Dopamine D4 Receptor Is a Regulator of Morphine-Induced Plasticity in the Rat Dorsal Striatum
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug Administration
2.3. Electrophysiology
2.3.1. Slice Preparation
2.3.2. Whole-Cell Current-Clamp Recordings
2.4. Cell Reconstruction and Morphometric Analysis
2.5. Immunohistochemistry
2.5.1. Single Immunohistochemical Labeling
2.5.2. Double Immunohistochemical Labeling
2.5.3. Microscopic Analysis and Semi-Quantification
2.6. Western Blot
2.7. Statistical Analysis
3. Results
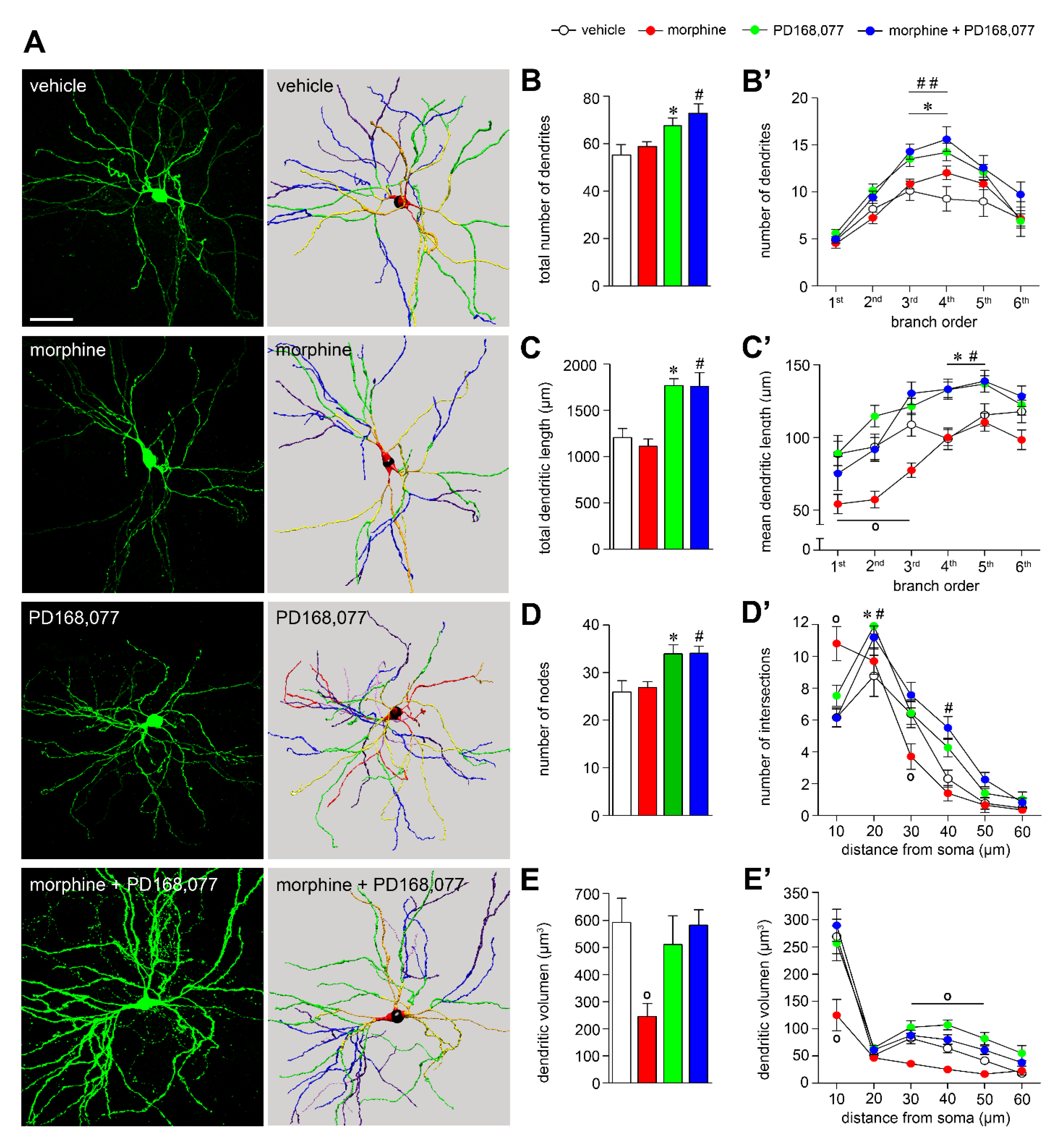
3.1. D4R Activation Results in MSNs Dendritic Arbors Stretching and Prevention of Morphine-Induced MSNs Dendritic Contraction
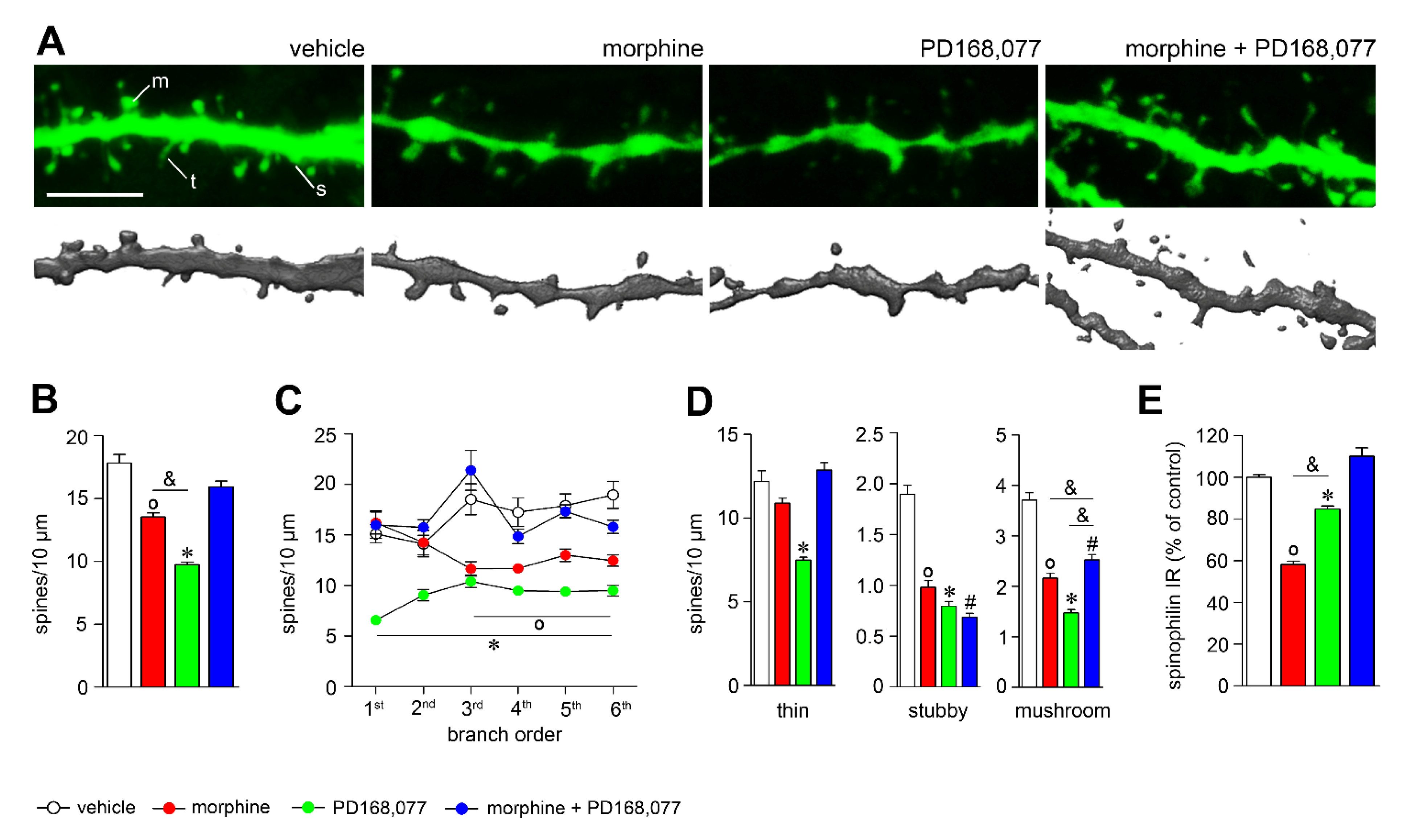
3.2. D4R Activation Counteracts Dendritic Spine Depletion Induced by Morphine
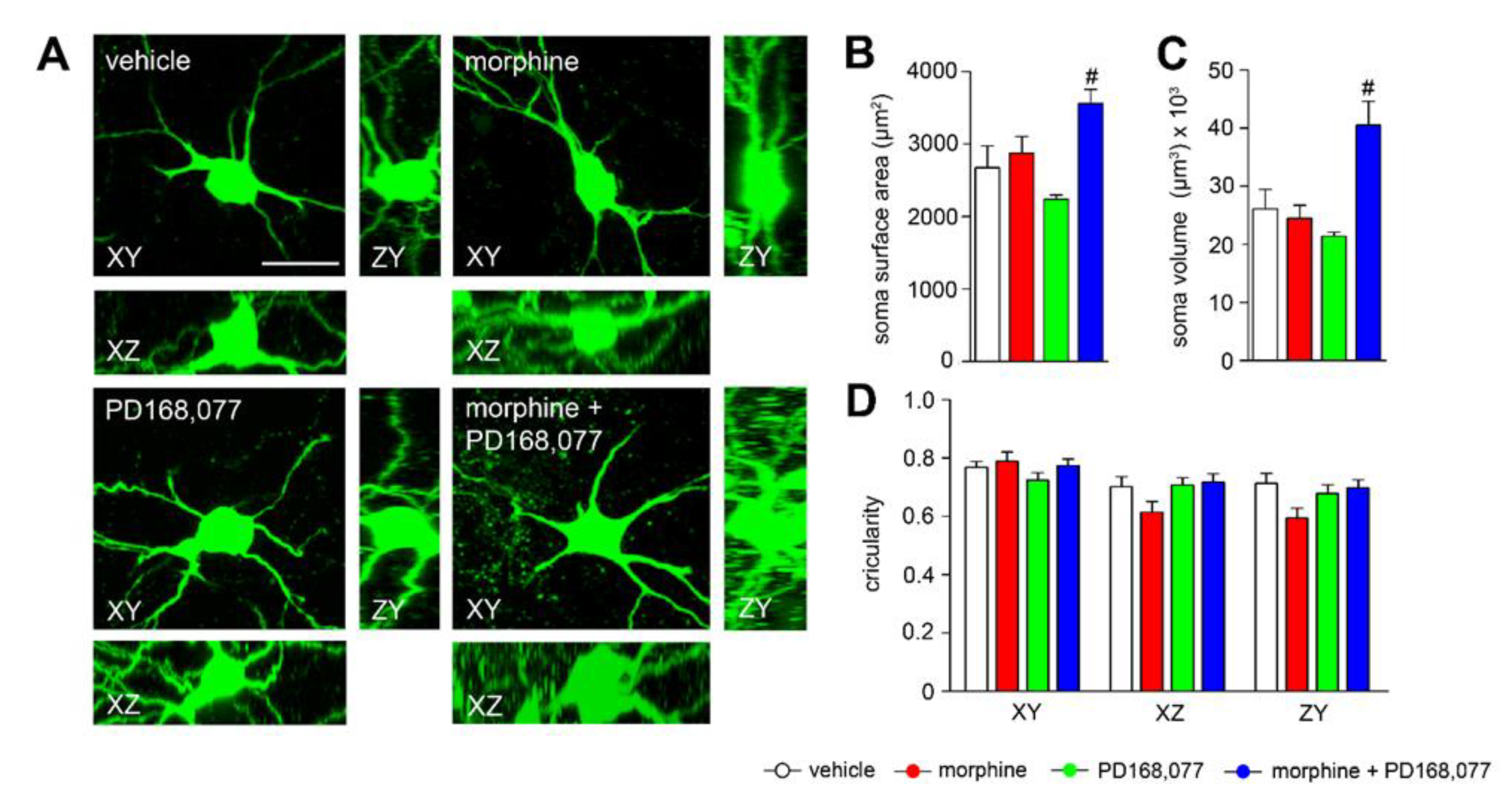
3.3. Changes in Striatal MSN Cell Bodies after Continuous Administration of Morphine and/or PD168,077
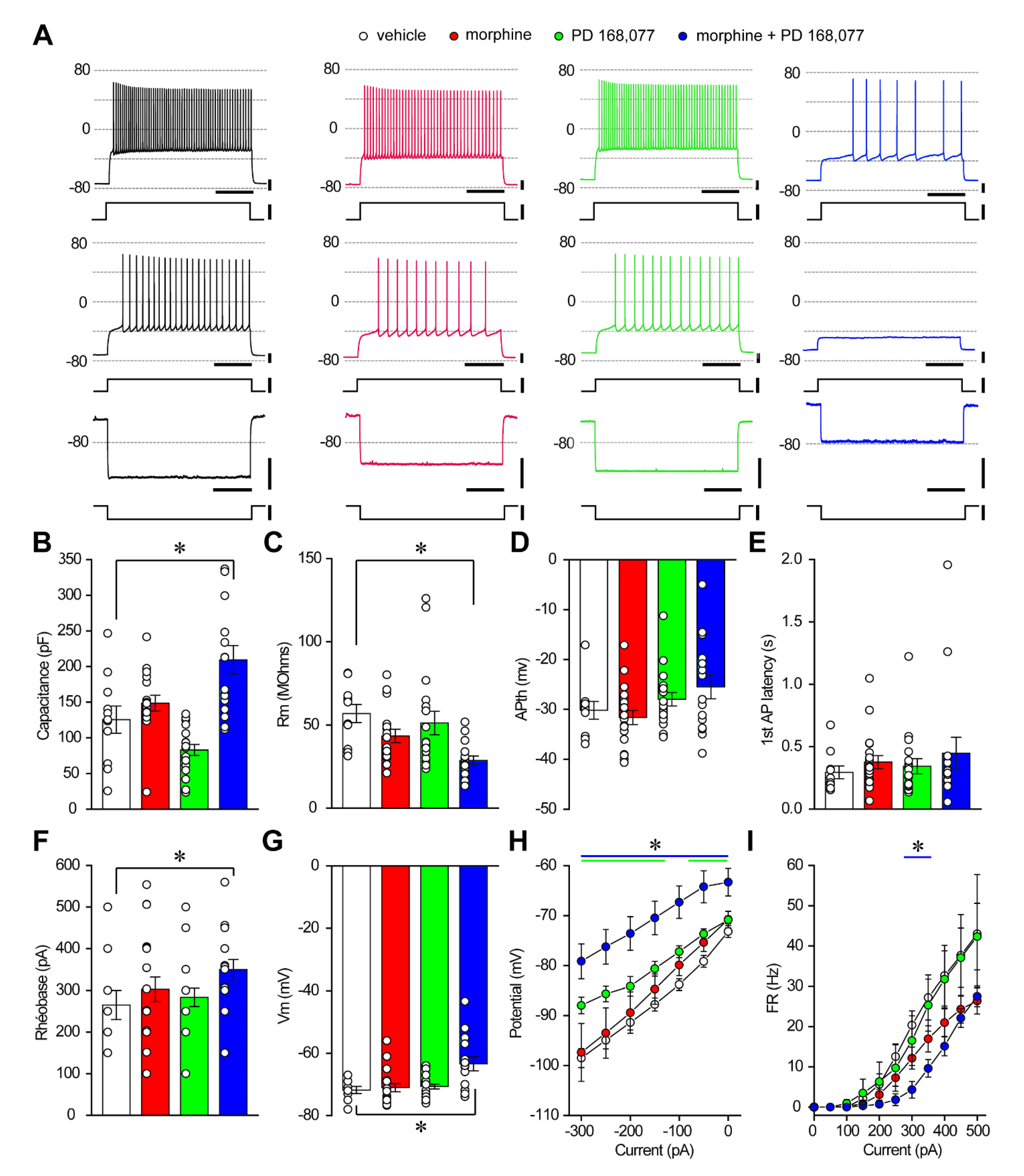
3.4. D4R Activation during Continuous Treatment with Morphine Changes the Passive Properties and Excitability of Striatal MSNs
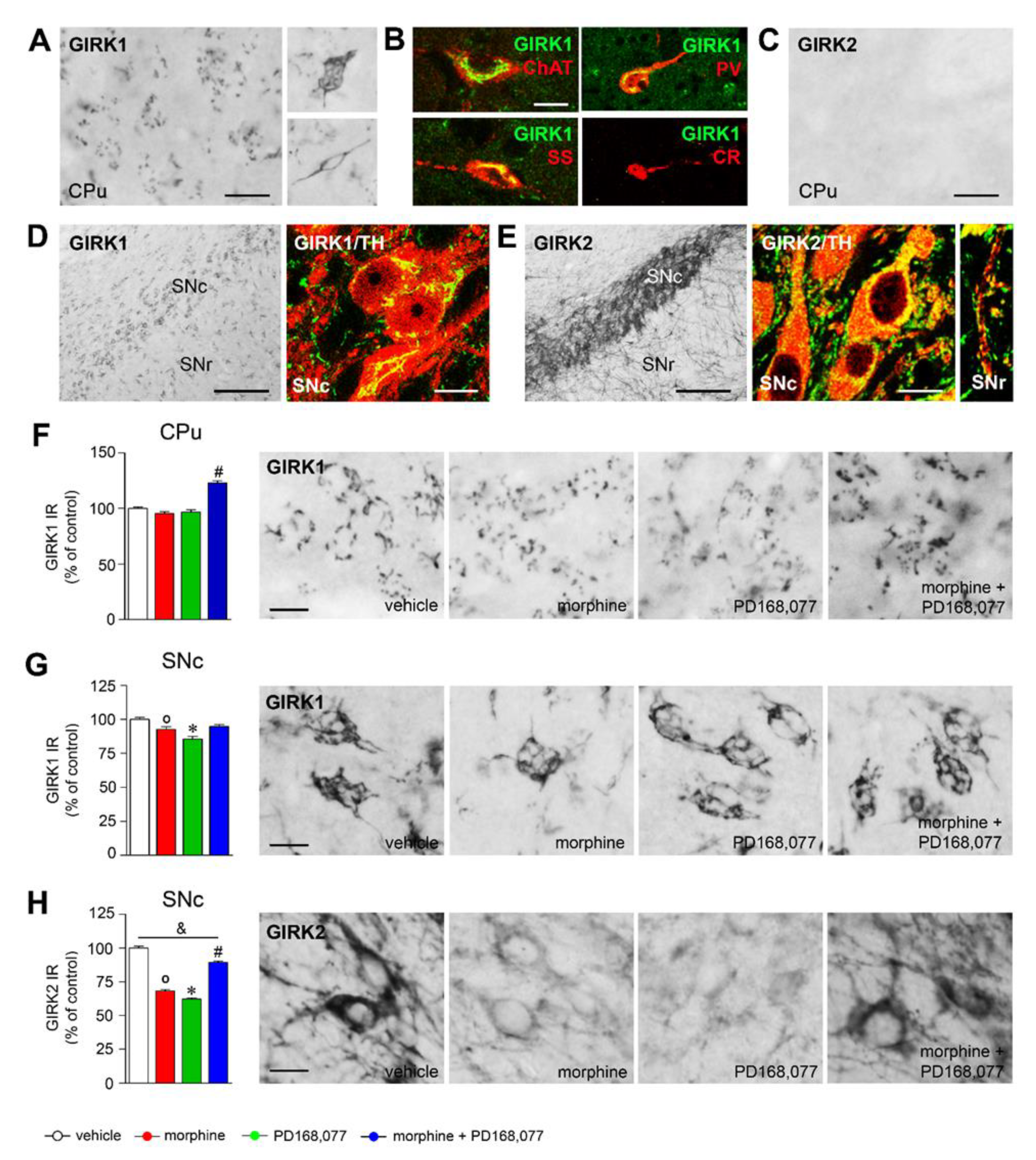
3.5. Regulation of GIRK1 and GIRK2 Expression in the Striatal MSNs and Nigral Dopamine Neurons by Morphine and/or PD168,077 Treatments
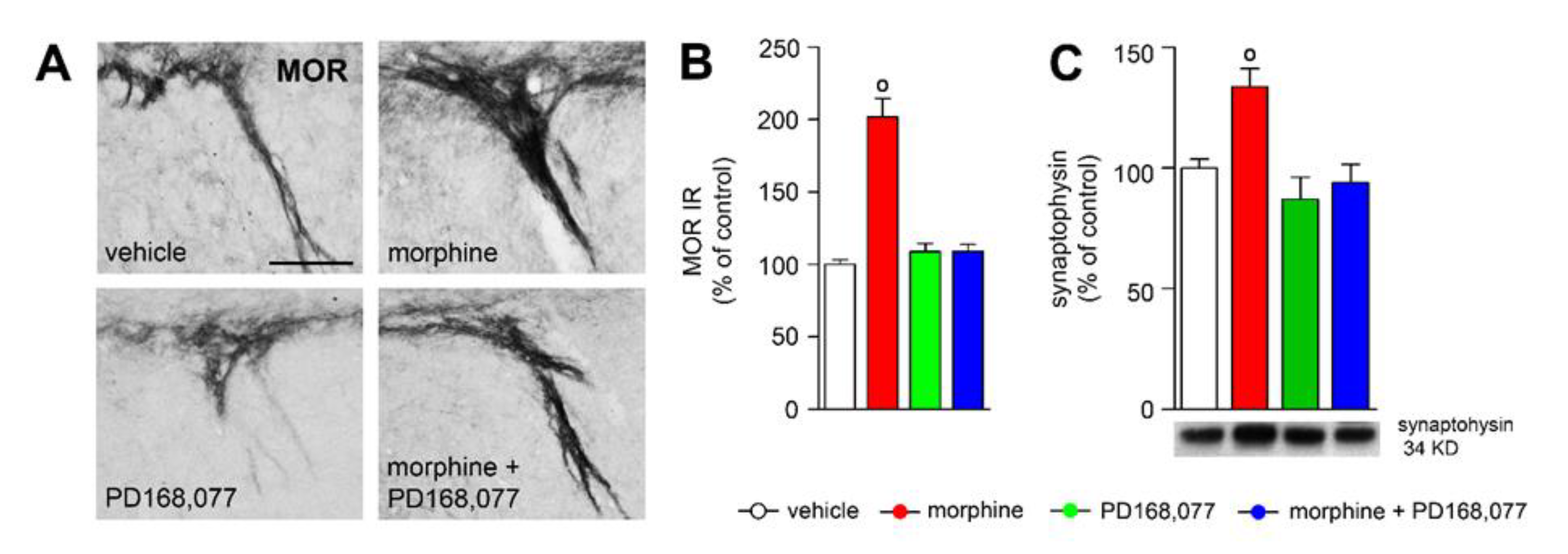
3.6. D4R Activation Counteracts Morphine-Induced Upregulation of MOR IR in the Striosome-Dendron Bouquets
4. Discussion
4.1. Morphine-Induced Structural Plasticity in Striatal MSNs
4.2. D4R Activation Alter Dendritic Arbor Complexity and Spine Density of the Striatal MSNs
4.3. D4R Activation Modulates Morphine-Induced Plasticity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Kolb, B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 2004, 47, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W.; O’Brien, C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 2008, 33, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Salery, M.; Godino, A.; Nestler, E.J. Drug-activated cells: From immediate early genes to neuronal ensembles in addiction. In Advances in Pharmacology; Academic Press Inc.: Cambridge, MA, USA, 2021; Volume 90, pp. 173–216. [Google Scholar] [CrossRef]
- Russo, S.J.; Dietz, D.M.; Dumitriu, D.; Morrison, J.H.; Malenka, R.C.; Nestler, E.J. The addicted synapse: Mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010, 33, 267–276. [Google Scholar] [CrossRef]
- Lüscher, C.; Malenka, R.C. Drug-Evoked Synaptic Plasticity in Addiction: From Molecular Changes to Circuit Remodeling. Neuron 2011, 69, 650–663. [Google Scholar] [CrossRef]
