Concomitant Retinal Alterations in Neuronal Activity and TNFα Pathway Are Detectable during the Pre-Symptomatic Stage in a Mouse Model of Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Electroretinography (ERG) and Component Analysis
2.3. Tissue Processing
2.4. Western Blot
2.5. ELISA
2.6. RT-qPCR
2.7. Immunohistochemistry (IHC)
2.8. Image Acquisition and Analysis
2.9. Statistical Analysis
3. Results
3.1. βCTF, But Not Aβ, Is Expressed in the Retina of Pre-Symptomatic APP/PS1 Mice
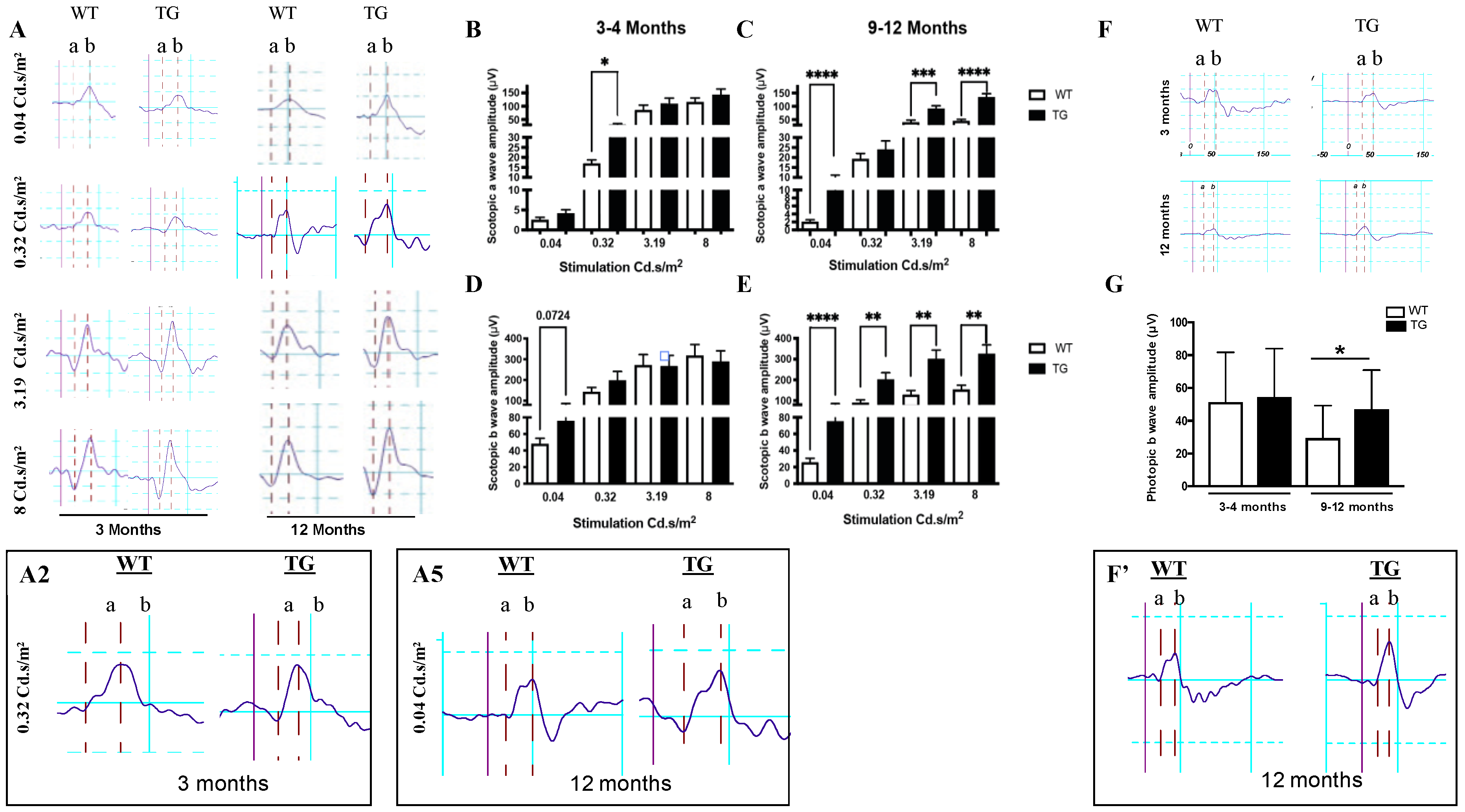
3.2. Retina of Pre-Symptomatic APP/PS1 Mice Displays Early Neuronal Hyperexcitability
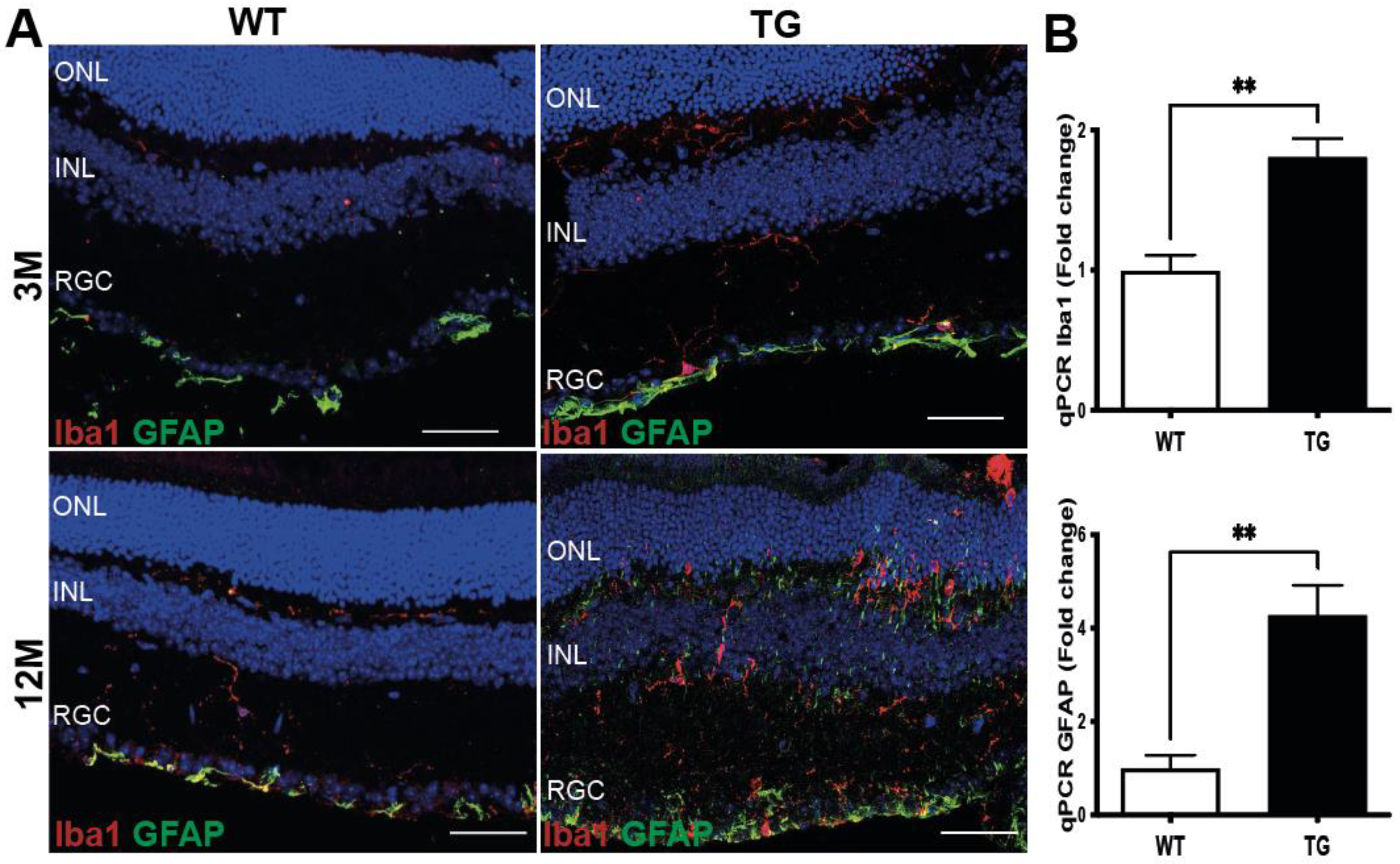
3.3. Early Signs of Gliosis in the Retina of Pre-Symptomatic APP/PS1 Mice
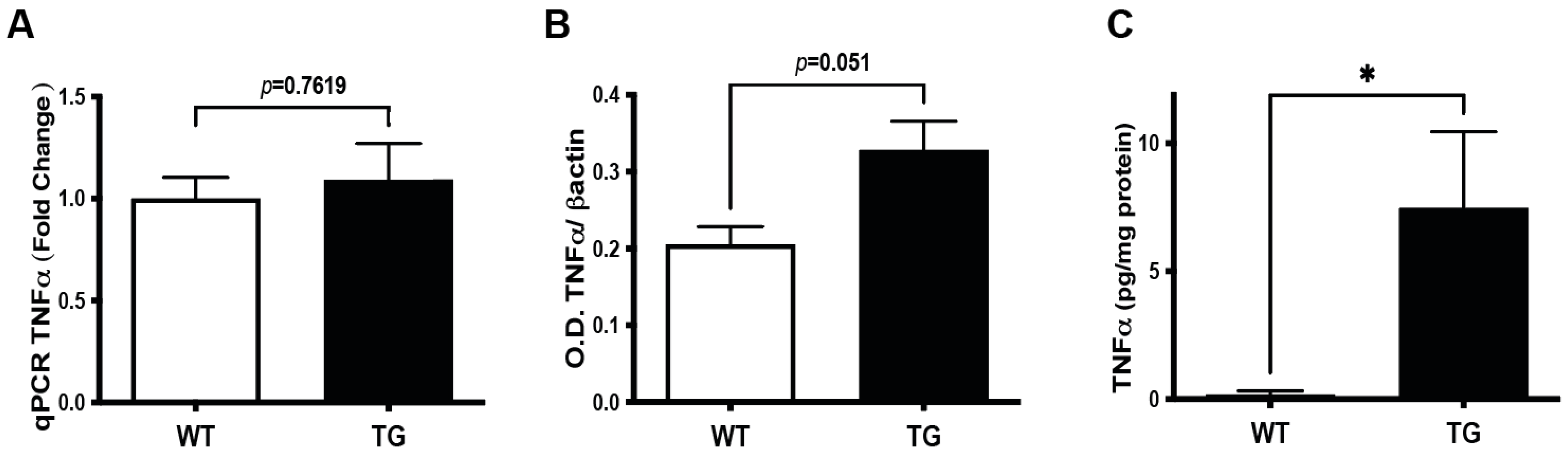
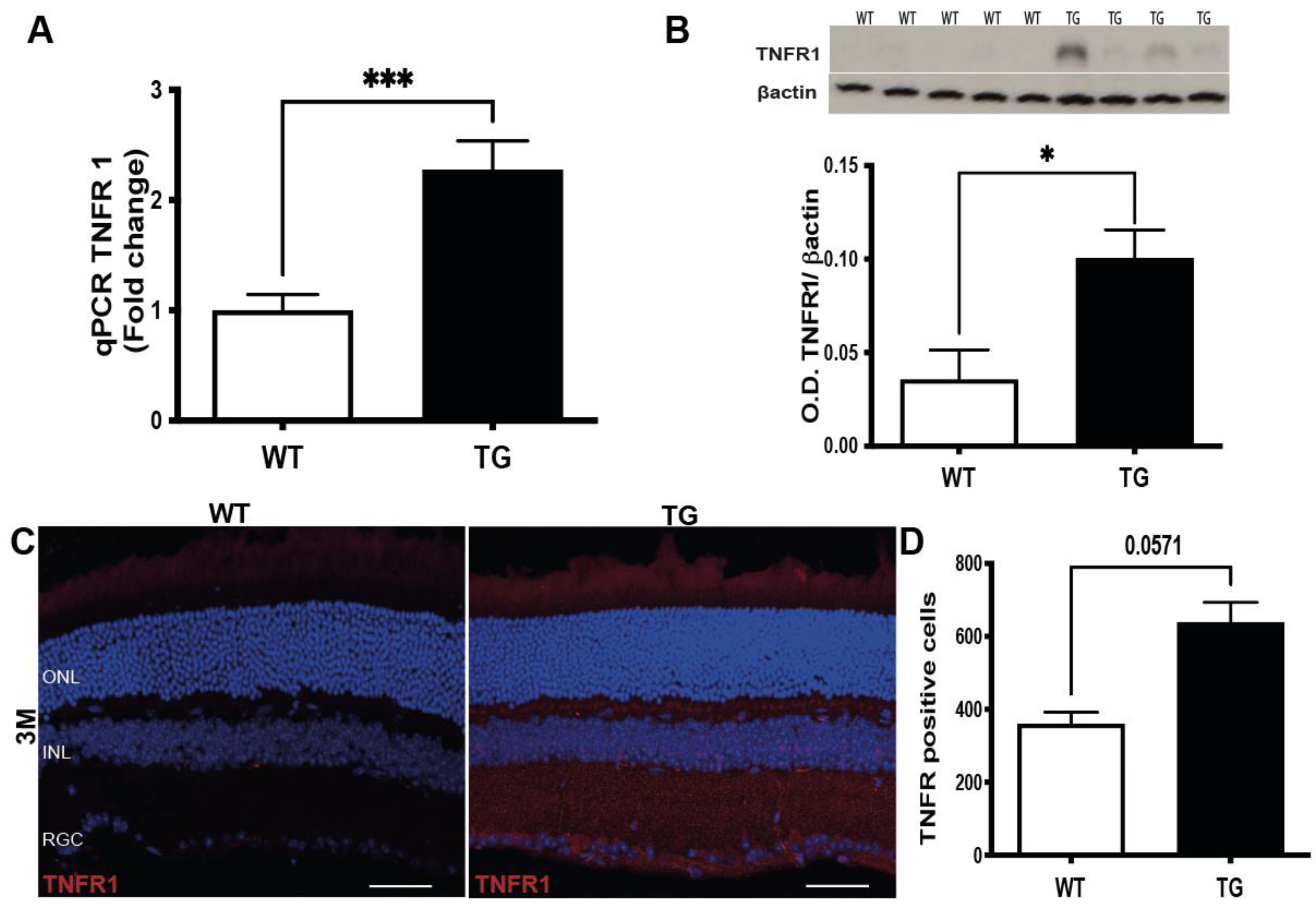
3.4. Induction of TNFα Signaling Pathway in the Retina of Pre-Symptomatic APP/PS1 Mice
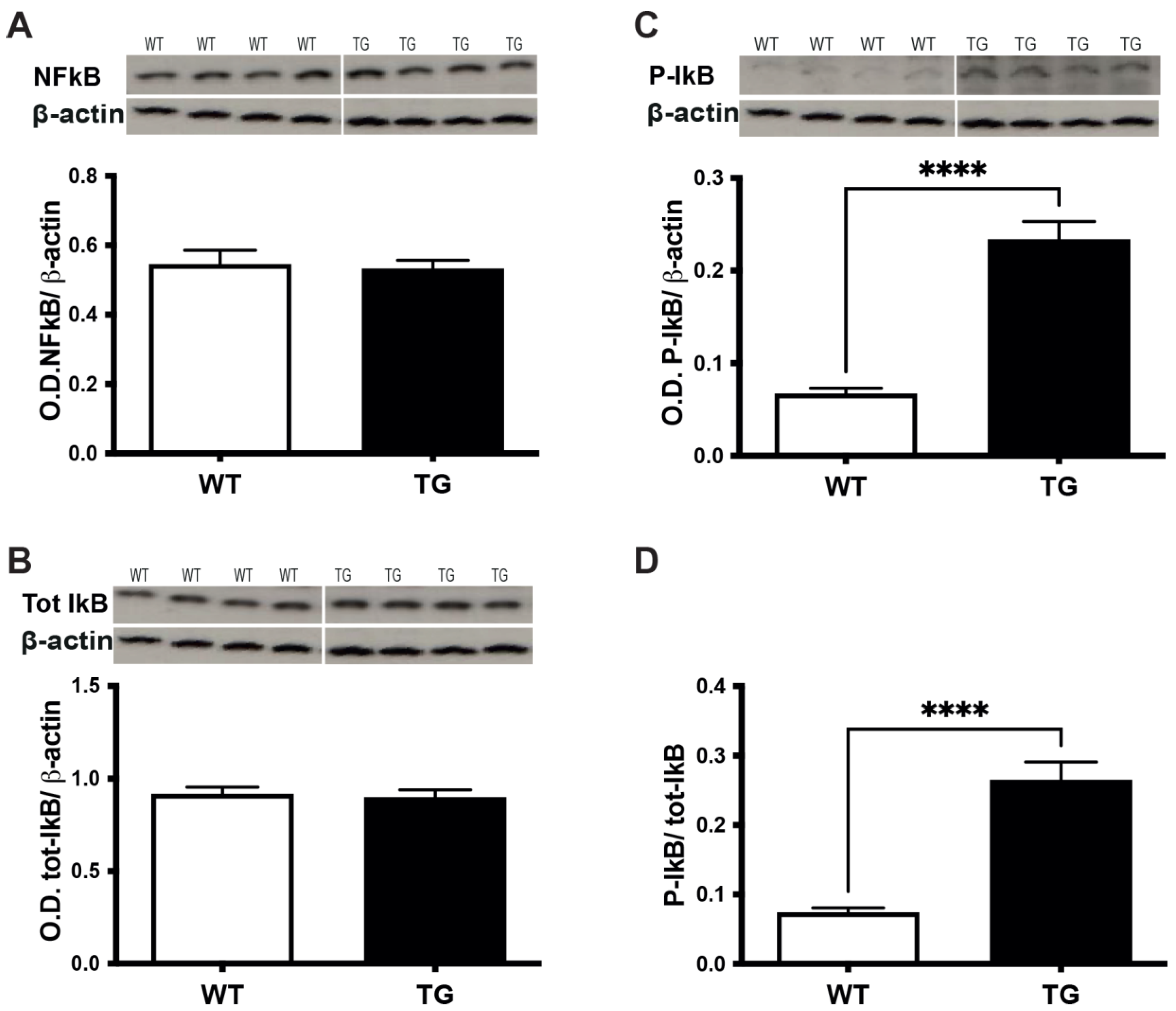
3.5. TNFα/TNFR1 Induction Correlates with the Phosphorylation of IkBα in the Retina of Pre-Symptomatic APP/PS1 Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An Unbiased Descriptive Classification Scheme for Alzheimer Disease Biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R.; Kaye, J.; Montine, T.J.; et al. Toward Defining the Preclinical Stages of Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zott, B.; Busche, M.A.; Sperling, R.A.; Konnerth, A. What Happens with the Circuit in Alzheimer’s Disease in Mice and Humans? Annu. Rev. Neurosci. 2018, 41, 277–297. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, C.; Colby-Milley, J.; Bouvier, D.; Farso, M.; Chabot, J.-G.; Quirion, R.; Krantic, S. ΒCTF-Correlated Burst of Hippocampal TNFα Occurs at a Very Early, Pre-Plaque Stage in the TgCRND8 Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2013, 36, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, C.; Tse, Y.C.; Nguyen, H.-B.; Krantic, S.; Breitner, J.C.S.; Quirion, R.; Wong, T.P. Inhibiting Tumor Necrosis Factor-α before Amyloidosis Prevents Synaptic Deficits in an Alzheimer’s Disease Model. Neurobiol. Aging 2016, 47, 41–49. [Google Scholar] [CrossRef]
