A Small Peptide Targeting the Ligand-Induced Androgen Receptor/Filamin a Interaction Inhibits the Invasive Phenotype of Prostate Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Constructs
2.2. Cell Cultures
2.3. Transfection, Transactivation and siRNA Experiments
2.4. Immunofluorescence (IF), DNA Synthesis, WST-1 and Prostate Specific Antigen (PSA) Assays
2.5. Wound Scratch and Transwell Assays
2.6. Establishment of Spheroids in ECM and Spheroid’s Viability Analysis
2.7. Lysates, Immune-Precipitation (IP), Co-Immune-Precipitation (Co-IP), and Western Blot (WB)
3. Results
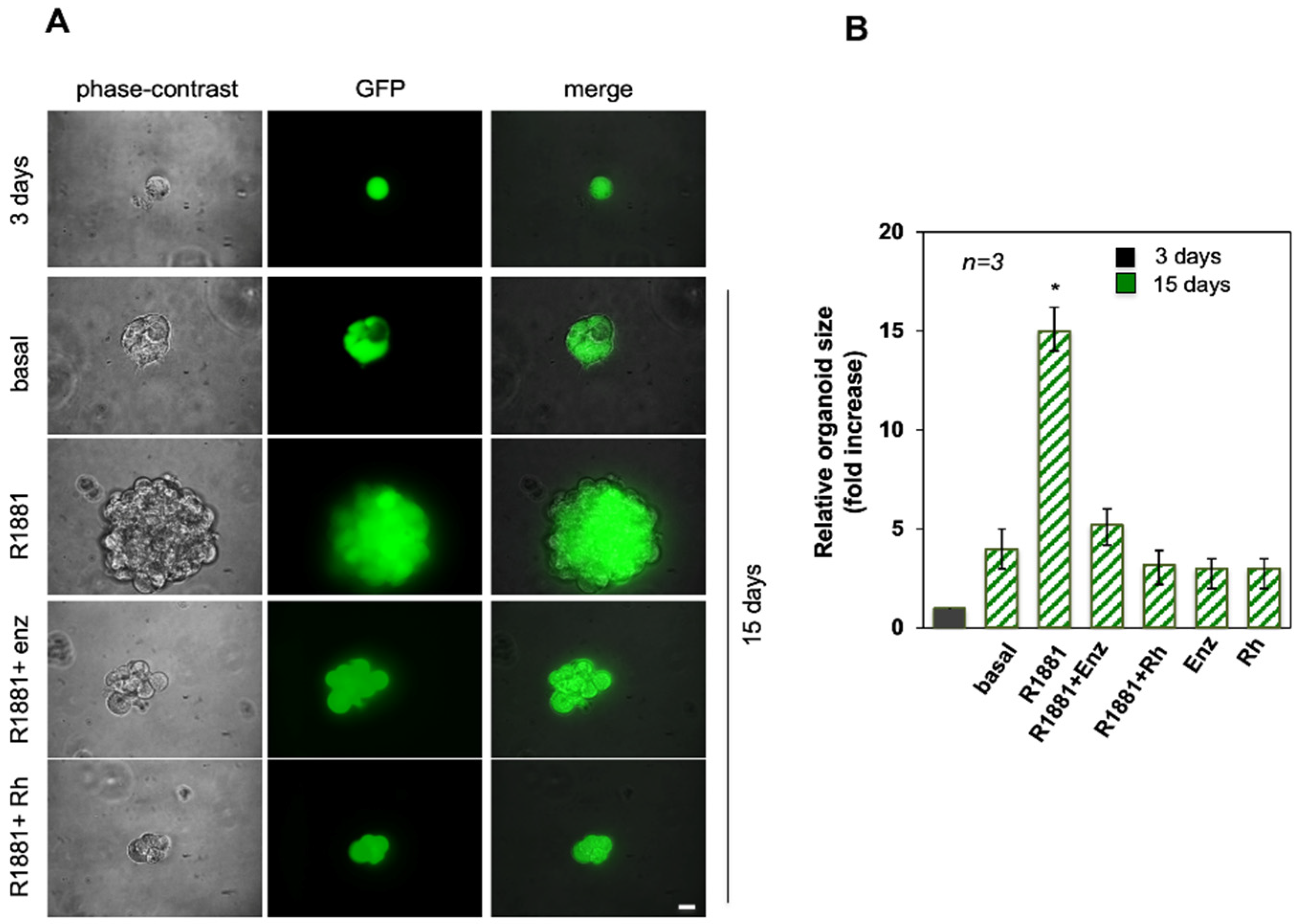
3.1. Androgen-Increased Growth of LNCaP Cell Spheroid Is Inhibited by the RH-2025u Peptide
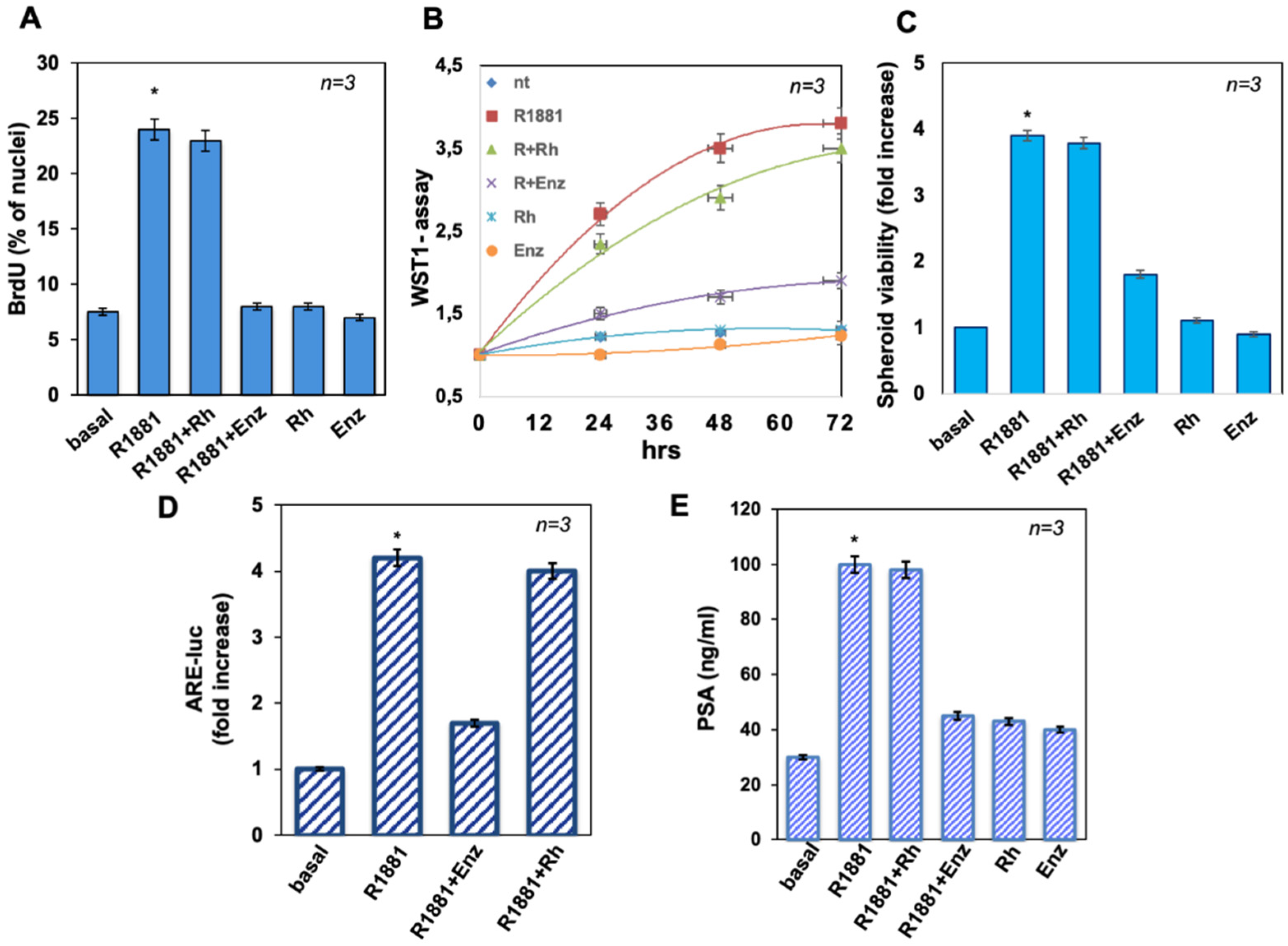
3.2. The Peptide Treatment Does Not Affect LNCaP Cell Proliferation in 2D and 3D Models Nor AR Induced Transcriptional Activity
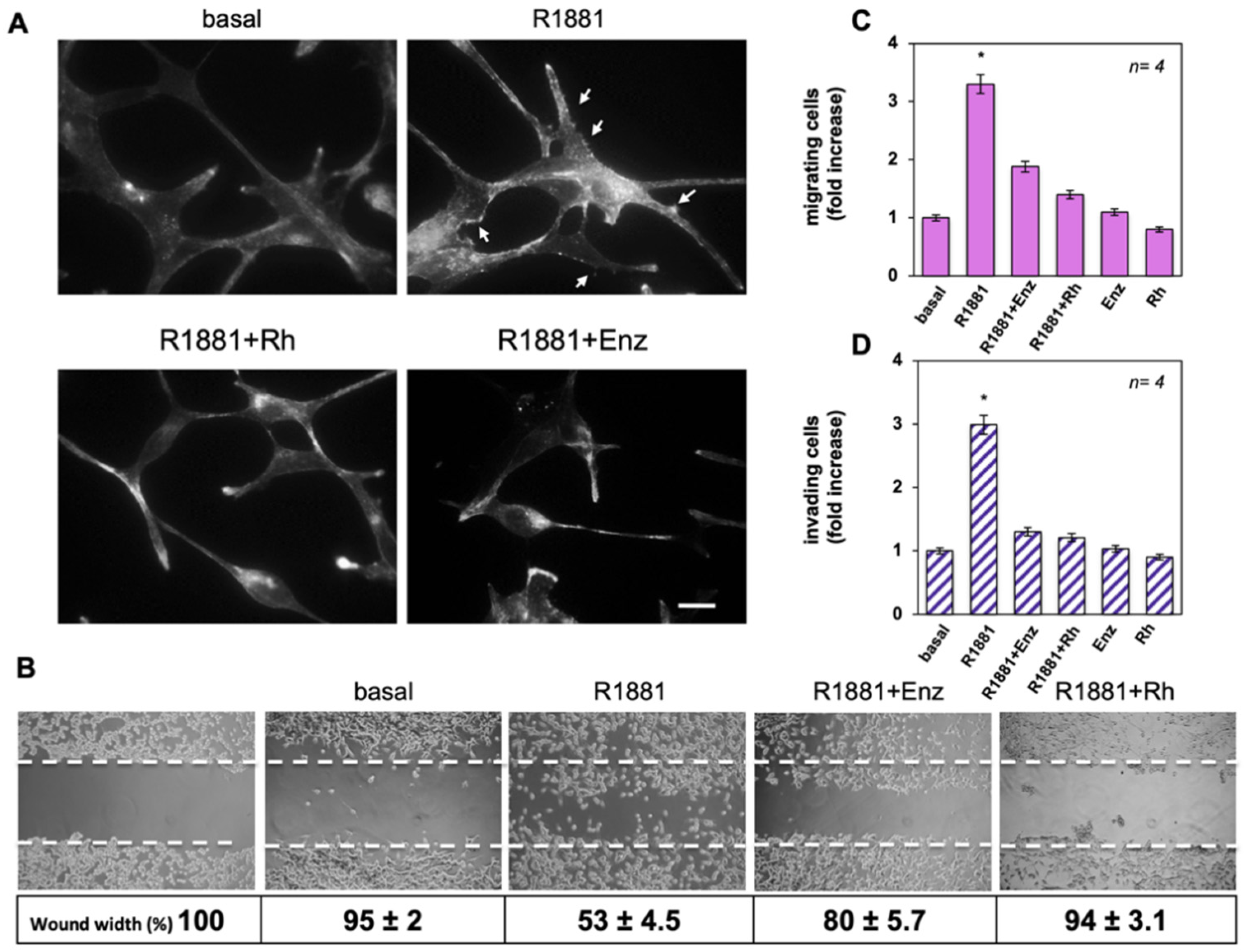
3.3. The Rh-2025u Abolishes the Androgen Stimulated Motility and Invasion of LNCaP Cells
3.4. The Rh-2025u Abolishes the Androgen Stimulated Motility and Invasion of AR Expressing Cells but Not of PC Cells That Do Not Express AR
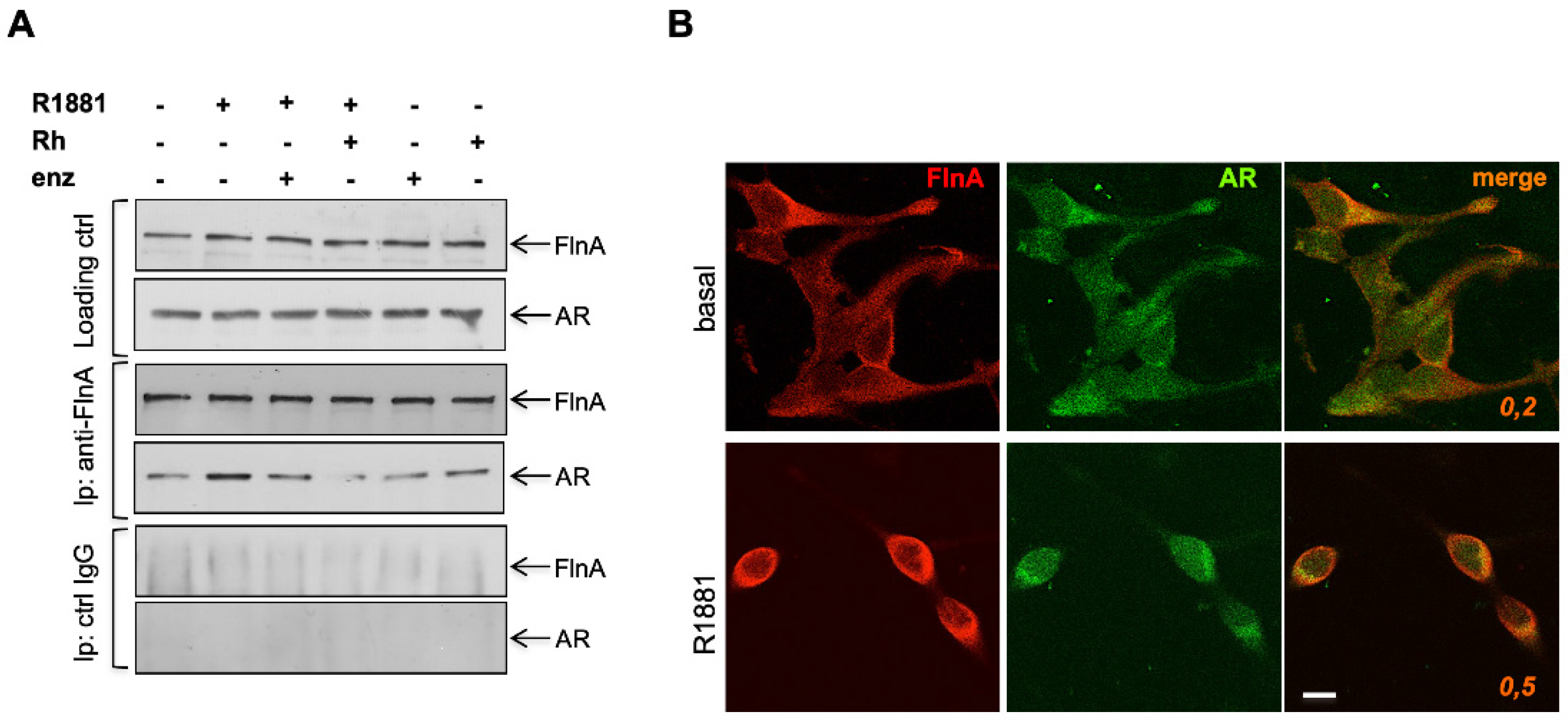
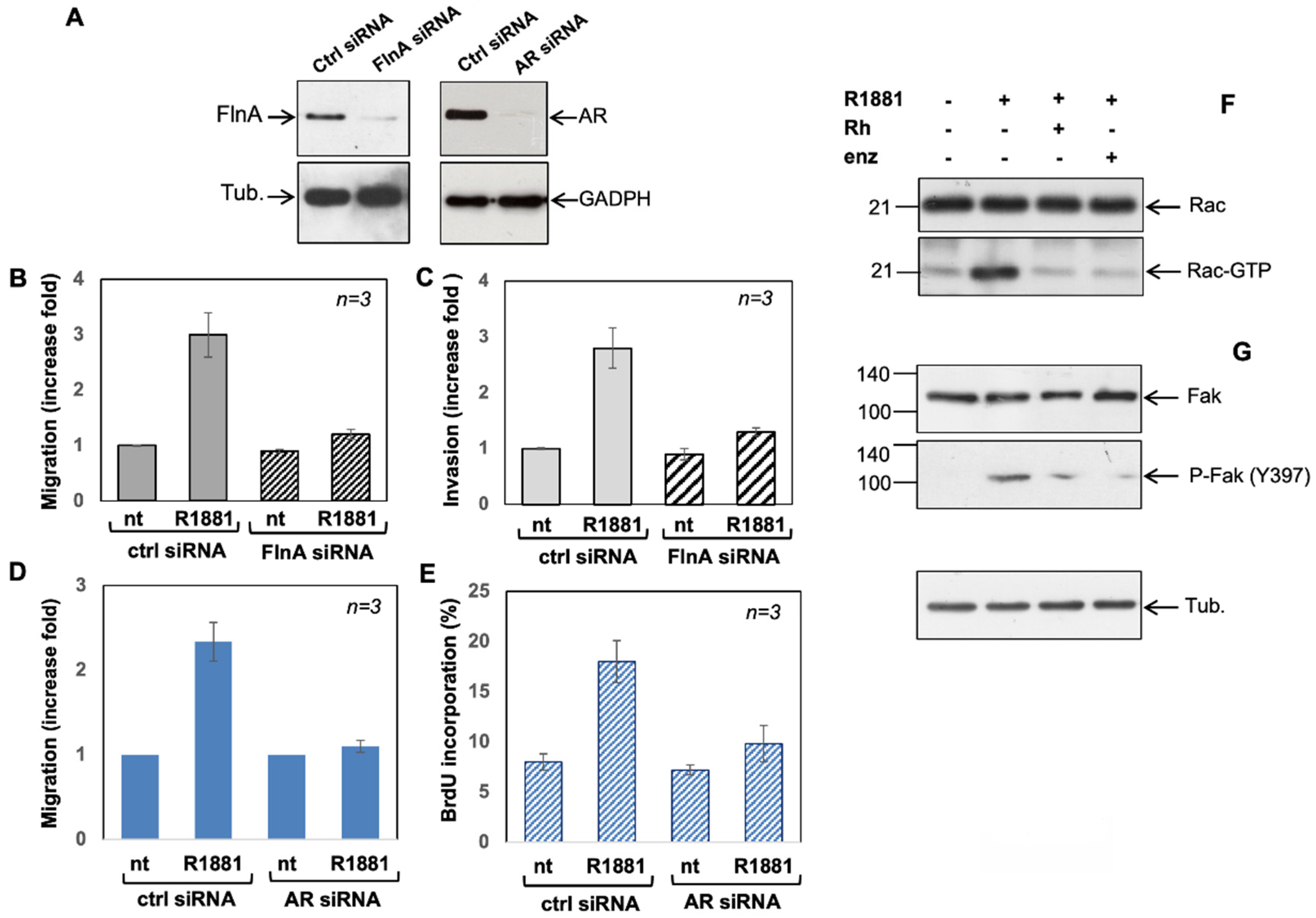
3.5. Filamin A and AR Are Both Required for LNCaP Cell Motility Induction by a Mechanism Involving Rac Activation and Fak Phosphorylation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eikenberry, S.E.; Nagy, J.D.; Kuang, Y. The Evolutionary Impact of Androgen Levels on Prostate Cancer in a Multi-Scale Mathematical Model. Biol. Direct. 2010, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Ku, S.-Y.; Gleave, M.E.; Beltran, H. Towards Precision Oncology in Advanced Prostate Cancer. Nat. Rev. Urol. 2019, 16, 645–654. [Google Scholar] [CrossRef]