- Graybiel, A.M.; Grafton, S.T. The striatum: Where skills and habits meet. Cold Spring Harb. Perspect. Biol. 2015, 7, a021691. [Google Scholar] [CrossRef]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef]
- Everitt, B.J.; Robbins, T.W. Drug addiction: Updating actions to habits to compulsions ten years on. Annu. Rev. Psychol. 2016, 67, 23–50. [Google Scholar] [CrossRef]
- Lipton, D.M.; Gonzales, B.J.; Citri, A. Dorsal striatal circuits for habits, compulsions and addictions. Front. Syst. Neurosci. 2019, 13, 28. [Google Scholar] [CrossRef]
- Dacher, M.; Nugent, F.S. Opiates and plasticity. Neuropharmacology 2011, 6, 1088–1096. [Google Scholar] [CrossRef]
- Robinson, T.E.; Kolb, B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse 1999, 33, 160–162. [Google Scholar] [CrossRef]
- Pal, A.; Das, S. Chronic morphine exposure and its abstinence alters dendritic spine morphology and upregulates Shank1. Neurochem. Int. 2013, 62, 956–964. [Google Scholar] [CrossRef]
- Geoffroy, H.; Canestrelli, C.; Marie, N.; Noble, F. Morphine-induced dendritic spine remodeling in rat nucleus accumbens is corticosterone dependent. Int. J. Neuropsychopharmacol. 2019, 22, 394–401. [Google Scholar] [CrossRef]
- Thompson, B.L.; Oscar-Berman, M.; Kaplan, G.B. Opioid-induced structural and functional plasticity of medium-spiny neurons in the nucleus accumbens. Neurosci. Biobehav. Rev. 2021, 120, 417–430. [Google Scholar] [CrossRef]
- Rivera, A.; Gago, B.; Suárez-Boomgaard, D.; Yoshitake, T.; Roales-Buján, R.; Valderrama-Carvajal, A.; Bilbao, A.; Medina-Luque, J.; Díaz-Cabiale, Z.; van Craenenbroeck, K.; et al. Dopamine D4 receptor stimulation prevents nigrostriatal dopamine pathway activation by morphine: Relevance for drug addiction. Addict. Biol. 2017, 22, 1232–1245. [Google Scholar] [CrossRef]
- Mazei-Robison, M.S.; Koo, J.W.; Friedman, A.K.; Lansink, C.S.; Robison, A.J.; Vinish, M.; Krishnan, V.; Kim, S.; Siuta, M.A.; Galli, A.; et al. Role for mTOR signaling and neuronal activity in morphine-induced adaptations in ventral tegmental area dopamine neurons. Neuron 2011, 72, 977–990. [Google Scholar] [CrossRef]
- Sklair-Tavron, L.; Shi, W.X.; Lane, S.B.; Harris, H.W.; Bunney, B.S.; Nestler, E.J. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc. Natl. Acad. Sci. USA 1996, 93, 11202–11207. [Google Scholar] [CrossRef]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar] [CrossRef]
- Pereira, F.C.; Lourenço, E.; Milhazes, N.; Morgadinho, T.; Ribeiro, C.F.; Ali, S.F.; Macedo, T.R. Methamphetamine, morphine, and their combination: Acute changes in striatal dopaminergic transmission evaluated by microdialysis in awake rats. Ann. N. Y. Acad. Sci. 2006, 1074, 160–173. [Google Scholar] [CrossRef]
