Dysfunctional Heteroreceptor Complexes as Novel Targets for the Treatment of Major Depressive and Anxiety Disorders
Abstract
:1. Introduction
2. Clinical Characteristics and Epidemiological Aspects Related to Depression and Anxiety
2.1. Major Depressive Disorder
2.2. Anxiety
2.3. Clinical Responses Associated to the Use of Pharmacological Compounds in Patients Suffering from Depression and Anxiety Disorders
3. Emergent Concepts of Receptor Heterodimerization and Their Impact on Nervous System Function
4. Receptor Homo and Heterodimerization with Relevance for MDD
4.1. Role of 5-HT Transporters and Receptor Systems in MDD
4.1.1. Role of 5-HT1AR-5-HT2AR Heteroreceptor Complexes in MDD
4.1.2. 5-HT1AR-FGFR1 Heteroreceptor Complex in MDD
4.1.3. 5-HT-Galanin Heterocomplexes in MDD
4.1.4. 5-HTR-OXTR Heterocomplexes in Depression
4.1.5. 5-HT1AR-D2LR Heterocomplexes in MDD
4.2. Influence of Other Heteroreceptor Complexes in MDD
4.2.1. Dopamine D1R-D2R Heterocomplexes in MDD
4.2.2. GalaninR2 (GalR2)-NPYY1R Heterocomplexes in MDD
4.2.3. OpioidR Heterocomplexes in MDD
5. Receptor-Receptor Interactions with Relevance for Anxiety
5.1. 5-HT1AR-5-HT2AR Isoreceptor Complexes in Anxiety
5.2. Opioid Heteroreceptor Complexes in Anxiety
5.3. Galanin Heteroreceptor Complexes in Anxiety
5.4. Gal-NPY Heteroreceptor Complex in Anxiety
5.5. Dopamine D1R-D2R Heterocomplexes in Anxiety
5.6. D2LR-5-HT1AR Heterocomplexes in Anxiety
5.7. D2R-OXTR Heterocomplexes in Anxiety
6. Ghrelin
6.1. Growth Hormone Secretagogue Receptor 1a (GHSR1a)-Containing Heteromer Complexes as Links between Stress-Induced Eating Behavior with Depression/Anxiety
6.2. GHSR1a-DA Receptor Heteroreceptor Complexes
6.3. GHSR1a-D1R Heteromers
6.4. GHSR1a-D2R Heteromers
7. Novel Therapeutic Strategies for Treatment of MDD and Anxiety Based on GPCR Heteroreceptor Complexes
Potential Allosteric Downstream-Mediated Receptor-Receptor Interactions in the Action of Fast Antidepressant Effects of Ketamine
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuxe, K.; Borroto-Escuela, D.O. Volume Transmission and Receptor-Receptor Interactions in Heteroreceptor Complexes: Understanding the Role of New Concepts for Brain Communication. Neural Regen. Res. 2016, 11, 1220–1223. [Google Scholar] [CrossRef]
- Borroto Escuela, D.O.; Calvo, F.; Narvaez, M.; Romero-Fernandez, W.; Millon, C.; Di Palma, M.; Pérez-Alea, M.; Tena, M.; Agnati, L.F.; Tarakanov, A.O.; et al. The GalR1–GalR2 Heteroreceptor Complex Can Be the Receptor for Galanin Fragment 1–15. Eur. Neuropsychopharmacol. 2014, 24, S242–S243. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Pintsuk, J.; Schäfer, T.; Friedland, K.; Ferraro, L.; Tanganelli, S.; Liu, F.; Fuxe, K. Multiple D2 Heteroreceptor Complexes: New Targets for Treatment of Schizophrenia. Ther. Adv. Psychopharmacol. 2016, 6, 77–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Lopez, A.D. Evidence-Based Health Policy-Lessons from the Global Burden of Disease Study. Science 1996, 274, 740–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, L.; Caraveo-Anduaga, J.J.; Berglund, P.; Bijl, R.V.; De Graaf, R.; Vollebergh, W.; Dragomirecka, E.; Kohn, R.; Keller, M.; Kessler, R.C.; et al. The Epidemiology of Major Depressive Episodes: Results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int. J. Methods Psychiatr. Res. 2003, 12, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ruan, M.; Chen, J.; Fang, Y. Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications. Neurosci. Bull. 2021, 37, 863–880. [Google Scholar] [CrossRef]
- Kessler, R.C.; Walters, E.E.; Forthofer, M.S. The Social Consequences of Psychiatric Disorders, III: Probability of Marital Stability. Am. J. Psychiatry 1998, 155, 1092–1096. [Google Scholar] [CrossRef]
- Moussavi, S.; Chatterji, S.; Verdes, E.; Tandon, A.; Patel, V.; Ustun, B. Depression, Chronic Diseases, and Decrements in Health: Results from the World Health Surveys. Lancet 2007, 370, 851–858. [Google Scholar] [CrossRef]
- Yang, Y.; Ligthart, L.; Terwindt, G.M.; Boomsma, D.I.; Rodriguez-Acevedo, A.J.; Nyholt, D.R. Genetic Epidemiology of Migraine and Depression. Cephalalgia 2016, 36, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Craske, M.G.; Stein, M.B. Anxiety. Lancet 2016, 388, 3048–3059. [Google Scholar] [CrossRef]
- Murrough, J.W.; Yaqubi, S.; Sayed, S.; Charney, D.S. Emerging Drugs for the Treatment of Anxiety. Expert Opin. Emerg. Drugs 2015, 20, 393–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maron, E.; Nutt, D. Biological Markers of Generalized Anxiety Disorder. Dialogues Clin. Neurosci. 2017, 19, 147–158. [Google Scholar] [CrossRef]
- Belon, J.P. L’anxiété et Les Troubles Anxieux (Anxiety and Anxiety Disorders). Actual. Pharm. 2019, 58, 18–22. [Google Scholar] [CrossRef]
- Kessler, R.C.; Petukhova, M.; Sampson, N.A.; Zaslavsky, A.M.; Wittchen, H.-U. Twelve-Month and Lifetime Prevalence and Lifetime Morbid Risk of Anxiety and Mood Disorders in the United States. Int. J. Methods Psychiatr. Res. 2012, 21, 169–184. [Google Scholar] [CrossRef]
- Salehi, M.; Amanat, M.; Mohammadi, M.; Salmanian, M.; Rezaei, N.; Saghazadeh, A.; Garakani, A. The Prevalence of Post-Traumatic Stress Disorder Related Symptoms in Coronavirus Outbreaks: A Systematic-Review and Meta-Analysis. J. Affect. Disord. 2021, 282, 527–538. [Google Scholar] [CrossRef]
- Bandelow, B.; Michaelis, S. Epidemiology of Anxiety Disorders in the 21st Century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [CrossRef]
- Ruscio, A.M.; Hallion, L.S.; Lim, C.C.W.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Andrade, L.H.; Borges, G.; Bromet, E.J.; Bunting, B.; et al. Cross-Sectional Comparison of the Epidemiology of DSM-5 Generalized Anxiety Disorder across the Globe. JAMA Psychiatry 2017, 74, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Broeyer, F.; de Kam, M.; Baas, J.; Cohen, A.; van Gerven, J. Pharmacodynamic Response Profiles of Anxiolytic and Sedative Drugs. Br. J. Clin. Pharmacol. 2017, 83, 1028–1038. [Google Scholar] [CrossRef]
- Katz, M.M.; Tekell, J.L.; Bowden, C.L.; Brannan, S.; Houston, J.P.; Berman, N.; Frazer, A. Onset and Early Behavioral Effects of Pharmacologically Different Antidepressants and Placebo in Depression. Neuropsychopharmacology 2004, 29, 566–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Reitt, M.; Röver, C.; Michaelis, S.; Görlich, Y.; Wedekind, D. Efficacy of Treatments for Anxiety Disorders: A Meta-Analysis. Int. Clin. Psychopharmacol. 2015, 30, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, S.G.; Smits, J.A.J. Cognitive-Behavioral Therapy for Adult Anxiety Disorders: A Meta-Analysis of Randomized Placebo-Controlled Trials. J. Clin. Psychiatry 2008, 69, 621–632. [Google Scholar] [CrossRef]
- Craske, M.G.; Stein, M.B.; Eley, T.C.; Milad, M.R.; Holmes, A.; Rapee, R.M.; Wittchen, H.U. Anxiety Disorders. Nat. Rev. Dis. Prim. 2017, 3, 17024. [Google Scholar] [CrossRef]
- Agnati, L.F.; Fuxe, K.; Zoli, M.; Rondanini, C.; Ogren, S.O. New Vistas on Synaptic Plasticity: The Receptor Mosaic Hypothesis of the Engram. Med. Biol. 1982, 60, 183–190. [Google Scholar]
- Fuxe, K.; Agnati, L.F.; Benfenati, F.; Cimmino, M.; Algeri, S.; Hòkfelt, T.; Mutt, V. Modulation by Cholecystokinins of 3H-Spiroperidol Binding in Rat Striatum: Evidence for Increased Affinity and Reduction in the Number of Binding Sites. Acta Physiol. Scand. 1981, 113, 567–569. [Google Scholar] [CrossRef]
- Fuxe, K.; Agnati, L.F.; Benfenati, F.; Celani, M.; Zini, I.; Zoli, M.; Mutt, V. Evidence for the Existence of Receptor—Receptor Interactions in the Central Nervous System. Studies on the Regulation of Monoamine Receptors by Neuropeptides. J. Neural. Transm. Suppl. 1983, 18, 165–179. [Google Scholar]
- Lefkowitz, R.J.; Cotecchia, S.; Samama, P.; Costa, T. Constitutive Activity of Receptors Coupled to Guanine Nucleotide Regulatory Proteins. Trends Pharmacol. Sci. 1993, 14, 303–307. [Google Scholar] [CrossRef]
- Baldwin, J.M. Structure and Function of Receptors Coupled to G Proteins. Curr. Opin. Cell Biol. 1994, 6, 180–190. [Google Scholar] [CrossRef]
- Zoli, M.; Agnati, L.F.; Hedlund, P.B.; Li, X.M.; Ferré, S.; Fuxe, K. Receptor-Receptor Interactions as an Integrative Mechanism in Nerve Cells. Mol. Neurobiol. 1993, 7, 293–334. [Google Scholar] [CrossRef] [PubMed]
- Milligan, G. G Protein-Coupled Receptor Dimerization: Function and Ligand Pharmacology. Mol. Pharmacol. 2004, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Terrillon, S.; Bouvier, M. Roles of G-Protein-Coupled Receptor Dimerization. EMBO Rep. 2004, 5, 30–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuxe, K.; Marcellino, D.; Rivera, A.; Diaz-Cabiale, Z.; Filip, M.; Gago, B.; Roberts, D.C.S.; Langel, U.; Genedani, S.; Ferraro, L.; et al. Receptor-Receptor Interactions within Receptor Mosaics. Impact on Neuropsychopharmacology. Brain Res. Rev. 2008, 58, 415–452. [Google Scholar] [CrossRef]
- Szidonya, L.; Cserzo, M.; Hunyady, L. Dimerization and Oligomerization of G-Protein-Coupled Receptors: Debated Structures with Established and Emerging Functions. J. Endocrinol. 2008, 196, 435–453. [Google Scholar] [CrossRef] [Green Version]
- Jordan, B.A.; Devi, L.A. G-Protein-Coupled Receptor Heterodimerization Modulates Receptor Function. Nature 1999, 399, 697–700. [Google Scholar] [CrossRef]
- Marshall, F.H.; Jones, K.A.; Kaupmann, K.; Bettler, B. GABA(B) Receptors—The First 7TM Heterodimers. Trends Pharmacol. Sci. 1999, 20, 396–399. [Google Scholar] [CrossRef]
- Franco, R.; Ferré, S.; Agnati, L.; Torvinen, M.; Ginés, S.; Hillion, J.; Casadó, V.; Lledó, P.M.; Zoli, M.; Lluis, C.; et al. Evidence for Adenosine/Dopamine Receptor Interactions: Indications for Heteromerization. Neuropsychopharmacology 2000, 23, S50–S59. [Google Scholar] [CrossRef]
- Marshall, F.H. Heterodimerization of G-Protein-Coupled Receptors in the CNS. Curr. Opin. Pharmacol. 2001, 1, 40–44. [Google Scholar] [CrossRef]
- George, S.R.; O’Dowd, B.F.; Lee, S.P. G-Protein-Coupled Receptor Oligomerization and Its Potential for Drug Discovery. Nat. Rev. Drug Discov. 2002, 1, 808–820. [Google Scholar] [CrossRef]
- Angers, S.; Salahpour, A.; Bouvier, M. Dimerization: An Emerging Concept for G Protein-Coupled Receptor Ontogeny and Function. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 409–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnati, L.F.; Ferré, S.; Lluis, C.; Franco, R.; Fuxe, K. Molecular Mechanisms and Therapeutical Implications of Intramembrane Receptor/Receptor Interactions among Heptahelical Receptors with Examples from the Striatopallidal GABA Neurons. Pharmacol. Rev. 2003, 55, 509–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikbin, N.; Edwards, C.; Reynolds, C. G-Proein Coupled Receptor Dimerization. Iran. J. Pharmac. Ter. 2003, 2, 1–11. [Google Scholar]
- White, J.H.; Wise, A.; Main, M.J.; Green, A.; Fraser, N.J.; Disney, G.H.; Barnes, A.A.; Emson, P.; Foord, S.M.; Marshall, F.H. Heterodimerization Is Required for the Formation of a Functional GABA(B) Receptor. Nature 1998, 396, 679–682. [Google Scholar] [CrossRef]
- Kunishima, N.; Shimada, Y.; Tsuji, Y.; Sato, T.; Yamamoto, M.; Kumasaka, T.; Nakanishi, S.; Jingami, H.; Morikawa, K. Structural Basis of Glutamate Recognition by a Dimeric Metabotropic Glutamate Receptor. Nature 2000, 407, 971–977. [Google Scholar] [CrossRef]
- Paglin, S.; Jamieson, J.D. Covalent Crosslinking of Angiotensin II to Its Binding Sites in Rat Adrenal Membranes. Proc. Natl. Acad. Sci. USA 1982, 79, 3739–3743. [Google Scholar] [CrossRef] [Green Version]
- Hebert, T.E.; Loisel, T.P.; Adam, L.; Ethier, N.; St. Onge, S.; Bouvier, M. Functional Rescue of a Constitutively Desensitized Beta2AR through Receptor Dimerization. Biochem. J. 1998, 330, 287–293. [Google Scholar] [CrossRef]
- Sekar, R.B.; Periasamy, A. Fluorescence Resonance Energy Transfer (FRET) Microscopy Imaging of Live Cell Protein Localizations. J. Cell Biol. 2003, 160, 629–633. [Google Scholar] [CrossRef] [Green Version]
- Fuxe, K.; Borroto-Escuela, D.O.; Marcellino, D.; Romero-Fernandez, W.; Frankowska, M.; Guidolin, D.; Filip, M.; Ferraro, L.; Woods, A.S.; Tarakanov, A.; et al. GPCR Heteromers and Their Allosteric Receptor-Receptor Interactions. Curr. Med. Chem. 2012, 19, 356–363. [Google Scholar] [CrossRef]
- Xu, X.; Soutto, M.; Xie, Q.; Servick, S.; Subramanian, C.; Von Arnim, A.G.; Johnson, C.H. Imaging Protein Interactions with Bioluminescence Resonance Energy Transfer (BRET) in Plant and Mammalian Cells and Tissues. Proc. Natl. Acad. Sci. USA 2007, 104, 10264–10269. [Google Scholar] [CrossRef] [Green Version]
