Dlx5/6 Expression Levels in Mouse GABAergic Neurons Regulate Adult Parvalbumin Neuronal Density and Anxiety/Compulsive Behaviours
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Behavioural Tests
- −
- Open Field Test with object exploration
- −
- Marble burying test (MBT)
- −
- Nest building test
2.3. Immunohistochemistry
2.4. In Situ Hybridisation
2.5. Quantification of PV Neuronal Density
2.6. Reverse Transcription Quantitative PCR (RT-qPCR)
2.7. Statistical Analysis
3. Results
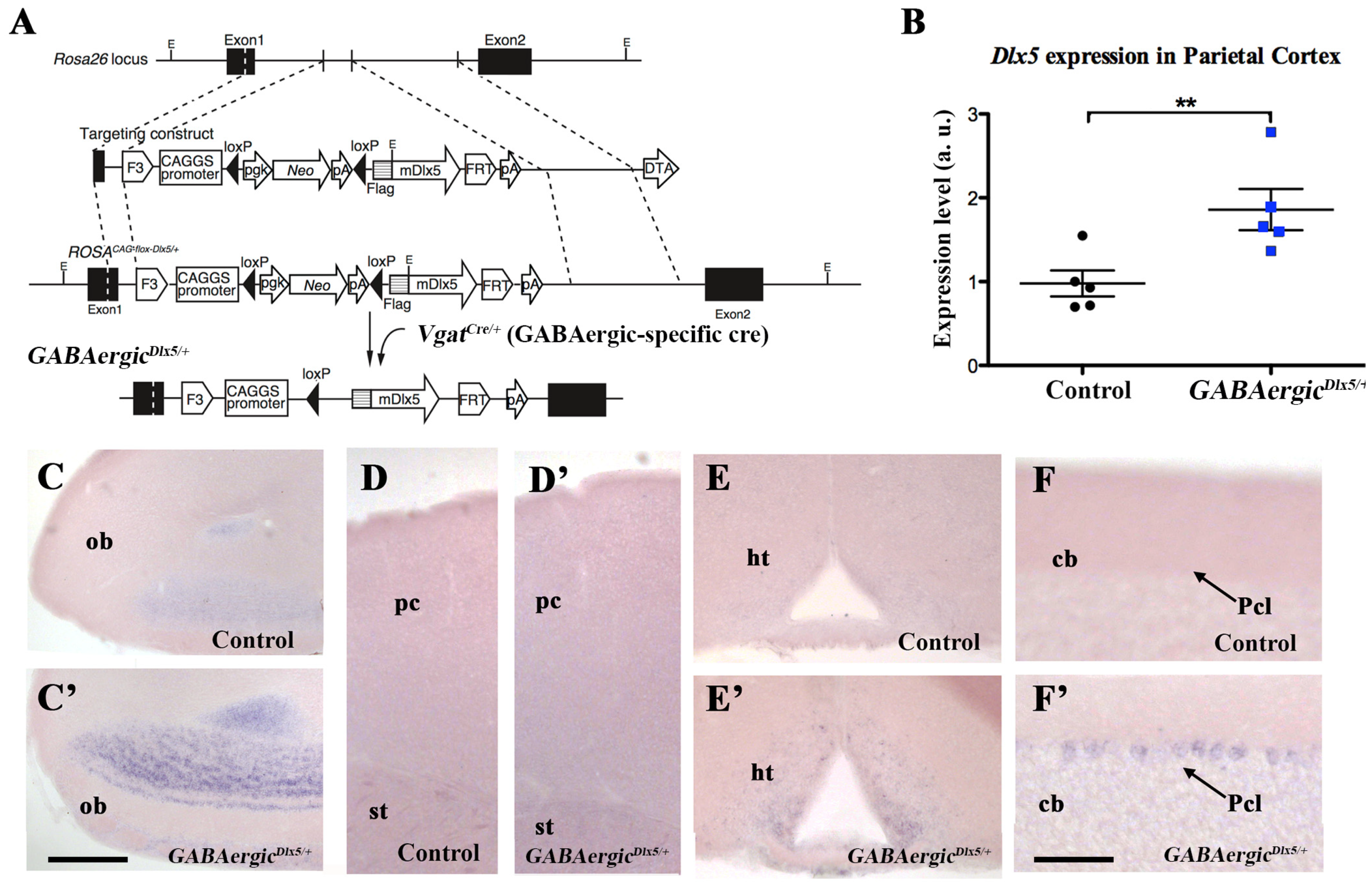
3.1. Deregulation of Dlx5/6 Expression in Mouse GABAergic Neurons
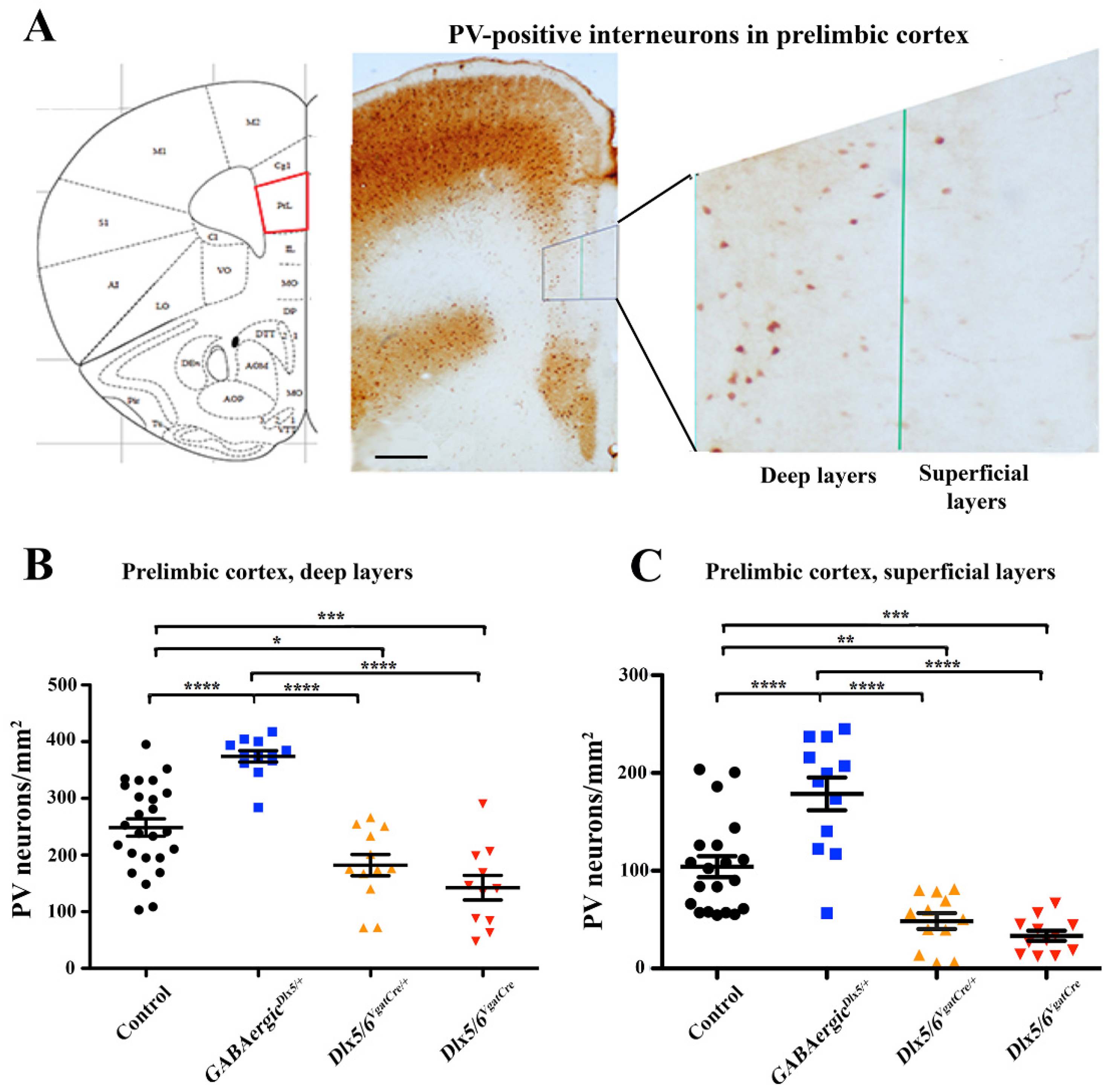
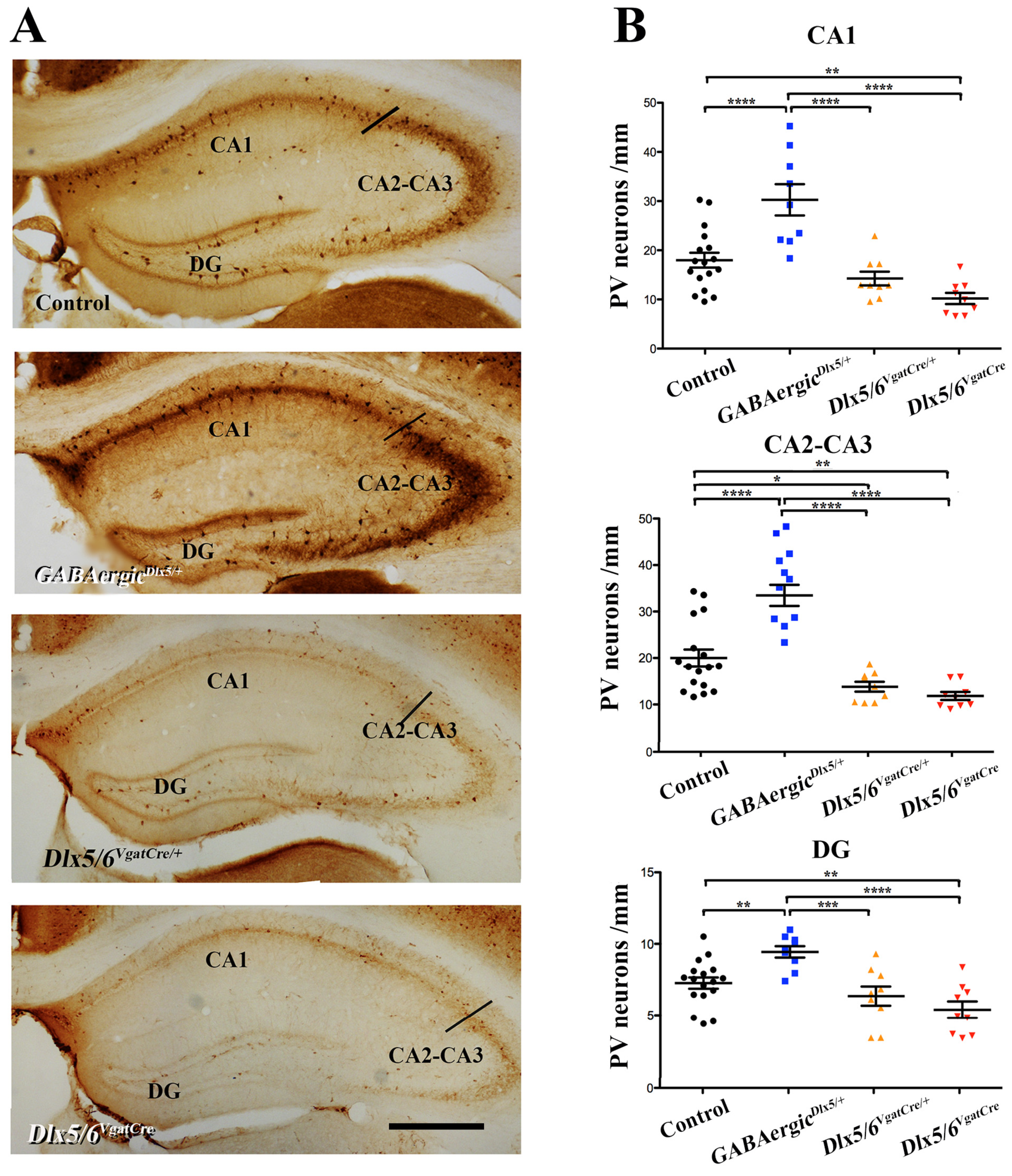
3.2. Effects of Dlx5/6 Deregulation in GABAergic Neurons on Adult PV-Interneurons Density
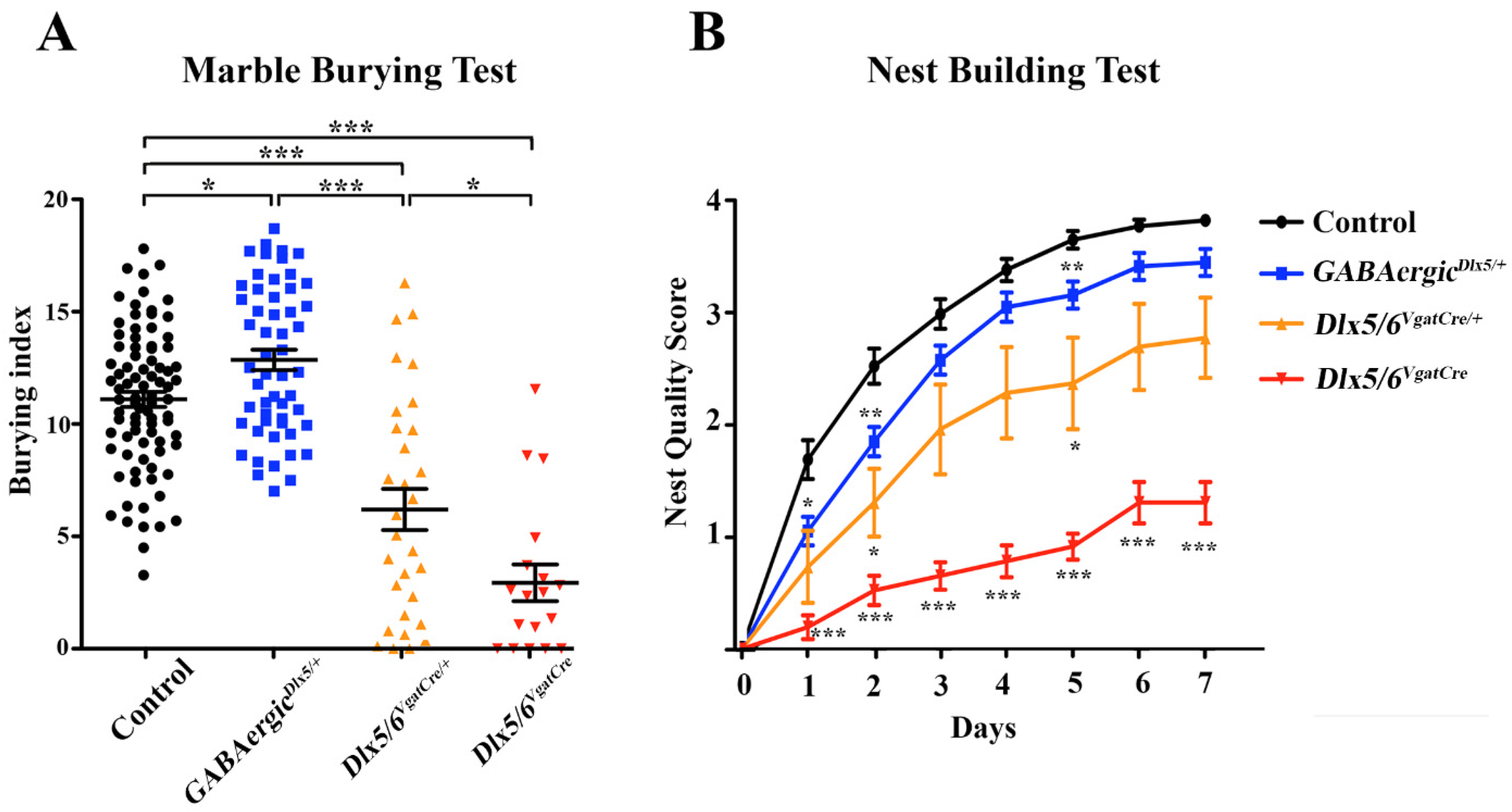
3.3. Behavioural Consequences of Dlx5/6 Expression Deregulation in GABAergic Neurons
- −
- Open Field Test with object exploration
- −
- Marble burying test (MBT)
- −
- Nest building test
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, B.; Yang, X.; Ye, L.; Liu, R.; Ye, B.; Du, W.; Shen, F.; Li, Q.; Guo, F.; Liu, J.; et al. Ketamine activated glutamatergic neurotransmission by GABAergic disinhibition in the medial prefrontal cortex. Neuro Pharmacol. 2021, 194, 108382. [Google Scholar] [CrossRef] [PubMed]
- Suyama, S.; Yada, T. New insight into GABAergic neurons in the hypothalamic feeding regulation. J. Physiol. Sci. 2018, 68, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Mi, D.; Llorca, A.; Marín, O. Development and Functional Diversification of Cortical Interneurons. Neuron 2018, 100, 294–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef] [Green Version]
- Tasic, B.; Yao, Z.; Graybuck, L.T.; Smith, K.A.; Nguyen, T.N.; Bertagnolli, D.; Goldy, J.; Garren, E.; Economo, M.N.; Viswanathan, S.; et al. Shared and distinct transcriptomic cell types across neocortical areas. Nature 2018, 563, 72–78. [Google Scholar] [CrossRef]