- Stossel, T.P.; Condeelis, J.; Cooley, L.; Hartwig, J.H.; Noegel, A.; Schleicher, M.; Shapiro, S.S. Filamins as Integrators of Cell Mechanics and Signalling. Nat. Rev. Mol. Cell Biol. 2001, 2, 138–145. [Google Scholar] [CrossRef]
- Savoy, R.M.; Ghosh, P.M. The Dual Role of Filamin A in Cancer: Can’t Live with (Too Much of) It, Can’t Live without It. Endocr. Relat. Cancer 2013, 20, R341–R356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castoria, G.; D’Amato, L.; Ciociola, A.; Giovannelli, P.; Giraldi, T.; Sepe, L.; Paolella, G.; Barone, M.V.; Migliaccio, A.; Auricchio, F. Androgen-Induced Cell Migration: Role of Androgen Receptor/Filamin A Association. PLoS ONE 2011, 6, e17218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castoria, G.; Giovannelli, P.; Di Donato, M.; Ciociola, A.; Hayashi, R.; Bernal, F.; Appella, E.; Auricchio, F.; Migliaccio, A. Role of Non-Genomic Androgen Signalling in Suppressing Proliferation of Fibroblasts and Fibrosarcoma Cells. Cell Death. Dis. 2014, 5, e1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Donato, M.; Bilancio, A.; D’Amato, L.; Claudiani, P.; Oliviero, M.A.; Barone, M.V.; Auricchio, A.; Appella, E.; Migliaccio, A.; Auricchio, F.; et al. Cross-Talk between Androgen Receptor/Filamin A and TrkA Regulates Neurite Outgrowth in PC12 Cells. Mol. Biol. Cell 2015, 26, 2858–2872. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Zamagni, A.; Galasso, G.; Di Zazzo, E.; Giovannelli, P.; Barone, M.V.; Zanoni, M.; Gunelli, R.; Costantini, M.; Auricchio, F.; et al. The Androgen Receptor/Filamin A Complex as a Target in Prostate Cancer Microenvironment. Cell Death. Dis. 2021, 12, 127. [Google Scholar] [CrossRef]