- Angers, S.; Salahpour, A.; Joly, E.; Hilairet, S.; Chelsky, D.; Dennis, M.; Bouvier, M. Detection of B2-Adrenergic Receptor Dimerization in Living Cells Using Bioluminescence Resonance Energy Transfer (BRET). Proc. Natl. Acad. Sci. USA 2000, 97, 3684–3689. [Google Scholar] [CrossRef] [PubMed]
- Carriba, P.; Navarro, G.; Ciruela, F.; Ferré, S.; Casadó, V.; Agnati, L.; Cortés, A.; Mallol, J.; Fuxe, K.; Canela, E.I.; et al. Detection of Heteromerization of More than Two Proteins by Sequential BRET-FRET. Nat. Methods 2008, 5, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Resina, I.; Martínez-Pinilla, E.; Borroto-Escuela, D.O.; Fuxe, K.; Navarro, G.; Franco, R. Methods to Identify the Signature of Trimers Formed by Three g Protein-Coupled Receptors or by Two G Protein-Coupled and One Ionotropic Receptor with Special Emphasis in the Functional Role in the Central Nervous System. In Receptor-Receptor Interactions in the Central Nervous System; Humana Press: New York, NY, USA, 2018; Volume 140, pp. 187–203. [Google Scholar] [CrossRef]
- Fredriksson, S.; Gullberg, M.; Jarvius, J.; Olsson, C.; Pietras, K.; Gústafsdóttir, S.M.; Östman, A.; Landegren, U. Protein Detection Using Proximity-Dependent DNA Ligation Assays. Nat. Biotechnol. 2002, 20, 473–477. [Google Scholar] [CrossRef]
- Trifilieff, P.; Rives, M.L.; Urizar, E.; Piskorowski, R.A.; Vishwasrao, H.D.; Castrillon, J.; Schmauss, C.; Slättman, M.; Gullberg, M.; Javitch, J.A. Detection of Antigen Interactions Ex Vivo by Proximity Ligation Assay: Endogenous Dopamine D2-Adenosine A2A Receptor Complexes in the Striatum. Biotechniques 2011, 51, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borroto-Escuela, D.O.; Hagman, B.; Woolfenden, M.; Pinton, L.; Jiménez-Beristain, A.; Oflijan, J.; Narvaez, M.; Di Palma, M.; Feltmann, K.; Sartini, S.; et al. In Situ Proximity Ligation Assay to Study and Understand the Distribution and Balance of GPCR Homo- and Heteroreceptor Complexes in the Brain. Neuromethods 2016, 110, 109–124. [Google Scholar]
- Borroto-Escuela, D.O.; Narvaez, M.; Valladolid-Acebes, I.; Shumilov, K.; Di Palma, M.; Wydra, K.; Schaefer, T.; Reyes-Resina, I.; Navarro, G.; Mudó, G.; et al. Detection, Analysis, and Quantification of GPCR Homo- and Heteroreceptor Complexes in Specific Neuronal Cell Populations Using the In Situ Proximity Ligation Assay. Neuromethods 2018, 140, 299–315. [Google Scholar] [CrossRef]
- Grisshammer, R. New Approaches towards the Understanding of Integral Membrane Proteins: A Structural Perspective on G Protein-Coupled Receptors. Protein Sci. 2017, 26, 1493–1504. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Moreira, I.S.; Urizar, E.; Weinstein, H.; Javitch, J.A. Allosteric Communication between Protomers of Dopamine Class A GPCR Dimers Modulates Activation. Nat. Chem. Biol. 2009, 5, 688–695. [Google Scholar] [CrossRef] [Green Version]
- Kenakin, T.; Agnati, L.F.; Caron, M.; Fredholm, B.; Guidoli, D.; Kobilka, B.; Lefkowitz, R.W.; Lohse, M.; Woods, A.; Fuxe, K. International Workshop at the Nobel Forum, Karolinska Institutet on G Protein-Coupled Receptors: Finding the Words to Describe Monomers, Oligomers, and Their Molecular Mechanisms and Defining Their Meaning. Can a Consensus Be Reached? J. Recept. Signal Transduct. 2010, 30, 284–286. [Google Scholar] [CrossRef]
- Milligan, G. Oligomerisation of G-Protein-Coupled Receptors. J. Cell Sci. 2001, 114, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Scarselli, M.; Novi, F.; Schallmach, E.; Lin, R.; Baragli, A.; Colzi, A.; Griffon, N.; Corsini, G.U.; Sokoloff, P.; Levenson, R.; et al. D2/D3 Dopamine Receptor Heterodimers Exhibit Unique Functional Properties. J. Biol. Chem. 2001, 276, 30308–30314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuxe, K.; Marcellino, D.; Leo, G.; Agnati, L.F. Molecular Integration via Allosteric Interactions in Receptor Heteromers. A Working Hypothesis. Curr. Opin. Pharmacol. 2010, 10, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Mora, M.; Pérez-Carrera, D.; Crespo-Ramírez, M.; Tarakanov, A.; Fuxe, K.; Borroto-Escuela, D.O. Signaling in Dopamine D2 Receptor-Oxytocin Receptor Heterocomplexes and Its Relevance for the Anxiolytic Effects of Dopamine and Oxytocin Interactions in the Amygdala of the Rat. Biochim. Biophys. Acta 2016, 1862, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wan, Q.; Pristupa, Z.B.; Yu, X.M.; Wang, Y.T.; Niznik, H.B. Direct Protein-Protein Coupling Enables Cross-Talk between Dopamine D5 and Gamma-Aminobutyric Acid A Receptors. Nature 2000, 403, 274–280. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, R.; Yan, H.; Yin, H.; Wu, X.; Tan, Y.; Li, L. Metabotropic Glutamate Receptor 5 Modulates Calcium Oscillation and Innate Immune Response Induced by Lipopolysaccharide in Microglial Cell. Neuroscience 2014, 281, 24–34. [Google Scholar] [CrossRef]
- Pérez de la Mora, M.; Ferré, S.; Fuxe, K. GABA-Dopamine Receptor-Receptor Interactions in Neostriatal Membranes of the Rat. Neurochem. Res. 1997, 22, 1051–1054. [Google Scholar] [CrossRef]
- Wang, M.; Wong, A.H.; Liu, F. Interactions between NMDA and Dopamine Receptors: A Potential Therapeutic Target. Brain Res. 2012, 1476, 154–163. [Google Scholar] [CrossRef]
- Lavine, N.; Ethier, N.; Oak, J.N.; Pei, L.; Liu, F.; Trieu, P.; Rebois, R.V.; Bouvier, M.; Hébert, T.E.; Van Tol, H.H.M. G Protein-Coupled Receptors Form Stable Complexes with Inwardly Rectifying Potassium Channels and Adenylyl Cyclase. J. Biol. Chem. 2002, 277, 46010–46019. [Google Scholar] [CrossRef] [Green Version]
- Lee, F.J.S.; Pei, L.; Moszczynska, A.; Vukusic, B.; Fletcher, P.J.; Liu, F. Dopamine Transporter Cell Surface Localization Facilitated by a Direct Interaction with the Dopamine D2 Receptor. EMBO J. 2007, 26, 2127–2136. [Google Scholar] [CrossRef] [Green Version]
- Lee, F.J.S.; Pei, L.; Liu, F. Disruption of the Dopamine Transporter-Dopamine D2 Receptor Interaction in Schizophrenia. Synapse 2009, 63, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Flajolet, M.; Wang, Z.; Futter, M.; Shen, W.; Nuangchamnong, N.; Bendor, J.; Wallach, I.; Nairn, A.C.; Surmeier, D.J.; Greengard, P. FGF Acts as a Co-Transmitter through Adenosine A(2A) Receptor to Regulate Synaptic Plasticity. Nat. Neurosci. 2008, 11, 1402–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borroto-Escuela, D.O.; Li, X.; Tarakanov, A.O.; Savelli, D.; Narváez, M.; Shumilov, K.; Andrade-Talavera, Y.; Jimenez-Beristain, A.; Pomierny, B.; Díaz-Cabiale, Z.; et al. Existence of Brain 5-HT1A–5-HT2A Isoreceptor Complexes with Antagonistic Allosteric Receptor–Receptor Interactions Regulating 5-HT1A Receptor Recognition. ACS Omega 2017, 2, 4779–4789. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Romero-Fernandez, W.; Pérez-Alea, M.; Narvaez, M.; Tarakanov, A.O.; Mudó, G.; Agnati, L.F.; Ciruela, F.; Belluardo, N.; Fuxe, K. The Existence of FGFR1–5-HT1A Receptor Heterocomplexes in Midbrain 5-HT Neurons of the Rat: Relevance for Neuroplasticity. J. Neurosci. 2012, 32, 6295–6303. [Google Scholar] [CrossRef] [Green Version]
- Di Palma, M.; Sartini, S.; Lattanzi, D.; Cuppini, R.; Pita-Rodriguez, M.; Diaz-Carmenate, Y.; Narvaez, M.; Fuxe, K.; Borroto-Escuela, D.O.; Ambrogini, P. Evidence for the Existence of A2AR-TrkB Heteroreceptor Complexes in the Dorsal Hippocampus of the Rat Brain: Potential Implications of A2AR and TrkB Interplay upon Ageing. Mech. Ageing Dev. 2020, 190, 111289. [Google Scholar] [CrossRef]
- Ressler, K.J.; Nemeroff, C.B. Role of Serotonergic and Noradrenergic Systems in the Pathophysiology of Depression and Anxiety Disorders. Depress. Anxiety 2000, 12, 2–19. [Google Scholar] [CrossRef]
- Nemeroff, C.B. Comorbidity of Mood and Anxiety Disorders: The Rule, Not the Exception? Am. J. Psychiatry 2002, 159, 3–4. [Google Scholar] [CrossRef]
- Fuxe, K.; Ungerstedt, U. Localization of 5-hydroxytryptamine Uptake in Rat Brain after Intraventricular Injection. J. Pharm. Pharmacol. 1967, 19, 335–337. [Google Scholar] [CrossRef]
- Carlsson, A.; Fuxe, K.; Ungerstedt, U. The Effect of Imipramine of Central 5-hydroxytryptamine Neurons. J. Pharm. Pharmacol. 1968, 20, 150–151. [Google Scholar] [CrossRef]
- Barnes, N.M.; Sharp, T. A Review of Central 5-HT Receptors and Their Function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Renner, U.; Zeug, A.; Woehler, A.; Niebert, M.; Dityatev, A.; Dityateva, G.; Gorinski, N.; Guseva, D.; Abdel-Galil, D.; Fröhlich, M.; et al. Heterodimerization of Serotonin Receptors 5-HT1A and 5-HT7 Differentially Regulates Receptor Signalling and Trafficking. J. Cell Sci. 2012, 125, 2486–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, S.; MacQueen, G. The Role of the Hippocampus in the Pathophysiology of Major Depression. J. Psychiatry Neurosci. 2004, 29, 417–426. [Google Scholar] [PubMed]
- Drevets, W.C.; Savitz, J.; Trimble, M. The Subgenual Anterior Cingulate Cortex in Mood Disorders. CNS Spectr. 2008, 13, 663–681. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Jiang, Y.; Hu, Z.; Yang, Y.; Du, X.; Botchway, B.O.; Fang, M. Serotonin Receptors 2A and 1A Modulate Anxiety-like Behavior in Post-Traumatic Stress Disordered Mice. Am. J. Transl. Res. 2019, 11, 2288–2303. [Google Scholar] [PubMed]
- Artigas, F. Serotonin Receptors Involved in Antidepressant Effects. Pharmacol. Ther. 2013, 137, 119–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuxe, K.; Ögren, S.O.; Agnati, L.; Gustafsson, J.Å.; Jonsson, G. On the Mechanism of Action of the Antidepressant Drugs Amitriptyline and Nortriptyline. Evidence for 5-Hydroxytryptamine Receptor Blocking Activity. Neurosci. Lett. 1977, 6, 339–343. [Google Scholar] [CrossRef]
- Ögren, S.O.; Fuxe, K.; Agnati, L.F.; Gustafssons, J.Å.; Jonsson, G.; Holm, A.C. Reevaluation of the Indoleamine Hypothesis of Depression. Evidence for a Reduction of Functional Activity of Central 5-HT Systems by Antidepressant Drugs. J. Neural Transm. 1979, 46, 85–103. [Google Scholar] [CrossRef]
- Zhang, G.; Stackman, R.W. The Role of Serotonin 5-HT2A Receptors in Memory and Cognition. Front. Pharmacol. 2015, 6, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Borroto-Escuela, D.O.; Agnati, L.F.; Bechter, K.; Jansson, A.; Tarakanov, A.O.; Fuxe, K. The Role of Transmitter Diffusion and Flow versus Extracellular Vesicles in Volume Transmission in the Brain Neural–Glial Networks. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140183. [Google Scholar] [CrossRef] [Green Version]
- Fuxe, K.; Dahlström, A.; Höistad, M.; Marcellino, D.; Jansson, A.; Rivera, A.; Diaz-Cabiale, Z.; Jacobsen, K.; Tinner-Staines, B.; Hagman, B.; et al. From the Golgi–Cajal Mapping to the Transmitter-Based Characterization of the Neuronal Networks Leading to Two Modes of Brain Communication: Wiring and Volume Transmission. Brain Res. Rev. 2007, 55, 17–54. [Google Scholar] [CrossRef]
- Fuxe, K.; Agnati, L.F. Two Principal Modes of Electrochemical Communication in the Brain: Volume Versus Wiring Transmission. In Volume Transmission in the Brain: Novel Mechanisms for Neural Transmission; Fuxe, K., Agnati, L.F., Eds.; Raven Press: New York, NY, USA, 1991; pp. 1–9. [Google Scholar]
- Jansson, A.; Tinner, B.; Bancila, M.; Vergé, D.; Steinbusch, H.W.; Agnati, L.; Fuxe, K. Relationships of 5-Hydroxytryptamine Immunoreactive Terminal-like Varicosities to 5-Hydroxytryptamine-2A Receptor-Immunoreactive Neuronal Processes in the Rat Forebrain. J. Chem. Neuroanat. 2001, 22, 185–203. [Google Scholar] [CrossRef]
- Jansson, A.; Goldstein, M.; Tinner, B.; Zoli, M.; Meador-Woodruff, J.H.; Lew, J.Y.; Levey, A.I.; Watson, S.; Agnati, L.F.; Fuxe, K. On the Distribution Patterns of D1, D2, Tyrosine Hydroxylase and Dopamine Transporter Immunoreactivities in the Ventral Striatum of the Rat. Neuroscience 1999, 89, 473–489. [Google Scholar] [CrossRef]
- Fuxe, K.; Borroto-Escuela, D.O.; Romero-Fernandez, W.; Palkovits, M.; Tarakanov, A.O.; Ciruela, F.; Agnati, L.F. Moonlighting Proteins and Protein-Protein Interactions as Neurotherapeutic Targets in the G Protein-Coupled Receptor Field. Neuropsychopharmacology 2014, 39, 131–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuxe, K.; Borroto-Escuela, D.O.; Tarakanov, A.O.; Romero-Fernandez, W.; Ferraro, L.; Tanganelli, S.; Perez-Alea, M.; Di Palma, M.; Agnati, L.F. Dopamine D2 Heteroreceptor Complexes and Their Receptor-Receptor Interactions in Ventral Striatum. Prog. Brain Res. 2014, 211, 113–139. [Google Scholar] [PubMed]