- Laclef, C.; Métin, C. Conserved rules in embryonic development of cortical interneurons. Semin. Cell Dev. Biol. 2018, 76, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Hussman, J.P. Suppressed GABAergic inhibition as a common factor in suspected etiologies of autism. J. Autism. Dev. Disord. 2001, 31, 247–248. [Google Scholar] [CrossRef]
- Rubenstein, J.L.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Contractor, A.; Ethell, I.M.; Portera-Cailliau, C. Cortical interneurons in autism. Nat. Neurosci. 2021, 24, 1648–1659. [Google Scholar] [CrossRef]
- Acampora, D.; Merlo, G.R.; Paleari, L.; Zerega, B.; Postiglione, M.P.; Mantero, S.; Bober, E.; Barbieri, O.; Simeone, A.; Levi, G. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development 1999, 126, 3795–3809. [Google Scholar] [CrossRef]
- Merlo, G.R.; Zerega, B.; Paleari, L.; Trombino, S.; Mantero, S.; Levi, G. Multiple functions of Dlx genes. Int. J. Dev. Biol. 2000, 44, 619–626. [Google Scholar] [PubMed]
- Perera, M.; Merlo, G.R.; Verardo, S.; Paleari, L.; Corte, G.; Levi, G. Defective neuronogenesis in the absence of Dlx5. Mol. Cell Neurosci. 2004, 25, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Testa, J.R. DLX Genes: Roles in Development and Cancer. Cancers 2021, 13, 3005. [Google Scholar] [CrossRef] [PubMed]
- Eisenstat, D.D.; Liu, J.K.; Mione, M.; Zhong, W.; Yu, G.; Anderson, S.A.; Ghattas, I.; Puelles, L.; Rubenstein, J.L. DLX-1, DLX-2, and DLX-5 expression define distinct stages of basal forebrain differentiation. J. Comp. Neurol. 1999, 414, 217–237. [Google Scholar] [CrossRef]
- de Lombares, C.; Heude, E.; Alfama, G.; Fontaine, A.; Hassouna, R.; Vernochet, C.; de Chaumont, F.; Olivo-Marin, C.; Ey, E.; Parnaudeau, S.; et al. Dlx5 and Dlx6 expression in GABAergic neurons controls behavior, metabolism, healthy aging and lifespan. Aging 2019, 11, 6638–6656. [Google Scholar] [CrossRef] [PubMed]
- Depew, M.J.; Lufkin, T.; Rubenstein, J.L. Specification of jaw subdivisions by Dlx genes. Science 2002, 298, 381–385. [Google Scholar] [CrossRef]