- Chang, C.S.; Kokontis, J.; Liao, S. Molecular Cloning of Human and Rat Complementary DNA Encoding Androgen Receptors. Science 1988, 240, 324–326. [Google Scholar] [CrossRef] [Green Version]
- Verrijdt, G.; Schoenmakers, E.; Haelens, A.; Peeters, B.; Verhoeven, G.; Rombauts, W.; Claessens, F. Change of Specificity Mutations in Androgen-Selective Enhancers: Evidence for a role of differential dna binding by the androgen receptor. J. Biol. Chem. 2000, 275, 12298–12305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeiffer, M.J.; Mulders, P.F.; Schalken, J.A. An in Vitro Model for Preclinical Testing of Endocrine Therapy Combinations for Prostate Cancer. Prostate 2010, 70, 1524–1532. [Google Scholar] [CrossRef]
- Di Donato, M.; Ostacolo, C.; Giovannelli, P.; Di Sarno, V.; Monterrey, I.M.G.; Campiglia, P.; Migliaccio, A.; Bertamino, A.; Castoria, G. Therapeutic Potential of TRPM8 Antagonists in Prostate Cancer. Sci. Rep. 2021, 11, 23232. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Cernera, G.; Migliaccio, A.; Castoria, G. Nerve Growth Factor Induces Proliferation and Aggressiveness in Prostate Cancer Cells. Cancers 2019, 11, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castoria, G.; Lombardi, M.; Barone, M.V.; Bilancio, A.; Di Domenico, M.; Bottero, D.; Vitale, F.; Migliaccio, A.; Auricchio, F. Androgen-Stimulated DNA Synthesis and Cytoskeletal Changes in Fibroblasts by a Nontranscriptional Receptor Action. J. Cell Biol. 2003, 161, 547–556. [Google Scholar] [CrossRef]
