Revisiting the Old Data of Heat Shock Protein 27 Expression in Squamous Cell Carcinoma: Enigmatic HSP27, More Than Heat Shock
Abstract
1. Introduction
2. Patterns of HSP27 Expression in SCC
3. Clinicopathological Meaning
4. Prognostic Significance
5. Participation in Proliferation
6. Involvement in Metastases
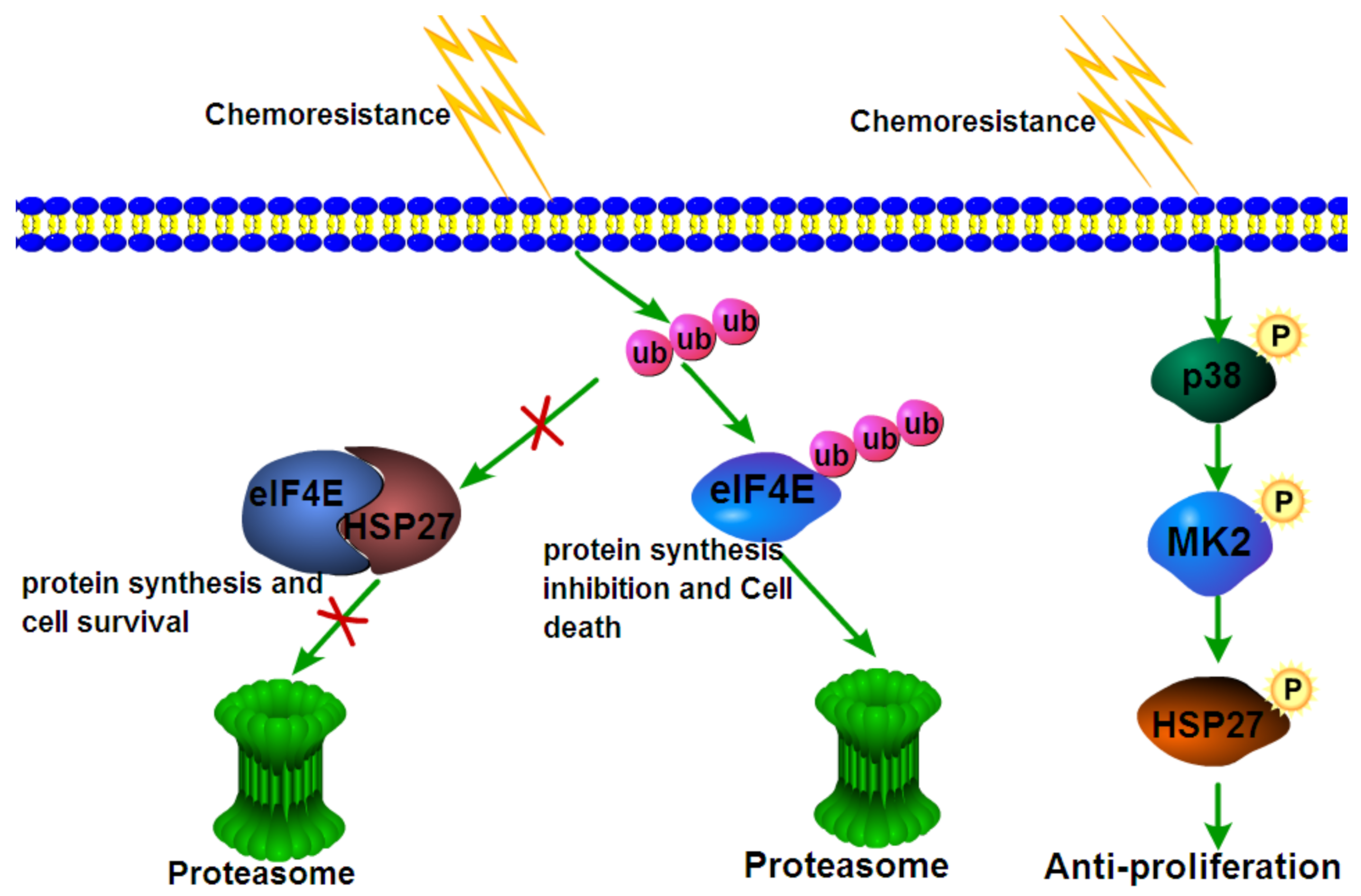
7. Engagement in Chemoresistance
8. Engagement in Radioresistance
9. Protection against Oxidative Stress
10. Participation in Epithelial-Mesenchymal Transition (EMT)
11. Extracellular HSP27
12. Inhibitors of HSP27 as a Therapeutic Strategy for Cancer
13. In Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandour-Edwards, R.; McClaren, M.; Isseroff, R.R. Immunolocalization of Low-Molecular-Weight Stress Protein HSP 27 in Normal Skin and Common Cutaneous Lesions. Am. J. Dermatopathol. 1994, 16, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Trautinger, F.; Kindas-Mugge, I.; Dekrout, B.; Knobler, R.M.; Metze, D. Expression of the 27-kDa heat shock protein in human epidermis and in epidermal neoplasms: An immunohistological study. Br. J. Dermatol. 1995, 133, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Lambot, M.A.; Peny, M.O.; Fayt, I.; Haot, J.; Noel, J.C. Overexpression of 27-kDa heat shock protein relates to poor histological differentiation in human oesophageal squamous cell carcinoma. Histopathology 2000, 36, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Y.; Xiong, G.; Zhang, J.; Wang W., J. Screening of differentially expressed proteins from human esophageal cancer and esophageal tissues by two-dimensional difference gel electrophoresis and mass spectrometry. Nan Fang Yi Ke Da Xue Xue Bao 2007, 27, 1406–1409. [Google Scholar]
- Liu, Z.; Feng, J.-G.; Tuersun, A.; Liu, T.; Liu, H.; Liu, Q.; Zheng, S.-T.; Huang, C.-G.; Lv, G.-D.; Sheyhidin, I.; et al. Proteomic identification of differentially-expressed proteins in esophageal cancer in three ethnic groups in Xinjiang. Mol. Biol. Rep. 2010, 38, 3261–3269. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Zheng, S.; Liu, Q.; Shen, T.; Han, X.; Zhang, Q.; Yang, L.; Lu, X. SUMOylation of HSP27 regulates PKM2 to promote esophageal squamous cell carcinoma progression. Oncol. Rep. 2020, 44, 1355–1364. [Google Scholar] [CrossRef]
- Xue, L.; Yang, L.; Jin, Z.-A.; Gao, F.; Kang, J.-Q.; Xu, G.-H.; Liu, B.; Li, H.; Wang, X.-J.; Liu, L.-J.; et al. Increased expression of HSP27 inhibits invasion and metastasis in human esophageal squamous cell carcinoma. Tumor Biol. 2014, 35, 6999–7007. [Google Scholar] [CrossRef]
- Kawanishi, K.; Shiozaki, H.; Doki, Y.; Sakita, I.; Inoue, M.; Yano, M.; Tsujinaka, T.; Shamma, A.; Monden, M. Prognostic significance of heat shock proteins 27 and 70 in patients with squamous cell carcinoma of the esophagus. Cancer 1999, 85, 1649–1657. [Google Scholar] [CrossRef]
- Suzuki, H.; Sugimura, H.; Hashimoto, K. Overexpression of heat shock protein 27 is associated with good prognosis in the patient with oral squamous cell carcinoma. Br. J. Oral Maxillofac. Surg. 2007, 45, 123–129. [Google Scholar] [CrossRef]
- Han, J.; Kioi, M.; Chu, W.-S.; Kasperbauer, J.L.; Strome, S.E.; Puri, R.K. Identification of potential therapeutic targets in human head & neck squamous cell carcinoma. Head Neck Oncol. 2009, 1, 27. [Google Scholar] [CrossRef][Green Version]
