Exogenous Ceramide Serves as a Precursor to Endogenous Ceramide Synthesis and as a Modulator of Keratinocyte Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. C17 Cer Synthesis
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Lipid Analysis
2.5. Western Blot Analysis
2.6. Quantitative RT-PCR
2.7. Statistical Analysis
3. Results
3.1. dC17/C18:0 and tC17/C18:0 Cer Preparation
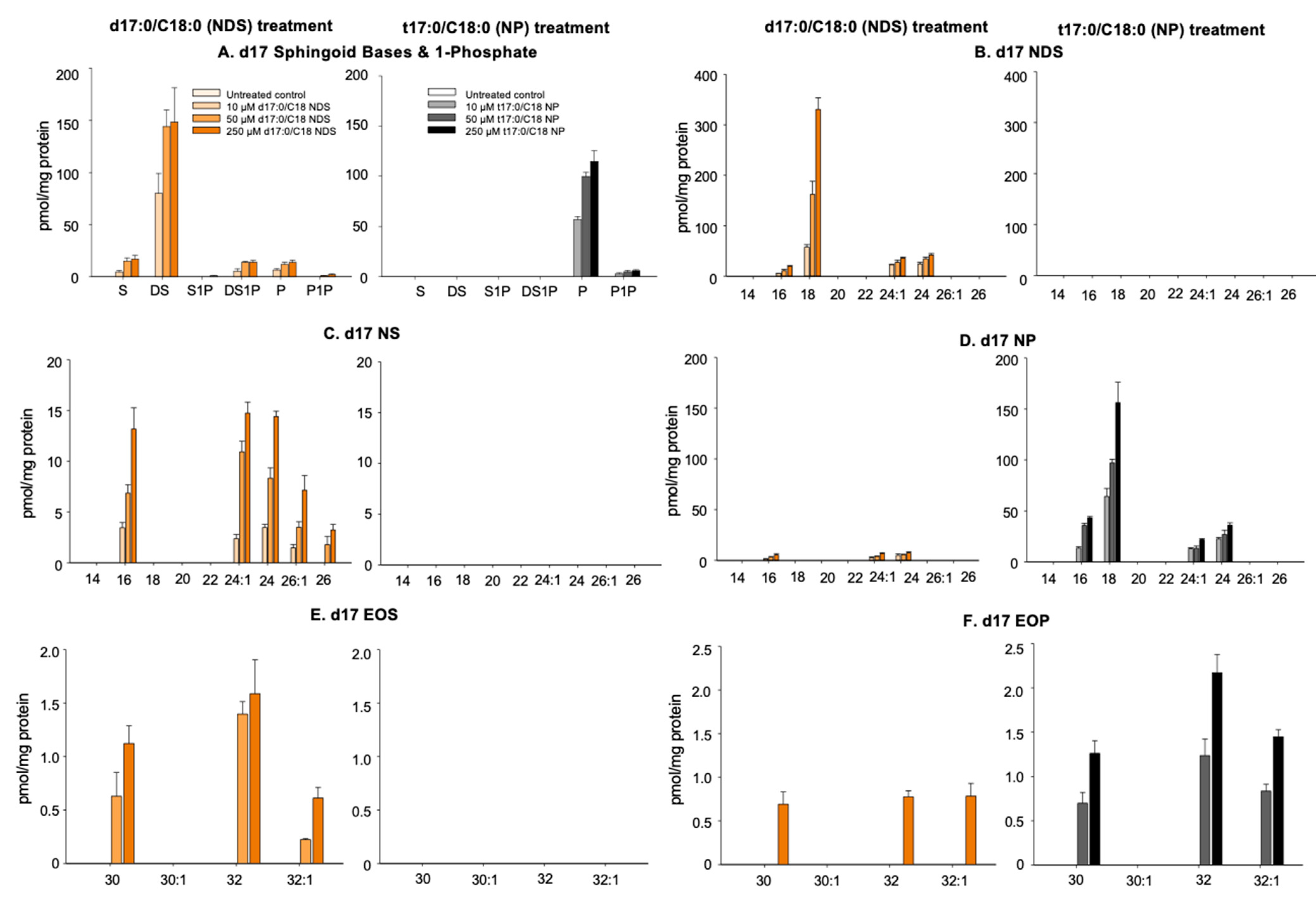
3.2. Conversion of 17NDS and 17NP to Other Cer Species
3.2.1. N-Deacylation of 17NDS and 17NP
3.2.2. Production of 17NS and 17NP
3.2.3. Production of Omega-O-AcylCers
3.3. Alterations of Cer Levels in KC Incubated with NDS and NP
3.4. Modulation of KC Differentiation by Exogenous Cer
3.5. Modulation of Cathelicidin Antimicrobial Peptide by Exogenous Cer
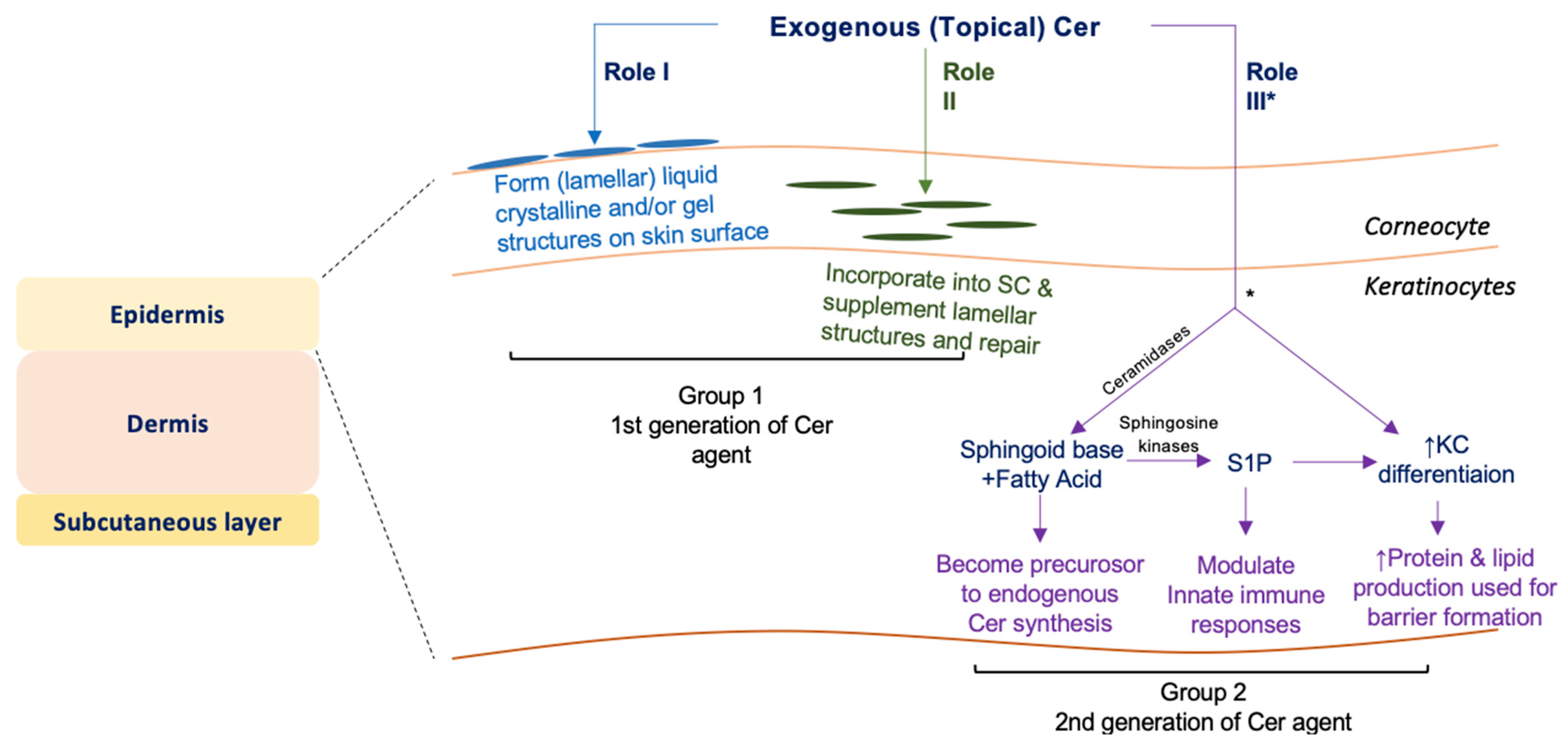
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schürer, N.Y.; Plewig, G.; Elias, P.M. Stratum corneum lipid function. Dermatology 1991, 183, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Kawana, M.; Miyamoto, M.; Ohno, Y.; Kihara, A. Comparative profiling and comprehensive quantification of stratum corneum ceramides in humans and mice by LC/MS/MS. J. Lipid Res. 2020, 61, 884–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. SKI Barrier Funct. 2016, 49, 8–26. [Google Scholar] [CrossRef]
- Uchida, Y.; Park, K. Ceramides in Skin Health and Disease: An Update. Am. J. Clin. Dermatol. 2021, 22, 853–866. [Google Scholar] [CrossRef]
- Bleck, O.; Abeck, D.; Ring, J.; Hoppe, U.; Vietzke, J.P.; Wolber, R.; Brandt, O.; Schreiner, V. Two ceramide subfractions detectable in Cer(AS) position by HPTLC in skin surface lipids of non-lesional skin of atopic eczema. J. Investig. Dermatol. 1999, 113, 894–900. [Google Scholar] [CrossRef] [Green Version]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, J.; Narita, H.; Kondo, N.; Hotta, M.; Takagi, Y.; Masukawa, Y.; Kitahara, T.; Takema, Y.; Koyano, S.; Yamazaki, S.; et al. Changes in the ceramide profile of atopic dermatitis patients. J. Investig. Dermatol. 2010, 130, 2511–2514. [Google Scholar] [CrossRef] [Green Version]
- Janssens, M.; van Smeden, J.; Gooris, G.S.; Bras, W.; Portale, G.; Caspers, P.J.; Vreeken, R.J.; Hankemeier, T.; Kezic, S.; Wolterbeek, R.; et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J. Lipid Res. 2012, 53, 2755–2766. [Google Scholar] [CrossRef] [Green Version]