- Nestler, E.J.; Barrot, M.; Self, D.W. ΔFosB: A sustained molecular switch for addiction. Proc. Natl. Acad. Sci. USA 2001, 98, 11042–11046. [Google Scholar] [CrossRef]
- Volkow, N.D.; McLellan, A.T. Opioid Abuse in Chronic Pain—Misconceptions and Mitigation Strategies. N. Engl. J. Med. 2016, 374, 1253–1263. [Google Scholar] [CrossRef]
- Botticelli, L.; di Bonaventura, E.M.; del Bello, F.; Giorgioni, G.; Piergentili, A.; Romano, A.; Quaglia, W.; Cifani, C.; di Bonaventura, M.V.M. Underlying susceptibility to eating disorders and drug abuse: Genetic and pharmacological aspects of dopamine D4 receptors. Nutrients 2020, 12, 2288. [Google Scholar] [CrossRef]
- Rubinstein, M.; Phillips, T.J.; Bunzow, J.R.; Falzone, T.L.; Dziewczapolski, G.; Zhang, G.; Fang, Y.; Larson, J.L.; McDougall, J.A.; Chester, J.A.; et al. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell 1997, 90, 991–1001. [Google Scholar] [CrossRef][Green Version]
- Gago, B.; Suárez-Boomgaard, D.; Fuxe, K.; Brené, S.; Reina-Sánchez, M.D.; Rodríguez-Pérez, L.M.; Agnati, L.F.; de la Calle, A.; Rivera, A. Effect of acute and continuous morphine treatment on transcription factor expression in subregions of the rat caudate putamen. Marked modulation by D 4 receptor activation. Brain Res. 2011, 1407, 47–61. [Google Scholar] [CrossRef]
- Suárez-Boomgaard, D.; Gago, B.; Valderrama-Carvajal, A.; Roales-Buján, R.; van Craenenbroeck, K.; Duchou, J.; Borroto-Escuela, D.O.; Medina-Luque, J.; de la Calle, A.; Fuxe, K.; et al. Dopamine D4 receptor counteracts morphine-induced changes in μ opioid receptor signaling in the striosomes of the rat caudate putamen. Int. J. Mol. Sci. 2014, 15, 1481–1498. [Google Scholar] [CrossRef]
- Negrete-Díaz, J.V.; Shumilov, K.; Real, M.Á.; Medina-Luque, J.; Valderrama-Carvajal, A.; Flores, G.; Rodríguez-Moreno, A.; Rivera, A. Pharmacological activation of dopamine D4 receptor modulates morphine-induced changes in the expression of GAD65/67 and GABAB receptors in the basal ganglia. Neuropharmacology 2019, 152, 22–29. [Google Scholar] [CrossRef]
- Rivera, A.; Cuéllar, B.; Girón, F.J.; Grandy, D.K.; de la Calle, A.; Moratalla, R. Dopamine D4 receptors are heterogeneously distributed in the striosomes/matrix compartments of the striatum. J. Neurochem. 2002, 80, 219–229. [Google Scholar] [CrossRef]
- Fuxe, K.; Marcellino, D.; Rivera, A.; Diaz-Cabiale, Z.; Filip, M.; Gago, B.; Roberts, D.C.S.; Langel, U.; Genedani, S.; Ferraro, L.; et al. Receptor-receptor interactions within receptor mosaics. Impact on neuropsychopharmacology. Brain Res. Rev. 2008, 58, 415–452. [Google Scholar] [CrossRef]