- Lo Muzio, L.; Leonardi, R.; Mariggio, M.A.; Mignogna, M.D.; Rubini, C.; Vinella, A.; Pannone, G.; Giannetti, L.; Serpico, R.; Testa N., F.; et al. HSP 27 as possible prognostic factor in patients with oral squamous cell carcinoma. Histol. Histopathol. 2004, 119–128. [Google Scholar] [CrossRef]
- Lo Muzio, L.; Campisi, G.; Farina, A.; Rubini, C.; Ferrari, F.; Falaschini, S.; Leonardi, R.; Carinci, F.; Stalbano, S.; De Rosa, G. Prognostic value of HSP27 in head and neck squamous cell carcinoma: A retrospective analysis of 57 tumours. Anticancer Res. 2006, 26, 1343–1349. [Google Scholar] [PubMed]
- Trautinger, F.; Kokesch, C.; Herbacek, I.; Knobler, R.M.; Kindås-Mügge, I. Overexpression of the small heat shock protein, hsp27, confers resistance to hyperthermia, but not to oxidative stress and UV-induced cell death, in a stably transfected squamous cell carcinoma cell line. J. Photochem. Photobiol. B Biol. 1997, 39, 90–95. [Google Scholar] [CrossRef]
- Ito, T.; Kawabe, R.; Kurasono, Y.; Hara, M.; Kitamura, H.; Fujita, K.; Kanisawa, M. Expression of heat shock proteins in squamous cell carcinoma of the tongue: An immunohistochemical study. J. Oral Pathol. Med. 1998, 27, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Pannone, G.; Magro, G.; Kudo, Y.; Takata, T.; Lo Muzio, L. Differential expression of heat shock protein 27 in normal oral mucosa, oral epithelial dysplasia and squamous cell carcinoma. Oncol. Rep. 2002, 9, 261–266. [Google Scholar] [CrossRef]
- Ono, A.; Kumai, T.; Koizumi, H.; Nishikawa, H.; Kobayashi, S.; Tadokoro, M. Overexpression of heat shock protein 27 in squamous cell carcinoma of the uterine cervix: A proteomic analysis using archival formalin-fixed, paraffin-embedded tissues. Hum. Pathol. 2009, 40, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Liu, X.; Sheng, S.; Ye, H.; Peng, T.; Shi, F.; Crowe, D.L.; Zhou, X. Dysregulation of heat shock protein 27 expression in oral tongue squamous cell carcinoma. BMC Cancer 2009, 9, 167. [Google Scholar] [CrossRef]
- Tozawa-Ono, A.; Yoshida, A.; Yokomachi, N.; Handa, R.; Koizumi, H.; Kiguchi, K.; Ishizuka, B.; Suzuki, N. Heat shock protein 27 and p16 immunohistochemistry in cervical intraepithelial neoplasia and squamous cell carcinoma. Hum. Cell 2012, 25, 24–28. [Google Scholar] [CrossRef]
- Mese, H.; Sasaki, A.; Nakayama, S.; Yoshioka, N.; Yoshihama, Y.; Kishimoto, K.; Matsumura, T. Prognostic significance of heat shock protein 27 (HSP27) in patients with oral squamous cell carcinoma. Oncol. Rep. 2002, 9, 341–344. [Google Scholar] [CrossRef]
- Karri, R.L.; Subramanyam, R.V.; Venigella, A.; Babburi, S.; Pinisetti, S.; Rudraraju, A. Differential expression of heat shock protein 27 in oral epithelial dysplasias and squamous cell carcinoma. J. Microsc. Ultrastruct. 2020, 8, 62–68. [Google Scholar] [CrossRef]
- Mohtasham, N.; Babakoohi, S.; Montaser-Kouhsari, L.; Memar, B.; Salehinejad, J.; Rahpeyma, A.; Khageh-Ahmady, S.; Marouzi, P.; Firooz, A.; Pazoki-Toroudi, H.; et al. The expression of heat shock proteins 27 and 105 in squamous cell carcinoma of the tongue and relationship with clinicopathological index. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e730–e735. [Google Scholar] [CrossRef] [PubMed]
- Luz, C.C.F.; Noguti, J.; de Araújo, L.B.; Dos Santos, G.M.S.; Gomes, T.S.; Neto, R.A. HSP27 and HSP70 Expression in Esophageal Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2017, 18, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Z.; Wang, W.; Dong, J.; Gong, X.; Pu, H.; Chen, X. Expression of Heat Shock Protein-27 (Hsp27) and P38MAPK in Esophageal Squamous Cell Carcinoma. Med. Sci. Monit. 2017, 23, 5246–5253. [Google Scholar] [CrossRef] [PubMed]