- van Smeden, J.; Janssens, M.; Kaye, E.C.; Caspers, P.J.; Lavrijsen, A.P.; Vreeken, R.J.; Bouwstra, J.A. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp. Dermatol. 2014, 23, 45–52. [Google Scholar] [CrossRef]
- Cho, Y.; Lew, B.L.; Seong, K.; Kim, N.I. An inverse relationship between ceramide synthesis and clinical severity in patients with psoriasis. J. Korean Med. Sci. 2004, 19, 859–863. [Google Scholar] [CrossRef] [Green Version]
- Motta, S.; Monti, M.; Sesana, S.; Caputo, R.; Carelli, S.; Ghidoni, R. Ceramide composition of the psoriatic scale. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 1993, 1182, 147–151. [Google Scholar] [CrossRef]
- Tawada, C.; Kanoh, H.; Nakamura, M.; Mizutani, Y.; Fujisawa, T.; Banno, Y.; Seishima, M. Interferon-gamma decreases ceramides with long-chain fatty acids: Possible involvement in atopic dermatitis and psoriasis. J. Investig. Dermatol. 2014, 134, 712–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyumvuhore, R.; Michael-Jubeli, R.; Verzeaux, L.; Boudier, D.; Le Guillou, M.; Bordes, S.; Libong, D.; Tfayli, A.; Manfait, M.; Closs, B. Lipid organization in xerosis: The key of the problem? Int. J. Cosmet. Sci. 2018, 40, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.L.S.; Chen, S.C.; Osterberg, L.; Brandt, S.; von Grote, E.C.; Meckfessel, M.H. A daily skincare regimen with a unique ceramide and filaggrin formulation rapidly improves chronic xerosis, pruritus, and quality of life in older adults. Geriatr. Nurs. 2018, 39, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Danby, S.G.; Brown, K.; Higgs-Bayliss, T.; Chittock, J.; Albenali, L.; Cork, M.J. The Effect of an Emollient Containing Urea, Ceramide NP, and Lactate on Skin Barrier Structure and Function in Older People with Dry Skin. SKI Pharmacol. Physiol. 2016, 29, 135–147. [Google Scholar] [CrossRef]
- Lueangarun, S.; Tragulplaingam, P.; Sugkraroek, S.; Tempark, T. The 24-hr, 28-day, and 7-day post-moisturizing efficacy of ceramides 1,3,6-II containing moisturizing cream compared with hydrophilic cream on skin dryness and barrier disruption in senile xerosis treatment. Dermatol. Ther. 2019, 32, e13090. [Google Scholar] [CrossRef]