- Doméné, A.; Cavanagh, C.; Page, G.; Bodard, S.; Klein, C.; Delarasse, C.; Chalon, S.; Krantic, S. Expression of Phenotypic Astrocyte Marker Is Increased in a Transgenic Mouse Model of Alzheimer’s Disease versus Age-Matched Controls: A Presymptomatic Stage Study. Int. J. Alzheimers Dis. 2016, 2016, 5696241. [Google Scholar] [CrossRef] [Green Version]
- Goutagny, R.; Gu, N.; Cavanagh, C.; Jackson, J.; Chabot, J.-G.; Quirion, R.; Krantic, S.; Williams, S. Alterations in Hippocampal Network Oscillations and Theta-Gamma Coupling Arise before Aβ Overproduction in a Mouse Model of Alzheimer’s Disease. Eur. J. Neurosci. 2013, 37, 1896–1902. [Google Scholar] [CrossRef]
- Mahar, I.; Albuquerque, M.S.; Mondragon-Rodriguez, S.; Cavanagh, C.; Davoli, M.A.; Chabot, J.-G.; Williams, S.; Mechawar, N.; Quirion, R.; Krantic, S. Phenotypic Alterations in Hippocampal NPY- and PV-Expressing Interneurons in a Presymptomatic Transgenic Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2016, 8, 327. [Google Scholar] [CrossRef] [Green Version]
- Stellwagen, D.; Malenka, R.C. Synaptic Scaling Mediated by Glial TNF-Alpha. Nature 2006, 440, 1054–1059. [Google Scholar] [CrossRef]
- Krantic, S. Editorial: From Current Diagnostic Tools and Therapeutics for Alzheimer’s Disease Towards Earlier Diagnostic Markers and Treatment Targets. Curr. Alzheimer Res. 2017, 14, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.T.; Behar-Cohen, F.; Krantic, S. Seeing Early Signs of Alzheimer’s Disease through the Lens of the Eye. Curr. Alzheimer Res. 2017, 14, 6–17. [Google Scholar] [CrossRef] [PubMed]
- den Haan, J.; Morrema, T.H.J.; Verbraak, F.D.; de Boer, J.F.; Scheltens, P.; Rozemuller, A.J.; Bergen, A.A.B.; Bouwman, F.H.; Hoozemans, J.J. Amyloid-Beta and Phosphorylated Tau in Post-Mortem Alzheimer’s Disease Retinas. Acta Neuropathol. Commun. 2018, 6, 147. [Google Scholar] [CrossRef]