- Gandour-Edwards, R.; Trock, B.J.; Gumerlock, P.; Donald, P.J. Heat Shock Protein and p53 Expression in Head and Neck Squamous Cell Carcinoma. Otolaryngol. Head. Neck Surg. 1998, 118, 610–624. [Google Scholar] [CrossRef]
- Shiozaki, H.; Doki, Y.; Kawanishi, K.; Shamma, A.; Yano, M.; Inoue, M.; Monden, M. Clinical application of malignancy potential grading as a prognostic factor of human esophageal cancers. Surgery 2000, 127, 552–561. [Google Scholar] [CrossRef]
- Takeno, S.; Noguchi, T.; Takahashi, Y.; Kikuchi, R.; Uchida, Y.; Yokoyama, S. Immunohistochemical and clinicopathologic analysis of response to neoadjuvant therapy for esophageal squamous cell carcinoma. Dis. Esophagus 2001, 14, 149–154. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kato, H.; Faried, A.; Sohda, M.; Nakajima, M.; Fukai, Y.; Masuda, N.; Manda, R.; Fukuchi, M.; Ojima, H.; et al. Predictors of response to chemo-radiotherapy and radiotherapy for esophageal squamous cell carcinoma. Anticancer Res. 2005, 25, 2749–2755. [Google Scholar]
- Ajalyakeen, H.; Almohareb, M.; Al-Assaf, M. Overexpression of heat shock protein 27 (HSP-27) is associated with bad prognosis in oral squamous cell carcinoma. Dent. Med. Probl. 2020, 57, 227–231. [Google Scholar] [CrossRef]
- I Lomnytska, M.; Becker, S.; Bodin, I.; Olsson, A.; Hellman, K.; Hellström, A.-C.; Mints, M.; Hellman, U.; Auer, G.; Andersson, S. Differential expression of ANXA6, HSP27, PRDX2, NCF2, and TPM4 during uterine cervix carcinogenesis: Diagnostic and prognostic value. Br. J. Cancer 2010, 104, 110–119. [Google Scholar] [CrossRef]
- Wang, X.-W.; Shi, X.-H.; Tong, Y.-S.; Cao, X.-F. The Prognostic Impact of Heat Shock Proteins Expression in Patients with Esophageal Cancer: A Meta-Analysis. Yonsei Med. J. 2015, 56, 1497–1502. [Google Scholar] [CrossRef]
- Kaigorodova, E.V.; Zavyalova, M.V.; Bychkov, V.A.; Perelmuter, V.M.; Choynzonov, E.L. Functional state of the Hsp27 chaperone as a molecular marker of an unfavorable course of larynx cancer. Cancer Biomark. 2016, 17, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kindas-Mugge, I.; Herbacek, I.; Jantschitsch, C.; Micksche, M.; Trautinger, F. Modification of growth and tumorigenicity in epidermal cell lines by DNA-mediated gene transfer of M(r) 27,000 heat shock protein (hsp27). Cell Growth Differ. 1996, 7, 1167–1174. [Google Scholar]
- Zhu, Z.; Xu, X.; Yu, Y.; Graham, M.; Prince, M.E.; Carey, T.E.; Sun, D.; Liu, G. Silencing Heat Shock Protein 27 Decreases Metastatic Behavior of Human Head and Neck Squamous Cell Cancer Cells in Vitro. Mol. Pharm. 2010, 7, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-K.; Wang, Y.-S.; Xu, X. Biology behavior of head and neck squamous cell cancer cells changes after knocking down heat shock protein 27. Hua Xi Kou Qiang Yi Xue Za Zhi 2020, 38, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, Y.; Chen, Y.; Tang, L.; Wang, G.; Jiang, H.; Wang, X.; Tao, Y.; Zhuang, W. Reduced LINC00551 expression promotes proliferation and invasion of esophageal squamous cancer by increase in HSP27 phosphorylation. J. Cell. Physiol. 2020, 236, 1418–1431. [Google Scholar] [CrossRef]
