Leonurine Protects Bone Mesenchymal Stem Cells from Oxidative Stress by Activating Mitophagy through PI3K/Akt/mTOR Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Preparation and Establishment of an Oxidative Stress Model In Vitro
2.2. Cell Vitality
2.2.1. CCK-8 Assays
2.2.2. Double Live/Dead Staining
2.2.3. Cell Apoptosis Analysis
2.2.4. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.2.5. Extraction of Proteins and Western Blot Analysis
2.3. Cell Differentiation
2.3.1. Alkaline Phosphatase (ALP) and Alizarin Red Staining
2.3.2. RNA and Protein Level Analysis
2.4. Intracellular ROS Measurements
2.4.1. Intracellular ROS Determination
2.4.2. Analysis of Intracellular ROS related mRNA Level
2.5. Mitophagy Accessibility
2.5.1. Measurement of Mitochondrial Membrane Potential
2.5.2. Colocalization of Mitochondria and Lysosome
2.5.3. Analysis of Protein Level
2.6. Pathway Investigation
2.6.1. Bibliometric Evaluation
2.6.2. PI3K/AKT/mTOR Pathway Analysis and Molecular Docking
2.7. Experiments on Animals
2.7.1. Animal Preparation
2.7.2. MicroCT and Histological Examination
2.8. Statistical Analysis
3. Results
3.1. Establishment of the Oxidative Stress Model In Vivo
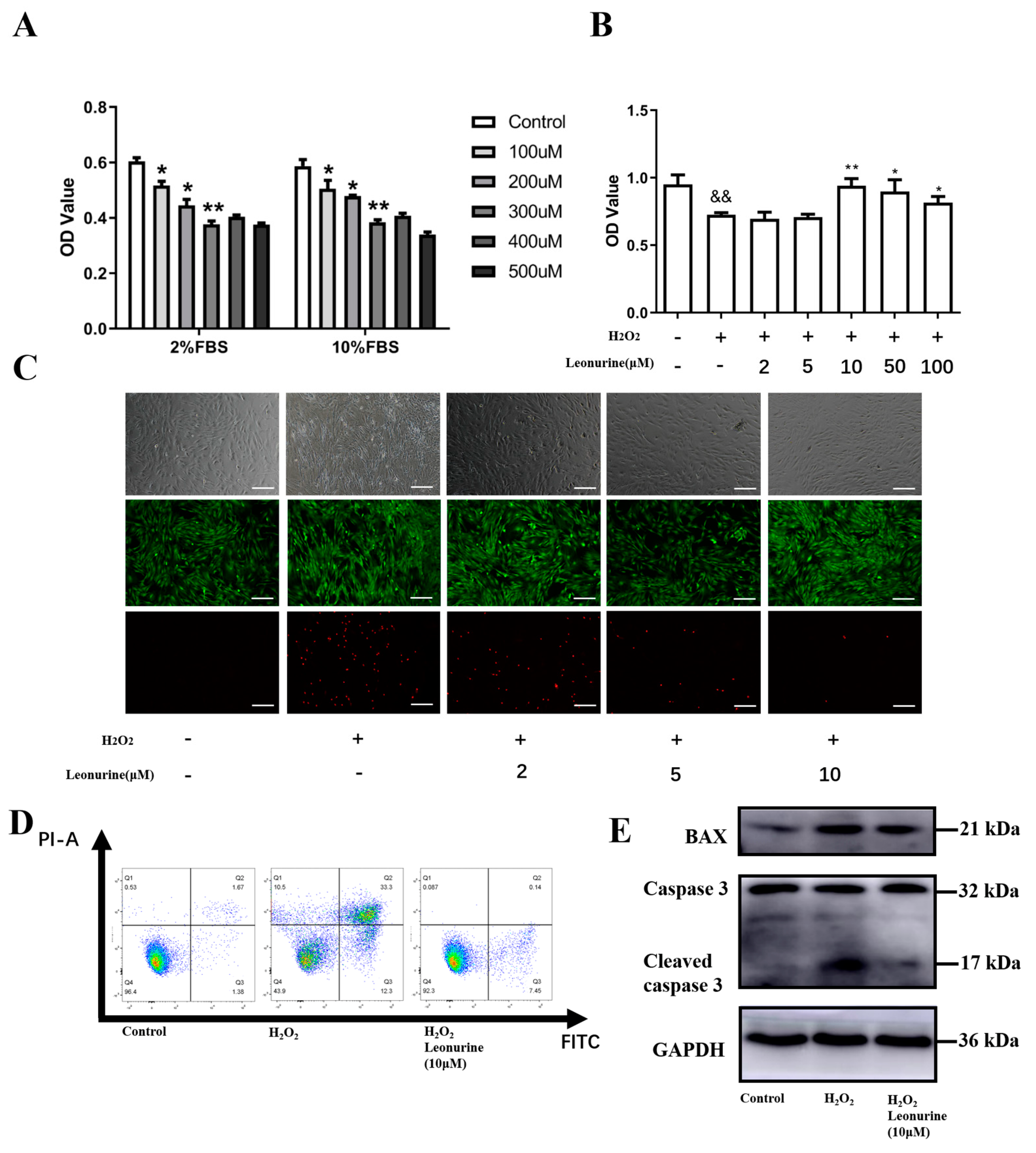
3.2. Leonurine Can Protect the Vitality of BMSCs from Oxidative Stress Damage
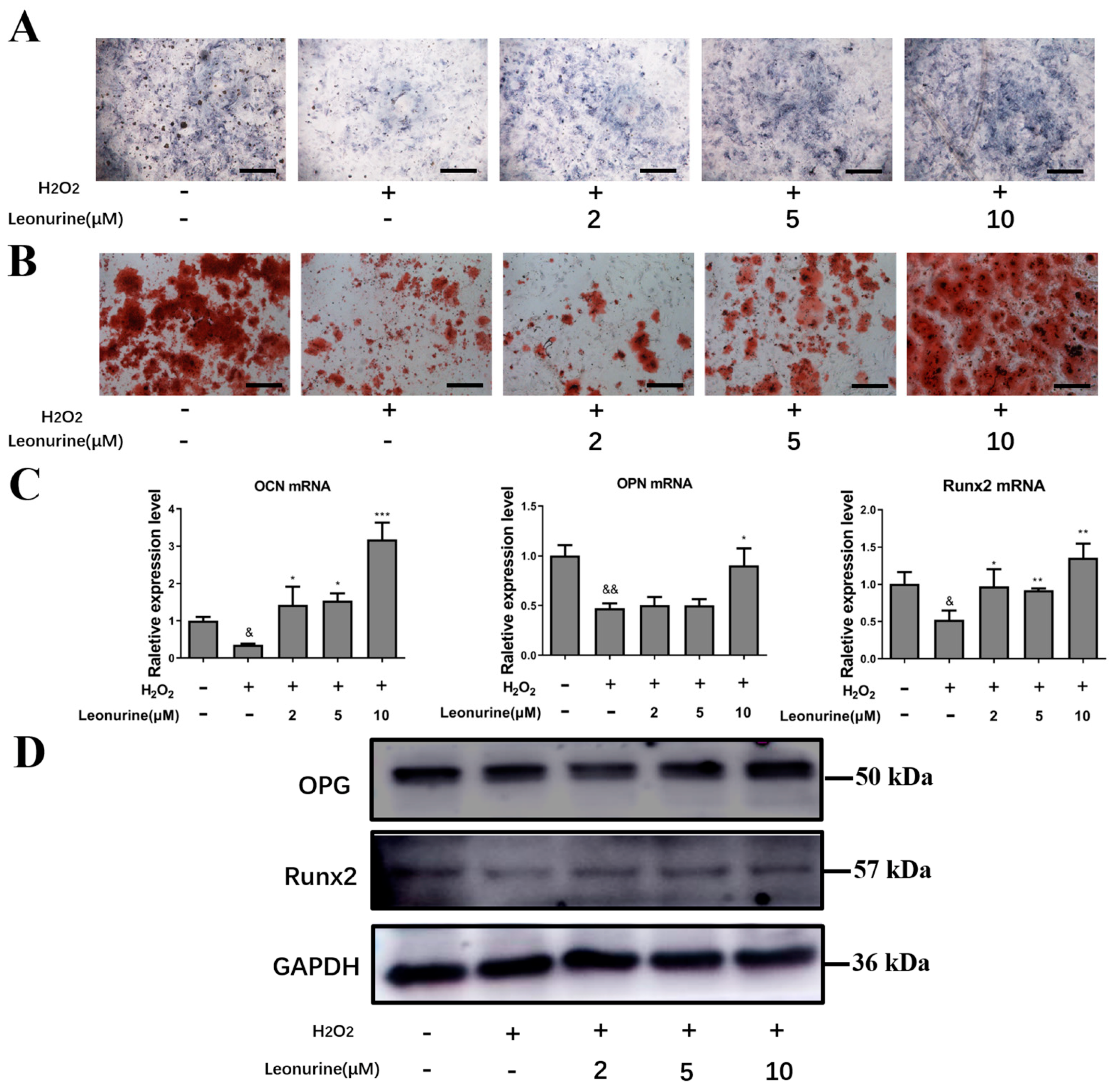