- Koronyo, Y.; Biggs, D.; Barron, E.; Boyer, D.S.; Pearlman, J.A.; Au, W.J.; Kile, S.J.; Blanco, A.; Fuchs, D.-T.; Ashfaq, A.; et al. Retinal Amyloid Pathology and Proof-of-Concept Imaging Trial in Alzheimer’s Disease. JCI Insight 2017, 2, 93621. [Google Scholar] [CrossRef] [PubMed]
- Koronyo-Hamaoui, M.; Koronyo, Y.; Ljubimov, A.V.; Miller, C.A.; Ko, M.K.; Black, K.L.; Schwartz, M.; Farkas, D.L. Identification of Amyloid Plaques in Retinas from Alzheimer’s Patients and Noninvasive In Vivo Optical Imaging of Retinal Plaques in a Mouse Model. Neuroimage 2011, 54 Suppl 1, S204–S217. [Google Scholar] [CrossRef] [Green Version]
- Hampel, H.; Toschi, N.; Babiloni, C.; Baldacci, F.; Black, K.L.; Bokde, A.L.W.; Bun, R.S.; Cacciola, F.; Cavedo, E.; Chiesa, P.A.; et al. Revolution of Alzheimer Precision Neurology. Passageway of Systems Biology and Neurophysiology. J. Alzheimers Dis. 2018, 64, S47–S105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowsky, J.L.; Slunt, H.H.; Gonzales, V.; Jenkins, N.A.; Copeland, N.G.; Borchelt, D.R. APP Processing and Amyloid Deposition in Mice Haplo-Insufficient for Presenilin 1. Neurobiol. Aging 2004, 25, 885–892. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, M.; Xi, Y.; Hao, J.; Zhang, Z.; Su, C.; Lei, G.; Miao, J.; Li, Z. Multiple Inflammatory Pathways Are Involved in the Development and Progression of Cognitive Deficits in APPswe/PS1dE9 Mice. Neurobiol. Aging 2012, 33, 2661–2677. [Google Scholar] [CrossRef]
- Minkeviciene, R.; Rheims, S.; Dobszay, M.B.; Zilberter, M.; Hartikainen, J.; Fülöp, L.; Penke, B.; Zilberter, Y.; Harkany, T.; Pitkänen, A.; et al. Amyloid Beta-Induced Neuronal Hyperexcitability Triggers Progressive Epilepsy. J. Neurosci. 2009, 29, 3453–3462. [Google Scholar] [CrossRef]