- Chen, S.-F.; Nieh, S.; Jao, S.-W.; Liu, C.-L.; Wu, C.-H.; Chang, Y.-C.; Yang, C.-Y.; Lin, Y.-S. Quercetin Suppresses Drug-Resistant Spheres via the p38 MAPK–Hsp27 Apoptotic Pathway in Oral Cancer Cells. PLoS ONE 2012, 7, e49275. [Google Scholar] [CrossRef]
- Liu, C.; Chou, K.-T.; Hsu, J.; Lin, J.; Hsu, T.; Yen, D.H.; Hung, S.; Hsu, H. High metabolic rate and stem cell characteristics of esophageal cancer stem-like cells depend on the Hsp27–AKT–HK2 pathway. Int. J. Cancer 2019, 145, 2144–2156. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, Z.; Liu, H.; Xiong, Y.; Luo, L.; Jia, X.; Peng, C.; Zhang, Q.; Li, N.; Gu, Y.; et al. HSP27-Mediated Extracellular and Intracellular Signaling Pathways Synergistically Confer Chemoresistance in Squamous Cell Carcinoma of Tongue. Clin. Cancer Res. 2018, 24, 1163–1175. [Google Scholar] [CrossRef]
- Kai, H.; Oba, M.; Yano, S.; Shuto, T.; Suico, M.A.; Eguma, A. IFN-γ down-regulates Hsp27 and enhances hyperthermia-induced tumor cell death in vitro and tumor suppression in vivo. Int. J. Oncol. 2008, 32, 1317–1324. [Google Scholar] [CrossRef]
- Fortin, A.; Raybaud-Diogène, H.; Têtu, B.; Huot, J.; Landry, J. Overexpression of the 27 KDa heat shock protein is associated with thermoresistance and chemoresistance but not with radioresistance. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 1259–1266. [Google Scholar] [CrossRef]
- Nakata, B.; Hom, D.; Barton, R.; Howell, S.; Los, G. mRNA levels of molecular chaperones hsp27, hsp60 and hsp70 in cisplatin resistant squamous cell carcinomas. Int. J. Oncol. 1996, 8, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Asada, Y.; Tsuruta, M.; Okabayashi, K.; Shigeta, K.; Ishida, T.; Shimada, T.; Suzumura, H.; Koishikawa, K.; Akimoto, S.; Hasegawa, H.; et al. Inhibition of Heat-shock Protein 27 Reduces 5-Fluorouracil-acquired Resistance in Human Colon Cancer Cells. Anticancer Res. 2021, 41, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, C.; Taieb, D.; Baylot, V.; Ettinger, S.; Soubeyran, P.; De-Thonel, A.; Nelson, C.; Garrido, C.; So, A.; Fazli, L.; et al. Heat shock protein 27 confers resistance to androgen ablation and chemotherapy in prostate cancer cells through eIF4E. Oncogene 2010, 29, 1883–1896. [Google Scholar] [CrossRef]
- Hsu, H.-S.; Lin, J.-H.; Huang, W.-C.; Hsu, T.-W.; Su, K.; Chiou, S.-H.; Tsai, Y.-T. Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer 2010, 117, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Stope, M.B.; Weiss, M.; Preuss, M.; Streitbörger, A.; Ritter, C.A.; Zimmermann, U.; Walther, R.; Burchardt, M. Immediate and transient phosphorylation of the heat shock protein 27 initiates chemoresistance in prostate cancer cells. Oncol. Rep. 2014, 32, 2380–2386. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Adachi, S.; Yasuda, I.; Yamauchi, T.; Kawaguchi, J.; Itani, M.; Yoshioka, T.; Matsushima-Nishiwaki, R.; Hirose, Y.; Kozawa, O.; et al. Phosphorylation status of heat shock protein 27 plays a key role in gemcitabine-induced apoptosis of pancreatic cancer cells. Cancer Lett. 2011, 313, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, W.; Lan, Q. The apoptosis-resistance in t-AUCB-treated glioblastoma cells depends on activation of Hsp27. J. Neuroncol. 2012, 110, 187–194. [Google Scholar] [CrossRef]