- Gago, B.; Fuxe, K.; Agnati, L.; Peñafiel, A.; de la Calle, A.; Rivera, A. Dopamine D4 receptor activation decreases the expression of μ-opioid receptors in the rat striatum. J. Comp. Neurol. 2007, 502, 358–366. [Google Scholar] [CrossRef]
- Rodriguez, A.; Ehlenberger, D.B.; Dickstein, D.L.; Hof, P.R.; Wearne, S.L. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS ONE 2008, 3, 1997. [Google Scholar] [CrossRef]
- Gago, B.; Fuxe, K.; Brené, S.; Díaz-Cabiale, Z.; Reina-Sánchez, M.D.; Suárez-Boomgaard, D.; Roales-Buján, R.; Valderrama-Carvajal, A.; de la Calle, A.; Rivera, A. Early modulation by the dopamine D4 receptor of morphine-induced changes in the opioid peptide systems in the rat caudate putamen. J. Neurosci. Res. 2013, 91, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yan, Z.; Ferreira, A.; Tomizawa, K.; Liauw, J.A.; Zhuo, M.; Allen, P.B.; Ouimet, C.C.; Greengard, P. Spinophilin regulates the formation and function of dendritic spines. Proc. Natl. Acad. Sci. USA 2000, 97, 9287–9292. [Google Scholar] [CrossRef] [PubMed]
- Nisenbaum, E.S.; Wilson, C.J. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection-neurons. J. Neurosci. 1995, 15, 4449–4463. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, C.; Slesinger, P.A. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat. Rev. Neurosci. 2010, 11, 301–315. [Google Scholar] [CrossRef]
- Valderrama-Carvajal, A.; Irizar, H.; Gago, B.; Jiménez-Urbieta, H.; Fuxe, K.; Rodríguez-Oroz, M.C.; Otaegui, D.; Rivera, A. Transcriptomic integration of D4R and MOR signaling in the rat caudate putamen. Sci. Rep. 2018, 8, 7337. [Google Scholar] [CrossRef]
- Fujiyama, F.; Sohn, J.; Nakano, T.; Furuta, T.; Nakamura, K.C.; Matsuda, W.; Kaneko, T. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: A single neuron-tracing study using a viral vector. Eur. J. Neurosci. 2011, 33, 668–677. [Google Scholar] [CrossRef]
- Watabe-Uchida, M.; Zhu, L.; Ogawa, S.K.; Vamanrao, A.; Uchida, N. Whole-Brain Mapping of Direct Inputs to Midbrain Dopamine Neurons. Neuron 2012, 74, 858–873. [Google Scholar] [CrossRef]
- Shumilov, K.; Real, M.A.; Valderrama-Carvajal, A.; Rivera, A. Selective ablation of striatal striosomes produces the deregulation of dopamine nigrostriatal pathway. PLoS ONE 2018, 13, e0203135. [Google Scholar] [CrossRef]
- Crittenden, J.R.; Tillberg, P.W.; Riad, M.H.; Shima, Y.; Gerfen, C.R.; Curry, J.; Housman, D.E.; Nelson, S.B.; Boyden, E.S.; Graybiel, A.M. Striosome-dendron bouquets highlight a unique striatonigral circuit targeting dopamine-containing neurons. Proc. Natl. Acad. Sci. USA 2016, 113, 11318–11323. [Google Scholar] [CrossRef]