- Borroto-Escuela, D.O.; Wydra, K.; Filip, M.; Fuxe, K. A2AR-D2R Heteroreceptor Complexes in Cocaine Reward and Addiction. Trends Pharmacol. Sci. 2018, 39, 1008–1020. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Narvaez, M.; Pérez-Alea, M.; Tarakanov, A.O.; Jiménez-Beristain, A.; Mudó, G.; Agnati, L.F.; Ciruela, F.; Belluardo, N.; Fuxe, K. Evidence for the Existence of FGFR1-5-HT1A Heteroreceptor Complexes in the Midbrain Raphe 5-HT System. Biochem. Biophys. Res. Commun. 2015, 456, 489–493. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Pérez-Alea, M.; Narvaez, M.; Tarakanov, A.O.; Mudó, G.; Jiménez-Beristain, A.; Agnati, L.F.; Ciruela, F.; Belluardo, N.; Fuxe, K. Enhancement of the FGFR1 Signaling in the FGFR1-5-HT1A Heteroreceptor Complex in Midbrain Raphe 5-HT Neuron Systems. Relevance for Neuroplasticity and Depression. Biochem. Biophys. Res. Commun. 2015, 463, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Borroto-Escuela, D.O.; Corrales, F.; Narvaez, M.; Oflijan, J.; Agnati, L.F.; Palkovits, M.; Fuxe, K. Dynamic Modulation of FGFR1-5-HT1A Heteroreceptor Complexes. Agonist Treatment Enhances Participation of FGFR1 and 5-HT1A Homodimers and Recruitment of β-Arrestin2. Biochem. Biophys. Res. Commun. 2013, 441, 387–392. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Romero-Fernandez, W.; Mudó, G.; Pérez-Alea, M.; Ciruela, F.; Tarakanov, A.O.; Narvaez, M.; Di Liberto, V.; Agnati, L.F.; Belluardo, N.; et al. Fibroblast Growth Factor Receptor 1-5-Hydroxytryptamine 1A Heteroreceptor Complexes and Their Enhancement of Hippocampal Plasticity. Biol. Psychiatry 2012, 79, 84–91. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Dupont, C.M.; Li, X.; Savelli, D.; Lattanzi, D.; Srivastava, I.; Narváez, M.; Di Palma, M.; Barbieri, E.; Andrade-Talavera, Y.; et al. Disturbances in the FGFR1-5-HT1A Heteroreceptor Complexes in the Raphe-Hippocampal 5-HT System Develop in a Genetic Rat Model of Depression. Front. Cell. Neurosci. 2017, 11, 309. [Google Scholar] [CrossRef] [Green Version]
- Lüscher, C.; Jan, L.Y.; Stoffel, M.; Malenka, R.C.; Nicoll, R.A. G Protein-Coupled Inwardly Rectifying K+ Channels (GIRKs) Mediate Postsynaptic but Not Presynaptic Transmitter Actions in Hippocampal Neurons. Neuron 1997, 19, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Borroto-Escuela, D.O.; Fuxe, K. Diversity and Bias through Dopamine D2R Heteroreceptor Complexes. Curr. Opin. Pharmacol. 2017, 32, 16–22. [Google Scholar] [CrossRef]
- Tatemoto, K.; Rökaeus, Å.; Jörnvall, H.; McDonald, T.J.; Mutt, V. Galanin—A Novel Biologically Active Peptide from Porcine Intestine. FEBS Lett. 1983, 164, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Branchek, T.A.; Smith, K.E.; Gerald, C.; Walker, M.W. Galanin Receptor Subtypes. Trends Pharmacol. Sci. 2000, 21, 109–117. [Google Scholar] [CrossRef]
- Mitsukawa, K.; Lu, X.; Bartfai, T. Galanin, Galanin Receptors and Drug Targets. Cell. Mol. Life Sci. 2008, 65, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Andersson, K.; Locatelli, V.; Mutt, V.; Lundberg, J.; Hökfelt, T.; Agnati, L.F.; Eneroth, P.; Bolme, P. Neuropeptides and Central Catecholamine Systems: Interactions in Neuroendocrine and Central Cardiovascular Regulation. Adv. Biochem. Psychopharmacol. 1980, 22, 37–50. [Google Scholar]
- Lu, X.; Barr, A.M.; Kinney, J.W.; Sanna, P.; Conti, B.; Behrens, M.M.; Bartfai, T. A Role for Galanin in Antidepressant Actions with a Focus on the Dorsal Raphe Nucleus. Proc. Natl. Acad. Sci. USA 2005, 102, 874–879. [Google Scholar] [CrossRef] [Green Version]
- Millón, C.; Flores-Burgess, A.; Narváez, M.; Borroto-Escuela, D.O.; Santín, L.; Parrado, C.; Narváez, J.A.; Fuxe, K.; Díaz-Cabiale, Z. A Role for Galanin N-Terminal Fragment (1-15) in Anxiety-and Depression-Related Behaviors in Rats. Int. J. Neuropsychopharmacol. 2015, 18, pyu064. [Google Scholar] [CrossRef] [Green Version]
- Millón, C.; Flores-Burgess, A.; Narváez, M.; Borroto-Escuela, D.O.; Santín, L.; Gago, B.; Narváez, J.A.; Fuxe, K.; Díaz-Cabiale, Z. Galanin (1–15) Enhances the Antidepressant Effects of the 5-HT1A Receptor Agonist 8-OH-DPAT: Involvement of the Raphe-Hippocampal 5-HT Neuron System. Brain Struct. Funct. 2016, 221, 4491–4504. [Google Scholar] [CrossRef]
- Juhasz, G.; Hullam, G.; Eszlari, N.; Gonda, X.; Antal, P.; Anderson, I.M.; Hökfelt, T.G.M.; Deakin, J.F.W.; Bagdy, G. Brain Galanin System Genes Interact with Life Stresses in Depression-Related Phenotypes. Proc. Natl. Acad. Sci. USA 2014, 111, E1666–E1673. [Google Scholar] [CrossRef] [Green Version]
- Swanson, C.J.; Blackburn, T.P.; Zhang, X.; Zheng, K.; David Xu, Z.-Q.; Hökfelt, T.; Wolinsky, T.D.; Konkel, M.J.; Chen, H.; Zhong, H.; et al. Anxiolytic-and Antidepressant-like Profiles of the Galanin-3 Receptor (Gal 3) Antagonists SNAP 37889 and SNAP 398299. Proc. Natl. Acad. Sci. USA 2005, 29, 17489–17494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Burgess, A.; Millón, C.; Gago, B.; Narváez, J.A.; Fuxe, K.; Díaz-Cabiale, Z. Small Interference RNA Knockdown Rats in Behavior. In Neuromethods; Wolfgang, W., Ed.; Springer Nature: Basel, Switzerland, 2018; pp. 133–148. ISBN 9781493989447. [Google Scholar]
- Kehr, J.; Yoshitake, T.; Wang, F.H.; Razani, H.; Gimenez-Llort, L.; Jansson, A.; Yamaguchi, M.; Ögren, S.O. Galanin Is a Potent in Vivo Modulator of Mesencephalic Serotonergic Neurotransmission. Neuropsychopharmacology 2002, 27, 341–356. [Google Scholar] [CrossRef] [Green Version]
- Jacobowitz, D.M.; Kresse, A.; Skofitsch, G. Galanin in the Brain: Chemoarchitectonics and Brain Cartography—A Historical Review. Peptides 2004, 25, 433–464. [Google Scholar] [CrossRef] [PubMed]
- Skofitsch, G.; Jacobowitz, D.M. Immunohistochemical Mapping of Galanin-like Neurons in the Rat Central Nervous System. Peptides 1985, 6, 509–546. [Google Scholar] [CrossRef]
- Skofitsch, G.; Sills, M.A.; Jacobowitz, D.M.; Sills, M.A.; Jacobowitz, D.M. Autoradiographic Distribution of 125I-Galanin Binding Sites in the Rat Central Nervous System. Peptides 1986, 7, 1029–1042. [Google Scholar] [CrossRef]
- Melander, T.; Hökfelt, T.; Rökaeus, Å.; Cuello, A.C.; Oertel, W.H.; Verhofstad, A.; Goldstein, M. Coexistence of Galanin-like Immunoreactivity with Catecholamines, 5-Hydroxytryptamine, GABA and Neuropeptides in the Rat CNS. J. Neurosci. 1986, 6, 3640–3654. [Google Scholar] [CrossRef] [Green Version]
- Magara, S.; Holst, S.; Lundberg, S.; Roman, E.; Lindskog, M. Altered Explorative Strategies and Reactive Coping Style in the FSL Rat Model of Depression. Front. Behav. Neurosci. 2015, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Kuteeva, E.; Wardi, T.; Lundström, L.; Sollenberg, U.; Langel, Ü.; Hökfelt, T.; Ögren, S.O. Differential Role of Galanin Receptors in the Regulation of Depression-like Behavior and Monoamine/Stress-Related Genes at the Cell Body Level. Neuropsychopharmacology 2008, 33, 2573–2585. [Google Scholar] [CrossRef]
- Kuteeva, E.; Wardi, T.; Hökfelt, T.; Ögren, S.O. Galanin Enhances and a Galanin Antagonist Attenuates Depression-like Behaviour in the Rat. Eur. Neuropsychopharmacol. 2007, 17, 64–69. [Google Scholar] [CrossRef]
- Fuxe, K.; Borroto-Escuela, D.O.; Romero-Fernandez, W.; Tarakanov, A.O.; Calvo, F.; Garriga, P.; Tena, M.; Narvaez, M.; Millón, C.; Parrado, C.; et al. On the Existence and Function of Galanin Receptor Heteromers in the Central Nervous System. Front. Endocrinol. 2012, 3, 127. [Google Scholar] [CrossRef] [Green Version]
- Lundström, L.; Elmquist, A.; Bartfai, T.; Langel, Ü. Galanin and Its Receptors in Neurological Disorders. Neuromol. Med. 2005, 7, 157–180. [Google Scholar] [CrossRef]
- Kuteeva, E.; Hökfelt, T.; Wardi, T.; Ögren, S.O. Galanin, Galanin Receptor Subtypes and Depression-like Behaviour. Cell. Mol. Life Sci. 2008, 65, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Saar, I.; Runesson, J.; Järv, J.; Kurrikoff, K.; Langel, Ü. Novel Galanin Receptor Subtype Specific Ligand in Depression like Behavior. Neurochem. Res. 2013, 38, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Saar, I.; Lahe, J.; Langel, K.; Runesson, J.; Webling, K.; Järv, J.; Rytkönen, J.; Närvänen, A.; Bartfai, T.; Kurrikoff, K.; et al. Novel Systemically Active Galanin Receptor 2 Ligands in Depression-like Behavior. J. Neurochem. 2013, 127, 114–123. [Google Scholar] [CrossRef]
- Wardi Le Maître, T.; Xia, S.; Le Maitre, E.; Dun, X.P.; Lu, J.; Theodorsson, E.; Ögren, S.O.; Hökfelt, T.; Xu, Z.Q.D. Galanin Receptor 2 Overexpressing Mice Display an Antidepressive-like Phenotype: Possible Involvement of the Subiculum. Neuroscience 2011, 190, 270–288. [Google Scholar] [CrossRef]
- Brunner, S.M.; Farzi, A.; Locker, F.; Holub, B.S.; Drexel, M.; Reichmann, F.; Lang, A.A.; Mayr, J.A.; Vilches, J.J.; Navarro, X.; et al. GAL3 Receptor KO Mice Exhibit an Anxietylike Phenotype. Proc. Natl. Acad. Sci. USA 2014, 111, 7138–7143. [Google Scholar] [CrossRef] [Green Version]
- Hedlund, P.; von Euler, G.; Fuxe, K. Activation of 5-Hydroxytryptamine1A Receptors Increases the Affinity of Galanin Receptors in Di- and Telencephalic Areas of the Rat. Brain Res. 1991, 560, 251–259. [Google Scholar] [CrossRef]
- García-Durán, L.; Flores-Burgess, A.; Cantero-García, N.; Puigcerver, A.; Narváez, J.Á.; Fuxe, K.; Santín, L.; Millón, C.; Díaz-Cabiale, Z. Galanin(1-15) Potentiates the Antidepressant-like Effects Induced by Escitalopram in a Rat Model of Depression. Int. J. Mol. Sci. 2021, 22, 10848. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Brito, I.; Romero-Fernandez, W.; Di Palma, M.; Oflijan, J.; Skieterska, K.; Duchou, J.; Van Craenenbroeck, K.; Suárez-Boomgaard, D.; Rivera, A.; et al. The G Protein-Coupled Receptor Heterodimer Network (GPCR-HetNet) and Its Hub Components. Int. J. Mol. Sci. 2014, 15, 8570–8590. [Google Scholar] [CrossRef]
- Akhtar, S.; Benter, I.F. Nonviral Delivery of Synthetic SiRNAs in Vivo. J. Clin. Invest. 2007, 117, 3623–3632. [Google Scholar] [CrossRef]
- Kole, R.; Krainer, A.R.; Altman, S. RNA Therapeutics: Beyond RNA Interference and Antisense Oligonucleotides. Nat. Rev. Drug Discov. 2012, 11, 125–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millón, C.; Flores-Burgess, A.; Narváez, M.; Borroto-Escuela, D.O.; Gago, B.; Santín, L.; Castilla-Ortega, E.; Narváez, J.Á.; Fuxe, K.; Díaz-Cabiale, Z. The Neuropeptides Galanin and Galanin(1–15) in Depression-like Behaviours. Neuropeptides 2017, 64, 39–45. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Narváez, M.; Ambrogini, P.; Ferraro, L.; Brito, I.; Romero-Fernandez, W.; Andrade-Talavera, Y.; Flores-Burgess, A.; Millon, C.; Gago, B.; et al. Receptor-eceptor Interactions in Multiple 5-HT1A Heteroreceptor Complexes in Raphe-Hippocampal 5-HT Transmission and Their Relevance for Depression and Its Treatment. Molecules 2018, 23, 1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuxe, K.; Ferré, S.; Zoli, M.; Agnati, L.F. Integrated Events in Central Dopamine Transmission as Analyzed at Multiple Levels. Evidence for Intramembrane Adenosine A2A/Dopamine D2 and Adenosine A1/Dopamine D1 Receptor Interactions in the Basal Ganglia. Brain Res. Rev. 1998, 26, 258–273. [Google Scholar] [CrossRef]
- Fuxe, K.; von Euler, G.; Agnati, L.F.; Ögren, S.O. Galanin Selectively Modulates 5-Hydroxytryptamine 1A Receptors in the Rat Ventral Limbic Cortex. Neurosci. Lett. 1988, 85, 163–167. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Ambrogini, P.; Narvaez, M.; Di Liberto, V.; Beggiato, S.; Ferraro, L.; Fores-Pons, R.; Alvarez-Contino, J.E.; Lopez-Salas, A.; Mudò, G.; et al. Serotonin Heteroreceptor Complexes and Their Integration of Signals in Neurons and Astroglia—Relevance for Mental Diseases. Cells 2021, 10, 1902. [Google Scholar] [CrossRef]
- Chruścicka, B.; Wallace Fitzsimons, S.E.; Borroto-Escuela, D.O.; Druelle, C.; Stamou, P.; Nally, K.; Dinan, T.G.; Cryan, J.F.; Fuxe, K.; Schellekens, H. Attenuation of Oxytocin and Serotonin 2A Receptor Signaling through Novel Heteroreceptor Formation. ACS Chem. Neurosci. 2019, 10, 3225–3240. [Google Scholar] [CrossRef]
- Chruścicka, B.; Cowan, C.S.M.; Wallace Fitzsimons, S.E.; Borroto-Escuela, D.O.; Druelle, C.M.; Stamou, P.; Bergmann, C.A.; Dinan, T.G.; Slattery, D.A.; Fuxe, K.; et al. Molecular, Biochemical and Behavioural Evidence for a Novel Oxytocin Receptor and Serotonin 2C Receptor Heterocomplex. Neuropharmacology 2021, 183. [Google Scholar] [CrossRef] [PubMed]
- Dölen, G.; Darvishzadeh, A.; Huang, K.W.; Malenka, R.C. Social Reward Requires Coordinated Activity of Nucleus Accumbens Oxytocin and Serotonin. Nature 2013, 501, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Moutkine, I.; Quentin, E.; Guiard, B.P.; Maroteaux, L.; Doly, S. Heterodimers of Serotonin Receptor Subtypes 2 Are Driven by 5-HT2C Protomers. J. Biol. Chem. 2017, 292, 6352–6368. [Google Scholar] [CrossRef] [Green Version]
