Abstract
Endothelial progenitor cells (EPC) may influence the integrity and stability of the vascular endothelium. The association of an altered total EPC number and function with cardiovascular diseases (CVD) and risk factors (CVF) was discussed; however, their role and applicability as biomarkers for clinical purposes have not yet been defined. Endothelial dysfunction is one of the key mechanisms in CVD. The assessment of endothelial dysfunction in vivo remains a major challenge, especially for a clinical evaluation of the need for therapeutic interventions or for primary prevention of CVD. One of the main challenges is the heterogeneity of this particular cell population. Endothelial cells (EC) can become senescent, and the majority of circulating endothelial cells (CEC) show evidence of apoptosis or necrosis. There are a few viable CECs that have properties similar to those of an endothelial progenitor cell. To use EPC levels as a biomarker for vascular function and cumulative cardiovascular risk, a correct definition of their phenotype, as well as an update on the clinical application and practicability of current isolation methods, are an urgent priority.
1. Introduction
Cardiovascular diseases (CVD) such as myocardial infarction (MI), cerebral and peripheral arterial disease (PAD) and arterial hypertension are the leading causes of globalmortality [1,2].
Coronary artery disease (CAD), leading to narrowing or complete blockage of arterial blood supply to the myocardium, is the most prevalent heart disease [2]. Pharmacological agents, interventional and surgical procedures, as well as diet and lifestyle-related concepts to better control established and newly discovered cardiovascular risk factors, are still not sufficient to prevent the millions of disease-related deaths worldwide every year [1,2,3,4].
Known cardiovascular risk factors (CVF) contribute to the cascade of atherogenesis especially by inducing injury and dysfunction in endothelial cells. Endothelial integrity is highly reliable due to repair and renewal by endothelial progenitor cells (EPC) derived from different sources, e.g., bone marrow (BM), circulating endothelial progenitor cells (CEPC) or adventitial residents [5]. The impaired mobilization or depletion of these cells contributes to endothelial dysfunction and CVD progression [6].
Ross’ classic paradigm already stated that endothelial cells (EC) injury is one of the most important stimuli for the development of atherosclerotic plaque [7]. In 1997, Asahara et al. reported the isolation of putative EPC from human peripheral blood, based on the cell-surface expression of CD34 and other endothelial markers and introduced the novel concept of CEPCs. These specific cells were reported to further differentiate, at least in vitro, into endothelial cells [8]. They could be identified at sites of active angiogenesis as well as in various animal models of ischemia [8]. CEPCs contribute to on-going endothelial repair through their ability to form layers of neo-endothelium at the site of injury or to serve as a cellular reservoir to replace dysfunctional endothelium [9].
Triggers for EPC recruitment in neo-angiogenesis or vascular injury include the increased availability of angiogenic growth factors or chemokines, such as the vascular endothelial growth factor (VEGF), as well as angiopoietin or stromal cell-derived factor (SDF)-1 bonding with the chemokine receptor (CXCR-4), thereby expanding its expression on EPCs [10,11,12,13]. ECs on the other hand, participate in different physiological processes, such as vasomotor tone, cellular trafficking, or innate and adaptive immunity [5]. The perioperative effects of endothelial progenitor cells, in patients with acute ischemia of the lower limbs undergoing surgical revascularization, with a plausible biological mechanism was generated for applications as a biomarker. A sound understanding and clear definition of endothelial-regenerating cells and their role in the differentiation into mature endothelium [14] is required for the establishment of an ideal test to measure EC function and enhancement of our understanding of the pathophysiology of atherosclerosis [15].
Adiponectin, adipocyte fatty acid-binding protein, heart-type fatty acid-binding protein, lipocalin-2, fibroblast growth factor 19 and 21, retinol-binding protein 4, plasminogen activator inhibitor-1, and 25-hydroxyvitamin D are just a few of the already known biomarkers linked to cardiovascular and metabolic diseases. These biomarkers may help predict CVD risk. More research is needed to assess biomarkers’ validity and their potential to improve clinical decision-making and therapy management. The endothelium has a unique position as both a sensor and participant in the atherosclerotic process [16]. Biomarkers that can be considered as indicators of biological states or conditions are progressively incorporated into cardiovascular risk assessments [17,18]. EPCs are considered the ideal target for inducing the neovascularization of ischemic tissue and can serve as a biomarkers for the surveillance of tissue damage and therapeutical outcomes [19].
In this review, we focus on current definitions of EPCs, discuss the individual relevance of circulating and adventitial resident progenitors in endothelial and vascular integrity, function, rejuvenation and restoration and address some promising therapeutic approaches and remaining questions.
2. Characterisation and Various Origins of Ecs/ Epcs in Humans
A consensus on the ideal marker for the identification of EPC cell types is lacking. This marker may originate from multiple precursors, such as a haemangioblast (HPC), BM progenitors, tissue-resident mesenchymal stem cells (MSC), and especially, from adipose tissue [20], impeding fast and simple isolation [5].
The three existing techniques used to isolate and cultivate human EPCs [21,22] lead to phenotypically differing cell types, and as such, potentially vague findings when analysing EPC’s role in cardiovascular repair (Figure 1) and cardiac outcome [23].
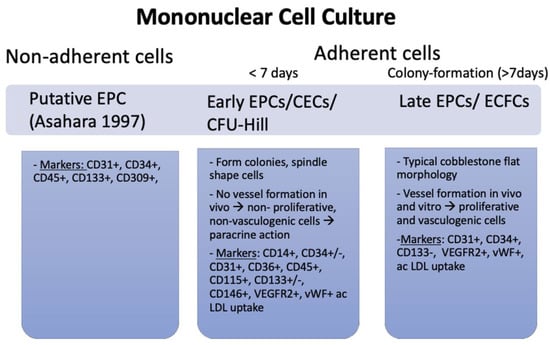
Figure 1.
Three different types of EPCs, their function and associated markers. In Asahara et al.’s method, non-adherent cells were cultivated, whereas in the protocols by CFU-Hill and Ingram, only adherent cells were used. The three cell cultures each have a distinct morphology, specific functions in vivo and display discriminating markers [8].
The use of density barrier centrifugation is one method that can be utilised in order to separate mononuclear cells from peripheral blood mononuclear cells (PBMNCs). In most cases, cells are sown onto plates that have been coated with fibronectin and then grown using endothelial growth factors. The remaining spindle-shaped cells not only endocytose acetylated low-density lipoprotein (LDL) but also express EC markers and possess other characteristics of ECs. This is, in addition to the fact that EC indicators are present in these cells [24], acquired via antigen transfer from platelets that contaminate isolates of PBMNCs [25]. Platelets on mononuclear cell cultures degrade into micro-particles (vesicles that retain specific antigens from the cell of origin) within 7 days, and CD31 expression (along with platelet-specific markers) was present at that time on EPCs, now called putative EPC’-aggregates‘, whereas EPCs were CD31-negative on day 1 [25]. Attempts to further purify the cell cultures led to the establishment of new protocols.