- Beverdam, A.; Merlo, G.R.; Paleari, L.; Mantero, S.; Genova, F.; Barbieri, O.; Janvier, P.; Levi, G. Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: Mirror of the past? Genesis 2002, 34, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Depew, M.J.; Liu, J.K.; Long, J.E.; Presley, R.; Meneses, J.J.; Pedersen, R.A.; Rubenstein, J.L. Dlx5 regulates regional development of the branchial arches and sensory capsules. Development 1999, 126, 3831–3846. [Google Scholar] [CrossRef]
- Wang, Y.; Dye, C.A.; Sohal, V.; Long, J.E.; Estrada, R.C.; Roztocil, T.; Lufkin, T.; Deisseroth, K.; Baraban, S.C.; Rubenstein, J.L. Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical interneurons. J. Neurosci. 2010, 30, 5334–5345. [Google Scholar] [CrossRef]
- Cho, K.K.; Hoch, R.; Lee, A.T.; Patel, T.; Rubenstein, J.L.; Sohal, V.S. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/-) mice. Neuron 2015, 85, 1332–1343. [Google Scholar] [CrossRef] [Green Version]
- Levi, G.; de Lombares, C.; Giuliani, C.; Iannuzzi, V.; Aouci, R.; Garagnani, P.; Franceschi, C.; Grimaud-Hervé, D.; Narboux-Nême, N. DLX5/6 GABAergic Expression Affects Social Vocalization: Implications for Human Evolution. Mol. Biol. Evol. 2021, 38, 4748–4764. [Google Scholar] [CrossRef] [PubMed]
- Kuwajima, T.; Nishimura, I.; Yoshikawa, K. Necdin promotes GABAergic neuron differentiation in cooperation with Dlx homeodomain proteins. J. Neurosci. 2006, 26, 5383–5392. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Masuda, Y.; Iwai, K.; Ikeda, K.; Watanabe, K. A RING finger protein Praja1 regulates Dlx5-dependent transcription through its ubiquitin ligase activity for the Dlx/Msx-interacting MAGE/Necdin family protein, Dlxin-1. J. Biol. Chem. 2002, 277, 22541–22546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandal, M.J.; Zhang, P.; Hadjimichael, E.; Walker, R.L.; Chen, C.; Liu, S.; Won, H.; van Bakel, H.; Varghese, M.; Wang, Y.; et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 2018, 362, eaat8127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okita, C.; Meguro, M.; Hoshiya, H.; Haruta, M.; Sakamoto, Y.K.; Oshimura, M. A new imprinted cluster on the human chromosome 7q21-q31, identified by human-mouse monochromosomal hybrids. Genomics 2003, 81, 556–559. [Google Scholar] [CrossRef]