- Insel, T.R. The Challenge of Translation in Social Neuroscience: A Review of Oxytocin, Vasopressin, and Affiliative Behavior. Neuron 2010, 65, 768–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamal, M.; Gbahou, F.; Guillaume, J.L.; Daulat, A.M.; Benleulmi-Chaachoua, A.; Luka, M.; Chen, P.; Anaraki, D.K.; Baroncini, M.; La Cour, C.M.; et al. Convergence of Melatonin and Serotonin (5-HT) Signaling at MT2/5-HT2C Receptor Heteromers. J. Biol. Chem. 2015, 290, 11537–11546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochran, D.M.; Fallon, D.; Hill, M.; Frazier, J.A. The Role of Oxytocin in Psychiatric Disorders: A Review of Biological and Therapeutic Research Findings. Harv. Rev. Psychiatry 2013, 21, 219–247. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.; Alhassen, L.; Argelagos, A.; Alhassen, W.; Vachirakorntong, B.; Lin, Z.; Sanathara, N.; Alachkar, A. Mating and Parenting Experiences Sculpture Mood-Modulating Effects of Oxytocin-MCH Signaling. Sci. Rep. 2020, 10, 13611. [Google Scholar] [CrossRef]
- Scantamburlo, G.; Ansseau, M.; Geenen, V.; Legros, J.-J. Intranasal Oxytocin as an Adjunct to Escitalopram in Major Depression. J. Neuropsychiatry Clin. Neurosci. 2011, 23, E5. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Błasiak, E.; Szafran-Pilch, K.; Dziedzicka-Wasylewska, M. Dopamine D2 and Serotonin 5-HT1A Receptor Interaction in the Context of the Effects of Antipsychotics—In Vitro Studies. J. Neurochem. 2016, 137, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Shioda, N.; Imai, Y.; Yabuki, Y.; Sugimoto, W.; Yamaguchi, K.; Wang, Y.; Hikida, T.; Sasaoka, T.; Mieda, M.; Fukunaga, K. Dopamine D2L Receptor Deficiency Causes Stress Vulnerability through 5-HT1A Receptor Dysfunction in Serotonergic Neurons. J. Neurosci. 2019, 39, 7551–7563. [Google Scholar] [CrossRef] [Green Version]
- Lukasiewicz, S.; Faron-Górecka, A.; Dziedzicka-Wasylewska, M. A Biophysical Approach for the Study of Dopamine Receptor Oligomerization. Methods Mol. Biol. 2013, 964, 79–94. [Google Scholar] [CrossRef]
- Björklund, A.; Dunnett, S.B. Fifty Years of Dopamine Research. Trends Neurosci. 2007, 30, 185–187. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Schwarcz, R.; Fuxe, K.; Agnati, L.F.; Gustafsson, J.Å. Effects of Bromocriptine on 3H-Spiroperidol Binding Sites in Rat Striatum. Evidence for Actions of Dopamine Receptors Not Linked to Adenylate Cyclase. Life Sci. 1978, 23, 465–469. [Google Scholar] [CrossRef]
- Kebabian, J.W.; Calne, D.B. Multiple Receptors for Dopamine. Nature 1979, 277, 93–96. [Google Scholar] [CrossRef]
- Seeman, P.; Van Tol, H.H.M. Dopamine Receptor Pharmacology. Trends Pharmacol. Sci. 1994, 15, 264–270. [Google Scholar] [CrossRef]
- Gingrich, J.A.; Caron, M.G. Recent Advances in the Molecular Biology of Dopamine Receptors. Annu. Rev. Neurosci. 1993, 16, 299–321. [Google Scholar] [CrossRef] [PubMed]
- Randrup, A.; Munkvad, I.; Fog, R.; Gerlach, J.; Molander, L.; Kielberg, B.; Scheelkruger, J. Mania, Depression, and Brain Dopamine. In Current Developments in Psychopharmacology; Essman, W.B., Valzelli, L., Eds.; Spectrum Publications: New York, NY, USA, 1975; Volume 2, pp. 206–248. [Google Scholar]
- Mendels, J.; Frazer, A.; Fitzgerald, R.G.; Ramsey, T.A.; Stokes, J.W. Biogenic Amine Metabolites in Cerebrospinal Fluid of Depressed and Manic Patients. Science 1972, 175, 1380–1382. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Jong, J.; Linnoila, M. Cerebrospinal Fluid Monoamine Metabolites and Suicidal Behavior in Depressed Patients: A 5-Year Follow-up Study. Arch. Gen. Psychiatry 1989, 46, 609–612. [Google Scholar] [CrossRef]
- Van Praag, H.M.; Korf, J.; Lakke, J.P.W.F.; Schut, T. Dopamine Metabolism in Depressions, Psychoses, and Parkinson’s Disease: The Problem of the Specificity of Biological Variables in Behaviour Disorders. Psychol. Med. 1975, 5, 138–146. [Google Scholar] [CrossRef]
- Kapur, S.; John Mann, J. Role of the Dopaminergic System in Depression. Biol. Psychiatry 1992, 32, 1–17. [Google Scholar] [CrossRef]
- D’Aquila, P.S.; Collu, M.; Gessa, G.L.; Serra, G. The Role of Dopamine in the Mechanism of Action of Antidepressant Drugs. Eur. J. Pharmacol. 2000, 405, 365–373. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Nemeroff, C.B. The Role of Dopamine in the Pathophysiology of Depression. Arch. Gen. Psychiatry 2007, 64, 327–337. [Google Scholar] [CrossRef]
- Schildkraut, J.J.; Gordon, E.K.; Durell, J. Catecholamine Metabolism in Affective Disorders. I. Normetanephrine and VMA Excretion in Depressed Patients Treated with Imipramine. J. Psychiatr. Res. 1965, 3, 213–228. [Google Scholar] [CrossRef]
- Hirschfeld, R.M.A. History and Evolution of the Monoamine Hypothesis of Depression. J. Clin. Psychiatry 2000, 61, 4–6. [Google Scholar] [PubMed]
- Arbuthnott, G.W.; Crow, T.J.; Fuxe, K.; Olson, L.; Ungerstedt, U. Depletion of Catecholamines in Vivo Induced by Electrical Stimulation of Central Monoamine Pathways. Brain Res. 1970, 24, 471–483. [Google Scholar] [CrossRef]
- Bressan, R.A.; Crippa, J.A. The Role of Dopamine in Reward and Pleasure Behaviour—Review of Data from Preclinical Research. Acta Psychiatr. Scand. Suppl. 2005, 111, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef] [Green Version]
- Hasler, G.; Drevets, W.C.; Manji, H.K.; Charney, D.S. Discovering Endophenotypes for Major Depression. Neuropsychopharmacology 2004, 29, 1765–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perreault, M.L.; Hasbi, A.; O’dowd, B.F.; George, S.R. Heteromeric Dopamine Receptor Signaling Complexes: Emerging Neurobiology and Disease Relevance. Neuropsychopharmacology 2014, 39, 156–168. [Google Scholar] [CrossRef] [Green Version]
- Misganaw, D. Heteromerization of Dopaminergic Receptors in the Brain: Pharmacological Implications. Pharmacol. Res. 2021, 170, 105600. [Google Scholar] [CrossRef]
- Hasbi, A.; O’Dowd, B.F.; George, S.R. Dopamine D1-D2 Receptor Heteromer Signaling Pathway in the Brain: Emerging Physiological Relevance. Mol. Brain 2011, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Hasbi, A.; Nguyen, T.; Rahal, H.; Manduca, J.D.; Miksys, S.; Tyndale, R.F.; Madras, B.K.; Perreault, M.L.; George, S.R. Sex Difference in Dopamine D1-D2 Receptor Complex Expression and Signaling Affects Depression-and Anxiety-like Behaviors. Biol. Sex Differ. 2020, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Heyl, D.L.; Champion, M.; Muterspaugh, R.; Connolly, M.; Baraka, A.; Khazaei, P.; Moe, B.; Al-Sheemary, Z.; Jaber, N.; Guy-Evans, H. Characterizing the Binding of Dopamine D1-D2 Receptors in Vitro and in Temporal and Frontal Lobe Tissue Total Protein. FEBS Lett. 2019, 593, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Corrales, E.; Navarro, A.; Cuenca, P.; Campos, D. Candidate Gene Study Reveals DRD1 and DRD2 as Putative Interacting Risk Factors for Youth Depression. Psychiatry Res. 2016, 244, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P. Brain Dopamine Receptors. Pharmacol. Rev. 1980, 32, 229–313. [Google Scholar]
- Lee, S.P.; So, C.H.; Rashid, A.J.; Varghese, G.; Cheng, R.; Lança, A.J.; O’Dowd, B.F.; George, S.R. Dopamine D1 and D2 Receptor Co-Activation Generates a Novel Phospholipase C-Mediated Calcium Signal. J. Biol. Chem. 2004, 279, 35671–35678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, A.J.; So, C.H.; Kong, M.M.C.; Furtak, T.; El-Ghundi, M.; Cheng, R.; O’Dowd, B.F.; George, S.R. D1-D2 Dopamine Receptor Heterooligomers with Unique Pharmacology Are Coupled to Rapid Activation of Gq/11 in the Striatum. Proc. Natl. Acad. Sci. USA 2007, 104, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasbi, A.; Fan, T.; Alijaniaram, M.; Nguyen, T.; Perreault, M.L.; O’Dowd, B.F.; George, S.R. Calcium Signaling Cascade Links Dopamine D1-D2 Receptor Heteromer to Striatal BDNF Production and Neuronal Growth. Proc. Natl. Acad. Sci. USA 2009, 106, 21377–21382. [Google Scholar] [CrossRef] [Green Version]
- Friedman, A.K.; Walsh, J.J.; Juarez, B.; Ku, S.M.; Chaudhury, D.; Wang, J.; Li, X.; Dietz, D.M.; Pan, N.; Vialou, V.F.; et al. Enhancing Depression Mechanisms in Midbrain Dopamine Neurons Achieves Homeostatic Resilience. Science 2014, 344, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Nestler, E.J.; Carlezon, W.A. The Mesolimbic Dopamine Reward Circuit in Depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- Tye, K.M.; Mirzabekov, J.J.; Warden, M.R.; Ferenczi, E.A.; Tsai, H.C.; Finkelstein, J.; Kim, S.Y.; Adhikari, A.; Thompson, K.R.; Andalman, A.S.; et al. Dopamine Neurons Modulate Neural Encoding and Expression of Depression-Related Behaviour. Nature 2013, 493, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Pei, L.; Li, S.; Wang, M.; Diwan, M.; Anisman, H.; Fletcher, P.J.; Nobrega, J.N.; Liu, F. Uncoupling the Dopamine D1-D2 Receptor Complex Exerts Antidepressant-like Effects. Nat. Med. 2010, 16, 1393–1395. [Google Scholar] [CrossRef]
- Shen, M.Y.F.; Perreault, M.L.; Bambico, F.R.; Jones-Tabah, J.; Cheung, M.; Fan, T.; Nobrega, J.N.; George, S.R. Rapid Anti-Depressant and Anxiolytic Actions Following Dopamine D1-D2 Receptor Heteromer Inactivation. Eur. Neuropsychopharmacol. 2015, 25, 2437–2448. [Google Scholar] [CrossRef]
- Duman, R.S. Pathophysiology of Depression and Innovative Treatments: Remodeling Glutamatergic Synaptic Connections. Dialogues Clin. Neurosci. 2014, 16, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Eisch, A.J.; Bolaños, C.A.; De Wit, J.; Simonak, R.D.; Pudiak, C.M.; Barrot, M.; Verhaagen, J.; Nestler, E.J. Brain-Derived Neurotrophic Factor in the Ventral Midbrain-Nucleus Accumbens Pathway: A Role in Depression. Biol. Psychiatry 2003, 54, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K.; Carlquist, M.; Mutt, V. Neuropeptide Y—A Novel Brain Peptide with Structural Similarities to Peptide YY and Pancreatic Polypeptide. Nature 1982, 296, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Sajdyk, T.J.; Shekhar, A.; Gehlert, D.R. Interactions between NPY and CRF in the Amygdala to Regulate Emotionality. Neuropeptides 2004, 38, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sperk, G. Changes in GABAA Receptors in Status Epilepticus. Epilepsia 2007, 48, 11–13. [Google Scholar] [CrossRef]
- Dumont, Y.; Fournies, A.; St-Pierre, S.; Quirion, R. Autoradiographic Distribution of [125I]Leu31,Pro34]PYY and [125I]PYY3-36 Binding Sites in the Rat Brain Evaluated with Two Newly Developed Y1 and Y2 Receptor Radioligands. Synapse 1996, 2, 139–158. [Google Scholar] [CrossRef]
- Paredes, M.F.; Greenwood, J.; Baraban, S.C. Neuropeptide Y Modulates a G Protein-Coupled Inwardly Rectifying Potassium Current in the Mouse Hippocampus. Neurosci. Lett. 2003, 340, 9–12. [Google Scholar] [CrossRef]
- Mathé, A.A.; Jimenez, P.A.; Theodorsson, E.; Stenfors, C. Neuropeptide Y, Neurokinin A and Neurotensin in Brain Regions of Fawn Hooded “Depressed”, Wistar, and Sprague Dawley Rats. Effects of Electroconvulsive Stimuli. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1998, 22, 529–546. [Google Scholar] [CrossRef]
- Jiménez-Vasquez, P.A.; Diaz-Cabiale, Z.; Caberlotto, L.; Bellido, I.; Overstreet, D.; Fuxe, K.; Mathé, A.A. Electroconvulsive Stimuli Selectively Affect Behavior and Neuropeptide Y (NPY) and NPY Y1 Receptor Gene Expressions in Hippocampus and Hypothalamus of Flinders Sensitive Line Rat Model of Depression. Eur. Neuropsychopharmacol. 2007, 17, 298–308. [Google Scholar] [CrossRef]
- Catena-Dell’Osso, M.; Fagiolini, A.; Marazziti, D.; Baroni, S.; Bellantuono, C. Non-Monoaminergic Targets for the Development of Antidepressants: Focus on Neuropeptides. Mini Rev. Med. Chem. 2013, 13, 2–10. [Google Scholar] [CrossRef]
- Redrobe, J.P.; Dumont, Y.; Fournier, A.; Quirion, R. The Neuropeptide Y (NPY) Y1 Receptor Subtype Mediates NPY-Induced Antidepressant-like Activity in the Mouse Forced Swimming Test. Neuropsychopharmacology 2002, 26, 615–624. [Google Scholar] [CrossRef]
- Stogner, K.A.; Holmes, P.V. Neuropeptide-Y Exerts Antidepressant-like Effects in the Forced Swim Test in Rats. Eur. J. Pharmacol. 2000, 387, 9–10. [Google Scholar] [CrossRef]