Using markers such as CD133, combined with CD34 and VEGFR2, ensured that only progenitor cells with vasculogenic properties were identified [26,27]. Cell labelling with antigen-specific antibodies and fluorescence-activated cell sorting (FACS) to select EPCs was applied [8], while still culturing the cells on fibronectin [25]. The CD34+ cells were surrounded by spindle-shaped cells expressing increased EC markers. Through the use of this marker combination, this cell type was successfully isolated from adult peripheral blood, umbilical cord blood, and foetal liver [28]. Recent research shows that human CD34+/CD133+/VEGFR2-positive cells are separate primitive haematopoietic progenitors lacking vessel formation ability and expressing CD45, a haemangioblast marker [29,30]
Thereafter, in vitro colony-forming cell assays allowed for the isolation of two cell types: colony-forming unit (CFU)-Hill cells and endothelial colony-forming cells (ECFC). In the ‘Hill assay’ and the ECFC, or ‘Ingram protocol’, monocytic cells isolated from blood samples were cultured for two days on fibronectin-coated dishes and then replated for further cultivation [6,27]. CFU-Hill cells are phagocytic and express EC-like markers (CD14, CD45, and CD115) but lack proliferative and vasculogenic activity. While lacking CD14, CD45, and CD115, ECFCs express EC markers and have the ability to form capillary-like structures in vitro and vessels in vivo [21]. ECFCs have been shown to reside in the arterial wall suggesting that this may be the main origin of these cells [26].
In summary, EPCs seem to represent two distinct populations with overlapping antigen expressions (e.g., CD34/VEGFR2): hematopoietic-derived spindle-shaped cells from isolation method I/II, also referred to as circulating angiogenic cells or early EPCs (CFU-Hill colonies), and ECFCs, or late EPCs [31]. Late EPCs have a vasculogenic ability in vitro and are well-integrated into membranes, whereas early EPCs act via a paracrine mechanisms [32] and might even protect late EPCs from oxidative stress [33].
3. Influence on Vascular Pathologies and Role as a Biomarker
At present, there is a lack of information or knowledge regarding cell phenotypes in different diseases as the cells originate from different vascular beds and sources [34]. Half of the CECs from healthy controls express CD36, a marker for cells of microvascular origin, whereas in sickle cell anaemia, this percentage increases to 80% [35]. Contrastingly, no CD36 could be stained in CECs from patients with acute coronary syndrome, consistent with the macro-vascular origin of these cells [36].
Investigating the role of CECs in endothelial injury with regard to plasma markers of endothelial injury (vWf, tissue plasminogen activator, soluble E-selectin) led to a correlation between CECs and vWf in heart failure [37].
Almost all types of CVD were associated with hypertension, diabetes, smoking and high cholesterol. These CVFs can contribute to endothelial dysfunction [38,39,40,41,42,43,44,45,46,47,48]. High homocysteine and ADMA values also showed a negative effect on EPC count [49,50]. On the other hand, high HDL cholesterol and TG levels correlated with CFU but not with CD34/133+ cell count [51]. (Table 1) Statin [52] and Angiotensin receptor II inhibitors [53], as well as oestrogen levels (high oestrogen levels in women were associated with an increased EPC count [54] in animal carotid injury oestrogen-enhanced EPC function [55]), glitazones [56], erythropoietin [53,57,58,59], and PDE5 inhibitors [60] all showed beneficial effects. EPC count was also dependent on SDF-1 [61,62], VEGF [10,11], NO [63], GCS-F and GM-CSF [64,65] levels.

Table 1.
Correlations of EPC count, EPC function and EPC apoptosis with cardiovascular risk and protective factors, pathophysiologic state, physiologic mediators and common drugs in cardiovascular disease.
Physical activity at a moderate level was identified to be potentially beneficial for preventing CVD. The increase in EPC count was found to be mediated by eNOS and VEGF, and apoptosis was reduced in the cells [66]. Physical activity lead to a higher amount of circulating CD34-positive EPCs in CVD patients [67]. Furthermore, this study identified an association of CD34-positive cell count with lower all-cause and cardiovascular mortality [67]. Vascular damage progression correlated with EPC count [68] just as a decreased amount of CD34-positive but increased amount of CD34+CD133+CD309+ and CD34+CD133+ cells suggested the progression of cerebral small vessel disease [69]. EPC count could, therefore, serve as a biomarker for CVD course. A correlation with CAD progression was also found for osteocalcin, a regulator of early EPC. A higher number of CVRFs was associated with a decreased total osteocalcin count. Osteocalcin positivity in EPCs was related to LDL, total cholesterol and TGs in both early and, significantly, in late CAD [70]. EPC count could also be used as a marker in treatment monitoring, such as in chronic total coronary artery occlusion since an association with Rentrop grade at baseline and 1 year post operation was discovered [71].
Some data still suggest that those “monitoring effects” are mostly seen in the young population. Aging by itself is another depriving factor of EPC [6,63]. Age directly limits EPC mobilisation but also via VEGF depletion and physiologically lowered NO levels, which contribute to the bad survival and proliferation of EPCs [63,72]. Moreover, several mechanisms, including the co-existence of CVF, lead to the impaired maintenance of endothelial integrity [73]. In elderly CAD patients with stable disease, EPC count was significantly reduced compared to younger patients [74]. The mobilisation of EPCs was also lower after a coronary bypass grafting in advanced age [75,76]. The severity of stable CAD shows an inverse correlation with the total/early EPC number [62,77]. Additionally, chronic vascular disease appears to have opposite effects on early and late EPC numbers and does not influence their functional capacity [62,77].
Using EPC as a therapeutic target for CVD may therefore underlay an age-related effect. Early EPC implantation increased neovascularisation in young mice, but not in older mice with elevated cholesterol or other CVD risk factors [78].
4. The Role of Epc in Congenital Heart Disease Heart Failure
Mechanisms of heart failure in adult heart disease recently received a lot of research attention, but its pathogenic and prognostic significance in single-ventricle physiology is still unknown [79,80,81]. Congenital cardiac malformations with a single ventricle have a high risk of mortality in the first year of life in such patients and frequently result in late complications developing during this stage of palliative repair [82]. Even though single-ventricle reconstruction trials have sought to identify predictors of poor outcomes at three years in patients with single-ventricle physiology based on the types of initial shunt (Norwood procedure with ventriculo-pulmonary Sano shunt or with modified subclavio-pulmonary Blalock shunt) and the timing of stage 2 palliation, a 12-year longitudinal cohort study in patients with Fontan (stage 3 procedure) circulation found that the risk of death or cardiac transplantation was closely associated with poorer ventricular function [83,84]. No definitive therapy was shown to improve heart function with a chronic volume or pressure overload, which may worsen prognosis for single-ventricle patients [85].
However, early phase 1/2 clinical trials utilizing the intracoronary delivery of derived progenitor cells demonstrated dependable and safe outcomes in patients with single ventricle physiology. Except for all-cause mortality after staged procedures, derived cardiac progenitors cells administration improved ventricular function and was linked with fewer late problems in patients with single ventricles. Patients treated with cardiac progenitors cells and those who suffered from heart failure with reduced EF but not heart failure with intact EF may experience a substantial improvement in clinical outcomes [86,87].