- Ruan, L.; Kang, Z.; Pei, G.; Le, Y. Amyloid Deposition and Inflammation in APPswe/PS1dE9 Mouse Model of Alzheimer’s Disease. Curr. Alzheimer Res. 2009, 6, 531–540. [Google Scholar] [CrossRef]
- Probert, L. TNF and Its Receptors in the CNS: The Essential, the Desirable and the Deleterious Effects. Neuroscience 2015, 302, 2–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prescott, J.A.; Mitchell, J.P.; Cook, S.J. Inhibitory Feedback Control of NF-ΚB Signalling in Health and Disease. Biochem. J. 2021, 478, 2619–2664. [Google Scholar] [CrossRef] [PubMed]
- Georgevsky, D.; Retsas, S.; Raoufi, N.; Shimoni, O.; Golzan, S.M. A Longitudinal Assessment of Retinal Function and Structure in the APP/PS1 Transgenic Mouse Model of Alzheimer’s Disease. Transl. Neurodegener. 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, C.; Cerri, E.; Fabiani, C.; Capsoni, S.; Cattaneo, A.; Domenici, L. The Retina as a Window to Early Dysfunctions of Alzheimer’s Disease Following Studies with a 5xFAD Mouse Model. Neurobiol. Aging 2018, 67, 181–188. [Google Scholar] [CrossRef]
- McAnany, J.J.; Matei, N.; Chen, Y.-F.; Liu, K.; Park, J.C.; Shahidi, M. Rod Pathway and Cone Pathway Retinal Dysfunction in the 5xFAD Mouse Model of Alzheimer’s Disease. Sci. Rep. 2021, 11, 4824. [Google Scholar] [CrossRef]
- Gupta, V.K.; Chitranshi, N.; Gupta, V.B.; Golzan, M.; Dheer, Y.; Wall, R.V.; Georgevsky, D.; King, A.E.; Vickers, J.C.; Chung, R.; et al. Amyloid β Accumulation and Inner Retinal Degenerative Changes in Alzheimer’s Disease Transgenic Mouse. Neurosci. Lett. 2016, 623, 52–56. [Google Scholar] [CrossRef]
- Perez, S.E.; Lumayag, S.; Kovacs, B.; Mufson, E.J.; Xu, S. β-Amyloid Deposition and Functional Impairment in the Retina of the APPswe/PS1ΔE9 Transgenic Mouse Model of Alzheimer’s Disease. Investig. Ophthalmol. Vis. Sci. 2009, 50, 793–800. [Google Scholar] [CrossRef]