- Fornasiero, E.F.; Bonanomi, D.; Benfenati, F.; Valtorta, F. The role of synapsins in neuronal development. Cell. Mol. Life Sci. 2010, 67, 1383–1396. [Google Scholar] [CrossRef]
- Mazei-Robison, M.S.; Nestler, E.J. Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harb. Perspect. Med. 2012, 2, a012070. [Google Scholar] [CrossRef]
- Drastichova, Z.; Hejnova, L.; Moravcova, R.; Novotny, J. Proteomic analysis unveils expressional changes in cytoskeleton-and synaptic plasticity-associated proteins in rat brain six months after withdrawal from morphine. Life 2021, 11, 683. [Google Scholar] [CrossRef]
- Gerdeman, G.L.; Partridge, J.G.; Lupica, C.R.; Lovinger, D.M. It could be habit forming: Drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003, 26, 184–192. [Google Scholar] [CrossRef]
- Robinson, T.E.; Gorny, G.; Savage, V.R.; Kolb, B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse 2002, 46, 271–279. [Google Scholar] [CrossRef]
- Jia, M.; Wang, X.; Zhang, H.; Wang, X.; Ma, H.; Yang, M.; Li, Y.; Cui, C. MicroRNA-132 is involved in morphine dependence via modifying the structural plasticity of the dentate gyrus neurons in rats. Addict. Biol. 2021, 12, e13086. [Google Scholar] [CrossRef]
- Vetter, P.; Roth, A.; Häusser, M. Propagation of action potentials in dendrites depends on dendritic morphology. J. Neurophysiol. 2001, 85, 926–937. [Google Scholar] [CrossRef]
- Van Elburg, R.A.J.; van Ooyen, A. Impact of dendritic size and dendritic topology on burst firing in pyramidal cells. PLoS Comput. Biol. 2010, 6, 1–19. [Google Scholar] [CrossRef]
- Cuntz, H.; Bird, A.D.; Mittag, M.; Beining, M.; Schneider, M.; Mediavilla, L.; Hoffmann, F.Z.; Deller, T.; Jedlicka, P. A general principle of dendritic constancy: A neuron’s size- and shape-invariant excitability. Neuron 2021, 109, 3647–3662. [Google Scholar] [CrossRef]
- Mel, B.W.; Schiller, J.; Poirazi, P. Synaptic plasticity in dendrites: Complications and coping strategies. Curr. Opin. Neurobiol. 2017, 43, 177–186. [Google Scholar] [CrossRef]
- Tønnesen, J.; Nägerl, U.V. Dendritic spines as tunable regulators of synaptic signals. Front. Psychiatry 2016, 7, 101. [Google Scholar] [CrossRef]
