjit, F. Alpha2β1 integrin promotes T cell survival and migration through the concomitant activation of ERK/Mcl-1 and p38 MAPK pathways. Cell. Signal. 2014, 26, 2008–2015. [Google Scholar] [CrossRef]
- Liu, C.C.; Leclair, P.; Yap, S.Q.; Lim, C.J. The membrane-proximal KXGFFKR motif of α-integrin mediates chemoresistance. Mol. Cell. Biol. 2013, 33, 4334–4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gendron, S.; Couture, J.; Aoudjit, F. Integrin α2β1 inhibits Fas-mediated apoptosis in T lymphocytes by protein phosphatase 2A-dependent activation of the MAPK/ERK pathway. J. Biol. Chem. 2003, 278, 48633–48643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachsais, M.; Naddaf, N.; Yacoub, D.; Salti, S.; Alaaeddine, N.; Aoudjit, F.; Hassan, G.S.; Mourad, W. The Interaction of CD154 with the α5β1 Integrin Inhibits Fas-Induced T Cell Death. PLoS ONE 2016, 11, e0158987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, T.; Findhammer, S.; Nolte, B.; Helm, E.B.; Brodt, H.R. Inhibition of TNF-α mediated cell death by HIV-1 specific protease inhibitors. Eur. J. Med. Res. 2003, 8, 17–24. [Google Scholar]
- Shakibaei, M.; Sung, B.; Sethi, G.; Aggarwal, B.B. TNF-α-induced mitochondrial alterations in human T cells requires FADD and caspase-8 activation but not RIP and caspase-3 activation. Antioxid. Redox Signal. 2010, 13, 821–831. [Google Scholar] [CrossRef] [Green Version]
- Back, J.; Chalifour, A.; Scarpellino, L.; Held, W. Stable masking by H-2Dd cis ligand limits Ly49A relocalization to the site of NK cell/target cell contact. Proc. Natl. Acad. Sci. USA 2007, 104, 3978–3983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, K.E.; Williams, G.S.; Davis, D.M.; Hoglund, P. Quantifying the reduction in accessibility of the inhibitory NK cell receptor Ly49A caused by binding MHC class I proteins in cis. Eur. J. Immunol. 2007, 37, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Doucey, M.A.; Scarpellino, L.; Zimmer, J.; Guillaume, P.; Luescher, I.F.; Bron, C.; Held, W. Cis association of Ly49A with MHC class I restricts natural killer cell inhibition. Nat. Immunol. 2004, 5, 328–336. [Google Scholar] [CrossRef]
- Saggu, G.; Okubo, K.; Chen, Y.; Vattepu, R.; Tsuboi, N.; Rosetti, F.; Cullere, X.; Washburn, N.; Tahir, S.; Rosado, A.M.; et al. Cis interaction between sialylated FcγRIIA and the αI-domain of Mac-1 limits antibody-mediated neutrophil recruitment. Nat. Commun. 2018, 9, 5058. [Google Scholar] [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Weis, S.M.; Cheresh, D.A. αV integrins in angiogenesis and cancer. Cold Spring Harb. Perspect. Med. 2011, 1, a006478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, B.S.; Kessler, H.; Kossatz, S.; Reuning, U. RGD-Binding Integrins Revisited: How Recently Discovered Functions and Novel Synthetic Ligands (Re-)Shape an Ever-Evolving Field. Cancers 2021, 13, 1711. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.J. Integrin structure. Biochem. Soc. Trans. 2000, 28, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, M.; Takada, Y.K.; Takada, Y. The chemokine fractalkine can activate integrins without CX3CR1 through direct binding to a ligand-binding site distinct from the classical RGD-binding site. PLoS ONE 2014, 9, e96372. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Davari, P.; Takada, Y.K.; Takada, Y. Stromal cell-derived factor-1 (CXCL12) activates integrins by direct binding to an allosteric ligand-binding site (site 2) of integrins without CXCR4. Biochem. J. 2018, 475, 723–732. [Google Scholar] [CrossRef]
- Fujita, M.; Zhu, K.; Fujita, C.K.; Zhao, M.; Lam, K.S.; Kurth, M.J.; Takada, Y.K.; Takada, Y. Proinflammatory secreted phospholipase A2 type IIA (sPLA-IIA) induces integrin activation through direct binding to a newly identified binding site (site 2) in integrins αvβ3, α4β1, and α5β1. J. Biol. Chem. 2015, 290, 259–271. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, G.S.; Salti, S.; Mourad, W. Novel Functions of Integrins as Receptors of CD154: Their Role in Inflammation and Apoptosis. Cells 2022, 11, 1747. https://doi.org/10.3390/cells11111747
Hassan GS, Salti S, Mourad W. Novel Functions of Integrins as Receptors of CD154: Their Role in Inflammation and Apoptosis. Cells. 2022; 11(11):1747. https://doi.org/10.3390/cells11111747
Chicago/Turabian StyleHassan, Ghada S., Suzanne Salti, and Walid Mourad. 2022. "Novel Functions of Integrins as Receptors of CD154: Their Role in Inflammation and Apoptosis" Cells 11, no. 11: 1747. https://doi.org/10.3390/cells11111747
APA StyleHassan, G. S., Salti, S., & Mourad, W. (2022). Novel Functions of Integrins as Receptors of CD154: Their Role in Inflammation and Apoptosis. Cells, 11(11), 1747. https://doi.org/10.3390/cells11111747