- Muschter, D.; Geyer, F.; Bauer, R.; Ettl, T.; Schreml, S.; Haubner, F. A comparison of cell survival and heat shock protein expression after radiation in normal dermal fibroblasts, microvascular endothelial cells, and different head and neck squamous carcinoma cell lines. Clin. Oral Investig. 2018, 22, 2251–2262. [Google Scholar] [CrossRef]
- Hadchity, E.; Aloy, M.-T.; Paulin, C.; Armandy, E.; Watkin, E.; Rousson, R.; Gleave, M.; Chapet, O.; Rodriguez-Lafrasse, C. Heat Shock Protein 27 as a New Therapeutic Target for Radiation Sensitization of Head and Neck Squamous Cell Carcinoma. Mol. Ther. 2009, 17, 1387–1394. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, G.; Huang, S.; Feng, X. Expression of HSP70/HSP27 protein in residual lesion after NPC radiotherapy. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2009, 34, 1091–1095. [Google Scholar]
- Arrigo, A.-P.; Firdaus, W.J.; Mellier, G.; Moulin, M.; Paul, C.; Diaz-Latoud, C.; Kretz-Remy, C. Cytotoxic effects induced by oxidative stress in cultured mammalian cells and protection provided by Hsp27 expression. Methods 2005, 35, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jia, K.; Sun, D.; Yang, M. Protective effect of HSP27 in atherosclerosis and coronary heart disease by inhibiting reactive oxygen species. J. Cell. Biochem. 2018, 120, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Ramani, S.; Park, S. HSP27 role in cardioprotection by modulating chemotherapeutic doxorubicin-induced cell death. J. Mol. Med. 2021, 99, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Tanaka, R.; Shimura, H.; Yamashiro, K.; Urabe, T.; Hattori, N. Phosphorylation enhances recombinant HSP27 neuroprotection against focal cerebral ischemia in mice. Neuroscience 2014, 278, 113–121. [Google Scholar] [CrossRef]
- Gaitanaki, C.; Konstantina, S.; Chrysa, S.; Beis, I. Oxidative stress stimulates multiple MAPK signalling pathways and phosphorylation of the small HSP27 in the perfused amphibian heart. J. Exp. Biol. 2003, 206, 2759–2769. [Google Scholar] [CrossRef]
- Mymrikov, E.V.; Seit-Nebi, A.S.; Gusev, N.B. Large Potentials of Small Heat Shock Proteins. Physiol. Rev. 2011, 91, 1123–1159. [Google Scholar] [CrossRef]
- Mehlen, P.; Hickey, E.; Weber, L.A.; Arrigo, A.P. Large Unphosphorylated Aggregates as the Active Form of hsp27 which Controls Intracellular Reactive Oxygen Species and Glutathione Levels and Generates a Protection against TNFalpha in NIH-3T3-Ras Cells. Biochem. Biophys. Res. Commun. 1997, 241, 187–192. [Google Scholar] [CrossRef]
- Rogalla, T.; Ehrnsperger, M.; Préville, X.; Kotlyarov, A.; Lutsch, G.; Ducasse, C.; Paul, C.; Wieske, M.; Arrigo, A.P.; Buchner, J.; et al. Regulation of Hsp27 Oligomerization, Chaperone Function, and Protective Activity Against Oxidative stress/tumor Necrosis Factor Alpha by Phosphorylation. J. Biol. Chem. 1999, 274, 18947–18956. [Google Scholar] [CrossRef]
- Huot, J.; Houle, F.; Spitz, D.R.; Landry, J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996, 56, 273–279. [Google Scholar]
- Preville, X.; Gaestel, M.; Arrigo, A.P. Phosphorylation is not essential for protection of L929 cells by Hsp25 against H2O2-mediated disruption actin cytoskeleton, a protection which appears related to the redox change mediated by Hsp25. Cell Stress Chaperones 1998, 3, 177–187. [Google Scholar] [CrossRef]
- Dávila, D.; Jiménez-Mateos, E.M.; Mooney, C.M.; Velasco, G.; Henshall, D.C.; Prehn, J.H.M. Hsp27 binding to the 3′UTR of bimmRNA prevents neuronal death during oxidative stress–induced injury: A novel cytoprotective mechanism. Mol. Biol. Cell 2014, 25, 3413–3423. [Google Scholar] [CrossRef] [PubMed]