- Meckfessel, M.H.; Brandt, S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J. Am. Acad. Dermatol. 2014, 71, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Opálka, L.; Kováčik, A.; Pullmannová, P.; Maixner, J.; Vávrová, K. Effects of omega-O-acylceramide structures and concentrations in healthy and diseased skin barrier lipid membrane models. J. Lipid Res. 2020, 61, 219–228. [Google Scholar] [CrossRef]
- Pullmannová, P.; Ermakova, E.; Kováčik, A.; Opálka, L.; Maixner, J.; Zbytovská, J.; Kučerka, N.; Vávrová, K. Long and very long lamellar phases in model stratum corneum lipid membranes. J. Lipid Res. 2019, 60, 963–971. [Google Scholar] [CrossRef]
- Kaneko, T.; Tanaka, T.; Nagase, M. Agent for Protecting Skin and Hair Moisture. US Patent 635532, 2002. [Google Scholar]
- Berkers, T.; Visscher, D.; Gooris, G.S.; Bouwstra, J.A. Topically Applied Ceramides Interact with the Stratum Corneum Lipid Matrix in Compromised Ex Vivo Skin. Pharm. Res. 2018, 35, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahle, F.F.; Metz, H.; Wohlrab, J.; Neubert, R.H. Polyglycerol fatty acid ester surfactant-based microemulsions for targeted delivery of ceramide AP into the stratum corneum: Formulation, characterisation, in vitro release and penetration investigation. Eur. J. Pharm. Biopharm. 2012, 82, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Tessema, E.N.; Gebre-Mariam, T.; Frolov, A.; Wohlrab, J.; Neubert, R.H.H. Development and validation of LC/APCI-MS method for the quantification of oat ceramides in skin permeation studies. Anal. Bioanal. Chem. 2018, 410, 4775–4785. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y. Ceramide signaling in mammalian epidermis. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2014, 1841, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Robson, K.J.; Stewart, M.E.; Michelsen, S.; Lazo, N.D.; Downing, D.T. 6-Hydroxy-4-sphingenine in human epidermal ceramides. J. Lipid Res. 1994, 35, 2060–2068. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr.; Wang, E.; Mullins, R.E. Kinetics of long-chain (sphingoid) base biosynthesis in intact LM cells: Effects of varying the extracellular concentrations of serine and fatty acid precursors of this pathway. Biochemistry 1988, 27, 340–345. [Google Scholar] [CrossRef]