- Lim, J.K.H.; Li, Q.-X.; He, Z.; Vingrys, A.J.; Chinnery, H.R.; Mullen, J.; Bui, B.V.; Nguyen, C.T.O. Retinal Functional and Structural Changes in the 5xFAD Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 862. [Google Scholar] [CrossRef]
- Joly, S.; Lamoureux, S.; Pernet, V. Nonamyloidogenic Processing of Amyloid Beta Precursor Protein Is Associated with Retinal Function Improvement in Aging Male APPswe/PS1ΔE9 Mice. Neurobiol. Aging 2017, 53, 181–191. [Google Scholar] [CrossRef]
- Liu, B.; Rasool, S.; Yang, Z.; Glabe, C.G.; Schreiber, S.S.; Ge, J.; Tan, Z. Amyloid-Peptide Vaccinations Reduce {beta}-Amyloid Plaques but Exacerbate Vascular Deposition and Inflammation in the Retina of Alzheimer’s Transgenic Mice. Am. J. Pathol. 2009, 175, 2099–2110. [Google Scholar] [CrossRef] [Green Version]
- Vandenabeele, M.; Veys, L.; Lemmens, S.; Hadoux, X.; Gelders, G.; Masin, L.; Serneels, L.; Theunis, J.; Saito, T.; Saido, T.C.; et al. The AppNL-G-F Mouse Retina Is a Site for Preclinical Alzheimer’s Disease Diagnosis and Research. Acta Neuropathol. Commun. 2021, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Merlin, S.; Lim, J.K.H.; Wong, V.H.Y.; Nguyen, C.T.O.; Morley, J.W.; Bui, B.V.; Tayebi, M. Age-Specific Retinal and Cerebral Immunodetection of Amyloid-β Plaques and Oligomers in a Rodent Model of Alzheimer’s Disease. J. Alzheimers Dis. 2020, 76, 1135–1150. [Google Scholar] [CrossRef] [PubMed]
- Ning, A.; Cui, J.; To, E.; Ashe, K.H.; Matsubara, J. Amyloid-Beta Deposits Lead to Retinal Degeneration in a Mouse Model of Alzheimer Disease. Invest. Ophthalmol. Vis. Sci. 2008, 49, 5136–5143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, M.M.; Rodríguez, J.J.; Gutierrez-Lanza, R.; Yates, J.; Verkhratsky, A.; Lutty, G.A. Retinal Macroglia Changes in a Triple Transgenic Mouse Model of Alzheimer’s Disease. Exp. Eye Res. 2014, 127, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Grimaldi, A.; Brighi, C.; Peruzzi, G.; Ragozzino, D.; Bonanni, V.; Limatola, C.; Ruocco, G.; Di Angelantonio, S. Inflammation, Neurodegeneration