- Beevi, S.S.; Rasheed, M.H.; Geetha, A. Evidence of oxidative and nitrosative stress in patients with cervical squamous cell carcinoma. Clin. Chim. Acta 2007, 375, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Bentz, B. Head and neck squamous cell carcinoma as a model of oxidative-stress and cancer. J. Surg. Oncol. 2007, 96, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Salzman, R.; Pácal, L.; Kaňková, K.; Tomandl, J.; Horáková, Z.; Tóthová, E.; Kostřica, R. High perioperative level of oxidative stress as a prognostic tool for identifying patients with a high risk of recurrence of head and neck squamous cell carcinoma. Int. J. Clin. Oncol. 2010, 15, 565–570. [Google Scholar] [CrossRef]
- Ye, X.; Brabletz, T.; Kang, Y.; Longmore, G.D.; Nieto, M.A.; Stanger, B.Z.; Yang, J.; Weinberg, R.A. Upholding a role for EMT in breast cancer metastasis. Nature 2017, 547, E1–E3. [Google Scholar] [CrossRef]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.C.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.-C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef]
- Wei, L.; Liu, T.-T.; Wang, H.-H.; Hong, H.-M.; Yu, A.L.; Feng, H.-P.; Chang, W.-W. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-κB. Breast Cancer Res. 2011, 13, R101. [Google Scholar] [CrossRef]
- Shiota, M.; Bishop, J.L.; Nip, K.M.; Zardan, A.; Takeuchi, A.; Cordonnier, T.; Beraldi, E.; Bazov, J.; Fazli, L.; Chi, K.; et al. Hsp27 Regulates Epithelial Mesenchymal Transition, Metastasis, and Circulating Tumor Cells in Prostate Cancer. Cancer Res. 2013, 73, 3109–3119. [Google Scholar] [CrossRef]
- Cordonnier, T.; Bishop, J.L.; Shiota, M.; Nip, K.M.; Thaper, D.; Vahid, S.; Heroux, D.; Gleave, M.; Zoubeidi, A. Hsp27 regulates EGF/β-catenin mediated epithelial to mesenchymal transition in prostate cancer. Int. J. Cancer 2015, 136, E496–E507. [Google Scholar] [CrossRef]
- Choi, S.-H.; Nam, J.-K.; Kim, B.-Y.; Jang, J.; Jin, Y.-B.; Lee, H.-J.; Park, S.; Ji, Y.H.; Cho, J.; Lee, Y.-J. HSPB1 Inhibits the Endothelial-to-Mesenchymal Transition to Suppress Pulmonary Fibrosis and Lung Tumorigenesis. Cancer Res. 2016, 76, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, G.; Bellaye, P.; Kolb, M.; Hammann, A.; Crestani, B.; Soler, P.; Marchal-Somme, J.; Hazoume, A.; Gauldie, J.; Gunther, A.; et al. Inhibition of HSP27 blocks fibrosis development and EMT features by promoting Snail degradation. FASEB J. 2013, 27, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Molto, M.D.; Pascual, L.; De Frutos, R. Puff activity after heat shock in two species of the Drosophila obscura group. Experientia 1987, 43, 1225–1227. [Google Scholar] [CrossRef] [PubMed]
- Tissiéres, A.; Mitchell, H.K.; Tracy, U.M. Protein synthesis in salivary glands of Drosophila melanogaster: Relation to chromosome puffs. J. Mol. Biol. 1974, 84, 389–398. [Google Scholar] [CrossRef]
- De Maio, A. Heat shock proteins: Facts, thoughts, and dreams. Shock 1999, 11, 1–12. [Google Scholar] [CrossRef]
- Tytell, M.; Greenberg, S.G.; Lasek, R.J. Heat shock-like protein is transferred from glia to axon. Brain Res. 1986, 363, 161–164. [Google Scholar] [CrossRef]
- Hightower, L.E.; Guidon, P.T., Jr. Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J. Cell. Physiol. 1989, 138, 257–266. [Google Scholar] [CrossRef]