5. Targeting of Treatment for CVD
Nonetheless, increasing the EPC count is a promising strategy. Early information [8] on EPC’s contribution to neo-angiogenesis could be secured in mice models of ischemia, where CD34- and stem cell antigen–1 (Sca-1)-enriched populations of mononuclear cells promoted new blood vessels and enhanced the perfusion and initiation of recovery in ischaemic tissue [64]. Kong et al. (2004) observed endothelial repair and neointima development after the cytokine-induced mobilization of circulating progenitors [88]. After EC injury, spleen-derived mononuclear cells and cultivated early EPCs, but not BM-derived EPCs, increased re-endothelialisation and decreased neointima formation [89]. In several models of vascular graft atherosclerosis, recipient-circulating progenitor cells were required to create an endothelial monolayer. An allograft of mouse Balb/c aorta into the carotid artery of chimera mice with BM from Tie2-LacZ mice showed activity 4 weeks following surgery. The quantification of the obtained data indicated that more than 70% of the regenerated endothelium was derived from non-BM tissues [90,91,92]. In humans, the implantation of bone marrow mononuclear cells (BMMCs) was found to be an effective treatment for PAD in an attempt to alleviate limb ischemia through the use of stem cells. Angiogenesis occurred as a result of the stimulation of EPC and the release of VEGF and cytokines [93]. The promotion of EPC and the release of VEGF and cytokines alleviated the symptoms and collateral circulation [93].
Yet, there are dangers associated with the direct implantation of EPCs mainly triggered by the high number of cells with inflammatory potential that could be coinjected [94]. Despite different routes of administration that are considered safe, such as intracardiac, intrahepatic or intramuscular [95], indirectly targeting EPC count and function might be a promising treatment option, lowering the associated risks of cell transplantation. CD-34-coated stents were applied and compared to paclitaxel, with good outcomes and significantly less post-discharge thrombotic events after 12 months follow-up [96]. In diabetic cardiomyopathy, treatment with BMP7, an anti-inflammatory protein, led to an increase in EPC markers and neovascularisation, ultimately improving cardiac remodelling [97]. Blocking hormonal pathways that are important in CVD development, such as gonadotropin, an enhancer of ECFC angiogenesis, and thrombin might also be beneficial [98]. Using EPC-derived vesicles [99] or exosomes [100] further helps to mitigate the problematic isolation and culturing process of EPCs, thereby providing more efficient therapeutic options.
Additionally, the transfer of plasmid DNA for VEGF to young and senescent EPCs via ultrasonic microbubble transfection could enhance the angiogenic effects of both older and young cells, thereby leading to better outcomes and solving two problems at once [101]
Other new therapeutic options with EPC as a target include the treatment of ischemic stroke in patients with pre-existent CVD [102].
6. Ongoing Challenges
There is a high risk of postoperative morbidity for patients following major surgery. Endothelial dysfunction in the perioperative period may increase the risk of surgical complications through altered vascular homeostasis and, consequently, decrease tissue perfusion. Targeting the endothelium and optimising natural physiological function in the perioperative phase is becoming a more popular as a way to improve postoperative outcomes. With an estimated surface area of more than 1000 m2, the human vascular system constantly maintains a balance between coagulation and bleeding, inflammation and immuno-protection rendering difficult the identification of one single area of activation or damage contributing to the release of a specific set of ECs [103]. MSCs and EPCs differ in their immunomodulatory and immunosuppressive effects. MSCs and EPCs reduce T-cell proliferation, activation and cytokine production and MSCs, in comparison to EPCs, may induce regulatory T-cells [104]. EPCs with haemangioblast properties (CD34+ and CD45+) or “early EPCs” do not differentiate into ECs supporting the fact that “true EPCs” reside in the vasculature [105]. Their role in vascular repair of ischemic tissues might rather be via paracrine mechanisms, such as the secretion of angiogenic cytokines (e.g., VEGF) [5,32].
Differentiating EPCs from CECs remains difficult [34]. Endothelial markers, such as CD-146 and UEA-1 are present on EPCs and severely ‘morphology-damaged’ cells obtained by CD-146-driven immune–magnetic isolation alike. The inability to grow these cells in culture is still regarded as an implication that these cells are not EPCs [106]. The activation of ECs occurs by pro-inflammatory cytokines, growth factors, lipoproteins, or even oxidative stress. Therefore, detected detached cells might be apoptotic or necrotic, distorting the set of detected circulating cells [107] and further confounding isolates of EPCs.
While the identification of resident-tissue EPCs is advancing somewhat, the mechanism of progenitor cell release and the distinct role of different EPC types are still not understood. Differentiating between the role of early and late EPCs in CVD is important as, in CAD patients, a decreased amount of early EPCs was found [108], accompanied with higher density of late EPCs [109].
Most studies to date describe factors which promote/inhibit either total (CD34+ or CD34/VEGFR2) or early EPC (CD34+/VEGFR2 +/CD133+ or VEGFR2+/CD133+) mobilisation and homing from the BM under physiological and pathological drug therapy conditions [110]. Changes in EPC count could be registered in patients displaying an onset of acute ischemia following MI [111,112,113], unstable angina, coronary artery bypass grafting [75,76] or stent implantation [114,115].
Within the identification of all of these influencing factors, a single clear mechanistic explanation to understand EPC function, mobilisation and endothelial integrity is lacking. Improvements in risk classification and the ability to regulate thrombotic and inflammatory cascades should enhance perioperative outcomes. The underlying subclinical microvascular endothelial dysfunction seems to have a greater impact on perioperative morbidity, contributing more to complications, such as impaired wound healing and end organ dysfunction, than the less common but more devastating macrovascular endothelial dysfunction.
Ongoing studies address sheer stress as another key factor since low or disturbed shear stress, which occurs in vessel branch points, the outer wall of bifurcations, and the inner wall of curvatures are considered pro-atherogenic [116]. Cell turnover rates in the arteries are very low, but in some areas this corresponds to increased permeability to plasma proteins [117], in atherosclerotic-lesion-prone site [118], and in areas of low shear stress, a high endothelial death rate and overall high turnover rate is needed to maintain vessel homeostasis [116]. Therefore, recent theories addressed the role of different flow patterns on EPC function [119]. A disturbed flow was associated with a higher mitotic and apoptotic activity of ECs and lessened eNOS expression [119]. Long-time outcomes of stenting were determined by endothelial cell turnover or number [120]. Histone deacetylases (HDAC) were identified as key players behind this finding [120]. A disturbed flow induced the transient stabilization of the HDAC3 protein in ECs by stimulating VEGFR2 [121]. HDAC3 belongs to the class I HDACs that enhances the removal of acetyl groups from histone and non-histone proteins [120,122]. HDAC3 is critical for EC survival and acts as a flow-pattern-dependent pro-survival molecule [123]. However, HDAC7 is modulated by VEGF and turbulent flow and is involved in endothelial homeostasis and differentiation, as well as vascular SMC proliferation [124]. SMC play the main role in the pathogenesis of vascular-disease-mediated restenosis [124]. Aside from being a potential drug target, HDACs could help explain post-interventional outcome differences. Trichostatin, for example, inhibits HDAC, which may be useful in future CVD treatments [125]. Such important players in CVD pathophysiology should be studied more extensively within the context of EC research in order to better understand the mechanisms involved and ultimately find new targets of treatment and biomarkers to monitor disease and measure therapeutic outcomes.
7. Conclusions
EC architecture and mechanisms of endothelial homeostasis are potential sensors and target therapeutic interventions in the atherosclerotic process. While current studies assessing EC subunits, phenotype and function enabled a general understanding of the pathophysiology of atherosclerosis, an ideal test of endothelial function for use in a clinical setting has yet to be established. A secure method for counting cells and differentiating cell kinds and compositions is urgently required, as is a deeper understanding of other modulators such as flow pattern, growth and transcription factors, and gene expression. CECs may serve as sensitive indicators of pre-existing damage and disease development, whereas EPCs may act as a biomarker of repair and a promising therapeutic target. Regenerative medicine has the potential to expand the therapeutic window for a variety of diseases, where surgical and medicamentous alternatives have run out. In CVD, safe cell replacement with new ones could usher in a new era of therapy.