3.3. Leonurine Can Protect the Differentiation Capacity of BMSCs from Oxidative Stress Damage
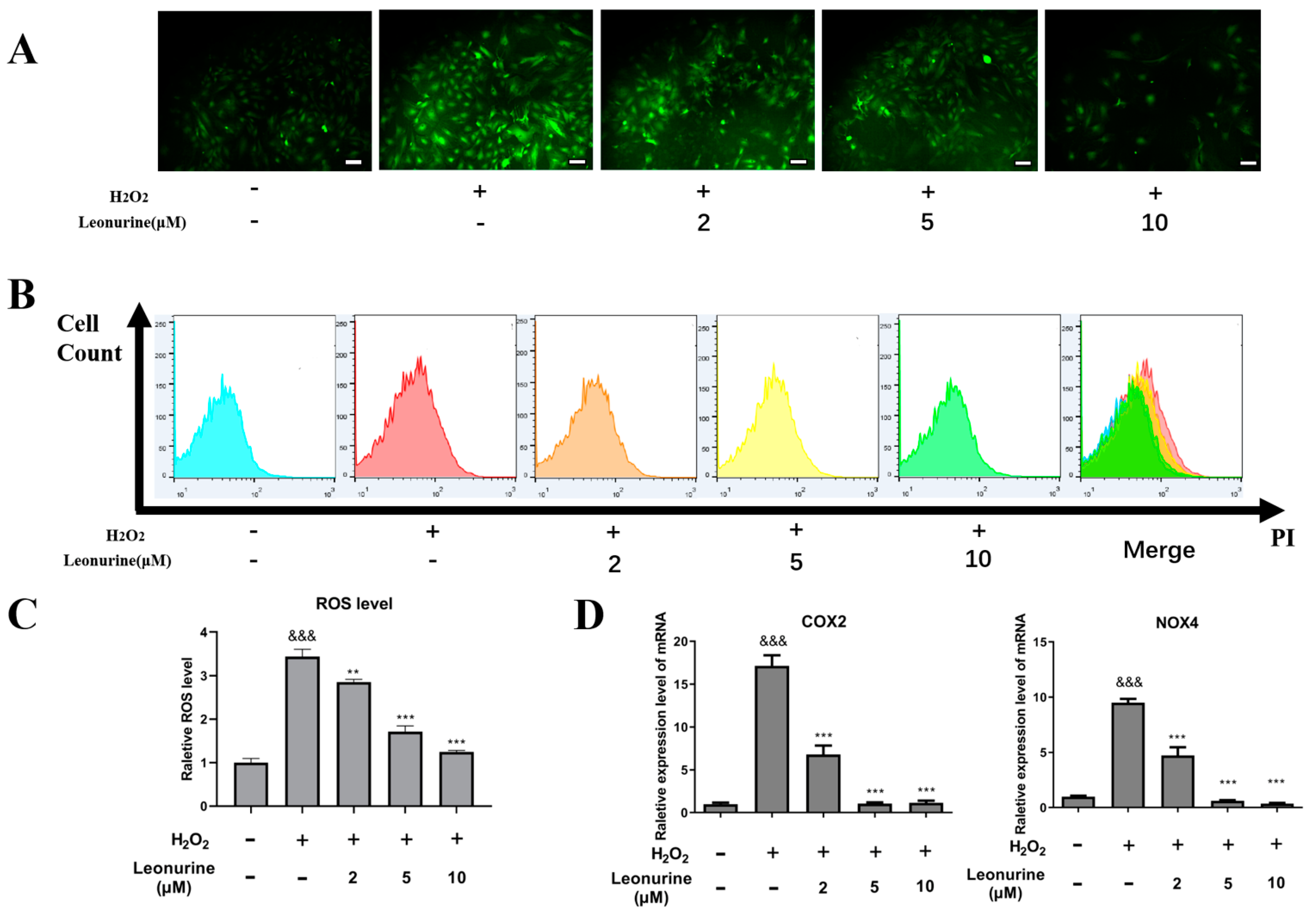
3.4. Leonurine Can Alleviate Intracellular Oxidative Stress of BMSCs
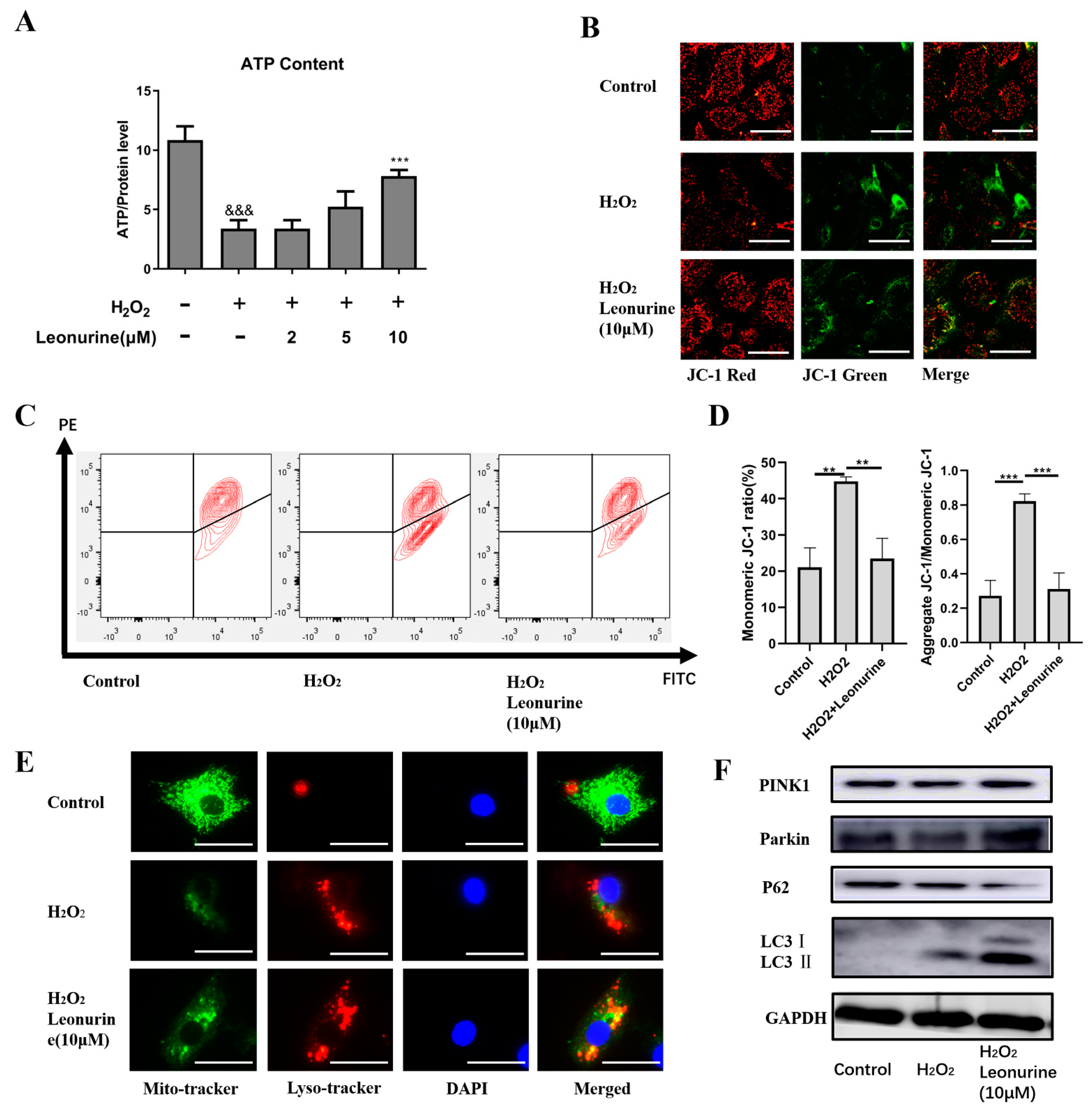
3.5. Leonurine Maintains Mitochondrial Quality Control by Activating Mitophagy
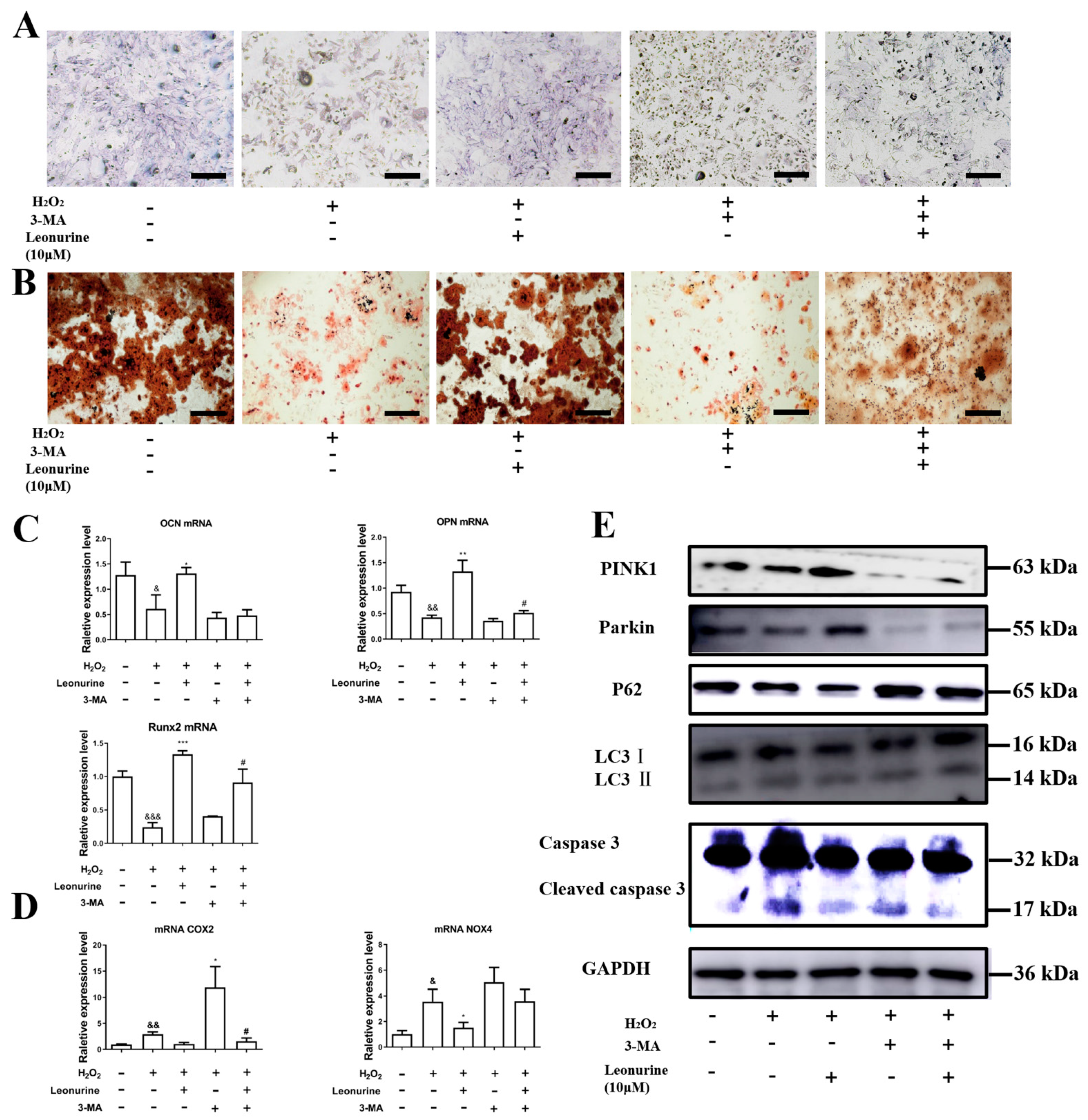
3.6. Inhibition of Mitophagy Will Block Leonurine Function
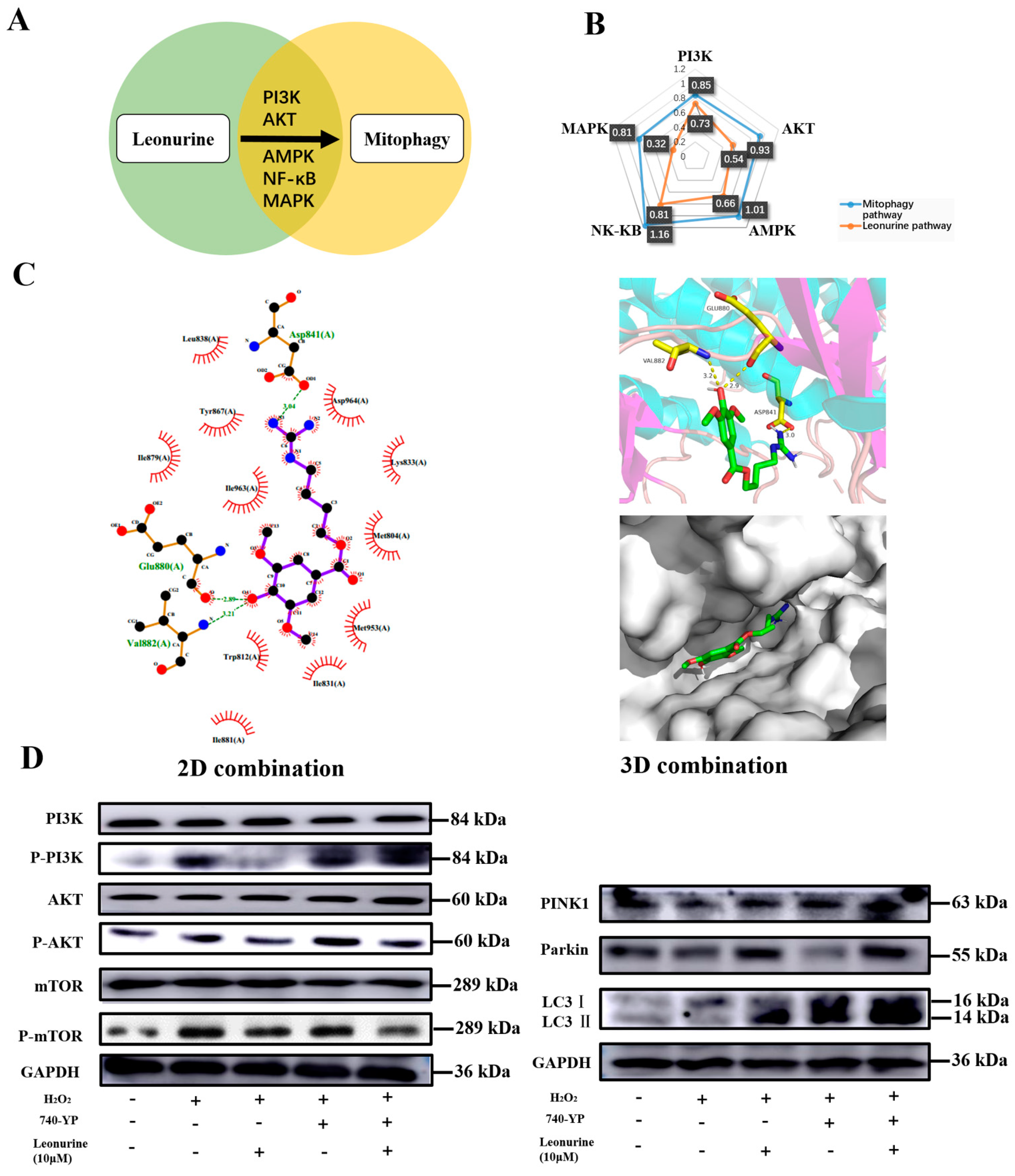