- Nafar, F.; Williams, J.B.; Mearow, K.M. Astrocytes Release HspB1 in Response to Amyloid-β Exposure in vitro. J. Alzheimer’s Dis. 2015, 49, 251–263. [Google Scholar] [CrossRef]
- Thuringer, D.; Jego, G.; Wettstein, G.; Terrier, O.; Cronier, L.; Yousfi, N.; Hébrard, S.; Bouchot, A.; Hazoumé, A.; Joly, A.; et al. Extracellular HSP27 mediates angiogenesis through Toll-like receptor 3. FASEB J. 2013, 27, 4169–4183. [Google Scholar] [CrossRef]
- Didelot, C.; Lanneau, D.; Brunet, M.; Joly, A.-L.; Thonel, A.; Chiosis, G.; Garrido, C. Anti-Cancer Therapeutic Approaches Based on Intracellular and Extracellular Heat Shock Proteins. Curr. Med. Chem. 2007, 14, 2839–2847. [Google Scholar] [CrossRef]
- Schmitt, E.; Gehrmann, M.; Brunet, M.; Multhoff, G.; Garrido, C. Intracellular and extracellular functions of heat shock proteins: Repercussions in cancer therapy. J. Leukoc. Biol. 2006, 81, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Stope, M.B.; Klinkmann, G.; Diesing, K.; Koensgen, D.; Burchardt, M.; Mustea, A. Heat Shock Protein HSP27 Secretion by Ovarian Cancer Cells Is Linked to Intracellular Expression Levels, Occurs Independently of the Endoplasmic Reticulum Pathway and HSP27’s Phosphorylation Status, and Is Mediated by Exosome Liberation. Dis. Markers 2017, 2017, 1575374. [Google Scholar] [CrossRef] [PubMed]
- Dietze, R.; Hammoud, M.K.; Gómez-Serrano, M.; Unger, A.; Bieringer, T.; Finkernagel, F.; Sokol, A.M.; Nist, A.; Stiewe, T.; Reinartz, S.; et al. Phosphoproteomics identify arachidonic-acid-regulated signal transduction pathways modulating macrophage functions with implications for ovarian cancer. Theranostics 2021, 11, 1377–1395. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Yu, E.Y.; Jacobs, C.; Bazov, J.; Kollmannsberger, C.; Higano, C.S.; Mukherjee, S.D.; Gleave, M.E.; Stewart, P.S.; Hotte, S.J. A phase I dose-escalation study of apatorsen (OGX-427), an antisense inhibitor targeting heat shock protein 27 (Hsp27), in patients with castration-resistant prostate cancer and other advanced cancers. Ann. Oncol. 2016, 27, 1116–1122. [Google Scholar] [CrossRef]
- Bellmunt, J.; Eigl, B.J.; Senkus, E.; Loriot, Y.; Twardowski, P.; Castellano, D.; Blais, N.; Sridhar, S.S.; Sternberg, C.N.; Retz, M.; et al. Borealis-1: A randomized, first-line, placebo-controlled, phase II study evaluating apatorsen and chemotherapy for patients with advanced urothelial cancer. Ann. Oncol. 2017, 28, 2481–2488. [Google Scholar] [CrossRef]
| First Author | Type of SCC | Approach Taken | Number of Cases | Associated with Differentiation | Conclusion | ||||
|---|---|---|---|---|---|---|---|---|---|
| Normal Mucosa | Dysplasia | EIN | CIS | Invasive SCC | |||||
| Trautinger F et al. [2] | skin | IHC | 10 all positive | 6 all negative | supporting | HSP27 in the upper epidermal layers may be a marker for epidermal malignancy | |||
| Ito T et al. [14] | Tongue | IHC | Negative | positive | Positive 18/24 | supporting | related to poor histological differentiation. | ||
| Lambot MA et al. [3] | Esophagus | IHC | 5 all positive | 21 positive | none | Hsp27 increases with the anaplasia of the ESCC. | |||
| Leonardi R et al. [15] | Esophagus | IHC | Intense staining | No or low staining | High and low staining | supporting | It is reduced in poorly differentiated areas and elevated in highly differentiated areas | ||