- Narváez, M.; Borroto-Escuela, D.O.; Millón, C.; Gago, B.; Flores-Burgess, A.; Santín, L.; Fuxe, K.; Narváez, J.A.; Díaz-Cabiale, Z. Galanin Receptor 2-Neuropeptide Y Y1 Receptor Interactions in the Dentate Gyrus Are Related with Antidepressant-like Effects. Brain Struct. Funct. 2016, 221, 4129–4139. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Pita-Rodriguez, M.; Fores-Pons, R.; Barbancho, M.A.; Fuxe, K.; Narváez, M. Galanin and Neuropeptide Y Interactions Elicit Antidepressant Activity Linked to Neuronal Precursor Cells of the Dentate Gyrus in the Ventral Hippocampus. J. Cell. Physiol. 2020, 236, 3565–3578. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Karjalainen, T.; Isojärvi, J.; Kantonen, T.; Tuisku, J.; Kaasinen, V.; Joutsa, J.; Nuutila, P.; Kalliokoski, K.; Hirvonen, J.; et al. Lowered Endogenous Mu-Opioid Receptor Availability in Subclinical Depression and Anxiety. Neuropsychopharmacology 2020, 45, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Browne, C.A.; Lucki, I. Targeting Opioid Dysregulation in Depression for the Development of Novel Therapeutics. Pharmacol. Ther. 2019, 201, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, A.; Inui, A.; Momose, K.; Ueno, N.; Fujino, M.; Kasuga, M. Endomorphins Have Orexigenic and Anxiolytic Activities in Mice. Neuroreport 1998, 9, 2265–2267. [Google Scholar] [CrossRef]
- Calenco-Choukroun, G.; Daugé, V.; Gacel, G.; Féger, J.; Roques, B.P. Opioid δ Agonists and Endogenous Enkephalins Induce Different Emotional Reactivity than μ Agonists after Injection in the Rat Ventral Tegmental Area. Psychopharmacology 1991, 103, 493–502. [Google Scholar] [CrossRef]
- Beardsley, P.M.; Howard, J.L.; Shelton, K.L.; Carroll, F.I. Differential Effects of the Novel Kappa Opioid Receptor Antagonist, JDTic, on Reinstatement of Cocaine-Seeking Induced by Footshock Stressors vs Cocaine Primes and Its Antidepressant-like Effects in Rats. Psychopharmacology 2005, 183, 118–126. [Google Scholar] [CrossRef]
- Huang, P.; Tunis, J.; Parry, C.; Tallarida, R.; Liu-Chen, L.Y. Synergistic Antidepressant-like Effects between a Kappa Opioid Antagonist (LY2444296) and a Delta Opioid Agonist (ADL5859) in the Mouse Forced Swim Test. Eur. J. Pharmacol. 2016, 781, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenza, M.; Butelman, E.R.; Kreek, M.J. Effects of the Novel Relatively Short-Acting Kappa Opioid Receptor Antagonist LY2444296 in Behaviors Observed after Chronic Extended-Access Cocaine Self-Administration in Rats. Psychopharmacology 2017, 234, 2219–2231. [Google Scholar] [CrossRef] [PubMed]
- Filliol, D.; Ghozland, S.; Chluba, J.; Martin, M.; Matthes, H.W.D.; Simonin, F.; Befort, K.; Gavériaux-Ruff, C.; Dierich, A.; LeMeur, M.; et al. Mice Deficient for δ- and μ-Opioid Receptors Exhibit Opposing Alterations of Emotional Responses. Nat. Genet. 2000, 25, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Jutkiewicz, E.M.; Traynor, J.R.; Woods, J.H.; Rice, K.C. Separation of the Convulsions and Antidepressant-like Effects Produced by the Delta-Opioid Agonist SNC80 in Rats. Psychopharmacology 2005, 182, 588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, I.; Sierra, S.; Lueptow, L.; Gupta, A.; Gouty, S.; Margolis, E.B.; Cox, B.M.; Devi, L.A. Biased Signaling by Endogenous Opioid Peptides. Proc. Natl. Acad. Sci. USA 2020, 117, 11820–11828. [Google Scholar] [CrossRef]
- George, S.R.; Fan, T.; Xie, Z.; Tse, R.; Tam, V.; Varghese, G.; O’Dowd, B.F. Oligomerization of μ- and δ-Opioid Receptors: Generation of Novel Functional Properties. J. Biol. Chem. 2000, 275, 26128–26135. [Google Scholar] [CrossRef] [Green Version]
- Gomes, I.; Gupta, A.; Filipovska, J.; Szeto, H.H.; Pintar, J.E.; Devi, L.A. A Role for Heterodimerization of μ and δ Opiate Receptors in Enhancing Morphine Analgesia. Proc. Natl. Acad. Sci. USA 2004, 101, 5135–5139. [Google Scholar] [CrossRef] [Green Version]
- Kabli, N.; Martin, N.; Fan, T.; Nguyen, T.; Hasbi, A.; Balboni, G.; O’dowd, B.F.; George, S.R. Agonists at the δ-Opioid Receptor Modify the Binding of μ-Receptor Agonists to the μ-δ Receptor Hetero-Oligomer. Br. J. Pharmacol. 2010, 161, 1122–1136. [Google Scholar] [CrossRef] [Green Version]
- Erbs, E.; Faget, L.; Veinante, P.; Kieffer, B.L.; Medicine, T. In Vivo Neuronal Co-Expression of Mu and Delta Opioid Receptors Uncovers New Therapeutic Perspectives. Recept. Clin. Investig. 2014, 1, 210. [Google Scholar] [CrossRef]
- Jordan, B.A.; Gomes, I.; Rios, C.; Filipovska, J.; Devi, L.A. Functional Interactions between μ Opioid and α 2A-Adrenergic Receptors. Mol. Pharmacol. 2003, 64, 1317–1324. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Limbird, L.E. Hetero-Oligomers of α 2A-Adrenergic and µ-Opioid Receptors Do Not Lead to Transactivation of G-Proteins or Altered Endocytosis Profiles. Biochem Soc. Trans. 2004, 32, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Vilardaga, J.-P.; Nikolaev, V.; Lorenz, K.; Ferrandon, S.; Lohse, M.J. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. FASEB J. 2008, 22, 126–131. [Google Scholar] [CrossRef]
- Jordan, B.A.; Trapaidze, N.; Gomes, I.; Nivarthi, R.; Devi, L.A. Oligomerization of Opioid Receptors with B2-Adrenergic Receptors: A Role in Trafficking and Mitogen-Activated Protein Kinase Activation. Proc. Natl. Acad. Sci. USA 2001, 98, 343–348. [Google Scholar] [CrossRef]
- McVey, M.; Ramsay, D.; Kellett, E.; Rees, S.; Wilson, S.; Pope, A.J.; Milligan, G. Monitoring Receptor Oligomerization Using Time-Resolved Fluorescence Resonance Energy Transfer and Bioluminescence Resonance Energy Transfer: The Human δ-Opioid Receptor Displays Constitutive Oligomerization at the Cell Surface, Which Is Not Regulated By. J. Biol. Chem. 2001, 276, 14092–14099. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, D.; Kellett, E.; Mcvey, M.; Rees, S.; Milligan, G. Homo-and Hetero-Oligomeric Interactions between G-Protein-Coupled Receptors in Living Cells Monitored by Two Variants of Bioluminescence Resonance Energy Transfer (BRET): Hetero-Oligomers between Receptor Subtypes Form More Efficiently than between Less Closely Related Sequences. Biochem. J. 2002, 365, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Cussac, D.; Rauly-Lestienne, I.; Heusler, P.; Finana, F.; Cathala, C.; Bernois, S.; De Vries, L. μ-Opioid and 5-HT1A Receptors Heterodimerize and Show Signalling Crosstalk via G Protein and MAP-Kinase Pathways. Cell. Signal. 2012, 24, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gimenez, J.F.; Vilaró, M.T.; Milligan, G. Morphine Desensitization, Internalization, and down-Regulation of the μ Opioid Receptor Is Facilitated by Serotonin 5-Hydroxytryptamine2A Receptor Coactivation. Mol. Pharmacol. 2008, 74, 1278–1291. [Google Scholar] [CrossRef] [Green Version]
- Vasudevan, L.; Borroto-Escuela, D.O.; Huysentruyt, J.; Fuxe, K.; Saini, D.K.; Stove, C. Heterodimerization of MU Opioid Receptor Protomer with Dopamine D2 Receptor Modulates Agonist- Induced Internalization of MU Opioid Receptor. Biomolecules 2019, 9, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, M.; Vasudevan, L.; Huysentruyt, J.; Risseeuw, M.D.P.; Stove, C.; Vanderheyden, P.M.L.; Van Craenenbroeck, K.; Van Calenbergh, S. Design, Synthesis, and Biological Evaluation of Bivalent Ligands Targeting Dopamine D2-Like Receptors and the μ-Opioid Receptor. ChemMedChem 2018, 13, 944–956. [Google Scholar] [CrossRef] [Green Version]
- Prinster, S.C.; Hague, C.; Hall, R.A. Heterodimerization of G Protein-Coupled Receptors: Specificity and Functional Significance. Pharmacol. Rev. 2005, 57, 289–298. [Google Scholar] [CrossRef]
- Ugur, M.; Derouiche, L.; Massotte, D. Heteromerization Modulates Mu Opioid Receptor Functional Properties in Vivo. Front. Pharmacol. 2018, 9, 1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torregrossa, M.M.; Jutkiewicz, E.M.; Mosberg, H.I.; Balboni, G.; Watson, S.J.; Woods, J.H. Peptidic Delta Opioid Receptor Agonists Produce Antidepressant-like Effects in the Forced Swim Test and Regulate BDNF MRNA Expression in Rats. Brain Res. 2006, 1069, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Kabli, N.; Nguyen, T.; Balboni, G.; O’Dowd, B.F.; George, S.R. Antidepressant-like and Anxiolytic-like Effects Following Activation of the μ-δ Opioid Receptor Heteromer in the Nucleus Accumbens. Mol. Psychiatry 2014, 19, 986–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bewernick, B.H.; Hurlemann, R.; Matusch, A.; Kayser, S.; Grubert, C.; Hadrysiewicz, B.; Axmacher, N.; Lemke, M.; Cooper-Mahkorn, D.; Cohen, M.X.; et al. Nucleus Accumbens Deep Brain Stimulation Decreases Ratings of Depression and Anxiety in Treatment-Resistant Depression. Biol. Psychiatry 2010, 67, 110–116. [Google Scholar] [CrossRef]
- Alexander, B.; Warner-Schmidt, J.; Eriksson, T.; Tamminga, C.; Arango-Lievano, M.; Ghose, S.; Vernov, M.; Stavarache, M.; Musatov, S.; Flajolet, M.; et al. Reversal of Depressed Behaviors in Mice by P11 Gene Therapy in the Nucleus Accumbens. Sci. Transl. Med. 2010, 2, 54ra76. [Google Scholar] [CrossRef] [Green Version]
- Borroto-Escuela, D.O.; Ambrogini, P.; Chruścicka, B.; Lindskog, M.; Crespo-Ramirez, M.; Hernández-Mondragón, J.C.; Pérez de la Mora, M.; Schellekens, H.; Fuxe, K. The Role of Central Serotonin Neurons and 5-HT Heteroreceptor Complexes in the Pathophysiology of Depression: A Historical Perspective and Future Prospects. Int. J. Mol. Sci. 2021, 22, 1927. [Google Scholar] [CrossRef]
- Parks, C.L.; Robinson, P.S.; Sibille, E.; Shenk, T.; Toth, M. Increased Anxiety of Mice Lacking the Serotonin1A Receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 10734–10739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisstaub, N.V.; Zhou, M.; Lira, A.; Lambe, E.; González-Maeso, J.; Hornung, J.P.; Sibille, E.; Underwood, M.; Itohara, S.; Dauer, W.T.; et al. Cortical 5-HT2A Receptor Signaling Modulates Anxiety-like Behaviors in Mice. Science 2006, 313, 536–540. [Google Scholar] [CrossRef] [Green Version]
- De Matos Feijó, F.; Bertoluci, M.C.; Reis, C. Serotonin and Hypothalamic Control of Hunger: A Review. Rev. Assoc. Med. Bras. 2011, 57, 74–77. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Kim, J.J.; Young, S.L.; Calcagnetti, D.J.; DeCola, J.P.; Helmstetter, F.J.; Landeira-Fernandez, J. Differential Effects of Selective Opioid Peptide Antagonists on the Acquisition of Pavlovian Fear Conditioning. Peptides 1991, 12, 1033–1037. [Google Scholar] [CrossRef] [Green Version]
- Szczytkowski-Thomson, J.L.; Lebonville, C.L.; Lysle, D.T. Morphine Prevents the Development of Stress-Enhanced Fear Learning. Pharmacol. Biochem. Behav. 2013, 103, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, K.; Korostynski, M.; Cieslak, P.E.; Wawrzczak-Bargiela, A.; Przewlocki, R. Opioid-Dependent Regulation of High and Low Fear Responses in Two Inbred Mouse Strains. Behav. Brain Res. 2015, 292, 95–101. [Google Scholar] [CrossRef]
- Holbrook, T.L.; Galarneau, M.R.; Dye, J.L.; Quinn, K.; Dougherty, A.L. Morphine Use after Combat Injury in Iraq and Post-Traumatic Stress Disorder. N. Engl. J. Med. 2010, 362, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, S.; McNally, G.P. Complementary Roles for Amygdala and Periaqueductal Gray in Temporal-Difference Fear Learning. Learn. Mem. 2009, 16, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomares-Castillo, E.; Hernández-Pérez, O.R.; Pérez-Carrera, D.; Crespo-Ramírez, M.; Fuxe, K.; Pérez de la Mora, M. The Intercalated Paracapsular Islands as a Module for Integration of Signals Regulating Anxiety in the Amygdala. Brain Res. 2012, 1476, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Bruchas, M.R.; Land, B.B.; Lemos, J.C.; Chavkin, C. CRF1-R Activation of the Dynorphin/Kappa Opioid System in the Mouse Basolateral Amygdala Mediates Anxiety-like Behavior. PLoS ONE 2009, 4, e8528. [Google Scholar] [CrossRef] [Green Version]
- Knoll, A.T.; Meloni, E.G.; Thomas, J.B.; Carroll, F.I.; Carlezon, W.A. Anxiolytic-like Effects of κ-Opioid Receptor Antagonists in Models of Unlearned and Learned Fear in Rats. J. Pharmacol. Exp. Ther. 2007, 323, 838–845. [Google Scholar] [CrossRef]