and Protein Aggregation in the Retina as Ocular Biomarkers for Alzheimer’s Disease in the 3xTg-AD Mouse Model. Cell Death Dis 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lei, C.; Lin, R.; Wang, J.; Tao, L.; Fu, X.; Qiu, Y.; Lei, B. Amelioration of Amyloid β-Induced Retinal Inflammatory Responses by a LXR Agonist TO901317 Is Associated with Inhibition of the NF-ΚB Signaling and NLRP3 Inflammasome. Neuroscience 2017, 360, 48–60. [Google Scholar] [CrossRef]
- Xu, W.; Fitzgerald, S.; Nixon, R.A.; Levy, E.; Wilson, D.A. Early Hyperactivity in Lateral Entorhinal Cortex Is Associated with Elevated Levels of AβPP Metabolites in the Tg2576 Mouse Model of Alzheimer’s Disease. Exp. Neurol. 2015, 264, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Bourgeois, A.; Lauritzen, I.; Lorivel, T.; Bauer, C.; Checler, F.; Pardossi-Piquard, R. Intraneuronal Accumulation of C99 Contributes to Synaptic Alterations, Apathy-like Behavior, and Spatial Learning Deficits in 3×TgAD and 2×TgAD Mice. Neurobiol. Aging 2018, 71, 21–31. [Google Scholar] [CrossRef]
- Pera, M.; Alcolea, D.; Sánchez-Valle, R.; Guardia-Laguarta, C.; Colom-Cadena, M.; Badiola, N.; Suárez-Calvet, M.; Lladó, A.; Barrera-Ocampo, A.A.; Sepulveda-Falla, D.; et al. Distinct Patterns of APP Processing in the CNS in Autosomal-Dominant and Sporadic Alzheimer Disease. Acta Neuropathol. 2013, 125, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Lauritzen, I.; Pardossi-Piquard, R.; Bauer, C.; Brigham, E.; Abraham, J.-D.; Ranaldi, S.; Fraser, P.; St-George-Hyslop, P.; Le Thuc, O.; Espin, V.; et al. The β-Secretase-Derived C-Terminal Fragment of ΒAPP, C99, but Not Aβ, Is a Key Contributor to Early Intraneuronal Lesions in Triple-Transgenic Mouse Hippocampus. J. Neurosci. 2012, 32, 16243–16255. [Google Scholar] [CrossRef]