Author Contributions
Conceptualisation, P.P.H., C.B., B.W. and M.M.L.; methodology, P.P.H., C.B., M.Y.E., T.C., M.D., J.H., B.W. and M.M.L.; validation, M.M.L.; resources, P.P.H., C.B., B.W. and M.M.L.; writing—original draft, P.P.H., C.B., M.Y.E., T.C., M.D., J.H., B.W. and M.M.L.; preparation, P.P.H., C.B., M.Y.E., T.C., M.D., J.H., B.W. and M.M.L.; writing—review and editing, P.P.H., C.B., M.Y.E., T.C., M.D., J.H., B.W. and M.M.L.; visualisation, P.P.H., C.B., B.W. and M.M.L.; supervision, P.P.H., B.W. and M.M.L.; project administration, M.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not available.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BM | bone marrow |
| BMMCs | bone marrow mononuclear cells |
| CAD | carotid artery disease |
| CD | cluster of differentiation |
| CEC | circulating endothelial cell |
| CFU | colony-forming unit |
| CVD | cardiovascular disease |
| CVRF | cardiovascular risk factors |
| CXCR-4 | chemokine receptor |
| EC | endothelial cell |
| ECFC | endothelial colony-forming cells |
| eNOS | endothelial nitric oxide synthase |
| EPC | endothelial progenitor cell |
| FACS | fluorescence-activated cell sorting |
| HDAC | histone deacetylase |
| HPC | haemangioblast |
| LDL | low-density lipoprotein |
| MSC | mesenchymal stem cell |
| PAD | peripheral artery disease |
| PBMNC | peripheral blood mononuclear cells |
| SCA | stem cell antigen |
| SDF | stromal cell-derived factor |
| VEGF | vascular epidermal growth factor |
| VEGFR | vascular epidermal growth factor receptor |
References
- Shung-King, M.; Weimann, A.; McCreedy, N.; Tatah, L.; Mapa-Tassou, C.; Muzenda, T.; Govia, I.; Were, V.; Oni, T. Protocol for a Multi-Level Policy Analysis of Non-Communicable Disease Determinants of Diet and Physical Activity: Implications for Low-and Middle-Income Countries in Africa and the Caribbean. Int. J. Environ. Res. Public Health 2021, 18, 13061. [Google Scholar] [CrossRef]
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Heinisch, P.P.; Mihalj, M.; Huber, M.; Schefold, J.C.; Hartmann, A.; Walter, M.; Steinhagen-Thiessen, E.; Schmidli, J.; Stüber, F.; Räber, L.; et al. Impact of Lipoprotein(a) Levels on Perioperative Outcomes in Cardiac Surgery. Cells 2021, 10, 2829. [Google Scholar] [CrossRef] [PubMed]
- Mihalj, M.; Heinisch, P.P.; Huber, M.; Schefold, J.C.; Hartmann, A.; Walter, M.; Steinhagen-Thiessen, E.; Schmidli, J.; Stüber, F.; Räber, L.; et al. Effect of Perioperative Lipid Status on Clinical Outcomes after Cardiac Surgery. Cells 2021, 10, 2717. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Liu, X.; Ding, H.; Zhang, W. Paracrine mechanisms of endothelial progenitor cells in vascular repair. Acta Histochem. 2021, 124, 151833. [Google Scholar] [CrossRef]
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef]
- Walter, D.H.; Rittig, K.; Bahlmann, F.H.; Kirchmair, R.; Silver, M.; Murayama, T.; Nishimura, H.; Losordo, D.W.; Asahara, T.; Isner, J.M. Statin therapy accelerates reendothelialization: A novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 2002, 105, 3017–3024. [Google Scholar] [CrossRef]
- Hattori, K.; Dias, S.; Heissig, B.; Hackett, N.R.; Lyden, D.; Tateno, M.; Hicklin, D.J.; Zhu, Z.; Witte, L.; Crystal, R.G.; et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J. Exp. Med. 2001, 193, 1005–1014. [Google Scholar] [CrossRef]
- Iwaguro, H.; Yamaguchi, J.; Kalka, C.; Murasawa, S.; Masuda, H.; Hayashi, S.; Silver, M.; Li, T.; Isner, J.M.; Asahara, T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation 2002, 105, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, J.; Kusano, K.F.; Masuo, O.; Kawamoto, A.; Silver, M.; Murasawa, S.; Bosch-Marce, M.; Masuda, H.; Losordo, D.W.; Isner, J.M.; et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 2003, 107, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Mohle, R.; Bautz, F.; Rafii, S.; Moore, M.A.; Brugger, W.; Kanz, L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood 1998, 91, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Buchanan, M.R.; Anderson, T.J. Endothelial function testing as a biomarker of vascular disease. Circulation 2003, 108, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Wang, C.H.; Li, S.H.; Dumont, A.S.; Fedak, P.W.; Badiwala, M.V.; Dhillon, B.; Weisel, R.D.; Li, R.K.; Mickle, D.A.; et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 2002, 106, 913–919. [Google Scholar] [CrossRef]
- Sen, S.; McDonald, S.P.; Coates, P.T.; Bonder, C.S. Endothelial progenitor cells: Novel biomarker and promising cell therapy for cardiovascular disease. Clin. Sci. 2011, 120, 263–283. [Google Scholar] [CrossRef]
- Grisar, J.C.; Haddad, F.; Gomari, F.A.; Wu, J.C. Endothelial progenitor cells in cardiovascular disease and chronic inflammation: From biomarker to therapeutic agent. Biomark. Med. 2011, 5, 731–744. [Google Scholar] [CrossRef]
- Matsuda, T.; Miyagawa, S.; Fukushima, S.; Kitagawa-Sakakida, S.; Akimaru, H.; Horii-Komatsu, M.; Kawamoto, A.; Saito, A.; Asahara, T.; Sawa, Y. Human cardiac stem cells with reduced notch signaling show enhanced therapeutic potential in a rat acute infarction model. Circ. J. 2014, 78, 222–231. [Google Scholar] [CrossRef]
- Saito, N.; Shirado, T.; Funabashi-Eto, H.; Wu, Y.; Mori, M.; Asahi, R.; Yoshimura, K. Purification and characterization of human adipose-resident microvascular endothelial progenitor cells. Sci. Rep. 2022, 12, 1775. [Google Scholar] [CrossRef]
- Yoder, M.C.; Mead, L.E.; Prater, D.; Krier, T.R.; Mroueh, K.N.; Li, F.; Krasich, R.; Temm, C.J.; Prchal, J.T.; Ingram, D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007, 109, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Yoder, M.C. Defining human endothelial progenitor cells. J. Thromb. Haemost. 2009, 7 (Suppl. S1), 49–52. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.M.; Cornet, A.; Emmerich, J.; Aiach, M.; Gaussem, P. Endothelial progenitor cells: Characterization, in vitro expansion, and prospects for autologous cell therapy. Cell Biol. Toxicol. 2007, 23, 223–239. [Google Scholar] [CrossRef]
- Zhang, S.J.; Zhang, H.; Hou, M.; Zheng, Z.; Zhou, J.; Su, W.; Wei, Y.; Hu, S. Is it possible to obtain “true endothelial progenitor cells” by in vitro culture of bone marrow mononuclear cells? Stem Cells Dev. 2007, 16, 683–690. [Google Scholar] [CrossRef]