- Pagano, M.; Naviglio, S.; Spina, A.; Chiosi, E.; Castoria, G.; Romano, M.; Sorrentino, A.; Illiano, F.; Illiano, G. Differentiation of H9c2 Cardiomyoblasts: The Role of Adenylate Cyclase System. J. Cell Physiol. 2004, 198, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, P.; Di Donato, M.; Auricchio, F.; Castoria, G.; Migliaccio, A. Androgens Induce Invasiveness of Triple Negative Breast Cancer Cells Through AR/Src/PI3-K Complex Assembly. Sci. Rep. 2019, 9, 4490. [Google Scholar] [CrossRef] [Green Version]
- Gingrich, J.R.; Tucker, J.A.; Walther, P.J.; Day, J.W.; Poulton, S.H.M.; Webb, K.S. Establishment and Characterization of a New Human Prostatic Carcinoma Cell Line (DuPro-1). J. Urol. 1991, 146, 915–919. [Google Scholar] [CrossRef]
- Sharp, A.; Coleman, I.; Yuan, W.; Sprenger, C.; Dolling, D.; Rodrigues, D.N.; Russo, J.W.; Figueiredo, I.; Bertan, C.; Seed, G.; et al. Androgen Receptor Splice Variant-7 Expression Emerges with Castration Resistance in Prostate Cancer. J. Clin. Investig. 2019, 129, 192–208. [Google Scholar] [CrossRef] [Green Version]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [Green Version]
- Kensler, K.H.; Rebbeck, T.R. Cancer Progress and Priorities: Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, M.; Paller, C.; Kyprianou, N. Mechanisms of Therapeutic Resistance in Prostate Cancer. Curr. Oncol. Rep. 2017, 19, 13. [Google Scholar] [CrossRef] [Green Version]
- Fontana, F.; Limonta, P. Dissecting the Hormonal Signaling Landscape in Castration-Resistant Prostate Cancer. Cells 2021, 10, 1133. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, X.; Liang, X.; Jiang, G. Molecular and Cellular Mechanisms of Castration Resistant Prostate Cancer (Review). Oncol. Lett. 2018, 15, 6063–6076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, N.; Clementino, M.; Kim, D.; Wang, L.; Verma, A.; Shi, X.; Zhang, Z.; DiPaola, R.S. New