- Horike, S.; Cai, S.; Miyano, M.; Cheng, J.F.; Kohwi-Shigematsu, T. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat. Genet. 2005, 37, 31–40. [Google Scholar] [CrossRef]
- Rasmussen, M.B.; Kreiborg, S.; Jensen, P.; Bak, M.; Mang, Y.; Lodahl, M.; Budtz-Jorgensen, E.; Tommerup, N.; Tranebjaerg, L.; Rendtorff, N.D. Phenotypic subregions within the split-hand/foot malformation 1 locus. Hum. Genet. 2016, 135, 345–357. [Google Scholar] [CrossRef]
- Fazel Darbandi, S.; Poitras, L.; Monis, S.; Lindtner, S.; Yu, M.; Hatch, G.; Rubenstein, J.L.; Ekker, M. Functional consequences of I56ii Dlx enhancer deletion in the developing mouse forebrain. Dev. Biol. 2016, 1606, 30263–30269. [Google Scholar] [CrossRef]
- Assali, A.; Harrington, A.J.; Cowan, C.W. Emerging roles for MEF2 in brain development and mental disorders. Curr. Opin. Neurobiol. 2019, 59, 49–58. [Google Scholar] [CrossRef]
- Hamilton, S.P.; Woo, J.M.; Carlson, E.J.; Ghanem, N.; Ekker, M.; Rubenstein, J.L. Analysis of four DLX homeobox genes in autistic probands. BMC Genet. 2005, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, N.; Yamagata, T.; Mori, M.; Kuwajima, M.; Suwa, K.; Momoi, M.Y. Expression analysis and mutation detection of DLX5 and DLX6 in autism. Brain Dev. 2010, 32, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Poitras, L.; Yu, M.; Lesage-Pelletier, C.; Macdonald, R.B.; Gagne, J.P.; Hatch, G.; Kelly, I.; Hamilton, S.P.; Rubenstein, J.L.; Poirier, G.G.; et al. An SNP in an ultraconserved regulatory element affects Dlx5/Dlx6 regulation in the forebrain. Development 2010, 137, 3089–3097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.C.; Pauls, D.L.; Lange, C.; Sasanfar, R.; Santangelo, S.L. Common genetic variation in the GAD1 gene and the entire family of DLX homeobox genes and autism spectrum disorders. Am. J. Med. Genetics. Part. B Neuro Psychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2011, 156, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazel Darbandi, S.; Esau, C.; Lesage-Pelletier, C.; Monis, S.; Poitras, L.; Yu, M.; Perin, S.; Hatch, G.; Ekker, M. Increased Sociability in Mice Lacking Intergenic Dlx Enhancers. Front. Neurosci. 