3.7. Analysis of the Leonurine-Implied Signaling Pathway
3.8. Leonurine Improves Bone Healing under Osteoporosis Conditions In Vitro
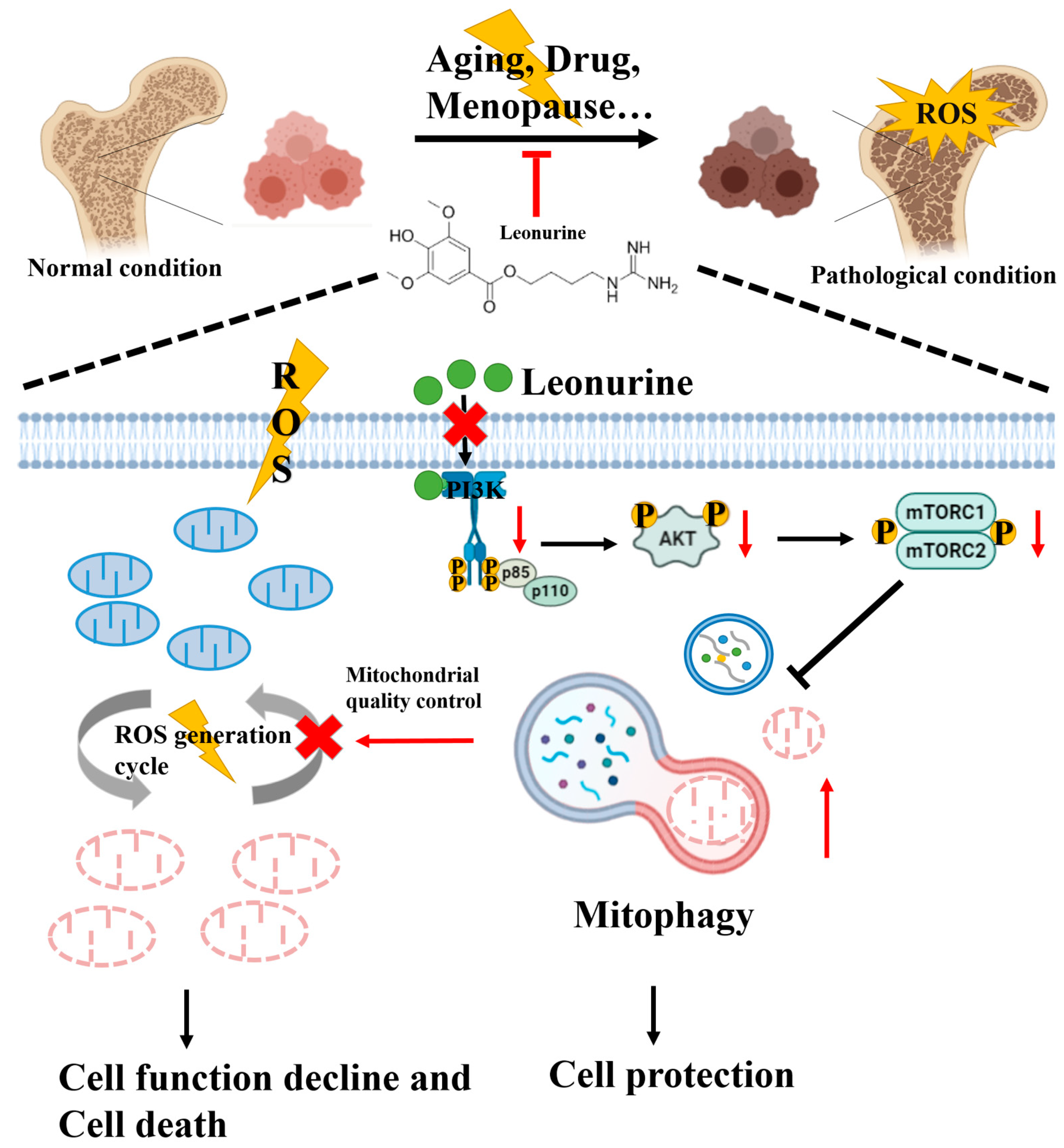
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leslie, W.D.; Morin, S.N. Osteoporosis epidemiology 2013: Implications for diagnosis, risk assessment, and treatment. Curr. Opin. Rheumatol. 2014, 26, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 1994; Volume 843, pp. 1–129.