- Knoll, A.T.; Muschamp, J.W.; Sillivan, S.E.; Ferguson, D.; Dietz, D.M.; Meloni, E.G.; Carroll, F.I.; Nestler, E.J.; Konradi, C.; Carlezon, W.A. Kappa Opioid Receptor Signaling in the Basolateral Amygdala Regulates Conditioned Fear and Anxiety in Rats. Biol. Psychiatry 2011, 70, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Browne, C.A.; Falcon, E.; Robinson, S.A.; Berton, O.; Lucki, I. Reversal of Stress-Induced Social Interaction Deficits by Buprenorphine. Int. J. Neuropsychopharmacol. 2018, 21, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Carr, G.V.; Lucki, I. Comparison of the Kappa-Opioid Receptor Antagonist DIPPA in Tests of Anxiety-like Behavior between Wistar Kyoto and Sprague Dawley Rats. Psychopharmacology 2010, 210, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Van’T Veer, A.; Bechtholt, A.J.; Onvani, S.; Potter, D.; Wang, Y.; Liu-Chen, L.Y.; Schütz, G.; Chartoff, E.H.; Rudolph, U.; Cohen, B.M.; et al. Ablation of Kappa-Opioid Receptors from Brain Dopamine Neurons Has Anxiolytic-like Effects and Enhances Cocaine-Induced Plasticity. Neuropsychopharmacology 2013, 38, 1585–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jutkiewicz, E.M.; Eller, E.B.; Folk, J.E.; Rice, K.C.; Traynor, J.R.; Woods, J.H. δ-Opioid Agonists: Differential Efficacy and Potency of SNC80, Its 3-OH (SNC86) and 3-Desoxy (SNC162) Derivatives in Sprague-Dawley Rats. J. Pharmacol. Exp. Ther. 2004, 309, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, A.; Kimura, Y.; Suzuki, T.; Kawai, K.; Nagase, H.; Kamei, J. Potential Anxiolytic and Antidepressant-Like Activities of SNC80, a Selective-Opioid Agonist, in Behavioral Models in Rodents. J. Pharmacol. Sci. 2004, 95, 374–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitoh, A.; Nagase, H. Delta Opioid Receptor (DOR) Ligands and Pharmacology: Development of Indolo- and Quinolinomorphinan Derivatives Based on the Message-Address Concept. Handb. Exp. Pharmacol. 2018, 247, 3–19. [Google Scholar] [PubMed]
- Perrine, S.A.; Hoshaw, B.A.; Unterwald, E.M. Delta Opioid Receptor Ligands Modulate Anxiety-like Behaviors in the Rat. Br. J. Pharmacol. 2006, 147, 864–872. [Google Scholar] [CrossRef]
- Roberts, A.J.; Gold, L.H.; Polis, I.; Mcdonald, J.S.; Filliol, D.; Kieffer, B.L.; Koob, G.F. Increased Ethanol Self-Administration in-Opioid Receptor Knockout Mice. Alcohol Clin. Exp. Res. 2001, 25, 1249–1256. [Google Scholar]
- Karlsson, R.M.; Holmes, A. Galanin as a Modulator of Anxiety and Depression and a Therapeutic Target for Affective Disease. Amino Acids 2006, 31, 231–239. [Google Scholar] [CrossRef]
- Bing, O.; Möller, C.; Engel, J.A.; Söderpalm, B.; Heilig, M. Anxiolytic-like Action of Centrally Administered Galanin. Neurosci. Lett. 1993, 164, 17–20. [Google Scholar] [CrossRef]
- Möller, C.; Sommer, W.; Thorsell, A.; Heilig, M. Anxiogenic-Like Action of Galanin after Intra-Amygdala Administration in the Rat. Neuropsychopharmocology 1999, 21, 507–512. [Google Scholar] [CrossRef]
- Bailey, K.R.; Pavlova, M.N.; Rohde, A.D.; Hohmann, J.G.; Crawley, J.N. Galanin Receptor Subtype 2 (GalR2) Null Mutant Mice Display an Anxiogenic-like Phenotype Specific to the Elevated plus-Maze. Pharmacol. Biochem. Behav. 2007, 86, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Khoshbouei, H.; Cecchi, M.; Dove, S.; Javors, M.; Morilak, D.A. Behavioral Reactivity to Stress: Amplification of Stress-Induced Noradrenergic Activation Elicits a Galanin-Mediated Anxiolytic Effect in Central Amygdala. Pharmacol. Biochem. Behav. 2002, 71, 407–417. [Google Scholar] [CrossRef]
- Barrera, G.; Echevarria, D.J.; Poulin, J.F.; Laforest, S.; Drolet, G.; Morilak, D.A. One for All or One for One: Does Co-Transmission Unify the Concept of a Brain Galanin “System” or Clarify Any Consistent Role in Anxiety? Neuropeptides 2005, 39, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.; Kinney, J.W.; Wrenn, C.C.; Li, Q.; Yang, R.J.; Ma, L.; Vishwanath, J.; Saavedra, M.C.; Innerfield, C.E.; Jacoby, A.S.; et al. Galanin GAL-RI Receptor Null Mutant Mice Display Increased Anxiety-like Behavior Specific to the Elevated plus-Maze. Neuropsychopharmacology 2003, 28, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Narvaez, M.; Di Palma, M.; Calvo, F.; Rodriguez, D.; Millon, C.; Carlsson, J.; Agnati, L.F.; Garriga, P.; Díaz-Cabiale, Z.; et al. Preferential Activation by Galanin 1-15 Fragment of the GalR1 Protomer of a GalR1-GalR2 Heteroreceptor Complex. Biochem. Biophys. Res. Commun. 2014, 452, 347–353. [Google Scholar] [CrossRef]
- Heilig, M.; McLeod, S.; Brot, M.; Heinrichs, S.C.; Menzaghi, F.; Koob, G.F.; Britton, K.T. Anxiolytic-like Action of Neuropeptide Y: Mediation by Y1 Receptors in Amygdala, and Dissociation from Food Intake Effects. Neuropsychopharmacology 1993, 8, 357–363. [Google Scholar] [CrossRef]
- Rotzinger, S.; Lovejoy, D.A.; Tan, L.A. Behavioral Effects of Neuropeptides in Rodent Models of Depression and Anxiety. Peptides 2010, 31, 736–756. [Google Scholar] [CrossRef]
- Narváez, M.; Borroto-Escuela, D.O.; Santín, L.; Millón, C.; Gago, B.; Flores-Burgess, A.; Barbancho, M.A.; Pérez de la Mora, M.; Narváez, J.A.; Díaz-Cabiale, Z.; et al. A Novel Integrative Mechanism in Anxiolytic Behavior Induced by Galanin 2/Neuropeptide Y Y1 Receptor Interactions on Medial Paracapsular Intercalated Amygdala in Rats. Front. Cell. Neurosci. 2018, 12, 119. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, R.M.; Choe, J.S.; Cameron, H.A.; Thorsell, A.; Crawley, J.N.; Holmes, A.; Heilig, M. The Neuropeptide Y Y1 Receptor Subtype Is Necessary for the Anxiolytic-like Effects of Neuropeptide Y, but Not the Antidepressant-like Effects of Fluoxetine, in Mice. Psychopharmacology 2008, 195, 547–557. [Google Scholar] [CrossRef]
- Lach, G.; de Lima, T.C.M. Role of NPY Y1 Receptor on Acquisition, Consolidation and Extinction on Contextual Fear Conditioning: Dissociation between Anxiety, Locomotion and Non-Emotional Memory Behavior. Neurobiol. Learn. Mem. 2013, 103, 26–33. [Google Scholar] [CrossRef]
- Royer, S.; Martina, M.; Paré, D. Polarized Synaptic Interactions between Intercalated Neurons of the Amygdala. J. Neurophysiol. 2000, 83, 3509–3518. [Google Scholar] [CrossRef]
- Gross, C.T.; Canteras, N.S. The Many Paths to Fear. Nat. Rev. Neurosci. 2012, 13, 651–658. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J. Rethinking the Emotional Brain. Neuron 2012, 73, 653–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezze, M.A.; Feldon, J. Mesolimbic Dopaminergic Pathways in Fear Conditioning. Prog. Neurobiol. 2004, 74, 301–320. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Mora, M.; Gallegos-Cari, A.; Arizmendi-García, Y.; Marcellino, D.; Fuxe, K. Role of Dopamine Receptor Mechanisms in the Amygdaloid Modulation of Fear and Anxiety: Structural and Functional Analysis. Prog. Neurobiol. 2010, 90, 198–216. [Google Scholar] [CrossRef]
- Zarrindast, M.R.; Khakpai, F. The Modulatory Role of Dopamine in Anxiety-like Behavior. Arch. Iran. Med. 2015, 18, 591–603. [Google Scholar]
- Pérez de la Mora, M.; Hernandez-Mondragon, C.; Crespo-Ramirez, M.; Rejon-Orantes, J.; Borroto-Escuela, D.O.; Fuxe, K. Conventional and Novel Pharmacological Approaches to Treat Dopamine-Related Disorders: Focus on Parkinson’s Disease and Schizophrenia. Neuroscience 2020, 439, 301–318. [Google Scholar] [CrossRef]
- Guarraci, F.A.; Frohardt, R.J.; Young, S.L.; Kapp, B.S. A Functional Role for Dopamine Transmission in the Amygdala during Condtioned Fear. Ann. N. Y. Acad. Sci. 1999, 877, 732–736. [Google Scholar] [CrossRef]
- Guarraci, F.A.; Frohardt, R.J.; Kapp, B.S. Amygdaloid D1 Dopamine Receptor Involvement in Pavlovian Fear Conditioning. Brain Res. 1999, 827, 28–40. [Google Scholar] [CrossRef]
- Lamont, E.W.; Kokkinidis, L. Infusion of the Dopamine D1 Receptor Antagonist SCH 23390 into the Amygdala Blocks Fear Expression in a Potentiated Startle Paradigm. Brain Res. 1998, 795, 128–136. [Google Scholar] [CrossRef]
- Nader, K.; LeDoux, J. The Dopaminergic Modulation of Fear: Quinpirole Impairs the Recall of Emotional Memories in Rats. Behav. Neurosci. 1999, 113, 152–165. [Google Scholar] [CrossRef]
- Pérez de la Mora, M.; Cárdenas-Cachón, L.; Vázquez-García, M.; Crespo-Ramírez, M.; Jacobsen, K.; Höistad, M.; Agnati, L.; Fuxe, K. Anxiolytic Effects of Intra-Amygdaloid Injection of the D1 Antagonist SCH23390 in the Rat. Neurosci. Lett. 2005, 377, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Guarraci, F.A.; Frohardt, R.J.; Falls, W.A.; Kapp, B.S. The Effects of Intra-Amygdaloid Infusions of a D2 Dopamine Receptor Antagonist on Pavlovian Fear Conditioning. Behav. Neurosci. 2000, 114, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Mora, M.; Gallegos-Cari, A.; Crespo-Ramirez, M.; Marcellino, D.; Hansson, A.C.; Fuxe, K. Distribution of Dopamine D2-like Receptors in the Rat Amygdala and Their Role in the Modulation of Unconditioned Fear and Anxiety. Neuroscience 2012, 201, 252–266. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. Emotion Circuits in the Brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J. The Amygdala. Curr. Biol. 2007, 17, 868–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, I.; Humeau, Y.; Grenier, F.; Ciocchi, S.; Herry, C.; Lüthi, A. Amygdala Inhibitory Circuits and the Control of Fear Memory. Neuron 2009, 62, 757–771. [Google Scholar] [CrossRef] [Green Version]
- Bodnoff, S.R.; Suranyi-Cadotte, B.; Quirion, R.; Meaney, M.J. A Comparison of the Effects of Diazepam versus Several Typical and Atypical Anti-Depressant Drugs in an Animal Model of Anxiety. Psychopharmacology 1989, 97, 277–279. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Neumann, I.D.; Landgraf, R. Balance of Brain Oxytocin and Vasopressin: Implications for Anxiety, Depression, and Social Behaviors. Trends Neurosci. 2012, 35, 649–659. [Google Scholar] [CrossRef]
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Cetin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked Axonal Oxytocin Release in the Central Amygdala Attenuates Fear Response. Neuron 2012, 73, 553–566. [Google Scholar] [CrossRef] [Green Version]
- Condés-Lara, M.; Veinante, P.; Rabai, M.; Freund-Mercier, M.J. Correlation between Oxytocin Neuronal Sensitivity and Oxytocin-Binding Sites in the Amygdala of the Rat: Electrophysiological and Histoautoradiographic Study. Brain Res. 1994, 637, 277–286. [Google Scholar] [CrossRef]
- Bale, T.L.; Davis, A.M.; Auger, A.P.; Dorsa, D.M.; McCarthy, M.M. CNS Region-Specific Oxytocin Receptor Expression: Importance in Regulation of Anxiety and Sex Behavior. J. Neurosci. 2001, 21, 2546–2552. [Google Scholar] [CrossRef] [PubMed]
- Viviani, D.; Charlet, A.; Van Den Burg, E.; Robinet, C.; Hurni, N.; Abatis, M.; Magara, F.; Stoop, R. Oxytocin Selectively Gates Fear Responses through Distinct Outputs from the Central Amygdala. Science 2011, 333, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Stoop, R. Neuromodulation by Oxytocin and Vasopressin. Neuron 2012, 76, 142–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blume, A.; Bosch, O.J.; Miklos, S.; Torner, L.; Wales, L.; Waldherr, M.; Neumann, I.D. Oxytocin Reduces Anxiety via ERK1/2 Activation: Local Effect within the Rat Hypothalamic Paraventricular Nucleus. Eur. J. Neurosci. 2008, 27, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- László, K.; Kovács, A.; Zagoracz, O.; Ollmann, T.; Péczely, L.; Kertes, E.; Lacy, D.G.; Lénárd, L. Positive Reinforcing Effect of Oxytocin Microinjection in the Rat Central Nucleus of Amygdala. Behav. Brain Res. 2016, 296, 279–285. [Google Scholar] [CrossRef]
- László, K.; Péczely, L.; Géczi, F.; Kovács, A.; Zagoracz, O.; Ollmann, T.; Kertes, E.; Kállai, V.; László, B.; Berta, B.; et al. The Role of D2 Dopamine Receptors in Oxytocin Induced Place Preference and Anxiolytic Effect. Horm. Behav. 2020, 124, 104777. [Google Scholar] [CrossRef]
- Romero-Fernandez, W.; Borroto-Escuela, D.O.; Agnati, L.F.; Fuxe, K. Evidence for the Existence of Dopamine D2-Oxytocin Receptor Heteromers in the Ventral and Dorsal Striatum with Facilitatory Receptor-Receptor Interactions. Mol. Psychiatry 2013, 18, 849–850. [Google Scholar] [CrossRef] [Green Version]
- Schellekens, H.; McNamara, O.; Dinan, T.G.; McCarthy, J.V.; McGlacken, G.P.; Cryan, J.F. Semagacestat, a γ-Secretase Inhibitor, Activates the Growth Hormone Secretagogue (GHS-R1a) Receptor. J. Pharm. Pharmacol. 2013, 65, 528–538. [Google Scholar] [CrossRef]
- Kojima, M.; Kangawa, K. Ghrelin: Structure and Function. Physiol. Rev. 2005, 85, 495–522. [Google Scholar] [CrossRef]
- Schellekens, H.; Finger, B.C.; Dinan, T.G.; Cryan, J.F. Ghrelin Signalling and Obesity: At the Interface of Stress, Mood and Food Reward. Pharmacol. Ther. 2012, 135, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Zigman, J.M.; Jones, J.E.; Lee, C.E.; Saper, C.B.; Elmquist, J.K. Expression of Ghrelin Receptor MRNA in the Rat and the Mouse Brain. J. Comp. Neurol. 2006, 494, 528–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, G.E.; Von Korff, M.; Saunders, K.; Miglioretti, D.L.; Crane, P.K.; Van Belle, G.; Kessler, R.C. Association between Obesity and Psychiatric Disorders in the US Adult Population. Arch. Gen. Psychiatry 2006, 63, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElroy, S.L.; Kotwal, R.; Malhotra, S.; Nelson, E.B.; Keck, P.E.; Nemeroff, C.B. Are Mood Disorders and Obesity Related? A Review for the Mental Health Professional. J. Clin. Psychiatry 2004, 65, 634–651. [Google Scholar] [CrossRef]