- Atwood, B.K.; Kupferschmidt, D.A.; Lovinger, D.M. Opioids induce dissociable forms of long-term depression of excitatory inputs to the dorsal striatum. Nat. Neurosci. 2014, 17, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, B.; Haggerty, D.L.; Atwood, B.K. Synapse-specific expression of mu opioid receptor long-term depression in the dorsomedial striatum. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bolam, J.P.; Hanley, J.J.; Booth, P.A.C.; Bevan, M.D. Synaptic Organisation of the Basal Ganglia. J. Anat. 2000, 196, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.D.; Wood, R.; Gurney, K. Reconstructing the three-dimensional gabaergic microcircuit of the striatum. PLoS Comput. Biol. 2010, 6, e1001011. [Google Scholar] [CrossRef]
- McDevitt, D.S.; Jonik, B.; Graziane, N.M. Morphine Differentially Alters the Synaptic and Intrinsic Properties of D1R- and D2R-Expressing Medium Spiny Neurons in the Nucleus Accumbens. Front. Synaptic Neurosci. 2019, 11, 35. [Google Scholar] [CrossRef]
- Heng, L.J.; Yang, J.; Liu, Y.H.; Wang, W.T.; Hu, S.J.; Gao, G.D. Repeated morphine exposure decreased the nucleus accumbens excitability during short-term withdrawal. Synapse 2008, 62, 775–782. [Google Scholar] [CrossRef]
- Mansour, A.; Fox, C.A.; Burke, S.; Meng, F.; Thompson, R.C.; Akil, H.; Watson, S.J. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: An in situ hybridization study. J. Comp. Neurol. 1994, 350, 412–438. [Google Scholar] [CrossRef]
- Yagishita, S.; Hayashi-Takagi, A.; Ellis-Davies, G.C.R.; Urakubo, H.; Ishii, S.; Kasai, H. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 2014, 345, 1616–1620. [Google Scholar] [CrossRef]
- Shen, W.; Flajolet, M.; Greengard, P.; Surmeier, D.J. Dichotomous dopaminergic control of striatal synaptic plasticity. Science 2008, 321, 848–851. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Ding, J.; Day, M.; Wang, Z.; Shen, W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007, 30, 228–235. [Google Scholar] [CrossRef]
- Planert, H.; Berger, T.K.; Silberberg, G. Membrane Properties of Striatal Direct and Indirect Pathway Neurons in Mouse and Rat Slices and Their Modulation by Dopamine. PLoS ONE 2013, 8, e57054. [Google Scholar] [CrossRef]
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional neuroanatomy of the basal ganglia. Cold Spring Harb. Perspect. Med. 2012, 2, a009621. [Google Scholar] [CrossRef]
- Suarez, L.M.; Solis, O.; Sanz-Magro, A.; Alberquilla, S.; Moratalla, R. Dopamine D1 Receptors Regulate Spines in Striatal Direct-Pathway and Indirect-Pathway Neurons. Mov. Disord. 2020, 35, 1810–1821. [Google Scholar] [CrossRef]
- Yan, Z.; Flores-Hernandez, J.; Surmeier, D.J. Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience 2001, 103, 1017–1024. [Google Scholar] [CrossRef]
- Aizman, O.; Brismar, H.; Uhlén, P.; Zettergren, E.; Levey, A.I.; Forssberg, H.; Greengard, P.; Aperia, A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat. Neurosci. 2000, 3, 226–230. [Google Scholar] [CrossRef]