2021, 15, 718948. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Narboux-Neme, N.; Gitton, Y.; de Lombares, C.; Fontaine, A.; Alfama, G.; Kitazawa, T.; Kawamura, Y.; Heude, E.; Marshall, L.; et al. Probing the origin of matching functional jaws: Roles of Dlx5/6 in cranial neural crest cells. Sci. Rep. 2018, 8, 14975. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Mao, X.; Zhu, C.; Zou, X.; Peng, F.; Yang, W.; Li, B.; Li, G.; Ge, T.; Cui, R. GABAergic System Dysfunction in Autism Spectrum Disorders. Front. Cell Dev. Biol. 2021, 9, 781327. [Google Scholar] [CrossRef]
- Liu, Y.; Ouyang, P.; Zheng, Y.; Mi, L.; Zhao, J.; Ning, Y.; Guo, W. A Selective Review of the Excitatory-Inhibitory Imbalance in Schizophrenia: Underlying Biology, Genetics, Microcircuits, and Symptoms. Front. Cell Dev. Biol. 2021, 9, 664535. [Google Scholar] [CrossRef]
- Oh, W.J.; Noggle, S.A.; Maddox, D.M.; Condie, B.G. The mouse vesicular inhibitory amino acid transporter gene: Expression during embryogenesis, analysis of its core promoter in neural stem cells and a reconsideration of its alternate splicing. Gene 2005, 351, 39–49. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Grandy, D.K.; Low, M.J.; Paulus, M.P.; Geyer, M.A. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J. Neurosci. 1999, 19, 9550–9556. [Google Scholar] [CrossRef]
- Bally-Cuif, L.; Wassef, M. Ectopic induction and reorganization of Wnt-1 expression in quail/chick chimeras. Development 1994, 120, 3379–3394. [Google Scholar] [CrossRef]