- Prokopi, M.; Pula, G.; Mayr, U.; Devue, C.; Gallagher, J.; Xiao, Q.; Boulanger, C.M.; Westwood, N.; Urbich, C.; Willeit, J.; et al. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood 2009, 114, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Mead, L.E.; Moore, D.B.; Woodard, W.; Fenoglio, A.; Yoder, M.C. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 2005, 105, 2783–2786. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef]
- Timmermans, F.; Plum, J.; Yoder, M.C.; Ingram, D.A.; Vandekerckhove, B.; Case, J. Endothelial progenitor cells: Identity defined? J. Cell Mol. Med. 2009, 13, 87–102. [Google Scholar] [CrossRef]
- Case, J.; Mead, L.E.; Bessler, W.K.; Prater, D.; White, H.A.; Saadatzadeh, M.R.; Bhavsar, J.R.; Yoder, M.C.; Haneline, L.S.; Ingram, D.A. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp. Hematol. 2007, 35, 1109–1118. [Google Scholar] [CrossRef]
- Timmermans, F.; Van Hauwermeiren, F.; De Smedt, M.; Raedt, R.; Plasschaert, F.; De Buyzere, M.L.; Gillebert, T.C.; Plum, J.; Vandekerckhove, B. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1572–1579. [Google Scholar] [CrossRef]
- Kirton, J.P.; Xu, Q. Endothelial precursors in vascular repair. Microvasc. Res. 2010, 79, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Yoon, C.H.; Kim, H.S.; Choi, J.H.; Kang, H.J.; Hwang, K.K.; Oh, B.H.; Lee, M.M.; Park, Y.B. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; von Ballmoos, M.W.; Faessler, D.; Voelzmann, J.; Ortmann, J.; Diehm, N.; Kalka-Moll, W.; Baumgartner, I.; Di Santo, S.; Kalka, C. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis 2010, 211, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.J.; Barber, C.L.; Sabatier, F.; Dignat-George, F.; Melero-Martin, J.M.; Khosrotehrani, K.; Ohneda, O.; Randi, A.M.; Chan, J.K.Y.; Yamaguchi, T.; et al. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem Cells Transl. Med. 2017, 6, 1316–1320. [Google Scholar] [CrossRef]
- Solovey, A.; Lin, Y.; Browne, P.; Choong, S.; Wayner, E.; Hebbel, R.P. Circulating activated endothelial cells in sickle cell anemia. N. Engl. J. Med. 1997, 337, 1584–1590. [Google Scholar] [CrossRef]
- Mutin, M.; Canavy, I.; Blann, A.; Bory, M.; Sampol, J.; Dignat-George, F. Direct evidence of endothelial injury in acute myocardial infarction and unstable angina by demonstration of circulating endothelial cells. Blood 1999, 93, 2951–2958. [Google Scholar] [CrossRef]
- Lee, K.W.; Lip, G.Y.; Tayebjee, M.; Foster, W.; Blann, A.D. Circulating endothelial cells, von Willebrand factor, interleukin-6, and prognosis in patients with acute coronary syndromes. Blood 2005, 105, 526–532. [Google Scholar] [CrossRef]
- Forgione, M.A.; Leopold, J.A.; Loscalzo, J. Roles of endothelial dysfunction in coronary artery disease. Curr. Opin. Cardiol. 2000, 15, 409–415. [Google Scholar] [CrossRef]
- Jambrik, Z.; Venneri, L.; Varga, A.; Rigo, F.; Borges, A.; Picano, E. Peripheral vascular endothelial function testing for the diagnosis of coronary artery disease. Am. Heart J. 2004, 148, 684–689. [Google Scholar] [CrossRef]
- Brevetti, G.; Silvestro, A.; Schiano, V.; Chiariello, M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: Additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation 2003, 108, 2093–2098. [Google Scholar] [CrossRef]
- Perticone, F.; Ceravolo, R.; Pujia, A.; Ventura, G.; Iacopino, S.; Scozzafava, A.; Ferraro, A.; Chello, M.; Mastroroberto, P.; Verdecchia, P.; et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 2001, 104, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.E.; Weihprecht, H.; Schobel, H.; John, S.; Weidinger, G.; Gatzka, C.; Veelken, R. Is endothelial function of the radial artery altered in human essential hypertension? Am. J. Hypertens. 1997, 10, 323–331. [Google Scholar] [CrossRef][Green Version]
- Zvan, B.; Zaletel, M.; Pogacnik, T.; Kiauta, T. Testing of cerebral endothelium function with L-arginine after stroke. Int. Angiol. 2002, 21, 256–259. [Google Scholar]
- Landmesser, U.; Spiekermann, S.; Dikalov, S.; Tatge, H.; Wilke, R.; Kohler, C.; Harrison, D.G.; Hornig, B.; Drexler, H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: Role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 2002, 106, 3073–3078. [Google Scholar] [CrossRef] [PubMed]
- de Jong, R.M.; Blanksma, P.K.; Cornel, J.H.; Van den Heuvel, A.F.; Siebelink, H.M.; Vaalburg, W.; van Veldhuisen, D.J. Endothelial dysfunction and reduced myocardial perfusion reserve in heart failure secondary to coronary artery disease. Am. J. Cardiol. 2003, 91, 497–500. [Google Scholar] [CrossRef]
- Marin, F.; Roldan, V.; Climent, V.E.; Ibáñez, A.; García, A.; Marco, P.; Sogorb, F.; Lip, G.Y. Plasma von Willebrand factor, soluble thrombomodulin, and fibrin D-dimer concentrations in acute onset non-rheumatic atrial fibrillation. Heart 2004, 90, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.S.; Pearce, L.A.; Chin, B.S.; Hart, R.G.; Lip, G.Y. Prognostic value of plasma von Willebrand factor and soluble P-selectin as indices of endothelial damage and platelet activation in 994 patients with nonvalvular atrial fibrillation. Circulation 2003, 107, 3141–3145. [Google Scholar] [CrossRef][Green Version]
- Conway, D.S.; Pearce, L.A.; Chin, B.S.; Hart, R.G.; Lip, G.Y. Plasma von Willebrand factor and soluble p-selectin as indices of endothelial damage and platelet activation in 1321 patients with nonvalvular atrial fibrillation: Relationship to stroke risk factors. Circulation 2002, 106, 1962–1967. [Google Scholar] [CrossRef]
- Chen, J.Z.; Zhu, J.H.; Wang, X.X.; Zhu, J.H.; Xie, X.D.; Sun, J.; Shang, Y.P.; Guo, X.G.; Dai, H.M.; Hu, S.J. Effects of homocysteine on number and activity of endothelial progenitor cells from peripheral blood. J. Mol. Cell Cardiol. 2004, 36, 233–239. [Google Scholar] [CrossRef]
- Thum, T.; Tsikas, D.; Stein, S.; Schultheiss, M.; Eigenthaler, M.; Anker, S.D.; Poole-Wilson, P.A.; Ertl, G.; Bauersachs, J. Suppression of endothelial progenitor cells in human coronary artery disease by the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine. J. Am. Coll. Cardiol. 2005, 46, 1693–1701. [Google Scholar] [CrossRef]