- Rivera, A.; Alberti, I.; Martín, A.B.; Narváez, J.A.; de la Calle, A.; Moratalla, R. Molecular phenotype of rat striatal neurons expressing the dopamine D5 receptor subtype. Eur. J. Neurosci. 2002, 16, 2049–2058. [Google Scholar] [CrossRef]
- Cazorla, M.; Shegda, M.; Ramesh, B.; Harrison, N.L.; Kellendonk, C. Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels. J. Neurosci. 2012, 32, 2398–2409. [Google Scholar] [CrossRef]
- Kim, A.; di Ciano, P.; Pushparaj, A.; Leca, J.; le Foll, B. The effects of dopamine D4 receptor ligands on operant alcohol self-administration and cue- and stress-induced reinstatement in rats. Eur. J. Pharmacol. 2020, 867, 172838. [Google Scholar] [CrossRef]
- Ananth, M.; Hetelekides, E.M.; Hamilton, J.; Thanos, P.K. Dopamine D4 receptor gene expression plays important role in extinction and reinstatement of cocaine-seeking behavior in mice. Behav. Brain Res. 2019, 365, 1–6. [Google Scholar] [CrossRef]
- Yan, Y.; Pushparaj, A.; le Strat, Y.; Gamaleddin, I.; Barnes, C.; Justinova, Z.; Goldberg, S.R.; le Foll, B. Blockade of dopamine D4 receptors attenuates reinstatement of extinguished nicotine-seeking behavior in rats. Neuropsychopharmacology 2012, 37, 685–696. [Google Scholar] [CrossRef]
- Rivera, A.; Trías, S.; Peñafiel, A.; Narváez, J.Á.; Díaz-Cabiale, Z.; Moratalla, R.; de la Calle, A.; Angel Narváez, J.; Díaz-Cabiale, Z.; Moratalla, R.; et al. Expression of D4 dopamine receptors in striatonigral and striatopallidal neurons in the rat striatum. Brain Res. 2003, 989, 35–41. [Google Scholar] [CrossRef][Green Version]
- Michaelsen, K.; Murk, K.; Zagrebelsky, M.; Dreznjak, A.; Jockusch, B.M.; Rothkegel, M.; Korte, M. Fine-tuning of neuronal architecture requires two profilin isoforms. Proc. Natl. Acad. Sci. USA 2010, 107, 15780–15785. [Google Scholar] [CrossRef]
- Beaubien, F.; Raja, R.; Kennedy, T.E.; Fournier, A.E.; Cloutier, J.F. Slitrk1 is localized to excitatory synapses and promotes their development. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Yoon, S.; Piguel, N.H.; Khalatyan, N.; Dionisio, L.E.; Savas, J.N.; Penzes, P. Homer1 promotes dendritic spine growth through ankyrin-G and its loss reshapes the synaptic proteome. Mol. Psychiatry 2021, 26, 1775–1789. [Google Scholar] [CrossRef]
- Fox, P.D.; Hentges, S.T. Differential Desensitization Observed at Multiple Effectors of Somatic μ-Opioid Receptors Underlies Sustained Agonist-Mediated Inhibition of Proopiomelanocortin Neuron Activity. J. Neurosci. 2017, 37, 8667–8677. [Google Scholar] [CrossRef]