- More, S.S.; Vince, R. Hyperspectral Imaging Signatures Detect Amyloidopathy in Alzheimer’s Mouse Retina Well before Onset of Cognitive Decline. ACS Chem. Neurosci. 2015, 6, 306–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Zhong, L.; Han, X.; Xiong, G.; Xu, D.; Zhang, S.; Cheng, H.; Chiu, K.; Xu, Y. Brain and Retinal Abnormalities in the 5xFAD Mouse Model of Alzheimer’s Disease at Early Stages. Front. Neurosci. 2021, 15, 681831. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Yang, M.; Zhu, L.; Zhou, Q.; Li, X.; Chen, Z.; Zou, C. Retinal Levels of Amyloid Beta Correlate with Cerebral Levels of Amyloid Beta in Young APPswe/PS1dE9 Transgenic Mice before Onset of Alzheimer’s Disease. Behav. Neurol. 2020, 2020, 1574816. [Google Scholar] [CrossRef] [PubMed]
- Asanad, S.; Felix, C.M.; Fantini, M.; Harrington, M.G.; Sadun, A.A.; Karanjia, R. Retinal Ganglion Cell Dysfunction in Preclinical Alzheimer’s Disease: An Electrophysiologic Biomarker Signature. Sci. Rep. 2021, 11, 6344. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-B.; Lin, Z.-J.; Li, N.; Yu, H.; Wu, Y.-L.; Shen, X. Evaluation of Retinal and Choroidal Changes in Patients with Alzheimer’s Type Dementia Using Optical Coherence Tomography Angiography. Int. J. Ophthalmol. 2021, 14, 860–868. [Google Scholar] [CrossRef] [PubMed]

| Antibodies | Ref. | Company | IHC Dilution | Western Blot Dilution |
|---|---|---|---|---|
| TNFα | AB2148P | Merck Millipore (Fontenay sous Bois, France) | - | 1:1000 |
| TNFR1 | ab19139 | Abcam (Paris, France) | 1:500 | 1:5000 |
| TNFR1 | Clone H-5 sc-8436 | Santa Cruz Biotechnology, CliniScience, (Nanterre, France) | - | 1:2000 |
| AβPP C-Ter fragment CT20 | 171610 | Merck Millipore (Fontenay sous Bois, France) | - | 1:5000 |
| Aβ 1-16 monoclonal 6E10 | SIG-39320 | BioLegend (London, UK) | - | 1:2000 |
| NFkB p65 (phospho S529) | ab 195838 | Abcam (Paris, France) | - | 1:1000 |
| IkBα (phospho S32 + S36) | ab 12135 | Abcam (Paris, France) | - | 1:1000 |
| total IkB | ab 32518 | Abcam (Paris, France) | - | 1:1000 |