- Sjövall, P.; Skedung, L.; Gregoire, S.; Biganska, O.; Clément, F.; Luengo, G.S. Imaging the distribution of skin lipids and topically applied compounds in human skin using mass spectrometry. Sci. Rep. 2018, 8, 16683. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.B.; Sung, M.; Noh, M.; Kim, J.E.; Jang, J.; Kim, S.J.; Kim, J.W. Effective association of ceramide-coassembled lipid nanovehicles with stratum corneum for improved skin barrier function and enhanced skin penetration. Int. J. Pharm. 2020, 579, 119162. [Google Scholar] [CrossRef]
- Tessema, E.N.; Gebre-Mariam, T.; Paulos, G.; Wohlrab, J.; Neubert, R.H.H. Delivery of oat-derived phytoceramides into the stratum corneum of the skin using nanocarriers: Formulation, characterization and in vitro and ex-vivo penetration studies. Eur. J. Pharm. Biopharm. 2018, 127, 260–269. [Google Scholar] [CrossRef]
- Shin, K.O.; Kim, K.P.; Cho, Y.; Kang, M.K.; Kang, Y.H.; Lee, Y.M.; Ikushiro, H.; Yokota, M.; Yano, T.; Choe, S.J.; et al. Both sphingosine kinase 1 and 2 coordinately regulate cathelicidin antimicrobial peptide production during keratinocyte differentiation. J. Investig. Dermatol. 2019, 139, 492–494. [Google Scholar] [CrossRef] [Green Version]
- Uchida, Y.; Behne, M.; Quiec, D.; Elias, P.M.; Holleran, W.M. Vitamin C stimulates sphingolipid production and markers of barrier formation in submerged human keratinocyte cultures. J. Investig. Dermatol. 2001, 117, 1307–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.; Elias, P.M.; Shin, K.O.; Lee, Y.M.; Hupe, M.; Borkowski, A.W.; Gallo, R.L.; Saba, J.; Holleran, W.M.; Uchida, Y. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol. Cell. Biol. 2013, 33, 752–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.; Ikushiro, H.; Seo, H.S.; Shin, K.O.; Kim, Y.I.; Kim, J.Y.; Lee, Y.M.; Yano, T.; Holleran, W.M.; Elias, P.; et al. ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex. Proc. Natl. Acad. Sci. USA 2016, 113, E1334–E1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijesinghe, D.S.; Allegood, J.C.; Gentile, L.B.; Fox, T.E.; Kester, M.; Chalfant, C.E. Use of high performance liquid chromatography-electrospray ionization-tandem mass spectrometry for the analysis of ceramide-1-phosphate levels. J. Lipid Res. 2010, 51, 641–651. [Google Scholar] [CrossRef] [Green Version]
- Uchida, Y.; Celli, A. A Method to Investigate the Epidermal Permeability Barrier In Vitro. In Molecular Dermatology Methods and Protocols; Methods in Molecular, Biology; Botchkareva, N.V., Westgate, G.E., Walker, J.M., Eds.; Springer: New York, NY, USA, 2020; pp. 73–90. [Google Scholar]