- Vong, L.; Ye, C.; Yang, Z.; Choi, B.; Chua, S., Jr.; Lowell, B.B. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 2011, 71, 142–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellessort, B.; Le Cardinal, M.; Bachelot, A.; Narboux-Neme, N.; Garagnani, P.; Pirazzini, C.; Barbieri, O.; Mastracci, L.; Jonchere, V.; Duvernois-Berthet, E.; et al. Dlx5 and Dlx6 control uterine adenogenesis during post-natal maturation: Possible consequences for endometriosis. Hum. Mol. Genet. 2016, 25, 97–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, H.C.; Odriozola, P.; Cohodes, E.M.; Mandell, J.D.; Li, A.; Yang, R.; Hall, B.S.; Haberman, J.T.; Zacharek, S.J.; Liston, C.; et al. Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans. Proc. Natl. Acad. Sci. USA 2019, 116, 26970–26979. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, K.C. The relation between fear induced by novel stimulation and exploratory behavior. J. Comp. Physiol. Psychol. 1955, 48, 254–260. [Google Scholar] [CrossRef]
- Berlyne, D.E. Conflict, Arousal, and Curiosity; McGraw-Hill Book Company: New York, NY, USA, 1960; pp. xii, 350-xii, 350. [Google Scholar]
- Belzung, C.; Le Pape, G. Comparison of different behavioral test situations used in psychopharmacology for measurement of anxiety. Physiol. Behav. 1994, 56, 623–628. [Google Scholar] [CrossRef]
- Selten, M.; van Bokhoven, H.; Nadif Kasri, N. Inhibitory control of the excitatory/inhibitory balance in psychiatric disorders. F1000Research 2018, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Penzes, P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr. Mol. Med. 2015, 15, 146–167. [Google Scholar] [CrossRef]
- Hu, H.; Gan, J.; Jonas, P. Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science 2014, 345, 1255263. [Google Scholar] [CrossRef]
- Varga, C.; Oijala, M.; Lish, J.; Szabo, G.G.; Bezaire, M.; Marchionni, I.; Golshani, P.; Soltesz, I. Functional fission of parvalbumin interneuron classes during fast network events. Elife 2014, 3, e04006. [Google Scholar] [CrossRef]
- Marín, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 107–120. [Google Scholar] [CrossRef]
- Hashimoto, T.; Volk, D.W.; Eggan, S.M.; Mirnics, K.; Pierri, J.N.; Sun, Z.; Sampson, A.R.; Lewis, D.A. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J. Neurosci. 2003, 23, 6315–6326. [Google Scholar] [CrossRef] [PubMed]