- Newman-Tancredi, A.; Heusler, P.; Martel, J.C.; Ormière, A.M.; Leduc, N.; Cussac, D. Agonist and antagonist properties of antipsychotics at human dopamine D4.4 receptors: G-protein activation and K+ channel modulation in transfected cells. Int. J. Neuropsychopharmacol. 2008, 11, 293–307. [Google Scholar] [CrossRef]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef]
- De Velasco, E.M.F.; McCall, N.; Wickman, K. GIRK Channel Plasticity and Implications for Drug Addiction. Int. Rev. Neurobiol. 2015, 123, 201–238. [Google Scholar] [CrossRef]
| Antibody | Type | Specie | Source / Reference | Dilution |
|---|---|---|---|---|
| Calretinin (CR) | Poly- | G | Swant (CG1) | 1:10,000 (IF) |
| Choline acetyltransferase (ChAT) | Poly- | G | Millipore (AB144P) | 1:750 (IF) |
| GIRK1 (Kir3.1) | Poly- | R | Alomone Labs (AB2040113) | 1:500 (IMQ, IF) |
| GIRK2 (Kir3.2) | Poly- | R | Alomone Labs (AB2040115) | 1:1000 (IMQ, IF) |
| μ opioid receptor (MOR) | Poly- | R | Millipore (PC165L) | 1:50,000 (IMQ) |
| Parvalbumin (PV) | Mono- | M | Sigma-Aldrich (P3171) | 1:5000 (IF) |
| Somatostatin (SS) | Poly- | G | Santa Cruz (sc-7819) | 1:5000 (IF) |
| Spinophilin | Poly- | R | Millipore (06-852) | 1:200 (IMQ) |
| Synaptophysin | Mono- | M | Abcam (ab8049) | 1:2000 (WB) |
| Tyrosine hydroxylase (TH) | Mono- | M | InmunoStar (P22941) | 1:1000 (IF) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, A.; Suárez-Boomgaard, D.; Miguelez, C.; Valderrama-Carvajal, A.; Baufreton, J.; Shumilov, K.; Taupignon, A.; Gago, B.; Real, M.Á. Dopamine D4 Receptor Is a Regulator of Morphine-Induced Plasticity in the Rat Dorsal Striatum. Cells 2022, 11, 31. https://doi.org/10.3390/cells11010031
Rivera A, Suárez-Boomgaard D, Miguelez C, Valderrama-Carvajal A, Baufreton J, Shumilov K, Taupignon A, Gago B, Real MÁ. Dopamine D4 Receptor Is a Regulator of Morphine-Induced Plasticity in the Rat Dorsal Striatum. Cells. 2022; 11(1):31. https://doi.org/10.3390/cells11010031
Chicago/Turabian StyleRivera, Alicia, Diana Suárez-Boomgaard, Cristina Miguelez, Alejandra Valderrama-Carvajal, Jérôme Baufreton, Kirill Shumilov, Anne Taupignon, Belén Gago, and M. Ángeles Real. 2022. "Dopamine D4 Receptor Is a Regulator of Morphine-Induced Plasticity in the Rat Dorsal Striatum" Cells 11, no. 1: 31. https://doi.org/10.3390/cells11010031
APA StyleRivera, A., Suárez-Boomgaard, D., Miguelez, C., Valderrama-Carvajal, A., Baufreton, J., Shumilov, K., Taupignon, A., Gago, B., & Real, M. Á. (2022). Dopamine D4 Receptor Is a Regulator of Morphine-Induced Plasticity in the Rat Dorsal Striatum. Cells, 11(1), 31. https://doi.org/10.3390/cells11010031