- Dlugosz, A.A.; Yuspa, S.H. Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase C. J. Cell. Biol. 1993, 120, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Lampe, M.A.; Williams, M.L.; Elias, P.M. Human epidermal lipids: Characterization and modulations during differentiation. J. Lipid Res. 1983, 24, 131–140. [Google Scholar] [CrossRef]
- Ponec, M.; Weerheim, A.; Kempenaar, J.; Mommaas, A.M.; Nugteren, D.H. Lipid composition of cultured human keratinocytes in relation to their differentiation. J. Lipid Res. 1988, 29, 949–961. [Google Scholar] [CrossRef]
- Howell, M.D.; Gallo, R.L.; Boguniewicz, M.; Jones, J.F.; Wong, C.; Streib, J.E.; Leung, D.Y. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity 2006, 24, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, K.; Gallo, R.L. Antimicrobial peptides in human skin disease. Eur. J. Dermatol. 2008, 18, 11–21. [Google Scholar] [CrossRef]
- Uchida, Y.; Park, K. Anti-microbial Peptides in Skin Barrier Functions. J. SKI Barrier Res. 2013, 15, 87–88. [Google Scholar]
- Park, K.; Elias, P.M.; Oda, Y.; Mackenzie, D.; Mauro, T.; Holleran, W.M.; Uchida, Y. Regulation of Cathelicidin Antimicrobial Peptide Expression by an Endoplasmic Reticulum (ER) Stress Signaling, Vitamin D Receptor-independent Pathway. J. Biol. Chem. 2011, 286, 34121–34130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, Y.; Nardo, A.D.; Collins, V.; Elias, P.M.; Holleran, W.M. De novo ceramide synthesis participates in the ultraviolet B irradiation-induced apoptosis in undifferentiated cultured human keratinocytes. J. Investig. Dermatol. 2003, 120, 662–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, Y.; Murata, S.; Schmuth, M.; Behne, M.J.; Lee, J.D.; Ichikawa, S.; Elias, P.M.; Hirabayashi, Y.; Holleran, W.M. Glucosylceramide synthesis and synthase expression protect against ceramide-induced stress. J. Lipid Res. 2002, 43, 1293–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Hong, I.; Hwang, J.S.; Choi, J.K.; Rho, H.S.; Kim, D.H.; Chang, I.; Lee, S.H.; Lee, M.O.; Hwang, J.S. Phytosphingosine stimulates the differentiation of human keratinocytes and inhibits TPA-induced inflammatory epidermal hyperplasia in hairless mouse skin. Mol. Med. 2006, 12, 17–24. [Google Scholar] [CrossRef]