- Amina, S.; Falcone, C.; Hong, T.; Wolf-Ochoa, M.W.; Vakilzadeh, G.; Allen, E.; Perez-Castro, R.; Kargar, M.; Noctor, S.; Martínez-Cerdeño, V. Chandelier Cartridge Density Is Reduced in the Prefrontal Cortex in Autism. Cereb. Cortex 2021, 31, 2944–2951. [Google Scholar] [CrossRef] [PubMed]
- Kaar, S.J.; Angelescu, I.; Marques, T.R.; Howes, O.D. Pre-frontal parvalbumin interneurons in schizophrenia: A meta-analysis of post-mortem studies. J. Neural. Transm. 2019, 126, 1637–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filice, F.; Vörckel, K.J.; Sungur, A.; Wöhr, M.; Schwaller, B. Reduction in parvalbumin expression not loss of the parvalbumin-expressing GABA interneuron subpopulation in genetic parvalbumin and shank mouse models of autism. Mol. Brain 2016, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umeda, K.; Iritani, S.; Fujishiro, H.; Sekiguchi, H.; Torii, Y.; Habuchi, C.; Kuroda, K.; Kaibuchi, K.; Ozaki, N. Immunohistochemical evaluation of the GABAergic neuronal system in the prefrontal cortex of a DISC1 knockout mouse model of schizophrenia. Synapse 2016, 70, 508–518. [Google Scholar] [CrossRef]
- Wöhr, M.; Orduz, D.; Gregory, P.; Moreno, H.; Khan, U.; Vörckel, K.J.; Wolfer, D.P.; Welzl, H.; Gall, D.; Schiffmann, S.N.; et al. Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Transl. Psychiatry 2015, 5, e525. [Google Scholar] [CrossRef]
- Lauber, E.; Filice, F.; Schwaller, B. Dysregulation of Parvalbumin Expression in the Cntnap2−/− Mouse Model of Autism Spectrum Disorder. Front. Mol. Neurosci. 2018, 11, 262. [Google Scholar] [CrossRef] [Green Version]
- Dienel, S.J.; Lewis, D.A. Alterations in cortical interneurons and cognitive function in schizophrenia. Neurobiol. Dis. 2019, 131, 104208. [Google Scholar] [CrossRef]
- Caballero, A.; Flores-Barrera, E.; Cass, D.K.; Tseng, K.Y. Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Struct. Funct. 2014, 219, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Harkness, J.H.; Gonzalez, A.E.; Bushana, P.N.; Jorgensen, E.T.; Hegarty, D.M.; Di Nardo, A.A.; Prochiantz, A.; Wisor, J.P.; Aicher, S.A.; Brown, T.E.; et al. Diurnal changes in perineuronal nets and parvalbumin neurons in the rat medial prefrontal cortex. Brain Struct. Funct. 2021, 226, 1135–1153. [Google Scholar] [CrossRef]