- Pellegatta, F.; Bragheri, M.; Grigore, L.; Raselli, S.; Maggi, F.M.; Brambilla, C.; Reduzzi, A.; Pirillo, A.; Norata, G.D.; Catapano, A.L. In vitro isolation of circulating endothelial progenitor cells is related to the high density lipoprotein plasma levels. Int. J. Mol. Med. 2006, 17, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Aicher, A.; Vasa, M.; Mildner-Rihm, C.; Adler, K.; Tiemann, M.; Rütten, H.; Fichtlscherer, S.; Martin, H.; Zeiher, A.M. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J. Clin. Investig. 2001, 108, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Bahlmann, F.H.; de Groot, K.; Mueller, O.; Hertel, B.; Haller, H.; Fliser, D. Stimulation of endothelial progenitor cells: A new putative therapeutic effect of angiotensin II receptor antagonists. Hypertension 2005, 45, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Strehlow, K.; Werner, N.; Berweiler, J.; Link, A.; Dirnagl, U.; Priller, J.; Laufs, K.; Ghaeni, L.; Milosevic, M.; Böhm, M.; et al. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation 2003, 107, 3059–3065. [Google Scholar] [CrossRef]
- Iwakura, A.; Luedemann, C.; Shastry, S.; Hanley, A.; Kearney, M.; Aikawa, R.; Isner, J.M.; Asahara, T.; Losordo, D.W. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation 2003, 108, 3115–3121. [Google Scholar] [CrossRef]
- Pistrosch, F.; Herbrig, K.; Oelschlaegel, U.; Richter, S.; Passauer, J.; Fischer, S.; Gross, P. PPARgamma-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis 2005, 183, 163–167. [Google Scholar] [CrossRef]
- Heeschen, C.; Aicher, A.; Lehmann, R.; Fichtlscherer, S.; Vasa, M.; Urbich, C.; Mildner-Rihm, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 2003, 102, 1340–1346. [Google Scholar] [CrossRef]
- Bahlmann, F.H.; DeGroot, K.; Duckert, T.; Niemczyk, E.; Bahlmann, E.; Boehm, S.M.; Haller, H.; Fliser, D. Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int. 2003, 64, 1648–1652. [Google Scholar] [CrossRef]
- Bahlmann, F.H.; De Groot, K.; Spandau, J.M.; Landry, A.L.; Hertel, B.; Duckert, T.; Boehm, S.M.; Menne, J.; Haller, H.; Fliser, D. Erythropoietin regulates endothelial progenitor cells. Blood 2004, 103, 921–926. [Google Scholar] [CrossRef]
- Foresta, C.; Lana, A.; Cabrelle, A.; Ferigo, M.; Caretta, N.; Garolla, A.; Palù, G.; Ferlin, A. PDE-5 inhibitor, Vardenafil, increases circulating progenitor cells in humans. Int. J. Impot. Res. 2005, 17, 377–380. [Google Scholar] [CrossRef]
- Cun, Y.; Diao, B.; Zhang, Z.; Wang, G.; Yu, J.; Ma, L.; Rao, Z. Role of the stromal cell derived factor-1 in the biological functions of endothelial progenitor cells and its underlying mechanisms. Exp. Ther. Med. 2021, 21, 39. [Google Scholar] [CrossRef]
- Xiao, Q.; Ye, S.; Oberhollenzer, F.; Mayr, A.; Jahangiri, M.; Willeit, J.; Kiechl, S.; Xu, Q. SDF1 gene variation is associated with circulating SDF1alpha level and endothelial progenitor cell number: The Bruneck Study. PLoS ONE 2008, 3, e4061. [Google Scholar] [CrossRef] [PubMed]
- Aicher, A.; Heeschen, C.; Mildner-Rihm, C.; Urbich, C.; Ihling, C.; Technau-Ihling, K.; Zeiher, A.M.; Dimmeler, S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat. Med. 2003, 9, 1370–1376. [Google Scholar] [CrossRef]
- Takahashi, T.; Kalka, C.; Masuda, H.; Chen, D.; Silver, M.; Kearney, M.; Magner, M.; Isner, J.M.; Asahara, T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999, 5, 434–438. [Google Scholar] [CrossRef]
- Qiu, C.; Xie, Q.; Zhang, D.; Chen, Q.; Hu, J.; Xu, L. GM-CSF induces cyclin D1 expression and proliferation of endothelial progenitor cells via PI3K and MAPK signaling. Cell Physiol. Biochem. 2014, 33, 784–795. [Google Scholar] [CrossRef]
- Laufs, U.; Werner, N.; Link, A.; Endres, M.; Wassmann, S.; Jürgens, K.; Miche, E.; Böhm, M.; Nickenig, G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 2004, 109, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Muggeridge, D.; Dodd, J.; Ross, M.D. CD34(+) progenitors are predictive of mortality and are associated with physical activity in cardiovascular disease patients. Atherosclerosis 2021, 333, 108–115. [Google Scholar] [CrossRef]
- Cassano, V.; Tripepi, G.; Perticone, M.; Miceli, S.; Scopacasa, I.; Armentaro, G.; Greco, M.; Maio, R.; Hribal, M.L.; Sesti, G.; et al. Endothelial progenitor cells predict vascular damage progression in naive hypertensive patients according to sex. Hypertens. Res. 2021, 44, 1451–1461. [Google Scholar] [CrossRef]
- Huang, Z.X.; Fang, J.; Zhou, C.H.; Zeng, J.; Yang, D.; Liu, Z. CD34(+) cells and endothelial progenitor cell subpopulations are associated with cerebral small vessel disease burden. Biomark. Med. 2021, 15, 191–200. [Google Scholar] [CrossRef]
- Shahrour, H.E.; Al Fahom, S.; Al-Massarani, G.; AlSaadi, A.R.; Magni, P. Osteocalcin-expressing endothelial progenitor cells and serum osteocalcin forms are independent biomarkers of coronary atherosclerotic disease severity in male and female patients. J. Endocrinol. Investig. 2022, 45, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Flores-Umanzor, E.J.; Ortega-Paz, L.; Cepas-Guillen, P.L.; Giacchi, G.; Padro, T.; Badimon, L.; Sabaté, M.; Brugaletta, S. Endothelial Progenitor Cell Function in Patients with Coronary Chronic Total Occlusion and its Relationship With Collateral Circulation. J. Invasive Cardiol. 2021, 33, E809–E816. [Google Scholar] [PubMed]
- Hoffmann, J.; Haendeler, J.; Aicher, A.; Rössig, L.; Vasa, M.; Zeiher, A.M.; Dimmeler, S. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: Important role of nitric oxide. Circ. Res. 2001, 89, 709–715. [Google Scholar] [CrossRef]
- Murasawa, S.; Llevadot, J.; Silver, M.; Isner, J.M.; Losordo, D.W.; Asahara, T. Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation 2002, 106, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Scheubel, R.J.; Zorn, H.; Silber, R.E.; Kuss, O.; Morawietz, H.; Holtz, J.; Simm, A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J. Am. Coll. Cardiol. 2003, 42, 2073–2080. [Google Scholar] [CrossRef]
- Hamano, K.; Nishida, M.; Hirata, K.; Mikamo, A.; Li, T.S.; Harada, M.; Miura, T.; Matsuzaki, M.; Esato, K. Local implantation of autologous bone marrow cells for therapeutic angiogenesis in patients with ischemic heart disease: Clinical trial and preliminary results. Jpn. Circ. J. 2001, 65, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Stamm, C.; Westphal, B.; Kleine, H.D.; Petzsch, M.; Kittner, C.; Klinge, H.; Schümichen, C.; Nienaber, C.A.; Freund, M.; Steinhoff, G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 2003, 361, 45–46. [Google Scholar] [CrossRef]