Developments in Mechanisms of Prostate Cancer Progression. Semin. Cancer Biol. 2019, 57, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Melekhova, A.; Baniahmad, A. ING Tumour Suppressors and ING Splice Variants as Coregulators of the Androgen Receptor Signalling in Prostate Cancer. Cells 2021, 10, 2599. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-J.; Lan, S.-W.; Lu, Y.-C.; Cheng, T.-S.; Lai, P.-F.; Tsai, C.-H.; Hsu, T.-W.; Lin, H.-Y.; Shyu, H.-Y.; Wu, S.-R.; et al. Inhibition of Cyclooxygenase-2-Mediated Matriptase Activation Contributes to the Suppression of Prostate Cancer Cell Motility and Metastasis. Oncogene 2017, 36, 4597–4609. [Google Scholar] [CrossRef] [PubMed]
- Voll, E.A.; Ogden, I.M.; Pavese, J.M.; Huang, X.K.; Xu, L.; Jovanovic, B.D.; Bergan, R.C. Heat Shock Protein 27 Regulates Human Prostate Cancer Cell Motility and Metastatic Progression. Oncotarget 2014, 5, 2648–2663. [Google Scholar] [CrossRef] [Green Version]
- Gkika, D.; Flourakis, M.; Lemonnier, L.; Prevarskaya, N. PSA Reduces Prostate Cancer Cell Motility by Stimulating TRPM8 Activity and Plasma Membrane Expression. Oncogene 2010, 29, 4611–4616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drivalos, A.; Chrisofos, M.; Efstathiou, E.; Kapranou, A.; Kollaitis, G.; Koutlis, G.; Antoniou, N.; Karanastasis, D.; Dimopoulos, M.A.; Bamias, A. Expression of A5-Integrin, A7-Integrin, Ε-Cadherin, and N-Cadherin in Localized Prostate Cancer. Urol. Oncol. 2016, 34, 165.e11–165.e18. [Google Scholar] [CrossRef]