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardura, J.A.; Alonso, V.; Esbrit, P.; Friedman, P.A. Oxidation inhibits PTH receptor signaling and trafficking. Biochem. Biophys. Res. Commun. 2017, 482, 1019–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhang, Q.; Yuan, Y.; Xin, N.; He, K.; Huang, Y.; Tang, H.; Gong, P. Sema3a inhibits the differentiation of Raw264.7 cells to osteoclasts under 2Gy radiation by reducing inflammation. PLoS ONE 2018, 13, e0200000. [Google Scholar] [CrossRef] [Green Version]
- Yishake, M.; Yasen, M.; Jiang, L.; Liu, W.; Xing, R.; Chen, Q.; Lin, H.; Dong, J. Effects of combined teriparatide and zoledronic acid on posterior lumbar vertebral fusion in an aged ovariectomized rat model of osteopenia. J. Orthop. Res. 2018, 36, 937–944. [Google Scholar] [CrossRef]
- Jun, S.K.; Yoon, J.Y.; Mahapatra, C.; Park, J.H.; Kim, H.W.; Kim, H.R.; Lee, J.H.; Lee, H.H. Ceria-incorporated MTA for accelerating odontoblastic differentiation via ROS downregulation. Dent. Mater. 2019, 35, 1291–1299. [Google Scholar] [CrossRef]
- Gao, J.; Feng, Z.; Wang, X.; Zeng, M.; Liu, J.; Han, S.; Xu, J.; Chen, L.; Cao, K.; Long, J.; et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018, 25, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, I.; Tomita, T.; Miyazaki, S.; Ozawa, E.; Yamamoto, L.A.; Sugimoto, T. Bazedoxifene Ameliorates Homocysteine-Induced Apoptosis and Accumulation of Advanced Glycation End Products by Reducing Oxidative Stress in MC3T3-E1 Cells. Calcif. Tissue Int. 2017, 100, 286–297. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. Quercetin as an Agent for Protecting the Bone: A Review of the Current Evidence. Int. J. Mol. Sci. 2020, 21, 6448. [Google Scholar] [CrossRef]
- Minamikawa, H.; Yamada, M.; Deyama, Y.; Suzuki, K.; Kaga, M.; Yawaka, Y.; Ogawa, T. Effect of N-acetylcysteine on rat dental pulp cells cultured on mineral trioxide aggregate. J. Endod. 2011, 37, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Tsukimura, N.; Ikeda, T.; Sugita, Y.; Att, W.; Kojima, N.; Kubo, K.; Ueno, T.; Sakurai, K.; Ogawa, T. N-acetyl cysteine as an osteogenesis-enhancing molecule for bone regeneration. Biomaterials 2013, 34, 6147–6156. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, R.; Wang, L.; Zhu, R.; Liu, H.; Guo, Y.; Zhao, B.; Zhao, S.; Tang, J.; Li, Y.; et al. Rehmanniae Radix in osteoporosis: A review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology. J. Ethnopharmacol. 2017, 198, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Xue, L.; Severino, R.P.; Gao, S.; Niu, J.; Qin, L.P.; Zhang, D.; Brömme, D. Salvia miltiorrhiza: An ancient Chinese herbal medicine as a source for anti-osteoporotic drugs. J. Ethnopharmacol. 2014, 155, 1401–1416. [Google Scholar] [CrossRef]
- Xi, H.R.; Ma, H.P.; Yang, F.F.; Gao, Y.H.; Zhou, J.; Wang, Y.Y.; Li, W.Y.; Xian, C.J.; Chen, K.M. Total flavonoid extract of Epimedium herb increases the peak bone mass of young rats involving enhanced activation of the AC10/cAMP/PKA/CREB pathway. J. Ethnopharmacol. 2018, 223, 76–87. [Google Scholar] [CrossRef]
- Loh, K.P.; Qi, J.; Tan, B.K.; Liu, X.H.; Wei, B.G.; Zhu, Y.Z. Leonurine protects middle cerebral artery occluded rats through antioxidant effect and regulation of mitochondrial function. Stroke 2010, 41, 2661–2668. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Guo, W.; Wen, Y.; Xiong, Q.; Liu, H.; Wu, J.; Zou, Y.; Zhu, Y. SCM-198 attenuates early atherosclerotic lesions in hypercholesterolemic rabbits via modulation of the inflammatory and oxidative stress pathways. Atherosclerosis 2012, 224, 43–50. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, Z.; Hu, N.; Liang, X.; Huang, W. Leonurine Hydrochloride Suppresses Inflammatory Responses and Ameliorates Cartilage Degradation in Osteoarthritis via NF-kappaB Signaling Pathway. Inflammation 2020, 43, 146–154. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Mu, S.; Man, X.; Ba, G.; Guo, R.; Li, Y.; Zhou, L.; Yang, L.; Fu, Q. Leonurine hydrochloride promotes osteogenic differentiation and increases osteoblastic bone formation in ovariectomized mice by Wnt/beta-catenin pathway. Biochem. Biophys. Res. Commun. 2018, 504, 941–948. [Google Scholar] [CrossRef]
- Yuan, F.L.; Xu, R.S.; Jiang, D.L.; He, X.L.; Su, Q.; Jin, C.; Li, X. Leonurine hydrochloride inhibits osteoclastogenesis and prevents osteoporosis associated with estrogen deficiency by inhibiting the NF-kappaB and PI3K/Akt signaling pathways. Bone 2015, 75, 128–137. [Google Scholar] [CrossRef]