- Kloiber, S.; Ising, M.; Reppermund, S.; Horstmann, S.; Dose, T.; Majer, M.; Zihl, J.; Pfister, H.; Unschuld, P.G.; Holsboer, F.; et al. Overweight and Obesity Affect Treatment Response in Major Depression. Biol. Psychiatry 2007, 62, 321–326. [Google Scholar] [CrossRef]
- Marijnissen, R.M.; Bus, B.A.A.; Holewijn, S.; Franke, B.; Purandare, N.; De Graaf, J.; Den Heijer, M.; Buitelaar, J.K.; Oude Voshaar, R.C. Depressive Symptom Clusters Are Differentially Associated with General and Visceral Obesity. J. Am. Geriatr. Soc. 2011, 59, 67–72. [Google Scholar] [CrossRef]
- Gariepy, G.; Nitka, D.; Schmitz, N. The Association between Obesity and Anxiety Disorders in the Population: A Systematic Review and Meta-Analysis. Int. J. Obes. 2010, 34, 407–419. [Google Scholar] [CrossRef] [Green Version]
- Pallister, E.; Waller, G. Anxiety in the Eating Disorders: Understanding the Overlap. Clin. Psychol. Rev. 2008, 28, 366–386. [Google Scholar] [CrossRef]
- Rebolledo-Solleiro, D.; Roldán-Roldán, G.; Díaz, D.; Velasco, M.; Larqué, C.; Rico-Rosillo, G.; Vega-Robledo, G.B.; Zambrano, E.; Hiriart, M.; Pérez de la Mora, M. Increased Anxiety-like Behavior Is Associated with the Metabolic Syndrome in Non-Stressed Rats. PLoS ONE 2017, 12, e0176554. [Google Scholar] [CrossRef] [Green Version]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin Is a Growth-Hormone-Releasing Acylated Peptide from Stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The Distribution and Mechanism of Action of Ghrelin in the CNS Demonstrates a Novel Hypothalamic Circuit Regulating Energy Homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Delgado, M.; Ganea, D. Anti-Inflammatory Neuropeptides: A New Class of Endogenous Immunoregulatory Agents. Brain. Behav. Immun. 2008, 22, 1146–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abizaid, A.; Hougland, J.L. Ghrelin Signaling: GOAT and GHS-R1a Take a LEAP in Complexity. Trends Endocrinol. Metab. 2020, 31, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, H.; Van Oeffelen, W.E.P.A.; Dinan, T.G.; Cryan, J.F. Promiscuous Dimerization of the Growth Hormone Secretagogue Receptor (GHS-R1a) Attenuates Ghrelin-Mediated Signaling. J. Biol. Chem. 2013, 288, 181–191. [Google Scholar] [CrossRef] [Green Version]
- M’Kadmi, C.; Leyris, J.P.; Onfroy, L.; Galés, C.; Sauliére, A.; Gagne, D.; Damian, M.; Mary, S.; Maingot, M.; Denoyelle, S.; et al. Agonism, Antagonism, and Inverse Agonism Bias at the Ghrelin Receptor Signaling. J. Biol. Chem. 2015, 290, 27021–27039. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.M.; Yu, H.; Palyha, O.C.; McKee, K.K.; Feighner, S.D.; Sirinathsinghji, D.J.S.; Smith, R.G.; Van Der Ploeg, L.H.T.; Howard, A.D. Distribution of MRNA Encoding the Growth Hormone Secretagogue Receptor in Brain and Peripheral Tissues. Mol. Brain Res. 1997, 48, 23–29. [Google Scholar] [CrossRef]
- Alvarez-Crespo, M.; Skibicka, K.P.; Farkas, I.; Molnár, C.S.; Egecioglu, E.; Hrabovszky, E.; Liposits, Z.; Dickson, S.L. The Amygdala as a Neurobiological Target for Ghrelin in Rats: Neuroanatomical, Electrophysiological and Behavioral Evidence. PLoS ONE 2012, 7, e46321. [Google Scholar] [CrossRef]
- Perello, M.; Cabral, A.; Cornejo, M.P.; De Francesco, P.N.; Fernandez, G.; Uriarte, M. Brain Accessibility Delineates the Central Effects of Circulating Ghrelin. J. Neuroendocrinol. 2019, 31, e12677. [Google Scholar] [CrossRef]
- Kineman, R.D.; Gahete, M.D.; Luque, R.M. Identification of a Mouse Ghrelin Gene Transcript That Contains Intron 2 and Is Regulated in the Pituitary and Hypothalamus in Response to Metabolic Stress. J. Mol. Endocrinol. 2007, 38, 511–521. [Google Scholar] [CrossRef] [Green Version]
- Holst, B.; Cygankiewicz, A.; Jensen, T.H.; Ankersen, M.; Schwartz, T.W. High Constitutive Signaling of the Ghrelin Receptor—Identification of a Potent Inverse Agonist. Mol. Endocrinol. 2003, 17, 2201–2210. [Google Scholar] [CrossRef]
- Petersen, P.S.; Woldbye, D.P.D.; Madsen, A.N.; Egerod, K.L.; Jin, C.; Lang, M.; Rasmussen, M.; Beck-Sickinger, A.G.; Holst, B. In Vivo Characterization of High Basal Signaling from the Ghrelin Receptor. Endocrinology 2009, 150, 4920–4930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schellekens, H.; Dinan, T.G.; Cryan, J.F. Taking Two to Tango: A Role for Ghrelin Receptor Heterodimerization in Stress and Reward. Front. Neurosci. 2013, 7, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Betancourt, L.; Smith, R.G. Ghrelin Amplifies Dopamine Signaling by Cross Talk Involving Formation of Growth Hormone Secretagogue Receptor/Dopamine Receptor Subtype 1 Heterodimers. Mol. Endocrinol. 2006, 20, 1772–1785. [Google Scholar] [CrossRef] [PubMed]
- Kern, A.; Mavrikaki, M.; Ullrich, C.; Albarran-Zeckler, R.; Brantley, A.F.; Smith, R.G. Hippocampal Dopamine/DRD1 Signaling Dependent on the Ghrelin Receptor. Cell 2015, 163, 1176–1190. [Google Scholar] [CrossRef] [Green Version]
- Rediger, A.; Tarnow, P.; Bickenbach, A.; Schaefer, M.; Krude, H.; Grüters, A.; Biebermann, H. Heterodimerization of Hypothalamic G-Protein-Coupled Receptors Involved in Weight Regulation. Obes. Facts 2009, 2, 80–86. [Google Scholar] [CrossRef]
- Wallace Fitzsimons, S.E.; Chruścicka, B.; Druelle, C.; Stamou, P.; Nally, K.; Dinan, T.G.; Cryan, J.F.; Schellekens, H. A Ghrelin Receptor and Oxytocin Receptor Heterocomplex Impairs Oxytocin Mediated Signalling. Neuropharmacology 2019, 152, 90–101. [Google Scholar] [CrossRef]
- Dallman, M.F.; Pecoraro, N.; Akana, S.F.; La Fleur, S.E.; Gomez, F.; Houshyar, H.; Bell, M.E.; Bhatnagar, S.; Laugero, K.D.; Manalo, S. Chronic Stress and Obesity: A New View of “Comfort Food”. Proc. Natl. Acad. Sci. USA 2003, 100, 11696–11701. [Google Scholar] [CrossRef] [Green Version]
- Fritz, E.M.; Singewald, N.; De Bundel, D. The Good, the Bad and the Unknown Aspects of Ghrelin in Stress Coping and Stress-Related Psychiatric Disorders. Front. Synaptic Neurosci. 2020, 12, 594484. [Google Scholar] [CrossRef]
- Schellekens, H.; Dinan, T.G.; Cryan, J.F. Lean Mean Fat Reducing “Ghrelin” Machine: Hypothalamic Ghrelin and Ghrelin Receptors as Therapeutic Targets in Obesity. Neuropharmacology 2010, 58, 2–16. [Google Scholar] [CrossRef]
- Lutter, M.; Sakata, I.; Osborne-Lawrence, S.; Rovinsky, S.A.; Anderson, J.G.; Jung, S.; Birnbaum, S.; Yanagisawa, M.; Elmquist, J.K.; Nestler, E.J.; et al. The Orexigenic Hormone Ghrelin Defends against Depressive Symptoms of Chronic Stress. Nat. Neurosci. 2008, 11, 752–753. [Google Scholar] [CrossRef] [Green Version]
- Wise, R.A. Role of Brain Dopamine in Food Reward and Reinforcement. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1149–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Wang, G.J.; Baler, R.D. Reward, Dopamine and the Control of Food Intake: Implications for Obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.S.; Gershon, S. Dopamine and Depression. J. Neural Transm. Gen. Sect. 1993, 91, 75–109. [Google Scholar] [CrossRef] [PubMed]
- Valant, C.; Robert Lane, J.; Sexton, P.M.; Christopoulos, A. The Best of Both Worlds? Bitopic Orthosteric/Allosteric Ligands of G Protein-Coupled Receptors. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 153–178. [Google Scholar] [CrossRef]
- Ezkurdia, I.; Juan, D.; Rodriguez, J.M.; Frankish, A.; Diekhans, M.; Harrow, J.; Vazquez, J.; Valencia, A.; Tress, M.L. Multiple Evidence Strands Suggest That There May Be as Few as 19,000 Human Protein-Coding Genes. Hum. Mol. Genet. 2014, 23, 5866–5878. [Google Scholar] [CrossRef] [Green Version]
- Gloriam, D.E.; Fredriksson, R.; Schiöth, H.B. The G Protein-Coupled Receptor Subset of the Rat Genome. BMC Genom. 2007, 8, 338. [Google Scholar] [CrossRef] [Green Version]
- Alexander, S.P.H.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. The Concise Guide to Pharmacology 2019/20: G Protein-Coupled Receptors. Br. J. Pharmacol. 2019, 176, S21–S141. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Liu, J.; Shi, Q.; Tan, Q.; Wu, D.; Skinner, J.J.; Walker, A.L.; Zhao, L.; Gu, X.; Chen, N.; et al. In Vitro Expression and Analysis of the 826 Human G Protein-Coupled Receptors. Protein Cell 2016, 7, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Lagerström, M.C.; Schiöth, H.B. Structural Diversity of G Protein-Coupled Receptors and Significance for Drug Discovery. Nat. Rev. Drug Discov. 2008, 7, 339–357. [Google Scholar] [CrossRef]
- Drews, J. Genomic Sciences and the Medicine of Tomorrow. Nat. Biotechnol. 1996, 14, 1516–1518. [Google Scholar] [CrossRef]
- Smith, J.S.; Lefkowitz, R.J.; Rajagopal, S. Biased Signalling: From Simple Switches to Allosteric Microprocessors. Nat. Rev. Drug Discov. 2018, 17, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Wootten, D.; Christopoulos, A.; Marti-Solano, M.; Babu, M.M.; Sexton, P.M. Mechanisms of Signalling and Biased Agonism in G Protein-Coupled Receptors. Nat. Rev. Mol. Cell Biol. 2018, 19, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Hillion, J.; Canals, M.; Torvinen, M.; Casadó, V.; Scott, R.; Terasmaa, A.; Hansson, A.; Watson, S.; Olah, M.E.; Mallol, J.; et al. Coaggregation, Cointernalization, and Codesensitization of Adenosine A2A Receptors and Dopamine D2 Receptors. J. Biol. Chem. 2002, 277, 18091–18097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canals, M.; Marcellino, D.; Fanelli, F.; Ciruela, F.; De Benedetti, P.; Goldberg, S.R.; Neve, K.; Fuxe, K.; Agnati, L.F.; Woods, A.S.; et al. Adenosine A2A-Dopamine D2 Receptor-Receptor Heteromerization: Qualitative and Quantitative Assessment by Fluorescence and Bioluminescence Energy Transfer. J. Biol. Chem. 2003, 278, 46741–46749. [Google Scholar] [CrossRef] [Green Version]
- Fuxe, K.; Ferré, S.; Snaprud, P.; von Euler, G.; Johansson, B.; Ferdholm, B. Antagonistic A2a/D2 Receptor Interactions in the Striatum as a Basis for Adenosine/Dopamine Interactions in the Central Nervous System. Drug Dev. Res. 1993, 28, 374–380. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Fuxe, K. Adenosine Heteroreceptor Complexes in the Basal Ganglia Are Implicated in Parkinson’s Disease and Its Treatment. J. Neural Transm. 2019, 126, 455–471. [Google Scholar] [CrossRef] [Green Version]
- Bara-Jimenez, W.; Sherzai, A.; Dimitrova, T.; Favit, A.; Bibbiani, F.; Gillespie, M.; Morris, M.J.; Mouradian, M.M.; Chase, T.N. Adenosine A(2A) Receptor Antagonist Treatment of Parkinson’s Disease. Neurology 2003, 61, 293–296. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Wydra, K.; Pintsuk, J.; Narvaez, M.; Corrales, F.; Zaniewska, M.; Agnati, L.F.; Franco, R.; Tanganelli, S.; Ferraro, L.; et al. Understanding the Functional Plasticity in Neural Networks of the Basal Ganglia in Cocaine Use Disorder: A Role for Allosteric Receptor-Receptor Interactions in A2A-D2 Heteroreceptor Complexes. Neural Plast. 2016, 2016, 4827268. [Google Scholar] [CrossRef] [Green Version]
- Portoghese, P.S.; Ronsisvalle, G.; Larson, D.L.; Yim, C.B.; Sayre, L.M.; Takemori, A.E. Opioid Agonist and Antagonist Bivalent Ligands as Receptor Probes. Life Sci. 1982, 31, 1283–1286. [Google Scholar] [CrossRef]
- Erez, M.; Takemori, A.E.; Portoghese, P.S. Narcotic Antagonistic Potency of Bivalent Ligands Which Contain Beta-Naltrexamine. Evidence for Bridging between Proximal Recognition Sites. J. Med. Chem. 1982, 25, 847–849. [Google Scholar] [CrossRef]
- Shonberg, J.; Scammells, P.J.; Capuano, B. Design Strategies for Bivalent Ligands Targeting GPCRs. ChemMedChem 2011, 6, 963–974. [Google Scholar] [CrossRef]
- Dietis, N.; Guerrini, R.; Calo, G.; Salvadori, S.; Rowbotham, D.J.; Lambert, D.G. Simultaneous Targeting of Multiple Opioid Receptors: A Strategy to Improve Side-Effect Profile. Br. J. Anaesth. 2009, 103, 38–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; St. Onge, C.M.; Ma, H.; Zhang, Y. Design of Bivalent Ligands Targeting Putative GPCR Dimers. Drug Discov. Today 2021, 26, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Mantas, I.; Saarinen, M.; Xu, Z.Q.D.; Svenningsson, P. Update on GPCR-Based Targets for the Development of Novel Antidepressants. Mol. Psychiatry 2021, 27, 534–558. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.H.; Battiti, F.O.; Bonifazi, A. 2016 Philip, S. Portoghese Medicinal Chemistry Lectureship: Designing Bivalent or Bitopic Molecules for G-Protein Coupled Receptors. The Whole Is Greater Than the Sum of Its Parts. J. Med. Chem. 2020, 63, 1779–1797. [Google Scholar] [CrossRef] [PubMed]