- Burdelski, C.; Fitzner, M.; Hube-Magg, C.; Kluth, M.; Heumann, A.; Simon, R.; Krech, T.; Clauditz, T.; Büscheck, F.; Steurer, S.; et al. Overexpression of the A Disintegrin and Metalloproteinase ADAM15 Is Linked to a Small but Highly Aggressive Subset of Prostate Cancers. Neoplasia 2017, 19, 279–287. [Google Scholar] [CrossRef]
- Bilgin Doğru, E.; Dizdar, Y.; Akşit, E.; Ural, F.; Şanlı, Ö.; Yasasever, V. EMMPRIN and ADAM12 in Prostate Cancer: Preliminary Results of a Prospective Study. Tumor. Biol. 2014, 35, 11647–11653. [Google Scholar] [CrossRef]
- Baspinar, S.; Bircan, S.; Ciris, M.; Karahan, N.; Bozkurt, K.K. Expression of NGF, GDNF and MMP-9 in Prostate Carcinoma. Pathol. Res. Pract. 2017, 213, 483–489. [Google Scholar] [CrossRef]
- Dalal, R.; Djakiew, D. Molecular Characterization of Neurotrophin Expression and the Corresponding Tropomyosin Receptor Kinases (Trks) in Epithelial and Stromal Cells of the Human Prostate. Mol. Cell Endocrinol. 1997, 134, 15–22. [Google Scholar] [CrossRef]
- Di Donato, M.; Cernera, G.; Auricchio, F.; Migliaccio, A.; Castoria, G. Cross-Talk between Androgen Receptor and Nerve Growth Factor Receptor in Prostate Cancer Cells: Implications for a New Therapeutic Approach. Cell Death. Discov. 2018, 4, 5. [Google Scholar] [CrossRef]
- Sharma, A.; Mendonca, J.; Ying, J.; Kim, H.-S.; Verdone, J.E.; Zarif, J.C.; Carducci, M.; Hammers, H.; Pienta, K.J.; Kachhap, S. The Prostate Metastasis Suppressor Gene NDRG1 Differentially Regulates Cell Motility and Invasion. Mol. Oncol. 2017, 11, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mao, Y.; Wang, H.; Yin, W.; Zhu, S.; Wang, W. miR-124 Suppresses Cell Motility and Adhesion by Targeting Talin 1 in Prostate Cancer Cells. Cancer Cell Int. 2015, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Wu, D.; Xu, B.; Qian, W.; Li, P.; Lu, Q.; Yin, C.; Zhang, W. MicroRNA-133 