| Iba1 | ab5076 | Abcam (Paris, France) | 1:500 | - |
| GFAP | LS-B4775-50 | LSBio (Paris, France) | 1:500 | - |
| ß Actin | MBS8533374 | CliniScience, (Nanterre, France) | - | 1:2000 |
| Gene | Sense 5′-3′ Primer | Anti-Sense 5′-3′ Primer |
|---|---|---|
| HPRT | TCT AAC TTT AAC TGG AAA GAA TGT C | TCC TTT TCA CCA GCA AGC T |
| TNFα | TCT CAA AAT TCG AGT GAC AAG C | ACT CCA GCT GCT CCT CCA C |
| TNFR1 | GAG AAA GTG AGT GCG TCC CT | TGA CAT TTG CAA GCG GAG GA |
| Iba1 | CCT GAT TGG AGG TGG ATG TCA C | GGC TCA CGA CTG TTT CTT TTT TCC |
| GFAP | CAG CTG GGC TGT ACA AAC CTT | CAT TGG AAG TGA AGC GTT TCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinet, V.; Arouche-Delaperche, L.; Dégardin, J.; Naud, M.-C.; Picaud, S.; Krantic, S. Concomitant Retinal Alterations in Neuronal Activity and TNFα Pathway Are Detectable during the Pre-Symptomatic Stage in a Mouse Model of Alzheimer’s Disease. Cells 2022, 11, 1650. https://doi.org/10.3390/cells11101650
Dinet V, Arouche-Delaperche L, Dégardin J, Naud M-C, Picaud S, Krantic S. Concomitant Retinal Alterations in Neuronal Activity and TNFα Pathway Are Detectable during the Pre-Symptomatic Stage in a Mouse Model of Alzheimer’s Disease. Cells. 2022; 11(10):1650. https://doi.org/10.3390/cells11101650
Chicago/Turabian StyleDinet, Virginie, Louiza Arouche-Delaperche, Julie Dégardin, Marie-Christine Naud, Serge Picaud, and Slavica Krantic. 2022. "Concomitant Retinal Alterations in Neuronal Activity and TNFα Pathway Are Detectable during the Pre-Symptomatic Stage in a Mouse Model of Alzheimer’s Disease" Cells 11, no. 10: 1650. https://doi.org/10.3390/cells11101650
APA StyleDinet, V., Arouche-Delaperche, L., Dégardin, J., Naud, M.-C., Picaud, S., & Krantic, S. (2022). Concomitant Retinal Alterations in Neuronal Activity and TNFα Pathway Are Detectable during the Pre-Symptomatic Stage in a Mouse Model of Alzheimer’s Disease. Cells, 11(10), 1650. https://doi.org/10.3390/cells11101650