- Sigruener, A.; Tarabin, V.; Paragh, G.; Liebisch, G.; Koehler, T.; Farwick, M.; Schmitz, G. Effects of sphingoid bases on the sphingolipidome in early keratinocyte differentiation. Exp. Dermatol. 2013, 22, 677–679. [Google Scholar] [CrossRef]
- Loiseau, N.; Obata, Y.; Moradian, S.; Sano, H.; Yoshino, S.; Aburai, K.; Takayama, K.; Sakamoto, K.; Holleran, W.M.; Elias, P.M.; et al. Altered sphingoid base profiles predict compromised membrane structure and permeability in atopic dermatitis. J. Dermatol. Sci. 2013, 72, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Houben, E.; Holleran, W.M.; Yaginuma, T.; Mao, C.; Obeid, L.M.; Rogiers, V.; Takagi, Y.; Elias, P.M.; Uchida, Y. Differentiation-associated expression of ceramidase isoforms in cultured keratinocytes and epidermis. J. Lipid Res. 2006, 47, 1063–1070. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.K.; Crumrine, D.; Ackerman, L.D.; Santiago, J.L.; Roelandt, T.; Uchida, Y.; Hupe, M.; Fabriàs, G.; Abad, J.L.; Rice, R.H.; et al. Cellular Changes that Accompany Shedding of Human Corneocytes. J. Investig. Dermatol. 2012, 132, 2430–2439. [Google Scholar] [CrossRef] [Green Version]
- Stiban, J.; Fistere, D.; Colombini, M. Dihydroceramide hinders ceramide channel formation: Implications on apoptosis. Apoptosis 2006, 11, 773–780. [Google Scholar] [CrossRef]
- Fabrias, G.; Muñoz-Olaya, J.; Cingolani, F.; Signorelli, P.; Casas, J.; Gagliostro, V.; Ghidoni, R. Dihydroceramide desaturase and dihydrosphingolipids: Debutant players in the sphingolipid arena. Prog. Lipid Res. 2012, 51, 82–94. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, K.-O.; Mihara, H.; Ishida, K.; Uchida, Y.; Park, K. Exogenous Ceramide Serves as a Precursor to Endogenous Ceramide Synthesis and as a Modulator of Keratinocyte Differentiation. Cells 2022, 11, 1742. https://doi.org/10.3390/cells11111742
Shin K-O, Mihara H, Ishida K, Uchida Y, Park K. Exogenous Ceramide Serves as a Precursor to Endogenous Ceramide Synthesis and as a Modulator of Keratinocyte Differentiation. Cells. 2022; 11(11):1742. https://doi.org/10.3390/cells11111742
Chicago/Turabian StyleShin, Kyong-Oh, Hisashi Mihara, Kenya Ishida, Yoshikazu Uchida, and Kyungho Park. 2022. "Exogenous Ceramide Serves as a Precursor to Endogenous Ceramide Synthesis and as a Modulator of Keratinocyte Differentiation" Cells 11, no. 11: 1742. https://doi.org/10.3390/cells11111742