| Ono A et al. [16] | Cervix | IHC | Negative or low | positive | positive | positive | supporting | suggesting the role of Hsp27 in tumor development and progression. | |
| Wang A et al. [17]. | Tongue | IHC | positive | positive | positive | positive | supporting | HSP27 was associated with poor differentiation. | |
| Tozawa-Ono A et al. [18] | Cervix | IHC | Positive 32 out of 51 | Positive 44 out of 57 | All positive | No | HSP27 may be a useful tool in diagnosing IN of the cervix. | ||
| First Author | Type of SCC Used | Approach Taken | Stage | Grade | Metastasis | Therapeutic Effect | Prognosis | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Gandour-Edwards R et al. [24] | Head and neck | IHC | Non associated | Non associated | Non associated | Not mentioned | Non | Remains unclear. |
| Ito T et al. [14] | Tongue | IHC | Non | Non | Non | Not mentioned | Non | Remains unclear. |
| Shiozaki H et al. [25] | Esophagus | IHC | Non | Non | Non | Not mentioned | associated | Associated with poor survival. |
| Takeno S et al. [26] | Esophagus | IHC | Non | Non | Non | Associated | Non | Hsp27 can predict the therapeutic effect. |
| Mese H et al. [19]. | Esophagus | IHC | Non | Non | Not mentioned | Not mentioned | associated | Independent prognostic factor. |
| Lo Muzio L et al. [11]. | Oral | IHC | associated | associated | Not mentioned | Not mentioned | associated | Could be a novel diagnostic and prognostic factor. |
| Miyazaki T et al. [27] | Esophagus | IHC | Non | Non | Non | Most reliable predictor | Non | Hsp27 was the most reliable predictor for chemo-or radiotherapy. |
| Lo Muzio L et al. [12] | Head and neck | IHC | Non | Non | Non | associated | Reduced expression of HSP27 is an early marker of poor prognosis. | |
| Wang A et al. [17] | Tongue | IHC | Non | Non | Non | Not mentioned | Associated | Hsp27 appears to be an independent prognostic marker. |
| Luz CC et al. [22] | Esophagus | IHC | Non | Non | Non | Not mentioned | Non | HSP27 was not a good prognostic factor for ESCC. |
| Zhang Y et al. [23] | Esophagus | IHC | Non | Non | associated | Not mentioned | Non | HSP27 could be used as a prognostic factor in ESCC. |
| Ajalyakeen H et al. [28] | Oral | IHC | Not mentioned | associated | Not mentioned | Not mentioned | Not mentioned | HSP27 may be an indicator of the biological behavior of the tumor. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Liang, Y.; Li, L.; Tan, Y.; Liu, Q.; Liu, T.; Lu, X. Revisiting the Old Data of Heat Shock Protein 27 Expression in Squamous Cell Carcinoma: Enigmatic HSP27, More Than Heat Shock. Cells 2022, 11, 1665. https://doi.org/10.3390/cells11101665
Zheng S, Liang Y, Li L, Tan Y, Liu Q, Liu T, Lu X. Revisiting the Old Data of Heat Shock Protein 27 Expression in Squamous Cell Carcinoma: Enigmatic HSP27, More Than Heat Shock. Cells. 2022; 11(10):1665. https://doi.org/10.3390/cells11101665
Chicago/Turabian StyleZheng, Shutao, Yan Liang, Lu Li, Yiyi Tan, Qing Liu, Tao Liu, and Xiaomei Lu. 2022. "Revisiting the Old Data of Heat Shock Protein 27 Expression in Squamous Cell Carcinoma: Enigmatic HSP27, More Than Heat Shock" Cells 11, no. 10: 1665. https://doi.org/10.3390/cells11101665
APA StyleZheng, S., Liang, Y., Li, L., Tan, Y., Liu, Q., Liu, T., & Lu, X. (2022). Revisiting the Old Data of Heat Shock Protein 27 Expression in Squamous Cell Carcinoma: Enigmatic HSP27, More Than Heat Shock. Cells, 11(10), 1665. https://doi.org/10.3390/cells11101665