- Lane, R.K.; Hilsabeck, T.; Rea, S.L. The role of mitochondrial dysfunction in age-related diseases. Biochim. Biophys. Acta 2015, 1847, 1387–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shum, L.C.; White, N.S.; Nadtochiy, S.M.; Bentley, K.L.D.M.; Brookes, P.S.; Jonason, J.H.; Eliseev, R.A. Cyclophilin D Knock-Out Mice Show Enhanced Resistance to Osteoporosis and to Metabolic Changes Observed in Aging Bone. PLoS ONE 2016, 11, e0155709. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirotti, C.; Rizza, S.; Giglio, P.; Poerio, N.; Allega, M.F.; Claps, G.; Pecorari, C.; Lee, J.H.; Benassi, B.; Barilà, D.; et al. Redox activation of ATM enhances GSNOR translation to sustain mitophagy and tolerance to oxidative stress. EMBO Rep. 2020, 22, e50500. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Pickles, S.; Vigie, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef] [Green Version]
- Cirotti, C.; Filomeni, G. ATM plays antioxidant, boosting mitophagy via denitrosylation. Autophagy 2020, 17, 590–592. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, L.; Qin, D.; Xia, Y.; Zhou, Z.; Zhang, X.; Wu, X. Carbon black suppresses the osteogenesis of mesenchymal stem cells: The role of mitochondria. Part. Fibre Toxicol. 2018, 15, 16. [Google Scholar] [CrossRef]
- Jing, X.; Du, T.; Yang, X.; Zhang, W.; Wang, G.; Liu, X.; Li, T.; Jiang, Z. Desferoxamine protects against glucocorticoid-induced osteonecrosis of the femoral head via activating HIF-1alpha expression. J. Cell. Physiol. 2020, 235, 9864–9875. [Google Scholar] [CrossRef]
- Zhang, F.; Peng, W.; Zhang, J.; Dong, W.; Wu, J.; Wang, T.; Xie, Z. P53 and Parkin co-regulate mitophagy in bone marrow mesenchymal stem cells to promote the repair of early steroid-induced osteonecrosis of the femoral head. Cell Death Dis. 2020, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Xu, X.; Tong, Z.; Yu, Q.; Lin, Y.; Kuang, W. Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: A possible mechanism of age related osteoporosis. Bone Res. 2015, 3, 15003. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Wu, S.; Wang, J.; Wang, J.; Yan, Y.; Zhu, M.; Zhang, D.; Jiang, C.; Liu, T. Oxidative stress induced by NOX2 contributes to neuropathic pain via plasma membrane translocation of PKCepsilon in rat dorsal root ganglion neurons. J. Neuroinflammation 2021, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; Carracedo, J.; de Sequera, P.; Bodega, G.; Perez, R.; Alique, M.; Ramírez, R. A high magnesium concentration in citrate dialysate prevents oxidative stress and damage in human monocytes in vitro. Clin. Kidney J. 2021, 14, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Ulla, A.; Uchida, T.; Miki, Y.; Sugiura, K.; Higashitani, A.; Kobayashi, T.; Ohno, A.; Nakao, R.; Hirasaka, K.; Sakakibara, I.; et al. Morin attenuates dexamethasone-mediated oxidative stress and atrophy in mouse C2C12 skeletal myotubes. Arch. Biochem. Biophys. 2021, 704, 108873. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Gea, V.; Hilscher, M.; Rozenfeld, R.; Lim, M.P.; Nieto, N.; Werner, S.; Devi, L.A.; Friedman, S.L. Endoplasmic reticulum stress induces fibrogenic activity in hepatic stellate cells through autophagy. J. Hepatol. 2013, 59, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Schroder, K. NADPH oxidases in bone homeostasis and osteoporosis. Free Radic. Biol. Med. 2019, 132, 67–72. [Google Scholar] [CrossRef]
- Lomovsky, A.; Baburina, Y.; Odinokova, I.; Kobyakova, M.; Evstratova, Y.; Sotnikova, L.; Krestinin, R.; Krestinina, O. Melatonin Can Modulate the Effect of Navitoclax (ABT-737) in HL-60 Cells. Antioxidants 2020, 9, 1143. [Google Scholar] [CrossRef]
- Rowlands, D.J. Mitochondria dysfunction: A novel therapeutic target in pathological lung remodeling or bystander? Pharmacol. Ther. 2016, 166, 96–105. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Wu, W.; Zhu, Q.; Liu, X. Discovery of Leonuri and therapeutical applications: From bench to bedside. Pharmacol. Ther. 2018, 188, 26–35. [Google Scholar] [CrossRef]