- Xiao, Q.; Kiechl, S.; Patel, S.; Oberhollenzer, F.; Weger, S.; Mayr, A.; Metzler, B.; Reindl, M.; Hu, Y.; Willeit, J.; et al. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis—Results from a large population-based study. PLoS ONE 2007, 2, e975. [Google Scholar] [CrossRef]
- Rauscher, F.M.; Goldschmidt-Clermont, P.J.; Davis, B.H.; Wang, T.; Gregg, D.; Ramaswami, P.; Pippen, A.M.; Annex, B.H.; Dong, C.; Taylor, D.A. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 2003, 108, 457–463. [Google Scholar] [CrossRef]
- Vedin, O.; Lam, C.S.P.; Koh, A.S.; Benson, L.; Teng, T.H.K.; Tay, W.T.; Braun, O.Ö.; Savarese, G.; Dahlström, U.; Lund, L.H. Significance of Ischemic Heart Disease in Patients with Heart Failure and Preserved, Midrange, and Reduced Ejection Fraction: A Nationwide Cohort Study. Circ. Heart Fail. 2017, 10, e003875. [Google Scholar] [CrossRef]
- Ohuchi, H.; Hayama, Y.; Negishi, J.; Noritake, K.; Iwasa, T.; Miyazaki, A.; Yamada, O.; Shiraishi, I. Heart failure with preserved right ventricular ejection fraction in postoperative adults with congenital heart disease: A subtype of severe right ventricular pathophysiology. Int. J. Cardiol. 2016, 212, 223–231. [Google Scholar] [CrossRef]
- Sano, T.; Ousaka, D.; Goto, T.; Ishigami, S.; Hirai, K.; Kasahara, S.; Ohtsuki, S.; Sano, S.; Oh, H. Impact of Cardiac Progenitor Cells on Heart Failure and Survival in Single Ventricle Congenital Heart Disease. Circ. Res. 2018, 122, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Hinton, R.B.; Ware, S.M. Heart Failure in Pediatric Patients with Congenital Heart Disease. Circ. Res. 2017, 120, 978–994. [Google Scholar] [CrossRef] [PubMed]
- Newburger, J.W.; Sleeper, L.A.; Frommelt, P.C.; Pearson, G.D.; Mahle, W.T.; Chen, S.; Dunbar-Masterson, C.; Mital, S.; Williams, I.A.; Ghanayem, N.S.; et al. Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial. Circulation 2014, 129, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Atz, A.M.; Zak, V.; Mahony, L.; Uzark, K.; D’Agincourt, N.; Goldberg, D.J.; Williams, R.V.; Breitbart, R.E.; Colan, S.D.; Burns, K.M.; et al. Longitudinal Outcomes of Patients with Single Ventricle After the Fontan Procedure. J. Am. Coll. Cardiol. 2017, 69, 2735–2744. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Teerlink, J.R. Heart failure. Lancet 2017, 390, 1981–1995. [Google Scholar] [CrossRef]
- Ishigami, S.; Ohtsuki, S.; Eitoku, T.; Ousaka, D.; Kondo, M.; Kurita, Y.; Hirai, K.; Fukushima, Y.; Baba, K.; Goto, T.; et al. Intracoronary Cardiac Progenitor Cells in Single Ventricle Physiology: The PERSEUS (Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease) Randomized Phase 2 Trial. Circ. Res. 2017, 120, 1162–1173. [Google Scholar] [CrossRef]
- Ishigami, S.; Ohtsuki, S.; Tarui, S.; Ousaka, D.; Eitoku, T.; Kondo, M.; Okuyama, M.; Kobayashi, J.; Baba, K.; Arai, S.; et al. Intracoronary autologous cardiac progenitor cell transfer in patients with hypoplastic left heart syndrome: The TICAP prospective phase 1 controlled trial. Circ. Res. 2015, 116, 653–664. [Google Scholar] [CrossRef]
- Kong, D.; Melo, L.G.; Gnecchi, M.; Zhang, L.; Mostoslavsky, G.; Liew, C.C.; Pratt, R.E.; Dzau, V.J. Cytokine-induced mobilization of circulating endothelial progenitor cells enhances repair of injured arteries. Circulation 2004, 110, 2039–2046. [Google Scholar] [CrossRef]
- Werner, N.; Junk, S.; Laufs, U.; Link, A.; Walenta, K.; Bohm, M.; Nickenig, G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ. Res. 2003, 93, e17–e24. [Google Scholar] [CrossRef]
- Hu, Y.; Davison, F.; Zhang, Z.; Xu, Q. Endothelial replacement and angiogenesis in arteriosclerotic lesions of allografts are contributed by circulating progenitor cells. Circulation 2003, 108, 3122–3127. [Google Scholar] [CrossRef]
- Xu, Q. Mouse models of arteriosclerosis: From arterial injuries to vascular grafts. Am. J. Pathol. 2004, 165, 1–10. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Z.; Davison, F.; Hu, Y. Circulating progenitor cells regenerate endothelium of vein graft atherosclerosis, which is diminished in ApoE-deficient mice. Circ. Res. 2003, 93, e76–e86. [Google Scholar] [CrossRef] [PubMed]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H.; et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef]
- Kawamoto, A.; Iwasaki, H.; Kusano, K.; Murayama, T.; Oyamada, A.; Silver, M.; Hulbert, C.; Gavin, M.; Hanley, A.; Ma, H.; et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation 2006, 114, 2163–2169. [Google Scholar] [CrossRef]
- Keighron, C.; Lyons, C.J.; Creane, M.; O’Brien, T.; Liew, A. Recent Advances in Endothelial Progenitor Cells Toward Their Use in Clinical Translation. Front. Med. 2018, 5, 354. [Google Scholar] [CrossRef]
- Meluzin, J.; Janousek, S.; Mayer, J.; Groch, L.; Hornácek, I.; Hlinomaz, O.; Kala, P.; Panovský, R.; Prásek, J.; Kamínek, M.; et al. Three-, 6-, and 12-month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. Int. J. Cardiol. 2008, 128, 185–192. [Google Scholar] [CrossRef]
- Elmadbouh, I.; Singla, D.K. BMP-7 Attenuates Inflammation-Induced Pyroptosis and Improves Cardiac Repair in Diabetic Cardiomyopathy. Cells 2021, 10, 2640. [Google Scholar] [CrossRef]
- Detriche, G.; Gendron, N.; Philippe, A.; Gruest, M.; Billoir, P.; Rossi, E.; Guerin, C.L.; Lokajczyk, A.; Brabant, S.; Prié, D.; et al. Gonadotropins as novel active partners in vascular diseases: Insight from angiogenic properties and thrombotic potential of endothelial colony-forming cells. J. Thromb. Haemost. 2022, 20, 230–237. [Google Scholar] [CrossRef]
- Salybekov, A.A.; Kunikeyev, A.D.; Kobayashi, S.; Asahara, T. Latest Advances in Endothelial Progenitor Cell-Derived Extracellular Vesicles Translation to the Clinic. Front. Cardiovasc. Med. 2021, 8, 734562. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Xu, J.; Liu, X.; Lu, Y.Q. Cardioprotective Roles of Endothelial Progenitor Cell-Derived Exosomes. Front. Cardiovasc. Med. 2021, 8, 717536. [Google Scholar] [CrossRef]
- Lee, Y.N.; Wu, Y.J.; Lee, H.I.; Wang, H.H.; Chang, C.Y.; Tien, T.Y.; Lin, C.F.; Su, C.H.; Yeh, H.I. Ultrasonic microbubble VEGF gene delivery improves angiogenesis of senescent endothelial progenitor cells. Sci. Rep. 2021, 11, 13449. [Google Scholar] [CrossRef] [PubMed]