- Boogert, N.J.; Madden, J.R.; Morand-Ferron, J.; Thornton, A. Measuring and understanding individual differences in cognition. Philos Trans. R. Soc. Lond B Biol. Sci. 2018, 373, 20170280. [Google Scholar] [CrossRef] [PubMed]


| Dlx5 | Fw: 5′ TCT CTA GGA CTGACG CAA ACA 3′ |
| Rv: 5′ GTT ACA CGC CAT AGG GTC GC 3′ | |
| Pgk1 | Fw: 5′ AACCTCCGCTTTCATGTAGAG 3′ |
| Rv: 5′ GACATCTCCTAGTTTGGACAGTG 3′ | |
| Hprt | Fw: 5′ CTCATGGACTGATTATGGACAGGAC 3′ |
| Rv: 5′ GCAGGTCACCAAAGAACTTATAGCC 3′ | |
| Ppia | Fw: 5′ CAACCCCACCGTGTTCTTCG 3′ |
| Rv: 5′ GTGTAAAGTCCCACCCTGGC 3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aouci, R.; El Soudany, M.; Maakoul, Z.; Fontaine, A.; Kurihara, H.; Levi, G.; Narboux-Nême, N. Dlx5/6 Expression Levels in Mouse GABAergic Neurons Regulate Adult Parvalbumin Neuronal Density and Anxiety/Compulsive Behaviours. Cells 2022, 11, 1739. https://doi.org/10.3390/cells11111739
Aouci R, El Soudany M, Maakoul Z, Fontaine A, Kurihara H, Levi G, Narboux-Nême N. Dlx5/6 Expression Levels in Mouse GABAergic Neurons Regulate Adult Parvalbumin Neuronal Density and Anxiety/Compulsive Behaviours. Cells. 2022; 11(11):1739. https://doi.org/10.3390/cells11111739
Chicago/Turabian StyleAouci, Rym, Mey El Soudany, Zakaria Maakoul, Anastasia Fontaine, Hiroki Kurihara, Giovanni Levi, and Nicolas Narboux-Nême. 2022. "Dlx5/6 Expression Levels in Mouse GABAergic Neurons Regulate Adult Parvalbumin Neuronal Density and Anxiety/Compulsive Behaviours" Cells 11, no. 11: 1739. https://doi.org/10.3390/cells11111739
APA StyleAouci, R., El Soudany, M., Maakoul, Z., Fontaine, A., Kurihara, H., Levi, G., & Narboux-Nême, N. (2022). Dlx5/6 Expression Levels in Mouse GABAergic Neurons Regulate Adult Parvalbumin Neuronal Density and Anxiety/Compulsive Behaviours. Cells, 11(11), 1739. https://doi.org/10.3390/cells11111739