Inhibits Cell Proliferation, Migration and Invasion in Prostate Cancer Cells by Targeting the Epidermal Growth Factor Receptor. Oncol. Rep. 2012, 27, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- El Haibi, C.P.; Sharma, P.K.; Singh, R.; Johnson, P.R.; Suttles, J.; Singh, S.; Lillard, J.W. PI3Kp110-, Src-, FAK-Dependent and DOCK2-Independent Migration and Invasion of CXCL13-Stimulated Prostate Cancer Cells. Mol. Cancer 2010, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Liang, Y.; Jiang, L.; Wang, H.; Wang, S.; Dong, J. CX3CL1/Fractalkine Enhances Prostate Cancer Spinal Metastasis by Activating the Src/FAK Pathway. Int. J. Oncol. 2018, 53, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wu, J.; Teng, J.; Li, W.; Zheng, J.; Bai, J. HCRP-1 Regulates Cell Migration, Invasion and Angiogenesis via Src/FAK Signaling in Human Prostate Cancer. Int. J. Biol. Sci. 2020, 16, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Aurilio, G.; Cimadamore, A.; Mazzucchelli, R.; Lopez-Beltran, A.; Verri, E.; Scarpelli, M.; Massari, F.; Cheng, L.; Santoni, M.; Montironi, R. Androgen Receptor Signaling Pathway in Prostate Cancer: From Genetics to Clinical Applications. Cells 2020, 9, 2653. [Google Scholar] [CrossRef]
- Migliaccio, A.; Castoria, G.; Di Domenico, M.; de Falco, A.; Bilancio, A.; Lombardi, M.; Barone, M.V.; Ametrano, D.; Zannini, M.S.; Abbondanza, C.; et al. Steroid-Induced Androgen Receptor–Oestradiol Receptor β–Src Complex Triggers Prostate Cancer Cell Proliferation. EMBO J. 2000, 19, 5406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Dalrymple, S.L.; Coleman, I.; Zheng, S.L.; Xu, J.; Hooper, J.E.; Antonarakis, E.S.; De Marzo, A.M.; Meeker, A.K.; Nelson, P.S.; et al. Role of Androgen Receptor Splice Variant-7 (AR-V7) in Prostate Cancer Resistance to 2nd-Generation Androgen Receptor Signaling Inhibitors. Oncogene 2020, 39, 6935–6949. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Monaco, A.; Licitra, F.; Perillo, B.; Migliaccio, A.; Castoria, G. Communication between Cells: Exosomes as a Delivery System in Prostate Cancer. Cell Commun. Signal. 2021, 19, 110. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Donato, M.; Giovannelli, P.; Barone, M.V.; Auricchio, F.; Castoria, G.; Migliaccio, A. A Small Peptide Targeting the Ligand-Induced Androgen Receptor/Filamin a Interaction Inhibits the Invasive Phenotype of Prostate Cancer Cells. Cells 2022, 11, 14. https://doi.org/10.3390/cells11010014
Di Donato M, Giovannelli P, Barone MV, Auricchio F, Castoria G, Migliaccio A. A Small Peptide Targeting the Ligand-Induced Androgen Receptor/Filamin a Interaction Inhibits the Invasive Phenotype of Prostate Cancer Cells. Cells. 2022; 11(1):14. https://doi.org/10.3390/cells11010014
Chicago/Turabian StyleDi Donato, Marzia, Pia Giovannelli, Maria Vittoria Barone, Ferdinando Auricchio, Gabriella Castoria, and Antimo Migliaccio. 2022. "A Small Peptide Targeting the Ligand-Induced Androgen Receptor/Filamin a Interaction Inhibits the Invasive Phenotype of Prostate Cancer Cells" Cells 11, no. 1: 14. https://doi.org/10.3390/cells11010014
APA StyleDi Donato, M., Giovannelli, P., Barone, M. V., Auricchio, F., Castoria, G., & Migliaccio, A. (2022). A Small Peptide Targeting the Ligand-Induced Androgen Receptor/Filamin a Interaction Inhibits the Invasive Phenotype of Prostate Cancer Cells. Cells, 11(1), 14. https://doi.org/10.3390/cells11010014