- Loiola, R.A.; Garcia-Gabilondo, M.; Grayston, A.; Bugno, P.; Kowalska, A.; Duban-Deweer, S.; Rizzi, E.; Hachani, J.; Sano, Y.; Shimizu, F.; et al. Secretome of endothelial progenitor cells from stroke patients promotes endothelial barrier tightness and protects against hypoxia-induced vascular leakage. Stem Cell Res. Ther. 2021, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Koc, M.; Bihorac, A.; Segal, M.S. Circulating endothelial cells as potential markers of the state of the endothelium in hemodialysis patients. Am. J. Kidney Dis. 2003, 42, 704–712. [Google Scholar] [CrossRef]
- Razazian, M.; Khosravi, M.; Bahiraii, S.; Uzan, G.; Shamdani, S.; Naserian, S. Differences and similarities between mesenchymal stem cell and endothelial progenitor cell immunoregulatory properties against T cells. World J. Stem Cells 2021, 13, 971–984. [Google Scholar] [CrossRef]
- Froehlich, H.; Simari, R.D.; Boilson, B.A. Differential phenotype and behavior in culture of CD34 positive cells from peripheral blood and adipose tissue. Heliyon 2021, 7, e07779. [Google Scholar] [CrossRef]
- Woywodt, A.; Streiber, F.; de Groot, K.; Regelsberger, H.; Haller, H.; Haubitz, M. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet 2003, 361, 206–210. [Google Scholar] [CrossRef]
- Woywodt, A.; Bahlmann, F.H.; De Groot, K.; Haller, H.; Haubitz, M. Circulating endothelial cells: Life, death, detachment and repair of the endothelial cell layer. Nephrol. Dial. Transpl. 2002, 17, 1728–1730. [Google Scholar] [CrossRef]
- Takada, K.; Toyokawa, G.; Kinoshita, F.; Jogo, T.; Kohashi, K.; Wakasu, S.; Ono, Y.; Tanaka, K.; Oba, T.; Osoegawa, A.; et al. Expression of PD-L1, PD-L2, and IDO1 on tumor cells and density of CD8-positive tumor-infiltrating lymphocytes in early-stage lung adenocarcinoma according to histological subtype. J. Cancer Res. Clin. Oncol. 2020, 146, 2639–2650. [Google Scholar] [CrossRef]
- Tagawa, S.; Nakanishi, C.; Mori, M.; Yoshimuta, T.; Yoshida, S.; Shimojima, M.; Yokawa, J.; Kawashiri, M.A.; Yamagishi, M.; Hayashi, K. Determination of Early and Late Endothelial Progenitor Cells in Peripheral Circulation and Their Clinical Association with Coronary Artery Disease. Int. J. Vasc. Med. 2015, 2015, 674213. [Google Scholar] [CrossRef]
- Heissig, B.; Hattori, K.; Dias, S.; Friedrich, M.; Ferris, B.; Hackett, N.R.; Crystal, R.G.; Besmer, P.; Lyden, D.; Moore, M.A.; et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 2002, 109, 625–637. [Google Scholar] [CrossRef]
- Schachinger, V.; Assmus, B.; Britten, M.B.; Honold, J.; Lehmann, R.; Teupe, C.; Abolmaali, N.D.; Vogl, T.J.; Hofmann, W.K.; Martin, H.; et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: Final one-year results of the TOPCARE-AMI Trial. J. Am. Coll. Cardiol. 2004, 44, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Assmus, B.; Schachinger, V.; Teupe, C.; Britten, M.; Lehmann, R.; Döbert, N.; Grünwald, F.; Aicher, A.; Urbich, C.; Martin, H.; et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 2002, 106, 3009–3017. [Google Scholar] [CrossRef] [PubMed]
- Britten, M.B.; Abolmaali, N.D.; Assmus, B.; Lehmann, R.; Honold, J.; Schmitt, J.; Vogl, T.J.; Martin, H.; Schächinger, V.; Dimmeler, S.; et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): Mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation 2003, 108, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.M.; Valgimigli, M.; Giannico, M.B.; Zaccone, V.; Perfetti, M.; D’Amario, D.; Rebuzzi, A.G.; Crea, F. From bone marrow to the arterial wall: The ongoing tale of endothelial progenitor cells. Eur. Heart J. 2009, 30, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, F.; Camoin-Jau, L.; Anfosso, F.; Sampol, J.; Dignat-George, F. Circulating endothelial cells, microparticles and progenitors: Key players towards the definition of vascular competence. J. Cell Mol. Med. 2009, 13, 454–471. [Google Scholar] [CrossRef]
- Tricot, O.; Mallat, Z.; Heymes, C.; Belmin, J.; Leseche, G.; Tedgui, A. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation 2000, 101, 2450–2453. [Google Scholar] [CrossRef]
- Schwartz, S.M.; Benditt, E.P. Clustering of replicating cells in aortic endothelium. Proc. Natl. Acad. Sci. USA 1976, 73, 651–653. [Google Scholar] [CrossRef]
- Schwartz, S.M.; Benditt, E.P. Cell replication in the aortic endothelium: A new method for study of the problem. Lab. Investig. 1973, 28, 699–707. [Google Scholar]
- Xu, Q. Biomechanical-stress-induced signaling and gene expression in the development of arteriosclerosis. Trends Cardiovasc. Med. 2000, 10, 35–41. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Fu, W.; Sahebkar, A.; Tan, Y.; Xu, S.; Li, H. Histone Deacetylases (HDACs) and Atherosclerosis: A Mechanistic and Pharmacological Review. Front. Cell Dev. Biol. 2020, 8, 581015. [Google Scholar] [CrossRef]
- Zampetaki, A.; Zeng, L.; Margariti, A.; Xiao, Q.; Li, H.; Zhang, Z.; Pepe, A.E.; Wang, G.; Habi, O.; deFalco, E.; et al. Histone deacetylase 3 is critical in endothelial survival and atherosclerosis development in response to disturbed flow. Circulation 2010, 121, 132–142. [Google Scholar] [CrossRef]
- Rossig, L.; Urbich, C.; Bruhl, T.; Dernbach, E.; Heeschen, C.; Chavakis, E.; Sasaki, K.; Aicher, D.; Diehl, F.; Seeger, F.; et al. Histone deacetylase activity is essential for the expression of HoxA9 and for endothelial commitment of progenitor cells. J. Exp. Med. 2005, 201, 1825–1835. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Effects of shear stress on endothelial cells: Go with the flow. Acta Physiol. 2017, 219, 382–408. [Google Scholar] [CrossRef]
- Zhou, B.; Margariti, A.; Zeng, L.; Habi, O.; Xiao, Q.; Martin, D.; Wang, G.; Hu, Y.; Wang, X.; Xu, Q. Splicing of histone deacetylase 7 modulates smooth muscle cell proliferation and neointima formation through nuclear beta-catenin translocation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2676–2684. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Peng, H.; Zhang, J.; Bo, L.; Wen, L.; Liu, W.; Bai, W.; Zhang, H. Histone Deacetylase Inhibitor Trichostatin A Reduces Endothelial Cell Proliferation by Suppressing STAT5A-Related Gene Transcription. Front. Oncol. 2021, 11, 746266. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).