- Cairns, G.; Thumiah-Mootoo, M.; Burelle, Y.; Khacho, M. Mitophagy: A New Player in Stem Cell Biology. Biology 2020, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.D.; Sun, J.L.; Zhu, C.H.; Tian, F.C.; Jiao, K.; Anderson, M.R.; Yiu, C.; Huang, C.; Jin, C.X.; Bergeron, B.E.; et al. Contribution of Mitophagy to Cell-Mediated Mineralization: Revisiting a 50-Year-Old Conundrum. Adv. Sci. 2018, 5, 1800873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Shi, X.; Weng, S.J.; Xie, J.; Tang, J.H.; Yan, D.Y.; Wang, B.Z.; Xie, Z.J.; Wu, Z.Y.; Yang, L. Vitamin K2 Can Rescue the Dexamethasone-Induced Downregulation of Osteoblast Autophagy and Mitophagy Thereby Restoring Osteoblast Function In Vitro and In Vivo. Front. Pharmacol. 2020, 11, 1209. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Deng, Z.; Ma, Y.; Jin, J.; Qi, F.; Li, S.; Liu, C.; Lyu, F.J.; Zheng, Q. The Role of Autophagy and Mitophagy in Bone Metabolic Disorders. Int. J. Biol. Sci. 2020, 16, 2675–2691. [Google Scholar] [CrossRef]
- Hu, Z.C.; Gong, L.F.; Li, X.B.; Fu, X.; Xuan, J.W.; Feng, Z.H.; Ni, W.F. Inhibition of PI3K/Akt/NF-kappaB signaling with leonurine for ameliorating the progression of osteoarthritis: In vitro and in vivo studies. J. Cell. Physiol. 2019, 234, 6940–6950. [Google Scholar] [CrossRef]
- Le Belle, J.E.; Orozco, N.M.; Paucar, A.A.; Saxe, J.P.; Mottahedeh, J.; Pyle, A.D.; Wu, H.; Kornblum, H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 2011, 8, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Dang, W.; Zhou, X.; Xu, L.; Wang, W.; Cao, W.; Chen, S.; Su, J.; Cai, X.; Xiao, S.; et al. PI3K-Akt-mTOR axis sustains rotavirus infection via the 4E-BP1 mediated autophagy pathway and represents an antiviral target. Virulence 2018, 9, 83–98. [Google Scholar] [CrossRef]
- Xie, C.; Yi, J.; Lu, J.; Nie, M.; Huang, M.; Rong, J.; Zhu, Z.; Chen, J.; Zhou, X.; Li, B.; et al. N-Acetylcysteine Reduces ROS-Mediated Oxidative DNA Damage and PI3K/Akt Pathway Activation Induced by Helicobacter pylori Infection. Oxid. Med. Cell. Longev. 2018, 2018, 1874985. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Feng, X.; Li, B.; Sha, J.; Wang, C.; Yang, T.; Cui, H.; Fan, H. Dexmedetomidine Protects Against Lipopolysaccharide-Induced Acute Kidney Injury by Enhancing Autophagy Through Inhibition of the PI3K/AKT/mTOR Pathway. Front. Pharmacol. 2020, 11, 128. [Google Scholar] [CrossRef]
- Singh, A.K.; Kashyap, M.P.; Tripathi, V.K.; Singh, S.; Garg, G.; Rizvi, S.I. Neuroprotection Through Rapamycin-Induced Activation of Autophagy and PI3K/Akt1/mTOR/CREB Signaling Against Amyloid-beta-Induced Oxidative Stress, Synaptic/Neurotransmission Dysfunction, and Neurodegeneration in Adult Rats. Mol. Neurobiol. 2017, 54, 5815–5828. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Gong, L.J.; Huang, J.M.; Jiang, C.; Yan, Z.Q. Pinocembrin alleviates glucocorticoid-induced apoptosis by activating autophagy via suppressing the PI3K/Akt/mTOR pathway in osteocytes. Eur. J. Pharmacol. 2020, 880, 173212. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Ren, H.; Qiu, T.; Zhang, Z.; Zhao, W.; Yu, X.; Huang, J.; Tang, J.; Liang, D.; Yao, Z.; et al. Mammalian target of rapamycin as a therapeutic target in osteoporosis. J. Cell. Physiol. 2018, 233, 3929–3944. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, D.P.; Praharaj, P.P.; Bhol, C.S.; Mahapatra, K.K.; Patra, S.; Behera, B.P.; Mishra, S.R.; Bhutia, S.K. The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics. Semin. Cancer Biol. 2020, 66, 45–58. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, J.; Li, H.; Yang, B.; Wang, J.; He, Q.; Weng, Q. Emerging views of OPTN (optineurin) function in the autophagic process associated with disease. Autophagy 2021, 18, 73–85. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Peng, Q.; Wang, D.; Zhou, R.; Wang, R.; Zhu, Y.; Qi, S. Leonurine Protects Bone Mesenchymal Stem Cells from Oxidative Stress by Activating Mitophagy through PI3K/Akt/mTOR Pathway. Cells 2022, 11, 1724. https://doi.org/10.3390/cells11111724
Zhao B, Peng Q, Wang D, Zhou R, Wang R, Zhu Y, Qi S. Leonurine Protects Bone Mesenchymal Stem Cells from Oxidative Stress by Activating Mitophagy through PI3K/Akt/mTOR Pathway. Cells. 2022; 11(11):1724. https://doi.org/10.3390/cells11111724
Chicago/Turabian StyleZhao, Bingkun, Qian Peng, Dan Wang, Rong Zhou, Raorao Wang, Yizhun Zhu, and Shengcai Qi. 2022. "Leonurine Protects Bone Mesenchymal Stem Cells from Oxidative Stress by Activating Mitophagy through PI3K/Akt/mTOR Pathway" Cells 11, no. 11: 1724. https://doi.org/10.3390/cells11111724
APA StyleZhao, B., Peng, Q., Wang, D., Zhou, R., Wang, R., Zhu, Y., & Qi, S. (2022). Leonurine Protects Bone Mesenchymal Stem Cells from Oxidative Stress by Activating Mitophagy through PI3K/Akt/mTOR Pathway. Cells, 11(11), 1724. https://doi.org/10.3390/cells11111724