- Sperk, G.; Hamilton, T.; Colmers, W.F. Neuropeptide Y in the Dentate Gyrus. Prog. Brain Res. 2007, 163, 285–297. [Google Scholar] [CrossRef]
- Hübner, H.; Schellhorn, T.; Gienger, M.; Schaab, C.; Kaindl, J.; Leeb, L.; Clark, T.; Moller, D.; Gmeiner, P. Structure-Guided Development of Heterodimer-Selective GPCR Ligands. Nat. Commun. 2016, 7, 12298. [Google Scholar] [CrossRef] [Green Version]
- Daniels, D.J.; Lenard, N.R.; Etienne, C.L.; Law, P.Y.; Roerig, S.C.; Portoghese, P.S. Opioid-Induced Tolerance and Dependence in Mice Is Modulated by the Distance between Pharmacophores in a Bivalent Ligand Series. Proc. Natl. Acad. Sci. USA 2005, 102, 19208–19213. [Google Scholar] [CrossRef] [Green Version]
- McRobb, F.M.; Crosby, I.T.; Yuriev, E.; Lane, J.R.; Capuano, B. Homobivalent Ligands of the Atypical Antipsychotic Clozapine: Design, Synthesis, and Pharmacological Evaluation. J. Med. Chem. 2012, 55, 1622–1634. [Google Scholar] [CrossRef]
- Hasbi, A.; Perreault, M.L.; Shen, M.Y.F.; Zhang, L.; To, R.; Fan, T.; Nguyen, T.; Ji, X.; O’Dowd, B.F.; George, S.R. A Peptide Targeting an Interaction Interface Disrupts the Dopamine D1-D2 Receptor Heteromer to Block Signaling and Function in Vitro and in Vivo: Effective Selective Antagonism. FASEB J. 2014, 28, 4806–4820. [Google Scholar] [CrossRef] [Green Version]
- Kamal, M.; Jockers, R. Bitopic Ligands: All-in-One Orthosteric and Allosteric. F1000 Biol. Rep. 2009, 1, 77. [Google Scholar] [CrossRef] [PubMed]
- Flores-Burgess, A.; Millón, C.; Gago, B.; García-Durán, L.; Cantero-García, N.; Puigcerver, A.; Narváez, J.A.; Fuxe, K.; Santín, L.; Díaz-Cabiale, Z. Galanin (1-15) Enhances the Behavioral Effects of Fluoxetine in the Olfactory Bulbectomy Rat, Suggesting a New Augmentation Strategy in Depression. Int. J. Neuropsychopharmacol. 2021, 25, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Wisler, J.W.; Rockman, H.A.; Lefkowitz, R.J. Biased G Protein-Coupled Receptor Signaling. Circulation 2018, 137, 2315–2317. [Google Scholar] [CrossRef] [PubMed]
- Anis, N.A.; Berry, S.C.; Burton, N.R.; Lodge, D. The Dissociative Anaesthetics, Ketamine and Phencyclidine, Selectively Reduce Excitation of Central Mammalian Neurones by N-methyl-aspartate. Br. J. Pharmacol. 1983, 79, 565–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krystal, J.H.; Karper, L.P.; Seibyl, J.P.; Freeman, G.K.; Delaney, R.; Bremner, J.D.; Heninger, G.R.; Bowers, M.B.; Charney, D.S. Subanesthetic Effects of the Noncompetitive NMDA Antagonist, Ketamine, in Humans: Psychotomimetic, Perceptual, Cognitive, and Neuroendocrine Responses. Arch. Gen. Psychiatry 1994, 51, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant Effects of Ketamine in Depressed Patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Zarate, C.A.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Dennis, M.; Charney, S.; Manji, H.K. A Randomized Trial of an N-Methyl-D-Aspartate Antagonist in Treatment-Resistant Major Depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef]
- Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Green, C.E.; Perez, A.M.; Iqbal, S.; Pillemer, S.; Foulkes, A.; Shah, A.; et al. Antidepressant Efficacy of Ketamine in Treatment-Resistant Major Depression: A Two-Site Randomized Controlled Trial. Am. J. Psychiatry 2013, 170, 1134–1142. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Sanacora, G.; Duman, R.S.; Krystal, J.H. Ketamine and Rapid-Acting Antidepressants: A Window into a New Neurobiology for Mood Disorder Therapeutics. Annu. Rev. Med. 2015, 66, 509–523. [Google Scholar] [CrossRef] [Green Version]
- Corriger, A.; Pickering, G. Ketamine and Depression: A Narrative Review. Drug Des. Devel. Ther. 2019, 13, 3051–3067. [Google Scholar] [CrossRef] [Green Version]
- Marcantoni, W.S.; Akoumba, B.S.; Wassef, M.; Mayrand, J.; Lai, H.; Richard-Devantoy, S.; Beauchamp, S. A Systematic Review and Meta-Analysis of the Efficacy of Intravenous Ketamine Infusion for Treatment Resistant Depression: January 2009–January 2019. J. Affect. Disord. 2020, 277, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Conley, A.A.; Norwood, A.E.Q.; Hatvany, T.C.; Griffith, J.D.; Barber, K.E. Efficacy of Ketamine for Major Depressive Episodes at 2, 4, and 6-Weeks Post-Treatment: A Meta-Analysis. Psychopharmacology 2021, 238, 1737–1752. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Brain-Derived Neurotrophic Factor-TrkB Signaling and the Mechanism of Antidepressant Activity by Ketamine in Mood Disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 137–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, K. Molecular Mechanisms of the Rapid-Acting and Long-Lasting Antidepressant Actions of (R)-Ketamine. Biochem. Pharmacol. 2020, 177, 113935. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.; Zarate, C.A.; Du, J.; Schloesser, R.J.; McCammon, J.; Chen, G.; Manji, H.K. Cellular Mechanisms Underlying the Antidepressant Effects of Ketamine: Role of α-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptors. Biol. Psychiatry 2008, 63, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Iijima, M.; Chaki, S. Involvement of AMPA Receptor in Both the Rapid and Sustained Antidepressant-like Effects of Ketamine in Animal Models of Depression. Behav. Brain Res. 2011, 224, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Monteggia, L.M. A Neurotrophic Model for Stress-Related Mood Disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Li, N.; Lee, B.; Liu, R.J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.Y.; Aghajanian, G.; Duman, R.S. MTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Lepack, A.E.; Fuchikami, M.; Dwyer, J.M.; Banasr, M.; Duman, R.S. BDNF Release Is Required for the Behavioral Actions of Ketamine. Int. J. Neuropsychopharmacol. 2014, 18, pyu033. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Song, Y.; Zhang, X.; Zhao, W.; Ma, T.; Liu, Y.; Ma, P.; Zhao, Y.; Zhang, H. Ketamine Relieves Depression-like Behaviors Induced by Chronic Postsurgical Pain in Rats through Anti-Inflammatory, Anti-Oxidant Effects and Regulating BDNF Expression. Psychopharmacology 2020, 237, 1657–1669. [Google Scholar] [CrossRef]
- Garcia, L.S.B.; Comim, C.M.; Valvassori, S.S.; Réus, G.Z.; Barbosa, L.M.; Andreazza, A.C.; Stertz, L.; Fries, G.R.; Gavioli, E.C.; Kapczinski, F.; et al. Acute Administration of Ketamine Induces Antidepressant-like Effects in the Forced Swimming Test and Increases BDNF Levels in the Rat Hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.F.; Kavalali, E.T.; Monteggia, L.M. NMDA Receptor Blockade at Rest Triggers Rapid Behavioural Antidepressant Responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant Drugs Act by Directly Binding to TRKB Neurotrophin Receptors. Cell 2021, 184, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Rybakowski, J.K.; Permoda-Osip, A.; Skibinska, M.; Adamski, R.; Bartkowska-Sniatkowska, A. Single Ketamine Infusion in Bipolar Depression Resistant to Antidepressants: Are Neurotrophins Involved? Hum. Psychopharmacol. 2013, 28, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Haile, C.N.; Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Foulkes, A.; Iqbal, S.; Mahoney, J.J.; De La Garza, R.; Charney, D.S.; et al. Plasma Brain Derived Neurotrophic Factor (BDNF) and Response to Ketamine in Treatment-Resistant Depression. Int. J. Neuropsychopharmacol. 2014, 17, 331–336. [Google Scholar] [CrossRef]
- Slipczuk, L.; Bekinschtein, P.; Katche, C.; Cammarota, M.; Izquierdo, I.; Medina, J.H. BDNF Activates MTOR to Regulate GluR1 Expression Required for Memory Formation. PLoS ONE 2009, 4, e6007. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Ahn, K.H.; Averill, L.A.; Nemati, S.; Averill, C.L.; Fouda, S.; Ranganathan, M.; Morgan, P.T.; D’Souza, D.C.; Mathalon, D.H.; et al. A Robust and Reproducible Connectome Fingerprint of Ketamine Is Highly Associated with the Connectomic Signature of Antidepressants. Neuropsychopharmacology 2021, 46, 478–485. [Google Scholar] [CrossRef]
- Segal, R.A. Selectivity in Neurotrophin Signaling: Theme and Variations. Annu. Rev. Neurosci. 2003, 26, 299–330. [Google Scholar] [CrossRef]
- Reichardt, L.F. Neurotrophin-Regulated Signalling Pathways. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; You, Y.; Gupta, V.B.; Klistorner, A.; Graham, S.L. TrkB Receptor Signalling: Implications in Neurodegenerative, Psychiatric and Proliferative Disorders. Int. J. Mol. Sci. 2013, 14, 10122–10142. [Google Scholar] [CrossRef]
- Yang, C.; Ren, Q.; Qu, Y.; Zhang, J.C.; Ma, M.; Dong, C.; Hashimoto, K. Mechanistic Target of Rapamycin—Independent Antidepressant Effects of (R)-Ketamine in a Social Defeat Stress Model. Biol. Psychiatry 2018, 83, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapur, S.; Seeman, P. NMDA Receptor Antagonists Ketamine and PCP Have Direct Effects on the Dopamine D2 and Serotonin 5-HT2 Receptors—Implications for Models of Schizophrenia. Mol. Psychiatry 2002, 7, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Hirota, K.; Hashimoto, Y.; Lambert, D.G. Interaction of Intravenous Anesthetics with Recombinant Human M1-M3 Muscarinic Receptors Expressed in Chinese Hamster Ovary Cells. Anesth. Analg. 2002, 95, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Uezono, Y. The Recent Progress in Research on Effects of Anesthetics and Analgesics on G Protein-Coupled Receptors. J. Anesth. 2013, 27, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Perez-Aguilar, J.M.; Gao, L.; Saven, J.G.; Matsunami, H.; Eckenhoff, R.G. Molecular Recognition of Ketamine by a Subset of Olfactory G Protein-Coupled Receptors. Sci. Signal. 2015, 8, ra33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saarelainen, T.; Hendolin, P.; Lucas, G.; Koponen, E.; Sairanen, M.; MacDonald, E.; Agerman, K.; Haapasalo, A.; Nawa, H.; Aloyz, R.; et al. Activation of the TrkB Neurotrophin Receptor Is Induced by Antidepressant Drugs and Is Required for Antidepressant-Induced Behavioral Effects. J. Neurosci. 2003, 23, 349–357. [Google Scholar] [CrossRef]
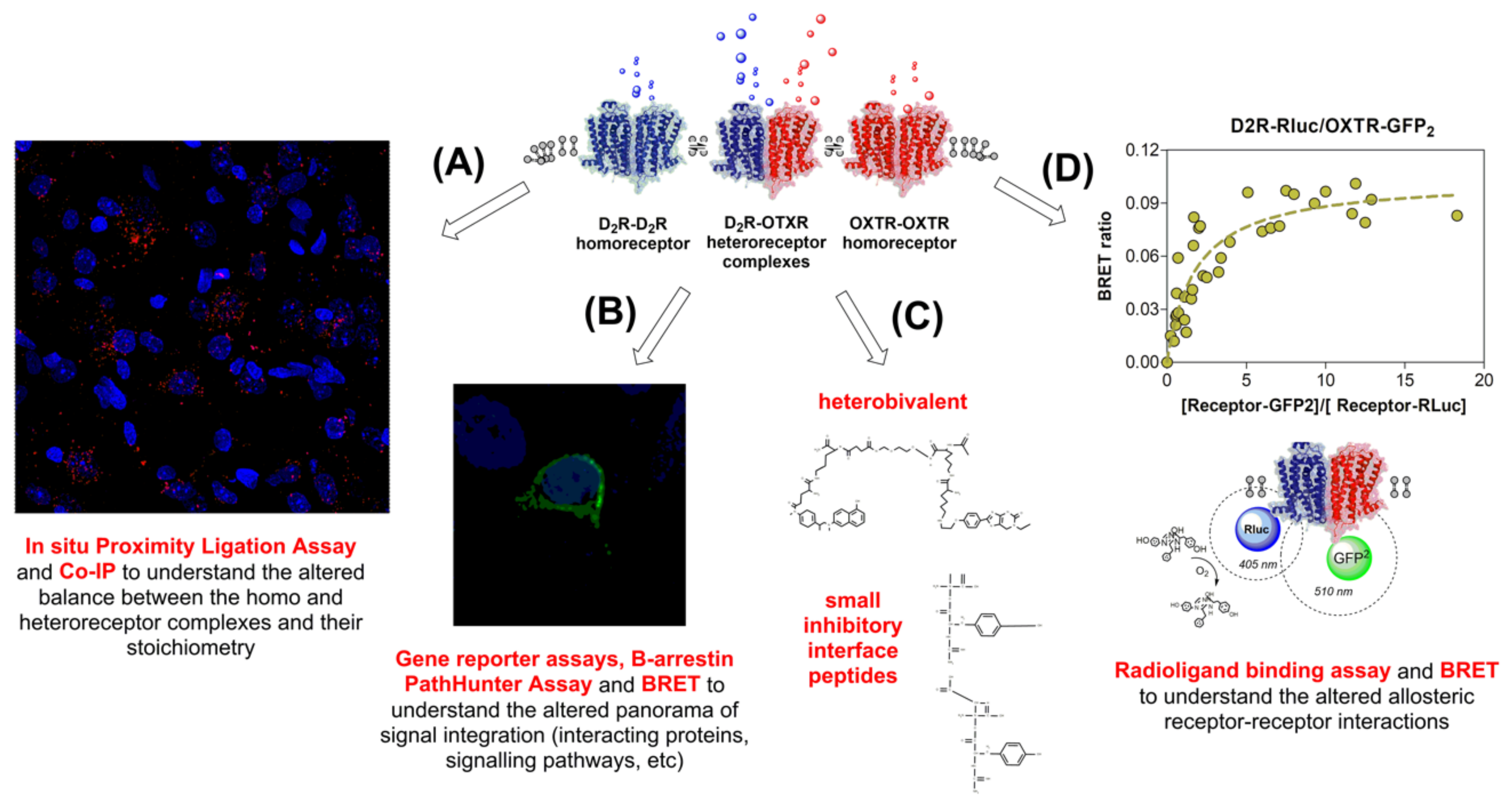
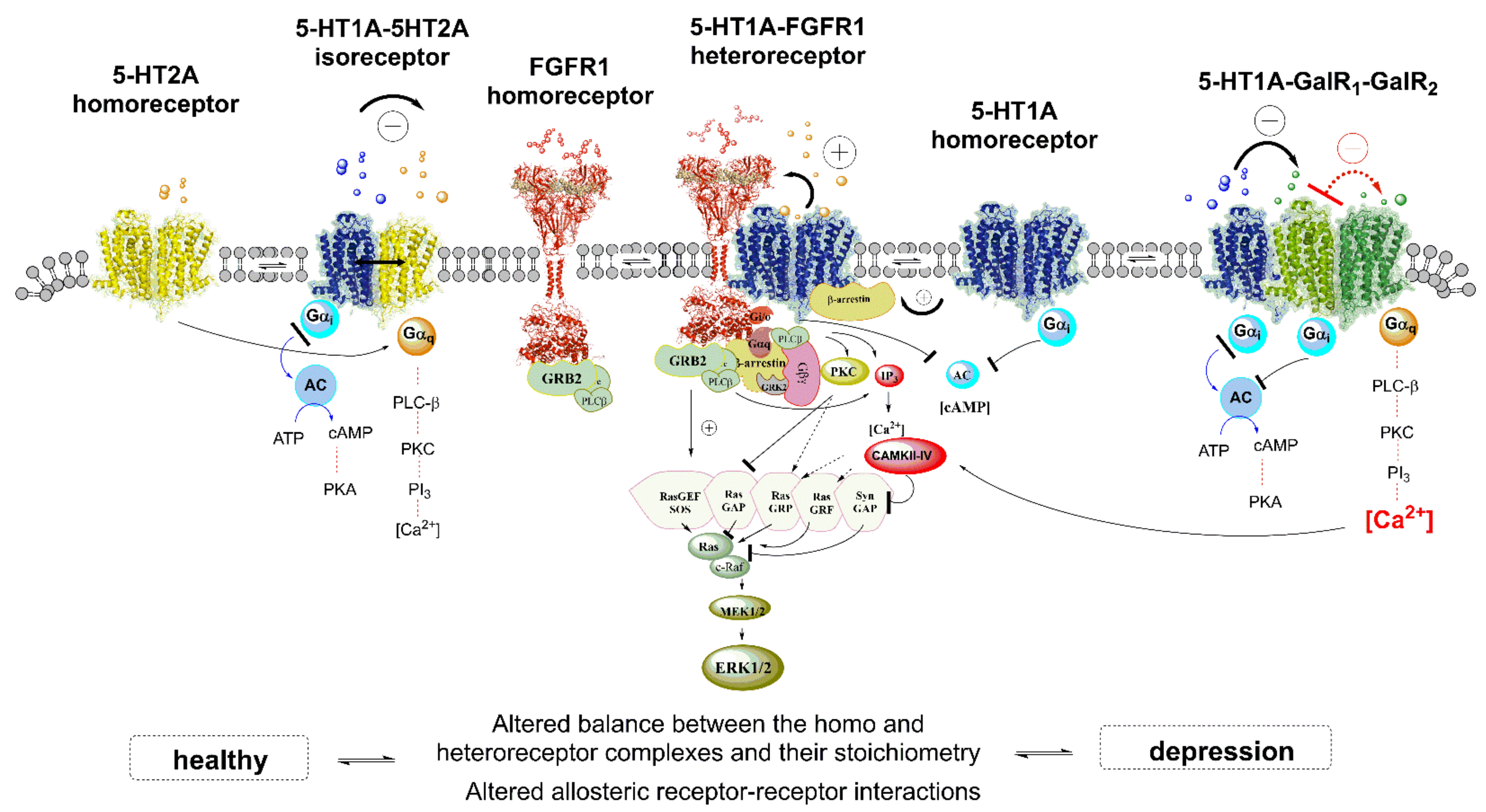
| Treatment Strategies Related to Heteroreceptor Complexes | ||||
|---|---|---|---|---|
| Strategy | Outcome | Putative Heteroreceptor Examples | Disorder | Reference |
| Selective protomer ligands | Induced oligomer formation | D2R-OXTR | Anxiety | [64] |
| Gal1R-Gal2R-5-HT1AR | Depression and anxiety | [40,110,130,135,229,355] | ||
| DOR-MOR | Depression, anxiety, and nociception | [205,206,207,208] | ||
| A2AR-D2R | Parkinson’s disease and schizophrenia | [75,90,95,198,338,339,340,341] | ||
| Biased ligands | Cross-talk effects between protomers | [103,334,335,356] | ||
| Homo-/Hetero-receptor bivalent compounds | Facilitatory/inhibitory effects by binding on both protomeric recognition sites | KOR-MOR | Nociception | [351] |
| MOR-mGluR5R | Nociception | [5] | ||
| Disrupting homo/heteroreceptor peptides | Disruption of constitutive heteroreceptor complexes | D1R-D2R | Depression and anxiety | [183,184,200] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez de la Mora, M.; Borroto-Escuela, D.O.; Crespo-Ramírez, M.; Rejón-Orantes, J.d.C.; Palacios-Lagunas, D.A.; Martínez-Mata, M.K.; Sánchez-Luna, D.; Tesoro-Cruz, E.; Fuxe, K. Dysfunctional Heteroreceptor Complexes as Novel Targets for the Treatment of Major Depressive and Anxiety Disorders. Cells 2022, 11, 1826. https://doi.org/10.3390/cells11111826
Pérez de la Mora M, Borroto-Escuela DO, Crespo-Ramírez M, Rejón-Orantes JdC, Palacios-Lagunas DA, Martínez-Mata MK, Sánchez-Luna D, Tesoro-Cruz E, Fuxe K. Dysfunctional Heteroreceptor Complexes as Novel Targets for the Treatment of Major Depressive and Anxiety Disorders. Cells. 2022; 11(11):1826. https://doi.org/10.3390/cells11111826
Chicago/Turabian StylePérez de la Mora, Miguel, Dasiel O. Borroto-Escuela, Minerva Crespo-Ramírez, José del Carmen Rejón-Orantes, Daniel Alejandro Palacios-Lagunas, Magda K. Martínez-Mata, Daniela Sánchez-Luna, Emiliano Tesoro-Cruz, and Kjell Fuxe. 2022. "Dysfunctional Heteroreceptor Complexes as Novel Targets for the Treatment of Major Depressive and Anxiety Disorders" Cells 11, no. 11: 1826. https://doi.org/10.3390/cells11111826
APA StylePérez de la Mora, M., Borroto-Escuela, D. O., Crespo-Ramírez, M., Rejón-Orantes, J. d. C., Palacios-Lagunas, D. A., Martínez-Mata, M. K., Sánchez-Luna, D., Tesoro-Cruz, E., & Fuxe, K. (2022). Dysfunctional Heteroreceptor Complexes as Novel Targets for the Treatment of Major Depressive and Anxiety Disorders. Cells, 11(11), 1826. https://doi.org/10.3390/cells11111826







